Abstract
Background:
The characteristics of the aggregation reaction that follows allergen binding to cell surface IgE on basophils and mast cells depend on a variety of factors that include the density of IgE and the affinity of the allergen for IgE. For simple bivalent stimuli, one prediction is that the location of the optimum for aggregation is not dependent on IgE density, only the affinity for IgE. However, this behavior doesn’t occur for stimulation with an anti-IgE Ab during treatment of patients with omalizumab.
Methods:
This study re-examined the stability of the optimum for histamine release, relative to cell surface IgE density, using the simple bivalent penicillin hapten (BPO2) or a bivalent monoclonal anti-IgE antibody.
Results:
The results validated one prediction for one bivalent hapten, BPO2; across a range of IgE densities, 270 to 23500 per cell, optimal histamine release remained constant (10 nM BPO2). In contrast, across a range of approximately 6000 to 110,000 IgE/cell, optimal histamine release shifted 8-30 fold for anti-IgE Ab. The distinguishing characteristic between the two bivalent stimuli was the difference in their crosslink re-equilibration. Recent modeling of histamine release suggested that the SYK to receptor ratio could determine the position of histamine release optimum. The current studies showed that there were significant shifts in the SYK-receptor ratios (from 1:6 to 5:1) but the basophil’s ability to sense this ratio was restricted to transient crosslinks, as occurred with anti-IgE Ab.
Conclusions:
The results suggest that ligand crosslinking dynamics couple with SYK and receptor expression levels to determine qualitative characteristics of the dose response curve for secretion.
Keywords: IgE, histamine release, signaling elements
Introduction
IgE-mediated secretion from mast cells and basophils is a reaction dependent on the ability of allergens to induce aggregation of cell surface-bound IgE. The reaction can be quite complex because branched aggregates of allergen --with its myriad of different epitopes -- and IgE antibody --with its myriad specificities and affinities for allergen epitopes— occur and aggregates can be quite large in size [1]. Allergic disease research often uses pan-crosslinking stimuli like anti-IgE or anti-FceRI antibodies to induce activation of basophils or mast cells. In early years of study, polyclonal anti-IgE antibodies were used to explore characteristics of basophil or mast cell secretion but often today, monoclonal antibodies are used. One monoclonal in common use behaved in ways that are not currently understood and this study sought to determine if there were properties of this reagent that revealed more subtle aspects of the IgE-mediated basophil response.
It is difficult to analytically model allergen-induced crosslinking reactions due to the branched nature of the aggregates formed on the cell surface. But it is possible to analytically model simple bivalent antigens [2,3]. For example, the simplest antigen derived from a known allergen would be bivalent penicillin (BPO2), two penicilloyl groups linked by a 6-10 carbon aliphatic linkage. This type of molecular species can only form linear aggregates, of variable size, of IgE. With a simple model in hand it is possible to make some predictions about the characteristics of secretion induced by this simple ligand. One such prediction is that the optimum for secretion occurs at 1/2Ka, where Ka is the intrinsic one-site affinity constant between hapten and IgE Ab [2]. This prediction has been shown to be correct [4]. A second expectation for the aggregate size distribution is that the position of the optimum for maximum crosslinks is not dependent on the density of antigen-specific IgE on the cell surface [3]. A very limited examination of this prediction suggested it was probably true as well [3].
In this context, recent studies on the responsiveness of peripheral blood basophils to stimulation with the crosslinking anti-IgE Ab, 6061P, during treatment with omalizumab, were not expected [5]. The 6061P monoclonal antibody is a monomeric mouse IgM (i.e., it is not a pentamer of the single antibody unit). As such, it should act as a bivalent crosslinker and therefore follow the rules described for other bivalent haptens. During treatment with omalizumab, the relative absence of free IgE reduces the density of receptor and bound IgE on basophils and mast cells [6–8]. The position of the peak response to stimulation with 6061P antibody shifts to 200 fold higher concentrations [5]. As noted, the position of the optimum should not be sensitive to the cell surface density of IgE. Therefore, the observed behavior is not understood and warranted closer study because it might be indicative of signal transduction reaction behavior that separates the characteristics of aggregate formation from the secretory reaction. Although the prediction of a non-shifting optimum was previously examined experimentally, only one experiment was done to test the prediction and the conditions were not well defined [3]. In fact, more recently, it was shown that a more complex aggregation reaction, stimulation with multivalent BPO-HSA was found to be sensitive the density of BPO-specific IgE with a rightward shift at lower densities [9]. This is a far more complex reaction and the predictions less clear for the dependency on IgE density, but it suggested that the original prediction needed a closer inspection. These studies began with this question and then explored one potential explanation for the optimum shift with 6061P antibody. The results suggested that there were other characteristics of the basophil response and aggregation that needed exploration and the second half of the report examines two candidate explanations for the experimental observations.
Methods
Materials
The following were purchased: PIPES, bovine serum albumin (BSA), EGTA, EDTA, D-glucose, NaF, Na4P2O7, Na3VO4, 2-ME, NP-40, FMLP (Sigma, St. Louis, MO); crystallized human serum albumin (HSA) (Miles Laboratories, Elkhart, IN); fetal calf serum (FCS) and RPMI 1640 containing 25 mM HEPES and L-glutamine (BioWhittaker, Walkersville, MD); Percoll, (Pharmacia, Piscataway, NJ); anti-SYK mAb, 4D10 (Santa Cruz Biotechnology, Santa Cruz, CA); a penicillin (BPO)-specific IgE was partially purified from the sera of penicillin allergic patients as previously described [7]; BPO2 (benzylpenicilloyl-di-octamine) was synthesized and purified as described previously [10,11]; monoclonal anti-human IgE Ab (6061P) (Hybridoma Labs, Baltimore, MD); antiHRP-conjugated donkey anti-rabbit Ig Ab, HRP-conjugated Sheep anti-mouse Ig Ab, protein G sepharose beads (Amersham Life Science, Arlington Heights, IL); monoclonals anti-FceRI antibodies 22E7 and 15A5 (gift of former Hoffman-LaRoche)(measured total FceRI and unoccupied FceRI, respectively). Countercurrent elutriation and labeling with antibodies for flow cytometry was conducted in PAG containing 0.25 % BSA in place of 0.003 % HSA. ESB is Novex electrophoresis sample buffer containing 5 % 2-mercaptoethanol. SDS stripping buffer for Western blots was 65 mM Tris (pH 6.7), 100 mM 2-mercaptoethanol and 2% SDS.
