Abstract
In the pathophysiology of neurodegenerative disorders, the role of misfolded protein deposition leading to neurodegeneration has been primarily discussed. In the last decade, however, it has been proposed a parallel involvement of innate immune activation, chronic inflammation and adaptive immunity in the neurodegeneration mechanisms triggered by proteinopathies. New insights in the neurodegenerative field strongly suggest a role for the immune system in the pathophysiology of neurodegenerative disorders. Therefore, the hypothesis underlining the modulation of the innate and the adaptive immune system in the events linked to brain deposition of misfolded proteins could open new perspectives in the setting of specific immunotherapeutic strategies for the treatment of neurodegenerative diseases. Therefore, we have reviewed the pathogenic hypothesis in neurodegenerative pathologies, underling the links between the deposition of misfolded protein mechanisms and the immune activation.
Keywords: adaptive immunity, choroid plexus, immunotherapy, innate immunity, neurodegenerative diseases, neuroinflammation, proteinopathies
Background
Neurodegenerative diseases is an umbrella expression including Alzheimer’s disease (AD), Parkinson’s disease (PD), Lewy body disease, amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD) and prion disease (Jellinger and Attems, 2015; Kumar et al., 2016; Chitnis and Weiner, 2017). Multimodal animal and cellular modelling studies suggest that misfolded protein aggregation plays a crucial role in the neuronal vulnerability linked to neurodegeneration (Golde and Miller, 2009; Hartl, 2017; Sami et al., 2017).
The pathophysiological hypothesis of neurodegenerative diseases relies on the fact that some proteins change their conformations, thereby gaining toxicity or losing their physiological functions, and generating small oligomeric or large fibrillary aggregates which, resulting in neurotoxicity, lead to neurodegeneration (Bayer, 2015; Sami et al., 2017; Soto and Pritzkow, 2018). Neurodegenerative proteinopathies were categorized as “pure” when composed by a single type of protein aggregates, or as “mixed” if characterized by the deposition of different misfolded protein classes (Bayer, 2015; Walker et al., 2015).
Recently, it has also been demonstrated a prominent role of the immune system in the neurodegenerative disease pathophysiology (López-Valdés and Martínez-Coria, 2016; McGeer and McGeer, 2002; Amor and Woodroofe, 2014; Schwartz and Baruch, 2014; Chitnis and Weiner, 2017). Accumulative data show the involvement of the innate, as well as, of the adaptive immune system in the inflammatory mechanisms associated with misfolded protein accumulation in the brain. Therefore, the employment of new immunotherapeutic strategies for the treatment of neurodegenerative diseases was proposed (McGeer and McGeer, 2011; Baruch et al., 2013, 2015; Amor and Woodroofe, 2014; Andreasson et al., 2016; Schwartz and Deczkowska, 2016). The present review analyzes different neurodegenerative disease pathogenic hypotheses recently proposed to explain the cascade of events starting with the deposition of misfolded proteins in the brain and leading to chronic immune activation.
Search Strategy and Selection Criteria
Databases (for published data): PubMed. The following electronic database was searched from inception up to March 2019. The search strategy was defined, agreed and carried by authors. The searches were undertaken using index terms and keywords relating to neurodegenerative diseases, proteinopathies, neuroinflammation, immunity and immunotherapy. Search results obtained were imported to Mendeley reference manager and duplicates were removed. Following this, the authors identified articles by screening titles and evaluated all full-text abstracts of potentially relevant articles for their eligibility.
Protein Misfolding and Deposition in Neurodegenerative Disorders
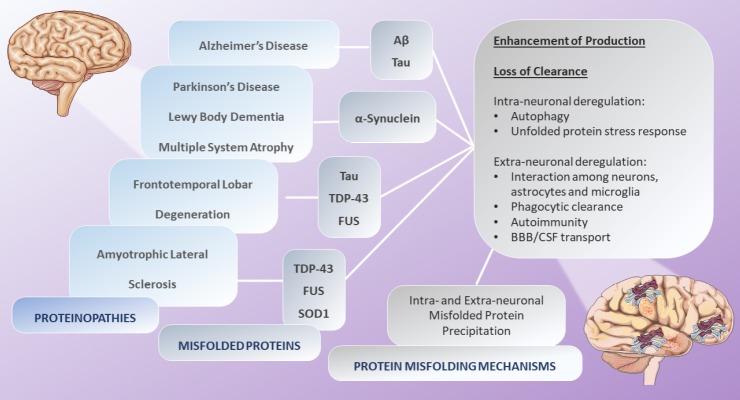
It is well known that the most frequent neurodegenerative proteinopathies are AD, LBD and PD. All neurodegenerative proteinopathies are characterized by intra- or extracellular protein accumulation in the central nervous system (CNS), as β-sheet rich aggregates (Walker et al., 2015; Ugalde et al., 2016). It is commonly recognized that some proteins that are unstructured in healthy brains, in neurodegenerative proteinopathies change their conformation, naturally undergoing profound modifications in their structural folding, thereby forming small oligomeric or large fibrillary aggregates (Golde and Miller, 2009; Bayer, 2015). These changes, in terms of size and three-dimensional shape, lead to their self-association, elongation and precipitation in specific brain regions, therefore producing the acquisition of pathological protein features. The molecular mechanisms resulting in misfolded protein conformational changes tend to be the same in all the proteinopathies and may include different mechanisms, such as post-translational modifications, the loss of protein clearance or the enhancement of protein production (Bayer, 2015). It has been suggested that the clearance of the proteins has a critical role in the preservation of neuronal cell integrity. Several publications described that, in most neurodegenerative conditions, compromised protein clearance might be able to modulate brain functions and structure, leading to clinical manifestations (Bae et al., 2012; Deleidi and Maetzler, 2012). In the brain, the deregulation of protein clearance mechanisms, both at the intra-neuronal (autophagy and unfolded protein stress response) and at the extra-neuronal level (interaction among neurons, astrocytes and microglia, phagocytic clearance, autoimmunity, cerebrospinal fluid transport, and transport across the blood-brain barrier), also lead to intra- and extra-neuronal misfolded protein precipitation, respectively (Alvarez-Erviti et al., 2010; Kumar et al., 2016; Chiti and Dobson, 2017; Hartl, 2017; Sami et al., 2017) (Figure 1).
