Key Points
Question
Does a behavioral intervention promoting vegetable consumption decrease cancer progression in men with early-stage prostate cancer on active surveillance?
Findings
In this randomized clinical trial that included 478 patients, there was no significant difference in prostate cancer progression over 2 years among men who participated in a counseling program that encouraged consumption of leafy green, carotenoid, and cruciferous vegetables compared with controls (hazard ratio, 0.96).
Meaning
A behavioral intervention that increased vegetable consumption did not significantly reduce the risk of prostate cancer progression among men with early-stage disease.
Abstract
Importance
Guidelines endorsing vegetable-enriched diets to improve outcomes for prostate cancer survivors are based on expert opinion, preclinical studies, and observational data.
Objective
To determine the effect of a behavioral intervention that increased vegetable intake on cancer progression in men with early-stage prostate cancer.
Design, Setting, and Participants
The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) was a randomized clinical trial conducted at 91 US urology and medical oncology clinics that enrolled 478 men aged 50 to 80 years with biopsy-proven prostate adenocarcinoma (International Society of Urological Pathology grade group = 1 in those <70 years and ≤2 in those ≥70 years), stage cT2a or less, and serum prostate-specific antigen (PSA) level less than 10 ng/mL. Enrollment occurred from January 2011 to August 2015; 24-month follow-up occurred from January 2013 to August 2017.
Interventions
Patients were randomized to a counseling behavioral intervention by telephone promoting consumption of 7 or more daily vegetable servings (MEAL intervention; n = 237) or a control group, which received written information about diet and prostate cancer (n = 241).
Main Outcomes and Measures
The primary outcome was time to progression; progression was defined as PSA level of 10 ng/mL or greater, PSA doubling time of less than 3 years, or upgrading (defined as increase in tumor volume or grade) on follow-up prostate biopsy.
Results
Among 478 patients randomized (mean [SD] age, 64 [7] years; mean [SD] PSA level, 4.9 [2.1] ng/mL), 443 eligible patients (93%) were included in the primary analysis. There were 245 progression events (intervention: 124; control: 121). There were no significant differences in time to progression (unadjusted hazards ratio, 0.96 [95% CI, 0.75 to 1.24]; adjusted hazard ratio, 0.97 [95% CI, 0.76 to 1.25]). The 24-month Kaplan-Meier progression-free percentages were 43.5% [95% CI, 36.5% to 50.6%] and 41.4% [95% CI, 34.3% to 48.7%] for the intervention and control groups, respectively (difference, 2.1% [95% CI, −8.1% to 12.2%]).
Conclusions and Relevance
Among men with early-stage prostate cancer managed with active surveillance, a behavioral intervention that increased vegetable consumption did not significantly reduce the risk of prostate cancer progression. The findings do not support use of this intervention to decrease prostate cancer progression in this population, although the study may have been underpowered to identify a clinically important difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT01238172
This randomized trial compares the effect of a counseling intervention to promote consumption of 7 or more daily vegetable servings vs an educational control on time to PSA- and biopsy-defined cancer progression in men with early-stage prostate cancer.
Introduction
Clinical guidelines for prostate cancer, circulated widely in the public domain, endorse the consumption of diets high in micronutrient-enriched vegetables. Drawing on expert opinion, epidemiological studies, and small preclinical experiments,1 these recommendations propose that vegetable-enriched diets may decrease cancer progression and death among prostate cancer survivors.
Indirect evidence suggests that consumption of micronutrient-enriched plants may promote genomic stability, induce expression of cytoprotective enzymes, and decrease the risk of lethal prostate cancer,2,3,4,5,6 but data from randomized clinical trials (RCTs) focused on actionable clinical end points are lacking. RCTs demonstrating the efficacy of vegetable-enriched diets to diminish cancer progression would justify the development of interventions to promote adoption of high-vegetable diets for prostate cancer survivors.
Most patients with prostate cancer present with early-stage disease.7 Active surveillance—in which patients with early-stage disease defer immediate treatment with surgery or radiation and instead pursue monitoring with prostate-specific antigen (PSA) and follow-up prostate biopsies—provides an alternative for many. However, at least 30% of active surveillance patients will progress or undergo definitive treatment within 2 years of follow-up.8,9,10,11,12 Prevention of disease progression in these patients would decrease treatment incidence, improve health-related quality of life, and reduce health care costs. Diet modification is one potential intervention to prevent progression of prostate cancer.
A behavioral intervention that promotes micronutrient-enriched plant intake among patients with prostate cancer was piloted and validated. This intervention uses motivational interviewing techniques and a centralized, telephone-based counseling system to remotely deliver effective behavioral change with high fidelity.13,14
The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]), a phase 3 study, tested the efficacy of this intervention to prevent disease progression in prostate cancer by increasing micronutrient-enriched vegetable consumption.13 The hypothesis was that this intervention would decrease the incidence of progression among patients with early-stage prostate cancer on active surveillance.