Buffers
PIPES-albumin-glucose (PAG) buffer consisted of 25 mM PIPES, 110 mM NaCl, 5 mM KCl, 0.1% glucose, and 0.003% HSA. PAGCM was PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2. PAG-EDTA consisted of PAG supplemented with 4 mM EDTA. Lactic acid buffer for removing endogenous cell bound IgE; 0.01 M lactic acid, 0.14 M NaCl, 0.005 M KCl, pH 3.9 [12].
Basophil isolation, sensitization, and histamine release reaction
Blood was partially enriched for basophils using single-step Percoll gradients. After washing to obtain a mononuclear cell preparation, cells were resuspended in saline one centrifugation before treatment for 15 seconds to 2 minutes with lactic acid stripping buffer. After washing the cells once, they were aliquoted and sensitized at 4°C for one hour with anti-BPO-IgE at several concentrations or with a monoclonal IgE (SE44, anti-gp120-specific IgE). After 1-3 washes (depending on the sensitization reagent), the cells were resuspended in PAG (to prepare for fixation and flow cytometry) or PAGCM buffer (to assess histamine release). Total histamine content was obtained by lysis of the cell preparation with 1.6% perchloric acid. These experiments included two different buffers for stimulation. The standard measurement is in PAGCM buffer but a second condition included 44% deuterium oxide to replace the water in the PAGCM. D2O is known to enhance secretion of any type of stimulation [13,14].
Histamine measurements
Histamine was measured by automated fluorimetry [15]. The percentage of total histamine release was calculated after subtraction of spontaneous histamine release.
Basophil cell surface IgE measurement
An aliquot of cells was used to determine the total basophil count by alcian blue staining and the remaining cells were treated with acetate buffer, pH 3.7, to dissociate endogenous IgE. The recovered supernatant was neutralized to pH 7 and IgE concentration measured by a total IgE assay (immunoCap). A calculation of the number of IgE molecules present and the cell number allowed the cell surface IgE density to be determined. Calibration of the antibody (22E7) used to detect FceRI by flow cytometry involved a comparison of flow cytometric results with explicit measurement of IgE eluted from a counted number of basophils, as described previously [16]. The flow cytometric results were then equated with this measured density of IgE/basophil. See reference [17] for a more complete description of the calibration procedure. To calibrate the signal that detects BPO-specific IgE, one aliquot of cells was sensitized with a high concentration of BPO-specific IgE and one at a lower concentration. The cells were labeled with either 15A5 (or appropriate isotype control Ab) or by the procedure for detecting BPO-specific IgE [18] (see below). By flow cytometry (and running with pre-established PMT settings and calibrating against a standard bead), the net difference in the BPO-specific signal between the two loading conditions and the net difference in the 15A5 signal was determined (as the BPO-signal increases, the 15A5 signal decreases). Since 15A5 has been calibrated against an absolute receptor measurement, the delta for BPO-specific signal and the delta for the 15A5 signal sets the equation; BPO = 15A5*(calibrated value for 15A5).
To assess the BPO-specific IgE density, basophils were sensitized with penicillin-specific IgE and its presence detected with a sandwich assay. Prior to fixing the cells, the suspension was chilled to 4°C, 10 μg/ml of BPO(32)-HSA added for 10 minutes, the cells washed twice in ice-cold buffer and fixed with 2% paraformaldehyde for 20 minutes prior to blocking and storage overnight. Before flow cytometry, the fixed cells were incubated with rabbit anti-BPO IgG ± BPO-EACA at 1mM. The presence of BPO-EACA (monovalent penicillin, penicillin coupled to ε-aminocaproic acid) generates a negative control for the measurements. A second background control was included; the labeling procedure above was also performed on cells not sensitized with BPO-specific IgE.
Assessing binding re-equilibration
Leukocytes were stimulated with either BPO2 or 6061P anti-IgE antibody. At intervals early in the reaction, the reaction mixtures were diluted 20-fold with buffer containing either 1) nothing (so bivalent ligand concentration decreased 20-fold), 2) stimulus at the same initiating concentration (therefore, no dilution of the stimulus), or 3) EDTA (4 mM final to immediately stop the release). In the case of BPO2 stimulation, an additional dilution series included 1 mM BPO-EACA (monovalent hapten). The reaction was allowed to proceed until the 30-minute time point, at which point the suspension was centrifuged to recover the supernatant for analysis of histamine.
Analysis of SYK protein expression by intracellular flow cytometry:
All analysis was performed on a BD FACSCalibur flow cytometer. The measurement of SYK protein expression by flow cytometry using the anti-SYK antibody 4D10 has been previously described and validated with respect to standard Western blotting [19,20]. This method has been used on both impure as well as purified cell fractions with equivalent results. Briefly, fixed mixed leukocytes were labeled with anti-IL-3R (BD Biosciences, Franklin Lakes, NJ) and anti-BDCA2 antibodies (phycoerythin and FITC, respectively) and permeabilized (Fix and Perm Kit, Caltag, Carlsbad, CA) in the presence of 4D10 or IgG2a antibody. Cells were then incubated with an anti-mIgG2a –alexa647 antibody (Molecular Probes, Eugene, OR) to complete the labeling. SYK protein expression is reported as normalized net MFI, the difference between 4D10 and isotype-labeled cells corrected for instrument variability using CaliBRITE APC calibration beads (BD Biosciences). As described previously, the flow cytometric method of determining SYK was calibrated against a quantitative Western blot that used a recombinant SYK protein as a standard.
Omalizumab study
There were two distinct omalizumab studies from which data was drawn. Since this report isn’t concerned with the primary outcome metrics of either the non-clinical or clinical part of studies, the details of the study design will not be presented. But the details can be found in the published study [5]. Informed consent was obtained via a protocol approved by the Johns Hopkins Hospital Institutional Review Board and the National Institute of Allergy and Infectious Diseases’ Data Safety Monitoring Boards. The cells from the 1-step Percoll gradient were analyzed by flow cytometry for cell surface levels of FcεRIα and IgE, internal levels of SYK, or used to assess histamine release in response to stimulation with anti-IgE antibody.
