Key Points
Question
What is the yield of primary care–based screening of children for islet autoantibodies?
Findings
In a program that screened 90 632 children aged 2 to 5 years in Bavaria, Germany, during primary care visits, 280 children (0.31%) had 2 or more islet autoantibodies, among whom 62 developed clinical type 1 diabetes and 2 had mild or moderate diabetic ketoacidosis. Mothers of children with presymptomatic type 1 diabetes reported more symptoms of depression at diagnosis than mothers of children without islet autoantibodies (median psychological stress score of 3 vs 2).
Meaning
These findings may inform considerations of population-based screening of children for islet autoantibodies.
Abstract
Importance
Public health screening for type 1 diabetes in its presymptomatic stages may reduce disease severity and burden on a population level.
Objective
To determine the prevalence of presymptomatic type 1 diabetes in children participating in a public health screening program for islet autoantibodies and the risk for progression to clinical diabetes.
Design, Setting, and Participants
Screening for islet autoantibodies was offered to children aged 1.75 to 5.99 years in Bavaria, Germany, between 2015 and 2019 by primary care pediatricians during well-baby visits. Families of children with multiple islet autoantibodies (presymptomatic type 1 diabetes) were invited to participate in a program of diabetes education, metabolic staging, assessment of psychological stress associated with diagnosis, and prospective follow-up for progression to clinical diabetes until July 31, 2019.
Exposures
Measurement of islet autoantibodies.
Main Outcomes and Measures
The primary outcome was presymptomatic type 1 diabetes, defined by 2 or more islet autoantibodies, with categorization into stages 1 (normoglycemia), 2 (dysglycemia), or 3 (clinical) type 1 diabetes. Secondary outcomes were the frequency of diabetic ketoacidosis and parental psychological stress, assessed by the Patient Health Questionnaire-9 (range, 0-27; higher scores indicate worse depression; ≤4 indicates no to minimal depression; >20 indicates severe depression).
Results
Of 90 632 children screened (median [interquartile range {IQR}] age, 3.1 [2.1-4.2] years; 48.5% girls), 280 (0.31%; 95% CI, 0.27-0.35) had presymptomatic type 1 diabetes, including 196 (0.22%) with stage 1, 17 (0.02%) with stage 2, 26 (0.03%) with stage 3, and 41 who were not staged. After a median (IQR) follow-up of 2.4 (1.0-3.2) years, another 36 children developed stage 3 type 1 diabetes. The 3-year cumulative risk for stage 3 type 1 diabetes in the 280 children with presymptomatic type 1 diabetes was 24.9% ([95% CI, 18.5%-30.7%]; 54 cases; annualized rate, 9.0%). Two children had diabetic ketoacidosis. Median (IQR) psychological stress scores were significantly increased at the time of metabolic staging in mothers of children with presymptomatic type 1 diabetes (3 [1-7]) compared with mothers of children without islet autoantibodies (2 [1-4]) (P = .002), but declined after 12 months of follow-up (2 [0-4]) (P < .001).
Conclusions and Relevance
Among children aged 2 to 5 years in Bavaria, Germany, a program of primary care–based screening showed an islet autoantibody prevalence of 0.31%. These findings may inform considerations of population-based screening of children for islet autoantibodies.
This study categorizes the prevalence of 2 or more islet autoantibodies identified in preschool children in a population screening program, and the progression from presymptomatic to clinical symptomatic type 1 diabetes.
Introduction
The identification of patients with a disease prior to its clinical manifestation provides opportunities for prevention and for counseling and preparation of individuals for the changes that arise with the disease. Type 1 diabetes in children is diagnosed following the onset of symptoms, including acutely elevated blood glucose concentrations and potentially life-threatening complications.1 Diabetic ketoacidosis is observed in more than 20% of children at the onset of type 1 diabetes2 and may be severe or even fatal in undiagnosed or misdiagnosed patients.3 Therefore, the possibility of diagnosing type 1 diabetes at an early disease stage could reduce morbidity.4,5
The preclinical period in patients who develop type 1 diabetes is characterized by the presence of autoantibodies against pancreatic islet cells. Most children with autoantibodies against 2 or more major islet autoantigens will develop overt diabetes,6 and the additional assessment of pancreatic islet β cell function enables the staging and stratification of the asymptomatic phase prior to clinical disease onset.7,8 These prognostic features of islet autoantibodies are used to recruit individuals to prevention trials and as early outcome markers in cohort studies and primary prevention trials.9,10,11 However, these uses are largely restricted to individuals with increased genetic risk, who represent a minority of future patients. The Fr1da study assessed the prevalence of presymptomatic type 1 diabetes in children participating in a public health screening program for islet autoantibodies, the risk for progression to clinical diabetes and diabetic ketoacidosis, and parental psychological stress.
Methods
Study Design
This study was approved by the institutional review board at Technical University Munich. From February 2015 to May 2019, children in Bavaria, Germany, aged 1.75 to 5.99 years without a previous diagnosis of diabetes were offered screening for multiple islet autoantibodies by primary care pediatricians in the context of well-baby visits (eFigure 1 in the Supplement). Participation by pediatricians and children was voluntary. Written informed consent was obtained from the children’s parents or legal guardians. The study design has been published.12
Capillary blood samples were collected by pediatricians and sent to the central laboratory for measurement of islet autoantibodies. Demographic data of the participating children (date of birth, sex, weight, height, date of blood collection, first-degree family history of type 1 diabetes) were collected using a questionnaire at the visit. If the screening sample was positive for multiple autoantibodies, the pediatrician was alerted and a confirmation venous blood sample was requested. At this second visit, the pediatrician often performed a random blood glucose measurement. If multiple islet autoantibodies were confirmed, the family was informed by the pediatrician. Families of children with presymptomatic type 1 diabetes were invited to participate in metabolic staging and an educational program at a pediatric diabetes clinic close to their residence. Depending on the staging outcome, a monitoring plan for future visits at 2- to 6-month intervals was implemented.12,13 Follow-up continued until July 31, 2019. Children with stage 1 type 1 diabetes (≥2 islet autoantibodies and normal glucose tolerance) were asked to participate in an intervention study (NCT02620072) with 1:1 randomization to receive either 12 months of treatment with oral insulin or placebo.
