Abstract
Background:
Lupus Low Disease Activity State (LLDAS) is a potential treat to target goal in systemic lupus erythematosus (SLE). This study determined predictors of time to reach LLDAS in a longitudinal cohort.
Methods:
Patients were grouped according to LLDAS status at cohort entry. Those who did not satisfy LLDAS at cohort entry were analyzed prospectively. The Kaplan Meier approach was used to estimate the time to LLDAS. Cox regression was used to identify patient characteristics that were associated with time to LLDAS.
Results:
The probability of LLDAS attainment within one year was 52% for Caucasians, 36% for African-Americans and 33% for SLE patients with renal involvement. The median time to LLDAS was 1.1 years. In multivariable models, African-American ethnicity, baseline prednisone >10 mg daily, hypocomplementemia, baseline damage, and baseline renal activity remained significant predictors of longer time to attain LLDAS, while disease duration <1 year and cutaneous activity were associated with earlier attainment.
Conclusions
LLDAS is potentially attainable in the majority of SLE patients. The time to LLDAS was found to be longer in African-American SLE patients. Characteristics of African-American SLE patients such as renal activity and hypocomplementemia were also independent predictors of slower attainment of LLDAS. These findings point to the need to include African-American SLE patients in both clinical and pharmaceutical research.
Introduction
Control of both SLE disease activity and corticosteroid use are important targets in the management of patients with SLE. In the principles of treat-to-target recommendations for SLE, the main target state was “remission”, but where remission could not be reached, the lowest acceptable disease activity might be the target (1). Thus, Franklyn et al.(2) developed and validated a less stringent targeted state than remission, the Lupus Low Disease Activity State (LLDAS). They found that SLE patients who were in LLDAS for more than half of the observation period had a lower risk of new damage (2). External validation of LLDAS included studies from Padova, Amsterdam and Pisa, which reported up to 86.7% of SLE patients attained LLDAS at a single point of time, and confirmed that attainment of LLDAS lowered the risk of new damage (3–5). The previous analysis of our cohort found that, if more than 50% of the follow-up time satisfied LLDAS, there was a 50% reduction in later organ damage (6). Furthermore, LLDAS has now been found to be a meaningful and discriminative endpoint in both primary and post-hoc analyses of several SLE randomized clinical trials (7–9).
Baseline characteristics that predicted the likelihood of attaining LLDAS were evaluated in several studies. Younger age, discoid rash, disease duration ≤1 year, elevated anti-dsDNA (10), renal disease and hypocomplementemia (10, 11) were found as negative independent predictors of attaining LLDAS, while cumulative prednisone dose, physician global assessment (PGA)>1 (3), higher SLEDAI score, joint and skin (3, 4) involvement were found to be negative predictors of sustained LLDAS.
The presentation and course of SLE is affected by ethnicity. African-American SLE patients are known to experience more severe SLE, more chronic disease activity pattern (12–14), and worse survival (15–18). African-Americans require a longer time to achieve remission (19) compared with other ethnicities. LLDAS in African-American patients has not been fully elucidated. In this study, we determined the time to LLDAS and predictors of time to LLDAS in the Hopkins Lupus Cohort, a United States cohort with both Caucasian and African-American representation.
Methods
The Hopkins Lupus Cohort is a prospective longitudinal single-center cohort of SLE patients ongoing since 1987, which was approved on an annual basis by the Johns Hopkins University School of Medicine Institutional Review Board. All patients gave written informed consent to participate. Visits were scheduled quarterly by protocol. Patients were seen by one rheumatologist (MP). This analysis was based on cohort data from its inception until January, 2019. A total of 2,512 SLE patients diagnosed according to the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria (20) or the classification criteria as defined by the American College of Rheumatology (21) (22) and as updated in 1997 (23), were included in the analyses. At each clinic visit, the physician global disease activity on a 0–3 visual analog scale (PGA) (24), the Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE disease activity index (SELENA-SLEDAI) (25, 26), SLICC/ACR Damage Index (SDI) (27), relevant serologies (anti-dsDNA, complement), and treatment were recorded.
In this study, we applied LLDAS (2) to the Hopkins Lupus Cohort. LLDAS was defined as a SELENA-SLEDAI score of ≤4 with no scores for the renal, central nervous system, cardiopulmonary, vasculitis, fever, no hemolytic anemia or gastrointestinal activity, no increase in any SELENA-SLEDAI component since the previous visit, a PGA of ≤1, and a prednisone dose of ≤7.5 mg/day. Immunosuppressant and hydroxychloroquine treatment were allowed for LLDAS. Patients were grouped according to LLDAS status at baseline.