Results
Sensitivity of Dose Response Curves to Cell Surface IgE Density
BPO2 dose response curve optimum and density
Basophils, either enriched on one-step or two-step Percoll gradients, were treated briefly with lactic acid to dissociate a portion of the surface IgE and to generate unoccupied receptors for sensitization with BPO-specific IgE. The density of BPO-specific IgE was assessed with a new flow cytometric methodology. However, this method had not been previously calibrated for absolute levels so this was done in these studies (see methods). The sensitization curve is fairly steep for BPO2 so that it is difficult to a priori choose two disparate densities of BPO-specifc IgE that capture a complete enough dose response curve to BPO2 that an optimum can be determined. A variety of relative concentrations of BPO-specific IgE for sensitization were tried. To maximize the difference in density ultimately required that cells sensitized with a high density of BPO-specific IgE be challenged in normal buffer while those sensitized with a much lower density were challenged in buffer containing 44% deuterium oxide. This experimental change is a well-described way to enhance secretion [13,21,22] but it was possible that the conditions could distort the results. Therefore, a variety of experiments were done to explore various relative densities of BPO-specific IgE where the buffer conditions were symmetric or asymmetric with respect to the presence of D2O, i.e., both conditions in standard buffer, both conditions in D2O-containing buffer or one condition with standard buffer and one with D2O-containing buffer. In no case was there an observed difference in the position of the optimum for secretion. Indeed, these studies generally showed that the position of the optimum for secretion always occurred at 10 nM BPO2, ranging from densities of 270 BPO-specific IgE/basophil to 23500/basophil across 10 experiments. Figure 1C plots the center point optimum as a function of the cell surface BPO-specific IgE density (the ordinate range is chosen to be similar to the range of shift observed with anti-IgE Ab 6061P below). A fit of the data suggests a slight inverse relationship but this trend is driven by the two data points at the low end; the heavy fit line excludes these two data points. But taking the results at face value, for a 100 fold range of density, at best the optimum (8-10 nM for most results) shifts 2-3 fold, or not at all.
Fig. 1.
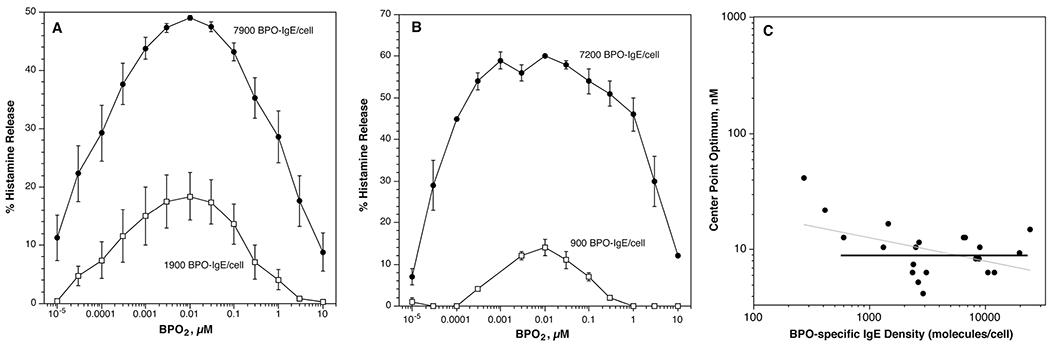
Concentration-dependence of histamine release from basophils sensitized with two densities of BPO (benzylpenicilloyl)-specific IgE and stimulated with BPO2. Panel A; average of all experiments, n=10, (●) high density sensitization vs. (○) low density sensitization. Panel B; subset of the experiments in panel A chosen to maximize the difference in BPO-specific IgE density (n=2). Panel C; using all the experimental results (±D2O), the relationship of BPO-specific IgE density and the optimum of the dose response curve plotted. The line fit (heavy) excludes the two lowest data points while the grey line includes all points.
For the analysis of paired experiments (two sensitization conditions for the same subject), figure 1 shows a couple of ways to average the results since the differential loading of the cells varied among experiments. Figure 1A averages all the experiments regardless of the buffer used (i.e., ±D20). In this plot there is an average 4 fold difference in BPO-specific IgE density. It is worth noting that the EC50 for the BPO2 response, estimated here as approximately 2500-3000 molecules/cell is similar to our previous measurements for BPO2 (and BPO-HSA, where the EC50 for BPO-HSA was 500-1000 molecules) [23], suggesting that the calibration of the flow cytometric method was yielding results similar to our older method of measurement BPO-specific IgE. Figure 1B isolates two experiments that produced the greatest paired difference in BPO-specific IgE density, on average, 8-fold in these two cases. The results were similar, with no significant difference in the optimums for release.
6061P dose response curve and density
The most direct way of testing whether the density of IgE alters the optimum for release when using 6061P anti-IgE Ab is to dissociate IgE from basophils, and resensitize the cells with different densities of IgE. In this design, most factors that can alter the IgE-mediated dose response curve stay constant. Basophils were treated for 2 minutes at room temperature with lactic acid and the cells divided into those sensitized with 10 μg/ml IgE (SE44, a monoclonal human IgE) in buffer containing 1 mM EDTA and 10 μg/ml heparin (to aid in sensitization) at 4°C or cells incubated in the same buffer but without IgE. After washing, a portion of the cells from each condition were set aside for flow cytometric measurement of cell surface IgE and the remainder were challenged with a range of concentrations of 6061P monoclonal anti-IgE Ab. In a pilot experiment a third condition, the dose response curve without prior dissociation, was included and demonstrated that the resensitization IgE behaved like the endogenous IgE with a similar optimum at similar high densities of cell surface IgE.
Figure 2A shows that there is a marked shift in the optimum for stimulation with 6061P anti-IgE Ab with an 13.8-fold difference in the density of cell surface IgE, 9400±1700/basophils vs. 130000±34000/basophil.
Fig. 2.