A control cohort was established from a sample of children whose screening test results were negative for islet autoantibodies between December 2015 and March 2018 and were living in the Munich area. In addition, children from the DiMelli study14 without prior screening for islet autoantibodies who were diagnosed with type 1 diabetes in the same pediatric clinics between February 2015 and February 2018 were included for the assessment of parental psychological stress.
Assessments
Islet autoantibodies were measured using a stepwise approach.12 First-line screening was performed with a sensitive enzyme-linked immunosorbent assay (3 Screen; RSR Ltd) to detect glutamic acid decarboxylase autoantibodies (GADAs), islet antigen 2 autoantibodies (IA-2As), and zinc transporter 8 autoantibodies (ZnT8As) in serum prepared from capillary blood.15 Screening samples positive for islet autoantibodies (>25 units/mL; 98th percentile of children samples) and confirmation venous blood samples were measured for GADAs, IA-2As, ZnT8As, and insulin autoantibodies (IAAs) using reference radiobinding assays.16,17,18 Oral glucose tolerance tests (OGTTs) were performed for staging using a glucose load containing the equivalent of 1.75 g/kg up to a maximum of 75 g anhydrous glucose dissolved in water.8 Blood gas analysis (pH) was performed to detect ketoacidosis in children diagnosed with clinical stage 3 type 1 diabetes. Genotyping on 46 single nucleotide polymorphisms was performed to calculate a genetic risk score19 if consent for ancillary research was provided. Psychological stress in parents/guardians was measured at the time of metabolic staging and at 6 and 12 months after presymptomatic diagnosis with the Patient Health Questionnaire-9 (PHQ-9; range, 0 [best] to 27 [worst]; scores ≤4 were interpreted as no to minimal depression and >20 as severe depression).20
Outcome Definitions
The primary outcome was presymptomatic type 1 diabetes, defined as positive for 2 or more islet autoantibodies (IAA, GADA, IA-2A, ZnT8A) in both the screening and confirmation samples or in the screening sample plus a diagnosis of diabetes prior to obtaining the second sample. Presymptomatic type 1 diabetes was classified as stage 1, 2, or 3, as previously advocated.7,8 Stage 1 type 1 diabetes was defined as 2 or more islet autoantibodies and normal glucose tolerance based on OGTT results. Stage 2 was defined as 2 or more islet autoantibodies accompanied by dysglycemia (fasting plasma glucose of 110-125 mg/dL or impaired 2-hour plasma glucose of 140-199 mg/dL and/or plasma glucose ≥200 mg/dL at intermediate time points [30, 60, 90 minutes]) based on OGTT results.8 Stage 3 type 1 diabetes was defined by the following American Diabetes Association criteria: fasting plasma glucose at least 126 mg/dL or a 2-hour plasma glucose of at least 200 mg/dL based on OGTT results or hemoglobin A1C greater than 6.5% or, in children with classic symptoms of hyperglycemia, a random plasma glucose at least 200 mg/dL (in the absence of unequivocal hyperglycemia, the first 3 criteria required confirmation by repeat testing).8 Stages 1 and 2 were diagnosed at clinical referral centers and stage 3 was diagnosed at referral centers or by the local pediatrician. Families of children who withdrew from the study or refused to allow OGTTs were contacted by telephone and asked if the child had developed diabetes. If participants were lost to follow-up, the local DiMelli register cohort study14 was used to obtain information on diabetes development of former study participants. Secondary outcomes of the study were the frequency of diabetic ketoacidosis and an assessment of parental psychological stress associated with a diagnosis of presymptomatic type 1 diabetes.
Statistical Analysis
Data were analyzed using SAS version 9.4 (SAS Institute) and R version 3.5.3 (R Foundation), using the survival and survminer packages. Prior to analysis, the body mass index (BMI) was transformed to a standardized BMI based on SD scores using World Health Organization reference values.21 Standardized BMI values less than −5 or greater than 5 were deemed implausible and were excluded. Overweight was defined as standardized BMI of 1 to 2 and obesity as BMI greater than 2, according to World Health Organization recommendations. Data were considered missing at random after examining relative frequency between categories of all analyzed variables and performing sensitivity analyses excluding subgroups with the highest proportion of missing values. Variables with missing values in more than 10% of children were imputed using regression predictions for deterministic imputation.22 For all other variables, missing values were not included in analyses.
Frequencies and relative risks (RRs) were calculated and are presented with 95% CIs. Exploratory bivariable analyses were performed using Pearson χ2 tests or Fisher exact tests to identify factors associated with increased RR for presymptomatic type 1 diabetes. Factors with significantly increased RR were tested in a multivariable generalized regression model adjusted for sex. At a prevalence of 0.3%, the study had 80% power to detect an uncorrected significant association at RRs greater than 2.5 for presymptomatic type 1 diabetes for categorical variables with frequencies between 3.4% and 96.6% (obesity), greater than 2.0 for variables with frequencies between 7% and 93% (region), and greater than 1.5 for variables with frequencies between 23% and 77%.
