Abstract
Chronic liver diseases, such as fibrosis and cancer, lead to a rigid or stiff liver because of perpetual activation of hepatic stellate cells or portal fibroblasts into matrix-producing myofibroblasts. Mechanical forces, as determined by the mechanical properties of extracellular matrix or pressure of circulating blood flow/shear stress, are sensed by mechanoreceptors at the plasma membrane and transmitted into a cell to impact cell function. This process is termed as mechanotransduction. This review includes basic knowledge regarding how external forces are sensed, amplified, and transmitted into the interior of a cell as far as the nucleus to regulate gene transcription and generate biological responses. It also reviews literatures to highlight the mechanisms by which mechanical forces in a normal or diseased liver influence the phenotype of hepatocytes, hepatic stellate cells, portal fibroblasts, and sinusoidal endothelial cells, and these cells in turn participate in the initiation and progression of liver diseases.
Keywords: integrins, focal adhesions, adherens junctions, cytoskeleton, epigenetics
Cells in our body experience various forces, such as gravity, tension or compression, pressure of circulating blood flow/shear stress, and static mechanical forces 1. These forces, in the form of mechanical cues, are sensed by mechanoreceptors and transmitted into the cell. This process is known as mechanotransduction. In addition to shear stress that constantly regulates the phenotype of liver sinusoidal endothelial cells (LSECs) by mechanotransduction, chronic liver diseases lead to increased deposition of extracellular matrix (ECM), which confers forces to liver resident cells, such as hepatocytes, portal fibroblasts, and hepatic stellate cells (HSCs). Elastic modulus (E) and shear modulus (G) are two common measures for rigidity or stiffness, with their relation expressed by the equation E = 3G 2. Real-time shear wave elastography and transient elastography of patients estimated that E was 5-6 kilopascal (kPa) for fibrosis stage of F0-1 liver and 7-20 kPa for F2-4 fibrotic liver 3. Similarly, magnetic resonance elastography demonstrated that G was < 2.5 kPa for normal liver, 2.9-5 kPa for stage F1-3, and > 5 kPa for F4 fibrotic or cirrhotic liver 2. Although hepatocyte swelling and shrinking can affect the results of elastography, the rigidity or stiffness of the liver increases with increased stages of fibrosis. Stiffness is not only a consequence of ECM accumulation and fibrosis, but also a significant contributor for liver diseases, such as fibrosis and cancer.
Myofibroblasts transdifferentiated from HSCs are a major source of ECM production in the liver and contribute to hepatic fibrosis 4. Interestingly, a stiff environment alone is able to drive myofibrobastic activation of HSCs; it promotes HSC to express α-SMA and fibronectin, markers of stellate cell activation, and induces the migratory and contractile phenotype of HSCs by activating focal adhesion kinase (FAK), proto-oncogene tyrosine-protein kinase Src (Src), Ras homolog gene family, member A (RhoA), and yes-associated protein 1 (YAP1) 1,5–7. Durotaxis or directed migration toward a stiffer substrate, has been characterized in the fibroblasts and it is mediated by FAK-YAP1 mechanosignaling 7,8. Thus, liver injury or a deregulated wound healing in the liver leads to local HSC activation and stiffness and the local stiffness creates a rigidity gradient. Through durotaxis and chemotaxis, the surrounding HSCs move toward this area and accumulate at the wound site so that a positive feedback loop forms to further increase fibrosis and liver stiffness 7. Tumor nodules are generally stiffer than the normal tissues because of a fibrotic response and ECM accumulation known as desmoplasia or tumor stiffening 9. Tumor stiffening can influence the malignant phenotype of cancer by enhancing extracellular signal–regulated kinases (ERK) activation, Rho/Rho-associated protein kinase (ROCK)-generated contractility, and focal adhesions (FAs) at the downstream of integrin clustering 10. Additionally, cancer-associated fibroblasts participate in the formation of distant metastatic sites through comigration with cancer cells and forming tracks within the tissues, aiding in cancer cell migration 11,12. Thus, ECM mechanics and myofibroblast activity contribute to the pathogenesis of liver fibrosis and cancer.
Mechanical forces can influence the behavior and function of liver resident cells by mechanostransduction. The transmission of a force from the exterior to the interior of a cell requires multiple cellular modules, including mechanosensing receptors at the plasma membrane, associated protein complexes, and mechanotransducers such as insoluble cytoskeleton. The mechanosensing receptors include integrins at cell-ECM junctions, cadherins and cell adhesion molecules (CAMs) at cell-cell contacts, stretch-activated ion channels, receptor tyrosine kinase and so on 13. These molecules detect the changes of forces and promote sensory protein complexes for force amplification and transmission. FAK, Src, talin, vinculin, β-catenin, and α-catenin are mechanosensitive molecules and they are recruited to FAs or cell-cell junctions where they convert mechanical forces to biochemical signals. The cell nucleus is coupled to the cytoskeleton by the linker of nucleoskeleton and cytoskeleton (LINC) so it directly receive forces through the cytoskeletal fibers 14 and respond to mechanotransduction by altering gene transcription. Below we review how forces are sensed, amplified, transmitted into a cell, and ultimately translated into gene transcription. We also focus on the molecular and cellular mechanisms by which forces in a normal or diseased liver influence the phenotype of hepatocytes, HSCs, portal fibroblasts, and endothelial cells, and these cells in turn participate in the initiation and progression of liver diseases, such as hepatic fibrosis and cancer.
