Keywords: apoptosis, Fis1, hypothermia, ischemia/reperfusion injury, mitochondria, mitochondrial fission, mitochondrial ultrastructure, neuroprotection, selective brain hypothermia, stroke
Abstract
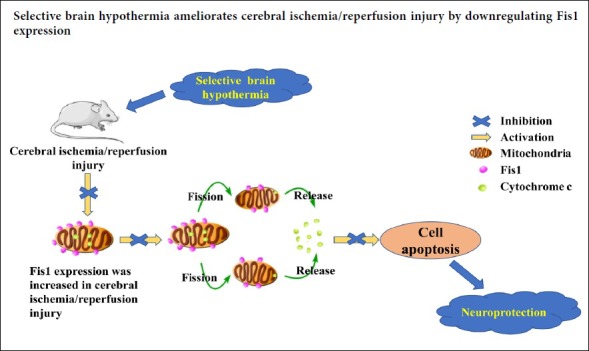
Selective brain hypothermia is considered an effective treatment for neuronal injury after stroke, and avoids the complications of general hypothermia. However, the mechanisms by which selective brain hypothermia affects mitochondrial fission remain unknown. In this study, we investigated the effect of selective brain hypothermia on the expression of fission 1 (Fis1) protein, a key factor in the mitochondrial fission system, during focal cerebral ischemia/reperfusion injury. Sprague-Dawley rats were divided into four groups. In the sham group, the carotid arteries were exposed only. In the other three groups, middle cerebral artery occlusion was performed using the intraluminal filament technique. After 2 hours of occlusion, the filament was slowly removed to allow blood reperfusion in the ischemia/reperfusion group. Saline, at 4°C and 37°C, were perfused through the carotid artery in the hypothermia and normothermia groups, respectively, followed by restoration of blood flow. Neurological function was assessed with the Zea Longa 5-point scoring method. Cerebral infarct volume was assessed by 2,3,5-triphenyltetrazolium chloride staining, and apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining. Fis1 and cytosolic cytochrome c levels were assessed by western blot assay. Fis1 mRNA expression was assessed by quantitative reverse transcription-polymerase chain reaction. Mitochondrial ultrastructure was evaluated by transmission electron microscopy. Compared with the sham group, apoptosis, Fis1 protein and mRNA expression and cytosolic cytochrome c levels in the cortical ischemic penumbra and cerebral infarct volume were increased after reperfusion in the other three groups. These changes caused by cerebral ischemia/reperfusion were inhibited in the hypothermia group compared with the normothermia group. These findings show that selective brain hypothermia inhibits Fis1 expression and reduces apoptosis, thereby ameliorating focal cerebral ischemia/reperfusion injury in rats. Experiments were authorized by the Ethics Committee of Qingdao Municipal Hospital of China (approval No. 2019008).
Chinese Library Classification No. R454.5; R363; R364
Introduction
Stroke is a leading cause of death and disability worldwide (de Rooij et al., 2013; Cuartero et al., 2016; Xie et al., 2018). Ischemic stroke caused by cerebral thrombosis or endovascular embolization accounts for the majority of strokes. Recanalization therapies are currently the primary treatment for ischemic stroke, and restore the supply of nutrients and oxygen and remove toxic metabolites (Vivien et al., 2011; Kraft et al., 2012). However, restoration of blood perfusion usually exacerbates tissue damage (Eltzschig and Eckle, 2011; Kalogeris et al., 2016), in a process termed cerebral ischemia/reperfusion (I/R) injury.
Recent studies suggest that morphological changes in the mitochondria might be relevant to I/R injury (Anzell et al., 2018; Murphy and Hartley, 2018). Within the cell, mitochondria exist in an ever-changing dynamic state—constant fission and fusion—to form mitochondrial networks that help maintain cell function and survival (Suliman and Piantadosi, 2016; Whitley et al., 2019). Notably, accumulating evidence indicates that I/R injury perturbs this balance to induce neuronal apoptosis (Sanchis-Gomar and Derbre, 2014; Liu et al., 2018; Yang et al., 2018). In addition, recent studies show that mitochondrial fission predominates in the early stage of apoptosis (Chen et al., 2014; Wang et al., 2014). Mitochondrial fission 1 (Fis1) protein, a 16-kDa protein anchored to the outer membrane of the mitochondria, mediates mitochondrial fission by recruiting cytoplasmic dynamin-related protein 1 (Drp1) into the mitochondrial outer membrane (Loson et al., 2013). In cells with Fis1 deletion, Drp1 is mostly localized to the cytoplasm, and mitochondrial fission is inhibited (Mozdy et al., 2000). Cytochrome c released from mitochondria plays a critical role in apoptosis (Chimenti et al., 2018). Fis1 overexpression increases the frequency of mitochondrial fission, which increases the release of cytochrome c and disrupts the mitochondrial membrane potential, thereby inducing apoptosis (Gomes and Scorrano, 2008; Geng et al., 2017).
Hypothermia is one of the most effective neuroprotective strategies, and can affect multiple pathological events in ischemic stroke, such as calcium overload, oxidative stress, mitochondrial dysfunction, and apoptosis (Yenari and Han, 2012; Wu and Grotta, 2013; Andresen et al., 2015; Kurisu and Yenari, 2017; Davies et al., 2019). Our previous study showed that hypothermia-induced neuroprotection against cerebral I/R injury is associated with the suppression of mitochondrial fission via inhibition of the translocation of Drp1 from the cytoplasm to the mitochondria in mice (Tang et al., 2016b). However, selective brain hypothermia, which involves selectively reducing brain temperature while maintaining core temperature, is strongly expected to become a preferred treatment for ischemic stroke because of its rapid cooling action and fewer systemic side effects (Dumitrascu et al., 2016; Kurisu et al., 2016a; Almekhlafi et al., 2019). However, it is currently unknown whether selective brain hypothermia alleviates I/R injury by regulating Fis1 expression. Therefore, in this study, we investigate whether selective brain hypothermia-induced neuroprotection is associated with Fis1 inhibition in rats with focal cerebral I/R injury.
Materials and Methods
Experimental animals and groups
A total of 160 specific-pathogen-free healthy male Sprague-Dawley rats weighing 200–250 g and aged 8–12 weeks were provided by the Qingdao Darenfucheng Animal Technology Co., Ltd., China (license No. SCXKL (Lu) 2014-007). Experiments were authorized by the Ethics Committee of Qingdao Municipal Hospital of China (approval No. 2019008). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996). All surgical interventions were performed under anesthesia with 1% pentobarbital sodium (30 mg/kg) by intraperitoneal injection. Animals were housed five per cage with free access to water and food. The rats were kept in a room under standard laboratory conditions of 18–24°C, 50–60% humidity, and a 12/12-hour light/dark cycle.
