Abstract
Background
Telerehabilitation offers an alternate way of delivering rehabilitation services. Information and communication technologies are used to facilitate communication between the healthcare professional and the patient in a remote location. The use of telerehabilitation is becoming more viable as the speed and sophistication of communication technologies improve. However, it is currently unclear how effective this model of delivery is relative to rehabilitation delivered face‐to‐face or when added to usual care.
Objectives
To determine whether the use of telerehabilitation leads to improved ability to perform activities of daily living amongst stroke survivors when compared with (1) in‐person rehabilitation (when the clinician and the patient are at the same physical location and rehabilitation is provided face‐to‐face); or (2) no rehabilitation or usual care.
Secondary objectives were to determine whether use of telerehabilitation leads to greater independence in self‐care and domestic life and improved mobility, balance, health‐related quality of life, depression, upper limb function, cognitive function or functional communication when compared with in‐person rehabilitation and no rehabilitation. Additionally, we aimed to report on the presence of adverse events, cost‐effectiveness, feasibility and levels of user satisfaction associated with telerehabilitation interventions.
Search methods
We searched the Cochrane Stroke Group Trials Register (June 2019), the Cochrane Central Register of Controlled Trials (the Cochrane Library, Issue 6, 2019), MEDLINE (Ovid, 1946 to June 2019), Embase (1974 to June 2019), and eight additional databases. We searched trial registries and reference lists.
Selection criteria
Randomised controlled trials (RCTs) of telerehabilitation in stroke. We included studies that compared telerehabilitation with in‐person rehabilitation or no rehabilitation. In addition, we synthesised and described the results of RCTs that compared two different methods of delivering telerehabilitation services without an alternative group. We included rehabilitation programmes that used a combination of telerehabilitation and in‐person rehabilitation provided that the greater proportion of intervention was provided via telerehabilitation.
Data collection and analysis
Two review authors independently identified trials on the basis of prespecified inclusion criteria, extracted data and assessed risk of bias. A third review author moderated any disagreements. The review authors contacted investigators to ask for missing information. We used GRADE to assess the quality of the evidence and interpret findings.
Main results
We included 22 trials in the review involving a total of 1937 participants. The studies ranged in size from the inclusion of 10 participants to 536 participants, and reporting quality was often inadequate, particularly in relation to random sequence generation and allocation concealment. Selective outcome reporting and incomplete outcome data were apparent in several studies. Study interventions and comparisons varied, meaning that, in many cases, it was inappropriate to pool studies. Intervention approaches included post‐hospital discharge support programs, upper limb training, lower limb and mobility retraining and communication therapy for people with post‐stroke language disorders. Studies were either conducted upon discharge from hospital or with people in the subacute or chronic phases following stroke.
Primary outcome: we found moderate‐quality evidence that there was no difference in activities of daily living between people who received a post‐hospital discharge telerehabilitation intervention and those who received usual care (based on 2 studies with 661 participants (standardised mean difference (SMD) ‐0.00, 95% confidence interval (CI) ‐0.15 to 0.15)). We found low‐quality evidence of no difference in effects on activities of daily living between telerehabilitation and in‐person physical therapy programmes (based on 2 studies with 75 participants: SMD 0.03, 95% CI ‐0.43 to 0.48). Secondary outcomes: we found a low quality of evidence that there was no difference between telerehabilitation and in‐person rehabilitation for balance outcomes (based on 3 studies with 106 participants: SMD 0.08, 95%CI ‐0.30 to 0.46). Pooling of three studies with 569 participants showed moderate‐quality evidence that there was no difference between those who received post‐discharge support interventions and those who received usual care on health‐related quality of life (SMD 0.03, 95% CI ‐0.14 to 0.20). Similarly, pooling of six studies (with 1145 participants) found moderate‐quality evidence that there was no difference in depressive symptoms when comparing post‐discharge tele‐support programs with usual care (SMD ‐0.04, 95% CI ‐0.19 to 0.11). We found no difference between groups for upper limb function (based on 3 studies with 170 participants: mean difference (MD) 1.23, 95% CI ‐2.17 to 4.64, low‐quality evidence) when a computer program was used to remotely retrain upper limb function in comparison to in‐person therapy. Evidence was insufficient to draw conclusions on the effects of telerehabilitation on mobility or participant satisfaction with the intervention. No studies evaluated the cost‐effectiveness of telerehabilitation; however, five of the studies reported health service utilisation outcomes or costs of the interventions provided within the study. Two studies reported on adverse events, although no serious trial‐related adverse events were reported.
Authors' conclusions
While there is now an increasing number of RCTs testing the efficacy of telerehabilitation, it is hard to draw conclusions about the effects as interventions and comparators varied greatly across studies. In addition, there were few adequately powered studies and several studies included in this review were at risk of bias. At this point, there is only low or moderate‐level evidence testing whether telerehabilitation is a more effective or similarly effective way to provide rehabilitation. Short‐term post‐hospital discharge telerehabilitation programmes have not been shown to reduce depressive symptoms, improve quality of life, or improve independence in activities of daily living when compared with usual care. Studies comparing telerehabilitation and in‐person therapy have also not found significantly different outcomes between groups, suggesting that telerehabilitation is not inferior. Some studies reported that telerehabilitation was less expensive to provide but information was lacking about cost‐effectiveness. Only two trials reported on whether or not any adverse events had occurred; these trials found no serious adverse events were related to telerehabilitation. The field is still emerging and more studies are needed to draw more definitive conclusions. In addition, while this review examined the efficacy of telerehabilitation when tested in randomised trials, studies that use mixed methods to evaluate the acceptability and feasibility of telehealth interventions are incredibly valuable in measuring outcomes.
Plain language summary
Telerehabilitation services for stroke
Review question This review aimed to gather evidence for the use of telerehabilitation after stroke. We aimed to compare telerehabilitation with therapy delivered face‐to‐face and with no therapy (usual care).
Background Stroke is a common cause of disability in adults. After a stroke, it is common for the individual to have difficulty managing everyday activities such as walking, showering, dressing, and participating in community activities. Many people need rehabilitation after stroke; this is usually provided by healthcare professionals in a hospital or clinic setting. Recent studies have investigated whether it is possible to use technologies such as the telephone or the Internet to help people communicate with healthcare professionals without having to leave their home. This approach, which is called telerehabilitation, may be a more convenient and less expensive way of providing rehabilitation. Telerehabilitation may be used to improve a range of outcomes including physical functioning and mood.
Study characteristics We searched for studies in June 2019 and identified 22 studies involving 1937 people after stroke. The studies used a wide range of treatments, including therapy programmes designed to improve arm function and ability to walk and programmes designed to provide counselling and support for people upon leaving hospital after stroke.
Key results As the studies were very different, it was rarely appropriate to combine results to determine overall effect. We found that people who received telerehabilitation had similar outcomes for activities of daily living function to those that received face‐to‐face therapy and those that received no therapy (usual care). At this point, not enough research has been done to show whether telerehabilitation is a more effective way to provide rehabilitation. Some studies report that telerehabilitation is less expensive to provide but information is lacking about cost‐effectiveness. Only two trials reported on whether or not any adverse events had occurred; these trials found no serious adverse events were related to telerehabilitation. Further trials are required.
Quality of the evidence The quality of the evidence was generally of low or moderate quality. The quality of the evidence for each outcome was limited due to small numbers of study participants and poor reporting of study details.