Figure 1.
Protein misfolding mechanisms.
In most neurodegenerative disorders, proteins that are unstructured in healthy brains, undergo modifications in their structural folding, forming small oligomeric or large fibrillary aggregates. These changes lead to their self-association, elongation and intra- and extra-neuronal precipitation. The molecular mechanisms resulting in misfolded protein conformational changes tend to be the same in all the proteinopathies and may include different mechanisms, such as post-translational modifications, the loss of protein clearance or the enhancement of protein production. Source: Servier Medical Art by Servier and modified under the following terms: Creative Commons Attribution 3.0 Unported license (CC BY 3.0). Aβ: Amyloid β protein; BBB: blood-brain barrier; CSF: cerebrospinal fluid; FUS: fused in sarcoma gene; SOD: superoxide dismutase; TDP-43: transactive response DNA binding protein with a molecular weight of 43 kDa.
Prion disease
Among the proteinopathies, the prion disease is characterized by a unique phenotype, given that the pathogenic mechanisms, in this case, involve the transmission of the prion (the causative agent of the disease) or the proteinaceous infective particles. Those particles are composed by an aberrant isoform (PrPSc) of the prion protein (PrPc). The PrPSc isoform aggregates and induces the conformational misfold of PrPC fragments, triggering its autocatalytic amplification and transmission in the CNS (Ugalde et al., 2016). Therefore, prion diseases are transmitted by highly infective misfolded prions (Brundin et al., 2010). The prion-like machinery relies on irreversible interactions among constitutive molecules, as well as on the resistance to protein clearance and on the ability to propagate to target cells (Bayer, 2015). Some studies conducted on animal models of AD and PD suggest a possible prion-like behavior of the β-amyloid protein (Aβ) and α-synuclein (Brundin et al., 2010; Ugalde et al., 2016; Dugger et al., 2017).
Parkinson’s disease
The α-synuclein is the major component of the intracytoplasmic fibrillar Lewy Bodies deposits and represents the hallmark of the synucleinopathy lesions (Surguchov, 2015). Parkinson’s disease is characterized by the deposition of α-synuclein aggregates in the brainstem, while Lewy body accumulation in the cortico-limbic system and the brainstem is typical of Lewy Body Dementia, and α-synuclein accumulation in basal ganglia, as well as in brainstem and cerebellum marks out Multiple System Atrophy (Emamzadeh and Surguchov, 2018). It is known that α-synuclein is a ubiquitous 140-amino acid protein of 18–20 kDa encoded by a gene on chromosome 4 that, together with β- and γ-synuclein is a member of a protein family with pleiotropic effects (Surguchov, 2015). It has been demonstrated that a mutation of the gene encoding for α-synuclein (SNCA) is linked to familial PD and the change of soluble α-synuclein peptides into amyloid fibrils and intermediate oligomers is a crucial mechanism in the synucleinopathy pathogenesis (Surguchev and Surguchov, 2017, 2018).
Alzheimer’s disease
In the AD pathogenesis, the extracellular amyloid plaques and neurofibrillary tangles represent the neuropathological hallmark. The major components of amyloid plaques are Aβ1–40 and Aβ1–42 peptides, which aggregate and form β-sheets. The Aβ peptides are produced by the proteolytic cleavage of the amyloid precursor protein by the beta-amyloid cleavage enzyme 1, and beta and gamma secretases (Bursavich et al., 2016). The Aβ40 is the predominant isoform of amyloid fragments detected in plasma and cerebrospinal fluid samples, while the Aβ42 isoform was mainly associated with nucleation, due to its aggregation tendency. In diffuse plaques, such as in Down’s syndrome, only the Aβ42 was found, while in senile plaques both the Aβ40 and the Aβ42 isoforms were described (Dickson, 1997). In parallel to the extracellular amyloid plaque deposition in AD brains, the Aβ intracellular deposition triggers the pathological cascade involving a second neurotoxic molecule: the Tau protein (Medina and Avila, 2014). Therefore, AD is also characterized by the deposition of the intra-neuronal neurofibrillary tangle of hyper-phosphorylated aggregated tau protein (Braak et al., 2006). Tau protein is involved in the formation and stabilization of microtubules (Medina and Avila, 2014). Besides the well-known concept related to the hyper-phosphorylated Tau-induced neurotoxicity, in which the process of tau phosphorylation is believed to be of critical relevance for tangle formation, recent data has shown that tau oligomers are linked to neurodegeneration, even in the absence of Aβ deposition (Kayed, 2010).
