Key Points
Question
How do patterns of blood pressure (BP) change over the life course and differ between sexes?
Findings
In this analysis of 4 community cohort studies, trajectories of BP elevation in 32 833 individuals (54% women) were examined serially over 4 decades (age span, 5 to 98 years). Women compared with men exhibited a steeper increase in BP measures that began as early as in the third decade and continued throughout the life course.
Meaning
Sex differences in BP trajectories, which begin early and persist with aging, may set the stage for later-life cardiovascular diseases that frequently present differently in women vs men.
Abstract
Importance
If we assume that women and men exhibit variations of the same fundamental vascular physiology, then conventional analyses of subclinical measures would suggest that women catch up to men by midlife in the extent of potentially important vascular disease. Alternatively, under the assumption that vascular physiology may fundamentally differ between women and men, a sex-specific analysis of existing data could offer new insights and augment our understanding of sex differences in cardiovascular diseases.
Objective
To evaluate whether longitudinal patterns of blood pressure (BP) elevation differ between women and men during the life course when considering baseline BP levels as the reference.
Design, Setting, and Participants
We conducted sex-specific analyses of longitudinal BP measures (144 599 observations) collected for a period of 43 years (1971 to 2014) in 4 community-based US cohort studies. The combined total included 32 833 participants (54% female) spanning ages 5 to 98 years. Data were analyzed between May 4, 2019, and August 5, 2019.
Exposures
Age and serially assessed longitudinal BP measures: systolic BP, diastolic BP, mean arterial pressure (MAP), and pulse pressure (PP).
Main Outcomes and Measures
Sex-specific change in each primary BP measure compared with baseline BP levels, derived from multilevel longitudinal models fitted over the age span, and new-onset cardiovascular disease events.
Results
Of the 32 833 participants, 17 733 were women (54%). Women compared with men exhibited a steeper increase in BP that began as early as in the third decade and continued through the life course (likelihood ratio test χ2 = 531 for systolic BP; χ2 = 123 for diastolic BP; χ2 = 325 for MAP; and χ2 = 572 for PP; P for all <.001). After adjustment for multiple cardiovascular disease risk factors, these between-sex differences in all BP trajectories persisted (likelihood ratio test χ2 = 314 for systolic BP; χ2 = 31 for diastolic BP; χ2 = 129 for MAP; and χ2 = 485 for PP; P for all <.001).
Conclusions and Relevance
In contrast with the notion that important vascular disease processes in women lag behind men by 10 to 20 years, sex-specific analyses indicate that BP measures actually progress more rapidly in women than in men, beginning early in life. This early-onset sexual dimorphism may set the stage for later-life cardiovascular diseases that tend to present differently, not simply later, in women compared with men.
This study evaluates whether longitudinal patterns of blood pressure elevation differ between women and men during the life course when considering baseline blood pressure levels as the reference.
Introduction
Over the last 2 decades, mounting evidence has highlighted differences between women and men in the manifestation of common cardiovascular diseases (CVDs). A prevailing perception is that women are affected by the same types of CVD that affect men, albeit with delayed onset and often atypical symptoms. However, with respect to ischemic heart disease (IHD) and heart failure (HF), it is now increasingly recognized that women are more likely than men to develop coronary microvascular dysfunction (CMD) and HF with preserved ejection fraction (HFpEF), especially in the setting of vascular risk factors such as hypertension.1,2,3 In effect, the broadening clinical experience of managing CVD conditions that manifest differently between women and men, combined with the accumulating data on sex-specific CVD presentations, suggest that cardiovascular pathophysiology is likely to be fundamentally different between the sexes. If true, then intrinsic sexual dimorphism in cardiovascular pathophysiology must plausibly extend from sexual dimorphism in cardiovascular physiology. To better understand how sex differences in earlier-life cardiovascular physiology may precede sex differences in later-life cardiovascular pathophysiology, we used population-based multicohort data to conduct a comprehensive sex-specific analysis of blood pressure (BP) trajectories over the life course, given that measures of BP elevation in the community represent the single most accessible metric of vascular aging as well as the largest contributor to IHD and HF risk in both women and men.4,5,6,7
Methods
Using approved access data from the National Heart, Lung, and Blood Institute BioLINCC repository, we aggregated serially examined BP measurements collected longitudinally from participants of 4 community cohorts whose study designs have been described previously: the Framingham Heart Study (FHS) offspring cohort,4 the Atherosclerosis Risk in Communities (ARIC) Study,8 the Coronary Artery Risk Development in Young Adults (CARDIA) Study,9 and the Multi-Ethnic Study of Atherosclerosis (MESA).10 Each participant provided written informed consent, and institutional review boards approved the study protocol at each site. A total of 32 845 unique participants from FHS (examinations 1-9), ARIC (visits 1-4), CARDIA (examinations 1-8), and MESA (examinations 1-4) were eligible for inclusion. We excluded individual-level observations from analyses if concurrent data were missing for BP measures or antihypertensive medication (eFigure 1 in the Supplement).