How are forces sensed and transmitted into a cell?
Mechanosensing receptors
Integrins and FAs.
Cells are anchored to ECM through specialized spots that are called FAs. FAs consist of integrin clusters and more than 150 proteins. Integrins are transmembrane receptors for ECM components, such as fibronectin, vitronectin and collagens 15,16. There are more than 20 integrin heterodimers comprised of different combinations of α subunits and β subunits. In human, there are 24 types of α subunits and 9 types of β subunits 17. Mechanical forces induce the conformation change of integrin β subunit to permit integrin clustering and binding of integrin to talin and vinculin, which are actin-binding proteins (Fig. 1, cell 1). Within a few minutes after integrin clustering is induced, FAK, Src, Phosphoinositide 3-kinases (PI3K), phospholipase C and other signaling molecules are recruited to FAs so that the forces are converted into biochemical signals. Forces can activate ERK, Jun N-terminal kinase (JNK) or Rho-family small GTPases signaling pathways to impact cell functions (Fig. 1, cell 1) 18. For example, FAK/Src relay forces to RhoA/ROCK and RhoA/Diaphanous-related formin-1 (Diaph1/mDia) to modulate actin polymerization and actomyosin contractility important for cell adhesion, spreading and migration 19. Mice homozygous for β1-intergin knockout were embryonic lethal and died at the stage of E6.0 20. Additionally, embryonic lethality was seen in mice null for either α4, α5, αv, or β8 and perinatal lethality in mice null for either α3, α6, α7, α8, α9, or β4 16, supporting that the contact of cells with ECM mediated by integrin promoted a proper supramolecular network of matrix proteins and intracellular mechanosignaling required for the tissue development and homeostasis. Interestingly, knockout of β1-intergin by cre/loxP-mediated gene deletion or knockdown of β1-intergin by siRNA in hepatocytes impaired growth factor-mediated signaling and liver regeneration in mice 21, highlighting a role of integrin-mediated mechanosignaling in the pathophysiology and homeostasis of the liver. In addition, an integrin-independent mechanosensing involving collagen, discoidin domain receptor 1 (DDR1) and myosin IIA has been described 22. DDR1 is a receptor of collagen and the binding of collagen to DDR1 leads to DDR1 clustering and association of DDR1 to the myosin IIA filaments so that the forces are transferred to the cytoskeleton filaments 22.
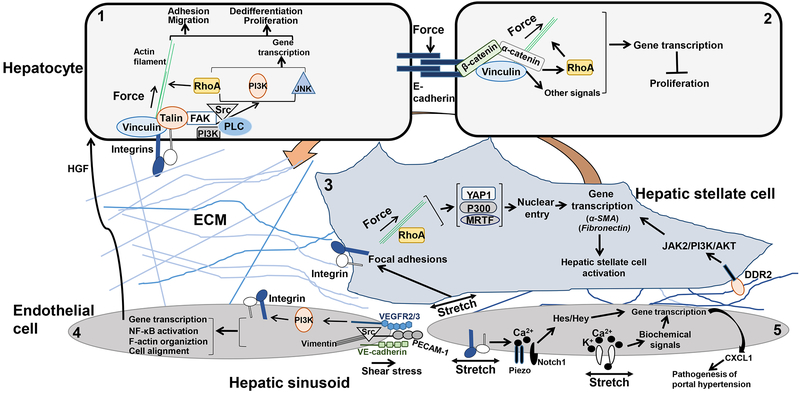
Figure 1.
Mechanotransduction in liver resident cells.
Cell 1. ECM-mediated mechanical forces induce activation of integrin and the formation of FAs comprised of actin-binding proteins, such as talin and vinculin, and signaling molecules, such as FAK, Src, PI3K and so on. Through a direct force transfer to the actin filaments and RhoA-mediated biochemical signaling, external forces are translated into actomyosin contractility, cytoskeleton remodeling, and gene transcription, culminating in a new phenotype of the cell.
Cell 2. Forces on E-cadherin induce the formation of cadherin complexes, which transmit forces into the interior of the cell by the actin filaments and signaling molecule such as RhoA. E-cadherin-mediated signaling is essential for the development of contact inhibition of cell proliferation of epithelial cells.
Cell 3. In HSCs, ECM-mediated forces or stretch of the plasma membrane activate integrin and its downstream signals leading to nuclear translocation of YAP1, p300 or MRTF, which subsequently turns on gene transcription for HSC activation. In addition, LSEC-dependent angiogenesis at the early-stage of fibrosis leads to ECM remodeling and mechanical forces that promote HSC activation by a collagen-DDR2/JAK2/PI3K/AKT mechanosignaling.