The rats were randomly divided into four groups (n = 40 per group). In the sham group, the carotid arteries were exposed only without middle cerebral artery occlusion (MCAO). In the I/R group, the rats received 2-hour MCAO followed by reperfusion without treatment. In the hypothermia group, ischemic rats received cold saline perfusion through the internal carotid artery at the onset of reperfusion. In the normothermia group, ischemic rats received warm saline perfusion through the internal carotid artery at the onset of reperfusion. The general layout of the experiment is described in Additional Figure 1 (546.7KB, tif) .
Production of the focal cerebral I/R injury model
Focal cerebral ischemia was induced by transient MCAO using the intraluminal filament technique as described by Longa et al. (1989), which is similar to the Bederson method (Bederson et al., 1986). Briefly, rats were intraperitoneally anesthetized with 1% pentobarbital sodium (30 mg/kg). A small incision (3–4 cm) was made along the midline of the neck to expose the right common, internal and external carotid arteries. The external carotid artery was ligated, and the internal and common carotid arteries were clamped with an artery clamp. A small incision was then made in the external carotid artery. A filament, which had a silicone-coated tip with a diameter of 0.22 mm, was inserted into the internal carotid artery approximately 18–20 mm from the bifurcation through the external carotid artery stump and advanced into the circle of Willis to occlude the origin of the middle cerebral artery. The carotid arteries were exposed without obstructing blood flow in the sham group. To reduce mortality rate, a suitable depth of anesthesia was obtained by assessing the lack of the corneal reflex, and this depth of anesthesia was maintained throughout the procedure. Oxygen (30% oxygen/70% air) was supplied during the perioperative period using a face mask. The rectal temperature of the rats was maintained at 36.8–37.2°C with a heating plate. Five rats died from the anesthesia or surgical procedure (two from deep anesthesia, three from excessive bleeding), and were replaced in subsequent experiments. After blocking the right middle cerebral artery for 2 hours, the filament was slowly removed to allow blood reperfusion. When the respiratory and heart rates were stable after reperfusion, neurological deficits were evaluated using the Zea Longa 5-point scoring method (Longa et al., 1989). The scores were calculated as follows: 0, no symptom of neurologic impairment; 1, the contralateral forelimb is unable to contract when the tail is lifted; 2, rotation inwards when walking; 3, tilted inwards when walking; 4, fails to spontaneously walk and loss of consciousness. Scores ranging from 1 to 3 points indicated successful production of the MCAO model. Rats with other scores were considered to indicate model failure, and were excluded. The excluded rats were replaced in subsequent experiments.
Selective cerebral hypothermia
Selective cerebral hypothermia was performed according to a previously published protocol (Kurisu et al., 2016a), Briefly, 4°C (cold) saline was infused (20 mL/kg) through a microcatheter placed in the right internal carotid artery via the external carotid artery for 15 minutes immediately after removal of the filament in the hypothermia group. To control for the effect of hemodilution by the infused saline, 37°C (warm) saline was infused in the same manner in the normothermia group. To ensure that selective brain hypothermia was successfully produced, cortical and rectal temperatures were monitored during the saline infusion. One rat died from hypothermia, and was replaced in subsequent experiments. Needle thermistor probes (BAT-12 Microprobe Thermometer; Physitemp Instruments, Inc., Clifton, NJ, USA) were placed into the cortex through holes 3 mm lateral to the bregma, 3 mm posterior to the bregma, and 3 mm lateral to the bregma on the ipsilateral side to monitor cortical temperature. Body temperatures were measured through the rectum. The rats were returned to their cages with free access to food and water and were closely monitored.
Evaluation of neurological deficits
At 6, 24 and 48 hours post-reperfusion, neurological deficits were evaluated using the Zea Longa 5-point scoring method (Longa et al., 1989), as described above.
Infarct volume analysis
After 24 hours of reperfusion, five rats in each group were sacrificed. The brains were quickly removed, placed in a refrigerator at –20°C for 20 minutes, and sectioned into coronal slices of 2 mm thickness. These sections were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (Amresco, Solong, CA, USA) at 37°C for 30 minutes in the dark to evaluate the infarct area. Normal brain tissue was stained red, whereas infarcted tissue was pale gray. The sections were imaged and analyzed using ImageJ software (NIH, Bethesda, MD, USA). Cerebral infarct size was calculated as: [(left hemisphere volume − right hemisphere non-infarcted volume)/left hemisphere volume] × 100%.
Hematoxylin-eosin and TUNEL stainings
At 6, 24 and 48 hours post-reperfusion, five rats from each group at each time point were euthanized with pentobarbital sodium and transcardially perfused with 0.9% NaCl, followed by 4% paraformaldehyde. The brains were quickly removed, and brain tissues in the coronal plane from 1 to 4 mm posterior to the optic chiasm were carefully dissected. The tissues were soaked in 10% paraformaldehyde/phosphate buffer overnight, dehydrated, cleared, dipped in paraffin wax, embedded, and cut into 5-μm-thick coronal sections. For hematoxylin-eosin staining, the sections were stained with hematoxylin for 10 minutes, immersed in 75% hydrochloric acid alcohol solution for 30 seconds, stained with eosin for 10 minutes, and immersed in 90% ethanol for 35 seconds. Six visual fields in the ischemic penumbra of each brain were randomly selected and observed under a 400× magnification objective lens (Olympus, Tokyo, Japan). Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was used to detect cell apoptosis. Briefly, paraffin sections were dewaxed, hydrated, and TUNEL-stained according to the instructions of a TUNEL kit (Merck Millipore, Darmstadt, Germany). Five visual fields of the ischemic penumbra of each brain slice were randomly selected for analysis under a 400× magnification objective lens (Olympus). The mean number of TUNEL-positive cells, identified by brownish yellow granules in the nucleus, was calculated. The percentage (%) of apoptotic cells = number of TUNEL-positive cells/total cells × 100%.
Western blot assay
At 6, 24 and 48 hours post-reperfusion, five rats from each group at each time point were sacrificed with pentobarbital sodium. The brains were quickly removed, and the ischemic cortices were rapidly dissected on ice, as previously described (Zhang et al., 2018). Mitochondrial and cytosolic fractions were separated using a cytosol/mitochondria fractionation kit (Beyotime Biotechnology, Beijing, China), according to the manufacturer’s instructions. The cytosolic fraction was stored at 4°C for cytochrome c assay. The expression of specific markers (Cox-IV for mitochondria) was used to ensure the purity of the cytosolic fraction. The ischemic penumbral cortices were homogenized in lysis buffer (RIPA and PMSF), and then centrifuged at 12,000 × g for 15 minutes at 4°C. The samples were used for Fis1 assays.
