Abstract
Background
Rosacea is a common inflammatory skin condition affecting approximately 5% of the world population. Therapeutic approaches to rosacea are focused on symptom suppression by means of anti-inflammatory agents. More recently, photodynamic therapy, especially light-emitting diodes, has been introduced as a valid alternative to conventional therapy.
Case presentation
In the present work, we reported the efficacy and safety of light-emitting diodes therapy combining blue (480 nm) and red (650 nm) light for the treatment of two patients with papulopustular rosacea: a 22-year-old Caucasian woman and a 68-year-old Caucasian man.
Conclusions
This kind of treatment could represent an effective, safer, and well-tolerated approach for the treatment of such conditions.
Keywords: Rosacea, Light-emitting diodes, LED, Photodynamic therapy
Background
Acne rosacea, usually referred to as rosacea, is a common inflammatory skin condition affecting mainly the central face [1]. The term was introduced by Thomas Bateman in the nineteenth century as an acne variety [2]. Its typical manifestations are generalized erythema, telangiectasia, and edema, then papules and pustules or a combination of all [3, 4].
In 2004, the National Rosacea Society (NRS) Expert Committee published a report on the classification and staging of rosacea that defined the criteria for rosacea classification and grading according to primary and secondary descriptors [5]. Four subtypes of rosacea can be recognized on the basis of different morphological characteristics: erythematotelangiectatic, papulopustular, phymatous, and ocular [5, 6]. The erythematotelangiectatic subtype is the most common one followed by papulopustular, phymatous and ocular types which are reported as less common [7]. Data from clinical practice show that patients often can harbor more than one rosacea subtype [7]; for this reason, incidence and prevalence evaluation is not simple. The latest data population, based on published data, refers to an incidence of 1.65 per 1000 persons per year [8] indicating approximately 5.46% of the worldwide population [9]. A stronger predominance for females was found for erythematotelangiectatic and papulopustular subtypes with a diagnosis, usually, after the fourth decade of life [8, 10].
The exact pathogenesis of rosacea remains unclear but the involvement of several external or endogenous factors is reported [1, 11]. In fact, recent findings highlighted the role of predisposing factors such as genetic predisposition and association with other diseases [12]. Microbial stimuli, especially colonization, ultraviolet (UV) radiation, stress, and environmental changes are also recognized as triggering factors both for the development and worsening of rosacea [12–14]. Therefore, dysregulation of innate immunity via the expression of higher amounts of toll-like receptor 2 (TLR2) in the skin [15] and augmentation of the inflammatory cascade have been reported [16] as abnormal expression of cathelicidin antimicrobial peptides [17].
More recently, rosacea and other skin diseases such as psoriasis and atopic dermatitis have been linked to intestinal dysbiosis [18, 19]. Authors reported the role of intestinal dysbiosis in promoting inflammation and impairment of normal lymphocyte function, potentially perpetuating chronic, low-grade inflammation [20]. Therefore, the potential role of microorganisms in the pathogenesis of rosacea has been hypothesized [21]. Parodi and colleagues [22] reported a higher incidence of small intestinal bacterial overgrowth (SIBO) when patients with rosacea were compared to controls. Most interesting, microbial unbalancing of the skin microbiota on the skin has been linked to rosacea clinical manifestations [23], even though the direct correlation between microbiota composition on the skin and the incidence of the pathology is still under investigation.
More recently, in a NRS-supported study in twins, Zaidi and colleagues [24] reported the first evidence highlighting the correlation between the severity of rosacea and microbial dysbiosis on the skin, but further study is needed to determine the species involved.
Historically, therapeutic approaches to rosacea focused on symptom suppression by means of anti-inflammatory agents such as doxycycline [25–27], metronidazole [28], topical azelaic acid [11, 29], sodium sulfacetamide [11, 30], and calcineurin inhibitors [31]. The use of serine protease inhibitors is to be considered an emerging therapy in rosacea [32].
Several concerns surround the use of tetracyclines, especially as long-term treatment is often necessary. Although it is commonly prescribed at a sub-antimicrobial dose, gastrointestinal side effects and photosensitivity are not uncommon and the risk of antimicrobial resistance increases with higher doses [33, 34].
Although not yet approved for the treatment of rosacea, efficacy of a low dose of isotretinoin has been reported in patients with papulopustular rosacea subtype [35].