Progression to higher stages of type 1 diabetes was analyzed using Kaplan-Meier plots. The time to event was calculated from the age of screening to the age at diagnosis of stage 3 type 1 diabetes or the age at last follow-up. Time-to-event analyses were also used to examine progression from stage 1 to stage 3 and from stage 2 to stage 3 and, in these instances, follow-up was calculated from the age of metabolic staging. Associations between risk of progression to higher stages of type 1 diabetes and variables were analyzed using bivariable Cox proportional hazards regression models. A sensitivity analysis that excluded the 98 children who participated in the intervention study was performed using a bivariable Cox proportional hazards regression model. The proportional hazards assumption was tested using the Schoenfeld residuals method and no violation was detected. The area under the curve of the OGTT results was calculated as previously described.23 Genetic risk scores, area under the curve of OGTT results, and 60-minute OGTT results were categorized in tertiles. PHQ-9 psychological scores were compared between groups using the Mann-Whitney U test or Wilcoxon signed rank test. No adjustment was made for multiple comparisons, so analyses should be interpreted as exploratory. Two-sided P values less than .05 were considered statistically significant.
Results
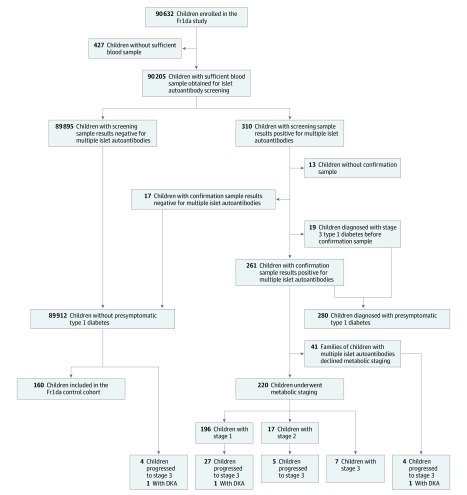
A total of 682 of 1027 (66.4%) primary care pediatricians in Bavaria enrolled 90 632 children (median [interquartile range {IQR}] age, 3.1 [2.1-4.2] years; 48.5% girls) in the study (Table and Figure 1). Approximately 420 000 eligible children aged 1.75 to 5.99 years lived in the respective area during the study period. Compared with a Bavarian preschool population survey of 108 637 children in 2014 to 2015,24 the children in this study were not significantly different in the frequency of boys (51.5% vs 51.3%) or obesity (3.2% vs 3.1%), as defined by German reference values. Sufficient blood for screening was collected from 90 205 children (99.5%; Figure 1).
Table. Description of the Study Population of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany (N = 90 632).
| Category | No. (%) | Presymptomatic Type 1 Diabetes | |
|---|---|---|---|
| No. | % (95% CI) | ||
| Sex | (n = 88 214) | ||
| Female | 42 815 (48.5) | 127 | 0.30 (0.25-0.35) |
| Male | 45 399 (51.5) | 153 | 0.34 (0.29-0.40) |
| Age | (n = 90 388) | ||
| <3 | 36 900 (40.8) | 82 | 0.22 (0.18-0.28) |
| 3-3.99 | 20 909 (23.1) | 57 | 0.27 (0.21-0.36) |
| 4-4.99 | 15 153 (16.8) | 63 | 0.42 (0.32-0.54) |
| 5-5.99 | 17 426 (19.3) | 78 | 0.45 (0.36-0.56) |
| First-degree relative with type 1 diabetes | (n = 88 253) | ||
| Yes | 3004 (3.4) | 32 | 1.07 (0.74-1.52) |
| No | 85 249 (96.6) | 248 | 0.29 (0.26-0.33) |
| BMIa | (n = 87 766) | ||
| Normal | 72 216 (82.3) | 226 | 0.31 (0.27-0.36) |
| Overweight | 11 968 (13.6) | 35 | 0.29 (0.21-0.41) |
| Obese | 3582 (4.1) | 19 | 0.54 (0.33-0.85) |
| Regionb | (n = 90 632) | ||
| Lower Bavaria | 4648 (5.1) | 12 | 0.26 (0.14-0.46) |
| Upper Bavaria | 36 139 (39.9) | 108 | 0.30 (0.25-0.36) |
| Swabia | 13 836 (15.3) | 54 | 0.39 (0.30-0.51) |
| Upper Palatinate | 6365 (7.0) | 18 | 0.28 (0.17-0.46) |
| Middle Franconia | 15 627 (17.2) | 45 | 0.29 (0.21-0.39) |
| Upper Franconia | 7018 (7.7) | 25 | 0.36 (0.24-0.53) |
| Lower Franconia | 6999 (7.7) | 18 | 0.26 (0.16-0.41) |
Standardized body mass index (BMI) was calculated using the World Health Organization Child Growth Standards based on height and weight and age.21 The 3 central parameters for the calculation are the Box-Cox power transformation (L), the median (M), and the variation coefficient (S). The formula for calculation is [(BMI/M)L − 1] / (L × S). Normal BMI is defined as a standardized value <1, overweight as 1 to 2, and obese as >2. There were 17 075 missing BMI values before imputation and for 2531 of these children, imputation of standardized BMI values was not possible due to missing sex or age.
See eFigure 1 in the Supplement for a map of the regions.
Figure 1. Flow of Participants in a Study of the Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany.
DKA indicates diabetic ketoacidosis. The control cohort was a sample of the children who did not have islet autoantibodies and were living in the Munich area.
Prevalence of Presymptomatic Type 1 Diabetes
In total, 280 of 90 632 (0.31% [95% CI, 0.27-0.35]) children were diagnosed with presymptomatic type 1 diabetes. This included 19 children whose screening sample results were positive for 2 or more islet autoantibodies and were diagnosed with stage 3 type 1 diabetes by the pediatrician before collection of the second blood sample. There were 120 children with 2 antibodies, 91 with 3 antibodies, and 69 with 4 antibodies (eTable 1 in the Supplement). The median (IQR) genetic risk score in 188 of 280 children (67%) was 12.9 (11.7-13.9). Thirteen children had screening sample results positive for islet antibodies but did not provide a second sample to complete the diagnosis. The other 89 912 children had screening sample results negative for multiple islet autoantibodies. Of those children with screening sample results negative for islet autoantibodies, 160 (median [IQR] age, 4.6 [3.3-5.6] years; 78 [48.8%] girls) were included in the study as control individuals.