Statistical Analysis System software was used (SAS Institute Inc. Cary, North Carolina, SAS 9.4). Chi-square test for categorical variables, the Wilcoxon Rank sum test, or the independent samples T test for continuous variables (where appropriate) were used to determine whether there was a significant difference between baseline characteristics of patients grouped according to LLDAS status at baseline.
Patients who did not satisfy LLDAS at cohort entry were analyzed prospectively. The time to LLDAS was defined as the time between the cohort entry and the first clinic visit at which LLDAS was attained. We used the Kaplan Meier approach to estimate the distribution of time to LLDAS and probability of patients achieving LLDAS after cohort entry, censoring patients who had a gap of 7 or more months in their follow-up time or who dropped out of the study before attaining LLDAS. We used Cox regression to identify patient characteristics that were associated with LLDAS. First, we assessed the relationship between each variable and time to LLDAS, one at a time. Those with significant association with time to LLDAS were then entered into the multivariable model and those that remained significant were retained in the final model. Variables that were highly collinear were included separately in multivariable model.
Results
Cohort Entry
Table 1 details the patient characteristics according to LLDAS status at baseline. The cumulative classification criteria were 49% malar rash, 19% discoid rash, 52% photosensitivity, 53% oral ulcer, 72% arthritis, 49% serositis, 45% renal disorder, 12% neurological disorder, 67% hematological disorder, 83% immunological disorder and 97% ANA positivity based on revised ACR classification criteria. Additional SLICC classification criteria included 21% direct Coombs test, 55% low C3, 48% low C4 and 16% low CH50. A total of 2,512 SLE patients were analyzed. 1086 (43.2%) patients were in LLDAS at the first cohort visit. Of these patients, 94% were female, 30.1% were African-American and 61.8% were Caucasian. The mean age at baseline was 40 years. Thirty-nine percent had been diagnosed with SLE within the past year, while 33.6% had SLE for 5 or more years. Patients, who were not in LLDAS at baseline, were significantly younger, and were more likely to be male and African-American. Disease duration was comparable between the groups.
Table 1.
Clinical and Demographic characteristics of the patients in the Hopkins Lupus Cohort, grouped according to LLDAS status at baseline
| LLDAS at cohort entry (n=1086) |
no LLDAS at cohort entry (n=1426) |
p-value | |
|---|---|---|---|
| Sex, Female | 1021 (94%) | 1294 (90.7%) | 0.0025 |
| Ethnicity | <0.0001 | ||
| Black | 327 (30.1%) | 663 (46.5%) | |
| White | 671 (61.8%) | 655 (45.9%) | |
| Other | 88 (8.1%) | 108 (7.6%) | |
| Age at baseline, years | <0.0001 | ||
| <30 | 284 (26.2%) | 526 (36.9%) | |
| 30–39 | 298 (27.4%) | 422 (29.6%) | |
| 40–49 | 252 (23.2%) | 266 (18.7%) | |
| 50≤ | 252 (23.2%) | 212 (14.9%) | |
| Mean (SD) | 39.8 (13.1) | 36.2 (12.6) | <0.0001 |
| History of smoking | 395 (36.6%) | 514 (36.1%) | 0.8256 |
| Duration of SLE prior to baseline | 0.199 | ||
| <1 year | 426 (39.2%) | 524 (36.7%) | |
| 1 to 5 years | 295 (27.2%) | 374 (26.2%) | |