Systolic BP (SBP) and diastolic BP (DBP) were assessed for seated participants who had been resting for at least 5 minutes using a mercury column sphygmomanometer in FHS, Hawksley random zero sphygmomanometer in ARIC and CARDIA (examinations 1-6), Omron model HEM907XL oscillometric BP monitor in CARDIA (examinations 7-8), and automated oscillometric device (Dinamap Monitor Pro 100) in MESA. To adjust for between-method heterogeneity, we used the mercury column sphygmomanometer (FHS) as reference and adjusted BP measures in ARIC by SBP plus 2.6 mm Hg and DBP plus 6.2 mm Hg and adjusted BP measures in MESA by SBP plus 0.5 mm Hg and DBP plus 2.9 mm Hg based on a previously described correction method.11,12 For CARDIA, we first calibrated oscillometric values to Hawksley random zero sphygmomanometer measures (step 1)13 and then adjusted BP measures to mercury column sphygmomanometer by SBP plus 2.6 mm Hg and DBP plus 6.2 mm Hg (step 2).11 To account for the treatment effects of antihypertensive therapy on blood pressure,14 we imputed untreated values by adding 10 mm Hg to SBP and 5 mm Hg to DBP values observed in the setting of antihypertensive therapy,15,16 and mean arterial pressure (MAP) and pulse pressure (PP) were calculated based on these imputed values: PP = SBP − DBP and MAP = DBP + (1/3) PP. In addition, we conducted a sensitivity analysis using alternate approaches to imputation, including (1) adding 15 mm Hg to SBP and 10 mm Hg to DBP and (2) adding 15 mm Hg to SBP and 5 mm Hg to DBP.
Mixed-effects regression models were used to display BP trajectories in women and men separately, with age used as the common timescale for all analyses. We used repeated BP measures to fit mixed-effects linear regression models with each BP measure as the outcome, participant indentifications as random intercepts, and age as a fixed effect expressed using restricted cubic splines with 4 knots to allow for nonlinear relationships. Mean values were estimated for each BP measure over an age range of 18 (0.5th percentile) to 85 (99.5th percentile) years for women and men separately. Within the premise of sex-specific physiology, we then calculated BP change from the baseline BP level as well as the differences in BP increment between women and men. Differences between sexes in the associations between BP measures and age were tested via likelihood ratio test between models with and without parameters representing the interaction between sex and the cubic spline variables representing age. Mixed-effects regression models were further adjusted for covariates including body mass index (calculated as weight in kilograms divided by height in meters squared), total cholesterol, diabetes mellitus, and current smoking status. In secondary analyses, we repeated all models stratified by race/ethnicity (white individuals vs black individuals) as well as by cohort and antihypertensive medication use (with vs without antihypertensive therapy).
To understand sex-specific BP trajectories in context, we examined sex-specific CVD incidence in our multicohort sample. We defined incident hard CVD as new-onset fatal or nonfatal myocardial infarction, HF, or stroke, adjudicated using established criteria that are known to be largely similar across all cohorts.9,17,18 We estimated the Kaplan-Meier cumulative incidence of hard CVD by sex. All analyses were performed using R, version 3.5.1 (R Foundation for Statistical Computing) and Stata, version 15 (StataCorp). All P values were 2-sided, with a significance level of 0.05.