Frontotemporal dementia and amyotrophic lateral sclerosis
Among tauopathies, the FTLD comprises a heterogeneous spectrum of clinical and neuropathological subtypes that are associated with cognitive and motor disorders. The neuropathology of the FTLD is characterised by abnormal intracellular accumulation of some disease-specific proteins that allow classifying FTLD into different categories (Rademakers et al., 2012). The three major recognized pathological FTLD subtypes are the FTLD-tau, FTLD-TDP, and FTLD-FUS. The FTLD-tau is characterized by the aggregation of hyperphosphorylated tau protein in neurons and glia. The FTLD-U has been initially recognized with ubiquitin immunohistochemistry, but it has been subsequently renamed FTLD-TDP considering that the ubiquitinated pathological protein in the majority of FTLD-U cases, was identified in the transactive response DNA binding protein with a molecular weight of 43 kDa (TDP-43). Finally, the FTLD-FUS is characterized by inclusions of fused in sarcoma gene (FUS). These findings suggest that FTD and ALS are strictly related disorders with overlapping neuropathologies (i.e., TDP-43 and FUS) (Rademakers et al., 2012; Onozato et al., 2016). An additional misfolded protein in ALS is the mutant superoxide dismutase 1 that represents the neuropathological hallmark of ALS, which is linked to motor neuron degeneration (Münch et al., 2011).
Recent literature also describes that the presence of TDP-43 pathology in AD enhances both the cognitive decline and the medial lobe atrophy (Josephs et al., 2014; Walker et al., 2015). Actually, loss of TDP-43 leads to specific proteomic patterns, indicating that TDP-43 is a relevant protein implicated in the regulation of RNA metabolism and intracellular transport, given that Ran-binding protein 1, DNA methyltransferase 3 alpha and chromogranin B are downregulated when TDP-43 is knockdown (Štalekar et al., 2015). Clinico-neuropthological studies, performed in AD and synucleinopathies, suggested that both Aβ and α-synuclein peptides interact each other through multiple molecular sites, producing common overlapping mechanisms in the pathophysiological cascades of different proteinopathies (Attems, 2017). Therefore, several proteins may be considered as neuropathological brain hallmarks, indicating the coexistence, in the same patients, of more than one neurodegenerative disease, and supporting the theory of the “mixed pathology” model (Jellinger and Attems, 2015). In this context, a distinctive misfolded protein often results predominant, acquiring a main role in the pathogenesis definition, while additional proteins resulted co-expressed, but to a lesser extent (Walker et al., 2015).
Immune Features of Neurodegenerative Diseases
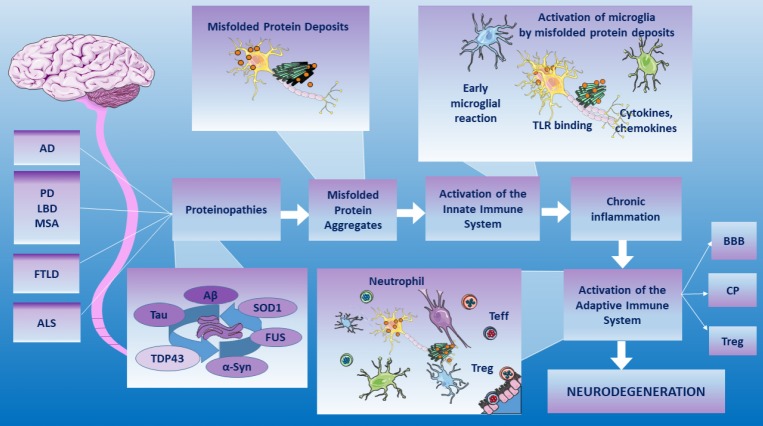
Several studies give light on the theory that combines the neurodegeneration with the immune activation (Golde and Miller, 2009; Schwartz and Baruch, 2014; Baruch et al., 2015; Schwartz and Deczkowska, 2016) (Figure 2). There is growing evidence on the fact that distinct immune responses, involving the adaptive as well as the innate immune system, are crucially implicated in neurodegenerative diseases (Chitnis and Weiner, 2017). Ageing leads to the change of the nervous and immune systems, thus resulting in a deregulation of immune responses in healthy brains, therefore triggering the neurodegenerative machinery. In neurological disorders, the role of adaptive and innate immune responses result beneficial or detrimental respect to their degree (Amor and Woodroofe, 2014; Schwartz and Baruch, 2014).
Figure 2.
Combined theory in the neurodegenerative diseases.