More recently, photodynamic therapy (PDT), especially light-emitting diodes (LED), has been introduced as a valid alternative to conventional therapy [36]. A few in vitro studies [37, 38] and a published in vivo study on patients with papulopustular rosacea with methyl ester aminolevulinate (MAL) coupled with PDT [39], reported efficacy of LEDs for treatment of rosacea.
Case presentations
Case report 1
A 22-year-old Caucasian woman presented to a dermatological clinic with a 5-year history of pink eruptions on her nose. She also reported a burning sensation. She was diagnosed as having papulopustular rosacea subtype, moderate grade, according to the classification and staging of rosacea developed by the NRS Expert Committee [5]. In the previous 2 years she was treated with two cycles of orally administered tetracycline (Lymecycline), 300 mg per day, for 12 weeks. Systemic therapy was associated with metronidazole cream 1% for cycles of 6 months. In the last 6 months before the visit, she also submitted to 40% pyruvic acid peeling every 25 days, with poor response and continuous relapses. A combined and sequential plan of blue (480 nm ± 15 nm, 300 J/minute) and red (650 ± 15 nm, 100 J/minute) LED therapy regimen was planned twice a week for a total of ten sessions. A quasi-monochromatic 120 LED system (Dermodinamica® instrument, ELISOR Srl, Milan, Italy) was used for 15 minutes (each wavelength).
Case report 2
A 68-year-old Caucasian man presented with a 7-year history of papulopustular rosacea, moderate grade [5], which extended over the entire surface of his face. He had experienced extended relapses on his face once a year in the past 6–7 years. He was previously treated with two cycles of Lymecycline (tetracycline) at 300 mg per day or azithromycin every 2 weeks in combination with 0.75% topical metronidazole. He was submitted to LED therapy twice a week for a total of ten sessions. Blue (480 nm ± 15 nm, 300 J/minute) and red (650 ± 15 nm, 300 J/minute) were sequentially irradiated for 15 minutes by means of LED system Dermodinamica® (ELISOR Srl, Milan, Italy). The therapy was coupled with topical 15% azelaic acid.
Outcome and follow-up
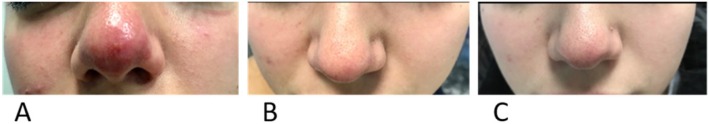
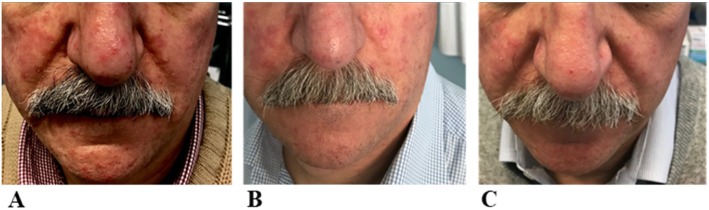
Erythema, burning sensation, and itching were assessed using a visual scale grading (0 = no symptoms, 4 = very severe). Erythema and papules were subjectively assessed by the dermatologist, whereas the intensity of itch and burning sensations was expressed by our patients. A good response was obtained for both patients after ten treatments with LEDs. Both patients reported a reduction of symptoms such as burning and itching. Also, a reduction of erythema and papules was observed after five sessions of LED therapy (Figs. 1b and 2b). Further improvement was observed at the end of treatment: ten sessions of LED therapy (Figs. 1c and 2c).
Fig. 1.
Papulopustular rosacea on the nose of case report 1 at the base time (a), after five sessions (b), and after ten sessions (c) with coupled blue (480 nm) and red (650 nm) light-emitting diodes therapy
Fig. 2.
Papulopustular rosacea with erythema and telangiectasias on the glabella, forehead, nose, cheeks, and chin of case report 2 at the base time (a), after five sessions (b), and after ten sessions (c) with coupled blue (480 nm) and red (650 nm) light-emitting diodes therapy
Discussion and Conclusions
Several therapeutic approaches are currently available for treating rosacea and they are mainly aimed at controlling disease symptoms [40, 41]. The therapeutic plan has to be adapted to the rosacea subtype and tailored according to the dominant manifestations of the patient [32, 35]. In general, the reduction of oral therapy in favor of topical or physical therapy is desirable in order to reduce side effects for patients and increasing the safety of treatment [5, 32].