Methods
This study was conducted through the Cancer and Leukemia Group B, now part of the Alliance for Clinical Trials in Oncology, a Clinical Trials Network group of the National Cancer Institute.13 The trial protocol and statistical plan are available in Supplement 1 and Supplement 2, respectively. Each participant signed an institutional review board–approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. This study was monitored twice annually by the Alliance Data and Safety Monitoring Board, a standing committee with members drawn from both within and outside of the Alliance. Data collection was conducted by the Alliance Statistics and Data Management Center (SDMC) and by UC San Diego Moores Comprehensive Cancer Center staff. Data quality was ensured by Alliance SDMC and by the study chairperson following Alliance policies. Patient-reported race/ethnicity, selected from fixed categories, was collected in compliance with National Cancer Institute reporting guidelines.15
From January 2011 to August 2015, patients were recruited from participating urology and medical oncology clinics. Eligible patients were randomized (1:1) to either the telephone-based counseling intervention (MEAL intervention) or a control condition. Instead of the counseling intervention, control patients received a publication on diet for prostate cancer.
Dynamic allocation (minimization method) was used for stratified randomization. Randomization was stratified by age (<70 years vs ≥70 years), race/ethnicity (African American vs other) and time since diagnostic prostate biopsy (0-12 months prior to registration vs >12 and ≤24 months prior to registration).
Study Participants
Eligible patients were 50 to 80 years of age and diagnosed as having stage cT2a or less prostate adenocarcinoma within 24 months of baseline with prostate biopsy (≥10 cores). Patients were required to have International Society of Urological Pathology (ISUP)16,17 grade group 1 (Gleason score 3 + 3 = 6) cancer in those younger than 70 years and group 2 or less (Gleason score ≤3 + 4 = 7) cancer in those aged 70 years and older and serum PSA level less than 10 ng/mL. Additional biopsy volume criteria included less than 25% of total cores and 50% or less of any single core involving adenocarcinoma. A single genitourinary pathologist (D.E.H.) performed centralized pathology review in a blinded manner to confirm eligibility.
Exclusion criteria included prior prostate cancer treatment with surgery, radiation, local ablation, or androgen deprivation therapy; metastatic disease; and baseline consumption of 6 or more servings per day of fruits and vegetables. Consumption of dietary supplements was permitted. Men taking 5-α reductase inhibitors for symptomatic benign prostatic hyperplasia were eligible after discontinuation of drug followed by a 90-day washout period.
Interventions
The telephone counseling intervention, provided through the UC San Diego Moores Comprehensive Cancer Center and previously described, used an approach adopted from social cognitive theory to achieve sustained behavior change.13,14 A similar program had successfully affected behavior change in breast cancer survivors.18 After randomization, each intervention participant was assigned to a counselor, who encouraged consumption of at least 7 daily vegetable-fruit servings (defined as a half-cup of raw or cooked vegetables or fruits or 100% vegetable juice), including at least 2 servings each of cruciferous vegetables and tomatoes.
Details of the intervention structure and content were previously reported13 and are provided in the trial protocol (Supplement 1). Briefly, the intervention was divided into 4 phases. The first phase (6 counseling telephone calls over 1 month) focused on building self-efficacy; the second (4 calls over 2 months) on consolidating the new dietary pattern; the third (4 calls over 4 months) on relapse prevention; and the fourth (8 calls over 16 months) on providing positive feedback and monitoring for declining interest. To ensure fidelity and minimize bias, counselors completed an intensive 80-hour training program and used a computer-assisted coaching protocol.
Control participants did not receive counseling calls and were instead provided printed materials from the Prostate Cancer Foundation encouraging consumption of a vegetable-rich diet. The latest version of these materials is available online.19
Primary Outcome
The protocol-specified primary outcome was time to progression (TTP), defined as the length of time from the date of random assignment to PSA level of 10 ng/mL or greater, PSA doubling time (PSADT) less than 3 years, or pathological progression on follow-up biopsy defined as any of the following: more than 25% of cores positive for cancer; more than 50% of any 1 core positive for cancer; or ISUP grade group above 1 in those younger than 70 years and ISUP grade group greater than 2 in those aged 70 years and older. Participants who died of any cause without experiencing progression were censored at the time of death. Additionally, participants who elected to pursue prostate cancer treatment (ie, surgery, radiation, tissue ablation, or androgen deprivation) during the study despite not meeting the criteria for progression were censored at the time of withdrawal for treatment.
Secondary Outcomes
A prespecified secondary outcome was the incidence of prostate cancer treatment among participants who otherwise did not meet the protocol-defined criteria for progression. Other secondary outcomes not reported in this article included the Personal Habits Questionnaire, the Functional Assessment of Cancer Therapy Scale–Prostate, the Memorial Anxiety Scale for Prostate Cancer, the International Prostate Symptom Score, the Expanded Prostate Cancer Index Composite 26, the Nutrition Self-Efficacy Scale, and Satisfaction with the MEAL Program (Supplement 1). These results will be reported in subsequent articles.
Preplanned Correlative Science Outcomes
Preplanned correlative science outcomes collected to assess fidelity to the intervention were diet composition and plasma carotenoid concentrations. For diet composition assessment, each participant was scheduled for a series of 4 separate diet assessment interviews (randomly selected and distributed evenly between weekends and weekdays) at baseline, 12-month follow-up, and 24-month follow-up. Each set of interviews was conducted over a 3-week period using the Nutrition Data System for Research software and nutrient database (NDS-R, version 2010, University of Minnesota Nutrition Coordinating Center, University of Minnesota, Minneapolis). At least 3 complete interviews at each time point were required for a valid diet assessment. Plasma carotenoids were measured from fasting blood samples collected at baseline and 12-month follow-up using high-performance liquid chromatography.13,14
Primary Outcome Evaluation
PSA was evaluated every 3 months starting from baseline. PSA assays were performed in the clinical laboratory of each study site using standard platforms; results were entered into the patient registration system. PSADT was calculated at the Alliance SDMC as the natural logarithm of 2 divided by the slope (the least squares estimator) of log (PSA) observations over time. The first 3 PSA measurements were used at the 6-month assessment (ie, at baseline and at months 3 and 6). From the 9-month assessment onward, all available PSA measurements were used to calculate PSADT, as long as the patients had at least 3 measurements.