Misfolded protein aggregates can bind and activate a whole range of PRRs triggering neuroinflammation. In the brain parenchyma, when the innate immunity microglia-mediated and adaptive immunity, regulated by the BBB, the CP and the Treg functionality, are not decisive, the toxic cascade and neuronal death have been demonstrated. Therefore, a relationship among amyloid-like deposition, immune mechanisms and neurodegenerative pathologies has been described. Source: Servier Medical Art by Servier and modified under the following terms: Creative Commons Attribution 3.0 Unported license (CC BY 3.0). AD: Alzheimer’s disease; Aβ: amyloid β protein; α-syn: α-synuclein; ALS: amyotrophic lateral sclerosis; BBB: blood-brain barrier; CP: choroid plexus; FTLD: Frontotemporal lobar degeneration; FUS: fused in sarcoma gene; LBD: Lewy body disease; MSA: multiple system atrophy; PD: Parkinson’s disease; PRRs: Pattern-recognition receptors; SOD1: superoxide dismutase 1; TDP-43: transactive response DNA binding protein with a molecular weight of 43 kDa; Teff: effector T cell; TLR: toll-like receptor; Treg: regulatory T cell.
Innate immunity in brain proteinopathies
The activation of the innate immune system is a crucial first line of defence and also recruits cells of the adaptive immune system in different ways: by inducing adhesion molecules on the blood-brain barrier (BBB) and by expressing co-stimulatory molecules on microglia (Amor et al., 2010). Inflammatory mechanisms can trigger, at various stages, the neurodegenerative cascade. Inflammation-mediated neurodegeneration can be linked to the dysfunction of endogenous or exogenous immune cells. In the central nervous system, the predominant population of endogenous immune cells are represented by astrocytes and mononuclear phagocytes, which include microglia and perivascular macrophages (Chitnis and Weiner, 2017). Misfolded protein aggregates look like pathogen-associated molecular patterns (PAMPs) and thymus-independent type 2 (TI-2) antigens (Takeda et al., 2003; Golde and Miller, 2009). The PAMPs are a group of molecules, such as proteins, polysaccharides, or nucleotides, that are able to activate the innate immune response, binding pattern-recognition receptors (PRRs). The TI-2 antigens are similar polymeric molecules that directly stimulate IgM secretion by B cells. PAMPs and TI-2 antigens resemble amyloid deposits (Glenner, 1980). Several groups and a plethora of experimental data support the hypothesis that extracellular and intracellular protein aggregates could act like PAMPs leading to chronic innate immune activation by PRRs (McGeer and McGeer, 2002; Salminen et al., 2009). Amyloid-like deposits can bind and activate a whole range of PRRs, including Toll-like receptors, formyl peptide receptors, the receptor for advanced glycation end products, scavenger receptors, complement and pentraxins (Golde and Miller, 2009). When this first innate immune-related process is not decisive, and microglia fails in the protein misfolded-deposit removal, the microglia-mediated mechanisms remain trapped in a vicious cycle characterized by pro-inflammatory cytokine production linked to a toxic cascade and neuronal death (Schwartz and Baruch, 2014).
Adaptive immunity in brain proteinopathies
In addition to microglial activation, the neuroinflammation is characterized by the presence of infiltrating leukocytes in the brain parenchyma (Schwartz and Deczkowska, 2016). The adaptive immunity, also known as acquired immunity, encloses the humoral and the cell-mediated immunity (Chen and Palmer, 2008). Under neurodegenerative conditions, T lymphocytes infiltrate specific brain regions (Engelhardt and Ransohoff, 2005) and high frequencies of reactive T lymphocytes have also been found in the bloodstream, suggesting their crucial role in the physiopathology of neurodegenerative diseases (Ciccocioppo et al., 2008; Huang et al., 2009; Miscia et al., 2009; Lanuti et al., 2012). In the central nervous system, CD4+ T cells orchestrate immune homeostasis through a complex network of cellular interactions (Huang et al., 2009). The involvement of cellular immune responses in neurodegenerative diseases has been demonstrated, as increased T-cell responses to CNS antigens, or shifts in CD4+ and CD8+ subsets both in the periphery and in the CNS have been shown (Amor et al., 2010). Therefore, a relationship among innate immunity, adaptive immunity and neurodegenerative pathologies has been demonstrated (McGeer and McGeer, 2011; Andreasson et al., 2016; Nataf, 2017). In the adaptive immunity, T cells play a key role both in cell-mediated immunity and in humoral immunity, participating in immunological homeostasis. The CD4+ T cells can be subtyped into effector T cells, memory T cells and regulatory T cells; their roles have mainly been emphasized in the neurodegenerative research field (Huang et al., 2009). After antigen exposure, naïve CD4+ T cells (Th0) undergo their clonal expansion. Depending on different stimuli as well as on antigen-presenting cell signals, CD4+ T cells become Th1 (IL-2, IFN-g, and TNF-a), Th2 (IL-4, IL-5, and IL-13) and Th17 (IL-17 and IL-22) cells (Bodles and Barger, 2004). During neuroinflammation, Th1, Th2 and Th17 cells provide both neuroprotection and neuronal loss. Specifically, Th1 and Th17 cells produce pro-inflammatory cytokines (IL-1, IL-6, IL-17, TNF-α and IFN-g) and directly contribute to neuroinflammation as well as indirectly enhance the secretion of reactive oxygen species and nitric oxide from microglia. Lymphocytes Th1 and Th17, together with Th2, up-regulate the release of insulin-like growth factor-1 (IGF-1) from microglia, enhancing microglia-mediated neuroprotection (Huang et al., 2009). Regulatory T cells (Tregs) express several markers, including CD4, CD25, CD62L, CD103, CD152, glucocorticoid-induced tumor necrosis factor receptor, latency-associated peptide and the forehead home box domain P3 transcription factor (Igarashi et al., 2008). During chronic neuroinflammation, Tregs display the ability to dampen down neuroinflammation by the suppression of Teff activation and induction of Teff apoptosis and also by the upregulation of brain-derived neurotrophic factors and glial cell-derived neurotrophic factors (O’Connor and Anderton, 2008). Regulatory T cells have been proposed as crucial players in neurodegenerative diseases, such as AD, PD, and ALS (Baruch et al., 2015; Dansokho et al., 2016; Ciccocioppo et al., 2019; Pieragostino et al., 2019). It has also been suggested, in AD transgenic animal models, that the adaptive immunity is impaired and that Tregs stimulation modulates brain inflammation in AD (Smigiel et al., 2014; Dansokho et al., 2016; Schwartz and Deczkowska, 2016).