Cell 4. Shear stress induces the formation of a mechanosensory protein complex comprised of PECAM-1, VE-cadherin, and VEGFR2. In this complex, VE-cadherin functions as an adaptor and PECAM-1 activates Src and binds to the intermediate vimentin filaments for force transmission. VEGFR2 activates PI3K, which leads to subsequent integrin activation and the biological responses, such as cell alignment in laminar shear and activation of NF-κB. In addition, shear stress-induced mechanosignaling leads to the release of hepatic growth factor (HGF) that triggers the proliferation and survival of adjacent hepatocytes.
Cell 5. Mechanical stretch of the cell membrane of LSECs activates cation channels. In addition, pulsatile forces on LSECs, as a result of hepatic congestion, activate integrin/Piezo/Notch to induce the release of CXCL1 that participates in the pathogenesis of portal hypertension.
Adhesion receptors and cell-cell junctions.
Cadherins, selectins, and CAMs are adhesion molecules that sense mechanical forces at the cell borders 23. E-cadherin plays a prominent role in forming adherens junctions in epithelial cells. To initiate adherens junctions, extracellular domains of cadherins engage in Ca2+-dependent hemophilic trans-interaction with identical cadherin molecules on an adjacent cell, while their cytoplasmic tails bind to p120- and β-catenin proteins 24. β-catenin interacts with α-catenin containing an actin-binding domain, so adherens junctions are physically linked to the actin cytoskeleton. Vinculin is phosphorylated at tyrosine 822 by Abelson tyrosine kinase in response to the clustering of cadherin-catenin molecules and it is recruited to the cadherin complexes as well (Figure 1, cell 2). The interaction of the cytoskeleton and adherens junctions is regulated by RhoA and stabilization of adherens junctions is essential for the development of contact inhibition of cell proliferation of epithelial cells. 24. E-Cadherin is frequently lost in human epithelial cancers, restoration of E-cadherin reduced cancer cell proliferation while disruption of E-cadherin promoted cell proliferation in a 3 dimensional (3D)-culture model 25. Additionally, the loss of E-cadherin-mediated cell adhesion was a rate-limiting step in the progression from β-cell adenoma to β-cell carcinoma in a murine pancreatic cancer model 26.
Platelet endothelial cell adhesion molecule (PECAM-1) is the main adhesion molecule of endothelial cells. In response to shear stress, PECAM-1, vascular endothelial cell cadherin (VE-cadherin), and vascular endothelial growth factor receptor 2 (VEGFR2) form a mechanosensory complex that senses and transmits fluid shear stress into endothelial cells (Fig. 1, cell 4) 27. In this protein complex, PECAM-1 is a direct force receptor and sensor by activating Src and binding to the type III intermediate filament (vimentin), whereas VE-cadherin functions as an adaptor 27,28. Activated VEGFR2 binds to PI3K to induce its activation, which subsequently leads to activation of integrin. Integrin activation in turn induces the alignment of endothelial cells in laminar shear and activation of NF-κB in endothelial cells (Fig. 1, cell 4). The endothelium of PECAM-1 knockout mice exhibited defects in both F-actin organization and activation of the NF-κB pathway 27, demonstrating the physiological relevance of this mechanosensory complex in vivo.
Ion channels.
Mechanical stretch of the cell membrane of bacteria induced activation of the large conductance mechanosensitive ion channel (MscL) and the small conductance mechanosensitive ion channel (MscS) directly in vitro, demonstrating for the first time that this type of ion channel was sensitive to mechanical forces 29. Later, TREK-1, a mammalian two pore domain K+ channel, was shown to be activated by mechanical stretch (Fig. 1, cell 5) 30. Similarly, stretch of the lipid bilayer induced activation of K+ channels, TRAAK and TREK-2 31,32. Cation channel Piezo1 senses shear-stress in endothelium (Fig. 1, cell 5) and red blood cells and Piezo2 senses touch, pain and proprioception in neuronal cells 33. Both Piezo1 and Piezo2 were activated by membrane tension and their activation led to electric signals in calcium-dependent signaling pathways in endothelial cells, sensory neurons, and other cell types 33. Together, these findings support that these ion channels are mechanosensitive and function as mechanosensing receptors.