Participants who did not receive definitive treatment underwent an end-of-study biopsy 24 months after baseline. Additional for-cause biopsies were performed as clinically indicated at the discretion of the treating physician.
Sample Size Calculation
The log-rank test with a 2-sided α = 5% and a sample size of 418 had at least 80% power to detect a difference in progression rate of 20% in the control vs 10% in the experimental group during the 24-month follow-up period. Fifty-seven events in the 2 groups combined were required to achieve the specified power for the test comparing TTP. Freedman’s formula for the required number of events was used.20
A 24-month progression rate of 20% in the control group was conservatively estimated. Because most patients who progress undergo treatment, it was determined that a 10% decrease in progression represented a clinically meaningful difference.21 Under the exponential distribution assumption for TTP, a 24-month progression rate of 20% vs 10% in the intervention group corresponds to a hazard ratio (HR) of 2.1. Assuming a 10% dropout rate, targeted enrollment was 464 participants. A conservative assumption was made that there would be a 10% dropout rate. In the pilot study, the dropout rate was 2%.14
Statistical Methods
Statistical analyses were conducted by the Alliance SDMC on a database frozen on April 9, 2018. The primary analysis set included all randomized participants; participants later deemed ineligible by review of eligibility criteria or centralized pathology of baseline prostate biopsy specimens were excluded from the primary analysis. Participants who did not have progression documented during the study period were censored at the date of the last follow-up. Participants who died of any cause without experiencing progression were censored at the time of death. Participants who elected to pursue treatment despite not meeting progression criteria were censored at the time of withdrawal for treatment.
TTP was analyzed using the Kaplan-Meier method. The log-rank test was used to determine superiority of the intervention group compared with the control group. A Cox proportional hazards model was used to estimate the HR and 95% CIs for the group comparison. As supportive analyses, Cox proportional hazards regression modeling was applied to adjust for the 3 stratification factors, and the primary analysis was repeated using the intention-to-treat analysis population, defined as all randomized participants.13,22 The Kolmogorov-type supremum test was applied to test the proportional hazards assumption for the treatment variable as well as graphical checks; based on these methods, the assumption of proportional hazards was satisfied (P = .46). As a protocol-defined sensitivity analysis, the primary analysis was repeated based on the Kaplan-Meier method in which progression was defined only as the Gleason score on repeat biopsy. In a post hoc analysis, a shared frailty model extension of the Cox proportional hazards regression model was fit to account for within-site homogeneity.
Time to treatment was defined as the length of time from the date of random assignment to receipt of alternative therapy; participants who died of any cause without receiving alternative therapy were censored at the time of death. Treatment-free percentages and intervention effect comparison were obtained from the Kaplan-Meier method and log-rank test, respectively. Additionally, the numbers and percentages of participants who pursued prostate cancer treatment were determined. A Fisher exact test of independence was conducted to assess whether the proportions were different between the study groups.
For the correlative end points of diet composition, mean (95% CI) daily intake of target dietary variables and dietary carotenoids at each study visit (baseline, 12 months, and 24 months) was calculated by study group. Linear mixed-effects models, which allowed for missing outcome data under the reasonable assumption of missing at random, were used to assess longitudinal changes for each targeted dietary variable.23 A major advantage of this approach is that partially complete records can be included in the analysis, thus avoiding the biases of completers-only analyses. In the models, repeated measures for each participant’s dietary intake at baseline, 12-month follow-up, and 24-month follow-up comprised the dependent variable. Study group, time, and group-by-time interactions were included as fixed-effect independent variables. A participant-specific intercept was included to account for between-person variation. Appropriate contrast terms were used to compare dietary changes within and between groups at each time point. Likelihood ratio tests and Wald statistics were used to test statistical significance of fixed effects. A statistically significant group-by-time interaction indicated that dietary change profiles differed between study groups. A similar mixed model was developed for the plasma carotenoid data. Residual plots were used to check model assumptions, with a log-transformation applied, as needed, to better approximate gaussian residuals. To further assess the robustness of the results, in sensitivity analysis, a series of alternative imputation models for the missing diet data (using the SAS PROC MI function), including multiple imputation, were considered. The differences in the results were negligible and, therefore, the reported results are based on the linear mixed-effects model.
Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary, correlative, and other end points should be interpreted as exploratory. P values are 2-sided and P ≤ .05 was deemed statistically significant.