The choroid plexus role in the neurodegenerative disorders
Expanding data support the hypothesis that, in neurodegenerative disorders, the recruitment of immunoregulatory cells to the brain areas of neuropathology is crucial for damping the inflammatory machinery (Schwartz and Baruch, 2014; Baruch et al., 2015). The BBB has the role in regulating the movement of the immunocompetent cells in the brain (Sardi et al., 2011; Baruch et al., 2013; Schwartz and Deczkowska, 2016). The chronic neuroinflammation status compromises the BBB integrity, increasing its permeability and leading to the loss of immunologically privileged status of the CNS. The migration and infiltration of T cells in the neuropathological areas of the brain is also a result of the BBB de-regulation and impairment (Montagne et al., 2015; Nelson et al., 2016). In this contest, the choroid plexus (CP) constitutes the blood-CSF barrier and represents a critical site for the neuroimmune crosstalk (Schwartz and Baruch, 2014). In the animal models of CNS inflammation, such as AD, ALS and the brain ageing, the CP is not stimulated to allow the trafficking of immune cells, probably because of the deficient IFN-g signalling levels (Baruch et al., 2013; Mesquita et al., 2015). In parallel, in ageing and neurodegenerative disorders, the CP does not allow the entrance of appropriate amounts of healing leukocytes, whereas compromised integrity of the BBB contributes to disease exacerbation through the passage of leukocytes. This supports the hypothesis that the CP is well equipped to regulate the trafficking of leukocytes for the maintenance of the CNS health (Schwartz and Baruch, 2014; Baruch et al., 2015; Schwartz and Deczkowska, 2016).
Immunotherapeutic Strategies in Neurodegenerative Diseases
Immunotherapy is one of the most studied therapeutic strategies in neurodegenerative disorders, and, in particular, in such a context, the vaccination against Aβ, α-synuclein, and tau has been extensively explored (Valera et al., 2016).
Immunotherapeutic approaches include both passive and active immunization. The passive immunization consists in the monoclonal antibody infusion directed against the target molecules, which, in this case, are the misfolded proteins; while the active immunization method employs specific antigens inducing specific adaptive immune responses (Arevalo-Villalobos et al., 2017).
Immunotherapy in synucleinopathies
Passive immunotherapy for treating synucleinopathies uses monoclonal antibodies directed against the C-terminus (Masliah et al., 2011) or the N-terminus or the central region of the α-synuclein. Those antibodies exerted neuroprotective effects on α-synuclein-induced nigral cell death and on the related innate immune activation (Shahaduzzaman et al., 2015). The active immunization with short peptides-AFFITOPEs®, has been studied in two mouse models of Lewy body disease; some studies showed a reduction in α-synuclein aggregation and an increase in anti-inflammatory cytokine production, resulting in an improvement of the motor functions (Mandler et al., 2015).
Immunotherapy in Alzheimer’s disease
Passive immunization against AD has strongly attracted the interest of scientists in recent years. Among humanized monoclonal antibodies, bapineuzumab, solanezumab, gantenerumab, crenezumab and monoclonal antibodies targeting phosphorylated Tau gave controversial results linked to their side effects. On the other hand, those monoclonal antibodies demonstrate an improvement in terms of cognitive dysfunctions only in patients with the mild form of the disease. These data suggest that this kind of immunotherapeutic approaches could be useful only when administrated at an early stage of the disease (Jia et al., 2014; Wisniewski and Goñi, 2014). As a consequence, this evidence remarks the need to recognize preclinical biomarkers that identify the early stage of the neuropathological mechanism, when the iceberg-like process is at its starting phase.
In the active immunization against the Aβ peptide, the AN-1792 vaccine (a full-length pre-aggregated amyloid peptide Aβ1–42) and the ACC-001 vaccine (a sequence of six amino acids (Aβ1–6)) phase II clinical trials have been stopped because their administration is associated with severe side effects, including aseptic meningoencephalitis (Arai et al., 2015). Another vaccine, the CAD106, consisting in Aβ1–6 fragments linked to the coat protein of bacteriophage Qβ, does not induce adverse effects; however, no clinical efficacy has been described so far (Farlow et al., 2015).