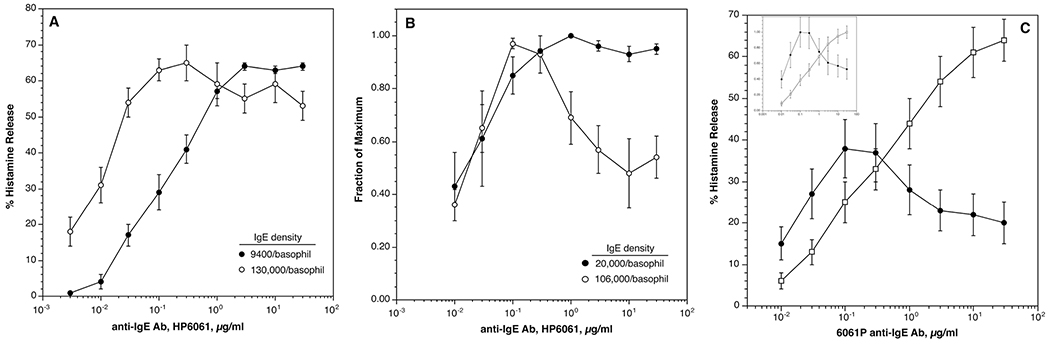
Concentration-dependence of histamine release from basophils stimulated with 6061P monoclonal anti-IgE Ab in two experimental designs. Panel A; endogenous IgE on basophils was dissociated with lactic acid treatment and the cells re-sensitized to obtain two densities of cell surface IgE and the cells stimulated with monoclonal anti-IgE Ab (n=4). Panel B; from a group of 14 basophil donors with a natural range of cell surface IgE densities, two groups of the extremes of density were formed (n=3) at high density vs. (n=4) at low density. The anti-IgE Ab concentration-dependence curves for histamine release for the two groups are shown as is the absolute density of cell surface IgE density. Panel C; n=13 6061P concentration-dependence curves obtained from patients being treated with omalizumab, (●) before and (□) during treatment [24].
A second method to determine the contribution of IgE density to the position of the optimum is to compare two extremes of the natural population density distribution. For this study, the basophils of 14 subjects were examined for the dose response optimum to 6061P anti-IgE antibody and for their surface IgE density as measured by flow cytometry. For the data analysis, 7 donors were chosen at the two extremes of the density distribution (n=4 low, n=3 high) in this group and the anti-IgE dose response curves for the two groups compared. Figure 2B shows the comparison. The difference in surface IgE expression was 6.5-fold (7200 ± 2700 vs. 46000± 2600 IgE/basophil) and the optimum shifts approximately 8-fold.
As part of another study [24], 13 patients were evaluated for changes in their response to 6061P prior to and 16 weeks after treatment with omalizumab. This data (not presented in the published study but similar to a previously published result with fewer samples [5]) is shown in figure 2C, cast in two ways, one absolute and one normalized for the change in maximum release. It shows a 200-fold shift in the optimum response to 6061P during treatment following an approximately 3 fold decrease in FceRI (see below for further analysis).
Basophil Characteristics that may Distinguish Aggregation Dynamics
Mathematical models of aggregation characteristics for simple bivalent stimuli indicate that the crosslinking curve is simple is structure, rising and falling according to classical solution phase aggregate formation long appreciated for antibody-antigen reactions. However, the signal transduction pathways sense non-equilibrium aspects of the aggregation reaction, such as the rate that aggregates form. A recent model, presented in further detail in the ‘Discussion’ section suggest that the ratio of SYK expression to FceRI density is a characteristic of the reaction that contributes to the shape of the dose response curve. This question of SYK:FceRI was considered next.
Evaluation of the SYK:FceRI Ratio
All four of the omalizumab studies performed in this laboratory [5,9,25] have included the measurement of SYK and receptor expression and the first study included measurement of cell surface IgE. From the results of this first study, it was possible to use the receptor density determined in the other studies to also estimate the surface IgE density for those studies. In addition, at the time of the first study [25], the flow cytometric measurement of SYK was cross-calibrated with a quantitative Western blot method so that we could estimate absolute SYK expression (molecules/basophil] from either the flow cytometric measurement of SYK or the average histamine release. With this information, an estimate of the ratio of SYK:IgE-occupied FceRI (labeled as SYK:FceRI) could be made for any experimental series. Figure 3 shows average SYK:FceRI ratios for 4 published omalizumab studies and the estimates of this ratio from the stripping and natural IgE differences studies (figure 2) in this report. Figure 3A shows the relative difference in this ratio for the 3 types of studies, with the greatest change occurring in the omalizumab studies. The rank-order is commensurate with the amount of dose response curve shift observed in figures 2 and the results in MacGlashan et al [5]. An estimate of the ratios is shown in figure 3B-D. It is notable that the ratio of SYK:IgE/FceRI flips in each of these experiments from SYK expression being less than IgE/FceRI density to the reverse.
Fig. 3.
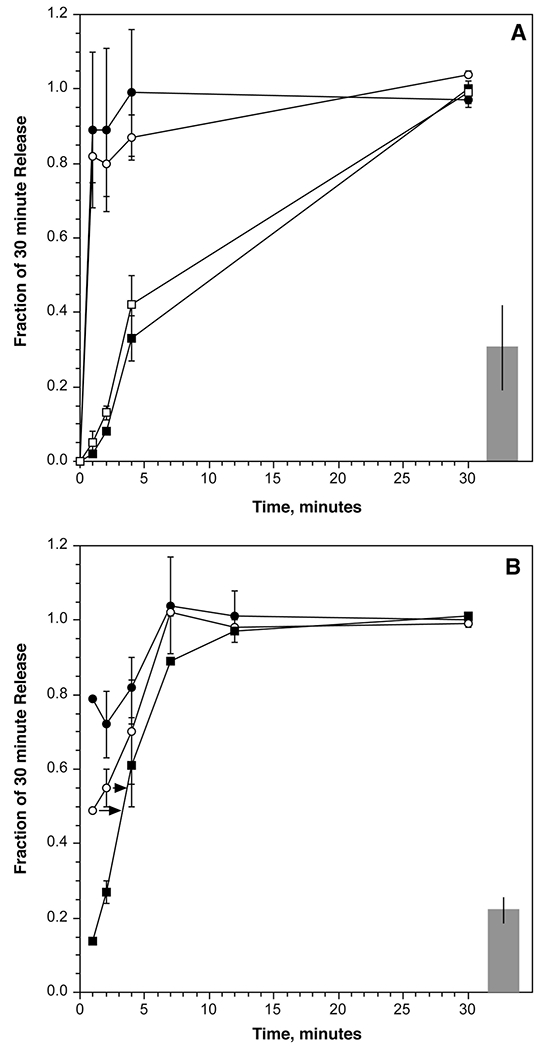
Using kinetic dilutions to assess the equilibration rate of an aggregating stimulus. Panel A: leukocytes were sensitized with BPO-specific IgE and stimulated with BPO2 (80 pM). At the time points shown, the reaction mixture was diluted as described in the methods and the reaction allowed to proceed until the 30 minute time before harvesting the supernatant (n=5); (▪) EDTA kinetic, (□) BPO-EACA (0.1 mM) kinetic, (○)dilution kinetic, (●)maintained concentration kinetic. Panel B: similar in design to the experiments in panel A but using 6061P anti-IgE Ab (0.8 μg/ml) to initiate the reaction (n=3); (▪) EDTA kinetic, (○)dilution kinetic, (●) maintained concentration kinetic. For both panels the data is expressed as a fraction of the release measured at the 30 minute time point without dilution. Also shown as the bar at the right of the plot is the release obtained with the concentration of diluted (20-fold) stimulus for the entire 30 minutes (relative to the initiating concentration release at 30 minutes). The initiating concentration, on average, induced 36±5% release for BPO2 and 70±12% release for 6061P. In panel B, the arrows indicate the time interval to that matches the dilution kinetic to the EDTA kinetic.