Results
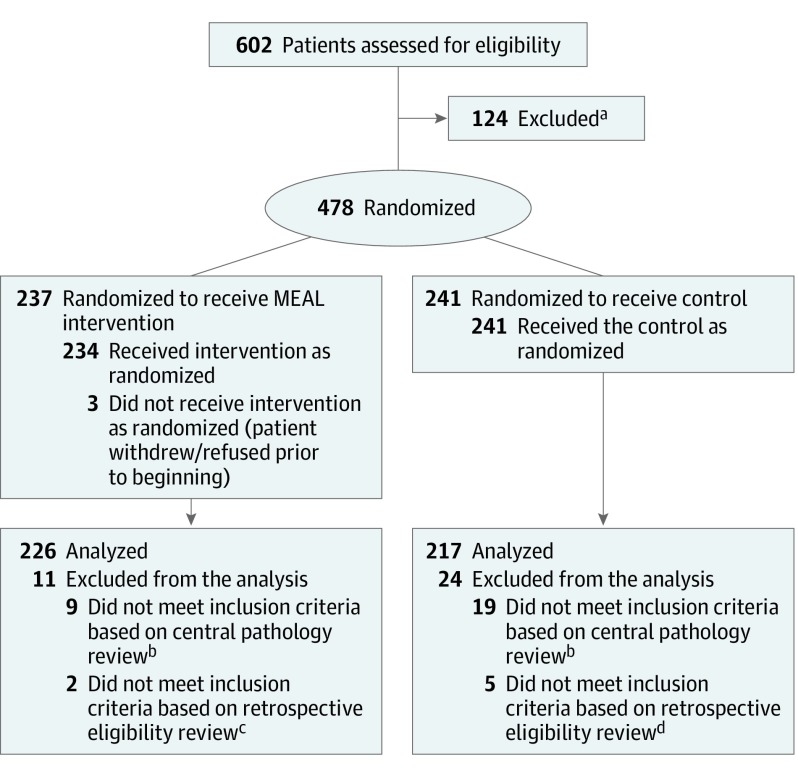
From 2011 to 2015, 478 participants were randomized at 91 study sites: 237 to the intervention and 241 to the control group (Figure 1). The number of participants deemed ineligible in the intervention and control groups was 11 (2 based on eligibility review and 9 based on central pathology review) and 24 (5 based on eligibility review and 19 based on central pathology review), respectively. Therefore, the primary analysis set included 443 participants: 226 intervention and 217 control (Figure 1). Of these 443 participants, 183 (81.7%) and 171 (79.5%) in the intervention and control groups, respectively, met the study’s per-protocol criteria. The reasons for not completing the protocol were withdrawal (intervention group: 36 [15.9%]; control group: 34 [15.8%]), alternative therapy (intervention group: 4 [1.8%]; control group: 4 [1.9%]), death (intervention group: 1 [0.4%]; control group: 3 [1.4%]); and other, including missing (intervention group: 2 [0.9%]; control group: 5 [2.3%]).
Figure 1. Recruitment, Randomization, and Patient Flow.
aThe unmet eligibility criteria were not captured as part of the protocol.
bCentralized pathology review was conducted by a single pathologist on tissue specimens collected at diagnosis to determine tumor volume and Gleason scores.
cThe unmet inclusion criteria included baseline prostate-specific antigen level greater than 10 ng/mL (n = 1) and more than 25% biopsy tissue cores positive for cancer (n = 1).
dThe unmet inclusion criteria included baseline prostate-specific antigen level greater than 10 ng/mL (n = 1), more than 25% biopsy tissue cores positive for cancer (n = 2), Gleason score of 7 for men aged 70 years or younger (n = 1), and consumed 6 or more servings per day of fruits and vegetables (n = 1).
Baseline characteristics were balanced across the 2 groups (Table 1). The mean (SD) age was 64 (7) years and the mean (SD) PSA level was 4.9 (2.1) ng/mL. Fifty participants (11%) were African American. There were no clinically significant differences between groups for age, race/ethnicity, region, time elapsed since prostate cancer diagnosis, PSA level, clinical stage, or tumor grade. A total of 9 participants (2%) had ISUP grade group 2 disease. The median number of PSA values collected for each participant was 7 in the intervention and 7 in the control group; 48.2% (95% CI, 41.7%-54.7%) in the intervention group and 46.5% (95% CI, 39.9%-53.1%) in the control group underwent the 24-month follow-up biopsy.
Table 1. Baseline Characteristics of Participants.
| Characteristic | No. (%) | |
|---|---|---|
| MEAL Intervention Group (n = 226) | Control Group (n = 217) | |
| Age, y | ||
| Mean (SD) | 63.7 (6.5) | 63.5 (6.6) |
| Median (IQR) | 64.0 (59.0-68.0) | 64.0 (59.0-68.0) |
| <70 y | 186 (82.3) | 173 (79.7) |
| Race and ethnicity | n = 226 | n = 216 |
| Non-Hispanic white | 186 (82.3) | 171 (78.8) |
| Non-Hispanic black or African American | 24 (10.6) | 26 (12.0) |
| Hispanic or Latino | 9 (4.0) | 7 (3.2) |
| Asian | 6 (2.7) | 9 (4.1) |
| More than 1 race/ethnicity | 0 (0.0) | 2 (0.9) |
| Native Hawaiian or Pacific Islander | 0 (0.0) | 1 (0.5) |
| American Indian or Alaska Native | 1 (0.4) | 0 (0.0) |
| Region | ||
| West | 85 (37.6) | 78 (35.9) |
| Northeast | 56 (24.8) | 48 (22.1) |
| South | 43 (19.0) | 39 (18.0) |
| Midwest | 42 (18.6) | 52 (24.0) |
| Body mass indexa | n = 55 | n = 53 |
| Mean (SD) | 28.7 (5.9) | 28.3 (3.7) |
| Median (IQR) | 27.5 (24.5-31.0) | 28.4 (26.0-30.8) |
| Prostate biopsy within 12 mob | 192 (85.0) | 183 (84.3) |
| Tumor stage | n = 225 | n = 217 |
| cT1a | 4 (1.8) | 1 (0.5) |
| cT1b | 1 (0.4) | 2 (0.9) |
| cT1c | 197 (87.6) | 187 (86.2) |
| cT2a | 23 (10.2) | 27 (12.4) |
| Serum PSA level, ng/mL | n = 224 | n = 217 |
| 0-2.5 | 25 (11.2) | 30 (13.8) |
| >2.5-5 | 99 (44.2) | 98 (45.2) |
Abbreviations: IQR, interquartile range; PSA, prostate-specific antigen.