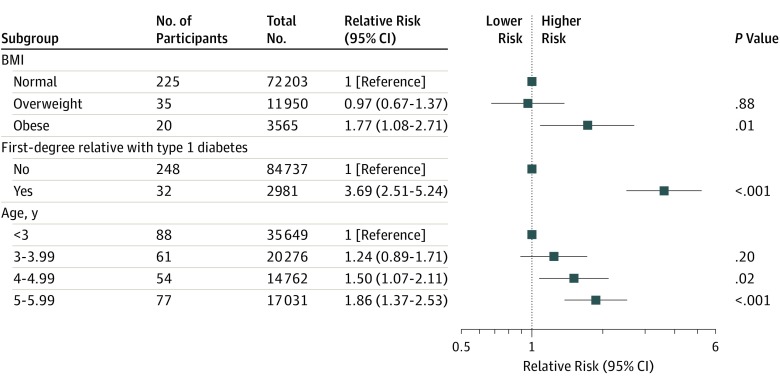
The unadjusted associations with presymptomatic type 1 diabetes are presented in eFigure 2 in the Supplement and the adjusted associations are shown in Figure 2. The adjusted RR for presymptomatic type 1 diabetes was greater in children with a first-degree relative with type 1 diabetes (RR, 3.69 [95% CI, 2.51-5.24]; P < .001), in obese children (RR, 1.77 [95% CI, 1.08-2.71]; P = .01), in children aged 4.00 to 4.99 years (RR, 1.50 [95% CI, 1.07-2.11]; P = .02), and in children aged 5.00 to 5.99 years (RR, 1.86 [95% CI, 1.37-2.53]; P < .001) (Figure 2). There were no differences in the prevalence of presymptomatic type 1 diabetes between girls and boys or between the 7 regions of Bavaria.
Figure 2. Multivariable Analysis of Relative Risks for Presymptomatic Type 1 Diabetes in a Study of the Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany.
The multivariable analysis includes the variables that were significantly associated with increased relative risk for presymptomatic type 1 diabetes in unadjusted analyses (see eFigure 2 in the Supplement).
Staging and Follow-up of Children With Presymptomatic Type 1 Diabetes
A total of 220 of 261 children (84.3%) with presymptomatic type 1 diabetes who had not already been diagnosed with stage 3 type 1 diabetes participated in metabolic staging, the diabetes education program, and follow-up at clinical referral centers. After staging, 196 of 90 632 children (0.22%) were diagnosed with stage 1 type 1 diabetes, 17 of 90 632 (0.02%) with stage 2 type 1 diabetes, and 26 of 90 632 (0.03%) with stage 3 type 1 diabetes (Figure 1). Another 36 children progressed to stage 3 and 25 progressed to stage 2 during follow-up (median [IQR] follow-up time of all children with presymptomatic type 1 diabetes, 2.4 [1.0-3.2] years). Stage 2 was detected prior to the diagnosis of stage 3 in 12 of these 36 children. Of the 196 children with stage 1 type 1 diabetes at the time of metabolic staging, 98 (50%) participated in the mechanistic intervention study.
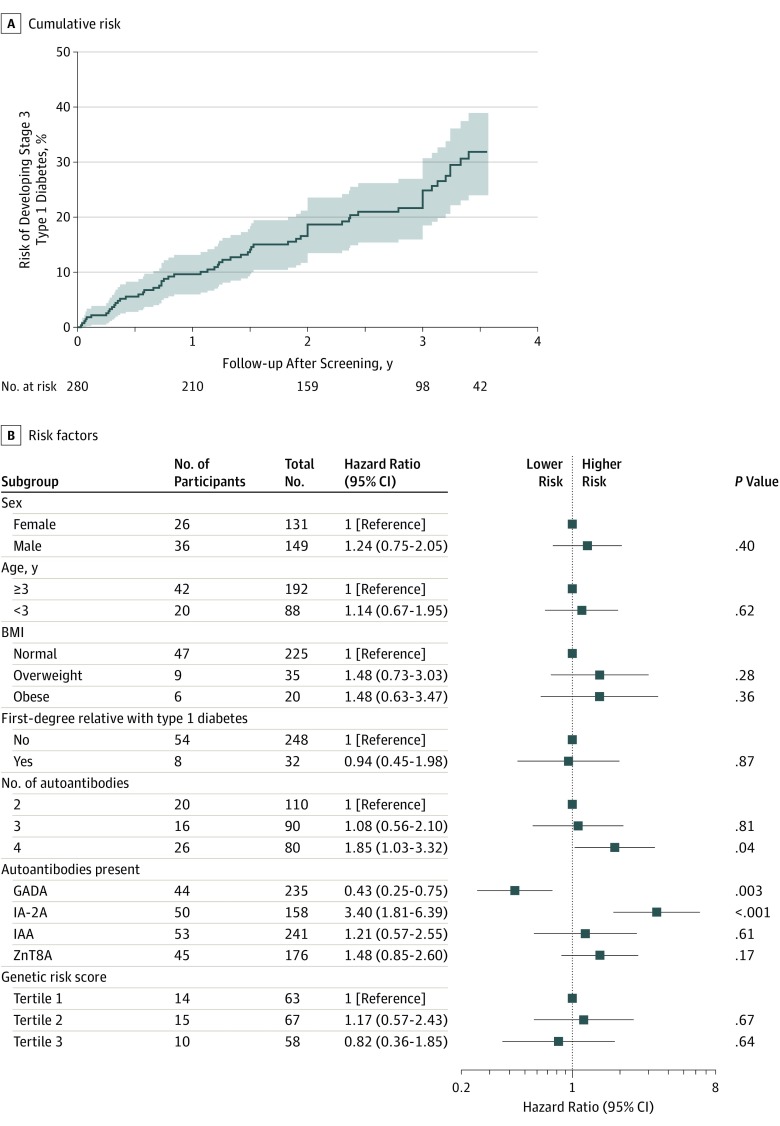
The cumulative risk of developing stage 3 type 1 diabetes for the 280 children with presymptomatic type 1 diabetes was 24.9% (95% CI, 18.5%-30.7%; 54 cases) over 3 years of follow-up, with an annualized risk of 9.0% (Figure 3A and eFigure 3 in the Supplement). There was a significantly greater risk associated with children who had 4 autoantibodies vs 2 autoantibodies (hazard ratio [HR], 1.85 [95% CI, 1.03-3.32]; P = .04) and with children whose screening results were positive for IA-2A (HR, 3.4 [95% CI, 1.81-6.39]; P < .001), and a significantly lower risk associated with children whose screening results were positive for GADA (HR, 0.43; [95% CI, 0.25-0.75]; P = .003; Figure 3B). The lower risk associated with GADA was observed in children with 2 autoantibodies and 3 autoantibodies (eFigure 4 in the Supplement). Sex, age, BMI, first-degree relative with type 1 diabetes, and genetic risk scores were not significantly associated with the development of stage 3 type 1 diabetes. Risk (24.7%) and risk factors were similar in a sensitivity analysis that excluded the 98 children who participated in the intervention study (eFigure 5 in the Supplement).