| >5 years | 365 (33.6%) | 528 (37%) | |
| Median (IQR) | 2.3 (0.3 – 7.5) | 2.5 (0.3 – 8.1) | 0.243 |
| Baseline Prednisone Dose | <0.0001 | ||
| ≤10 mg/day | 1086 (100%) | 495 (34.7%) | |
| >10 mg/day | 0 (0%) | 931 (65.3%) | |
| Baseline Hydroxychloroquine | 546 (50.3%) | 703 (49.3%) | 0.6274 |
| Baseline immunosuppressant | 120 (11.0%) | 425 (29.8%) | <0.0001 |
| Baseline Low C3 | 186 (17.7%) | 523 (37.9%) | <0.0001 |
| Baseline Low C4 | 170 (16.2%) | 431 (31.3%) | <0.0001 |
| Baseline Anti-dsDNA positivity | 201 (19.6%) | 586 (43.2%) | <0.0001 |
| Baseline PGA ≤1 | 1086 (100%) | 723 (50.7%) | <0.0001 |
| Baseline SLICC/ACR Damage Index Score >1 | 200 (18.6%) | 400 (28.2%) | <0.0001 |
| Baseline SELENA-SLEDAI | <0.0001 | ||
| ≤4 | 1086 (100%) | 767 (53.8%) | |
| >4 | 0 (0%) | 659 (46.2%) | |
| Baseline Musculoskeletal activity | 27 (2.5%) | 258 (18.1%) | <0.0001 |
| Baseline Cutaneous activity | 199 (18.3%) | 380 (26.6%) | <0.0001 |
| Baseline Renal activity | 0 (0%) | 384 (26.9%) | <0.0001 |
| Baseline Hematological activity | 59 (5.4%) | 146 (10.2%) | <0.0001 |
| Baseline Serositis activity | 0 (0%) | 87 (6.1%) | <0.0001 |
| Baseline Vasculitis | 0 (0%) | 43 (3.0%) | <0.0001 |
| Antiphospholipid antibodies | |||
| Anti-cardiolipin | 486 (46.2%) | 668 (48.2%) | 0.3385 |
| Lupus anti-coagulant (RVVT) | 271 (25.6%) | 367 (26.5%) | 0.617 |
LLDAS=lupus low disease activity state, SLE=Systemic Lupus Erythematosus, C3=Complement 3, C4=Complement 4, Anti-dsDNA=anti double stranded DNA, PGA=Physician Global Assessment, SLICC/ACR= Systemic Lupus International Collaborating Clinics/ American College of Rheumatology, SELENA-SLEDAI= the Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE disease activity index, RVVT=Russell viper venom time.
Follow Up
Figure 1 details the probability of patients achieving LLDAS at stated time points. Based on our Kaplan-Meier analysis, the estimated probability of LLDAS attainment within one year was 52% for Caucasian-Americans, and 36% for African-Americans. Among those with renal involvement, the estimated probability of achieving LLDAS within one year was 33%. Ninety-three percent of Caucasian-Americans, 82% of African-Americans and 89% of patients with renal involvement would achieve LLDAS at the end of 5 years of follow up.
Figure 1.
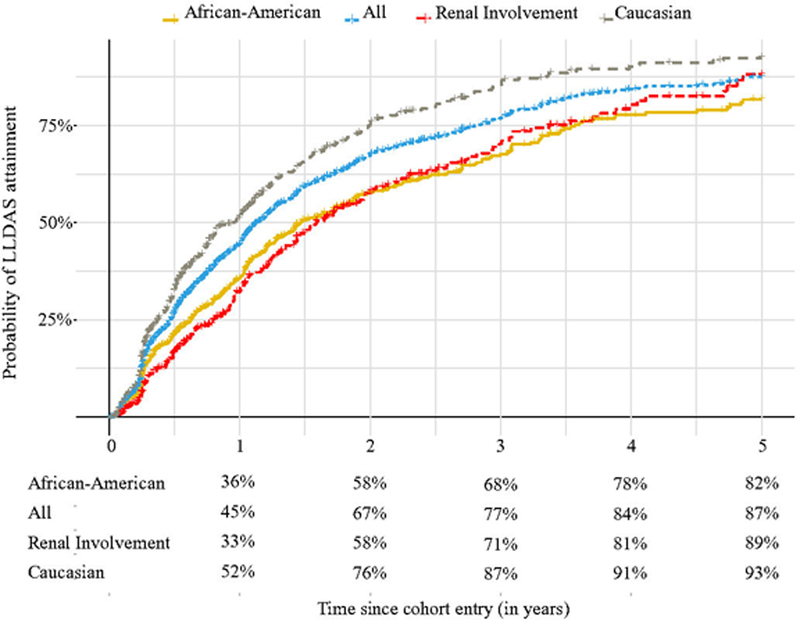
shows the probability of LLDAS attainment according to patients ethnicity and renal involvement at the stated time points.
Predictors of time to LLDAS
Table 2 shows the median time to LLDAS, with baseline characteristics of patients that were associated with time to LLDAS based on Kaplan-Meier models. Table 2 also shows estimated rate ratios for LLDAS attainment based on both univariate and multivariable Cox regression models. The median time to LLDAS was 1.1 years. We found that disease duration <1 year, taking prednisone <10 mg daily, taking hydroxychloroquine, normal level of C3, C4 and anti-dsDNA, PGA score of ≤1, SELENA-SLEDAI score of ≤4, and cutaneous activity were associated with attaining LLDAS faster, while African-American ethnicity, baseline renal activity, baseline damage accrual and presence of lupus anticoagulant were associated with later attainment of LLDAS.