Results
A total of 32 833 unique participants, each contributing at least 1 observation with both BP and antihypertensive medication data available (eFigure 1 in the Supplement), were included for analyses: 5120 participants from FHS (after 4 were excluded), 15 786 from ARIC (6 excluded), 5113 from CARDIA (2 excluded), and 6814 from MESA (0 excluded). These participants (32 833 total; 17 733 women [54%]) contributed 144 599 observations during a 43-year period (1971-2014), with ages spanning 5 to 98 years. During 4 decades of follow-up, 8130 participants (24.8%) had developed new-onset hard CVD events (eTable in the Supplement).
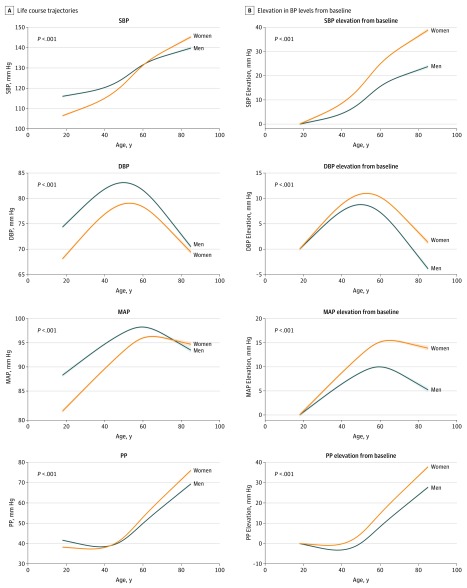
Figure 1A displays BP values for both sexes on the same vertical axis, with trends suggesting that BP levels in women appear to catch up to BP levels in men by midlife. When the data are displayed with sex-specific values set to represent change from baseline BP level (ie, elevation from baseline), allowing comparison of older individuals with their younger selves over time, another pattern emerges (Figure 1B). In effect, as early as the third decade of life, women compared with men exhibited faster rates of progressive BP elevation with aging (likelihood ratio test χ2 = 531 for systolic BP; χ2 = 123 for diastolic BP; χ2 = 325 for MAP; and χ2 = 572 for PP; P for all <.001). When considering women-to-men difference in BP change, as displayed in Figure 2, all BP components, including systolic BP, diastolic BP, PP, and MAP, increased more predominantly in women compared with men over the life course.
Figure 1. Sex-Specific Trajectories of Blood Pressure (BP).
Life course trajectories of BP elevation are displayed on the same vertical axis in panel A, suggesting that BP levels in women catch up to men after midlife. Under the premise of sex-specific physiology, elevation in BP levels from baseline for both sexes is shown in panel B, indicating that BP elevation in women begins earlier and progresses more rapidly in women than in men over the life course. For BP trajectory plots, restricted cubic splines were fitted from mixed-effects linear regression models. P values for sex differences in BP trajectories were derived from the likelihood ratio test for models with and without parameters representing the interaction between sex and the cubic spline variables representing age. Shaded regions denote 95% CI. DBP indicates diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
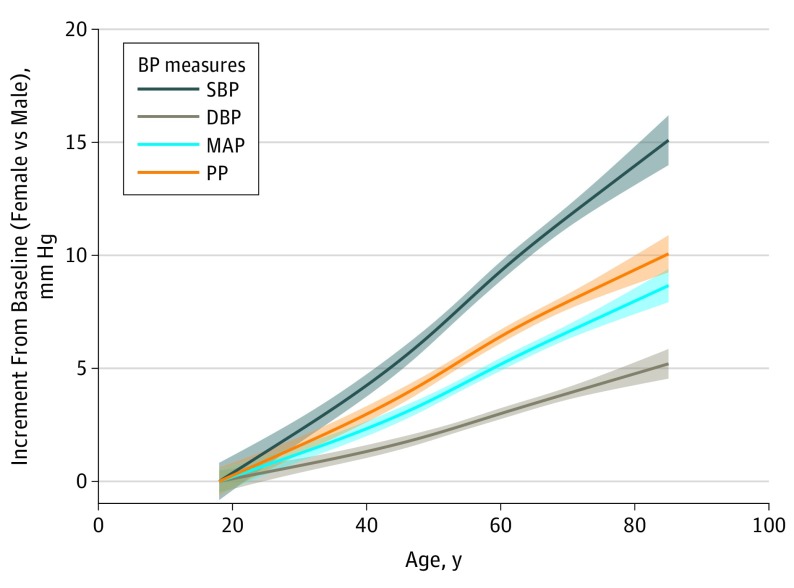
Figure 2. Differences Between Women and Men in Incremental Blood Pressure (BP) Elevation From Baseline Levels.
