Highlights
-
•
The concept of “efficiency” is often used to describe differences in brain activation between groups or individuals.
-
•
I argue that this concept is empty and simply redescribes the data.
-
•
I review different explanations for differences in activation and highlight the challenges in understanding these differences.
Keyword: Neuroimaging, Neural Energetics, Metabolism, fMRI, Response Time, Networks
Abstract
It is common in the cognitive neuroscience literature to explain differences in activation in terms of differences in the “efficiency” of neural function. I argue here that this usage of the concept of efficiency is empty and simply redescribes activation differences rather than providing a useful explanation of them. I examine a number of possible explanations for differential activation in terms of task performance, neuronal computation, neuronal energetics, and network organization. While the concept of “efficiency” is vacuous as it is commonly employed in the neuroimaging literature, an examination of brain development in the context of neural coding, neuroenergetics, and network structure provides a roadmap for future investigation, which is fundamental to an improved understanding of developmental effects and group differences in neuroimaging signals.
1. Introduction
As scientists we aim to help understand how the world works, which generally means providing mechanistic explanations of natural phenomena. For example, the synaptic plasticity theory of memory proposes that memories are created through the modulation of the strength of synapses between neurons via specific mechanisms such as NMDA-dependent long-term potentiation. Such an explanation tells us something about the putative causal structure of the mechanisms that generate the relevant data, which supports the generation of predictions about the effects of particular experimental interventions. For example, this theory predicts that manipulations that block NMDA receptor function should reduce the ability to form new memories (and indeed they do). In cognitive neuroscience, we possess only a small number of theories with similar explanatory power. For example, in the last 50 years memory research has moved from the initial establishment of the medial temporal lobe's role in memory (through the study of amnesic patients like H.M.) to a deep understanding of the circuitry of the medial temporal lobe and the computational role of different subregions (Milner et al., 1998). However, in many other domains of cognitive neuroscience, including developmental cognitive neuroscience, some very commonly used explanations are far less satisfying.
In the present paper I will focus on the concept of “efficiency” which is commonly used as an explanation in cognitive neuroscience. Perhaps the best known usage of the term comes from the “neural efficiency theory” of intelligence proposed by Haier and colleagues. For example:
A series of investigations in normal subjects indicate an inverse relationship between brain glucose metabolic rate (GMR) and psychometric measures of intelligence…These studies have been interpreted as evidence for a brain efficiency model of intelligence: Intelligence is not a function of how hard the brain works but rather how efficiently it works… This efficiency may derive from the disuse of many brain areas irrelevant for good task performance as well as the more focused use of specific task-relevant areas. (Haier et al., 1992)
Other examples are found throughout the literature across studies of development, aging, individual differences and learning.
-
•
“…improved handwriting is associated with increased computational efficiency or neural coding and hence reduced BOLD signal increase in the right IFG for reading-related functions.” (Gimenez et al., 2014).
-
•
“We show that increased neural efficiency and capacity, as reflected by more “youth-like” brain response patterns in regions of interest of the frontoparietal WM network, were associated with better behavioral training outcome” (Heinzel et al., 2014).
-
•
“These results suggest that lifelong bilingualism offsets age-related declines in the neural efficiency for cognitive control processes.” (Gold et al., 2013)
-
•
“…activity was significantly reduced for trained items so that a further increase from two to three items was observed. We interpret this difference as a correlate of a gain in neural efficiency…We assume that training causes a more efficient neural representation of trained items supported by long-term memory and this allows holding more items in working memory.” (Zimmer et al., 2012)
-
•
“These results indicate that a short regimen of [working memory] training is associated with lower prefrontal activation – a marker of neural efficiency – in divergent thinking.” (Vartanian et al., 2013)
In general, the term is used to describe situations where performance appears similar but activation is greater for one group (which is taken to be “less efficient”). It should be noted that there is a very different notion of efficiency that has arisen from the network analyses of brain connectivity; I discuss this further below, but here I am focused on the use of the concept to describe differences in univariate activation.