The therapeutic approach described in this report aims at reporting the efficacy and safety of combined blue (480 nm ± 15 nm) and red (650 ± 15 nm) LED light-based therapy in patients affected by rosacea.
Previous research reported the efficacy of red and blue light coupled for the treatment of mild to moderate acne lesions [42, 43]. Blue light (400–470 nm), due to its lower penetration, is useful in such skin conditions related to the epidermis layer of the skin [44]; therefore, it is also able to interfere with human sebocytes proliferation [45]. On the other hand, red light (630 nm) is reported to have a significant effect on sebum production [46, 47]. The benefits deriving from PDT using LEDs are not limited to its efficacy but are also related to its safety and tolerance by patients; therefore, its advantages can be extended to a broad range of dermatological conditions [48, 49].
In fact, PDT is routinely used by dermatologists in the treatment of moderate to severe acne vulgaris [50, 51] and perioral dermatitis [18]. Rosacea shares several features with other dermatological diseases, especially acne. In patients with acne, PDT has been supposed to act via modulation of the functionality of the pilosebaceous unit and this could probably also be applied to rosacea.
Previous to our work, several authors reported the efficacy of PDT therapy on patients with rosacea [36, 52–54]. Moreover, an in vitro study on rosacea-like mouse skin [55] reported the efficacy of LED at 630 and 940 nm on the down-regulation of key inflammatory mediators of rosacea, such as cathelicidin (LL-37), TLR2, and kallikreins (KLKs). These results are in line with reported evidence on the efficacy of LED therapy also to interact with the host immune system. LEDs may also interact with skin microbiome [56–58] and this could also have as significant an impact on the etiopathogenesis of rosacea as on immune response modulation. A deeper knowledge of the implications of both gut and skin microbiome in rosacea is still needed; our recent research is aimed at evaluating the real effect of blue and red light LEDs on skin microflora in patients with rosacea and patients with acne.
In addition, the safety deriving from the use of LED devices encourages their ever-increasing use for the treatment of many dermatological conditions, including rosacea.
Nowadays, the treatment of patients with rosacea still represents a challenge for dermatologists. Conventional treatment of rosacea is either ineffective or results in the dissatisfaction of patients due to the need for continuous treatment.
The case reports presented in the current work show, for the first time, the usefulness of LED therapy combining blue and red light benefits for the treatment of patients with rosacea. This kind of treatment could represent an effective, safer, and well-tolerated approach for the treatment of such kinds of condition.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Authors’ contributions
Conceptualization, methodology and investigations: FR, ES, MD. Data curation and formal analysis: ES. Resources: ES and MD. Wrote the paper: FR and ES. All authors read and approved the final manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The study was approved by the Ethical Independent Committee for Clinical, not pharmacological investigation in Genoa (Italy) and in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patients for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–758. doi: 10.1016/j.jaad.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Plewig G, Kligman AM. ACNE and ROSACEA. Berlin: Springer; 2000. History of Acne and Rosacea. [Google Scholar]