Protein concentration was measured with a bicinchoninic acid protein assay kit (Beyotime Biotechnology). Equal amounts of protein (30–50 µg) per sample were loaded into each well of 10% sodium dodecyl sulfate-polyacrylamide gels, separated by electrophoresis, and transferred onto a polyvinylidene fluoride membrane using the semi-dry method. After washing with 1× Tris-buffered saline/Tween-20 and blocking with 3% bovine serum albumin at room temperature for 2 hours, the membranes were incubated overnight at 4°C with the following primary antibodies: Fis1 monoclonal antibody (rabbit, 1:1000; Abcam, Cambridge, UK), cytochrome c monoclonal antibody (rabbit, 1:1000; Cell Signaling Technology, Boston, MA, USA), Cox-IV monoclonal antibody (rabbit, 1:2000; Abcam), and β-actin monoclonal antibody (rabbit, 1:5000; Zhongshan Goldenbridge Biotechnology, Beijing, China). After washing with 1× Tris-buffered saline/Tween-20, the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5000; Zhongshan Goldenbridge Biotechnology) in blocking solution for 1 hour at room temperature. The membranes were washed again, and the immunoreactive bands were detected using enhanced chemiluminescence (Beyotime Biotechnology). Images were quantified with ImageJ software (NIH). The levels of Fis1 and cytosolic cytochrome c were normalized to β-actin.
Quantitative reverse transcription-polymerase chain reaction
Total RNA from the ischemic penumbra-containing cortices was extracted using the TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. For cDNA synthesis, 1 μg of total RNA was reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit with gDNA Eraser (Perfect Real Time) (TaKaRa), according to the manufacturer’s instructions. Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) amplification and monitoring were performed using an ABI 7300 fast real time PCR system (Life Sciences, Carlsbad, CA, USA) and SYBR Premix Ex Taq (Tli RNaseH Plus) (TaKaRa). The primers used are listed in Table 1. Relative expression was determined using the 2–ΔΔCt method, with β-actin as an internal control.
Table 1.
Quantitative real-time polymerase chain reaction primer sequences
| Gene | Primer sequences | Product size (bp) |
|---|---|---|
| Fis1 | Forward:5′-CTG GAC TCA TTG GAC TGG CTG TG-3′ | 106 |
| Reverse:5′-AGG AAG GCG GTG GTG AGG ATG-3′ | ||
| β-Actin | Forward:5′-CAC CCG CGA GTA CAA CCT TC-3′ | |
| Reverse:5′-CCC ATA CCC ACC CAT CAC ACC-3′ | 125 |
Fis1: Fission 1.
Transmission electron microscopy and quantitative analysis of mitochondrial morphology
After 24 hours of reperfusion, five rats in each group were euthanized, and the brains were perfusion-fixed with 2.5% glutaraldehyde. Coronal brain tissue blocks (1 × 1 × 1 mm3) of the parietal lobe containing the cerebral ischemic region were postfixed with 4% glutaraldehyde at 4°C for 2 hours. The sections were then rinsed three times in 0.2 M PBS, soaked in 1% osmium tetroxide for 2 hours, rinsed in 0.2 M PBS again, dehydrated in an ascending ethanol series (to 100%), and embedded in epoxy resin. The sections were sectioned into 50 nm ultrathin sections with an ultramicrotome (UC6, Leica, Wetzlar, Germany) and placed on 200-mesh copper grids. Afterwards, the ultrathin sections were stained with lead citrate and observed under an H-7650 transmission electron microscope (Hitachi, Tokyo, Japan).
Four regions were randomly selected from a total of five slices from each sample under 100,000× magnification. The aspect ratio, vacuolation ratio and mean area density of vacuolated mitochondria were measured using ImageJ software (NIH, Bethesda, MD, USA) as morphological parameters. The aspect ratio was defined as the ratio of the major axes to the minor axes of the analyzed mitochondria. The vacuolation ratio was defined as the ratio of vacuolated mitochondria to the total number of mitochondria. The mean area density was defined as the ratio of the area of the vacuolated mitochondria to the total mitochondrial area (Wiemerslage and Lee, 2016).
Statistical analysis
All data are expressed as the mean ± SD, and were analyzed using SPSS 19.0 statistical software (IBM Corporation, Armonk, NY, USA). One-way analysis of variance was used for comparisons between different groups, followed by the least significant difference post hoc test. P < 0.05 was considered statistically significant.
Results
Perfusion of cold saline via the carotid artery selectively reduces cortical temperature
We first explored whether cold saline perfusion via the carotid artery induces selective brain hypothermia. Therefore, we continuously monitored cortical and rectal temperatures for 1 hour after reperfusion. In the hypothermia group, cortical temperature rapidly dropped from 34.39 ± 0.23°C to 32.22 ± 0.09°C (P < 0.01; Figure 1A) during the infusion, and was maintained below 33.0°C for at least 15 minutes after the infusion. In contrast, no significant changes in cortical temperature were found in the normothermia group (from 34.25 ± 0.11°C to 34.81 ± 0.44°C; P > 0.05; Figure 1A). Rectal temperature did not change during the observation period in either the hypothermia group or the normothermia group (P > 0.05; Figure 1B). These results suggest that cold saline treatment produces selective brain hypothermia.
Figure 1.
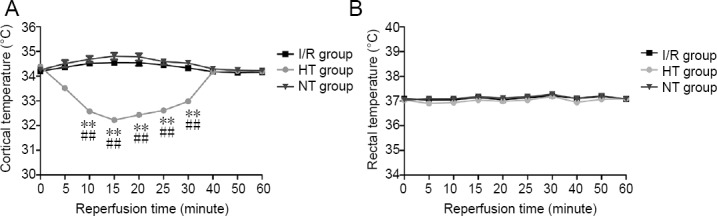
Cortical and rectal temperatures in rats with focal cerebral I/R injury 1 hour after reperfusion.
(A) Cortical temperature; (B) rectal temperature. Data are shown as the mean ± SD (n = 10; one-way analysis of variance followed by the least significant difference post hoc test). **P < 0.01, vs. I/R group; ##P < 0.01, vs. NT group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
Selective brain hypothermia decreases neurological deficit scores and cerebral infarct size
Neurological examination was conducted using the Zea Longa 5-point scoring method at 6, 24 and 48 hours post reperfusion (Figure 2). Cerebral infarct size was determined by TTC staining 24 hours after reperfusion (Figure 3A). Higher neurological deficit scores and cerebral infarct size were observed in the I/R, hypothermia and normothermia groups compared with the sham group (P < 0.05). Neurological deficit scores and cerebral infarct size were reduced in the hypothermia group compared with the I/R and normothermia groups (P < 0.05). There was little difference in neurological deficit score and cerebral infarct size between the normothermia and I/R groups (P > 0.05; Figures 2 and 3B).