Table 2.
Predictors of time to LLDAS
| Median time to LLDAS |
Univariate | Multivariable | |||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Ethnicity | |||||
| Non-African-American | 0.95 | Ref | Ref | ||
| African-American | 1.5 | 0.63(0.55–0.73) | <0.001 | 0.61(0.52–0.70 | <0.001 |
| Duration of SLE* | |||||
| <1 year | 1 | Ref | Ref | ||
| 1–5 years | 1.2 | 0.75(0.62–0.89 | 0.001 | 0.8(0.67–0.96 | 0.016 |
| >5 years | 1.4 | 0.72(0.62–0.85 | <0.001 | 0.79(0.66–0.94 | 0.007 |
| Prednisone dose* | |||||
| ≤10 mg | 0.6 | Ref | Ref | ||
| >10 mg | 1.4 | 0.56(0.48–0.64) | <0.001 | 0.57(0.49–0.66) | <0.001 |
| Hydroxychloroquine use* | |||||
| No | 1.3 | Ref | |||
| Yes | 1 | 1.23(1.07–1.41) | 0.004 | ||
| Hypocomplementemia* | |||||
| No | 0.9 | Ref | Ref | ||
| Yes | 1.5 | 0.66(0.57–0.76) | <0.001 | 0.68(0.59–0.79 | <0.001 |
| anti-dsDNA positivity* | |||||
| No | 1.1 | Ref | |||
| Yes | 1.3 | 0.85(0.73–0.98) | 0.026 | ||
| PGA* | |||||
| ≤1 | 1 | Ref | |||
| >1 | 1.3 | 0.78(0.68–0.90 | <0.001 | ||
| SLICC/ACR Damage Index Score* | |||||
| ≤1 | 1 | Ref | Ref | ||
| >1 | 1.4 | 0.79(0.67–0.92) | 0.003 | 0.84(0.71–0.99) | 0.041 |
| SELENA-SLEDAI* | |||||
| ≤4 | 1 | Ref | |||
| >4 | 1.4 | 0.81(0.70–0.92) | 0.002 | ||
| Cutaneous activity* | |||||
| Absent | 1.2 | Ref | Ref | ||
| Present | 0.9 | 1.23(1.06–1.44) | 0.007 | 1.19(1.01–1.39) | 0.035 |
| Renal activity* | |||||
| Absent | 1 | Ref | Ref | ||
| Present | 1.6 | 0.70(0.59–0.82) | <0.001 | 0.72(0.61–0.85) | <0.001 |
| Lupus Anticoagulant | |||||
| Never | 1.1 | Ref | |||
| Ever | 1.3 | 0.85(0.72–0.99) | 0.042 | ||
Median time to LLDAS was presented as years. There was no significant association between time to LLDAS and baseline age, sex, history of smoking, immunosuppressant use, musculoskeletal activity, hematological activity, serositis, and anticardiolipin antibody positivity. SELENA-SLEDAI and PGA were not included in the final multivariable model due to their collinearity with cutaneous and renal activity.
LLDAS=lupus low disease activity state, SLE=Systemic Lupus Erythematosus, C3=Complement 3, C4=Complement 4, Anti-dsDNA=anti double stranded DNA, PGA=Physician Global Assessment, SLICC/ACR= Systemic Lupus International Collaborating Clinics/ American College of Rheumatology, SELENA-SLEDAI= the Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE disease activity index, and *= baseline.
In the multivariable model, African-American ethnicity, taking prednisone >10 mg, baseline hypocomplementemia, baseline damage accrual, and baseline renal activity remained significant predictors of later attainment of LLDAS. Disease duration <1 year and cutaneous activity remained significant predictors of earlier attainment of LLDAS.
We also performed a subgroup analysis of inception patients. Five-hundred and thirty-six patients, who entered the cohort within 18 months of SLE diagnosis date and were not in LLDAS at cohort entry, were analyzed. African-American ethnicity (CI 0.50,0.76), taking prednisone >10 mg (CI 0.52,0.79), baseline hypocomplementemia (CI 0.61,0.92), and baseline renal activity (CI 0.59,0.94) were found to be predictors of later attainment of LLDAS.