Summary of findings
Background
Description of the condition
Stroke is one of the most common causes of death and acquired disability worldwide (Thrift 2017). Survivors of stroke commonly experience a range of symptoms affecting motor function, speech, swallowing, vision, sensation and cognition, and recovery can be slow and incomplete (Crichton 2016; Langhorne 2011). These symptoms often lead to difficulty managing activities and limited participation in home and community activities. Approximately half of stroke survivors access some form of rehabilitation on discharge from acute services (National Institutes of Health 2014; Stroke Foundation 2017). Rehabilitation programmes are often lengthy and resource intensive. Therefore, determining the most effective and efficient ways to deliver stroke rehabilitation services is a matter of priority.
Description of the intervention
Telerehabilitation is the provision of rehabilitation services to patients at a remote location using information and communication technologies (Brennan 2009). Communication between the patient and the rehabilitation professional may occur through a variety of technologies such as the telephone, Internet‐based videoconferencing and sensors (such as pedometers). Virtual reality programmes may also be used as a medium for therapy; the patient completes therapy tasks within a computer‐generated virtual environment, and data are transmitted to the therapist (Rogante 2010). Telerehabilitation consultations may include assessment, diagnosis, goal‐setting, therapy, education, and monitoring (Russell 2009).
Stemming from the broader approach of telehealth, telerehabilitation has been described as an alternative method of delivering conventional rehabilitation services rather than a subspecialty (Winters 2002). There has been increasing interest in the use of telerehabilitation over time as technologies have become increasingly prevalent and more sophisticated (Brochard 2010; Galea 2019); however, translation into clinical practice has been slow and barriers experienced early in the development of the field persist (Standing 2018).
Many examples in the current literature demonstrate the scope of telerehabilitation. For example, home assessments to determine the need for modifications have been completed remotely by occupational therapists using a combination of still photography, telephone calls, and videoconferencing technology (Ninnis 2019). Physiotherapists have used telerehabilitation for treatment of musculoskeletal conditions and post‐surgical care (Richardson 2017; Van Egmond 2018), and speech pathologists have demonstrated the feasibility of providing aphasia rehabilitation using asynchronous telerehabilitation (Hill 2018).
How the intervention might work
Telerehabilitaton has been described simply as an alternative method of providing rehabilitation. Therefore, in theory, the mechanisms leading to recovery should mirror those associated with conventional rehabilitation programmes. It is now well established that organised, interdisciplinary stroke care reduces the likelihood of institutional care and long‐term disability and increases independence in activities of daily living (Kalra 2007; Pollock 2014). Improvements in function after completion of rehabilitation programmes have been attributed to a combination of physiological recovery, reorganisation within the brain (known as neuroplasticity), and compensation (Kwakkel 2004).
One of the key advantages of telerehabilitation is that it provides the opportunity for people who are isolated to access rehabilitation services. This feature is particularly beneficial in vast countries such as Canada and Australia, where many people live long distances away from specialised rehabilitation centres. People in rural and remote areas are unlikely to have access to rehabilitation teams with expertise in stroke, and they may not have access to rehabilitation clinicians at all. Eliminating the need for travel to rehabilitation centres may also benefit people with severely restricted mobility who have difficulty travelling or are unable to travel. Telerehabilitation is also likely to be beneficial in low‐resource settings where access to health professionals is poor but access to devices such as mobile phones is present.
Telerehabilitation services may also be used to complement and enhance the quality of current rehabilitation services. Stroke survivors have expressed concern regarding the lack of available long‐term support and ongoing unmet rehabilitation needs (Ullberg 2016). It is possible that the use of telerehabilitation may help to address these gaps by supporting patients as they resume life roles on discharge from inpatient facilities.
Furthermore, the use of telerehabilitation may result in cost savings in various ways. Reduced travel time (for clinicians who visit patients in their own home) may mean that clinicians are able to fit more consultations into a single day. In addition, it may be possible to discharge patients from inpatient rehabilitation facilities earlier and offer telerehabilitation as a way of continuing the rehabilitation programme. Furthermore, telerehabilitation may provide a mechanism for increasing the dose of therapy without an increase in face‐to‐face supervision.
Despite its apparent advantages, the challenges associated with telerehabilitation are well documented (Standing 2018; Theodoros 2008). One of the key issues facing clinicians is how to conduct assessments or provide interventions that are typically 'hands on', for example, assessment of muscle strength. The inability to conduct hands‐on assessment or treatment means that therapists need to modify current techniques, for example, by utilising family members or teaching the patient ways to perform the intervention independently (Russell 2009).
Furthermore, clinicians and patients may not possess the technical expertise to establish systems and to troubleshoot information and communication technologies. It has been recommended that service providers ensure that technical requirements are met (such as having adequate bandwidth), provide access to technical support and provide training to all users (clinicians and patients). Concerns have also been raised about the security of data transfer and how patient confidentiality can be maintained (American Telemedicine Association 2010).
Why it is important to do this review
Changes in the demographics of the population mean that the burden of stroke is projected to increase (Feigin 2017). New approaches that are demonstrated to be clinically sound and cost‐effective will be required. Increasing interest in telerehabilitation suggests that this area will continue to grow (Brochard 2010; Galea 2019), and there is great potential to implement effective telehealth interventions in low and middle‐income countries. Furthermore, clinical guidelines for stroke recommend telerehabilitation for people without access to centre‐based rehabilitation services (Blacquiere 2017). However, establishment of telerehabilitation services may be expensive because of the costs of equipment, training, and ongoing technical support. Therefore, it is important to determine whether telerehabilitation services once established may result in the desired outcomes.
Our first version of this review, published in 2013, included 10 RCTs which were heterogeneous in terms of the aims of their intervention (Laver 2013). Based on the lack of information available at that time, we were unable to reach conclusions about the effectiveness of telerehabilitation after stroke. Another recently published systematic review examined the effectiveness of telerehabilitation after stroke and, using different inclusion criteria, identified 11 RCTs (Chen 2015). The authors concluded that despite the relatively small field of evidence from which to draw information, telerehabilitation was non‐inferior to conventional rehabilitation approaches in improving activities of daily living and motor function (Chen 2015).
Given the growth of research in this area and the potential for telerehabilitation to improve access to, and quality of, rehabilitation services while reducing costs, an update of our previous review was warranted. Furthermore, health services are increasingly offering telerehabilitation services for their clients so evaluation of this approach is important.
Objectives
To determine whether the use of telerehabilitation leads to improved ability to perform activities of daily living amongst stroke survivors when compared with (1) in‐person rehabilitation (when the clinician and the patient are at the same physical location and rehabilitation is provided face‐to‐face); or (2) no rehabilitation or usual care.
Secondary objectives were to determine whether use of telerehabilitation leads to greater independence in self‐care and domestic life and improved mobility, balance, health‐related quality of life, depression, upper limb function, cognitive function, or functional communication when compared with in‐person rehabilitation and no rehabilitation. Additionally, we aimed to report on the presence of adverse events, cost‐effectiveness, feasibility, and levels of user satisfaction associated with telerehabilitation interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs. We considered cross‐over trials as RCTs in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies if they compared telerehabilitation with in‐person rehabilitation or no rehabilitation, two different methods of delivering telerehabilitation services, different doses of telerehabilitation or telerehabilitation plus usual care compared with usual care alone.
Types of participants
All study participants had received a clinical diagnosis of stroke as defined by the World Health Organization ("a syndrome of rapidly developing symptoms and signs of focal, and at times global, loss of cerebral function lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin") (WHO 1989). We included people with all types of stroke, at all levels of severity, and at all stages post‐stroke (acute, subacute, or chronic). We also included participants with subarachnoid haemorrhage. We excluded studies with participants of mixed aetiology (e.g. stroke and traumatic brain injury) unless data were available for stroke survivors only. We set no age limits; however, we planned to acknowledge the inclusion of any participants who were younger than 18 years of age.