Figure 3. Risk of Stage 3 Type 1 Diabetes in a Study of the Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany.
A. The curve is truncated at 3.57 years of follow-up from screening as the number at risk reaches 15% (n = 42) of the included children. The median (interquartile range) observation time is 2.4 (1.0-3.3) years. GADA indicates glutamic acid decarboxylase autoantibody; IA-2A, islet antigen 2 autoantibody; IAA, insulin autoantibody; ZnT8A, zinc transporter 8 autoantibody.
Among 196 children with stage 1 type 1 diabetes, the risk of progression to stage 2 or 3 was 28.7% (95% CI, 21.0%-35.6%; 45 cases) by 3 years of follow-up, with an annualized risk of 10.6% (eFigures 6 and 7 in the Supplement). Factors significantly associated with disease stage progression were obesity, 4 autoantibodies, IA-2A, glycated hemoglobin of at least 5.7%, area under the curve of OGTT results, and 60-minute OGTT results. Sex, age, family history of type 1 diabetes, and genetic risk scores were not significantly associated with risk of progression. Loss of islet autoantibody positivity was observed in 1 child during follow-up.
A diagnosis of stage 3 type 1 diabetes was reported in 4 of the 89 912 children who did not have presymptomatic type 1 diabetes; at diagnosis, 3 had 1 autoantibody and 1 had 0 autoantibodies. In total, 66 known cases of stage 3 type 1 diabetes were observed during the 244 069 follow-up years of all children screened in the study, yielding a rate of 27 per 100 000 years (95% CI, 21.3-34.4). The sensitivity of a presymptomatic diagnosis for identifying stage 3 type 1 diabetes within 3 years of follow-up from screening was 54 of 56 (96.4%) and the specificity was 90 350 of 90 576 (99.8%).
Diabetes Severity and Psychological Stress Associated With Presymptomatic Type 1 Diabetes
Of the 62 children with presymptomatic type 1 diabetes who developed stage 3 type 1 diabetes, 2 (3.2%) had a laboratory diagnosis of mild (pH of 7.28) or moderate (pH of 7.14) diabetic ketoacidosis without clinical symptoms, and 60 (96.8%) did not have diabetic ketoacidosis. The children with diabetic ketoacidosis were hospitalized but did not require treatment in an intensive care unit. The family of 1 of these children declined to participate in the educational and staging program.
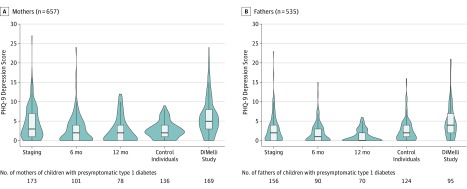
PHQ-9 questionnaires were administered to 432 parents at staging (76% response rate), 314 at 6 months (61% response rate), and 256 at 12 months (58% response rate). Median [IQR] PHQ-9 scores were significantly greater at the time of metabolic staging in mothers of children with presymptomatic type 1 diabetes than scores in mothers of children with negative results for islet autoantibodies in the control cohort (3 [1-7] vs 2 [1-4]; P = .002) (Figure 4 and eTable 2 in the Supplement). The scores were not significantly greater in fathers of children with presymptomatic type 1 diabetes vs fathers of children without autoantibodies (median [IQR], 2 [0-4] vs 2 [1-4]). The scores declined significantly over time in both in mothers (median [IQR] score at 12 months, 2 [0-4]; P < .001) and fathers (median [IQR] score at 12 months, 0 [0-2]; P < .001) of children with presymptomatic type 1 diabetes. The median (IQR) PHQ-9 score at metabolic staging was lower than in 169 mothers (5 [3-8]; P < .001) and 95 fathers (4 [2-7]; P < .001) of children diagnosed with type 1 diabetes without prior screening in the DiMelli study.14
Figure 4. Psychological Stress Scores and Diagnosis of Presymptomatic Type 1 Diabetes in a Study of the Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany.
Violin plots of Patient Health Questionnaire 9 (PHQ-9) depression scores of mothers and fathers of children with presymptomatic type 1 diabetes. Scores were assessed at the metabolic staging visit and at 6 and 12 months after diagnosis. Scores for children in the control cohort and children with symptomatic type 1 diabetes enrolled in the DiMelli study14 are also shown. Scores range from 0 to 27 and were interpreted as no to minimal depression if less than or equal to 4 and as severe depression if greater than 20. The violin plots display the density of PHQ-9 scores. The box plots display the median and interquartile range and extend to the upper and lower adjacent values.