Crosslink stablility of BPO2 and 6061P anti-IgE Ab
The studies above do resolve why two bivalent stimuli would sense the SYK:FceRI ratio differently. This ratio would be different for different densities of BPO-specific IgE and yet the optimum for release is fixed across densities. A distinguishing characteristic for the two stimuli may be found in the stability of the crosslinks formed. This is a characteristic that is dependent on the affinity of the stimulus for its IgE. Crosslink stability can be assessed by testing how a rapid change in ligand concentration alters an ongoing histamine release reaction. This approach accounts for several variables, including the cell’s ability to sense re-equilibration of crosslinks. The rate of equilibration could be directly demonstrated for the aggregates formed by initiating a crosslinking reaction on basophils and then rapidly diluting the reaction [26]. If the concentration of the BPO2 was chosen to be in the ascending limb of the dose response curve and the crosslinks initially formed re-equilibrated rapidly, then the dilution would re-establish crosslinks commensurate with the lower concentration of BPO2. The result is a rapid cessation of release that could be detected by comparing the kinetics of histamine release to the rate of histamine release stopped by the addition of EDTA, which itself immediately stops the reaction. For the anti-BPO-specific IgE Ab used for the density studies in figure 1, we performed this measurement. Figure 4A shows the results of this experiment. One advantage of working with the BPO-based system is that a monovalent BPO (BPO-EACA) could also be used to stop the reaction. The kinetics when using BPO1 and EDTA to stop the reaction are similar. However, for this set of reagents, bound BPO2 did not rapidly re-equilibrate; dilution had no effect on the continuation of the reaction. In contrast, when performing a similar experiment with 6061P anti-IgE Ab, figure 4B shows that this bivalent ligand does equilibrate, not fast enough to yield a kinetic curve precisely like that generated with EDTA but requiring between 1 and 2 minutes to slow the reaction.
Fig. 4.
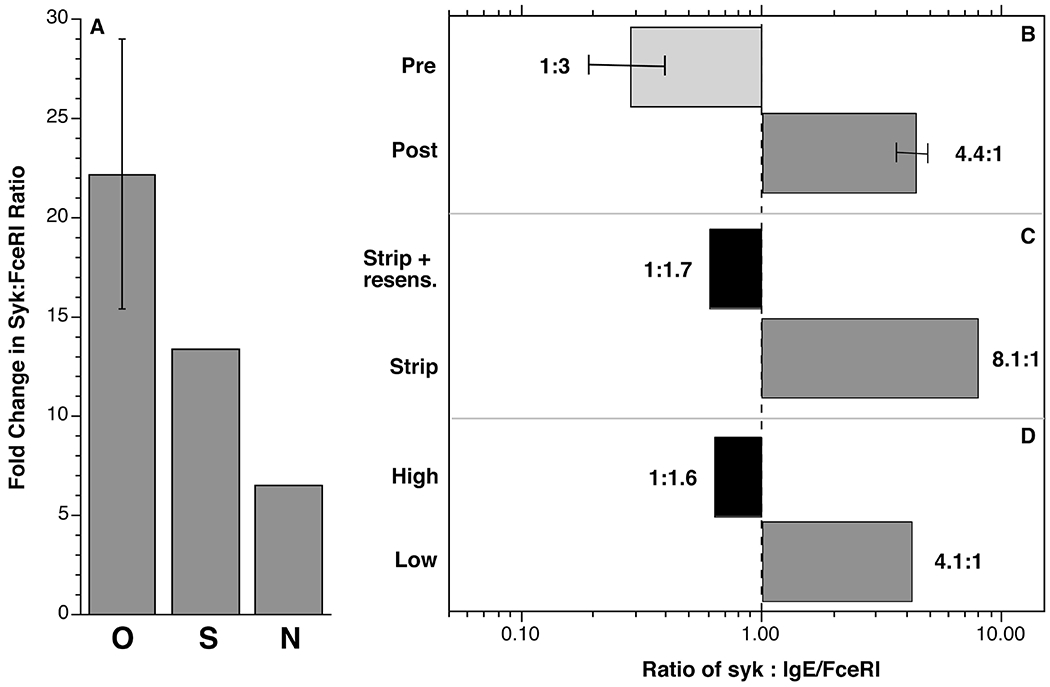
Ratio of SYK and FceRI expression in basophils from the 6061 experiments in this report. Panel A; changes in the ratio for the 3 experimental series, O = omalizumab study, S = IgE dissociation and resensitization series, N = natural variation in IgE density series. Panels B-D show the estimated absolute SYK:FceRI ratios for each of the 3 experimental series.
Discussion
Sensitivity of Dose Response Curves to Cell Surface IgE Density
The results shown in figure 1 demonstrate that over a broad range of densities (250-24000 IgE/basophil) that the position of optimum response for a simple bivalent ligand is nearly stable (at least relative to 6061P). This result anchors the idea that the variation observed for 6061P is unexpected.
