Abstract
Periacetabular osteotomy (PAO) is an effective surgical treatment for hip dysplasia. The goal of PAO is to reorient the acetabulum to improve joint stability, lessen contact stresses and slow the development of hip arthrosis. During PAO, the acetabulum is repositioned to adequately cover the femoral head. PAO preserves the weight-bearing posterior column of the pelvis, maintains the acetabular blood supply and retains the hip abductor musculature. The surgical technique needed to perform PAO is technically demanding, with correct repositioning of the acetabulum the most important—and challenging—aspect of the procedure. Imageless navigation has proven useful in other technically challenging surgeries, although its use in PAO has not yet been investigated. We have modified the standard technique for PAO to include the use of an imageless navigation system to confirm acetabular fragment position following osteotomy. Here, we describe the surgical technique and discuss the potential of this modified technique to improve patient-related outcomes.
BACKGROUND
Developmental hip dysplasia is the most common cause of secondary osteoarthritis [1]. The insufficient coverage of the femoral head and disproportionately shallow acetabulum, characteristic of hip dysplasia, are associated with labral hypertrophy, and altered forces on the acetabular labrum due to abnormal weight-bearing forces and decreased joint stability [2–6]. The incidence of hip dysplasia ranges between 1.7% and 20% in the general population, although most studies report an incidence between 3% and 5% [7–11]. The etiology of hip dysplasia is multifactorial, as both genetics and environmental factors are known to play a role [12]. If left untreated, hip dysplasia may cause substantial pain, decreased function and eventual progression to hip osteoarthritis.
For young, active patients with healthy hip cartilage, periacetabular osteotomy (PAO) is the preferred surgical option to improve mechanical loading conditions related to hip dysplasia. The procedure is technically demanding, with a substantial learning curve [13, 14]. Accurately reorienting the acetabulum to a position that normalizes cartilage loading is critically important, and is largely done by visual cues, without a reliable way to discretely measure intraoperative correction. Imageless navigation has proven useful in primary and revision hip arthroplasty [15, 16] to ensure precise and accurate position of acetabular components. Extrapolating the use of imageless navigation used for total hip arthroplasty (THA), we believe there is a potential role for use of similar navigation technology to guide acetabular re-orientation in PAO. We describe a technique for use of imageless navigation in PAO and the potential benefits of its incorporation into the standard surgical technique.
SURGICAL TECHNIQUE
Computer-assisted navigation
The Intellijoint HIP® (Intellijoint Surgical, Inc., Waterloo, ON, Canada) is a US Food and Drug Administration-cleared, 3D mini-optical navigation system approved for use in primary and revision THA. The system incorporates into a standard THA surgical workflow with minimal disruption [17]. Detailed descriptions of the use of the device in both lateral approach [18–20] and anterior approach [21] THA have been described previously. Successful off-label use of the device has also been documented in cases of THA for Legg-Calve-Perthes Disease, and for hip resurfacing [15, 16]. The navigation system consists of a camera, an optical tracker and a laptop workstation. The workstation is situated outside of the sterile field, while the camera and other related equipment remain in the sterile operative field. This device uses optical technology, infrared light and integrated microelectronics to provide data in the THA application regarding acetabular position, as well as leg length and offset, which are relayed to the workstation in real-time and are available for surgeon reference. During surgery, the camera is fixed to the iliac crest and captures the movements and position of the tracker, which can be magnetically fixed to the greater trochanter via a femoral platform (for posterolateral approach THA), or to various instruments during surgery, via a magnetic V-block. For example, attachment of the tracker to a surgical probe allows for specific measurement of objects or distances. All data are relayed from the tracker to the camera and are stored on the laptop workstation.