Types of interventions
We included Interventions if they matched the following definition of telerehabilitation: "the delivery of rehabilitation services via information and communication technologies" (Brennan 2009). Clinically, this term encompasses a range of rehabilitation services that include assessment, prevention, intervention, supervision, education, consultation, and counselling. Interventions must have lasted longer than one session. Interactive and communication technologies included the telephone, the Internet, virtual reality, and monitoring via sensors or wearable devices. We included rehabilitation programmes that used "store and forward" methods of communication, or real‐time interaction. Interventions were provided by one or more health disciplines (e.g. we planned to include studies involving only one health profession and studies which involved a multidisciplinary intervention). We included rehabilitation programmes that used a combination of telerehabilitation and in‐person rehabilitation to conduct assessment or intervention, provided that the greater proportion of intervention was provided via telerehabilitation. We did not include the use of telerehabilitation when the purpose was to provide education or support for healthcare professionals rather than patient care.
Types of outcome measures
Primary outcomes
The primary outcome of interest was independence in activities of daily living assessed post‐intervention. In the review, this encompassed the self‐care, mobility and domestic life activity and participation domains derived from the International Classification of Functioning, Disability and Health (WHO 2010). Included assessment tools were those such as the Functional Independence Measure, the Barthel Index, Lawton Instrumental Activities of Daily Living, Frenchay Activities Index, and the Nottingham Extended Activities of Daily Living Index.
Secondary outcomes
Self‐care and domestic life.
Mobility (e.g. Timed Up and Go test, walking speed, functional ambulation category).
Balance.
Participant satisfaction with the intervention.
Self‐reported health‐related quality of life.
Depression.
Upper limb function (e.g. Action Research Arm Test, Wolf Motor Function Test, Fugl‐Meyer Upper Extremity measure).
Cognitive function (global measures such as the Mini Mental State Examination, or specific measures such as tests of attention or executive functioning).
Functional communication.
Cost‐effectiveness (as measured by comparing the costs and outcomes of each intervention approach).
Adverse events.
We also aimed to provide information on the feasibility of telerehabilitation for use with people after stroke by reporting on participant eligibility criteria and recruitment methods used in the individual studies identified.
Search methods for identification of studies
See the 'Specialized register' information at the Cochrane Stroke Group's website. We searched for relevant trials in all languages and arranged translation of trial reports where necessary.
Electronic searches
The searches for studies in our previous reviews were conducted in November 2012. The searches for this update were completed in July 2017 and then updated in June 2018, and again in June 2019.
We searched the Cochrane Stroke Group Trials Register, which was searched by the Managing Editor in November 2012, and by the Information Specialist in July 2017, June 2018, and June 2019 using the intervention code, telemedicine. In addition, we searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 6) in the Cochrane Library (searched 4 June 2019) (Appendix 1), MEDLINE (Ovid, 1946 to 3 June 2019) (Appendix 2), Embase Ovid (1974 to Week 22, 2019) (Appendix 3), AMED Ovid (Allied and Complementary Medicine; 1985 to May 2019) (Appendix 4), CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1982 to 4 June 2019) (Appendix 5), PsycINFO Ovid (from 1806 to 4 June 2019) (Appendix 6), PsycBITE (Psychological Database for Brain Impairment Treatment Efficacy, www.psycbite.com/ to 20 September 2018) (Appendix 7), OTseeker (www.otseeker.com to 20 September 2018) (Appendix 8), Physiotherapy Evidence Database (www.pedro.org.au to 20 September 2018) (Appendix 9), REHABDATA (www.naric.com/research/rehab/ to 20 September 2018) (Appendix 10), and the Health Technology Assessment Database (HTA) (www.crd.york.ac.uk/crdweb/ to 20 September 2018) (Appendix 11). We developed the MEDLINE search strategy with the help of the Cochrane Information Specialist and used a combination of controlled vocabulary and text‐word terms. We adapted this strategy for use with the other databases. Search words for trial registers and for other Web‐based databases included telerehabilitation, telemedicine, telehealth, videoconferencing, and stroke.
We also:
searched the following ongoing trials registers: US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) to 4 June 2019 (Appendix 12);
used the Cited Reference Search within Science Citation Index (SCI) and Social Science Citation Index (SSCI) to track relevant references;
searched ProQuest Dissertations and Theses (to 4 June 2019) (Appendix 13);
searched the UK Telemedicine and E‐health Information Service (www.teis.port.ac.uk/) for the first version of this review. The website was discontinued and was not searched for this version of the review; and
searched the grey literature using Open Grey (www.opengrey.eu) and Google Scholar (www.scholar.google.com) on 4 June 2019.
Searching other resources
To identify further published, unpublished and ongoing trials, we:
scanned the reference lists of all identified studies and reviews; and
scanned the abstracts of non–English language studies if they were available in English.
Data collection and analysis
Selection of studies
Two review authors (KEL and ZA) independently reviewed titles and abstracts of the records identified through searches and excluded obviously irrelevant studies. We obtained the full text of the remaining studies, and two review authors (KEL and ZA) selected studies for inclusion based on the inclusion criteria of the review. When unsure regarding inclusion of a particular study, a third review author (MC, SG or CS) made the final decision. We contacted trial authors for further details when required and documented the reasons for exclusion.
Data extraction and management
Two review authors (KEL and ZA) independently extracted study data and recorded information on a predesigned data extraction form. We extracted the following study details.
Citation details: title, authors, source and year of publication.
Participant inclusion and exclusion criteria.
Participant details: age, gender, location of stroke, time since onset of stroke and level of disability.
Recruitment details: numbers of people screened, eligible, recruited and randomly assigned; withdrawals.
Methodological quality: the Cochrane Collaboration's tool for assessing risk of bias.
Intervention details: descriptions of procedures, personnel involved, duration, dose and comparison interventions.
Outcome measures: measures used, by whom, when they were administered and how they were administered (in‐person or via information and communication technologies).
We contacted trial authors to ask for missing information when required. We resolved differences by discussion or by consultation with a third review author when necessary.
Assessment of risk of bias in included studies
Two review authors (KEL and ZA) independently assessed the risk of bias of included studies using Cochrane's 'Risk of bias' tool (Higgins 2011). This tool allows assessment of the following possible sources of bias: random sequence generation; allocation concealment; blinding of outcome assessors; incomplete outcome data; selective reporting; and any other potential sources of bias. We did not report on whether studies were able to blind participants or personnel because of the difficulties involved in achieving this in rehabilitation trials. We compared each study against the tool and assessed it as 'low risk', 'high risk', or 'unclear risk' of bias, depending on whether it met the criteria for each aspect of the tool. A third review author resolved any disagreements.
Measures of treatment effect
Two review authors (KEL and ZA) independently assigned outcome measures to the domain assessed (activities of daily living, participant satisfaction, health‐related quality of life, depression, mobility, upper limb function, cognitive function, functional communication). If more than one outcome measure was used in the same domain from the same study, we included the measure most frequently used across included studies.
We intended to conduct separate analyses between short‐term (less than three months after intervention) and long‐term (three months or longer) outcomes.
We planned to calculate risk ratios (RRs) and 95% confidence intervals (CIs) for dichotomous outcomes and mean differences (MDs), or standardised mean differences (SMDs) and 95% CIs for continuous outcomes, as appropriate.
Unit of analysis issues
The unit of randomisation in these trials was the individual participant. For three‐armed trials in which telerehabilitation was compared with in‐person or no rehabilitation, we intended to enter half the sample size for the telerehabilitation group. Thus, each alternative intervention would be included in a separate comparison, and the number of participants in the telerehabilitation group would be divided equally between comparisons; the telerehabilitation group mean and standard deviation would remain unchanged.