Discussion
Public health screening found a 0.31% prevalence of presymptomatic type 1 diabetes in children aged 2 to 5 years, including 0.02% of children with multiple islet autoantibodies and dysglycemia (stage 2 type 1 diabetes) and 0.03% with previously undiagnosed stage 3 type 1 diabetes.
To our knowledge, this is the first study to introduce preschool screening for autoimmune disease in the general population. Smaller studies have performed screening of school-age children.25,26,27 The strengths of the current study include the introduction of screening in routine pediatric health care, the large number of children screened, inclusion of children without genetic preselection, systematic follow-up of the majority of children diagnosed with presymptomatic type 1 diabetes and their families, and inclusion of a control cohort of children whose screening results were negative for autoantibodies.
Screening of more than 90 000 children in 4 years was achieved in a period when there was no known effective prevention therapy. The success of a single 14-day course of teplizumab in delaying the progression to stage 3 type 1 diabetes28 may further increase the acceptance of screening. This study identified children with stage 2 diabetes who may benefit from this treatment and shows how primary care screening could rapidly disseminate access to therapies.
A potential clinical benefit of identifying type 1 diabetes in a presymptomatic stage may be a reduction in the prevalence of diabetic ketoacidosis, which is associated with detrimental neurocognitive outcomes, poor long-term glycemic control, and increased medical costs.5,29,30 Previous studies reported a low frequency of diabetic ketoacidosis at the diagnosis of type 1 diabetes in genetically at-risk individuals with islet autoantibodies in longitudinal natural history studies.4,31 In this study, the prevalence of diabetic ketoacidosis was less than 5%. The previously reported prevalence in unscreened children is more than 20% in Germany2 and 40% in the United States.32
The transient association with increased psychological stress scores observed in the mothers of children diagnosed with presymptomatic type 1 diabetes in this study is consistent with the findings of children diagnosed in natural history studies, which provided regular care of children whose test results were positive for autoantibodies.33,34 Also consistent in all studies is that the distress reported by the families of children diagnosed with presymptomatic type 1 diabetes was low or moderate in the majority of families. Therefore, with an appropriate education and care program, islet autoantibody screening and a presymptomatic diagnosis of type 1 diabetes appear unlikely to lead to a level of parental psychological stress that was observed in families of children diagnosed with clinical type 1 diabetes.
A diagnosis of type 1 diabetes in presymptomatic stages should be associated with an elevated risk for progression to stage 3 type 1 diabetes. The 9% annualized risk for progression observed in this study is similar to the 11% annualized risk found in children with multiple islet autoantibodies who have a family history of type 1 diabetes or a genetically defined increased susceptibility.6 Although it is possible that a few children may not progress to overt diabetes, it appears that young children with multiple islet autoantibodies identified by the screening strategy in the current study have a genetic susceptibility profile similar to that of children with diagnosed type 1 diabetes, and many are already at an advanced presymptomatic stage. Additional selection of children who rapidly progress to stage 3 type 1 diabetes might be achieved by targeting children with IA-2A and children with elevated hemoglobin A1c or glucose levels.35,36,37 Genetic risk was not associated with increased disease stage progression in children with presymptomatic type 1 diabetes, and their genetic risk score (12.9) was similar to that of children with type 1 diabetes in the DiMelli study14 (median [IQR], 13.1 [12.2-14.1]) and greater than that of the 20 668 infants from the general Bavarian population included in the Freder1k study38 (median [IQR], 10.6 [9.7-11.6]). Therefore, a priori genetic selection is unlikely to substantially increase specificity.
The screening strategy was based on islet autoantibody incidence data, which indicated a peak incidence of islet autoantibodies in the first 3 years of life.10,39 Screening was restricted to a single point between 2 and 5 years of age. Screening costs were kept low by using a relatively inexpensive and sensitive prescreening method that could exclude 98% of children from more expensive measurements. Further cost reductions could be achieved by screening with point-of-care islet autoantibody tests if they become available. Although the study did not include a measure of type 1 diabetes ascertainment, the screening strategy to identify children with multiple islet autoantibodies identified more than 95% of the reported cases of stage 3 type 1 diabetes among all screened children. These data suggest that public health screening had a high sensitivity for identifying future cases of childhood type 1 diabetes. New cases of presymptomatic type 1 diabetes occur after 5 years of age, but an age-based analysis of the remaining risk for multiple islet autoantibodies in prospectively followed genetically at-risk children suggests that the risk is reduced after the preschool period and may not be large enough to warrant a second screen in adolescence.40
Limitations
This study has several limitations. First, although it provides information on evaluating whether public health screening might eventually be of value in some populations, a randomized clinical trial would be required to assess whether screening reduces diabetic ketoacidosis or affects psychological stress. Such a trial should include measures of the number of families who refuse participation in screening and the cost-effectiveness of screening. Second, participation in the study was voluntary and screening was performed in a minority of the eligible children. Although the sex and BMI distribution of the screened children was not significantly different from that of all children of similar age living in Bavaria, the findings may not be representative of all children in Bavaria, other populations, or in children younger than 2 years and older than 5 years. Third, the offer to enroll in an intervention study to children with stage 1 presymptomatic type 1 diabetes may have influenced participation in the metabolic staging and education program and the psychological stress of the families. A sensitivity analysis without the children who participated in the 12-month intervention suggested that the findings on disease progression during follow-up were not substantially changed by the relatively short treatment period in a minority of the participants. Fourth, BMI was missing in more than 10% of participants. Missing values were assessed to be missing at random, but associations observed with BMI should be interpreted with caution. Fifth, 24% to 42% of parents did not return psychological stress questionnaires. More accurate assessment might be achieved if questionnaires could be administered at time points before and after notification of a diagnosis in the setting of a randomized trial.