Figure 2.
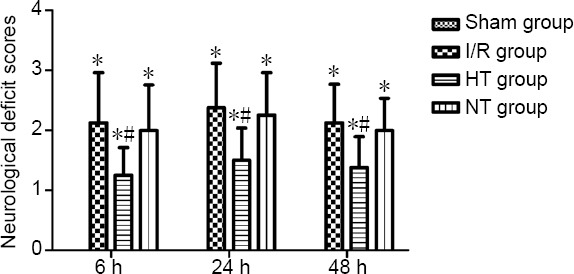
Effects of selective brain hypothermia on neurological deficit scores in rats with focal cerebral I/R injury.
Data are shown as the mean ± SD (n = 8; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
Figure 3.
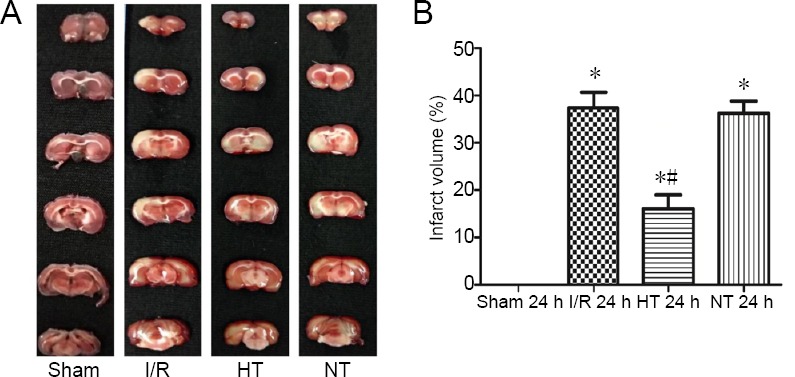
Effects of selective brain hypothermia on infarct volume after 24 hours of reperfusion in rats with focal cerebral I/R injury.
(A) Representative TTC-stained coronal brain sections. (B) Quantification of the percentage of cerebral infarct size among the four groups. Data are shown as the mean ± SD (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia; TTC: 2,3,5-triphenyltetrazolium chloride.
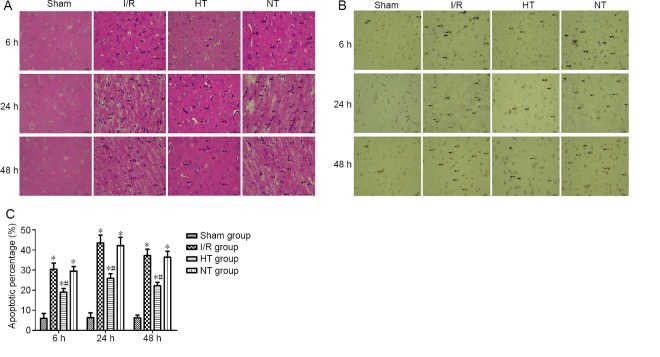
Selective brain hypothermia alleviates histopathological changes and apoptosis in the cortical ischemic penumbra in rats with I/R injury
At 6, 24 and 48 hours post-reperfusion, hematoxylin-eosin staining revealed no morphologically abnormal cells in the sham group, while shrunken cell bodies and nuclear pyknosis were present in the other three groups. These pathological changes were markedly ameliorated in the hypothermia group compared with the I/R and normothermia groups (Figure 4A). TUNEL analysis was used to detect apoptosis. As shown in Figure 4B and C, the percentage of TUNEL-positive cells was increased in the ischemic penumbra at 6, 24 and 48 hours post reperfusion in the three injury groups compared with the sham group (P < 0.05). The proportion of TUNEL-positive cells was lower in the hypothermia group than in the I/R group at 6, 24 and 48 hours post reperfusion (P < 0.05). There were no significant differences between the normothermia and I/R groups (P > 0.05).
Figure 4.
Effects of selective brain hypothermia on ischemia-induced histopathological changes and apoptosis in rats with focal cerebral I/R injury.
(A) Representative images of hematoxylin-eosin staining of the cortical ischemic penumbra. Shrunken cell bodies and nuclear pyknosis (black arrows) were observed in the I/R, HT and NT groups, but not in the sham group. The degree of histopathological changes was slightly less in the HT group than in the I/R group. (B) Representative images of TUNEL staining showing cellular apoptosis in the cortical ischemic penumbra. TUNEL-positive nuclei (with brownish yellow granules) were considered to indicate apoptotic cells (black arrows). Scale bars: 400 μm; light microscope. (C) Quantification of the percentage of TUNEL-positive cells in the four groups. The data are expressed as the mean ± SD (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
Selective brain hypothermia decreases the expression of Fis1 protein and mRNA in the ischemic penumbra following I/R
Fis1 expression in the ischemic penumbra was measured by western blot assay, and Fis1 mRNA expression was measured by qRT-PCR. Similar trends in the expression of Fis1 protein and mRNA were observed. The expression levels of Fis1 protein and mRNA were higher at 6, 24 and 48 hours post-reperfusion in the three injury groups compared with the sham group (P < 0.05). The expression levels of Fis1 protein and mRNA at 6, 24 and 48 hours post reperfusion were reduced in the hypothermia group (P < 0.05). Changes in the expression of Fis1 protein and mRNA were negligible between the normothermia and I/R groups (P > 0.05; Figure 5B and C).
Figure 5.
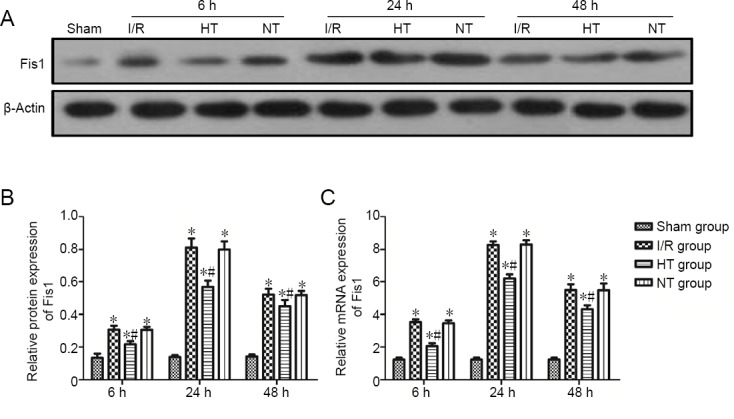
Fis1 protein expression in the cortical ischemic penumbra following focal cerebral I/R injury in rats.