2. Is “efficiency” really an explanation?
Upon closer examination it is clear that “efficiency” generally fails as a scientific explanation when used in this way. An analogy is instructive. Let's say that we are interested in understanding individual differences in gas mileage between different makes of automobiles. We perform an experiment in which we drive two cars (a hybrid Toyota Prius and a gas-only Porsche Carrera) from Los Angeles to San Francisco along exactly the same route, and we measure their fuel consumption. The results of the experiment show that the Prius uses 1/2 as much fuel as the Porsche to travel the same distance. Here are two possible explanations of this difference in fuel consumption:
-
1.
The Prius has a gas-electric hybrid engine (which uses surplus engine power to generate electricity which is then turned back into drive power) and regenerative braking (which captures energy that would otherwise be lost as heat).
-
2.
The Prius is more efficient.
None of us would accept the latter as a suitable explanation for the difference in fuel consumption; in fact, we would likely recognize immediately that the second “explanation” is not an explanation at all, but rather simply a redescription of the data.
I propose that the common usage of the concept of “efficiency” in cognitive neuroscience is equally vacuous. Looking back at the list of quotes above, in each case it is apparent that the term “efficiency” is simply a redescription of the phenomenon of reduced activation; although it sounds like it is explaining the result, it does not tell us any more about the mechanism, and implies no additional experiments that one might do to test the explanation. To the degree that we judge a scientific explanation with regard to its production of new testable hypotheses, the efficiency explanation fails completely when used in this way.
This is not to argue that concept of efficiency is intrinsically useless; to the contrary, a better understanding of the relation between energy expenditure and neural computations is fundamental to understanding developmental differences in neuroimaging signals. Take any developmental experiment where groups are compared and differences in activation are observed on a particular comparison of task conditions. These differences could reflect any of the following biological differences:
-
•
A different set of cognitive processes is being performed. For example, a child with Tourette syndrome participating in an fMRI study is effectively in a dual task situation, performing the specified cognitive task while also actively attempting to suppress their tics, whereas a healthy child would not experience this secondary response inhibition demand.
-
•
A different neural computation is performed to complete the same task. For example, in an pseudoword pronunciation task, one group pronounces the stimulus through grapheme-to-phoneme conversion, while a more skilled group pronounces it using analogy to known words. Alternatively, one group represents a stimulus domain using a dense coding scheme, whereas another represents it using sparse coding.
Neither of these captures what is generally meant by the concept of efficiency, i.e. reduced energy expenditure for the same work. However, there are several other potential explanations that come closer to this concept:
-
•
The same neural computation is being performed for the same amount of time, but with different intensity. For example, in one group neurons in the relevant region fire at 20 Hz for 100 ms, while in the other group neurons fire at 10 Hz for the same amount of time.
-
•
The same neural computation is performed at the same intensity, but for different lengths of time. For example, in one group neurons fire at 20 Hz for 100 ms, while in the other group the same neurons fire at 20 Hz for 50 ms.
Finally, there is one explanation that comes closest to capturing the essence of the concept of neural cost-efficiency:
-
•
The same neural computation is performed with identical time and intensity, but the metabolic expenditure differs between the groups. For example, the groups could differ in the amount of transmitter release, the nature of neurovascular coupling (e.g. due to differences in contact between astrocytes and neurons), or the degree to which they rely upon oxidative versus non-oxidative metabolism.
The point of laying out each of these other possibilities is to highlight that while there is a way in which the concept of efficiency can be well-specified as a plausible explanation of activation differences that makes non-trivial predictions, doing so requires confronting a number of difficult questions which are rarely if ever addressed in the cognitive neuroscience literature.
3. Explaining differences in activation
Because many researchers in cognitive neuroscience (including but not limited to developmental cognitive neuroscientists) are fundamentally interested in understanding differences in brain activity between individuals at different points in time, we desperately need better explanations for such differences (cf. Poldrack, 2000). Here I will outline in greater depth the potential explanations for differential activation mentioned above.