Dealing with missing data
We contacted trial authors to request missing data. We converted available data, when possible, using the procedures detailed in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We intended to deal with missing data as recommended by the Cochrane Handbook for Systematic Reviews of Interventions. When dropouts were clearly identified, we used the denominator of participants contributing data at the relevant outcome assessment.
Assessment of heterogeneity
When appropriate, we pooled results to present an estimate of treatment effect using a random‐effects model. We assessed heterogeneity by performing visual inspection of the forest plot along with the I2 statistic (Higgins 2011) where we considered that up to 40% heterogeneity might not be important, but higher levels indicated moderate or substantial heterogeneity.
Assessment of reporting biases
We sought to assess the impact of publication bias by searching clinical trials registers for studies. In addition, we investigated whether selective reporting occurred by comparing study protocols and the methods sections of papers with the results sections. We intended to assess small sample bias by preparing a funnel plot if we had 10 or more studies included in any meta‐analysis.
Data synthesis
We conducted a meta‐analysis based on a random‐effects model with 95% CIs using RevMan 5.3 (RevMan 2014). We explored heterogeneity as detailed below.
Subgroup analysis and investigation of heterogeneity
If we identified a sufficient number of comparable studies (eight or more), we planned to perform subgroup analyses to determine whether the effect on the primary outcome varied according to time since onset of stroke, severity of stroke, frequency of the intervention (occasions of service per week), intensity of the intervention (total hours of intervention), intervention approach selected (e.g. speech therapy, upper limb retraining), mode of delivery (e.g. telephone versus videoconferencing, real‐time communication versus 'store and forward'), and whether the intervention was provided by a multidisciplinary team or by members of a single discipline.
Sensitivity analysis
We intended to perform sensitivity analyses for all outcomes based on the methodological quality of studies (allocation concealment, blinding of outcome assessor, intention‐to‐treat analysis) to assess the impact of risk of bias in the included studies. We planned to conduct sensitivity analysis regardless of the level of heterogeneity detected. We also planned to conduct a sensitivity analysis to identify differences noted when a fixed‐effect versus a random‐effects model was used.
GRADE and Summary of findings
We used GRADE to interpret findings (Guyatt 2008), and presented 'Summary of findings' tables. The tables provide outcome‐specific information concerning the overall quality of evidence, magnitude of effect, and the sum of available data. When using GRADE, we downgraded the evidence from 'high quality' by one level for serious (or by two levels for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias. We presented the following outcomes in our 'Summary of findings' tables: activities of daily living, self‐care and domestic life, mobility, balance, self‐reported health‐related quality of life, depression, and upper limb function.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies; Characteristics of studies awaiting classification.
Results of the search
We identified 189 studies by searching the Cochrane Stroke Group trials register and clinical trial registries, and 25,234 references by searching electronic databases, totaling 25,423 references. We reviewed 416 articles in full text and contacted study authors to request more information when required, excluding articles that clearly did not meet the inclusion criteria. Details of 29 excluded studies are provided in the 'Characteristics of excluded studies' table. We listed studies in the excluded studies section of the review if they prompted conversations between the review authors regarding their eligibility. We identified 22 ongoing studies (Characteristics of ongoing studies), and six studies which are awaiting classification (Characteristics of studies awaiting classification). Search details are presented in the flow diagram (Figure 1).
1.
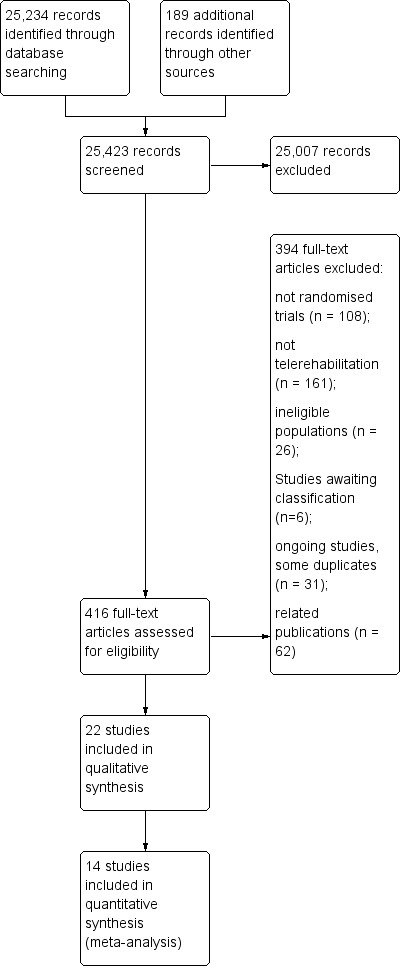
Study flow diagram.
Included studies
We included 22 RCTs, with a total of 1937 participants, in the review.
Sample characteristics
Included studies were conducted in the USA (n = 8), Canada (n = 3), the Netherlands (n = 2), Italy (n = 2), Germany (n = 2), China (n = 2), Taiwan (n = 1), Spain (n = 1), and Slovenia (n = 1). All studies were published within the previous 15 years (between 2004 and 2019). Sample sizes ranged from 10 to 536; most studies (71%, n = 16) included fewer than 100 participants (Table 3; Table 4).
1. Numbers of participants screened, recruited and followed up.
| Study | Screened | Randomised | Allocated to intervention group | Allocated to control group | Assessed at follow‐up |
| Bishop 2014 | Not reported | 49 | 23 | 26 | 41 |
| Bizovičar 2017 | Not reported | 10 | 5 | 5 | Unclear |
| Boter 2004 | 691 | 536 | 263 | 273 | 486 |
| Carey 2007 | 167 | 25 | 13 | 12 | 20 |
| Chen 2017 | 97 | 54 | 27 | 27 | 50 |
| Chumbler 2012 | 52 | 52 | 27 | 25 | 44 |
| Cramer 2019 | 232 | 124 | 62 | 62 | 124 analysed |
| Deng 2012 | 62 | 19 | 9 | 10 | 16 |
| Forducey 2012 | Not reported | 11 | Not reported | Not reported | 9 |
| Huijgen 2008 | Not reported | 16 | Not reported | Not reported | Not reported |