Force transmission by insoluble cytoskeleton and biochemical signaling
The cytoskeletal network of eukaryotic cells is mainly made up by 3 types of the cytoskeleton filaments: actin microfilaments, microtubules and intermediate filaments. They form a dynamic network linking the plasma membrane to the cell nucleus. The cytoskeletal network is important for keeping the shape of a cell, stabilizing a tissue, and providing resistance forces to cell deformation. When a FA or cell-cell junction forms, the actin filaments are coupled to the adhesion complex by talin, vinculin, or α-catenin. Actomyosin contractility and myosin motors are major contributors to the cytoskeletal tension that increases in response to mechanical forces. In turn, the increased cytoskeletal tension reinforces the adhesions. The actin and intermediate filaments also connect the adhesions to nesprins, a component of the LINC complex, or lamins at the nuclear envelop so the external forces can reach the nucleus 14. Three models have been proposed for force transmission within a cell: (a) direct force transmission by the cytoskeleton, (b) biochemical signaling, and (c) direct force transmission and signaling 14. While the direct force transmission along the actin fibers occurs fast (on the time scales of seconds), contributing to a rapid, large and global cellular deformation, the biochemical signaling-mediated force transfer is slower (minutes to hours) and mediates the effects of long-lasting mechanical stimuli. The third one combines both, which may be used to explain how a cell senses and responds to a variety of forces with different spatial and temporal scales 14.
Forces modulate gene transcription
Mechanostransduction can influence gene transcription. It has been shown that force-induced deformation of a cell nucleus led to the opening of the nuclear pores and increase of nuclear transport of proteins 34–36. For example, in response to the forces directly applied on the top of the nucleus by atomic force microscopy, the nuclear envelope became flattened, the nuclear pores were enlarged, and nuclear import of YAP1 was subsequently increased 36. Additionally, stretch of the nucleus and chromatin by magnetic twisting of cell surface resulted in gene transcription of a green fluorescent protein (GFP)-tagged transgene 37, supporting that the cell nucleus is a mechanosensitive organelle that connects external forces to gene transcription.
How do forces influence the phenotype of liver cells and liver diseases?
Hepatocytes.
The role of stiffness on the phenotype of hepatocytes has received ample attention because the correlation of fibrosis to the incidence rates of hepatocellular carcinoma (HCC). It has been shown that primary hepatocytes cultured on stiff (thin films of monomeric collagen adsorbed to a rigid dish) or soft gels of fibrillar collagen displayed contrasting phenotypes; hepatocytes remained differentiated and growth arrested on a soft substrate and they spread and proliferated on a stiff substrate 38. When primary hepatocytes isolated from rats or mice were cultured on the substrates with different stiffness, simulating non-fibrotic and fibrotic environments, the mRNA levels of albumin were markedly reduced by stiffness 39,40. These findings suggest that a stiff substrate leads to a dedifferentiation phenotype of hepatocytes (Fig. 1, cell 1).
How does stiffness induce a dedifferentiation phenotype of hepatocytes? Hepatocyte nuclear factor 4 alpha (HNF4α) is a transcription factor that plays a key role in the maintenance of hepatocyte-specific functions. Using collagen matrix with tunable stiffness, Desai, SS et al. found that a level of stiffness, resembling the fibrotic liver, reduced HNF4α protein level and the transcripts of the targets of HNF4α, such as Baat, F7, and Gys2, through the Rho/ROCK signaling pathway 40. Additionally, forced expression of HNF4α in hepatocytes cultured on a stiff matrix reduced the expression of mesenchymal genes such as Snail. However, it did not restore the expression of hepatic functional genes such as Baat, F7, and Gys2, indicating that stiffness had other negative effects on hepatocyte function in addition to inhibiting HNF4α expression 40. Using RNA sequencing, Xia et al. found that thousands of genes of hepatocytes were upregulated and downregulated by stiffness 41. For example, stiffness increased the expression of genes encoding the cytoskeleton regulatory proteins, such as ARP3 actin-related protein 3 homolog C (ACTR3C), tubulin tyrosine ligase-like family member 3 (TTLL3), and actin-related protein 2/3 complex, subunit 3 (ARPC3), and expression of genes encoding proteins related to ECM-receptor interaction, such as alpha 1 integrin (ITGA1), fibronectin 1 (FN1), sorting nexin 15 (SNX15), laminin, and alpha 4 (LAMA4). In addition, the expression of epithelial cell-related genes, such as claudin 12 (CLDN12), was downregulated and mesenchymal phenotype-related genes, such as CDH2 was upregulated by stiffness. Interestingly, Gortzen J et al. collected hepatocytes cultured on substrates with different stiffness for real-time quantitative reverse transcription-PCR (qRT-PCR) and found that stiffness reduced RhoA mRNA but increased Src mRNA level of hepatocytes 39. Immunoblotting revealed that stiffness increased activating phosphorylation level of Src (p-Src418) and decreased inactivating phosphorylation level of Src (p-Src530) 39. Using immunofluorescence, Desai SS et al. further demonstrated that in murine fibrotic liver induced by CCl4 or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC), the protein level of fibronectin, activated β1-integrin, and phosphorylated FAK (pFAKY397) increased in the hepatocytes adjacent to the fibrotic tracts 40, suggesting that stiffness indeed activated an integrin-FAK/Src mechanosignaling that led to gene transcription and a new phenotype of hepatocytes in vivo (Fig. 1, cell 1).
Hepatocellular carcinoma (HCC).