Under the premise of sex-specific physiology, we calculated between-sex differences (women minus men) in the change of BP values from baseline, and these data demonstrate faster rates of increase in women than men for all BP measures, particularly for systolic BP and pulse pressure (PP). Fitted lines denote mean sex differences in incremental BP (women vs men) from baseline, calculated from restricted cubic spline of mixed-effects linear regression models (Figure 1B). Shaded regions denote 95% CI. DBP indicates diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure.
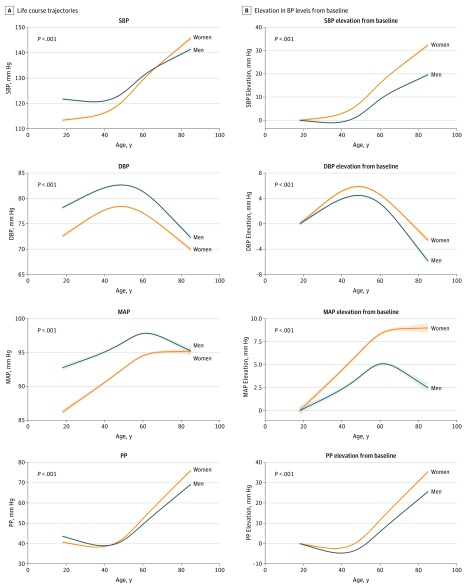
In analyses adjusting for clinical covariates (including BMI, total cholesterol, diabetes mellitus, and current smoking), the rates of increase for all BP measures were expectedly attenuated for both sexes when compared with unadjusted BP trajectories; notably, multivariable-adjusted BP trajectories remained higher in women than in men over the life course (Figure 3; likelihood ratio test χ2 = 314 for systolic BP; χ2 = 31 for diastolic BP; χ2 = 129 for MAP; and χ2 = 485 for PP; P for all <.001).
Figure 3. Multivariable-Adjusted Sex-Specific Trajectories of Blood Pressure (BP) With Aging.
The BP trajectories adjusted for multiple risk factors (body mass index, total cholesterol, diabetes, and smoking status) are displayed as bolder curves (with shading for error limits). Although BP trajectories in both sexes were attenuated with multivariable adjustment, consistent with the known contributions of risk factors to age-related BP elevation, between-sex differences in all BP trajectories persisted. P values are for sex differences in the BP trajectories. DBP indicates diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
In secondary analyses stratified by race/ethnicity, cohort, and antihypertensive medication use, we observed similar trends (eFigures 2-6 in the Supplement). Our main results were also unchanged when using alternative methods to impute the potential effects of antihypertensive therapy (eFigure 7 in the Supplement).
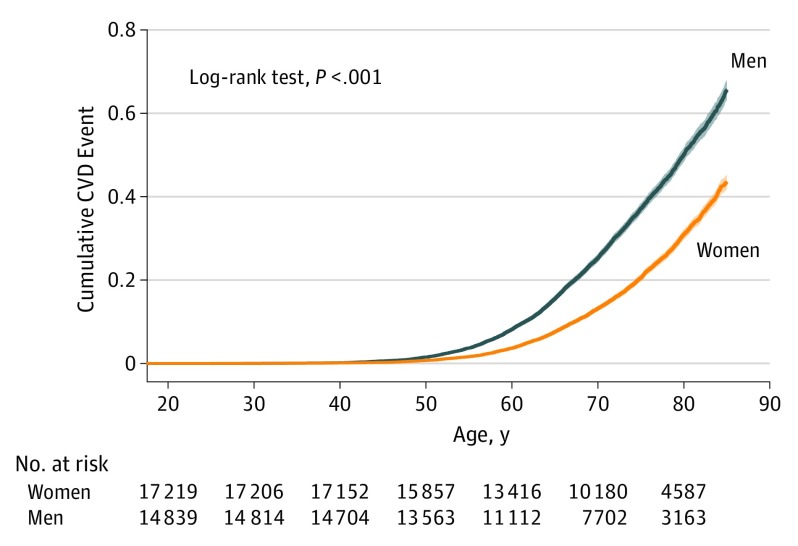
To further understand sex-specific BP trajectories in context, we examined incidence of new-onset hard CVD events in our study sample and observed the cumulative incidence to be higher in men than in women over the adult life course, as expected (Figure 4; 4486 of 15 100 men [29.7%] vs 3644 of 17 733 women [20.5%] developed incident CVD; HR, 1.61; 95% CI, 1.54-1.69; P < .001; log-rank P < .001).