Calculated as weight in kilograms divided by height in meters squared.
The remainder of participants underwent prostate biopsy within 13 to 24 months.
Primary Outcome
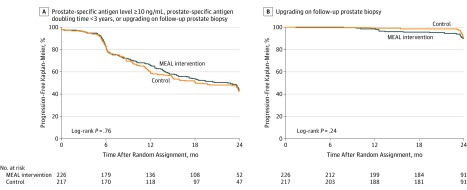
There were 245 progression events (intervention: 124; control: 121). There were 4 deaths (0.9%) during the study (intervention group: 1 [0.4%]; control group: 3 [1.4%]), and these participants were censored at the time of death. Five participants (1.1%) (intervention group: 3 [1.3%]; control group: 2 [0.9%]) withdrew from the study to pursue alternative treatment without experiencing clinical progression and, therefore, were censored at the time of study withdrawal. There was no significant difference in TTP comparing intervention vs control (unadjusted HR, 0.96 [95% CI, 0.75 to 1.24], P = .76; adjusted HR, 0.97 [95% CI, 0.76 to 1.25], P = .84) (Figure 2A), including the shared frailty model (unadjusted HR, 0.96 [95% CI, 0.75 to 1.24]; P = .76). The progression-free Kaplan-Meier percentages for the intervention and control groups were 43.5% (95% CI, 36.5% to 50.6%) and 41.4% (95% CI, 34.3% to 48.7%), respectively (difference, 2.1% [95% CI, −8.1% to 12.2%]).
Figure 2. Time to Progression Where Progression Was Defined as Prostate-Specific Antigen Level ≥10 ng/mL, Prostate-Specific Antigen Doubling Time <3 Years, or Upgrading on Follow-up Prostate Biopsy (A) and Upgrading on Follow-up Prostate Biopsy (B).
A, The median follow-up time was 24.1 months (interquartile range [IQR], 22.7-24.7) for the MEAL intervention group and 24.0 months (IQR, 22.9-24.6) for the control group. B, The median follow-up time was 23.9 months (IQR, 22.4-24.5) for the MEAL intervention group and 23.9 months (IQR, 22.1-24.6) for the control group.
There was no significant between-group difference for TTP by tumor grade on repeat biopsy (HR, 1.40 [95% CI, 0.79 to 2.46]; P = .24) (Figure 2B). There were 49 such events, including 28 in the intervention group and 21 in the control group. The progression-free Kaplan-Meier percentages for the intervention and control groups were 89.9% (95% CI, 84.4% to 94.1%) and 90.2% (95% CI, 84.4% to 94.8%), respectively (difference, −0.3% [95% CI, −7.3% to 6.7%]).
Secondary Outcomes
Eight participants withdrew from the study to pursue active treatment (intervention group: 4 [1.8%]; control group: 4 [1.9%]). Two additional participants in the intervention group pursued active treatment, although they completed the intervention per-protocol criteria. The type of active treatment pursued was not recorded. There was no significant difference in the total number of patients who pursued active treatment during the 24-month follow-up (intervention group: 6 [2.7%]; control group: 4 [1.8%]; P = .75). The HR for time to treatment was 1.38 (95% CI, 0.39 to 4.90; P = .61).
Correlative Outcomes
Table 2 shows the results of the diet composition analyses. At baseline, there were no significant between-group differences. At 12-month follow-up, intervention participants (n = 208) reported significant increases compared with controls (n = 199) in daily total vegetable servings (mean change, 2.43 [95% CI, 2.17 to 2.69] vs 0.45 [95% CI, 0.19 to 0.70]; P < .001), cruciferous servings (mean change, 43.10 g/d [95% CI, 35.21 to 50.99] vs 6.44 g/d [95% CI, −1.39 to 14.26]); P < .001), and total carotenoids (mean change, 13 839.31 µg/d [95% CI, 11 960.15 to 15 718.48] vs 2030.79 µg/d [167.32 to 3894.25]; P < .001). At 24-month follow-up, significant between-group differences (intervention group: 190; control group: 185) persisted for total vegetable servings (mean change, 2.01 [95% CI, 1.75 to 2.28] vs 0.37 [95% CI, 0.11 to 0.62]; P < .001), cruciferous servings (mean change, 0.50 [95% CI, 0.38 to 0.61] vs 0.01 [95% CI, −0.1 to 0.12]; P < .001), and total carotenoids (9687.87 µg/d [95% CI, 7801.18 to 11574.57] vs 324.73 µg/d [95% CI, −1543.61 to 2193.07]; P < .001). Additional diet composition data are presented in the eTable and eFigure in Supplement 3.