It has been shown that stiffness promoted HCC proliferation, motility, and drug resistance to cisplatin and Sorafenib42,43. Stiffness stimulated HCC proliferation by activating the β1-integrin/FAK signaling and Sorafenib resistance by enhancing nuclear translocation of YAP142,43. In HCC patient samples, tumor stiffness correlated positively with the expression of β1-integrin and the β1-integrin expression levels correlated with Edmondson pathologic grade, encapsulation, metastasis, and HBV infection 44. In a long-term follow-up of 1146 HCV infected patients, cumulative HCC incidence rates at 5 years were positively correlated with liver stiffness whereas overall patient survival rates were negatively correlated with liver stiffness 45. However, it is unclear whether or how stiffness of a fibrotic liver induces malignant transformation of liver cells and HCC development. We recently performed RNA sequencing and found that stiffness promoted HSC to release a panel of paracrine factors, including CXCL12, IL11, IL6, PDGFA and B, and VEGFA 46, which promoted colorectal liver metastasis in mice by a paracrine mechanism. It remains to be determined if liver fibrosis and stiffness indeed promote malignant transformation of hepatocytes in part through the effect on HSCs and the hepatic tumor microenvironment.
HSCs and portal fibroblasts.
Liver injury or cancer invasion of the liver induces differentiation of HSCs into myofibroblasts that contribute to fibrosis 47,48. HSCs and portal fibroblasts are mechanosensitive cells and they are two distinct cell populations that express different protein markers. HSCs were elastin-negative and desmin-positive in culture whereas portal fibroblasts were elastin-positive and desmin-negative 49. When HSCs and portal fibroblasts were plated on the polyacrylamide hydrogels with tunable stiffness, they both responded to stiffness and demonstrated an increasingly myofibroblastic phenotype as stiffness increased 5,49. It appeared that their myofibroblastic differentiation required mechanical forces, cell adhesions, and the generation of cellular tension, and that TGFβ enhanced but was not required 5,49.
How do forces promote HSC activation? Immunohisotochemistry revealed that YAP1 was in the cytoplasm of the fibroblasts of normal livers but entered the nucleus of the activated-HSC/myofibroblasts of fibrotic livers in murine and patient samples 50. Culturing HSCs on a regular culture plate (stiff substrate) for 10 hours led to nuclear localization of YAP1 50. Additionally, YAP1 knockdown or YAP1 inhibitor verteporfin inhibited stiffness-mediated HSC activation in vitro and verteporfin reduced murine liver fibrosis induced by CCl4 or bile duct ligation (BDL) 50,51. These data suggest YAP1 as a key factor for stiffness-induced HSC activation (Fig. 1, cell 3). Interestingly, 15 minutes after murine partial hepatectomy, YAP1 and its transcriptional targets were upregulated in HSCs, indicating that mechanical forces, as a result of an elevated blood flow/shear stress, led to YAP1 upregulation and HSC activation 52. Consistent with this, Simonetto DA et al. used cyclic stretch of HSCs to show that stretching forces applied to HSCs in vitro stimulated HSCs to produce fibronection by enhancing gene transcription and promoted fibril assembly by the β1-integrin/actin dependent mechanism 53 (Fig. 1, cell 3). Furthermore, Martin K. et al. found that the loss of β1-integrin in HSCs promoted YAP1 phosphorylation and its cytoplasmic retention, which led to downregulation of YAP1 52, supporting a key role of the β1-integrin/YAP1 mechanosignaling in force-mediated HSC activation.
Megakaryoblastic leukemia factor-1 (MKL1), also called myocardin related transcription factor (MRTFA), is another transcription coactivator that undergoes nuclear translocation in response to forces 54,55 (Fig. 1, cell 3). MKL1/MRTFA bound to actin and its nuclear translocation required force-mediated actin filament assembly 54,55. MRTF activity is elevated in cancer-associated fibroblasts and it is required for cell contractile and proinvasive properties. Interestingly, the expression of the direct MRTF targets and expression of YAP1 targets were mutually dependent, suggesting that MRTF-mediated and YAP1-mediated transcription pathways interacted indirectly for mechanotransduction of the fibroblasts 56. In addition, we found that disruption of transcription coactivator p300 by cre-mediated gene deletion, shRNA-mediated knockdown, or p300 inhibitor abolished stiffness-induced HSC activation 46. Mechanistically, stiffness induced a RhoA-Akt mechanosignaling to induce p300 phosphorylation and nuclear targeting so that p300 epigenetically turned on gene transcription for HSC activation 46 (Fig. 1, cell 3). In a separate study, we showed that p300 bound to TAZ and transported TAZ into the nucleus of HSCs under TGFβ1 stimulation 57. Although we could not detect p300/YAP1 binding in HSCs, overexpression of HA-tagged p300 and its related protein family member CREB-binding protein (CBP) promoted acetylation of YAP1 in HEK293T cells 58. It would be interesting to investigate if YAP1 is acetylated by CBP/p300 in HSCs and YAP1 acetylation indeed contributes to its nuclear translocation and mechanotransduction of HSCs.
LSECs.