Figure 4. Sex Differences in Cardiovascular Disease (CVD) Incidence.
Cumulative incidence of hard CVD events is higher in men than in women over the life course; log-rank P value is shown.
Discussion
If we assume that women and men exhibit variations of the same fundamental physiology, then conventional analyses of longitudinal BP data would suggest that women catch up to men by midlife in the extent of vascular disease manifesting as age-related elevations in BP.4 However, if we suspend this hypothesis, and consider the possibility that physiology, including vascular physiology, is fundamentally different between women and men, then sex-specific analyses of the data can reveal new insights. In particular, when we permit sex-stratified analyses of longitudinal data with serial BP measures compared with baseline levels, women compared with men clearly exhibit a steeper increase in BP that begins as early as the third decade and continues throughout the life course.
Despite multiple previous reports of age-related BP trends observed in women and men,4,19 relatively little work has been done to examine life course BP trajectories in relation to sex-specific baseline values. In a study of more than 30 000 predominantly white adults enrolled in 8 UK cohorts, Wills et al20 observed a steeper rise of SBP with aging in women compared with men. Our analysis expands from this prior work by comprehensively examining all BP measures, including PP and MAP, in a multiracial sample derived from several large US cohorts. Although our study did not include children or young adults, Shen et al21 examined serial BP data from the Bogalusa Heart Study and observed no sex differences from ages 5 to 14 years, but then higher slopes of SBP and DBP rise in male individuals than female individuals beginning at age 15 years (coinciding with peripubertal growth) followed by steeper slopes of SBP and DBP rise in women than men starting in the 20s to 30s. These findings are also consistent with our results, indicating that the sex differences in BP trajectories seen over adulthood begin early in life. Interestingly, when we adjusted our analyses for cardiometabolic risk factors, we found that the rates of increase for all BP measures were similarly attenuated in both sexes, as expected and consistent with the notion that risk factor exposures contribute substantially to progressive age-related rise in BP. Notably, BP trajectories of increase remained more pronounced in women than men in these adjusted analyses.
Sexual dimorphism in age-related BP elevation may be due to many causes, including differences in hormonal factors, chromosomal factors, and sex-biased nonchromosomal gene expression.22 Importantly, complex social, economic, and structural environmental factors lead to differences in the lived experience between women and men that can also affect physiology as well as vascular biology.23 Nonetheless, still the most commonly considered contributors to biological sex differences remain those associated with sex hormones (ie, type and timing of menarche, pregnancy, and menopause).24 However, the evidence to date would suggest that episodes of hormonal variation or perturbation are not sufficient to account for the sex differences in cardiovascular phenotypes that are consistently seen during the entire life course.25 Women compared with men have not only smaller total body size on average but also smaller organs, including the heart, and smaller vessel caliber, including the coronary arteries, even after adjusting for body surface area.26,27 These morphologic differences are likely coupled with intrinsic physiologic differences that become more evident as well as more persistent with aging and the age-related accumulation of common risk exposures. This construct is consistent with our finding that MAP (a measure that grossly reflects small artery compliance) increases at a higher rate in women than men over the life span. In turn, the higher rate of MAP increase in women, potentially indicative of small artery remodeling (media-to-lumen ratio), may contribute to the greater prevalence of CMD in women than men.28 The higher rate of PP increase in women, reflecting accelerated arterial stiffening and coinciding with greater concentric left ventricular remodeling, may contribute to the excess risk of HFpEF in women.29 Alternatively, or in addition, sex differences in associations of BP with systemic microvascular inflammation and oxidative stress30 may also contribute to coronary microvascular endothelial dysfunction and adverse myocardial remodeling, the putative subclinical precursors to CMD and HFpEF.31
Limitations
Notwithstanding strengths of this analysis, including the combined multicohort and multiethnic design, broad age range, and serially standardized BP measurements collected for up to 4 decades, certain limitations merit consideration. The fitted splines are unable to completely explain variation over the entire life course, especially at the individual level. While imputing for differences in methods for assessing BP, we could not account for all the heterogeneity existing between or within cohort study settings. Although we studied individuals across the age range from multiple racial/ethnic groups, generalizability to populations not represented herein remains unknown. Our findings could have been influenced by relative undertreatment of hypertension in women, which could not be fully captured by our analyses despite imputing for presence vs absence of antihypertensive medication use. It is known that less healthy men tend to be survived by healthier men, and this survival bias could have contributed to lower or more stable BP levels in older men compared with older women.