Table 2. Dietary Patterns of Study Participantsa.
| Baseline Values, Mean (SD) | 12-Month Change | 24-Month Change | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEAL Intervention Group | Control Group | MEAL Intervention Group | Control Group | Between-Group Difference | MEAL Intervention Group | Control Group | Between-Group Difference | |||||||
| Change (95% CI) | P Valueb,c | Change (95% CI) | P Valueb,c | Change (95% CI) | P Valueb,d | Change (95% CI) | P Valueb,c | Change (95% CI) | P Valueb,c | Change (95% CI) | P Valueb,d | |||
| Energy, kcal/d | 2145.93 (609.87) | 2099.53 (512.02) | −230.87 (−296.01 to −165.72) | <.001 | −174.44 (−239.04 to −109.84) | <.001 | −56.42 (−148.16 to 35.31) | .23 | −250.01 (−315.43 to −184.59 | <.001 | −130.3 (−195.08 to −65.52) | <.001 | −119.71 (−211.78 to −27.65) | .01 |
| Dark green vegetables, servings/d | 0.51 (0.63) | 0.42 (0.49) | 0.64 (0.52 to 0.76) | <.001 | 0.2 (0.08 to 0.32 | <.001 | 0.44 (0.27 to 0.6) | <.001 | 0.54 (0.42 to 0.66 | <.001 | 0.14 (0.03 to 0.26) | .02 | 0.4 (0.23 to 0.57) | <.001 |
| Deep yellow vegetables, servings/d | 0.18 (0.24) | 0.21 (0.31) | 0.30 (0.24 to 0.37) | <.001 | 0.06 (0 to 0.13) | .05 | 0.24 (0.15 to 0.33) | <.001 | 0.19 (0.12 to 0.25 | <.001 | 0.05 (−0.02 to 0.11) | .13 | 0.14 (0.05 to 0.23) | .004 |
| Tomatoes, servings/d | 0.54 (0.54) | 0.56 (0.55) | 0.18 (0.1 to 0.26) | <.001 | −0.04 (−0.13 to 0.04) | .3 | 0.23 (0.11 to 0.34) | <.001 | 0.06 (−0.02 to 0.14) | .17 | −0.09 (−0.17 to 0) | .04 | 0.14 (0.03 to 0.26) | .02 |
| Legumes, servings/d | 0.24 (0.38) | 0.22 (0.37) | 0.15 (0.08 to 0.22) | <.001 | 0.03 (−0.03 to 0.1) | .33 | 0.12 (0.02 to 0.21) | .02 | 0.18 (0.12 to 0.25) | <.001 | 0.03 (−0.03 to 0.1) | .32 | 0.15 (0.05 to 0.24) | .002 |
| Other vegetables, servings/d | 1.23 (0.9) | 1.23 (0.85) | 0.64 (0.5 to 0.79) | <.001 | 0.10 (−0.04 to 0.24 | .16 | 0.54 (0.34 to 0.74) | <.001 | 0.46 (0.31 to 0.6) | <.001 | 0.04 (−0.1 to 0.18) | .56 | 0.42 (0.22 to 0.62) | <.001 |
| Cruciferous, g/d | 22.5 (32.21) | 28.05 (37.78) | 43.10 (35.21 to 50.99) | <.001 | 6.44 (−1.39 to 14.26 | .11 | 36.66 (25.55 to 47.78) | <.001 | 29.86 (21.94 to 37.78) | <.001 | −0.40 (−8.24 to 7.45) | .92 | 30.26 (19.11 to 41.41) | <.001 |
| Cruciferous, servings/d | 0.32 (0.45) | 0.39 (0.53) | 0.71 (0.6 to 0.83) | <.001 | 0.12 (0.01 to 0.23) | .04 | 0.60 (0.44 to 0.75) | <.001 | 0.50 (0.38 to 0.61) | <.001 | 0.01 (−0.10 to 0.12) | .84 | 0.49 (0.33 to 0.64) | <.001 |
| Total vegetables, servings/d | 3.38 (1.58) | 3.39 (1.41) | 2.43 (2.17 to 2.69) | <.001 | 0.45 (0.19 to 0.70) | <.001 | 1.99 (1.62 to 2.35) | <.001 | 2.01 (1.75 to 2.28) | <.001 | 0.37 (0.11 to 0.62) | .006 | 1.65 (1.28 to 2.02) | <.001 |
| Red meat, g/d | 52.17 (52.5) | 52.64 (50.05) | −11.54 (−19.03 to −4.06) | .003 | −9.83 (−17.26 to −2.41) | <.001 | −1.71 (−12.25 to 8.83) | .75 | −7.34 (−14.85 to 0.17) | .06 | −3.04 (−10.48 to 4.4) | .42 | −4.30 (−14.88 to 6.27) | .42 |
| Fat, g/d | 35.47 (6.74) | 34.93 (6.62) | −2.09 (−3.01 to −1.17) | <.001 | −0.17 (−1.08 to 0.74) | .71 | −1.92 (−3.21 to −0.63) | .004 | −1.77 (−2.69 to −0.85) | <.001 | 0.21 (−0.7 to 1.13) | .65 | −1.98 (−3.28 to −0.68) | .003 |
| Saturated fat, g/d | 11.49 (3.01) | 11.38 (3.01) | −1.69 (−2.07 to −1.3) | <.001 | −0.44 (−0.82 to −0.06) | .02 | −1.25 (−1.79 to −0.7) | <.001 | −0.94 (−1.33 to −0.55) | <.001 | −0.12 (−0.50 to 0.27) | .56 | −0.82 (−1.37 to −0.28) | .003 |
| Lycopene, μg/d | 5455.24 (6443.04) | 5704.5 (6193.53) | 7125.95 (5682.17 to 8569.73) | <.001 | 135.55 (−1296.16 to 1567.27) | .85 | 6990.4 (4957.1 to 9023.7) | <.001 | 5179.06 (3729.67 to 6628.45) | <.001 | −466.25 (−1901.60 to 969.11) | .52 | 5645.31 (3605.46 to 7685.15) | <.001 |
| Total carotenoids (diet), μg/d | 12 103.3 (8390.14) | 12 284.62 (8687.44) | 13 839.31 (11 960.15 to 15718.48) | <.001 | 2030.79 (167.32 to 3894.25) | .03 | 11 808.53 (9162.07 to 14 454.99) | <.001 | 9687.87 (7801.18 to 11 574.57) | <.001 | 324.73 (−1543.61 to 2193.07) | .73 | 9363.14 (6707.9 to 12 018.38) | <.001 |
Results are based on a linear mixed-effects model that included diet group, time as a categorical variable (0 for baseline, 1 for 12 months, and 2 for 24 months), and the group-by-time interaction.