The experiments with 6061P anti-IgE Ab demonstrate that a shift in the optimum can be observed by directly reducing the density of IgE with lactic acid treatment or observed in the natural behavior of dose response curves among subject’s whose basophils express different densities of IgE but were otherwise similar in their maximal IgE-mediated response (which results from roughly equivalent SYK expression). The more recent omalizumab results [5] (and the results in figure 2C) re-capitulate a smaller sampling of omalizumab-treated patients from a prior study [25] and provide a better sense of the average behavior during treatment with this biological agent.
All three types of experiments lead to the conclusion that cell surface IgE density controls the concentration for an optimal response to 6061P. Alone, two of the tests for this conclusion could be interpreted differently. For example, after dissociating IgE to perform the experiments in figure 2A, the cells were incubated with and without exogenous IgE. Therefore one group has a mixture of endogenous bound IgE and exogenous IgE while the other group has only endogenous bound IgE. The affinity of 6061P for the two situations may be different. However, as noted, in the pilot studies not shown, the dose response curve after re-sensitization was similar to the dose response without dissociation of endogenous IgE.
In the comparison of subjects with different IgE densities (figure 2B), there is no guarantee that the surface IgE Abs were not different between the two groups. But unless there is a property of IgE generation the leads to IgE Abs that differ with respect to binding 6061P anti-IgE Ab when IgE titers are low vs. high, the mixing of different subjects would be expected to lead to an average IgE behavior similar for the two groups.
For the case of omalizumab treatment, the comparison is within the same subject at two free IgE titers. There is no evidence that omalizumab modifies the generation of IgE in such a way that could lead to different IgE Abs that would bind to 6061P with different affinities.
Therefore, these experiments demonstrated the dependence of the optimum for 6061P on the density of cell surface IgE. Indeed, in many subsequent experiments we have found that cell surface IgE density is broadly predictable from a simple histamine release study by noting the position of the optimum.
Why the dependency on IgE density?
As noted above, these results create a problem for the theoretical underpinnings of crosslinking reactions. But as also noted above, the predictions for where the optimum for crosslinking occurs and its predicted lack of dependency on IgE density are only relevant to the actual aggregation reaction, which is not necessarily concordant with the function of the cell. Secretion, in this case, is dependent on the entirety of the signal transduction reaction that senses the presence of crosslinks. Mathematical modeling of the early IgE-mediated reaction has suggested a possible explanation [27] for IgE density dependence with simple aggregating stimuli. Figure 5 presents a simplified view of the results from a recent model [27] (an abstraction of a similar figure in the published report) where the ratio of SYK and aggregated receptor could be varied as needed. This model and its results were based on a considerable amount of experimental data but this particular prediction from the model has not been examined experimentally. It can be seen in figure 5 that low ratios of SYK:receptor alter the dose response curve by creating a curve with 2 peaks. Overall, the curve retains symmetry but if one is only capable of testing one side of the full curve, illustrated by graying out the right half, a change in ratio produces a shift in the optimal functional response (not the crosslinking curve, see the report for this information). The various characteristics of this curve are largely re-capitulated in the 6061P dose response curve before and after treatment with omalizumab (it would be practically difficult to examine the full breadth of the 6061P dose response curve since concentrations >tens of mg/ml would be needed on the high concentration side of the curve). Note that the middle portion of the curve at low ratios of SYK:FceRI does not return to baseline but flattens at a level well above baseline. This is the characteristic of the 6061P dose response curve normally observed [5] and it disappears when the ratio increases as it does after treatment. The dip in the middle varies according to the ratio as well and natural individual 6061P curves show variant forms of this behavior.
Fig. 5.
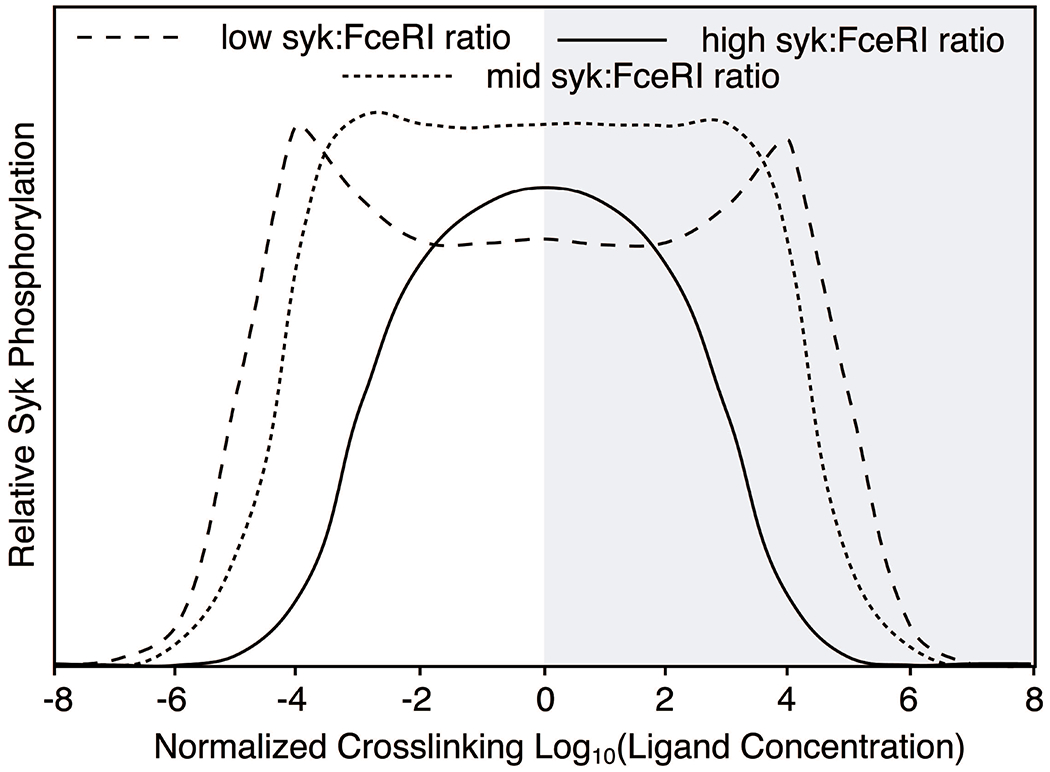
Predicted activation of a downstream signaling molecule in a model of the early IgE-medated reaction. The results were abstracted from data presented in reference [21] to highlight the differences in activation at 3 ratios of SYK:FceRI-IgE. The right half of the dose response curve is grayed-out to emphasize the portion of the curve that is practically accessible for 6061P anti-IgE Ab. Note that despite the “saddle” shape at low SYK:FceRI, the curve remains symmetric and that aggregate size is maximal at the center of the curve. It is only the downstream steps that experience the “saddle” characteristic.