- 3.Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 Suppl 1):S15–S26. doi: 10.1016/j.jaad.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 4.Tan J, Berg M. Rosacea: current state of epidemiology. J Am Acad Dermatol. 2013;69(6 Suppl 1):S27–S35. doi: 10.1016/j.jaad.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Wilkin J, Dahl M, Detmar M, Drake L, Liang MH, Odom R, Powell F, National Rosacea Society Expert Committee Standard grading system for rosacea: report of the National Rosacea Society Expert Committee on the classification and staging of rosacea. J Am Acad Dermatol. 2004;50(6):907–912. doi: 10.1016/j.jaad.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Wilkin J, Dahl M, Detmar M, Drake L, Feinstein A, Odom R, Powell F. Standard classification of rosacea: Report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46(4):584–587. doi: 10.1067/mjd.2002.120625. [DOI] [PubMed] [Google Scholar]
- 7.Berg M, Lidén S. An epidemiological study of rosacea. Acta Derm Venereol. 1989;69(5):419–423. [PubMed] [Google Scholar]
- 8.Spoendlin J, Voegel JJ, Jick SS, Meier CR. A study on the epidemiology of rosacea in the U.K. Br J Dermatol. 2012;167(3):598–605. doi: 10.1111/j.1365-2133.2012.11037.x. [DOI] [PubMed] [Google Scholar]
- 9.Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179(2):282–289. doi: 10.1111/bjd.16481. [DOI] [PubMed] [Google Scholar]
- 10.Rainer BM, Fischer AH, Luz Felipe da Silva D, Kang S, Chien AL. Rosacea is associated with chronic systemic diseases in a skin severity-dependent manner: results of a case-control study. J Am Acad Dermatol. 2015;73(4):604–608. doi: 10.1016/j.jaad.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Woo YR, Lim JH, Cho DH, Park HJ. Rosacea: Molecular Mechanisms and Management of a Chronic Cutaneous Inflammatory Condition. Int J Mol Sci. 2016;17(9) Review. PMID: 2764916 [DOI] [PMC free article] [PubMed]
- 12.Elewski BE, Draelos Z, Dréno B, Jansen T, Layton A, Picardo M. Rosacea - global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25(2):188–200. doi: 10.1111/j.1468-3083.2010.03751.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinhoff M, Buddenkotte J, Aubert J, Sulk M, Novak P, Schwab VD, Mess C, Cevikbas F, Rivier M, Carlavan I, Déret S, Rosignoli C, Metze D, Luger TA, Voegel JJ. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15(1):2–11. doi: 10.1038/jidsymp.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Rosso JQ, Gallo RL, Tanghetti E, Webster G, Thiboutot D. An evaluation of potential correlations between pathophysiologic mechanisms, clinical manifestations, and management of rosacea. Cutis. 2013;91(3 Suppl):1–8. [PubMed] [Google Scholar]
- 15.Cribier B. Pathophysiology of rosacea: redness, telangiectasia, and rosacea. Ann Dermatol Venereol. 2011;138 Suppl 3:S184–S191. doi: 10.1016/S0151-9638(11)70088-6. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki K, Gallo RL. Rosacea as a disease of cathelicidins and skin innate immunity. J Investig Dermatol Symp Proc. 2011;15(1):12–15. doi: 10.1038/jidsymp.2011.4. [DOI] [PubMed] [Google Scholar]
- 17.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122(2):261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughn AR, Notay M, Clark AK, Sivamani RK. Skin-gut axis: The relationship between intestinal bacteria and skin health. World J Dermatol. 2017;6(4):52–58. doi: 10.5314/wjd.v6.i4.52. [DOI] [Google Scholar]
- 19.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131(10):1974–1980. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng QX, Soh AYS, Loke W, Lim DY, Yeo WS. The role of inflammation in irritable bowel syndrome (IBS) J Inflamm Res. 2018;11:345–349. doi: 10.2147/JIR.S174982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes AD. Potential role of microorganisms in the pathogenesis of rosacea. J Am Acad Dermatol. 2013;69(6):1025–1032. doi: 10.1016/j.jaad.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Parodi A, Paolino S, Greco A, Drago F, Mansi C, Rebora A, Parodi A, Savarino V. Small intestinal bacterial overgrowth in rosacea: clinical effectiveness of its eradication. Clin Gastroenterol Hepatol. 2008;6(7):759–764. doi: 10.1016/j.cgh.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 23.Picardo M, Ottaviani M. Skin microbiome and skin disease: the example of rosacea. J Clin Gastroenterol. 2014;48(Suppl 1):S85–S86. doi: 10.1097/MCG.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 24.Zaidi AK, Spaunhurst K, Sprockett D, Thomason Y, Mann MW, Fu P, Ammons C, Gerstenblith M, Tuttle MS, Popkin DL. Characterization of the facial microbiome in twins discordant for rosacea. Exp Dermatol. 