Discussion
In view of the ethnic disparities in SLE outcomes, it is important to study results in cohorts which include different ethnicities. The Hopkins Lupus Cohort has a balanced representation of Caucasian and African-American ethnicities. Other studies of LLDAS (2–5, 10, 11) cannot generalize to patients with SLE within the United States. In general, though, studies of time to LLDAS are lacking. We applied the LLDAS definition to the Hopkins Lupus Cohort and identified the frequency of LLDAS, time to LLDAS and clinical determinants of time to LLDAS.
First, African-American SLE patients were found to require a longer time to achieve LLDAS. This is the first study confirming the association between African-American ethnicity and its effect on LLDAS. Most SLE cohorts do not contain different ethnicities (3, 11). One that was predominantly of Chinese patients (10), reported that ethnicity had no effect on LLDAS. However, it has been well established that disease prevalence, severity and mortality are increased in the African-American population compared to the white population (6, 12–14, 17, 28, 29). Moreover, lupus nephritis (LN), discoid lupus, hematologic, serologic and immunologic SLE manifestations are more common in African-Americans (13, 30–32). However, the longer time until LLDAS in African-Americans persisted even after adjustment for renal activity. It is established that African-Americans are significantly underrepresented in SLE clinical trials(33). Our findings further emphasize the importance of including African-Americans in clinical and pharmaceutical research studies considering heterogeneity in outcomes among ethnicities.
Second, among patients who were not in LLDAS at cohort entry, we estimated that 45% percent of all and 36% of African-American patients would achieve LLDAS within one year. Eighty-seven percent of all, and 82% of African-American patients would achieve LLDAS at the end of 5 years of follow up. Whether cross-sectional or longitudinal, cohorts that analyzed the frequency of LLDAS were in general agreement with our results (3–5, 10). LLDAS should be an achievable treat to target goal in the majority of SLE patients, as opposed to remission. Although remission should remain the ultimate goal, our current therapies are insufficient to establish remission as the treat to target goal for standard of care. Our findings clarify that LLDAS is an achievable target for both African-American as well as Caucasian patients.
Third, the median time to LLDAS was found to be 1.1 years. The range of the follow up time until LLDAS was 0.3 to 180 months. Indeed, the importance of faster attainment of LLDAS comes from what we know about the association between pre-existing damage and further damage accrual (34), between early damage and higher mortality (34, 35) and, between LLDAS and reduced risk of new damage. A desirable treat to target state should be reachable early in the disease course to prevent damage. We previously reported that the median time to remission ranged between 1.8–11.0 years depending on the definition of remission (19). This is noteworthy since the median time to LLDAS showed that, in many patients, LLDAS is attainable in time to actually prevent early damage and during the duration of randomized clinical trials.
Fourth, we found that renal activity independently predicted a longer time to LLDAS, which is in agreement with previous studies (10, 11). Lupus nephritis remains associated with higher health care costs and remains an indicator of high morbidity and mortality (36, 37). In particular, lupus nephritis is more common (38), develops earlier (39), and has worse outcomes (40, 41) in African-Americans (28). Achievement of LLDAS was found to predict statistically significant reductions in end stage renal disease (ESRD) in our previous analysis (6). Thirty-three percent of our patients with baseline renal involvement would attain LLDAS within 1 year. Although renal involvement is a predictor of later attainment of LLDAS, LLDAS is still a potential target for patients with renal involvement, because it is associated with a low risk of progression to ESRD.
Fifth, we found baseline cutaneous activity as an independent predictor for early LLDAS attainment. Skin activity in SLE is an umbrella term for a family of manifestations with a wide range of prognosis. Unfortunately, our cohort database (based on SLEDAI) did not sub-categorize cutaneous manifestations at baseline and did not define discoid rash as a distinct variable. Golder et al. found discoid rash as a negative predictor of LLDAS attainment (10).
Sixth, we showed that patients with disease duration <1 year were able to attain LLDAS faster. This is in contrast to Golder et al.’s multicenter cross-sectional study from 2016 (10), which reported a shorter disease duration as a negative predictor of LLDAS attainment. However, their mean disease duration at baseline was 8.64 years. Only 8% of their patients had a disease duration of <1 year at enrolment, as opposed to 38% of our cohort. In addition, their paper included patients that we excluded, those who were LLDAS at baseline. SLE disease activity decreases over time (42). The discrepancy between our result and previous studies might be explained by sample selection. We analyzed only patients who were not in LLDAS at cohort entry. Inception patients are much more likely not to be in LLDAS as their disease manifestations are evolving over time, irrespective of disease severity. However, patients with longer disease duration may have established (ingrained) SLE manifestations, and not being in LLDAS at cohort entry may implicate these patients had a more difficult disease to control.