3.1. Is the set of cognitive processes the same?
We nearly always assume that the cognitive processes being performed by the subject are strictly defined by the experimental paradigm that the subject is presented with, but it is clear that this is often an invalid assumption. In particular, the requirements of the fMRI acquisition environment (i.e. lying very still and quiet in a small tube for an extended period of time) represent a “meta-task” that the subject must perform in order to comply with the experimenter's demands. Whereas most adults have little trouble exerting the executive control necessary to accomplish this meta-task, for children or individuals with executive control disorders one might consider the meta-task demands to essentially be a demanding secondary task. If the primary task is one that engages cognitive processes that overlap with the meta-task (e.g. response inhibition, working memory), then one might expect interactions with the experimental task that could result in activation differences (e.g. enhanced activation due to overload, or reduced activation due to ceiling effects on activation). Similar concerns arise regarding differential task difficulty; if a task is much easier for one group than another, then differences could reflect the fact that subjects in the easy group are engaging in additional task-independent thought during performance of the experimental task.
3.2. Is the computation the same?
The next major question regarding the interpretation of activation differences is whether the same neural computations are being performed. There are numerous ways in which the computations underlying task performance can change with learning and development. Most notable is the fact that as experience accrues, it is often possible to perform a task based on memory for prior experiences rather than through application of rules or brute force computation (Logan, 1988). For example, there is a hypothesis in reading development that initial application of grapheme-to-phoneme conversion rules is supplanted by direct access to phonological word forms from visual forms for familiar words (Grainger et al., 2012). Similarly, acquisition of mirror-reading skill is thought to progress from initial visuospatial transformation to later use of direct visual recognition (Poldrack et al., 1998). If the computations being performed differ between groups, then labeling the differences as using “efficiency” is invalid because the work being performed differs; an analogy would be comparing the fuel expended to travel from Los Angeles to San Francisco, where one car travels via the interstate and the other follows the Pacific Coast Highway.
Determining empirically whether the same computation is being performed is challenging because it rests on accepting a null hypothesis. Strategies could include the use of behavioral manipulations to assess the effects of relevant variables on behavior. For example, in a mental rotation task where the subject compares two block figures (Shepard and Metzler, 1971), one could measure the slope of response times as a function of the angle of rotation; major changes in this slope would suggest that the task is being performed in a different manner. Similarly one might ask whether the same factors cause behavioral interference between the groups. Using fMRI one might attempt to establish whether different networks are active, or whether the same network is active to different degrees, though this will be statistically challenging. Overall, it is straightforward to show that different computations are being used but fundamentally challenging to show that the computation has not changed.
Changes in the nature of neuronal information coding could also result in differences in activation. In particular, the concept of “efficient coding” (Barlow, 1961) suggests that energy usage is minimized when information is coded in a way that reduces the redundancy of information across neurons, which leads to the development of sparse codes (cf. Olshausen and Field, 1996). There has been little study of how the sparseness of neuronal coding changes with development in mammalian cortex, but recent work has demonstrated that sparseness of coding in ferret visual cortex decreases rather than increases across development (Berkes et al., 2011), which is inconsistent with the decreased activation generally observed in the context of developmental neuroimaging. Nonetheless, this suggests that in order to fully identify whether the same computation is being performed, it would be necessary to examine the way in which neural coding may have changed. This is rarely possible in humans, but could be examined in animal models.