In the model, the maximum response doesn’t change and this was found with the stripping experiments. In figure 2, the maximum release remained constant but the optimum shifted rightward. During treatment with omalizumab, the optimum shifted rightward and the maximum increased. Another unexpected characteristic of the basophil during omalizumab treatment is an increase in the expression of SYK [25]. In conjunction with the decrease in cell surface receptor and its bound IgE, the ratio of SYK :IgE-FceRI is exaggerated and the cell acquires more of a critical signaling element, in this case, SYK. As we have previously demonstrated, SYK is a rate-limiting component of the IgE-mediated reaction in basophils, typically tuned to lie on the cusp of allowing an IgE-mediated response [28]. Thus, the response to anti-IgE Ab results in both shifts in its optimum and its maximum response, unlike the results following stripping where absolute SYK levels do not change (as noted in the methods section, SYK expression pre and post stripping was not found to change).
Therefore, 6061P reveals an interesting aspect of signal transduction, probably because it operates on such an excess of receptors. The typical basophil has 100,000-150,000 receptors [14] while only expressing 25,000 molecules of SYK [28] but normal allergens don’t interact with this density of receptors since the average ratio of allergen-specific IgE to total IgE is 1% [29]. In other words, allergens don’t create the conditions that a pan-crosslinker like anti-IgE creates and don’t typically probe the relationship between SYK and receptor density. The behavior is probably also unique to monoclonal anti-IgE antibodies since only they are bivalent linkers. A long-used goat polyclonal anti-IgE Ab does not show this characteristic despite being a pan-crosslinker [6] but we and others have found that these curves are quite complex in structure, showing partial engagement of CD32b [30] and creating observable super-aggregates that change the signaling dynamic [31]. The curve-shift effect may also be restricted to monoclonal antibodies that do not interact with CD32b. We have found that mouse IgG1 antibodies interact with CD32b and this would complicate the character of a dose response curve but 6061P is a monomeric mouse IgM and it doesn’t bind to CD32b [30].
The shift in the optimum was consistent with the relative difference in IgE density and SYK expression in group’s being compared; 1) the greatest shift occurring during omalizumab treatment (200 fold shift), 2) followed by what was possible to create with lactic acid treatment (11 fold shift) and 3) finally, the natural difference among subjects with different IgE densities (8 fold)(at least for the survey done for this study).
Why the difference in behavior between BPO2 and 6061P?
Therefore, the question that follows is why there is a density dependence with 6061P, a bivalent stimulus, and the behavior for another simple bivalent ligand, BPO2? The results shown in figure 4 differentiate these two stimuli; 6061P re-equilibrates rapidly after dilution while BPO2 (with the BPO-specific IgE in this study) does not. We found a similarly rapid re-equilibration of 6061P when stimulating human lung mast cells [32]. The BPO-EACA results show that the BPO ligand is dissociating rapidly but the crosslink remains ‘broken’ only if there is a vast excess of a monovalent version present; rapid re-binding disallows crosslink ‘breaking’ and retains a signaling-effective crosslink. This kind of rapid re-binding apparently doesn’t occur effectively for 6061P. This would be consistent with forward rate constants being slower for large proteins. It may also result from only dimeric aggregates being formed with this stimulus. If the forward binding is slow and the dissociation fast, smaller aggregates form. This difference in the dynamics of crosslink formation is interesting for its implications because stability of the crosslink is important in signal generation [33,34]. Kinetic proofreading of the initial signal determines the nature of downstream signaling and reveals sensitivity to conditions not as apparent with stable signals. The IgE-mediated aggregation reaction appears to sense crosslink transience [33,34]. This leads to the tentative speculation that an unstable crosslink exposes the relevance of SYK-receptor ratios, unlike BPO2, leading to shifting optimums for histamine release.
Summary
This study demonstrates the unique characteristics of a low affinity bivalent monomeric IgE antibody. Although it was unexpected for a bivalent ligand to show sensitivity to receptor density, a recent modeling of the reaction suggests a possible influence (SYK:IgE ratios) in the reaction. It provides support for some of the modeling of this complex reaction proposed by others [21]. The results also provide an additional indication that the expression of SYK in human basophils is a rate-limiting step in its activation cascade and that expression has functional consequence beyond determining the magnitude of the basophil response to IgE-mediated stimulation. On a practical level, the results demonstrate that interpretation of changes in dose response curves for commonly used reagents may require considerations not normally part of the analysis, in this instance, that changes in receptor density (bound to IgE in this case) change the optimal response.
Acknowledgements
The authors also thank Valerie Alexander for her excellent technical assistance.
Funding
This study was supported by NIH grant UO1-AI100952.