(A) Western blot assay for Fis1 protein expression in the cortical ischemic penumbra: Blots are representative images of all rats per group. (B) Relative quantities of Fis1 to β-actin: The results were normalized to β-actin expression. (C) qRT-PCR for Fis1 mRNA expression in the cortical ischemic penumbra. Relative quantities of Fis1 mRNA to β-actin. The results were normalized to β-actin expression. The data are shown as the mean ± SD (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group. Fis1: Fission 1; HT: hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
Selective brain hypothermia reduces cytosolic cytochrome c levels in the ischemic penumbra following I/R
Cytochrome c is released from mitochondria into the cytosol during mitochondrial fission, and induces apoptosis. Therefore, we analyzed the levels of cytosolic cytochrome c by western blot assay to assess the levels of mitochondrial fission. The expression of specific markers (Cox-IV for mitochondria) was used to confirm the purity of the cytosolic fraction. The blots revealed good purity of the cytosolic fraction (Figure 6A). As shown in Figure 6B, cytosolic cytochrome c levels were significantly increased at 6, 24 and 48 hours post-reperfusion in the three injury groups compared with the sham group (P < 0.05). By contrast, cytosolic cytochrome c levels at 6, 24 and 48 hours post-reperfusion were reduced in the hypothermia group (P < 0.05). No significant changes in cytosolic cytochrome c levels were found between the normothermia and I/R groups (P > 0.05).
Figure 6.
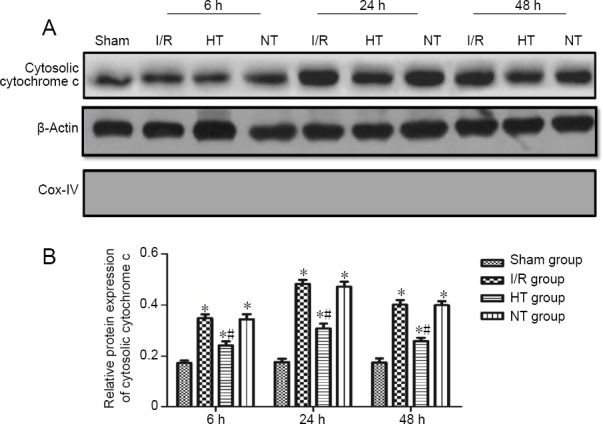
Cytosolic cytochrome c levels in the cortical ischemic penumbra of rats with focal cerebral I/R injury.
(A) Western blot assay of cytosolic cytochrome c levels in the cortical ischemic penumbra. Cox-IV was used to assess the purity of the cytosolic fraction. Blots are representative images of all rats per group. (B) Relative quantities of cytosolic cytochrome c to β-actin. The results were normalized to β-actin expression. The data are shown as the mean ± SD (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
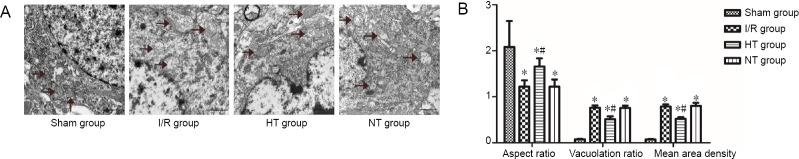
Effects of selective brain hypothermia on changes in mitochondrial ultrastructure
Transmission electron microscopy was used to evaluate mitochondrial ultrastructure at 24 hours after reperfusion. The mitochondria were well arranged and exhibited a complete bilayer membrane structure and normal cristae without swelling or vacuolar degeneration in the sham group (Figure 7A). In contrast, in the I/R and normothermia groups, mitochondria showed signs of pathological changes, such as disappearance of bilayer membrane structure, vacuolar degeneration and swelling, and loss of cristae. These observations suggest that excessive mitochondrial fission occurred 24 hours after reperfusion in these groups (Figure 7A). These pathomorphological changes were ameliorated in the hypothermia group, with mitochondria exhibiting a less swollen and relatively intact membrane (Figure 7A).
Figure 7.
Effects of selective brain hypothermia on changes in mitochondrial ultrastructure.
(A) Electron photomicrographs showing mitochondrial (black arrows) morphological structure in the cortical ischemic penumbra after 24 hours of reperfusion. Scale bars: 1 μm; transmission electron microscope. (B) Quantitative parameters for mitochondrial morphological changes, including aspect ratio, vacuolation ratio and mean area density of vacuolated mitochondria. Data are shown as the mean ± SD (n = 5; one-way analysis of variance followed by the least significant difference post hoc test). *P < 0.05, vs. sham group; #P < 0.05, vs. the I/R group. HT: Hypothermia; I/R: ischemia/reperfusion; NT: normothermia.
The aspect ratio, vacuolation ratio and mean area density of vacuolated mitochondria were used to quantify changes in mitochondrial morphology (Figure 7B). The aspect ratio was significantly higher in the sham group than in the other groups (P < 0.05). The vacuolation ratio and mean area density of vacuolated mitochondria were significantly lower in the sham group than in the other groups (P < 0.05). By contrast, the aspect ratio was increased and the vacuolation ratio and mean area density of vacuolated mitochondria were reduced in the hypothermia group compared with the IR group (P < 0.05). No significant differences in these parameters were found between the normothermia and I/R groups (P > 0.05).
Discussion
In this study, we used the MCAO model of focal cerebral I/R injury to simulate the pathophysiological changes in ischemic stroke. In addition, only male rats were used to eliminate the effect of estrogen, which has been shown to protect against cerebral ischemia (Xiao et al., 2018). Cerebral I/R injury is a common clinical pathophysiological phenomenon after the restoration of blood perfusion in stroke patients, and involves multiple pathogenic processes (Kalogeris et al., 2016; Leech et al., 2019). Mitochondria play an important role in I/R injury, and are involved in calcium homeostasis, oxidative phosphorylation, reactive oxygen species production, and apoptosis (Russo et al., 2018; Yang et al., 2018). Recent studies suggest that morphological changes in the mitochondria are relevant to I/R injury (Liu et al., 2012; Anzell et al., 2018; Li and Liu, 2018). In stress, the balance of mitochondrial fission and fusion is lost, and fission prevails, resulting in mitochondrial fragmentation and dysfunction (Twig and Shirihai, 2011; Calo et al., 2013; Kausar et al., 2018). Moreover, recent studies show that excessive mitochondrial fission or fragmentation occur in cerebral I/R injury (Kim et al., 2011; Tang et al., 2016a; Anzell et al., 2018). In addition, Fis1, a critical mitochondrial fission system protein, plays an important role in the regulation of mitochondrial fission. In our previous study, Drp1 expression in the mitochondrial outer membrane increased during cerebral I/R injury (Zhang et al., 2018). Based on this finding, we speculated that Fis1 expression would increase during cerebral I/R injury. Indeed, we found here that Fis1 expression is increased during cerebral I/R injury. Our findings are in agreement with other studies demonstrating that Fis1 expression is increased in cerebral I/R injury (Lu et al., 2017; Anzell et al., 2018).