Preoperative preparation
As in standard PAO, a thorough patient history containing the patient’s age, body mass index, level of activity and functional goals as well as a physical examination, are necessary prior to surgery. This should include an assessment of gait, leg length, joint stability and range of motion. Radiographs should include anteroposterior (AP) pelvic, oblique (e.g. false profile) and lateral views [22]. Kamath et al. [23] further recommend functional abduction and internal rotation radiographs to ensure the ability for containment. This information (patient history and preoperative radiographs) will allow the surgeon to produce a patient-specific, preoperative plan based on the patient’s own anatomy. Magnetic resonance imaging may also be considered, as assessment of secondary cartilage damage, labral tears, ruptures of the ligamentum teres, or other intraarticular pathologies may be prudent.
Positioning and navigation installation
The PAO is performed according to the original technique described by Ganz et al. [24] with some individual surgeon specific variations. The patient is placed in a supine position on a radiolucent table. The operative leg is draped free. Intraoperative fluoroscopy may be used to monitor PAO cuts and acetabular orientation. For intraoperative imaging, the pelvic position is adjusted until at neutral rotation is achieved with respect to fluoroscopic projection. The caudad/cephalad orientation is adjusted to recreate the position of the pelvis on preoperative AP pelvis view. The image may require minor rotation (e.g. 5° increments) adjustments to simulate the radiographic projection of an AP pelvic radiograph.
In this modified technique utilizing optical navigation, the navigation camera is installed on the contralateral iliac crest. Per the standard procedure for the navigation device, two surgical pins are inserted into the iliac crest through stab incisions. A small magnetic platform is fixed to the surgical pins and provides a mounting point for the camera, which is aimed at the planned incision on the contralateral hip. The patient’s initial position is then registered by touching a tracker probe to both anterior superior iliac spines (ASIS) and the pubic symphysis, which establishes the orientation of the anterior pelvic plane (Fig. 1). A non-sterile or sterile electrocardiogram lead may be placed on the symphysis or contralateral ASIS as necessary for precise landmarking. As the device is fixed to the patient during surgery, any patient movement is accounted for by the device and positional data are provided relative to the current position of the patient, not the registration position. Following registration, the camera can be detached from its platform as needed without disrupting the registered patient orientation.
Fig. 1.
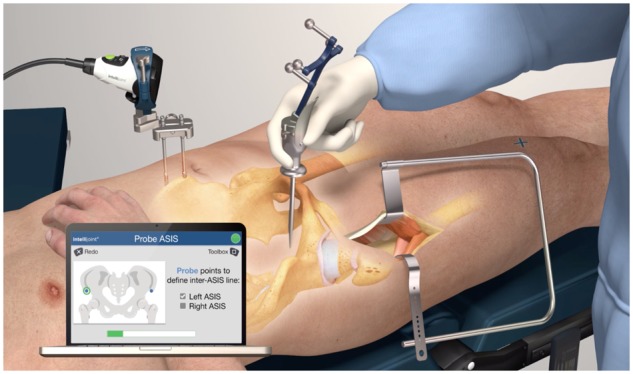
Animation of intra-operative utilization of the Intellijoint HIP® 3D mini-optical navigation tool.
Incision, exposure and osteotomies
The incision and approach have been well-described by Ganz et al. [24]. The first osteotomy is a complete cut of the superior pubic ramus. An Eva retractor is placed under the superior pubic ramus in the area of the obturator canal in order to protect the obturator neurovascular bundle. A straight osteotome or narrow saw is used to perform this cut. The tip of the osteotome is placed at the level of the teardrop of the acetabulum as seen on the AP view of the hip. The osteotome is directed at a 45° angle from anterolateral to posteromedial in line with the superior pubic ramus.