P values based on a mixed-model analysis.
For within-group changes, values at each follow-up compared with baseline.
Changes in intervention compared with changes in control.
Plasma carotenoids, measured at 12-month follow-up in a sample of participants as a biomarker of vegetable intake, validated the self-reported dietary data. There were no significant baseline differences in mean levels of the log-transformed carotenoid values between groups: 0.41 log-μmol/L (95% CI, 0.34-0.47) in the intervention group (n = 152) vs 0.40 log-μmol/L (95% CI, 0.34-0.47) in the control group (n = 142). At 12 months, the mean levels of the log-transformed carotenoid values between groups were 0.64 (95% CI, 0.56-0.72) in the intervention group vs 0.52 (95% CI, 0.45-0.60) in the control group, with a significant between-group difference in baseline to 12-month changes (intervention vs control difference in mean change scores, 0.1 [95% CI, 0.02-0.18]; P = .01).
Discussion
The behavioral intervention in this study produced robust, sustained increases in carotenoid, cruciferous-rich, and leafy green vegetable intake for 2 years, but did not significantly reduce the risk of clinical progression compared with control in patients with early-stage prostate cancer on active surveillance. These data fail to support prevailing assertions in evidence-based clinical guidelines and the popular media that diets high in micronutrient-enriched vegetables improve cancer-specific outcomes among prostate cancer survivors.
To our knowledge, this study is the first RCT to test the effect of a dietary intervention for prostate cancer and demonstrates that scalable, robust behavior modification is feasible in this population. The magnitudes of the dietary changes, which persisted through 24 months of follow-up, were substantial and suggest a clinically meaningful effect.24 While behavior modifications of diet or activity may potentially benefit patients with more advanced stages of disease,25,26,27 RCTs in this population would be needed to test this hypothesis.
These results are consistent with 2 RCTs, involving nearly 50 000 men, that observed that selenium and vitamin C supplements did not alter, and vitamin E supplements modestly increased, the risk of incident prostate cancer,28,29 as well as with an RCT of more than 12 000 men that demonstrated lack of efficacy of vitamin D or marine n-3 (omega-3) fatty acids to prevent prostate cancer.30,31 These results also failed to support the hypothesis that dietary interventions of macronutrients may have greater efficacy than those of micronutrients.32
Nevertheless, enthusiasm for diet-based cancer interventions remains high, driven by assumptions of causality made from epidemiological data.33 Inherent limitations in these data include small effect sizes and substantial confounding.33,34,35 The overdependence of prostate cancer nutrition guidelines on observational studies with uncertain clinical validity suggests a need to shift nutritional research toward definitive RCTs.
Limitations
This study has several limitations. First, biopsy undersampling of tumors at baseline may have led to misclassification of baseline Gleason grade. Second, somatic gene expression testing of biopsy tissue was not used as an additional method for determining suitability for active surveillance. Third, magnetic resonance imaging–guided biopsy was not routinely used. However, randomization would have minimized the potential for differential bias, and protocol-defined biopsy methodology was consistent with standard-of-care practices.
Conclusions
Among men with early-stage prostate cancer managed with active surveillance, a behavioral intervention to increase vegetable consumption did not significantly reduce the risk of prostate cancer progression. The findings do not support use of this intervention to decrease prostate cancer progression in this population, although the study may have been underpowered to identify a clinically important difference.