Conclusion
Neurodegenerative diseases have a substantial impact on global health and no curative treatments are available to date. In addition to classic immunotherapeutic approaches, new insights are pointing out multifaceted immunotherapeutic perspectives. The involvement of the adaptive immunity in the NDs, which manifestation relies on the involvement of the Treg compartment that affects the brain’s choroid plexus, a selective gateway for recruitment of immunoregulatory cells to cerebral sites of pathology, has been proposed as an additional factor in the modulation of neuroinflammatory mechanisms (Baruch et al., 2015; Fakhoury, 2015; Schwartz and Deczkowska, 2016; Arevalo-Villalobos et al., 2017). The emerging data support the immune involvement in neurodegenerative disorders. Further studies could be addressed to identify new immunotherapeutic prospective based on the adaptive immune modulation.
Additional file: Open peer review report 1 (88.4KB, pdf) .
Acknowledgments:
Thanks to Prof. Domenico Gambi and to Prof. Marco Onofrj for their precious supervision.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Andrei Surguchov, University of Kansas Medical Center, USA.
P-Reviewer: Surguchov A; C-Editors: Zhao M, Li JY; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 2.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amor S, Woodroofe MN. Innate and adaptive immune responses in neurodegeneration and repair. Immunology. 2014;141:287–291. doi: 10.1111/imm.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreasson KI, Bachstetter AD, Colonna M, Ginhoux F, Holmes C, Lamb B, Landreth G, Lee DC, Low D, Lynch MA, Monsonego A, O’Banion MK, Pekny M, Puschmann T, Russek-Blum N, Sandusky LA, Selenica ML, Takata K, Teeling J, Town T, et al. Targeting innate immunity for neurodegenerative disorders of the central nervous system. J Neurochem. 2016;138:653–693. doi: 10.1111/jnc.13667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai H, Suzuki H, Yoshiyama T. Vanutide cridificar and the QS-21 adjuvant in Japanese subjects with mild to moderate Alzheimer’s disease: results from two phase 2 studies. Curr Alzheimer Res. 2015;12:242–254. doi: 10.2174/1567205012666150302154121. [DOI] [PubMed] [Google Scholar]
- 6.Arevalo-Villalobos JI, Rosales-Mendoza S, Zarazua S. Immunotherapies for neurodegenerative diseases: current status and potential of plant-made biopharmaceuticals. Expert Rev Vaccines. 2017;16:151–159. doi: 10.1080/14760584.2016.1229602. [DOI] [PubMed] [Google Scholar]
- 7.Attems J. Alzheimer’s disease pathology in synucleinopathies. Lancet Neurol. 2017;16:22–23. doi: 10.1016/S1474-4422(16)30282-4. [DOI] [PubMed] [Google Scholar]
- 8.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, Lee SJ. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W, Mirlas-Neisberg N, Cardon M, Vaknin I, Cahalon L, Berkutzki T, Mattson MP, Gomez-Pinilla F, Friedman N, Schwartz M. CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci U S A. 2013;110:2264–2269. doi: 10.1073/pnas.1211270110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baruch K, Rosenzweig N, Kertser A, Deczkowska A, Sharif AM, Spinrad A, Tsitsou-Kampeli A, Sarel A, Cahalon L, Schwartz M. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967. doi: 10.1038/ncomms8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer TA. Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur Neuropsychopharmacol. 2015;25:713–724. doi: 10.1016/j.euroneuro.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Bodles AM, Barger SW. Cytokines and the aging brain - what we don’t know might help us. Trends Neurosci. 2004;27:621–626. doi: 10.1016/j.tins.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–307. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bursavich MG, Harrison BA, Blain JF. Gamma secretase modulators: new Alzheimer’s drugs on the horizon? J Med Chem. 2016;59:7389–7409. doi: 10.1021/acs.jmedchem.5b01960. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17:R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- 17.Chiti F, Dobson CM. Protein misfolding, amyloid formation, and human disease: A summary of progress over the last decade. Annu Rev Biochem. 2017;86:27–68. doi: 10.1146/annurev-biochem-061516-045115. [DOI] [PubMed] [Google Scholar]
- 18.Chitnis T, Weiner HL. CNS inflammation and neurodegeneration. J Clin Invest. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciccocioppo F, Lanuti P, Marchisio M, Gambi F, Santavenere E, Pierdomenico L, Bascelli A, Velluto L, Gambi D, Miscia S. Expression and phosphorylation of protein kinase C isoforms in Abeta(1-42) activated T lymphocytes from Alzheimers disease. Int J Immunopathol Pharmacol. 2008;21:23–33. doi: 10.1177/039463200802100104. [DOI] [PubMed] [Google Scholar]
- 20.Ciccocioppo F, Lanuti P, Pierdomenico L, Simeone P, Bologna G, Ercolino E, Buttari F, Fantozzi R, Thomas A, Onofrj M, Centonze D, Miscia S, Marchisio M. The characterization of regulatory T-cell profiles in Alzheimer’s disease and multiple sclerosis. Sci Rep. 2019;9:8788. doi: 10.1038/s41598-019-45433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dansokho C, Ait Ahmed D, Aid S, Toly-Ndour C, Chaigneau T, Calle V, Cagnard N, Holzenberger M, Piaggio E, Aucouturier P, Dorothée G. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain. 2016;139:1237–1251. doi: 10.1093/brain/awv408. [DOI] [PubMed] [Google Scholar]