| Kirkness 2017 | 416 | 100 | 37 | 35 (in‐person), 28 (usual care) | 91 |
| Lin 2014 | 94 | 24 | 12 | 12 | 23 |
| Llorens 2015 | 115 | 31 | 15 | 16 | 30 |
| Mayo 2008 | 294 | 190 | 96 | 94 | 157 |
| Meltzer 2018 | Not reported | 53 | 20 | 22 | Unclear |
| Piron 2008 | Not reported | 10 | 5 | 5 | 10 |
| Piron 2009 | Not reported | 36 | 18 | 18 | 36 |
| Rochette 2013 | 286 | 186 | 92 | 94 | 139 |
| Saal 2015 | 1045 | 265 | 130 | 135 | 230 |
| Smith 2012 | 161 | 38 | 19 | 19 | 32 |
| Vauth 2016 | Unclear | 17 | Unclear | Unclear | Unclear |
| Wan 2016 | 186 | 91 | 46 | 45 | 80 |
2. Comparison of characteristics of studies included within the review.
| Study | Intervention | Comparison | Time after stroke | Country of study |
| Bishop 2014 | Family therapy phone calls to assist with transition home (up to 26 phone calls over 6 months) | Usual care | Not reported however occurred on discharge from hospital to home | USA |
| Bizovičar 2017 | Web‐based exercises (posture and upper limb) and therapist weekly consultations | Provision of written exercises without additional therapist contact | Subacute | Slovenia |
| Boter 2004 | Case management via 3 telephone calls and a home visit up to 24 weeks after discharge from an acute hospital following stroke | Usual care | Not reported; however, intervention was provided on discharge from acute facility | Netherlands |
| Carey 2007 | Upper limb therapy targeting finger and wrist movements provided via a computerised programme in which explicit feedback on performance was provided. Regular teleconferencing occurred between participant and therapist. | Upper limb therapy targeting finger and wrist movements provided via a computerised programme whereby explicit feedback on performance was not provided. Regular teleconferencing occurred between participant and therapist. | Chronic phase | USA |
| Chen 2017 | Exercise programme and electrical stimulation which was supervised and monitored remotely via a telerehabilitation system | Same programme of exercises but provided face to face in an outpatient therapy service | Subacute phase | China |
| Chumbler 2012 | A programme designed to improve the person's functional mobility administered via televisits, use of an in‐home messaging device, and 5 telephone calls over a 3‐month period | Usual care | Subacute phase | USA |
| Cramer 2019 | Telehrehabilitaiton program designed to improve upper limb function and involving on‐screen games | Similar therapy content and same dose of therapy but provided in the clinic | Subacute | USA |
| Deng 2012 | Lower limb therapy targeting ankle movements provided via a computerised programme in which explicit feedback on performance was provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Lower limb therapy targeting ankle movements provided via a computerised programme whereby explicit feedback on performance was not provided. Teleconferencing was used regularly, and performance data were emailed to the therapist. | Chronic phase | USA |
| Forducey 2012 | A total of 12 therapy sessions (occupational therapy and physiotherapy) were conducted via a desktop videophone. Interventions included education, retraining of self‐care, functional mobility and posture, home modifications and therapy to improve function in impaired limbs. | The same intervention programme was delivered face‐to‐face. | Not reported | USA |
| Huijgen 2008 | Upper limb therapy using the Home Care Activity Device (computer‐based programme) for 1 month | Usual care and generic exercises were provided by a physician | Chronic phase | Netherlands |
| Kirkness 2017 | Living Well with Stroke intervention designed to reduce depressive symptoms in people with stroke and depression delivered via telephone | Living Well with Stroke (in‐person) Usual care |
Subacute | USA |
| Lin 2014 | Physical exercises especially balance and lower limbs provided remotely via telerehabilitation system | Similar exercises provided face‐to‐face | Chronic | Taiwan |
| Llorens 2015 | Virtual reality system used in the home with the aim of improving balance. Remote monitoring and phone call checks | Virtual reality system used in the clinic with the aim of improving balance | Chronic | Spain |
| Mayo 2008 | Case management intervention provided via home visits and telephone calls for 6 weeks following discharge from acute care | Participants were instructed to make an appointment with their general practitioner. | Acute phase | Canada |
| Meltzer 2018 | Aphasia rehabilitation provided remotely via telerehabilitation | Aphasia rehabilitation provided in‐person | Chronic phase | Canada |
| Piron 2008 | Upper limb therapy that was delivered using a virtual reality programme at home and supplemented by videoconferencing | Upper limb therapy that was delivered using a virtual reality programme and conducted in the clinic setting | Chronic phase | Italy |
| Piron 2009 | Upper limb therapy that was delivered using a virtual reality telerehabilitation programme and that took place in the home | A programme of conventional upper limb exercises | Chronic phase | Italy |
| Rochette 2013 | Regular phone calls to discuss family functioning and risk factors after discharge from hospital | Phone number to call health professional if queries | Acute phase | Canada |
| Saal 2015 | Stroke support service upon discharge from acute hospital | Usual care | Post‐acute phase | Germany |
| Smith 2012 | An intervention to support the caregivers of stroke survivors by enhancing knowledge, skills, and coping. Delivered via email, online chat sessions, and online resources | Participants had access to some of the online resources. | Not reported | USA |
| Vauth 2016 | Rehabilitation for people with aphasia after discharge from hospital | Conventional therapy | Chronic phase | Germany |
| Wan 2016 | Goal‐setting telephone follow‐up relating to health behaviours | Usual stroke education | Subacute phase | China |
Most participants in the included studies were aged in their 50s, 60s, and 70s. Similar numbers of men and women were included, with the exception of two studies (Chumbler 2012; Smith 2012), for which only men were recruited. Eight studies recruited participants in the acute stages post‐stroke and provided rehabilitation upon discharge from hospital (Bishop 2014; Boter 2004; Chen 2017; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Wan 2016), whereas the rest of the studies involved participants in subacute and chronic stages.
Criteria for participant inclusion and exclusion varied amongst studies. Thirteen studies stated that they excluded participants with significant cognitive impairment (Bishop 2014; Chen 2017; Chumbler 2012; Cramer 2019; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009; Rochette 2013; Wan 2016), although this condition was defined differently between studies; four studies stated that participants needed to have a caregiver available (Bishop 2014; Forducey 2012; Meltzer 2018; Smith 2012).
As seen in Table 3 (where screening rates were reported) 1611 out of 3666 screened were randomised, resulting in a participation rate of 44%. This rate varied widely between studies, ranging from 15% (Carey 2007), to 100% (Chumbler 2012).
Interventions
All interventions were delivered in the participant's own home with the exception of one study in which participants were residing in a long‐term care facility (Lin 2014). An additional study found that some participants either could not or preferred not to use the telerehabilitation equipment within their homes (Meltzer 2018). In these situations, participants in the telerehabilitation group used equipment at a local healthcare centre or at the study site; however, they used equipment in a separate room and avoided contact with the study team and interventionists.
The primary aim of the intervention varied across the studies. Eight of the studies aimed to enhance care and well‐being after discharge from hospital through interventions which included goal‐setting, education about secondary prevention, family therapy, and case management (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012; Wan 2016). Most of the remaining studies involved interventions which aimed to improve physical function (upper limb, lower limb), and mobility and balance (Bizovičar 2017; Carey 2007; Chen 2017; Chumbler 2012; Cramer 2019; Deng 2012; Forducey 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Piron 2009). Specifically, six studies aimed to improve upper limb function through the use of customised computer‐based training programmes (Bizovičar 2017; Carey 2007; Cramer 2019; Huijgen 2008; Piron 2008; Piron 2009); four studies aimed to improve balance and mobility using customised telerehabilitation systems and communication between the participant and the therapist (Chumbler 2012; Deng 2012; Lin 2014; Llorens 2015); and one study involved exercises delivered remotely plus electrical stimulation with the aim of improving limb function, mobility, and balance (Chen 2017). One study used a combination of occupational therapy and physiotherapy to provide rehabilitation that often focused on remediation of impaired limbs (Forducey 2012). Two studies provided speech and language therapy for people with aphasia (Meltzer 2018; Vauth 2016).
Several different types of information and communication technologies were used to deliver telerehabilitation interventions. These included the telephone (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Meltzer 2018; Rochette 2013; Saal 2015; Wan 2016), videoconferencing hardware and software (Bizovičar 2017; Carey 2007; Chen 2017; Cramer 2019; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Piron 2009), and desktop videophones (Forducey 2012). Some studies used a combination of technologies: Chumbler 2012 used a combination of telephone calls, an in‐home messaging device and video recordings taken by a research assistant to be reviewed by the teletherapist; Smith 2012 used a combination of email, an online chat program and an online resource room (a virtual online library) established for caregivers of stroke survivors; and Chen 2017 utilised a telerehabilitation system which integrated electrical stimulation, measured physiological performance, and enabled data and medical records storage. The system used by Lin 2014 enabled videoconferencing plus monitoring of heart rate, oxygen saturation, and blood pressure.