Apoptosis is a key pathological event in cerebral I/R injury (Bai et al., 2016; Gong et al., 2017; Radak et al., 2017; Long et al., 2019). Excessive mitochondrial fission promotes mitochondrial outer membrane permeability and increases cytochrome c release, thereby activating the apoptotic cascade and aggravating neurological damage (MacDougall et al., 2018; Yang et al., 2018). In a study by Wang et al. (2012), Fis1 overexpression increased mitochondrial fission and apoptosis, whereas Fis1 knockdown attenuated mitochondrial fission and apoptosis. Consistent with previous studies, our current findings suggest that Fis1 overexpression increases mitochondrial fission, causing cytochrome c release and apoptosis during cerebral I/R injury.
Numerous studies have focused on the neuroprotective effects of hypothermia, one of the most effective therapeutic strategies for ischemic stroke (Yenari and Han, 2012; Wu and Grotta, 2013; Andresen et al., 2015; Zheng and Gao, 2016; Kurisu and Yenari, 2017). Compared with systemic hypothermia, selective brain hypothermia is more suitable for neuroprotection after stroke, because it quickly achieves the target temperature and avoids the adverse effects of general hypothermia (Chen et al., 2013; Esposito et al., 2014; Seyedsaadat et al., 2019). Moreover, transarterial regional hypothermia, which produces selective brain hypothermia, may exert strong neuroprotective effects in the MCAO with transient collateral hypoperfusion model (Kurisu et al., 2016a). In addition, some pre-clinical and clinical studies show that short-duration infusion of cold saline through the artery is feasible and safe for acute ischemic stroke (Tang et al., 2013; Kurisu et al., 2016b; Wu et al., 2018). Therefore, in the present study, we successfully produced the hypothermia model following MCAO by perfusing cold saline through the internal carotid artery. To eliminate the effect of hemodilution by the infused saline, we used 37°C saline infusion in the same manner in the normothermia group. Neurological deficit scores and cerebral infarct volume decreased after cold saline perfusion in the hypothermia group. Furthermore, differences between the I/R and normothermia groups were negligible. These results show that selective brain hypothermia attenuates focal cerebral I/R injury.
Mitochondrial dysfunction is a key step of cerebral I/R injury, and mitochondria are a target of hypothermia-induced neuroprotection (Nguyen et al., 2018; Zhao et al., 2018). General hypothermia attenuates mitochondrial oxidative stress and reduces mitochondrial membrane permeability (Gong et al., 2012, 2013). In addition, in our previous study, general hypothermia reduced mitochondrial fission by inhibiting the translocation of Drp1 from the cytoplasm to the mitochondrial outer membrane (Tang et al., 2016b). Drp1 is mainly localized to the cytoplasm, and mitochondrial fission is inhibited in cells with Fis1 deletion mutations (Mozdy et al., 2000). However, it remained unclear whether selective brain hypothermia inhibits mitochondrial fission and reduces apoptosis by inhibiting Fis1 expression. Therefore, in this study, we analyzed the expression levels of Fis1 protein and mRNA and cytosolic cytochrome c, as well as apoptosis in the ischemic penumbra of the cerebral cortex in rats with focal cerebral I/R injury. The results clearly demonstrate that selective brain hypothermia inhibits the expression of Fis1 protein and mRNA, and reduces the levels of cytosolic cytochrome c and apoptosis at 6, 24 and 48 hours after reperfusion. Furthermore, mitochondrial ultrastructural analysis revealed that 24 hours after reperfusion, mitochondrial fission was reduced in the hypothermia group compared with the I/R and normothermia groups. Based on our present findings and a previous report (Tang et al., 2016b), we hypothesize that selective brain hypothermia downregulates Fis1 expression in the mitochondrial outer membrane, thereby inhibiting excessive mitochondrial fission induced by the binding of Drp1 to Fis1, in turn decreasing the mitochondrial release of cytochrome c into the cytosol, ultimately reducing apoptosis.
This study has some limitations. First, the mechanisms by which selective brain hypothermia inhibits Fis1 remain unclear. We will investigate the effects of Fis1 overexpression and knockdown in vitro and in vivo in future studies. Second, mitochondrial membrane potential perturbation and reactive oxygen species production, related to mitochondrial dysfunction following I/R injury need to be examined in greater detail. Third, further studies are needed to investigate the changes in the degree of binding of Drp1 to Fis1 after selective brain hypothermia.
In conclusion, although the mechanisms underlying the neuroprotection conferred by selective brain hypothermia remain to be further clarified, our current results nonetheless suggest that selective brain hypothermia exerts a neuroprotective effect, at least in part, by regulating Fis1 expression.
Additional files:
Additional Figure 1 (546.7KB, tif) : Experimental process.
Experimental process.
Neurological deficit score was assessed by Zea Longa 5-point scoring method, and the specific rating criteria are as follows in the text. The related indicator detection included that cerebral infarct volume was assessed by 5-triphenyltetrazolium chloride (TTC) staining; cell apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, Fis1 and cyto-Cyto c expression were assessed by western blot, Fis1 mRNA expression was assessed by qRT-PCR, and mitochondrial ultrastructure was assessed by transmission electron microscopy. HT: Hypothermia; I/R: ischemia/reperfusion; MCAO: middle cerebral artery occlusion; NT: normothermia; R: reperfusion.
Additional file 1: Open peer review report 1 (102.8KB, pdf) .
Acknowledgments:
We would like to thank Meng-Meng Zhang from Department of Pathology in Affiliated Qingdao Municipal Hospital of Qingdao University, China for helping us make paraffin sections of hematoxylin-eosin staining and TUNEL staining.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2015HM023 (to MSW); the Science and Technology Plan Project of Qingdao City of China, No. 19-6-1-50-nsh (to MSW). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: Experiments were authorized by the Ethics Committee of Qingdao Municipal Hospital of China (approval No. 2019008). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Muneeb Faiq, New York University School of Medicine, USA.
Funding: This study was supported by the Natural Science Foundation of Shandong Province of China, No. ZR2015HM023 (to MSW); the Science and Technology Plan Project of Qingdao City of China, No. 19-6-1-50-nsh (to MSW).