Conclusions
Among children aged 2 to 5 years in Bavaria, Germany, a program of primary care–based screening demonstrated an islet autoantibody prevalence of 0.31%. These findings may inform considerations of population-based screening of children for islet autoantibodies.
List of Fr1da Study Group
eFigure 1. Regions of Bavaria Participating in the Fr1da Study
eFigure 2. Violin and Box Plots of Type 1 Diabetes Genetic Risk Scores in the Fr1da, DiMelli, and Freder1k Studies
eFigure 3. Unadjusted Relative Risk Ratios for Presymptomatic Type 1 Diabetes
eFigure 4. Risk of Developing Stage 3 Type 1 diabetes
eFigure 5. Risk of Stage 3 Type 1 Diabetes According to GAD Autoantibody Status
eFigure 6. Risk of Stage 3 Type 1 Diabetes with Censoring of Children who Participated in the Fr1da Intervention Trial
eFigure 7. Risk of Developing Stage 2 or Stage 3 Type 1 Diabetes
eFigure 8. Risk of developing Stage 2 or Stage 3 Type 1 Diabetes with Censoring of Children who Participated in the Fr1da Intervention Trial
eTable 1. Combinations of Autoantibodies in Fr1da Children with Presymptomatic Type 1 Diabetes
eTable 2. Patient Health Questionnaire-9 Depression Scores of Mothers and Fathers from Children with Presymptomatic Type 1 Diabetes, Children in the Fr1da Control Cohort, and Children with Symptomatic New-Onset Type 1 Diabetes in the DiMelli Cohort
References
- 1.Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69-82. doi: 10.1016/S0140-6736(13)60591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with human development index (HDI): an updated systematic review, meta-analysis, and meta-regression. Horm Metab Res. 2018;50(3):209-222. doi: 10.1055/s-0044-102090 [DOI] [PubMed] [Google Scholar]
- 3.Wolfsdorf JI, Allgrove J, Craig ME, et al. ; International Society for Pediatric and Adolescent Diabetes . ISPAD Clinical Practice Consensus Guidelines 2014: diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014;15(suppl 20):154-179. doi: 10.1111/pedi.12165 [DOI] [PubMed] [Google Scholar]
- 4.Elding Larsson H, Vehik K, Bell R, et al. ; TEDDY Study Group; SEARCH Study Group; Swediabkids Study Group; DPV Study Group; Finnish Diabetes Registry Study Group . Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347-2352. doi: 10.2337/dc11-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. 2017;40(9):1249-1255. doi: 10.2337/dc17-0558 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler AG, Rewers M, Simell O, et al. . Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473-2479. doi: 10.1001/jama.2013.6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel RA, Dunne JL, Atkinson MA, et al. . Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. doi: 10.2337/dc15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang JL, Maahs DM, Garvey KC, et al. . Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care. 2018;41(9):2026-2044. doi: 10.2337/dci18-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skyler JS, Krischer JP, Wolfsdorf J, et al. . Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care. 2005;28(5):1068-1076. doi: 10.2337/diacare.28.5.1068 [DOI] [PubMed] [Google Scholar]
- 10.Krischer JP, Lynch KF, Schatz DA, et al. ; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia. 2015;58(5):980-987. doi: 10.1007/s00125-015-3514-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knip M, Åkerblom HK, Al Taji E, et al. ; Writing Group for the TRIGR Study Group . Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes: the TRIGR randomized clinical trial. JAMA. 2018;319(1):38-48. doi: 10.1001/jama.2017.19826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raab J, Haupt F, Scholz M, et al. ; Fr1da Study Group . Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da study. BMJ Open. 2016;6(5):e011144. doi: 10.1136/bmjopen-2016-011144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Insel RA, Dunne JL, Ziegler AG. General population screening for type 1 diabetes: has its time come? Curr Opin Endocrinol Diabetes Obes. 2015;22(4):270-276. doi: 10.1097/MED.0000000000000173 [DOI] [PubMed] [Google Scholar]
- 14.Warncke K, Krasmann M, Puff R, Dunstheimer D, Ziegler AG, Beyerlein A. Does diabetes appear in distinct phenotypes in young people? results of the diabetes mellitus incidence cohort registry (DiMelli). PLoS One. 2013;8(9):e74339. doi: 10.1371/journal.pone.0074339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amoroso M, Achenbach P, Powell M, et al. . 3 Screen islet cell autoantibody ELISA: a sensitive and specific ELISA for the combined measurement of autoantibodies to GAD65, to IA-2 and to ZnT8. Clin Chim Acta. 2016;462:60-64. doi: 10.1016/j.cca.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 16.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48(3):460-468. doi: 10.2337/diabetes.48.3.460 [DOI] [PubMed] [Google Scholar]
- 17.Achenbach P, Lampasona V, Landherr U, et al. . Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia. 2009;52(9):1881-1888. doi: 10.1007/s00125-009-1438-0 [DOI] [PubMed] [Google Scholar]
- 18.Bonifacio E, Yu L, Williams AK, et al. . Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360-3367. doi: 10.1210/jc.2010-0293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonifacio E, Beyerlein A, Hippich M, et al. ; TEDDY Study Group . Genetic scores to stratify risk of developing multiple islet autoantibodies and type 1 diabetes: a prospective study in children. PLoS Med. 2018;15(4):e1002548. doi: 10.1371/journal.pmed.1002548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Multicentre Growth Reference Study Group WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76-85. [DOI] [PubMed] [Google Scholar]
- 22.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 23.Sakaguchi K, Takeda K, Maeda M, et al. . Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2015;7(1):53-58. doi: 10.1007/s13340-015-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit Gesundheit der Vorschulkinder in Bayern: Ergebnisse der Schuleingangsuntersuchung zum Schuljahr 2014/2015. https://www.lgl.bayern.de/publikationen/gesundheit/doc/schuleingangsuntersuchung_2014_2015.pdf. Published August 2017. Accessed December 30, 2019.