The second osteotomy begins at the ischium, where an incomplete cut is made, as the posterior column of the innominate bone is to remain intact when the osteotomy is complete. A 30° forked osteotome is required for the procedure. We typically use a narrow-bladed osteotome. The osteotome is placed on the hip flexed to at least 70° and the iliopsoas tendon is retracted anteriorly. The blade of the osteotome is inserted through the elevated portion of the periosteum, along the inferior border of the superior pubic ramus. The osteotome runs under the psoas tendon, distal to the superior ramus on the anterior surface of the ischium. The cut is typically located in the subcotyloid groove, just inferior to the posterior horn of the acetabulum. This portion of the ischium has a broad cortical surface and generally requires two separate cuts. The first cut is on the medial cortical surface, while the second cut is on the lateral surface. The hip is extended slightly and abducted during this stage of the osteotomy to protect the sciatic nerve. Intraoperative fluoroscopy can be used to assist in visualization of the osteotomy at this stage. The tip of the osteotome can be palpated on the ischium by feeling the contour of the obturator ring along the inferior aspect of the quadrilateral surface. AP and iliac oblique fluoroscopic views can be used to ensure the ischial cut remains extra-articular and does not violate the posterior border of the innominate bone.
The third cut of the osteotomy is made from the anterior aspect of the iliac wing to a point approximately 1 cm superolateral to the brim of the true pelvis. This is the only cut performed with a large oscillating saw. The level of the osteotomy is determined on the AP view with intraoperative fluoroscopy. The cut is made to preserve a minimum of 3–5 cm of intact bone superior to the acetabulum on AP view. Performing this cut at the level of the ASIS may be satisfactory in some patients. In more severe cases of dysplasia, the level of this cut will need to be superior to the ASIS on the iliac crest to maintain adequate bone stock above the acetabulum for later fixation. A narrow strip of abductor musculature on the external surface of the iliac wing is elevated with cautery to allow passage of a blunt Hohmann retractor to protect the neurovascular structures when using the oscillating saw. In general, it is preferable to err by extending the cut more posteriorly into the iliac wing than leaving the cut too anterior, as an excessively short or anterior cut can result in difficultly completing the osteotomy proximally, and in extreme cases can lead to an intra-articular osteotomy. Note that bleeding is increased from the bone itself upon completion of this cut.
The fourth cut begins at the posterior-most extent of the third cut on the internal aspect of the iliac wing. In the original Ganz technique, the quadrilateral surface cut was a scoring of the bone. The technique has evolved to a formal cut of the bone of the posterior column. The cut is made by directing a straight or curved osteotome, depending on the body habitus of the patient, the width of the retro-acetabular space and the amount of dysplasia present, from this point across the pelvic brim and parallel to the greater sciatic notch. An iliac oblique view is used to view the trajectory of the osteotome. In many cases, performing this cut will complete the lateral portion of the osteotomy at the iliac wing. Note that when this occurs, there is distal and lateral displacement of the proximal portion of the acetabular segment. A curved osteotome can be used to redirect the path of the quadrilateral surface limb of the osteotomy.
The initial Bernese technique describes a fifth cut, made with an osteotome angled at 50°, and intended to connect the quadrilateral surface cuts with the subcotyloid groove cortex. With the use of the lateral cut of the subcotyloid groove of the ischium, it is now common that the fifth cut is unnecessary in the majority of cases. If this cut is made, the osteotome is directed from medial to lateral, best seen on an iliac oblique view projection. Attention to placement is critical during this step, as the posteriorly placed osteotome can unintentionally disrupt the posterior column.
Following all osteotomy cuts, a Schanz screw is placed at the level of the anterior inferior iliac spine (AIIS), directed into the intact bone of the mobile acetabular segment. In severely dysplastic hips, confirming placement of the Schanz pin on the fluoroscopy is useful, as occasionally the AIIS will be at an intra-articular level in advanced dysplasia. If the proximal portion of the acetabular fragment did not displace with the quadrilateral surface cut, the proximal portion of the osteotomy should be completed next. In that case, a wide flat osteotome is inserted into the full length of the third cut made in the iliac wing. A Farabeuf clamp, Weber clamp, ball-spike pusher, or pliers are used to rotate the osteotome to mobilize the proximal portion of the osteotomy. With the use of this technique, it is generally not necessary to perform a cut on the lateral surface of the iliac wing. The osteotome is then replaced into the full length of the quadrilateral surface cut and is rotated with simultaneous gentle flexion stress to the acetabular fragment with the Schanz pin. This combination of maneuvers completes the osteotomy. Completion can be confirmed with motion of the acetabular fragment with the Schanz pin. If the acetabulum does not move easily, all cuts are remobilized with the osteotome until the acetabular fragment is freely mobile.