Trial Protocol
Statistical Analysis Plan
eTable. Dietary Patterns in the Men’s Eating and Living (MEAL) Study
eFigure. Additional Analysis of Dietary Patterns of Study Participants
Data Sharing Statement
References
- 1.Resnick MJ, Lacchetti C, Bergman J, et al. . Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol. 2015;33(9):1078-1085. doi: 10.1200/JCO.2014.60.2557 [DOI] [PubMed] [Google Scholar]
- 2.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10(9):949-954. [PubMed] [Google Scholar]
- 3.Nordström T, Van Blarigan EL, Ngo V, et al. . Associations between circulating carotenoids, genomic instability and the risk of high-grade prostate cancer. Prostate. 2016;76(4):339-348. doi: 10.1002/pros.23125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zu K, Mucci L, Rosner BA, et al. . Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106(2):djt430. doi: 10.1093/jnci/djt430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frattaroli J, Weidner G, Dnistrian AM, et al. . Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319-1323. doi: 10.1016/j.urology.2008.04.050 [DOI] [PubMed] [Google Scholar]
- 6.Ornish D, Lin J, Chan JM, et al. . Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112-1120. doi: 10.1016/S1470-2045(13)70366-8 [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314(1):80-82. doi: 10.1001/jama.2015.6036 [DOI] [PubMed] [Google Scholar]
- 8.Bul M, Zhu X, Valdagni R, et al. . Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597-603. doi: 10.1016/j.eururo.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Vesprini D, Sethukavalan P, et al. . Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272-277. doi: 10.1200/JCO.2014.55.1192 [DOI] [PubMed] [Google Scholar]
- 10.Tosoian JJ, Trock BJ, Landis P, et al. . Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185-2190. doi: 10.1200/JCO.2010.32.8112 [DOI] [PubMed] [Google Scholar]
- 11.Welty CJ, Cowan JE, Nguyen H, et al. . Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193(3):807-811. doi: 10.1016/j.juro.2014.09.094 [DOI] [PubMed] [Google Scholar]
- 12.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415-1424. doi: 10.1056/NEJMoa1606220 [DOI] [PubMed] [Google Scholar]
- 13.Parsons JK, Pierce JP, Mohler J, et al. . A randomized trial of diet in men with early stage prostate cancer on active surveillance: rationale and design of the Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]). Contemp Clin Trials. 2014;38(2):198-203. doi: 10.1016/j.cct.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101(10):1227-1231. doi: 10.1111/j.1464-410X.2007.07365.x [DOI] [PubMed] [Google Scholar]
- 15.National Cancer Institute Cancer therapy evaluation program instructions and guidelines version 3.0, release 5. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/cdus_ig_3r5.pdf. Published January 11, 2011. Accessed September 12, 2019.
- 16.Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: prostate and bladder tumours. Eur Urol. 2016;70(1):106-119. doi: 10.1016/j.eururo.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 17.Epstein JI, Amin MB, Reuter VE, Humphrey PA. Contemporary Gleason grading of prostatic carcinoma: an update with discussion on practical issues to implement the 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2017;41(4):e1-e7. doi: 10.1097/PAS.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 18.Pierce JP, Natarajan L, Caan BJ, et al. . Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: the Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA. 2007;298(3):289-298. doi: 10.1001/jama.298.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prostate Cancer Foundation Prostate cancer diet. https://www.pcf.org/patient-resources/living-prostate-cancer/prostate-cancer-diet/. Accessed September 12, 2019.
- 20.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1(2):121-129. doi: 10.1002/sim.4780010204 [DOI] [PubMed] [Google Scholar]
- 21.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126-131. doi: 10.1200/JCO.2009.24.2180 [DOI] [PubMed] [Google Scholar]
- 22.Bang H, Jung SH, George SL. Sample size calculation for simulation-based multiple-testing procedures. J Biopharm Stat. 2005;15(6):957-967. doi: 10.1080/10543400500265710 [DOI] [PubMed] [Google Scholar]
- 23.Diggle PJ, Heagerty PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford, UK: Clarendon Press; 1996. [Google Scholar]
- 24.Thomson CA, Ravia J. A systematic review of behavioral interventions to promote intake of fruit and vegetables. J Am Diet Assoc. 2011;111(10):1523-1535. doi: 10.1016/j.jada.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 25.Sfanos KS, Markowski MC, Peiffer LB, et al. . Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis. 2018;21(4):539-548. doi: 10.1038/s41391-018-0061-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newton RU, Kenfield SA, Hart NH, et al. . Intense Exercise for Survival Among Men With Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): a multicentre, randomised, controlled phase III study protocol. BMJ Open. 2018;8(5):e022899. doi: 10.1136/bmjopen-2018-022899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner JR, Livingston PM, Fraser SF. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol. 2014;32(4):335-346. doi: 10.1200/JCO.2013.49.5523 [DOI] [PubMed] [Google Scholar]
- 28.Klein EA, Thompson IM Jr, Tangen CM, et al. . Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549-1556. doi: 10.1001/jama.2011.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaziano JM, Glynn RJ, Christen WG, et al. . Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2009;301(1):52-62. doi: 10.1001/jama.2008.862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23-32. doi: 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group . Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380(1):33-44. doi: 10.1056/NEJMoa1809944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyskens FL Jr, Szabo E. Diet and cancer: the disconnect between epidemiology and randomized clinical trials. Cancer Epidemiol Biomarkers Prev. 2005;14(6):1366-1369. doi: 10.1158/1055-9965.EPI-04-0666 [DOI] [PubMed] [Google Scholar]
- 33.Ioannidis JPA. The challenge of reforming nutritional epidemiologic research. JAMA. 2018;320(10):969-970. doi: 10.1001/jama.2018.11025 [DOI] [PubMed] [Google Scholar]
- 34.Trepanowski JF, Ioannidis JPA. Perspective: limiting dependence on nonrandomized studies and improving randomized trials in human nutrition research: why and how. Adv Nutr. 2018;9(4):367-377. doi: 10.1093/advances/nmy014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schoenfeld JD, Ioannidis JP. Is everything we eat associated with cancer? a systematic cookbook review. Am J Clin Nutr. 2013;97(1):127-134. doi: 10.3945/ajcn.112.047142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eTable. Dietary Patterns in the Men’s Eating and Living (MEAL) Study
eFigure. Additional Analysis of Dietary Patterns of Study Participants
Data Sharing Statement