- 25.Schatz D, Krischer J, Horne G, et al. . Islet cell antibodies predict insulin-dependent diabetes in United States school age children as powerfully as in unaffected relatives. J Clin Invest. 1994;93(6):2403-2407. doi: 10.1172/JCI117247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velluzzi F, Secci G, Sepe V, et al. ; Sardinian Autoimmunity Study Group . Prediction of type 1 diabetes in Sardinian schoolchildren using islet cell autoantibodies: 10-year follow-up of the Sardinian schoolchildren type 1 diabetes prediction study. Acta Diabetol. 2016;53(1):73-79. doi: 10.1007/s00592-015-0751-y [DOI] [PubMed] [Google Scholar]
- 27.Strebelow M, Schlosser M, Ziegler B, Rjasanowski I, Ziegler M. Karlsburg type i diabetes risk study of a general population: frequencies and interactions of the four major type i diabetes-associated autoantibodies studied in 9419 schoolchildren. Diabetologia. 1999;42(6):661-670. doi: 10.1007/s001250051213 [DOI] [PubMed] [Google Scholar]
- 28.Herold KC, Bundy BN, Long SA, et al. ; Type 1 Diabetes TrialNet Study Group . An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med. 2019;381(7):603-613. doi: 10.1056/NEJMoa1902226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cameron FJ, Scratch SE, Nadebaum C, et al. ; DKA Brain Injury Study Group . Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. 2014;37(6):1554-1562. doi: 10.2337/dc13-1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saydah SH, Shrestha SS, Zhang P, Zhou X, Imperatore G. Medical costs among youth younger than 20 years of age with and without diabetic ketoacidosis at the time of diabetes diagnosis. Diabetes Care. 2019;42(12):2256-2261. doi: 10.2337/dc19-1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308-313. doi: 10.1111/j.1399-5448.2011.00829.x [DOI] [PubMed] [Google Scholar]
- 32.Rewers A, Dong F, Slover RH, Klingensmith GJ, Rewers M. Incidence of diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado youth, 1998-2012. JAMA. 2015;313(15):1570-1572. doi: 10.1001/jama.2015.1414 [DOI] [PubMed] [Google Scholar]
- 33.Bennett Johnson S, Tercyak KP Jr. Psychological impact of islet cell antibody screening for IDDM on children, adults, and their family members. Diabetes Care. 1995;18(10):1370-1372. doi: 10.2337/diacare.18.10.1370 [DOI] [PubMed] [Google Scholar]
- 34.Hummel M, Ziegler AG, Roth R. Psychological impact of childhood islet autoantibody testing in families participating in the BABYDIAB study. Diabet Med. 2004;21(4):324-328. doi: 10.1111/j.1464-5491.2004.01142.x [DOI] [PubMed] [Google Scholar]
- 35.Decochez K, De Leeuw IH, Keymeulen B, et al. ; Belgian Diabetes Registry . IA-2 autoantibodies predict impending type I diabetes in siblings of patients. Diabetologia. 2002;45(12):1658-1666. doi: 10.1007/s00125-002-0949-8 [DOI] [PubMed] [Google Scholar]
- 36.Xu P, Wu Y, Zhu Y, et al. ; Diabetes Prevention Trial-Type 1 (DPT-1) Study Group . Prognostic performance of metabolic indexes in predicting onset of type 1 diabetes. Diabetes Care. 2010;33(12):2508-2513. doi: 10.2337/dc10-0802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobsen LM, Larsson HE, Tamura RN, et al. ; TEDDY Study Group . Predicting progression to type 1 diabetes from ages 3 to 6 in islet autoantibody positive TEDDY children. Pediatr Diabetes. 2019;20(3):263-270. doi: 10.1111/pedi.12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkler C, Haupt F, Heigermoser M, et al. ; GPPAD Study Group . Identification of infants with increased type 1 diabetes genetic risk for enrollment into Primary Prevention Trials-GPPAD-02 study design and first results. Pediatr Diabetes. 2019;20(6):720-727. doi: 10.1111/pedi.12870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziegler AG, Bonifacio E; BABYDIAB-BABYDIET Study Group . Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia. 2012;55(7):1937-1943. doi: 10.1007/s00125-012-2472-x [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann VS, Weiß A, Winkler C, et al. . Landmark models to define the age-adjusted risk of developing stage 1 type 1 diabetes across childhood and adolescence. BMC Med. 2019;17(1):125. doi: 10.1186/s12916-019-1360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of Fr1da Study Group
eFigure 1. Regions of Bavaria Participating in the Fr1da Study
eFigure 2. Violin and Box Plots of Type 1 Diabetes Genetic Risk Scores in the Fr1da, DiMelli, and Freder1k Studies
eFigure 3. Unadjusted Relative Risk Ratios for Presymptomatic Type 1 Diabetes
eFigure 4. Risk of Developing Stage 3 Type 1 diabetes
eFigure 5. Risk of Stage 3 Type 1 Diabetes According to GAD Autoantibody Status
eFigure 6. Risk of Stage 3 Type 1 Diabetes with Censoring of Children who Participated in the Fr1da Intervention Trial
eFigure 7. Risk of Developing Stage 2 or Stage 3 Type 1 Diabetes
eFigure 8. Risk of developing Stage 2 or Stage 3 Type 1 Diabetes with Censoring of Children who Participated in the Fr1da Intervention Trial
eTable 1. Combinations of Autoantibodies in Fr1da Children with Presymptomatic Type 1 Diabetes
eTable 2. Patient Health Questionnaire-9 Depression Scores of Mothers and Fathers from Children with Presymptomatic Type 1 Diabetes, Children in the Fr1da Control Cohort, and Children with Symptomatic New-Onset Type 1 Diabetes in the DiMelli Cohort