Correction
Following osteotomy, the correction begins by using the fluoroscopy to view the pelvis from an AP perspective. The correction begins with anterior rotation or flexion of the acetabular fragment, followed by lateral rotation and finally version correction. In general, for correction of dysplastic hips without retroversion, the pubic root of the acetabular fragment will rotate to a position superior to the intact remnant of the superior pubic ramus. The radiographic features of an adequate correction include: the teardrop is elevated or moves superiorly, the acetabular dome is made more horizontal, the anterior rim is medial to the posterior rim and the hip center is medialized.
The navigation device is used to confirm the orientation of the corrected acetabular fragment (Fig. 2). Using a magnetic V-block, the tracker is attached to a surgical probe, which is used to probe the acetabular rim. The rim is probed in three distinct locations (e.g. superior, inferolateral and inferomedial borders), which allows for calculation of the acetabular orientation (anteversion and inclination) in real-time. Once this step is completed, any movement of the acetabular rim is shown on the imageless navigation device screen and available for the surgeon to reference. Note that baseline measurements for comparison should be made prior to completion of the osteotomy cuts. Any necessary adjustments can be made intraoperatively, using the probe to reconfirm orientation until the desired positioning is achieved. Once in a suitable position, the V-block is removed and the acetabular fragment is fixed in position with cortical screws, per the standard PAO technique. Typically, two to three screws from the iliac wing into the acetabular fragment and a third screw from the acetabular fragment into the intact posterior iliac wing.
Fig. 2.
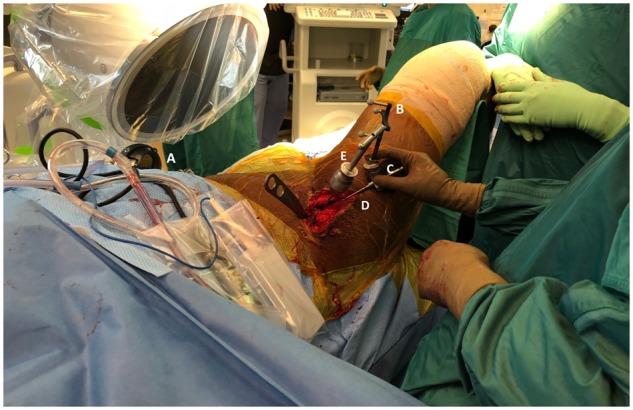
The Intellijoint HIP® 3D mini-optical navigation tool in clinical use during PAO surgery. The camera (A), enclosed in its sterile drape, is attached to the contralateral iliac crest via two screws (out of view). The tracker (B) is magnetically attached via a V-block adaptor (C) to a surgical probe (D) to allow for real-time measurement of acetabular orientation. Adjustments to acetabular fragment orientation can be made with the Schanz pin connected to a T-handle (E), with the probe and tracker used to independently confirm positioning.
Repositioning the acetabular fragment into the ideal position is the most important aspect of the PAO procedure. The following parameters are assessed by the image intensifier: (i) Tönnis acetabular angle of <10°; (ii) lateral center edge angle of 25°–40°; (iii) distance between the medial femoral head to the ilioischial line of 0–10 mm; (iv) anterior acetabular rim located on the superior medial third of the femoral head and the posterior acetabular rim located on the inferior lateral part of the femoral head (i.e. preservation of appropriate acetabular version); and (v) anterior center edge angle of 25°–40°. We also recommend using the preoperative morphologic character of the obturator foramen on the pelvic X-ray as a basic reference to avoid the malposition of the acetabular fragment. The imageless navigation system can be referenced for the achievement of these appropriate angular parameters. The imageless navigation system is used to remove the guesswork in relation to pelvic position and tilt and surgeons can reference that information to see in real-time the relation to the patient’s own tilt.