2018;27(3):295–298. doi: 10.1111/exd.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Nardo A, Holmes AD, Muto Y, Huang EY, Preston N, Winkelman WJ, Gallo RL. Improved clinical outcome and biomarkers in adults with papulopustular rosacea treated with doxycycline modified-release capsules in a randomized trial. J Am Acad Dermatol. 2016;74(6):1086–1092. doi: 10.1016/j.jaad.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Del Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, Bradshaw M. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56(5):791–802. doi: 10.1016/j.jaad.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Wise RD. Submicrobial doxycycline and rosacea. Compr Ther. 2007;33(2):78–81. doi: 10.1007/s12019-007-8003-x. [DOI] [PubMed] [Google Scholar]
- 28.Cardwell LA, Alinia H, Moradi Tuchayi S, Feldman SR. New developments in the treatment of rosacea - role of once-daily ivermectin cream. Clin Cosmet Investig Dermatol. 2016;9:71–77. doi: 10.2147/CCID.S98091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coda AB, Hata T, Miller J, Audish D, Kotol P, Two A, Shafiq F, Yamasaki K, Harper JC, Del Rosso JQ, Gallo RL. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol. 2013;69(4):570–577. doi: 10.1016/j.jaad.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Rosso JQ. Evaluating the role of topical therapies in the management of rosacea: focus on combination sodium sulfacetamide and sulfur formulations. Cutis. 2004;73(1 Suppl):29–33. [PubMed] [Google Scholar]
- 31.Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part II. Topical and systemic therapies in the treatment of rosacea. J Am Acad Dermatol. 2015;72(5):761–770. doi: 10.1016/j.jaad.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 32.van Zuuren EJ, Fedorowicz Z. Low-Dose Isotretinoin: An Option for Difficult-to-Treat Papulopustular Rosacea. J Invest Dermatol. 2016;136(6):1081–1083. doi: 10.1016/j.jid.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Mehrotra AN, Thomas Z, Goyal VP. Gastrointestinal side effects of tetracycline and its possible prevention by antifungal compounds. J Assoc Physicians India. 1970;18(7):627–629. [PubMed] [Google Scholar]
- 34.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65(2):232–260. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Two AM, Hata TR, Nakatsuji T, Coda AB, Kotol PF, Wu W, Shafiq F, Huang EY, Gallo RL. Reduction in serine protease activity correlates with improved rosacea severity in a small, randomized pilot study of a topical serine protease inhibitor. J Invest Dermatol. 2014;134(4):1143–1145. doi: 10.1038/jid.2013.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11(9):1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Guo H, Tian Q, Zheng G, Hu Y, Fu Y, Tan H. Effects of 5-aminolevulinic acid-mediated photodynamic therapy on antibiotic-resistant staphylococcal biofilm: an in vitro study. J Surg Res. 2013;184(2):1013–1021. doi: 10.1016/j.jss.2013.03.094. [DOI] [PubMed] [Google Scholar]
- 38.Bryld LE, Jemec GB. Photodynamic therapy in a series of rosacea patients. J Eur Acad Dermatol Venereol. 2007;21(9):1199–1202. doi: 10.1111/j.1468-3083.2007.02219.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee JB, Bae SH, Moon KR, Na EY, Yun SJ, Lee SC. Light-emitting diodes downregulate cathelicidin, kallikrein and toll-like receptor 2 expressions in keratinocytes and rosacea-like mouse skin. Exp Dermatol. 2016;25(12):956–961. doi: 10.1111/exd.13133. [DOI] [PubMed] [Google Scholar]
- 40.Feldman SR, Huang WW, Huynh TT. Current drug therapies for rosacea: a chronic vascular and inflammatory skin disease. J Manag Care Spec Pharm. 2014;20(6):623–629. doi: 10.18553/jmcp.2014.20.6.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asai Y, Tan J, Baibergenova A, Barankin B, Cochrane CL, Humphrey S, Lynde CW, Marcoux D, Poulin Y, Rivers JK, Sapijaszko M, Sibbald RG, Toole J, Ulmer M, Zip C. Canadian Clinical Practice Guidelines for Rosacea. J Cutan Med Surg. 2016;20(5):432–445. doi: 10.1177/1203475416650427. [DOI] [PubMed] [Google Scholar]