3.3. Intensity and timing are (nearly) indistinguishable with fMRI
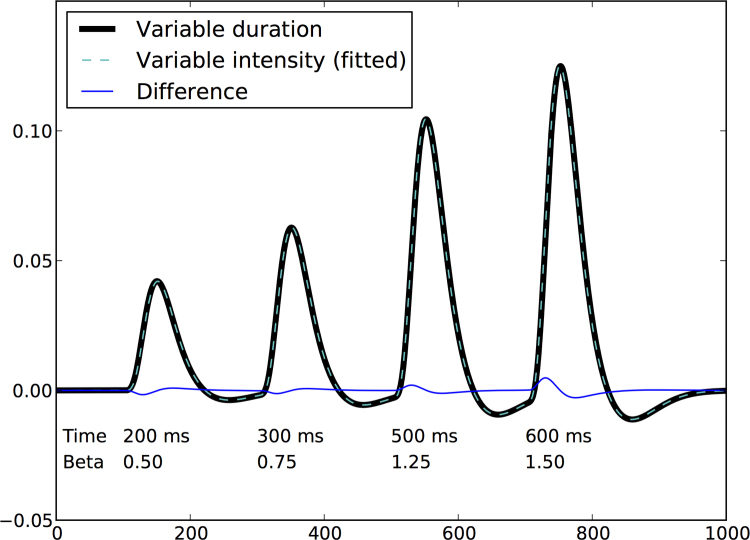
We can next ask how differences in intensity and timing of neural signaling affect fMRI signals, and whether they can be distinguished. This is particularly pertinent given that experience and maturation both lead to the ability to perform tasks more quickly, and it is rare to see a neuroimaging finding in these domains that is not accompanied by changes in response times, even in cases where one has good reason to believe that the same computation is being performed. Further, these differences in time on task are associated with regionally specific differences in activation regardless of the specific task. For example, (Yarkoni et al., 2009) found that differences in response time (RT) between task conditions were associated with differences in activation in prefrontal and other regions in five different studies using different tasks. Unfortunately, it is almost impossible to distinguish changes in activation due to differential intensity from changes due to time on task. Fig. 1 shows that differences in activation timing can be mimicked almost exactly by differences in intensity, which leads to a fundamental difficulty in the interpretation of activation changes. Fortunately, RT can be measured independently and used in the statistical model to correct for the effects of time on task (cf. Grinband et al., 2008), and the foregoing demonstration highlights the absolute necessity of such corrections. If RT is not modeled, then there is no way to know whether differences in activation are due to differences in time on task versus true differences in activation intensity. Conversely, if one shows activation differences after having properly removed the effects of time on task across groups, then this provides some degree of confidence that the results do indeed reflect differences in the level of activation intensity. However, even when RT has been modeled as a nuisance factor, it will be very difficult to fully remove its effects (e.g. due to nonlinearities) and thus it will always be difficult to interpret activation differences that are accompanied by behavioral differences in RT.
Fig. 1.
Differences in processing time are very difficult to distinguish from differences in intensity of activation. The thick black line reflects data generated for four trials that vary in processing times (as listed in the figure) through convolution with a standard SPM hemodynamic response function. The dashed gray line reflects the results of fitting a model to those data in which differences between trials are based on the intensity of the activation, using a fixed duration of 400 ms (with the fitted intensity for each trial presented as “beta” in the figure). The black and dashed gray lines are almost indistinguishable (difference between these functions is plotted in blue), highlighting the fact that the effects of processing time and activation intensity are for practical purposes indistinguishable, both resulting in similarly increased activation. Code to generate this figure is available from https://github.com/poldrack/rtmodel. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Neural energetics and activation differences
As noted above, another possible explanation of activation differences is that the computation remains the same in quality, time, and intensity, but the energy used to perform the computation differs. This most closely approaches the usual meaning of the concept of efficiency, i.e. the amount of energy needed to perform a given amount of work. There is a large number of biophysical mechanisms by which the same neuronal firing pattern could consume a differential amount of energy.
The energy “budget” for neuronal signaling in the cerebral cortex involves action potentials (21%), synaptic processes (59%), and resting potentials (20%) (Howarth et al., 2012). The energetic efficiency of action potentials lies largely in the overlap of the inward (Na+) and outward (K+) currents, and varies greatly between classes of neurons; for example, squid giant axons are highly inefficient, whereas cortical and thalamic neurons in rodents are highly efficient (Sengupta et al., 2010). Further, metabolic efficiency varies across different parts of the neuron (Hallermann et al., 2012). These data suggest that any changes in neuronal structure could potentially result in changes in energy usage; the time course of any such changes appears to be currently unknown. Similarly, changes in the nature of synaptic signaling, such as the well known changes in synaptic density (i.e. “pruning”) that accompany brain development along with changes in the relative abundance of NMDA receptors and metabotropic signaling, could impact the relative efficiency of signaling with development. These suggestions only scratch the surface of the potential biophysical causes for differential energy efficiency of neuronal signaling; my point is simply to highlight how little we currently understand about the potential causes of true differences in neural efficiency.