We also performed a subgroup analysis of an “inception cohort” of our SLE patients with a disease duration less than 18 months at cohort entry. The results did not deviate from our main findings. African-American ethnicity, taking prednisone >10 mg, baseline hypocomplementemia, and baseline renal activity were found to be predictors of later attainment of LLDAS in these patients, as well. As one might expect, damage accrual was similar between the groups, and thus did not enter in multivariable analysis. Cutaneous activity became insignificant in the multivariable analysis in these inception patients.
Seventh, hydroxychloroquine is the cornerstone of the treatment of SLE with multiple benefits including improved survival (43–45), decreased frequency of lupus flares (46) and reduced risk of damage accrual (47). In the univariate model, hydroxychloroquine was significantly associated with earlier LLDAS attainment. However, this association was lost in the multivariable model. Our result was likely underpowered due to the high frequency of nonadherence we have previously reported (48).
Baseline damage, hypocomplementemia and prednisone >10 mg daily were found to be independent predictors of longer time to LLDAS. This is expected, as they are associated with active or refractory disease. Furthermore, we found that patients with baseline lower SELENA-SLEDAI and PGA scores more frequently and rapidly attained LLDAS, compared to those with higher scores at baseline. To differentiate the effects of different organ system involvement to the time to LLDAS, we did not include SELENA-SLEDAI and PGA into the multivariable models because of the collinearity. Other multivariable models that did include SLENA-SLEDAI or PGA instead of renal and cutaneous activity, showed that SELENA-SLEDAI and PGA are independent negative predictors of time to LLDAS, which agree with previous studies (3, 4).
A limitation of our analysis is the lack of sufficient other ethnicities such as Hispanic-American and Asian-American. SLE is also more severe in Hispanic-American patients (13). Hispanic-American patients tend to have more acute disease onset, more lupus nephritis, higher disease activity and damage, compared to Caucasian patients (14, 28, 32, 49, 50). Our cohort represents the Baltimore area, with predominantly African-American and Caucasian patients.
This is the largest United States study to assess predictors of time to LLDAS. Besides the large population and long follow-up time, the Hopkins Lupus Cohort is the only ongoing cohort in which patients were followed quarterly by one rheumatologist (MP), and comprises both Caucasian and African-American patients. Moreover, we censored patients with a gap of > 7 months between visits to define time to LLDAS more accurately. It is the first to include a large number of African-Americans, and the first to analyze time to LLDAS. We demonstrated the achievability of LLDAS in both African-Americans and Caucasian patients, supporting the validity of LLDAS in multiple ethnicities. African-American SLE patients were found to take longer to achieve LLDAS. Characteristics of African-American SLE patients, such as renal activity and hypocomplementemia (38), were also independent predictors of longer time to LLDAS. These findings point to the need to include African-American SLE patients in both clinical and pharmaceutical research, as we cannot generalize from studies from Europe and Asia. LLDAS is an attainable and practical treatment target for both clinical trials and daily practice, as a part of the stepwise approach on the way to remission.
Significance and Innovation.
The achievability of LLDAS in both African-Americans and Caucasian patients was demonstrated, supporting the validity of LLDAS in multiple ethnicities.
African-American SLE patients were found to take longer to achieve LLDAS.
These findings point to the need to include African-American SLE patients in both clinical and pharmaceutical research, as we cannot generalize from studies from Europe and Asia.
Acknowledgments
Funding The Hopkins Lupus Cohort was funded by the US National Institutes of Health: R0-1 AR 43727.
Footnotes
Conflicts of Interests The authors have no related COIs to disclose.