- 22.Deleidi M, Maetzler W. Protein clearance mechanisms of alpha-synuclein and amyloid-beta in lewy body disorders. Int J Alzheimers Dis. 2012;2012:1–9. doi: 10.1155/2012/391438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–339. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Dugger BN, Perl DP, Carlson GA. Neurodegenerative disease transmission and transgenesis in mice. Cold Spring Harb Perspect Biol. 2017;9:a023549. doi: 10.1101/cshperspect.a023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emamzadeh FN, Surguchov A. Parkinson’s disease: biomarkers, treatment, and risk factors. Front Neurosci. 2018;12:612. doi: 10.3389/fnins.2018.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Fakhoury M. Role of immunity and inflammation in the pathophysiology of neurodegenerative diseases. Neurodegener Dis. 2015;15:63–69. doi: 10.1159/000369933. [DOI] [PubMed] [Google Scholar]
- 28.Farlow MR, Andreasen N, Riviere M-E, Vostiar I, Vitaliti A, Sovago J, Caputo A, Winblad B, Graf A. Long-term treatment with active Aβ immunotherapy with CAD106 in mild Alzheimer’s disease. Alzheimers Res Ther. 2015;7:23. doi: 10.1186/s13195-015-0108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glenner GG. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts) N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 30.Golde TE, Miller VM. Proteinopathy-induced neuronal senescence: a hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimers Res Ther. 2009;1:5. doi: 10.1186/alzrt5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartl FU. Protein misfolding diseases. Annu Rev Biochem. 2017;86:21–26. doi: 10.1146/annurev-biochem-061516-044518. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Reynolds AD, Mosley RL, Gendelman HE. CD 4+ T cells in the pathobiology of neurodegenerative disorders. J Neuroimmunol. 2009;211:3–15. doi: 10.1016/j.jneuroim.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Igarashi H, Cao Y, Iwai H, Piao J, Kamimura Y, Hashiguchi M, Amagasa T, Azuma M. GITR ligand-costimulation activates effector and regulatory functions of CD4+ T cells. Biochem Biophys Res Commun. 2008;369:1134–1138. doi: 10.1016/j.bbrc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 34.Jellinger KA, Attems J. Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm. 2015;122:505–521. doi: 10.1007/s00702-014-1288-x. [DOI] [PubMed] [Google Scholar]
- 35.Jia Q, Deng Y, Qing H. Potential therapeutic strategies for Alzheimer’s disease targeting or beyond β -amyloid: insights from clinical trials. Biomed Res Int. 2014;2014:1–22. doi: 10.1155/2014/837157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Jack CR, Parisi JE, Petersen RC, Dickson DW. TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol. 2014;127:811–824. doi: 10.1007/s00401-014-1269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kayed R. Anti-tau oligomers passive vaccination for the treatment of Alzheimer disease. Hum Vaccin. 2010;6:931–935. doi: 10.4161/hv.6.11.12689. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Sami N, Kashav T, Islam A, Ahmad F, Hassan MI. Protein aggregation and neurodegenerative diseases: From theory to therapy. Eur J Med Chem. 2016;124:1105–1120. doi: 10.1016/j.ejmech.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 39.Lanuti P, Ciccocioppo F, Bonanni L, Marchisio M, Lachmann R, Tabet N, Pierdomenico L, Santavenere E, Catinella V, Iacone A, Thomas A, Gambi D, Miscia S, Onofrj M, Kern F. Amyloid-specific T-cells differentiate Alzheimer’s disease from Lewy body dementia. Neurobiol Aging. 2012;33:2599–2611. doi: 10.1016/j.neurobiolaging.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 40.López-Valdés HE, Martínez-Coria H. The role of neuroinflammation in age-related dementias. Rev Invest Clin. 2016;68:40–48. [PubMed] [Google Scholar]
- 41.Mandler M, Valera E, Rockenstein E, Mante M, Weninger H, Patrick C, Adame A, Schmidhuber S, Santic R, Schneeberger A, Schmidt W, Mattner F, Masliah E. Active immunization against alpha-synuclein ameliorates the degenerative pathology and prevents demyelination in a model of multiple system atrophy. Mol Neurodegener. 2015;10:10. doi: 10.1186/s13024-015-0008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, Mueller-Steiner S, Seubert P, Barbour R, McConlogue L, Buttini M, Games D, Schenk D. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.McGeer PL, McGeer EG. Innate immunity, local inflammation, and degenerative disease. Sci Aging Knowl Environ. 2002;2002:re3. doi: 10.1126/sageke.2002.29.re3. [DOI] [PubMed] [Google Scholar]