Using a combination of neuronal recordings and 2DG, Picard et al. (2013) recently demonstrated the first evidence of experience-dependent changes in the energy usage of neural activity. Monkeys were trained to perform a internally generated motor task over an extended period (up to 6 years), and then compared performance of this task with an untrained (visually guided) motor task. Neuronal recordings in motor cortex revealed no differences in spike rates between the two tasks, but 2-deoxy-glucose uptake differed significantly between the conditions, suggesting a difference in energy usage in the face of seemingly identical neuronal computations. While this kind of approach is likely to remain unfeasible with human subjects (particularly with children), it does highlight the fact that specific hypotheses about the relation between neuronal computation and energetics can be addressed using existing methods in nonhuman animal models. It may also be possible to address some of these questions through combinations of imaging and stimulation techniques in humans.
Another potentially interesting source of developmental differences in energetic efficiency is the degree to which brain activity relies upon different energetic mechanisms across development. Neurons can rely upon a number of different energetic pathways, including oxidative phosphorylation (Kety, 1957, Sibson et al., 1998, Sokoloff, 1960), aerobic glycolysis (Fox et al., 1988), and metabolism of ketone bodies (Sokoloff, 1973), which vary greatly in their energetic efficiency (e.g. oxidative phosphorylation can generate 15 times more ATP than glycolysis using the same amount of glucose; Fox et al. (1988)). The brain's use of these different energetic pathways is known to vary with development. In particular, a recent meta-analysis by Goyal et al. (2014) showed that aerobic glycolysis is greatly increased during childhood, and that regional increases in glycolysis are associated with expression of genes related to synaptic development. There are also known changes in the expression of genes relevant to energetics, such as the glucose transporter (Vannucci and Vannucci, 2000). A better understanding of developmental changes in neural energetics across development and their relation to functional imaging signals could provide important calibration to help understand the degree to which development effects in imaging studies reflect neuronal versus energetic differences, in the same way that studies of neurovascular coupling in aging have helped provide guidance in the interpretation of aging-related imaging signals (e.g., Gazzaley and D’Esposito, 2004)
5. Network efficiency
The total amount of energy consumed by neuronal computations depends not just upon the function of individual neurons, but also on how those neurons are connected to one another. With the advent of graph-theoretical approaches to the analysis of neuroimaging data (Bullmore and Sporns, 2009), there is increasing evidence regarding the cost-efficiency of large-scale brain network structure (Bullmore and Sporns, 2012), where “efficiency” in this case is defined in terms of cost of transmitting information within the network. In particular, it appears that brain networks are organized in a way that approaches the maximum possible cost-efficiency, though the embedding of complex topological structure that maximizes complexity while minimizing transmission costs (Bassett et al., 2010). This approach provides an alternative but well-formulated conception of “efficiency” that can be tested using both structural and functional MRI data.
Recent work has begun to examine individual differences in the efficiency of brain network structure and function. Cao et al. (2014) measured the topological efficiency of resting state networks in a group of participants ranging from 7 to 87 years old, where efficiency is defined as the inverse of the mean path length between each pair of nodes in the network (Latora and Marchiori, 2001). They found no relation between age and the overall efficiency of brain networks, whereas there was an inverted-u shaped relation between age and local efficiency (which is the average efficiency of the subnetwork formed by each node). Similarly, a study of children from 5 to 18 years of age showed no relation between global efficiency and age but a positive relationship between local efficiency and age (Wu et al., 2013) (cf. also Supekar et al., 2009). Similar results have appeared for structural connectivity. For example, Dennis et al. (2013) examined global and local efficiency of structural networks identified using diffusion tensor imaging in a large sample ranging from 12 to 30 years of age, and found no relation between global efficiency and age but a significant relation between local efficiency in a number of regions (including hub-like regions such as posterior cingulate and medial prefrontal cortex). A study of the very early development of brain networks based on gray matter volume showed that global efficiency increases gradually from one month of age to two years (Fan et al., 2011). Together these data begin to suggest that while global efficiency reaches adult-like levels fairly early in development, local efficiency continues to change across the span of development. One current unknown is how these changes in local network efficiency might related to changes in task activation; this is an important question for future research. In addition, developmental differences in resting state connectivity may suffer from the same problems noted above regarding task activation; in particular, children may require greater effort to engage the meta-task of remaining still during the scan, which would presumably affect network structure.