- 42.Lee SY, You CE, Park MY. Blue and red light combination LED phototherapy for acne vulgaris in patients with skin phototype IV. Lasers Surg Med. 2007;39(2):180–188. doi: 10.1002/lsm.20412. [DOI] [PubMed] [Google Scholar]
- 43.Kwon HH, Lee JB, Yoon JY, Park SY, Ryu HH, Park BM, Kim YJ, Suh DH. The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: a double-blind, randomized controlled trial. Br J Dermatol. 2013;168(5):1088–1094. doi: 10.1111/bjd.12186. [DOI] [PubMed] [Google Scholar]
- 44.Friedmann DP, Goldman MP, Fabi SG, Guiha I. The effect of multiple sequential light sources to activate aminolevulinic Acid in the treatment of actinic keratoses: a retrospective study. J Clin Aesthet Dermatol. 2014;7(9):20–25. [PMC free article] [PubMed] [Google Scholar]
- 45.Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49(2):271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 46.Charakida A, Seaton ED, Charakida M, Mouser P, Avgerinos A, Chu AC. Phototherapy in the treatment of acne vulgaris: what is its role? Am J Clin Dermatol. 2004;5(4):211–216. doi: 10.2165/00128071-200405040-00001. [DOI] [PubMed] [Google Scholar]
- 47.Ablon G. Phototherapy with Light Emitting Diodes: Treating a Broad Range of Medical and Aesthetic Conditions in Dermatology. J Clin Aesthet Dermatol. 2018;11(2):21–27. [PMC free article] [PubMed] [Google Scholar]
- 48.Sorbellini E, Rucco M, Rinaldi F. Photodynamic and photobiological effects of light-emitting diode (LED) therapy in dermatological disease: an update. Lasers Med Sci. 2018;33(7):1431–1439. doi: 10.1007/s10103-018-2584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pei S, Inamadar AC, Adya KA, Tsoukas MM. Light-based therapies in acne treatment. Indian Dermatol Online J. 2015;6(3):145–157. doi: 10.4103/2229-5178.156379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong Y, Zhou G, Chen J, Shen L, Jianxin Z, Xu Q, Zhu Y. A new LED device used for photodynamic therapy in treatment of moderate to severe acne vulgaris. Photodiagn Photodyn Ther. 2016;13:188–195. doi: 10.1016/j.pdpdt.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 51.Schreiber S. A Novel Regimen for Perioral Dermatitis by Photodynamic Therapy. J Pharm Pharmacol. 2016;4:559–563. [Google Scholar]
- 52.Nybaek H, Jemec GB. Photodynamic therapy in the treatment of rosacea. Dermatology. 2005;211(2):135–138. doi: 10.1159/000086443. [DOI] [PubMed] [Google Scholar]
- 53.Katz B, Patel V. Photodynamic therapy for the treatment of erythema, papules, pustules, and severe flushing consistent with rosacea. J Drugs Dermatol. 2006;5(2 Suppl):6–8. [PubMed] [Google Scholar]
- 54.Cecic I, Sun J, Korbelik M. Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochem Photobiol. 2006;82(2):558–562. doi: 10.1562/2005-09-09-RA-681. [DOI] [PubMed] [Google Scholar]
- 55.Noborio R, Nishida E, Kurokawa M, Morita A. A new targeted blue light phototherapy for the treatment of acne. Photodermatol Photoimmunol Photomed. 2007;23(1):32–34. doi: 10.1111/j.1600-0781.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- 56.Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, Shalita AR, Lozada VT, Berson D, Finlay A, Goh CL, Herane MI, Kaminsky A, Kubba R, Layton A, Miyachi Y, Perez M, Martin JP, Ramos-E-Silva M, See JA, Shear N, Wolf J, Jr, Global Alliance to Improve Outcomes in Acne New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne group. J Am Acad Dermatol. 2009;60(5 Suppl):S1–50. doi: 10.1016/j.jaad.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 57.Kumar A, Ghate V, Kim MJ, Zhou W, Khoo GH, Yuk HG. Antibacterial efficacy of 405, 460 and 520 nm light emitting diodes on Lactobacillus plantarum, Staphylococcus aureus and Vibrio parahaemolyticus. J Appl Microbiol. 2016;120(1):49–56. doi: 10.1111/jam.12975. [DOI] [PubMed] [Google Scholar]
- 58.Cicarma E, Mørk C, Porojnicu AC, Juzeniene A, Tam TT, Dahlback A, Moan J. Influence of narrowband UVB phototherapy on vitamin D and folate status. Exp Dermatol. 2010;19(8):e67–e72. doi: 10.1111/j.1600-0625.2009.00987.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.