Most interventions were conducted entirely by using information and communication technologies (Bishop 2014; Bizovičar 2017; Carey 2007; Chen 2017; Cramer 2019; Deng 2012; Forducey 2012; Huijgen 2008; Kirkness 2017; Lin 2014; Meltzer 2018; Piron 2008; Piron 2009; Rochette 2013; Saal 2015; Wan 2016). Four studies used a combination of telephone calls and home or clinic visits (Boter 2004; Llorens 2015; Mayo 2008; Saal 2015). The remaining study used 'store and forward' methods in which the research assistant video‐recorded the participant in his or her home and transmitted the information to the teletherapist for review (Chumbler 2012). The teletherapist was almost always described as being a health professional although details of their professional background was lacking in some studies.
Comparisons
Our preplanned comparisons were:
telerehabilitation compared with in‐person rehabilitation. We identified nine studies which tested this comparison (Chen 2017; Cramer 2019; Forducey 2012; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009; Vauth 2016); and
telerehabilitation compared with no rehabilitation or usual care. Ten studies (Bizovičar 2017; Bishop 2014; Boter 2004; Chumbler 2012; Huijgen 2008; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012; Wan 2016) compared telerehabilitation with a level of care which would be considered as a usual level of care available to clients of the service.
We also included studies if they compared different forms of telerehabilitation. Two studies compared different models of telerehabilitation (Carey 2007; Deng 2012).
The remaining study was a three‐arm study which compared telerehabilitation with both in‐person intervention and no intervention (usual care) (Kirkness 2017).
A wide range of outcome measures were used to assess the effects of the range of intervention approaches. These included measures of physical function, independence in activities of daily living, quality of life, and participant satisfaction. All studies assessed outcome measures post‐intervention. Several studies included follow‐up at one month (Piron 2009; Smith 2012), three months (Carey 2007; Chumbler 2012; Llorens 2015), six months (Bishop 2014; Chen 2017; Mayo 2008; Wan 2016), or 12 months (Kirkness 2017; Rochette 2013; Saal 2015), after completion of the intervention.
Studies included in the meta‐analysis
We included data from 14 studies in the meta‐analysis. We could not use data from the remaining studies in our meta‐analysis. Reasons included: data were not presented in suitable format for pooling and not available from the author(s) (Bishop 2014; Forducey 2012; Huijgen 2008; Vauth 2016), studies compared two forms of telerehabilitation (Carey 2007; Deng 2012), or studies did not report on our primary or secondary outcomes (Meltzer 2018).
Excluded studies
We deemed 29 studies to be ineligible: five because of ineligible populations (e.g. traumatic brain injury or transient ischaemic attack), four because they were not randomised trials, and the remaining 20 because the intervention did not meet our definition of telerehabilitation (Characteristics of excluded studies).
Risk of bias in included studies
2.
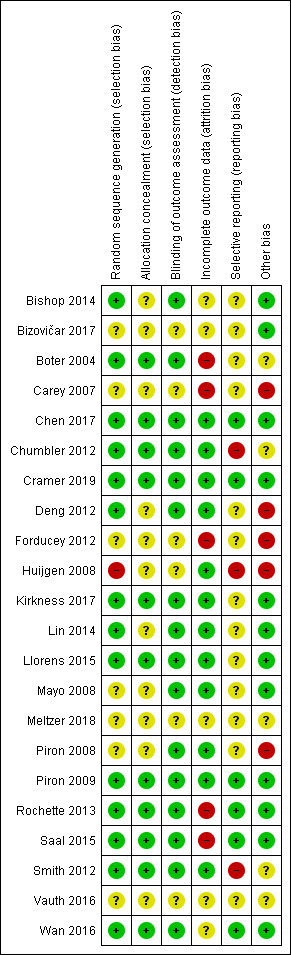
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
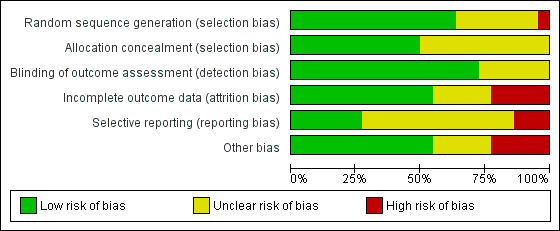
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Allocation concealment was adequate in 11 studies (50%), therefore, considered at low risk of bias but there was an unclear risk of bias in the reports of the remaining studies.
Blinding
Partial blinding of participants and personnel was performed in one of the studies, in which participants were masked to the study objectives because of postponed informed consent procedures (Boter 2004). It was unclear (unclear risk of bias) whether the outcome assessor was blinded to intervention group allocation in six studies. The remaining studies clearly stated that the assessor was blinded to allocation and we therefore considered them to be at low risk of bias.
Incomplete outcome data
For 10 studies it was unclear as to whether there was risk of bias in relation to incomplete outcome data. We deemed that there was low risk of attrition bias in the remaining studies.
Selective reporting
We identified a number of studies as being free of selective reporting and considered at low risk of bias (Chen 2017; Cramer 2019; Piron 2009; Rochette 2013; Saal 2015; Wan 2016). In three studies, we identified a high risk of bias due to selective reporting (Chumbler 2012; Huijgen 2008; Smith 2012). It was unclear (unclear risk of bias) whether selective reporting occurred in the remaining studies.
Other potential sources of bias
We identified several studies as being at risk of bias because of small sample sizes where there were less than 50 participants or differences between groups at baseline, or both (Bishop 2014; Bizovičar 2017; Carey 2007; Chen 2017; Deng 2012; Forducey 2012; Huijgen 2008; Lin 2014; Llorens 2015; Piron 2008; Meltzer 2018; Vauth 2016). It was unclear whether other studies were at risk of other sources of bias.
Effects of interventions
for the main comparison.
| Telerehabilitation compared with in‐person rehabilitation for stroke | ||||
|
Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: in‐person rehabilitation | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Independence in ADL post‐intervention |
No significant difference found on total ADL function score: MD 0.59 (‐5.50 to 6.68) (Analysis 1.1) | 75 (2 studies) |
⊕⊕⊝⊝ lowa,b | |
|
Self‐care and domestic life Post‐intervention |
Outcome not assessed in included studies | |||
|
Mobility post‐intervention |
Outcome not assessed in included studies | |||
|
Balance post‐intervention |
No significant difference found on balance outcomes: MD 0.48 (‐1.36 to 2.32) (Analysis 1.2) | 106 participants (3 studies) |
⊕⊕⊝⊝ lowa,b | |
|
Self‐reported health‐related quality of life post‐intervention |
Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
|
Depression post‐intervention |
Outcome not assessed in included studies (where the comparison was telerehabilitation versus in‐person rehabilitation) | |||
|
Upper limb function post‐intervention |
No significant difference found on total UL function score: MD 1.23 (‐2.17 to 4.64) (Analysis 1.3) | 170 (3 studies) | ⊕⊕⊝⊝ lowa,b | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; MD: mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aDowngraded one level due to risk of bias.
bDowngraded one level due to imprecision related to small sample size.