6. Conclusion
“Efficiency” is such a facile explanation that it is easy to miss the emptiness that is inherent in its usual application to neuroimaging data. This is not uncommon when everyday terms are used to describe psychological phenomena. For example, Navon (1984) made a very compelling argument that the concept of “mental resources” (which was commonly used by cognitive psychologists in the 1970s and remains in common usage amongst cognitive neuroscientists) was similarly non-explanatory, a “soup stone” in his parlance. Here I have laid out the problems with the concept of efficiency as it is usually applied within the neuroimaging literature, and examined a number of alternative explanations for differences in activation signals. This analysis uncovers deep problems for the interpretation of developmental changes in neuroimaging signals, and highlights the need for additional work using animal models to examine the ways in which neuronal energetics change in concert with developmental experience and brain maturation.
Conflict of interest statement
The author has no conflicts of interest to declare for this manuscript.
Acknowledgments
Thanks to Jessica Church-Lang, Sarah Helfinstein, Tom Schonberg, and Tal Yarkoni for helpful comments on an earlier draft.
Footnotes
Available online 13 June 2014
References
- Barlow H. Sensory Communication. 1961. Possible principles underlying the transformation of sensory messages; pp. 217–234. http://redwood.berkeley.edu/w/images/f/fd/02-barlow-pr-1954.pdf. [Google Scholar]
- Bassett D.S., Greenfield D.L., Meyer-Lindenberg A., Weinberger D.R., Moore S.W., Bullmore E.T. Efficient physical embedding of topologically complex information processing networks in brains and computer circuits. PLoS Comput. Biol. 2010;6(4):e1000748. doi: 10.1371/journal.pcbi.1000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkes P., Orb’an G., Lengyel M., Fiser J. Spontaneous cortical activity reveals hallmarks of an optimal internal model of the environment. Science. 2011;331(6013):83–87. doi: 10.1126/science.1195870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10(3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cao M., Wang J.-H., Dai Z.-J., Cao X.-Y., Jiang L.-L., Fan F.-M., Song X.-W., Xia M.-R., Shu N., Dong Q., Milham M.P., Castellanos F.X., Zuo X.-N., He Y. Topological organization of the human brain functional connectome across the lifespan. Dev. Cogn. Neurosci. 2014;7:76–93. doi: 10.1016/j.dcn.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E.L., Jahanshad N., McMahon K.L., de Zubicaray G.I., Martin N.G., Hickie I.B., Toga A.W., Wright M.J., Thompson P.M. Development of brain structural connectivity between ages 12 and 30: a 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013;64:671–684. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Shi F., Smith J.K., Lin W., Gilmore J.H., Shen D. Brain anatomical networks in early human brain development. Neuroimage. 2011;54(3):1862–1871. doi: 10.1016/j.neuroimage.2010.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.T., Raichle M.E., Mintun M.A., Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gazzaley A., D’Esposito M. BOLD functional MRI and cognitive aging. In: Cabeza R., Nyberg L., Park D., editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. Oxford University Press; New York, NY, US: 2005. pp. 107–131. [Google Scholar]
- Gimenez P., Bugescu N., Black J.M., Hancock R., Pugh K., Nagamine M., Kutner E., Mazaika P., Hendren R., McCandliss B.D., Hoeft F. Neuroimaging correlates of handwriting quality as children learn to read and write. Front. Hum. Neurosci. 2014;8:155. doi: 10.3389/fnhum.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.T., Kim C., Johnson N.F., Kryscio R.J., Smith C.D. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J. Neurosci. 2013;33(2):387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M.S., Hawrylycz M., Miller J.A., Snyder A.Z., Raichle M.E. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19(1):49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J., Lété B., Bertand D., Dufau S., Ziegler J.C. Evidence for multiple routes in learning to read. Cognition. 2012;123(2):280–292. doi: 10.1016/j.cognition.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Grinband J., Wager T.D., Lindquist M., Ferrera V.P., Hirsch J. Detection of time-varying signals in event-related fMRI designs. Neuroimage. 2008;43(3):509–520. doi: 10.1016/j.neuroimage.2008.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier R.J., Siegel B., Tang C., Abel L., Buchsbaum M.S. Intelligence and changes in regional cerebral glucose metabolic rate following learning. Intelligence. 1992;16(3–4):415–426. http://www.sciencedirect.com/science/article/pii/016028969290018M (Special Issue: Biology and Intelligence) [Google Scholar]