References
- 1.van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrom K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis 2014;73:958–67. [DOI] [PubMed] [Google Scholar]
- 2.Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Definition and initial validation of a Lupus Low Disease Activity State (LLDAS). Ann Rheum Dis 2016;75:1615–21. [DOI] [PubMed] [Google Scholar]
- 3.Zen M, Iaccarino L, Gatto M, Saccon F, Larosa M, Ghirardello A, et al. Lupus low disease activity state is associated with a decrease in damage progression in Caucasian patients with SLE, but overlaps with remission. Ann Rheum Dis 2018;77:104–10. [DOI] [PubMed] [Google Scholar]
- 4.Tani C, Vagelli R, Stagnaro C, Carli L, Mosca M. Remission and low disease activity in systemic lupus erythematosus: an achievable goal even with fewer steroids? Real-life data from a monocentric cohort. Lupus Sci Med 2018;5:e000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsang ASMW, Bultink IE, Heslinga M, Voskuyl AE. Both prolonged remission and Lupus Low Disease Activity State are associated with reduced damage accrual in systemic lupus erythematosus. Rheumatology (Oxford) 2017;56:121–8. [DOI] [PubMed] [Google Scholar]
- 6.Petri M, Magder LS. Comparison of Remission and Lupus Low Disease Activity State in Damage Prevention in a United States Systemic Lupus Erythematosus Cohort. Arthritis Rheumatol 2018;70:1790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morand EF, Trasieva T, Berglind A, Illei GG, Tummala R. Lupus Low Disease Activity State (LLDAS) attainment discriminates responders in a systemic lupus erythematosus trial: post-hoc analysis of the Phase IIb MUSE trial of anifrolumab. Ann Rheum Dis 2018;77:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oon S, Huq M, Golder V, Ong PX, Morand EF, Nikpour M. Lupus Low Disease Activity State (LLDAS) discriminates responders in the BLISS-52 and BLISS-76 phase III trials of belimumab in systemic lupus erythematosus. Ann Rheum Dis 2019;78:629–33. [DOI] [PubMed] [Google Scholar]
- 9.Ordi-Ros J, Saez-Comet L, Perez-Conesa M, Vidal X, Mitjavila F, Castro Salomo A, et al. Enteric-coated mycophenolate sodium versus azathioprine in patients with active systemic lupus erythematosus: a randomised clinical trial. Ann Rheum Dis 2017;76:1575–82. [DOI] [PubMed] [Google Scholar]
- 10.Golder V, Kandane-Rathnayake R, Hoi AY, Huq M, Louthrenoo W, An Y, et al. Frequency and predictors of the lupus low disease activity state in a multi-national and multi-ethnic cohort. Arthritis Res Ther 2016;18:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piga M, Floris A, Cappellazzo G, Chessa E, Congia M, Mathieu A, et al. Failure to achieve lupus low disease activity state (LLDAS) six months after diagnosis is associated with early damage accrual in Caucasian patients with systemic lupus erythematosus. Arthritis Res Ther 2017;19:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannakou I, Chatzidionysiou K, Magder LS, Gyori N, van Vollenhoven R, Petri MA. Predictors of persistent disease activity and long quiescence in systemic lupus erythematosus: results from the Hopkins Lupus Cohort. Lupus Sci Med 2018;5:e000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alarcon GS, Friedman AW, Straaton KV, Moulds JM, Lisse J, Bastian HM, et al. Systemic lupus erythematosus in three ethnic groups: III. A comparison of characteristics early in the natural history of the LUMINA cohort. LUpus in MInority populations: NAture vs. Nurture. Lupus 1999;8:197–209. [DOI] [PubMed] [Google Scholar]
- 14.Alarcon GS, Roseman J, Bartolucci AA, Friedman AW, Moulds JM, Goel N, et al. Systemic lupus erythematosus in three ethnic groups: II. Features predictive of disease activity early in its course. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998;41:1173–80. [DOI] [PubMed] [Google Scholar]
- 15.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 16.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85:147–56. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan E, Hubert HB. Ethnicity and mortality from systemic lupus erythematosus in the US. Ann Rheum Dis 2006;65:1500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korbet SM, Schwartz MM, Evans J, Lewis EJ, Collaborative Study G. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol 2007;18:244–54. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm TR, Magder LS, Petri M. Remission in systemic lupus erythematosus: durable remission is rare. Ann Rheum Dis 2017;76:547–53. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jolly M, Galicier L, Aumaitre O, Frances C, Le Guern V, Liote F, et al. Quality of life in systemic lupus erythematosus: description in a cohort of French patients and association with blood hydroxychloroquine levels. Lupus 2016;25:735–40. [DOI] [PubMed] [Google Scholar]
- 22.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 23.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 24.Petri M, Hellmann D, Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J Rheumatol 1992;19:53–9. [PubMed] [Google Scholar]