Footnotes
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Sullivan AL, Grimley PM, Metzger H: Electron microscopic localization of immunoglobulin E on the surface membrane of human basophils. Journal of Experimental Medicine 1971;134:1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dembo M, Goldstein B: Theory of equilibrium binding of symmetric bivalent haptens to cell surface antibody: application to histamine release from basophils. J Immunol 1978;121:345–353. [PubMed] [Google Scholar]
- 3.Dembo M, Goldstein B, Sobotka AK, Lichtenstein LM: Histamine release due to bivalent penicilloyl haptens: Control by the number of cross-linked IgE antibodies on the basophil plasma membrane. J Immunol 1978;121:354–358. [PubMed] [Google Scholar]
- 4.MacGlashan DW Jr., Dembo M, Goldstein B: Test of a theory relating to the cross-linking of IgE antibody on the surface of human basophils. J Immunol 1985;135:4129–4134. [PubMed] [Google Scholar]
- 5.Macglashan DW Jr., Saini SS: Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol 2013;132:906–911 e901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacGlashan J DW, Bochner BS, Adelman DC, Jardieu PM, Togias A, Mckenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM: Down-regulation of FceRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol 1997;158:1438–1445. [PubMed] [Google Scholar]
- 7.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S: Omalizumab-induced reductions in mast cell Fce psilon RI expression and function. J Allergy Clin Immunol 2004;114:527–530. [DOI] [PubMed] [Google Scholar]
- 8.Gomez G, Jogie-Brahim S, Shima M, Schwartz LB: Omalizumab reverses the phenotypic and functional effects of IgE-enhanced Fc epsilonRI on human skin mast cells. J Immunol 2007;179:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacGlashan DW Jr., Savage JH, Wood RA, Saini SS: Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol 2012;130:1130–1135 e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGlashan DW Jr., Mogowski M, Lichtenstein LM: Studies of antigen binding on human basophils. II. Continued expression of antigen-specific IgE during antigen-induced desensitization. J Immunol 1983;130:2337–2342. [PubMed] [Google Scholar]
- 11.Levine BB: The nature of the antigen-antibody complexes which initiate anaphylactic reactions. II. The effect of molecular size on the abilities of homologous multivalent benzylpenicilloyl haptens to evoke PCA and passive arthus reactions in the guinea pig. J Immunol 1965;94:121–131. [PubMed] [Google Scholar]
- 12.Pruzansky JJ, Grammer LC, Patterson R, Roberts M: Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J Immunol 1983;131:1949–1953. [PubMed] [Google Scholar]
- 13.Gillespie E, Lichtenstein LM: Histamine from human leukocytes: Studies with deuterium oxide, colchicine and cytochalasin B. J Clin Invest 1972;51:2941–2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tung R, Lichtenstein LM: In vitro histamine release from basophils of asthmatic and atopic individuals in D2O. J Immunol 1982;128:2067–2072. [PubMed] [Google Scholar]
- 15.Siraganian RP: An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem 1974;57:383–394. [DOI] [PubMed] [Google Scholar]
- 16.MacGlashan DW Jr.: Releasability of human basophils: Cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol 1993;91:605–615. [DOI] [PubMed] [Google Scholar]
- 17.MacGlashan DW Jr.: Endocytosis, Re-cycling and Degradation of Unoccupied FceRI in Human Basophils. J Leuk Biol 2007;82:1003–1010. [DOI] [PubMed] [Google Scholar]
- 18.MacGlashan D Jr.: Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcepsilonRI expression characterized by other methods of desensitization. Clin Exp Allergy 2012;42:1060–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGlashan DW Jr., Ishmael S, Macdonald SM, Langdon JM, Arm JP, Sloane DE: Induced Loss of Syk in Human Basophils by Non-IgE-Dependent Stimuli. J Immunol 2008;180:4208–4217. [DOI] [PubMed] [Google Scholar]
- 20.Ishmael S, MacGlashan D Jr.: Early signal protein expression profiles in basophils: a population study. J Leukoc Biol 2009;86:313–325. [DOI] [PubMed] [Google Scholar]
- 21.Urata C, Watanabe A, Ogawa Y, Takei N, Nomoto H, Mizobe M, Abe Y, Mano K, Urata S: Effect of deuterium oxide (D2O) on the IgE-mediated Ca2+ influx, arachidonic acid and histamine release in rat basophilic leukemia cells. Arerugi 1989;38:285–295. [PubMed] [Google Scholar]
- 22.MacGlashan DW Jr., Peters SP, Warner J, Lichtenstein LM: Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol 1986;136:2231–2239. [PubMed] [Google Scholar]
- 23.MacGlashan DW Jr., Lavens-Phillips S: Characteristics of the free cytosolic calcium timelag following IgE-mediated stimulation of human basophils: Significiance for the non-releasing basophil phenotype. J Leuk Biol 2001;69:224–232. [PubMed] [Google Scholar]
- 24.MacGlashan DW Jr., Saini SS: Syk expression and IgE-mediated histamine release in basophils as biomarkers for predicting the clinical efficacy of omalizumab. J Allergy Clin Immunol 2017;139:1680–1682 e1610. [DOI] [PubMed] [Google Scholar]
- 25.Zaidi AK, Saini SS, Macglashan DW Jr.: Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol 2010;125:902–908 e907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dembo M, Goldstein B, Sobotka AK, Lichtenstein LM: Histamine release due to bivalent penicilloyl haptens: the relation of activation and desensitization of basophils to dynamic aspects of ligand binding to cell surface antibody. J Immunol 1979;122:518–528. [PubMed] [Google Scholar]
- 27.Nag A, Faeder JR, Goldstein B: Shaping the response: the role of FcepsilonRI and Syk expression levels in mast cell signaling. IET systems biology 2010;4:334–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacGlashan DW Jr.: Relationship Between Syk and SHIP Expression and Secretion from Human Basophils in the General Population. Journal of Allergy and Clinical Immunology 2007;119:626–633. [DOI] [PubMed] [Google Scholar]
- 29.Erwin EA, Ronmark E, Wickens K, Perzanowski MS, Barry D, Lundback B, Crane J, Platts-Mills TA: Contribution of dust mite and cat specific IgE to total IgE: relevance to asthma prevalence. J Allergy Clin Immunol 2007;119:359–365. [DOI] [PubMed] [Google Scholar]
- 30.Macglashan D Jr., Moore G, Muchhal U: Regulation of IgE-mediated signalling in human basophils by CD32b and its role in Syk down-regulation: basic mechanisms in allergic disease. Clin Exp Allergy 2014;44:713–723. [DOI] [PubMed] [Google Scholar]
- 31.Becker KE, Ishizaka T, Metzger H, Ishizaka K, Grimley PM: Surface IgE on human basophils during histamine release. Journal of Experimental Medicine 1973;138:394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis A, MacGlashan DW Jr., Suvarna SK, Peachell PT: Recovery from desensitization of IgE-dependent responses in human lung mast cells. Clin Exp Allergy 2017;47:1022–1031. [DOI] [PubMed] [Google Scholar]
- 33.Torigoe C, Inman JK, Metzger H: An unusual mechanism for ligand antagonism. Science 1998;281:568–572. [DOI] [PubMed] [Google Scholar]
- 34.Liu ZJ, Haleem-Smith H, Chen H, Metzger H: Unexpected signals in a system subject to kinetic proofreading. Proc Natl Acad Sci U S A 2001;98:7289–7294. [DOI] [PMC free article] [PubMed] [Google Scholar]