- Hallermann S., de Kock C.P.J., Stuart G.J., Kole M.H.P. State and location dependence of action potential metabolic cost in cortical pyramidal neurons. Nat. Neurosci. 2012;15(7):1007–1014. doi: 10.1038/nn.3132. [DOI] [PubMed] [Google Scholar]
- Heinzel S., Lorenz R.C., Brockhaus W.-R., Wüstenberg T., Kathmann N., Heinz A., Rapp M.A. Working memory load-dependent brain response predicts behavioral training gains in older adults. J. Neurosci. 2014;34(4):1224–1233. doi: 10.1523/JNEUROSCI.2463-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C., Gleeson P., Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32(7):1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety S.S. Metabolism of the Nervous System. 1957. The general metabolism of the brain in vivo; pp. 221–237. [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Logan G.D. Toward an instance theory of automatization. Psychol. Rev. 1988;95:492–527. [Google Scholar]
- Milner B., Squire L.R., Kandel E.R. Cognitive neuroscience and the study of memory. Neuron. 1998;20(3):445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Navon D. Resources: a theoretical soup stone? Psychol. Rev. 1984;91:16–22. [Google Scholar]
- Olshausen B.A., Field D.J. Natural image statistics and efficient coding. Network. 1996;7(2):333–339. doi: 10.1088/0954-898X/7/2/014. [DOI] [PubMed] [Google Scholar]
- Picard N., Matsuzaka Y., Strick P.L. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat. Neurosci. 2013;16(9):1340–1347. doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A. Imaging brain plasticity: conceptual and methodological issues – a theoretical review. Neuroimage. 2000;12(1):1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Desmond J.E., Glover G.H., Gabrieli J.D. The neural basis of visual skill learning: an fMRI study of mirror reading. Cereb. Cortex. 1998;8(1):1–10. doi: 10.1093/cercor/8.1.1. [DOI] [PubMed] [Google Scholar]
- Sengupta B., Stemmler M., Laughlin S.B., Niven J.E. Action potential energy efficiency varies among neuron types in vertebrates and invertebrates. PLoS Comput. Biol. 2010;6:e1000840. doi: 10.1371/journal.pcbi.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard R.N., Metzler J. Mental rotation of three-dimensional objects. Science. 1971;171(3972):701–703. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Sibson N.R., Dhankhar A., Mason G.F., Rothman D.L., Behar K.L., Shulman R.G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. U.S.A. 1998;95(1):316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. vol. 3. 1960. The metabolism of the central nervous system in vivo; pp. 1843–1864. (Handbook of Physiology – Neurophysiology). [Google Scholar]
- Sokoloff L. Metabolism of ketone bodies by the brain. Annu. Rev. Med. 1973;24:271–280. doi: 10.1146/annurev.me.24.020173.001415. [DOI] [PubMed] [Google Scholar]
- Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci R.C., Vannucci S.J. Glucose metabolism in the developing brain. Semin. Perinatol. 2000;24(2):107–115. doi: 10.1053/sp.2000.6361. [DOI] [PubMed] [Google Scholar]
- Vartanian O., Jobidon M.-E., Bouak F., Nakashima A., Smith I., Lam Q., Cheung B. Working memory training is associated with lower prefrontal cortex activation in a divergent thinking task. Neuroscience. 2013;236:186–194. doi: 10.1016/j.neuroscience.2012.12.060. [DOI] [PubMed] [Google Scholar]
- Wu K., Taki Y., Sato K., Hashizume H., Sassa Y., Takeuchi H., Thyreau B., He Y., Evans A.C., Li X., Kawashima R., Fukuda H. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PLoS ONE. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Barch D.M., Gray J.R., Conturo T.E., Braver T.S. Bold correlates of trial-by-trial reaction time variability in gray and white matter: a multistudy fMRI analysis. PLoS ONE. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer H.D., Popp C., Reith W., Krick C. Gains of item-specific training in visual working memory and their neural correlates. Brain Res. 2012;1466:44–55. doi: 10.1016/j.brainres.2012.05.019. [DOI] [PubMed] [Google Scholar]