Conclusions
In summary, we analyzed BP trajectories over the life course in a sex-stratified fashion and observed that progressive BP elevation increased more rapidly in women than in men, beginning as early as in the third decade of life. In contrast with the notion that important vascular diseases in women lag behind men by 10 to 20 years, our findings indicate that certain vascular changes not only develop earlier but also progress faster in women than in men. In effect, sex differences in physiology, starting in early life, may well set the stage for later-life cardiac as well as vascular diseases that often present differently in women compared with men. Additional work is needed to further understand sexual dimorphism in cardiovascular risk to optimize prevention and management efforts in both women and men.
eFigure 1. Study Sampling Strategy
eTable. Baseline Characteristics of the Study Sample
eFigure 2. Sex-Specific Trajectories of Blood Pressure with Aging in Whites
eFigure 3. Sex-Specific Trajectories of Blood Pressure with Aging in Blacks
eFigure 4. Sex-Specific Trajectories of Blood Pressure with Aging, Stratified by Cohort
eFigure 5. Sex-Specific Trajectories of Blood Pressure with Aging in Participant-Observations without Concurrent Antihypertensive Medication Use
eFigure 6. Sex-Specific Trajectories of Blood Pressure with Aging in Participant-Observations with Concurrent Antihypertensive Medication Use
eFigure 7. Sex-Specific Trajectories of Blood Pressure with Aging, Using Alternate Approaches to Imputing the Effects of Antihypertensive Therapy
References
- 1.Dean J, Cruz SD, Mehta PK, Merz CN. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nat Rev Cardiol. 2015;12(7):406-414. doi: 10.1038/nrcardio.2015.72 [DOI] [PubMed] [Google Scholar]
- 2.Eaton CB, Pettinger M, Rossouw J, et al. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9(10):e002883. doi: 10.1161/CIRCHEARTFAILURE.115.002883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation. 2018;138(2):198-205. doi: 10.1161/CIRCULATIONAHA.118.034271 [DOI] [PubMed] [Google Scholar]
- 4.Cheng S, Xanthakis V, Sullivan LM, Vasan RS. Blood pressure tracking over the adult life course: patterns and correlates in the Framingham heart study. Hypertension. 2012;60(6):1393-1399. doi: 10.1161/HYPERTENSIONAHA.112.201780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165-182. doi: 10.1001/jama.2016.19043 [DOI] [PubMed] [Google Scholar]
- 6.Daubert MA, Douglas PS. Primary prevention of heart failure in women. JACC Heart Fail. 2019;7(3):181-191. doi: 10.1016/j.jchf.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 7.Mehta LS, Beckie TM, DeVon HA, et al. ; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916-947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 8.The ARIC investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687-702. doi: 10.1093/oxfordjournals.aje.a115184 [DOI] [PubMed] [Google Scholar]
- 9.Yano Y, Reis JP, Tedla YG, et al. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Cardiol. 2017;2(4):381-389. doi: 10.1001/jamacardio.2016.5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 11.O’Brien E, Mee F, Atkins N, O’Malley K. Inaccuracy of the Hawksley random zero sphygmomanometer. Lancet. 1990;336(8729):1465-1468. doi: 10.1016/0140-6736(90)93177-Q [DOI] [PubMed] [Google Scholar]
- 12.Ni H, Wu C, Prineas R, et al. Comparison of Dinamap PRO-100 and mercury sphygmomanometer blood pressure measurements in a population-based study. Am J Hypertens. 2006;19(4):353-360. doi: 10.1016/j.amjhyper.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs DR Jr, Yatsuya H, Hearst MO, et al. Rate of decline of forced vital capacity predicts future arterial hypertension: the Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012;59(2):219-225. doi: 10.1161/HYPERTENSIONAHA.111.184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24(19):2911-2935. doi: 10.1002/sim.2165 [DOI] [PubMed] [Google Scholar]