Closure
The practice of capsulotomy following correction and fixation in PAO is now commonly accepted and allows for both inspection for potential impingement with the proximal femur as well as access to inspect the labrum at the time of the procedure. Alternatively, arthroscopy or no intra-articular viewing may be chosen based on surgeon preference. Following correction, the ASIS on the operative side is typically prominent anteriorly. This prominence is trimmed prior to closure. A screw is placed to reduce the osteotomized ASIS fragment to the ilium (versus heavy suture fixation), thus reattaching the inguinal ligament, sartorius and tensor fascia lata. The navigation camera is detached, and the mounting pins are removed. Primary wound closure is completed per surgeon preference.
DISCUSSION
Since its introduction more than 30 years ago, the Bernese PAO has proven to be an effective procedure to address dysplasia and to restore hip function in patients long-term [25, 26]. However, execution of this technique can be technically challenging. The primary goal of PAO, and the main challenge of the surgery, is the adequate repositioning of the osteotomized acetabular fragment. Currently, intraoperative fluoroscopy and radiography provide the most reliable feedback regarding fragment orientation, although they do not provide any objective numerical information about the position of the acetabulum. Here, we discuss a modification of the standard PAO surgical technique to incorporate an imageless navigation system, which provides real-time data regarding acetabular orientation. Given the narrow range in which the hip joint orientation can be placed safely and the associated importance of intraoperative precision [27], the use of navigation during PAO may represent an important evolution of this technique. This modification has the potential to improve patient outcomes in both the short- and long-term.
There have been several contemporary reports of the use of computer-assisted navigation during PAO. One of the first was by Langlotz et al. [28], who reported 12 patients who underwent PAO with computed tomographic-based navigation. They reported no complications in this cohort up to 10.5 months of follow-up. Another randomized clinical trial by Hsieh et al. [29] compared two groups of 18 patients undergoing PAO: one group with image-guided navigation during surgery and the other performed with conventional surgery. The average number of intraoperative radiographs for the conventional group was 4.4, whereas the computer-assisted group had a mean of 0.6 radiographs per patient. Additionally, an average of 21 min less of operative time was seen in the computer-assisted group, with no significant differences with respect to operative blood loss, transfusion requirement, correction of deformity and functional improvement.
Previous studies have proven that the imageless navigation device used in this technique accurately has delivered intraoperative measurements of acetabular anteversion and inclination in both anterior and posterior/lateral surgical approach THA [18, 19, 21, 30–33]. Capabilities of imageless navigation may surpass the capabilities of traditional fluoroscopy-guided THA and provide the added benefit of reduced radiation exposure to the patient and surgeon [34].
To our knowledge, this is the first report of a surgical technique for PAO incorporating imageless—rather than an image-based—navigation. While image-based computer-assisted navigation systems may provide the surgeon with a detailed preoperative plan, they are unable to provide intraoperative information to the surgeon. This intraoperative information can be crucial to success in the PAO, as incorrect positioning of the acetabular fragment can have significant negative consequences. Additionally, the integration of navigation into the PAO technique may provide a benefit regarding surgeon training and may help to reduce the learning curve associated with PAO, while also potentially improving outcomes for patients.
CONCLUSION
Technology with the ability to reduce complications should be integrated into orthopedic practice to ensure high-quality patient care. The use of imageless navigation technology in challenging surgeries such as PAO should be an ongoing consideration for surgeons, as it provides vital information on acetabular position in real-time and allows the surgeon to precisely target a patient-specific position when repositioning the acetabular fragment. Intraoperative precision carries the potential for long-term durability of the PAO and improves patient outcomes.
ACKNOWLEDGEMENTS
The authors would like to thank Andre Hladio (M.ASc.) and Joseph Schipper (B.ASc.) for their assistance with the integration of the Intellijoint HIP system.