P-Reviewer: Faiq M; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Patel B, Pack M, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Almekhlafi MA, Colbourne F, Al Sultan AS, Goyal M, Demchuk AM. Selective brain cooling: let us have a moment of science. J Cereb Blood Flow Metab. 2019;39:182–183. doi: 10.1177/0271678X18800274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andresen M, Gazmuri JT, Marin A, Regueira T, Rovegno M. Therapeutic hypothermia for acute brain injuries. Scand J Trauma Resusc Emerg Med. 2015;23:42–50. doi: 10.1186/s13049-015-0121-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anzell AR, Maizy R, Przyklenk K, Sanderson TH. Mitochondrial quality control and disease: insights into ischemia-reperfusion injury. Mol Neurobiol. 2018;55:2547–2564. doi: 10.1007/s12035-017-0503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai S, Sun Y, Wu L, Wu Z, Fang M. Tripotolide ameliorates inflammation and apoptosis induced by focal cerebral ischemia/reperfusion in rats. Med JZU. 2016;45:493–500. doi: 10.3785/j.issn.1008-9292.2016.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- 6.Calo L, Dong Y, Kumar R, Przyklenk K, Sanderson TH. Mitochondrial dynamics: an emerging paradigm in ischemia-reperfusion injury. Curr Pharm Des. 2013;19:6848–6857. doi: 10.2174/138161281939131127110701. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Fredrickson V, Ding Y, Cheng H, Wang N, Ling F, Ji X. Enhanced neuroprotection by local intra-arterial infusion of human albumin solution and local hypothermia. Stroke. 2013;44:260–262. doi: 10.1161/STROKEAHA.112.675462. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Winger AJ, Knowlton AA. Mitochondrial dynamic changes in health and genetic diseases. Mol Biol Rep. 2014;41:7053–7062. doi: 10.1007/s11033-014-3663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimenti MS, Sunzini F, Fiorucci L, Botti E, Fonti GL, Conigliaro P, Triggianese P, Costa L, Caso F, Giunta A, Esposito M, Bianchi L, Santucci R, Perricone R. Potential role of cytochrome c and tryptase in psoriasis and psoriatic arthritis pathogenesis: focus on resistance to apoptosis and oxidative stress. Front Immunol. 2018;9:1–11. doi: 10.3389/fimmu.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuartero MI, de la Parra J, Garcia-Culebras A, Ballesteros I, Lizasoain I, Moro MA. The kynurenine pathway in the acute and chronic phases of cerebral ischemia. Curr Pharm Des. 2016;22:1060–1073. doi: 10.2174/1381612822666151214125950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies A, Wassink G, Bennet L, Gunn AJ, Davidson JO. Can we further optimize therapeutic hypothermia for hypoxic-ischemic encephalopathy? Neural Regen Res. 2019;14:1678–1683. doi: 10.4103/1673-5374.257512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Rooij NK, Rinkel GJ, Dankbaar JW, Frijns CJ. Delayed cerebral ischemia after subarachnoid hemorrhage: a systematic review of clinical, laboratory, and radiological predictors. Stroke. 2013;44:43–54. doi: 10.1161/STROKEAHA.112.674291. [DOI] [PubMed] [Google Scholar]
- 13.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: Meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36:1157–1164. doi: 10.1177/0271678X16645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esposito E, Ebner M, Ziemann U, Poli S. In cold blood: intraarteral cold infusions for selective brain cooling in stroke. J Cereb Blood Flow Metab. 2014;34:743–752. doi: 10.1038/jcbfm.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng HX, Li RP, Li YG, Wang XQ, Zhang L, Deng JB, Wang L. 14, 15-EET suppresses neuronal apoptosis in ischemia-reperfusion through the mitochondrial pathway. Neurochem Res. 2017;42:2841–2849. doi: 10.1007/s11064-017-2297-6. [DOI] [PubMed] [Google Scholar]
- 17.Gomes LC, Scorrano L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim Biophys Acta. 2008;1777:860–866. doi: 10.1016/j.bbabio.2008.05.442. [DOI] [PubMed] [Google Scholar]
- 18.Gong L, Tang Y, An R, Lin M, Chen L, Du J. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:465–475. doi: 10.1038/cddis.2017.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong P, Hua R, Zhang Y, Zhao H, Tang Z, Mei X, Zhang M, Cui J, Li C. Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. J Cereb Blood Flow Metab. 2013;33:928–934. doi: 10.1038/jcbfm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gong P, Li CS, Hua R, Zhao H, Tang ZR, Mei X, Zhang MY, Cui J. Mild hypothermia attenuates mitochondrial oxidative stress by protecting respiratory enzymes and upregulating MnSOD in a pig model of cardiac arrest. PLoS One. 2012;7:313–324. doi: 10.1371/journal.pone.0035313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Ischemia/reperfusion. Compr Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kausar S, Wang F, Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells. 2018;7:274–293. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, Dillin A, Mercola M, Ronai ZA. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft P, De Meyer SF, Kleinschnitz C. Next-generation antithrombotics in ischemic stroke: preclinical perspective on ‘bleeding-free antithrombosis’. J Cereb Blood Flow Metab. 2012;32:1831–1840. doi: 10.1038/jcbfm.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurisu K, Abumiya T, Ito M, Gekka M, Osanai T, Shichinohe H, Nakayama N, Kazumata K, Houkin K. Transarterial regional hypothermia provides robust neuroprotection in a rat model of permanent middle cerebral artery occlusion with transient collateral hypoperfusion. Brain Res. 2016a;1651:95–103. doi: 10.1016/j.brainres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Kurisu K, Abumiya T, Nakamura H, Shimbo D, Shichinohe H, Nakayama N, Kazumata K, Shimizu H, Houkin K. Transarterial regional brain hypothermia inhibits acute aquaporin-4 surge and sequential microvascular events in ischemia/reperfusion injury. Neurosurgery. 2016b;79:125–134. doi: 10.1227/NEU.0000000000001088. [DOI] [PubMed] [Google Scholar]
- 27.Kurisu K, Yenari MA. Therapeutic hypothermia for ischemic stroke; pathophysiology and future promise. Neuropharmacology. 2017;134:302–309. doi: 10.1016/j.neuropharm.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leech T, Chattipakorn N, Chattipakorn SC. The beneficial roles of metformin on the brain with cerebral ischaemia/reperfusion injury. Pharmacol Res. 2019;146:261–278. doi: 10.1016/j.phrs.2019.104261. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Liu X. Novel insights into the role of mitochondrial fusion and fission in cardiomyocyte apoptosis induced by ischemia/reperfusion. J Cell Physiol. 2018;233:5589–5597. doi: 10.1002/jcp.26522. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Lu J, Manaenko A, Tang J, Hu Q. Mitochondria in ischemic stroke: new insight and implications. Aging Dis. 2018;9:924–937. doi: 10.14336/AD.2017.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu W, Tian F, Kurata T, Morimoto N, Abe K. Dynamic changes of mitochondrial fission proteins after transient cerebral ischemia in mice. Brain Res. 2012;1456:94–99. doi: 10.1016/j.brainres.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Long YF, Wang XL, Wang MP, Tang XJ. Effects of androgen on the expression of Bcl-2, Bax and Cyt-C in brain tissue of adult rat models of middle cerebral artery occlusio. Zhongguo Zuzhi Gongcheng Yanjiu. 2019;23:4344–4349. [Google Scholar]