2.
| Telerehabilitation (post hospital discharge support) compared with usual care for stroke | ||||
|
Patient or population: people with stroke Settings: living in the community Intervention: telerehabilitation Comparison: usual care | ||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Independence in ADL post‐intervention |
No significant difference found on total ADL function score: SMD ‐0.00 (‐0.15 to 0.15) (Analysis 2.1) | 661 (2 studies) | ⊕⊕⊕⊝ moderatea | |
|
Self‐care and domestic life post‐intervention |
Outcome not assessed in included studies | |||
|
Mobility post‐intervention |
No significant difference found in gait speed: MD 0.01 (‐0.12 to 0.14) (Analysis 2.2) | 144 (1 study) |
⊕⊕⊝⊝ lowa,b | |
|
Balance post‐intervention |
Outcome not assessed in included studies | |||
|
Self‐reported health‐related quality of life post‐intervention |
No significant difference found in self‐reported quality of life: SMD 0.03 (‐0.14 to 0.20) (Analysis 2.3) | 569 (3 studies) | ⊕⊕⊕⊝ moderatea | |
|
Depression post‐intervention |
No significant difference found in depressive symptoms SMD ‐0.04 (‐0.19 to 0.11) (Analysis 2.4) | 1145 (6 studies) | ⊕⊕⊕⊝ moderatea | |
|
Upper limb function post‐intervention |
No significant difference found in upper limb function: SMD 0.33 (‐0.21 to 0.87) (Analysis 2.5) | 54 (2 studies) |
⊕⊕⊝⊝ lowa,b | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; SMD: standard mean difference; MD: mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
aDowngraded one level due to risk of bias.
bDowngraded one level due to imprecision related to small sample size.
Please refer also to the Table 1; and Table 2.
Telerehabilitation versus in‐person rehabilitation
Comparison 1.1: Telerehabilitation vs in‐person rehabilitation: activities of daily living
Three studies compared the effects of a physical therapy programme when delivered via telerehabilitation and when delivered in‐person (Chen 2017; Forducey 2012; Lin 2014). Two of these studies were pooled (Chen 2017; Lin 2014), whereas data from the third study was not reported in the paper in a way that could be used in the analysis and not available from the study authors (Forducey 2012). Pooling of the two studies with 75 participants showed no differences between groups post‐intervention (MD 0.59, 95% CI ‐5.5 to 6.68, I2 = 0%, low‐quality evidence) (Chen 2017; Lin 2014) (Analysis 1.1). We performed sensitivity analysis by removing Lin 2014 from the analysis due to unclear risk of bias for allocation concealment. This did not change the result (MD 1.6, 95% CI ‐5.32 to 8.52). The third study compared a telerehabilitation intervention delivered by physiotherapists and occupational therapists, in which the primary aim was restoration of physical function versus a more conventional rehabilitation approach delivered face‐to‐face (Forducey 2012). Both groups received the same dose of therapy. Participants receiving telerehabilitation communicated with the therapist via a desktop videophone connected to a standard home telephone line. The study authors reported that both telerehabilitation and control groups showed statistically significant improvement in activities of daily living. No significant differences in improvement were noted between groups.
1.1. Analysis.
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 1 Activities of daily living.
Comparison 1.2: Telerehabilitation vs in‐person rehabilitation: balance
Three studies (with 106 participants) compared the effect of a physical therapy programme delivered using telerehabilitation with a physical therapy programme delivered in‐person and reported on balance outcomes (Chen 2017; Lin 2014; Llorens 2015). The effect of telerehabilitation compared to in‐person rehabilitation was found to be equivalent (MD 0.48, 95% CI ‐1.36 to 2.32, I2 = 0%, low‐quality evidence) (Analysis 1.2). Sensitivity analysis, involving removal of Lin 2014 from the analysis due to unclear risk of bias for allocation concealment, did not change the result (MD 0.53, 95% CI ‐1.33 to 2.38).
1.2. Analysis.
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 2 Balance.
Telerehabilitation vs in‐person rehabilitation: participant satisfaction with the intervention
Two studies compared satisfaction between telerehabilitation and in‐person therapy (Cramer 2019; Piron 2008). We were unable to obtain the data required to pool these studies; however, both studies reported that participants in the intervention and control groups had high levels of satisfaction with the intervention, although Cramer 2019 reported that those in the telerehabilitation group reported slightly lower levels of satisfaction.
Telerehabilitation vs in‐person rehabilitation: health‐related quality of life
One study compared telehealth delivery of a programme of physiotherapy and occupational therapy with in‐person therapy (Forducey 2012). The investigators reported that although both groups reported improvement in health‐related quality of life, no differences between groups were evident. Data were not available in a form that could be presented in a forest plot.
Comparison 1.3: Telerehabilitation vs in‐person rehabilitation: upper limb function
We pooled three studies which consisted of a total of 170 participants and used a computer software program to retrain upper limb function (Cramer 2019; Piron 2008; Piron 2009). One of the studies compared the intervention versus the same intervention delivered in‐person (Piron 2008), another compared use of a virtual reality program provided via telerehabilitation versus conventional therapy delivered in‐person (Piron 2009), and the final study compared the same dose of therapy and similar content delivered via telerehabilitation or in the clinic (Cramer 2019). Participants in all studies were assessed with the Fugl‐Meyer Upper Extremity Scale post‐intervention. The impact of telerehabilitation on upper limb function was not different from the impact of the control intervention (MD 1.23, 95% CI ‐2.17 to 4.64, I2 = 42%, low‐quality evidence) (Analysis 1.3). Removal of Piron 2008 from the analysis (which was at risk of bias for allocation concealment) did not change the result (MD 1.47, 95% CI ‐2.99 to 5.93).
1.3. Analysis.
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 3 Upper limb function.
Comparison 1.4: Telerehabilitation vs in‐person rehabilitation: functional communication
Meltzer 2018 examined the difference between telerehabilitation and in‐person therapy for people with post‐stroke language disorders. The study authors reported that participants in both groups improved significantly on the Western Aphasia Battery aphasia quotient and Cognitive Linguistic Quick Test. There was no difference between groups (MD 1.10, 95% CI ‐2.52 to 4.72) (Analysis 1.4). An additional study looking at communication outcomes after stroke did not clearly present treatment outcomes and did not respond to our requests for more information (Vauth 2016).
1.4. Analysis.
Comparison 1 Telerehabilitation versus in‐person rehabilitation, Outcome 4 Functional communication.
Telerehabilitation versus usual care
Comparison 2.1: Telerehabilitation vs usual care: activities of daily living
Two studies, including 661 participants, delivered a post‐hospital discharge support programme, provided via a combination of telephone calls and home visits (Boter 2004; Mayo 2008). The control group received usual care, in which they did not receive any intervention within the research study; however, participants may or may not have received follow‐up from other sources. The estimated effect of telerehabilitation on activities of daily living, as measured by the Barthel Index, was SMD 0.00 (95% CI ‐0.15 to 0.15, I2 = 0%, moderate‐quality evidence) (Analysis 2.1). Removal of Mayo 2008 from the analysis, which was at risk of bias for allocation concealment, did not change the result (SMD 0.02, 95% CI ‐0.16 to 0.19). A third study found no difference between a post‐hospital discharge support programme and usual care on scores on the Frenchay Activities Index, which measures instrumental activities of daily living (Bishop 2014).
2.1. Analysis.
Comparison 2 Telerehabilitation versus usual care, Outcome 1 Activities of daily living.
A further study compared a combination of technologies (video recordings, in‐home messaging, and phone calls) in an intervention designed to improve functional mobility versus usual care and reported no statistically significant differences between groups after the intervention was provided (Chumbler 2012).
Comparison 2.2: Telerehabilitation vs usual care: mobility
One study, which offered post‐hospital discharge support and case management (Mayo 2008), assessed mobility post‐intervention using the Timed Up and Go test and gait speed and reported no significant differences between groups post‐intervention or at follow‐up six months after stroke (MD 0.01, 95% CI ‐0.12 to 0.14, low‐quality evidence) (Analysis 2.3).
2.3. Analysis.
Comparison 2 Telerehabilitation versus usual care, Outcome 3 Health‐related quality of life.