FUNDING
None.
CONFLICT OF INTEREST STATEMENT
R.R.M. and J.M.M. are employees of Intellijoint Surgical, Inc.
REFERENCES
- 1. Okano K, Yamada K, Takahashi K. et al. Joint congruency in abduction before surgery as an indication for rotational acetabular osteotomy in early hip osteoarthritis. Int Orthop 2010; 34: 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Domb BG, Lareau JM, Baydoun H, Botser I. et al. Is intraarticular pathology common in patients with hip dysplasia undergoing periacetabular osteotomy? Clin Orthop Relat Res 2014; 472: 674–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujii M, Nakashima Y, Jingushi S. et al. Intraarticular findings in symptomatic developmental dysplasia of the hip. J Pediatr Orthop 2009; 29: 9–13. [DOI] [PubMed] [Google Scholar]
- 4. Hartig-Andreasen C, Søballe K, Troelsen A.. The role of the acetabular labrum in hip dysplasia: a literature overview. Acta Orthop 2013; 84: 60–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCarthy JC, Lee JA.. Acetabular dysplasia: a paradigm of arthroscopic examination of chondral injuries. Clin Orthop Relat Res 2002; 405: 122–8. [DOI] [PubMed] [Google Scholar]
- 6. Ross JR, Zaltz I, Nepple JJ. et al. Arthroscopic disease classification and interventions as an adjunct in the treatment of acetabular dysplasia. Am J Sports Med 2011; 39: 72S–8S. [DOI] [PubMed] [Google Scholar]
- 7. Jacobsen S, Sonne-Holm S.. Hip dysplasia: a significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology (Oxford) 2005; 44: 211–8. [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen S, Sonne-Holm S, Soballe K. et al. Hip dysplasia and osteoarthrosis: a survey of 4151 subjects from the Osteoarthrosis Substudy of the Copenhagen City Heart Study. Acta Orthop 2005; 76: 149–58. [DOI] [PubMed] [Google Scholar]
- 9. Gosvig KK, Jacobsen S, Sonne-Holm S. et al. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. J Bone Joint Surg Am 2010; 92: 1162–9. [DOI] [PubMed] [Google Scholar]
- 10. Ortiz-Neira CL, Paolucci EO, Donnon T.. A meta-analysis of common risk factors associated with the diagnosis of developmental dysplasia of the hip in newborns. Eur J Radiol 2012; 81: e344–51. [DOI] [PubMed] [Google Scholar]
- 11. Engesæter IØ, Laborie LB, Lehmann TG. et al. Prevalence of radiographic findings associated with hip dysplasia in a population-based cohort of 2081 19-year-old Norwegians. Bone Joint J 2013; 95-b: 279–85. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Quality Improvement, Subcommittee on Developmental Dysplasia of the Hip. Clinical practice guideline: early detection of developmental dysplasia of the hip. American Academy of Pediatrics. Pediatrics 2000; 105: 896–905. [DOI] [PubMed] [Google Scholar]
- 13. McKinley TO. The Bernese Periacetabular Osteotomy: review of reported outcomes and the early experience at the University of Iowa. Iowa Orthop J 2003; 23: 23–8. [PMC free article] [PubMed] [Google Scholar]
- 14. Hussell JG, Rodriguez JA, Ganz R.. Technical complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res 1999; 363: 81–92. [PubMed] [Google Scholar]
- 15. Shah RR, Gobin V, Muir JM.. Imageless navigation improves intraoperative monitoring of leg length changes during total hip arthroplasty for Legg-Calve-Perthes disease: two case reports. Case Rep Orthop 2018; 2018: 4362367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vigdorchik JM, Elbuluk A, Benson JR, Muir JM.. Birmingham hip resurfacing using a novel mini-navigation system: a case report. J Orthop Case Rep 2018; 8: 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christ A, Ponzio D, Pitta M. et al. Minimal increase in total hip arthroplasty surgical procedural time with the use of a novel