Under physiological conditions, shear stress induces mechanosignaling of LSECs and stimulates LSEC to release paracrine factors that are termed as angiocrine factors. Angiocrine factors contribute to the development and growth of the liver as well as the maintenance of the function of the liver 59. For example, using ex vivo perfusion of adult mouse livers and in vitro stretch of human LSECs, Lorenz et al. found that shear stress on LSECs activated β1-integrin and vascular endothelial growth factors receptor 3 (VEGFR3) that led to the release of hepatic growth factor and that this angiocrine signal triggered the proliferation and survival of the adjacent hepatocytes 60 (Fig. 1, cell 4). This finding highlights the role of mechanotransduction of LSECs for the growth and maintenance of the liver, consistent with findings by others demonstrating that the endothelium itself formed specialized vascular niches for the production of angiocrine factors important for the induction, specification, patterning and guidance of organ development and regeneration, as well as the maintenance of homeostasis and metabolism 59,61.
Under pathological conditions, such as portal hypertension and fibrosis, mechanotransduction of LSECs leads to angiocrine and phenotypic changes that further promote the diseases. For example, using in vitro cyclic stretch of LSECs and a hepatic congestion mouse model by partial ligation of the mouse inferior vena cava, Hilscher et al. showed that stretch activated integrin/Notch/piezo1 of LSECs to promote expression of CXCL1 and that CXCL1, released from LSECs, attracted neutrophils to form neutrophil extracellular traps and microthrombi pivotal for portal hypertension via volume-pressure effects with the sinusoidal lumen 62 (Fig. 1, cell 5). It has been hypothesized that LSEC-dependent angiogenesis at the early-stage of liver fibrosis may stiffen ECM and generate forces to influence HSC activation and disease progression. To test this, Liu L et al. set up a series of fibrotic microniches by plating LSECs on 2D substrates with defined stiffness on the bottom and overlaying 3D collagen (type I) hydrogel embedded with HSCs on the top to mimic LSEC/HSC interactions 63. LSECs plated on the substrates with stiffness ranging from 140 Pa to 610 Pa formed capillary-like structures, simulating angiogenesis during the early-stage of liver fibrosis. LSEC-dependent angiogenesis induced the condensation of collagen fibers and the forces generated by collagen remodeling in turn promoted HSC activation by activating the collagen-DDR2-JAK2/PI3K/AKT-myocardin mechanosignling in HSCs 63 (Fig. 1, cell 5). Thus, under disease conditions, mechanical forces in the liver influence the phenotype of LSECs by mechanotransduction and LESCs in turn contribute to the disease development and progression by angiocrine- or force- dependent mechanism.
Conclusion
Chronic liver diseases lead to liver stiffness by inducing activation of HSCs and portal fibroblasts into ECM-producing myofibroblasts. ECM-mediated forces in turn influence the behavior and function of liver cells by mechanotransduction. Mechanotransduction is facilitated by the mechanosensing receptors at the plasma membrane, associated protein complexes (FAs and adheres junctions), force transmission by the mechanosensitive molecules and insoluble cytoskeleton, and lastly, gene transcription in the nucleus. Through mechanostransduction, external forces can reach the nucleus to modify gene transcription and generate a variety of biological responses, such as proliferation, migration, differentiation, polarity, or apoptosis. In addition to forces generated by ECM, shear stress is another type of force influencing LSECs constantly. Thus, hepatocytes, HSCs, portal fibroblasts, and LSECs are major cell types in the liver sensitive to mechanical forces. In response to forces, these cells alter their phenotypes and participate in the initiation and progression of liver diseases, including hepatic fibrosis and cancer.
Our research activities tend to characterize and separate the role of mechanotransduction from that of biochemical signaling induced by growth factors, cytokines, and chemokines. We need to take it into consideration that both mechanotransduction and the biochemical signaling in the liver actually interact and crosstalk to produce synergistic effects on liver diseases. The majority of the cell-based studies we performed were done with cells grown on cell culture plastic which was extremely rigid (stiffness > gigapascal). Because the plastic was much stiffer than a normal or diseased liver, this practice may be inappropriate for studying some cell behavior and function of liver cells 1. Additionally, 2D monolayer culture may fail to provide us with the pathophysiological relevant information because cells in vivo were actually surrounded by other cells and ECM in a 3D microenvironment. So the conclusions drawn from experiments with 2D-culture may be different from those drawn from 3D-culture. For example, to test the role of YAP1 for HSC activation, Mannaert I et al. transfected YAP1 siRNA into HSCs and cultured the cells as 3D-aggregates. After 4 days of 3D-culture, they transferred cells onto cell culture plastic to induce HSC activation 50. This 3D- to 2D-culture protocol allowed YAP1 be knocked down before YAP1 activation by stiffness, which demonstrated that YAP1 siRNA inhibited stiffness-mediated HSC activation 50. Interestingly, we used 2D-culture to test the role of YAP1 for TGFβ1-stimulated HSC activation and found that knockdown of both YAP1 and TAZ inhibited HSC activation while knockdown of YAP1 alone did not 57, suggesting that a stiff environment activated YAP1 and TAZ and that both contributed to HSC activation. Taken together, a right model or system is extremely important for our experiments and more complex systems that recapitulate in vivo ECM/cell and cell/cell interactions are urgently needed for our research field. Moreover, combined strategies and agents that target against both the biochemical signaling and mechanosignaling need to be considered in the future studies aimed at improving the clinical outcome of patients with liver diseases.