- 15.Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122(3):290-300. doi: 10.1016/j.amjmed.2008.09.038 [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Kraja AT, Oberman A, et al. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens. 2005;18(7):935-942. doi: 10.1016/j.amjhyper.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 17.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126(1):50-59. doi: 10.1161/CIRCULATIONAHA.111.057232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasan RS, Short MI, Niiranen TJ, et al. Interrelations between arterial stiffness, target organ damage, and cardiovascular disease outcomes. J Am Heart Assoc. 2019;8(14):e012141. doi: 10.1161/JAHA.119.012141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin SS, Gustin W IV, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation. 1997;96(1):308-315. doi: 10.1161/01.CIR.96.1.308 [DOI] [PubMed] [Google Scholar]
- 20.Wills AK, Lawlor DA, Matthews FE, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8(6):e1000440. doi: 10.1371/journal.pmed.1000440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen W, Zhang T, Li S, et al. Race and sex differences of long-term blood pressure profiles from childhood and adult hypertension: the Bogalusa Heart Study. Hypertension. 2017;70(1):66-74. doi: 10.1161/HYPERTENSIONAHA.117.09537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naqvi S, Godfrey AK, Hughes JF, Goodheart ML, Mitchell RN, Page DC. Conservation, acquisition, and functional impact of sex-biased gene expression in mammals. Science. 2019;365(6450):eaaw7317. doi: 10.1126/science.aaw7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heise L, Greene ME, Opper N, et al. ; Gender Equality, Norms, and Health Steering Committee . Gender inequality and restrictive gender norms: framing the challenges to health. Lancet. 2019;393(10189):2440-2454. doi: 10.1016/S0140-6736(19)30652-X [DOI] [PubMed] [Google Scholar]
- 24.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler Thromb Vasc Biol. 2017;37(5):746-756. doi: 10.1161/ATVBAHA.116.307301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaillard R, Steegers EA, Tiemeier H, Hofman A, Jaddoe VW. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: the generation R study. Circulation. 2013;128(20):2202-2210. doi: 10.1161/CIRCULATIONAHA.113.003881 [DOI] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 27.Dickerson JA, Nagaraja HN, Raman SV. Gender-related differences in coronary artery dimensions: a volumetric analysis. Clin Cardiol. 2010;33(2):E44-E49. doi: 10.1002/clc.20509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzoni D, Agabiti-Rosei C, Agabiti-Rosei E. Hemodynamic consequences of changes in microvascular structure. Am J Hypertens. 2017;30(10):939-946. doi: 10.1093/ajh/hpx032 [DOI] [PubMed] [Google Scholar]
- 29.Weber T, Wassertheurer S, O’Rourke MF, et al. Pulsatile hemodynamics in patients with exertional dyspnea: potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61(18):1874-1883. doi: 10.1016/j.jacc.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 30.Guzik TJ, Touyz RM. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension. 2017;70(4):660-667. doi: 10.1161/HYPERTENSIONAHA.117.07802 [DOI] [PubMed] [Google Scholar]
- 31.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271. doi: 10.1016/j.jacc.2013.02.092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Sampling Strategy
eTable. Baseline Characteristics of the Study Sample
eFigure 2. Sex-Specific Trajectories of Blood Pressure with Aging in Whites
eFigure 3. Sex-Specific Trajectories of Blood Pressure with Aging in Blacks
eFigure 4. Sex-Specific Trajectories of Blood Pressure with Aging, Stratified by Cohort
eFigure 5. Sex-Specific Trajectories of Blood Pressure with Aging in Participant-Observations without Concurrent Antihypertensive Medication Use
eFigure 6. Sex-Specific Trajectories of Blood Pressure with Aging in Participant-Observations with Concurrent Antihypertensive Medication Use
eFigure 7. Sex-Specific Trajectories of Blood Pressure with Aging, Using Alternate Approaches to Imputing the Effects of Antihypertensive Therapy