- 25.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005;353:2550–8. [DOI] [PubMed] [Google Scholar]
- 26.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 27.Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 28.Carter EE, Barr SG, Clarke AE. The global burden of SLE: prevalence, health disparities and socioeconomic impact. Nat Rev Rheumatol 2016;12:605–20. [DOI] [PubMed] [Google Scholar]
- 29.Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcon GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum 2013;65:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward MM, Studenski S. Clinical manifestations of systemic lupus erythematosus. Identification of racial and socioeconomic influences. Arch Intern Med 1990;150:849–53. [PubMed] [Google Scholar]
- 31.Cooper GS, Parks CG, Treadwell EL, St Clair EW, Gilkeson GS, Cohen PL, et al. Differences by race, sex and age in the clinical and immunologic features of recently diagnosed systemic lupus erythematosus patients in the southeastern United States. Lupus 2002;11:161–7. [DOI] [PubMed] [Google Scholar]
- 32.Alarcon GS, McGwin G Jr., Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP, et al. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 2002;11:95–101. [DOI] [PubMed] [Google Scholar]
- 33.Falasinnu T, Chaichian Y, Bass MB, Simard JF. The Representation of Gender and Race/Ethnic Groups in Randomized Clinical Trials of Individuals with Systemic Lupus Erythematosus. Curr Rheumatol Rep 2018;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruce IN, O’Keeffe AG, Farewell V, Hanly JG, Manzi S, Su L, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahman P, Gladman DD, Urowitz MB, Hallett D, Tam LS. Early damage as measured by the SLICC/ACR damage index is a predictor of mortality in systemic lupus erythematosus. Lupus 2001;10:93–6. [DOI] [PubMed] [Google Scholar]
- 36.Clarke AE, Panopalis P, Petri M, Manzi S, Isenberg DA, Gordon C, et al. SLE patients with renal damage incur higher health care costs. Rheumatology (Oxford) 2008;47:329–33. [DOI] [PubMed] [Google Scholar]
- 37.Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971–2015: A Systematic Review and Bayesian Meta-Analysis. Arthritis Rheumatol 2016;68:1432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hochberg MC, Boyd RE, Ahearn JM, Arnett FC, Bias WB, Provost TT, et al. Systemic lupus erythematosus: a review of clinico-laboratory features and immunogenetic markers in 150 patients with emphasis on demographic subsets. Medicine (Baltimore) 1985;64:285–95. [PubMed] [Google Scholar]
- 39.Burgos PI, McGwin G Jr., Pons-Estel GJ, Reveille JD, Alarcon GS, Vila LM. US patients of Hispanic and African ancestry develop lupus nephritis early in the disease course: data from LUMINA, a multiethnic US cohort (LUMINA LXXIV). Ann Rheum Dis 2011;70:393–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contreras G, Lenz O, Pardo V, Borja E, Cely C, Iqbal K, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006;69:1846–51. [DOI] [PubMed] [Google Scholar]
- 41.Nee R, Martinez-Osorio J, Yuan CM, Little DJ, Watson MA, Agodoa L, et al. Survival Disparity of African American Versus Non-African American Patients With ESRD Due to SLE. Am J Kidney Dis 2015;66:630–7. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Gonzalez LA, Roseman JM, Vila LM, Reveille JD, Alarcon GS. Predictors of the rate of change in disease activity over time in LUMINA, a multiethnic US cohort of patients with systemic lupus erythematosus: LUMINA LXX. Lupus 2010;19:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alarcon GS, McGwin G, Bertoli AM, Fessler BJ, Calvo-Alen J, Bastian HM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007;66:1168–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, Garmendia M, Villar I, Martinez-Berriotxoa A, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006;15:577–83. [DOI] [PubMed] [Google Scholar]
- 45.Mok CC, Tse SM, Chan KL, Ho LY. Effect of immunosuppressive therapies on survival of systemic lupus erythematosus: a propensity score analysis of a longitudinal cohort. Lupus 2018;27:722–7. [DOI] [PubMed] [Google Scholar]
- 46.Canadian Hydroxychloroquine Study G. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991;324:150–4. [DOI] [PubMed] [Google Scholar]
- 47.Fessler BJ, Alarcon GS, McGwin G Jr., Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum 2005;52:1473–80. [DOI] [PubMed] [Google Scholar]
- 48.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine Blood Levels in Systemic Lupus Erythematosus: Clarifying Dosing Controversies and Improving Adherence. J Rheumatol 2015;42:2092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alarcon GS, McGwin G Jr., Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001;44:2797–806. [DOI] [PubMed] [Google Scholar]
- 50.Alarcon GS, Calvo-Alen J, McGwin G, Uribe AG, Toloza SM, Roseman JM, et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006;65:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