Acknowledgments
Grant Support: NIH grant R01 CA160069
Footnotes
Conflict of Interest: No conflict of interest exists
References
- 1.Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47(4):1394–1400. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesh SK, Ehman RL. Magnetic resonance elastography of liver. Magn Reson Imaging Clin N Am. 2014;22(3):433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferraioli G, Tinelli C, Dal Bello B, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56(6):2125–2133. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1(2):98–105. [DOI] [PubMed] [Google Scholar]
- 5.Olsen AL, Bloomer SA, Chan EP, et al. Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301(1):G110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch-Fortea M, Martin-Belmonte F. Mechanosensitive adhesion complexes in epithelial architecture and cancer onset. Curr Opin Cell Biol. 2018;50:42–49. [DOI] [PubMed] [Google Scholar]
- 7.Lachowski D, Cortes E, Robinson B, Rice A, Rombouts K, Del Rio Hernandez AE. FAK controls the mechanical activation of YAP, a transcriptional regulator required for durotaxis. FASEB J. 2018;32(2):1099–1107. [DOI] [PubMed] [Google Scholar]
- 8.Lachowski D, Cortes E, Pink D, et al. Substrate Rigidity Controls Activation and Durotaxis in Pancreatic Stellate Cells. Sci Rep. 2017;7(1):2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gkretsi V, Stylianopoulos T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front Oncol. 2018;8:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. [DOI] [PubMed] [Google Scholar]
- 11.Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177(5):2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–1400. [DOI] [PubMed] [Google Scholar]
- 13.Lim CG, Jang J, Kim C. Cellular machinery for sensing mechanical force. BMB Rep. 2018;51(12):623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathieu S, Manneville JB. Intracellular mechanics: connecting rheology and mechanotransduction. Curr Opin Cell Biol. 2019;56:34–44. [DOI] [PubMed] [Google Scholar]
- 15.Tucker GC. Inhibitors of integrins. Curr Opin Pharmacol. 2002;2(4):394–402. [DOI] [PubMed] [Google Scholar]
- 16.Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Integrin inactivators: balancing cellular functions in vitro and in vivo. Nat Rev Mol Cell Biol. 2013;14(7):430–442. [DOI] [PubMed] [Google Scholar]
- 17.Alberts BJA, Lewis J, et al. Molecular Biology of the Cell. 4th edition . 2002. [Google Scholar]
- 18.Brakebusch C, Bouvard D, Stanchi F, Sakai T, Fassler R. Integrins in invasive growth. J Clin Invest. 2002;109(8):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur J Cell Biol. 2013;92(10-11):303–315. [DOI] [PubMed] [Google Scholar]
- 20.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9(15):1896–1908. [DOI] [PubMed] [Google Scholar]
- 21.Speicher T, Siegenthaler B, Bogorad RL, et al. Knockdown and knockout of beta1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun. 2014;5:3862. [DOI] [PubMed] [Google Scholar]
- 22.Coelho NM, McCulloch CA. Mechanical signaling through the discoidin domain receptor 1 plays a central role in tissue fibrosis. Cell Adh Migr. 2018;12(4):348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–599. [DOI] [PubMed] [Google Scholar]
- 24.Klezovitch O, Vasioukhin V. Cadherin signaling: keeping cells in touch. F1000Res. 2015;4(F1000 Faculty Rev):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1). J Cell Biol. 1998;142(2):557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392(6672):190–193. [DOI] [PubMed] [Google Scholar]
- 27.Tzima E, Irani-Tehrani M, Kiosses WB, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. [DOI] [PubMed] [Google Scholar]
- 28.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23(11):1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sukharev SI, Blount P, Martinac B, Blattner FR, Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368(6468):265–268. [DOI] [PubMed] [Google Scholar]
- 30.Patel AJ, Honore E, Maingret F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17(15):4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. J Biol Chem. 1999;274(3):1381–1387. [DOI] [PubMed] [Google Scholar]
- 32.Lesage F, Maingret F, Lazdunski M. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive K(+) channel. FEBS Lett. 2000;471(2-3):137–140. [DOI] [PubMed] [Google Scholar]
- 33.Parpaite T, Coste B. Piezo channels. Curr Biol. 2017;27(7):R250–R252. [DOI] [PubMed] [Google Scholar]
- 34.Ingber DE, Madri JA, Folkman J. Endothelial growth factors and extracellular matrix regulate DNA synthesis through modulation of cell and nuclear expansion. In Vitro Cell Dev Biol. 1987;23(5):387–394. [DOI] [PubMed] [Google Scholar]
- 35.Haase K, Macadangdang JK, Edrington CH, et al. Extracellular Forces Cause the Nucleus to Deform in a Highly Controlled Anisotropic Manner. Sci Rep. 2016;6:21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elosegui-Artola A, Andreu I, Beedle AEM, et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell. 2017;171(6):1397–1410 e1314. [DOI] [PubMed] [Google Scholar]