- 33.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 34.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CJ, Guo YZ, Zhang Y, Yang L, Chang Y, Zhang JW, Jing L, Zhang JZ. Coenzyme Q10 ameliorates cerebral ischemia reperfusion injury in hyperglycemic rats. Pathol Res Pract. 2017;213:1191–1199. doi: 10.1016/j.prp.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 36.MacDougall G, Anderton RS, Mastaglia FL, Knuckey NW, Meloni BP. Mitochondria and neuroprotection in stroke: cationic arginine-rich peptides (CARPs) as a novel class of mitochondria-targeted neuroprotective therapeutics. Neurobiol Dis. 2018;121:17–33. doi: 10.1016/j.nbd.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. Cell Biol. 2000;151:367–379. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865–886. doi: 10.1038/nrd.2018.174. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen H, Zarriello S, Rajani M, Tuazon J, Napoli E, Borlongan CV. Understanding the role of dysfunctional and healthy mitochondria in stroke pathology and its treatment. Int J Mol Sci. 2018;19:2127–2147. doi: 10.3390/ijms19072127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER. Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol. 2017;15:115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- 41.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, Napoli E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018;4:84–94. doi: 10.4103/bc.bc_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchis-Gomar F, Derbre F. Mitochondrial fission and fusion in human diseases. N Engl J Med. 2014;370:1073–1074. doi: 10.1056/NEJMc1316254. [DOI] [PubMed] [Google Scholar]
- 43.Seyedsaadat SM, Marasco SF, Daly DJ, McEgan R, Anderson J, Rodgers S, Kreck T, Kadirvel R, Kallmes DF. Selective brain hypothermia: feasibility and safety study of a novel method in five patients. Perfusion. 2019;10:1–8. doi: 10.1177/0267659119853950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suliman HB, Piantadosi CA. Mitochondrial quality control as a therapeutic target. Pharmacol Rev. 2016;68:20–48. doi: 10.1124/pr.115.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang J, Hu Z, Tan J, Yang S, Zeng L. Parkin protects against oxygen-glucose deprivation/reperfusion insult by promoting drp1 degradation. Oxid Med Cell Longev. 2016a;2016:1–10. doi: 10.1155/2016/8474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang XN, Liu L, Koike MA, Yenari MA. Mild hypothermia reduces tissue plasminogen activator-related hemorrhage and blood brain barrier disruption after experimental stroke. Ther Hypothermia Temp Manag. 2013;3:74–83. doi: 10.1089/ther.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Y, Liu X, Zhao J, Tan X, Liu B, Zhang G, Sun L, Han D, Chen H, Wang M. Hypothermia-induced ischemic tolerance is associated with Drp1 inhibition in cerebral ischemia-reperfusion injury of mice. Brain Res. 2016b;1646:73–83. doi: 10.1016/j.brainres.2016.05.042. [DOI] [PubMed] [Google Scholar]
- 48.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vivien D, Gauberti M, Montagne A, Defer G, Touze E. Impact of tissue plasminogen activator on the neurovascular unit: from clinical data to experimental evidence. J Cereb Blood Flow Metab. 2011;31:2119–2134. doi: 10.1038/jcbfm.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang K, Long B, Jiao J, Wang J, Liu J, Li Q, Li P. miR-484 regulates mitochondrial network through targeting Fis1. Nat Commun. 2012;3:781–789. doi: 10.1038/ncomms1770. [DOI] [PubMed] [Google Scholar]
- 51.Wang K, Long B, Zhou LY, Liu F, Zhou QY, Liu CY, Fan YY, Li PF. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 downregulation. Nat Commun. 2014;5:3596–3609. doi: 10.1038/ncomms4596. [DOI] [PubMed] [Google Scholar]
- 52.Whitley BN, Engelhart EA, Hoppins S. Mitochondrial dynamics and their potential as a therapeutic target. Mitochondrion. 2019;2:1567–1582. doi: 10.1016/j.mito.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wiemerslage L, Lee D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J Neurosci Methods. 2016;262:56–65. doi: 10.1016/j.jneumeth.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C, Zhao W, An H, Wu L, Chen J, Hussain M, Ding Y, Li C, Wei W, Duan J, Wang C, Yang Q, Wu D, Liu L, Ji X. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J Cereb Blood Flow Metab. 2018;38:2251–2260. doi: 10.1177/0271678X18790139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu TC, Grotta JC. Hypothermia for acute ischaemic stroke. Lancet Neurol. 2013;12:275–284. doi: 10.1016/S1474-4422(13)70013-9. [DOI] [PubMed] [Google Scholar]
- 56.Xiao H, Deng M, Yang B, Hu Z, Tang J. Pretreatment with 17beta-estradiol attenuates cerebral ischemia-induced blood-brain barrier disruption in aged rats: involvement of antioxidant signaling. Neuroendocrinology. 2018;106:20–29. doi: 10.1159/000455866. [DOI] [PubMed] [Google Scholar]
- 57.Xie W, Zhou P, Sun Y, Meng X, Dai Z, Sun G, Sun X. Protective effects and target network analysis of ginsenoside Rg1 in cerebral ischemia and reperfusion injury: a comprehensive overview of experimental studies. Cells. 2018;7:270–288. doi: 10.3390/cells7120270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang JL, Mukda S, Chen SD. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018;16:263–275. doi: 10.1016/j.redox.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 60.Zhang G, Sun L, Chen H, Wang M. Electroacupuncture preconditioning protects against focal cerebral ischemia reperfusion injury via suppression of dynamic-related protein 1. Neural Regen Res. 2018;13:86–93. doi: 10.4103/1673-5374.224373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H, Luo Y, Chen L, Zhang Z, Shen C, Li Y, Xu R. Sirt3 inhibits cerebral ischemia-reperfusion injury through normalizing Wnt/beta-catenin pathway and blocking mitochondrial fission. Cell Stress Chaperones. 2018;23:1079–1092. doi: 10.1007/s12192-018-0917-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng RJ, Gao YH. Effect of mild hypothermia on nerve regeneration microenvironment of infarcted area in rat models of cerebral infarction. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4013–4019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental process.
Neurological deficit score was assessed by Zea Longa 5-point scoring method, and the specific rating criteria are as follows in the text. The related indicator detection included that cerebral infarct volume was assessed by 5-triphenyltetrazolium chloride (TTC) staining; cell apoptosis was assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining, Fis1 and cyto-Cyto c expression were assessed by western blot, Fis1 mRNA expression was assessed by qRT-PCR, and mitochondrial ultrastructure was assessed by transmission electron microscopy. HT: Hypothermia; I/R: ischemia/reperfusion; MCAO: middle cerebral artery occlusion; NT: normothermia; R: reperfusion.