- 44.McGeer PL, McGeer EG. History of innate immunity in neurodegenerative disorders. Front Pharmacol. 2011;2:77. doi: 10.3389/fphar.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medina M, Avila J. The role of extracellular Tau in the spreading of neurofibrillary pathology. Front Cell Neurosci. 2014;8:113. doi: 10.3389/fncel.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mesquita SD, Ferreira AC, Gao F, Coppola G, Geschwind DH, Sousa JC, Correia-Neves M, Sousa N, Palha JA, Marques F. The choroid plexus transcriptome reveals changes in type I and II interferon responses in a mouse model of Alzheimer’s disease. Brain Behav Immun. 2015;49:280–292. doi: 10.1016/j.bbi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Miscia S, Ciccocioppo F, Lanuti P, Velluto L, Bascelli A, Pierdomenico L, Genovesi D, Di Siena A, Santavenere E, Gambi F, Ausili-Cèfaro G, Grimley PM, Marchisio M, Gambi D. Abeta(1-42) stimulated T cells express P-PKC-delta and P-PKC-zeta in Alzheimer disease. Neurobiol Aging. 2009;30:394–406. doi: 10.1016/j.neurobiolaging.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nataf S. Autoimmunity as a driving force of cognitive evolution. Front Neurosci. 2017;11:582. doi: 10.3389/fnins.2017.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim Biophys Acta. 2016;1862:887–900. doi: 10.1016/j.bbadis.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193:1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Onozato T, Nakahara A, Suzuki-Kouyama E, Hineno A, Yasude T, Nakamura T, Yahikozawa H, Watanabe M, Kayanuma K, Makishita H, Ohara S, Hashimoto T, Higuchi K, Sakai T, Asano K, Hashimoto T, Kanno H, Nakayama J, Oyanagi K. Axonal TDP-43 aggregates in sporadic amyotrophic lateral sclerosis. Neuropathol Appl Neurobiol. 2016;42:561–572. doi: 10.1111/nan.12310. [DOI] [PubMed] [Google Scholar]
- 54.Pieragostino D, Lanuti P, Cicalini I, Cufaro MC, Ciccocioppo F, Ronci M, Simeone P, Onofrj M, van der Pol E, Fontana A, Marchisio M, Del Boccio P. Proteomics characterization of extracellular vesicles sorted by flow cytometry reveals a disease-specific molecular cross-talk from cerebrospinal fluid and tears in multiple sclerosis. J Proteomics. 2019;204:103403. doi: 10.1016/j.jprot.2019.103403. [DOI] [PubMed] [Google Scholar]
- 55.Rademakers R, Neumann M, Mackenzie IR. Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol. 2012;8:423–434. doi: 10.1038/nrneurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Sami N, Rahman S, Kumar V, Zaidi S, Islam A, Ali S, Ahmad F, Hassan MI. Protein aggregation, misfolding and consequential human neurodegenerative diseases. Int J Neurosci. 2017;127:1047–1057. doi: 10.1080/00207454.2017.1286339. [DOI] [PubMed] [Google Scholar]
- 58.Sardi F, Fassina L, Venturini L, Inguscio M, Guerriero F, Rolfo E, Ricevuti G. Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev. 2011;11:149–153. doi: 10.1016/j.autrev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz M, Deczkowska A. Neurological disease as a failure of brain-immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol. 2016;37:668–679. doi: 10.1016/j.it.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Shahaduzzaman M, Nash K, Hudson C, Sharif M, Grimmig B, Lin X, Bai G, Liu H, Ugen KE, Cao C, Bickford PC. Anti-human α-synuclein N-terminal peptide antibody protects against dopaminergic cell death and ameliorates behavioral deficits in an AAV-α-synuclein rat model of Parkinson’s disease. PLoS One. 2015;10:e0116841. doi: 10.1371/journal.pone.0116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ. Regulatory T-cell homeostasis: steady-state maintenance and modulation during inflammation. Immunol Rev. 2014;259:40–59. doi: 10.1111/imr.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soto C, Pritzkow S. Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases. Nat Neurosci. 2018;21:1332–1340. doi: 10.1038/s41593-018-0235-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Štalekar M, Yin X, Rebolj K, Darovic S, Troakes C, Mayr M, Shaw CE, Rogelj B. Proteomic analyses reveal that loss of TDP-43 affects RNA processing and intracellular transport. Neuroscience. 2015;293:157–170. doi: 10.1016/j.neuroscience.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 65.Surguchev AA, Surguchov A. Synucleins and gene expression: ramblers in a crowd or cops regulating traffic? Front Mol Neurosci. 2017;10:224. doi: 10.3389/fnmol.2017.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surguchov A. Intracellular dynamics of synucleins: “here, there and everywhere”. Int Rev Cell Mol Biol. 2015;320:103–169. doi: 10.1016/bs.ircmb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 67.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 68.Ugalde CL, Finkelstein DI, Lawson VA, Hill AF. Pathogenic mechanisms of prion protein, amyloid-β and α-synuclein misfolding: the prion concept and neurotoxicity of protein oligomers. J Neurochem. 2016;139:162–180. doi: 10.1111/jnc.13772. [DOI] [PubMed] [Google Scholar]
- 69.Valera E, Spencer B, Masliah E. Immunotherapeutic approaches targeting amyloid-β, α-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics. 2016;13:179–189. doi: 10.1007/s13311-015-0397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, Colloby SJ, Jellinger K, Attems J. Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol. 2015;129:729–748. doi: 10.1007/s00401-015-1406-3. [DOI] [PubMed] [Google Scholar]
- 71.Wisniewski T, Goñi F. Immunotherapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:499–507. doi: 10.1016/j.bcp.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.