- 37.Tajik A, Zhang Y, Wei F, et al. Transcription upregulation via force-induced direct stretching of chromatin. Nat Mater. 2016;15(12):1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen LK, Wilhelm J, Fassett JT. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006;72:205–236. [DOI] [PubMed] [Google Scholar]
- 39.Görtzen J, Schierwagen R, Bierwolf J, et al. Interplay of matrix stiffness and c-SRC in hepatic fibrosis. Frontiers in physiology. 2015;6:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desai SS, Tung JC, Zhou VX, et al. Physiological ranges of matrix rigidity modulate primary mouse hepatocyte function in part through hepatocyte nuclear factor 4 alpha. Hepatology. 2016;64(1):261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xia T, Zhao R, Feng F, et al. Gene expression profiling of human hepatocytes grown on differing substrate stiffness. Biotechnol Lett. 2018;40(5):809–818. [DOI] [PubMed] [Google Scholar]
- 42.Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Rong Y, Huang Y, et al. Cirrhotic stiffness affects the migration of hepatocellular carcinoma cells and induces sorafenib resistance through YAP. J Cell Physiol. 2019;234(3):2639–2648. [DOI] [PubMed] [Google Scholar]
- 44.Zhao G, Cui J, Qin Q, et al. Mechanical stiffness of liver tissues in relation to integrin beta1 expression may influence the development of hepatic cirrhosis and hepatocellular carcinoma. J Surg Oncol. 2010;102(5):482–489. [DOI] [PubMed] [Google Scholar]
- 45.Nakagomi R, Tateishi R, Masuzaki R, et al. Liver stiffness measurements in chronic hepatitis C: Treatment evaluation and risk assessment. J Gastroenterol Hepatol. 2019;34(5):921–928. [DOI] [PubMed] [Google Scholar]
- 46.Dou C, Liu Z, Tu K, et al. P300 Acetyltransferase Mediates Stiffness-Induced Activation of Hepatic Stellate Cells Into Tumor-Promoting Myofibroblasts. Gastroenterology. 2018;154(8):2209–2221 e2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Billadeau DD, Abdelhakim H, et al. IQGAP1 suppresses TbetaRII-mediated myofibroblastic activation and metastatic growth in liver. J Clin Invest. 2013;123(3):1138–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46(4):1246–1256. [DOI] [PubMed] [Google Scholar]
- 50.Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015. [DOI] [PubMed] [Google Scholar]
- 51.Martin K, Pritchett J, Llewellyn J, et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat Commun. 2016;7:12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simonetto DA, Yang HY, Yin M, et al. Chronic passive venous congestion drives hepatic fibrogenesis via sinusoidal thrombosis and mechanical forces. Hepatology. 2015;61(2):648–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao XH, Laschinger C, Arora P, Szaszi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J Cell Sci. 2007;120(Pt 10):1801–1809. [DOI] [PubMed] [Google Scholar]
- 55.Huang X, Yang N, Fiore VF, et al. Matrix stiffness–induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. American journal of respiratory cell and molecular biology. 2012;47(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster CT, Gualdrini F, Treisman R. Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics. Genes Dev. 2017;31(23-24):2361–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Tu K, Liu D, et al. p300 Acetyltransferase Is a Cytoplasm-to-Nucleus Shuttle for SMAD2/3 and TAZ Nuclear Transport in Transforming Growth Factor beta-Stimulated Hepatic Stellate Cells. Hepatology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hata S, Hirayama J, Kajiho H, et al. A novel acetylation cycle of transcription co-activator Yes-associated protein that is downstream of Hippo pathway is triggered in response to SN2 alkylating agents. J Biol Chem. 2012;287(26):22089–22098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostallari E, Shah VH. Angiocrine signaling in the hepatic sinusoids in health and disease. Am J Physiol Gastrointest Liver Physiol. 2016;311(2):G246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenz L, Axnick J, Buschmann T, et al. Mechanosensing by beta1 integrin induces angiocrine signals for liver growth and survival. Nature. 2018;562(7725):128–132. [DOI] [PubMed] [Google Scholar]
- 61.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529(7586):316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilscher MB, Sehrawat T, Arab Verdugo JP, et al. Mechanical Stretch Increases Expression of CXCL1 in Liver Sinusoidal Endothelial Cells to Recruit Neutrophils, Generate Sinusoidal Microthombi, and Promote Portal Hypertension. Gastroenterology. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu L, You Z, Yu H, et al. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nat Mater. 2017;16(12):1252–1261. [DOI] [PubMed] [Google Scholar]