surgical navigation tool. Open Orthop J 2018; 12: 389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paprosky WG, Muir JM.. Intellijoint HIP((R)): a 3D mini-optical navigation tool for improving intraoperative accuracy during total hip arthroplasty. Med Dev (Auckland, NZ) 2016; 9: 401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grosso P, Snider M, Muir JM.. A smart tool for intraoperative leg length targeting in total hip arthroplasty: a retrospective cohort study. Open Orthop J 2016; 10: 490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vigdorchik JM, Cross MB, Bogner EA. et al. A cadaver study to evaluate the accuracy of a new 3D mini-optical navigation tool for total hip arthroplasty. Surg Technol Int 2017; 30: 447–54. [PubMed] [Google Scholar]
- 21. Parvizi J, Benson JR, Muir JM.. A new mini-navigation tool allows accurate component placement during anterior total hip arthroplasty. Med Dev (Auckland, NZ) 2018; 11: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henle P, Tannast M, KA S.. Bildgebende Diagnostik der Hüftdysplasie. Orthopade 2008; 1: 525–31. [DOI] [PubMed] [Google Scholar]
- 23. Kamath AF. Bernese periacetabular osteotomy for hip dysplasia: surgical technique and indications. World J Orthop 2016; 7:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganz R, Klaue K, Vinh TS, Mast JW.. A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop Relat Res 1988; 232: 26–36. [PubMed] [Google Scholar]
- 25. Siebenrock KA, Schöll E, Lottenbach M, Ganz R.. Bernese periacetabular osteotomy. Clin Orthop Relat Res 1999; 363: 9–20. [PubMed] [Google Scholar]
- 26. Steppacher SD, Tannast M, Ganz R, Siebenrock KA.. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res 2008; 466: 1633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou Y, Kang X, Li C. et al. Application of a 3-dimensional printed navigation template in Bernese periacetabular osteotomies: a cadaveric study. Medicine (Baltimore) 2016; 95: e5557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langlotz F, Stucki M, Bachler R. et al. The first twelve cases of computer assisted periacetabular osteotomy. Comput Aided Surg 1997; 2: 317–26. [DOI] [PubMed] [Google Scholar]
- 29. Hsieh PH, Chang YH, Shih CH.. Image-guided periacetabular osteotomy: computer-assisted navigation compared with the conventional technique: a randomized study of 36 patients followed for 2 years. Acta Orthop 2006; 77: 591–7. [DOI] [PubMed] [Google Scholar]
- 30. Vigdorchik JM, Muir JM, Buckland A. et al. Undetected intraoperative pelvic movement can lead to inaccurate acetabular cup component placement during total hip arthroplasty: a mathematical simulation estimating change in cup position. J Hip Surg 2017; 01: 186–93. [Google Scholar]
- 31. Cross MB, Schwarzkopf R, Miller TT. et al. Improving registration accuracy during total hip arthroplasty: a cadaver study of a new, 3-D mini-optical navigation system. Hip Int 2018; 28: 33–9. [DOI] [PubMed] [Google Scholar]
- 32. Schwarzkopf R, Muir JM, Paprosky WG. et al. Quantifying pelvic motion during total hip arthroplasty using a new surgical navigation device. J Arthroplasty 2017; 32: 3056–60. [DOI] [PubMed] [Google Scholar]
- 33. Schwarzkopf R, Vigdorchik JM, Miller TT. et al. Quantification of imaging error in the measurement of cup position: a cadaveric comparison of radiographic and computed tomography imaging. Orthopedics 2017; 40: e952–8. [DOI] [PubMed] [Google Scholar]
- 34. Bradley MP, Benson JR, Muir JM.. Accuracy of acetabular component positioning using computer-assisted navigation in direct anterior total hip arthroplasty. Cureus 2019; 11: e4478. doi: 10.7759/cureus.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]