Telerehabilitation vs usual care: participant satisfaction with the intervention
Three studies reported outcomes related to participant satisfaction with the intervention using different scales (Boter 2004; Huijgen 2008; Lin 2014). We were unable to pool data due to the different nature of the measures used and inability to extract data suitable for pooling. One study compared post‐hospital discharge case management provided for up to six months with usual care and reported no significant differences in satisfaction with care between intervention and control groups (Boter 2004). A further study compared telerehabilitation physical therapy with in‐person physical therapy for people residing in long‐term care and found that participants in both groups reported moderately high levels of satisfaction with the intervention and there were no significant differences between groups (Lin 2014).
Comparison 2.3: Telerehabilitation vs usual care: self‐reported health‐related quality of life
Four studies reported outcomes for health‐related quality of life (Boter 2004; Mayo 2008; Rochette 2013; Saal 2015). We were able to pool three of these studies, with 569 participants, which compared post‐hospital discharge support with usual care (Mayo 2008; Rochette 2013; Saal 2015). Analysis showed similar outcomes between groups post‐intervention (SMD 0.03, 95% CI ‐0.14 to 0.20, I 2 = 5%, moderate‐quality evidence) (Analysis 2.3). We did not conduct sensitivity analysis as we considered all studies to be at risk of bias.
We were unable to pool results for the remaining study due to the way in which data were presented. Boter 2004, reported that participants in the intervention group who received a case management intervention had better scores in the domain of 'role limitations due to emotional health' on the Short Form (SF)‐36; however, no other significant differences were noted between groups.
Comparison 2.4: Telerehabilitation vs usual care: depression
Seven studies compared post‐hospital discharge support with usual care and examined the effect on depressive symptoms (Bishop 2014; Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012). We were able to pool data for six of the studies but data were not available in the necessary format for meta‐analysis from the remaining paper or study author (Bishop 2014). Analysis showed the post‐intervention effect of telerehabilitation was not greater than that of usual care when measured using tools quantifying depressive symptoms (SMD ‐0.04, 95% CI ‐0.19 to 0.11. I2 = 31%, moderate‐quality evidence) (Analysis 2.4). Removal of three studies, which we considered at risk of bias due to incomplete outcomes, did not change the result (SMD ‐0.19, 95% CI ‐0.51 to 0.13) (Boter 2004; Rochette 2013; Saal 2015). The remaining paper reported that there was no difference between groups when comparing scores from the Geriatric Depression Scale (short form) (Bishop 2014).
2.4. Analysis.
Comparison 2 Telerehabilitation versus usual care, Outcome 4 Depression.
Comparison 2.5 Telerehabilitation vs usual care: upper limb function
Three studies compared telerehabilitation with usual care and assessed upper limb function (Bizovičar 2017; Chumbler 2012; Huijgen 2008). We were unable obtain data in a suitable format for one of the studies (Huijgen 2008). This particular study reported that there were no observed differences between groups on the Action Research Arm Test or the Nine‐Hole Peg Test after intervention (Huijgen 2008). We pooled the other two studies, with 54 participants; the result suggested similar outcomes in both groups (SMD 0.33, 95% CI ‐0.21 to 0.87, I2 = 0%, low‐quality evidence) (Bizovičar 2017; Chumbler 2012).
Costs and cost‐effectiveness of telerehabilitation
No studies included in our original review reported information about the cost‐effectiveness of telerehabilitation (Laver 2013). In this updated version of the review, four studies reported information about treatment costs or service utilisation, or both (Bishop 2014; Llorens 2015; Rochette 2013; Saal 2015). Bishop 2014 found that there was a significant reduction in visits to the doctor (for the stroke survivor and caregiver combined) in those receiving the intervention at the three‐month follow‐up assessment but this was not sustained at six months. Rochette 2013 found no significant differences between groups in unplanned use of health service, and Saal 2015 found no significant differences between groups in relation to medical care (over 3 months) or health service use (over 12 months). Llorens 2015 calculated the cost of the telerehabilitation intervention per participant to be $835.61 compared to $1490.23 for the in‐clinic programme, therefore, the difference in cost between the interventions was $654.72 per participant.
Adverse events
Two studies reported information about adverse events. One study reported that no adverse events occurred (Chen 2017). The other study reported that non‐serious adverse events considered related to the study occurred in six people in the telerehabilitation group (arm and shoulder pain) and five people in the control group (fatigue and arm and shoulder pain) (Cramer 2019).
Studies comparing two different telerehabilitation interventions
Two studies included in the review compared different forms of telerehabilitation (Carey 2007; Deng 2012). Although the main aim of the studies was different, with one study aiming to improve finger and wrist movement (Carey 2007), and the other study aiming to improve ankle movement (Deng 2012), these studies were similar with regard to the method of intervention and the comparison, and were conducted by the same research group. Both studies compared a computer program that provided feedback on movement and accuracy versus a program that provided less feedback. Teleconferencing was used in both studies to enable communication with the therapist. Carey 2007 found that both groups improved on measures of hand function after intervention, with no clear difference noted between the groups. Deng 2012 reported that, after intervention, both groups exhibited an increase in dorsiflexion during gait; this was significantly greater in the group that received more feedback.
Discussion
Summary of main results
We found 22 studies (with 1937 participants) that were eligible for inclusion in this review. Because of clinical heterogeneity between studies, there were few occasions where we were able to pool data.
Independence in activities of daily living
We pooled data from two trials with 661 participants that compared a post‐hospital discharge support programme with usual care. Data from these trials showed with moderate certainty that there was no evidence of a beneficial effect of telerehabilitation when compared with usual care. However, the moderate quality of evidence identified means that further research in this area is likely to change our confidence in the estimate of effect and may change the estimate . We pooled two trials (75 participants) that compared physical therapy provided using telerehabilitation with physical therapy provided in‐person and found only low certainty that there was no difference in outcome between groups. Two additional studies assessed independence in activities of daily living after telerehabilitation interventions (Chumbler 2012; Forducey 2012); one compared telerehabilitation versus face‐to‐face therapy, and the other compared telerehabilitation versus usual care, which may or may not have included any intervention. Both studies failed to find any significant differences in outcomes between the groups post‐intervention.
Secondary outcomes
We pooled three trials with 106 participants that compared telerehabilitation and in‐person rehabilitation and examined the effect on balance (Chen 2017; Lin 2014; Llorens 2015). There was low‐quality evidence of no difference between groups suggesting that neither approach was superior. Three trials that measured quality of life found, with moderate‐quality evidence, that those who received a post‐hospital discharge support programme did not have better outcomes than those that received usual care (Mayo 2008; Rochette 2013; Saal 2015). Similarly, pooling of six studies with 1145 participants found moderate‐quality evidence that those who received a post‐hospital discharge support program did not have lower levels of depression than those that had (Boter 2004; Kirkness 2017; Mayo 2008; Rochette 2013; Saal 2015; Smith 2012).
We pooled three trials with 170 participants that aimed to retrain upper limb function using a computer program administered via telerehabilitation (Cramer 2019; Piron 2008; Piron 2009). These studies were generally small; thus, evidence was insufficient to allow conclusions on whether the intervention was more effective than the comparison upper limb therapy programme.
It was inappropriate to conduct further analyses because of heterogeneity between studies. Limited information and insufficient evidence prevented conclusions regarding the effects of telerehabilitation on mobility, participant satisfaction, or functional communication. This update of the review identified studies that reported information about adverse events and costs; however, the information presented was limited and insufficient for us to reach conclusions about these outcomes.
We were unable to find any data related to our other secondary outcomes of self‐care and domestic life or cognitive function.
Overall completeness and applicability of evidence
The previous version of this review identified 10 studies and this update of the review included 22 randomised trials demonstrating that the field is building, albeit slowly. Furthermore, we noted significant heterogeneity between the included studies with regard to the intervention used, the information and communication technologies involved, and the comparison intervention and outcomes assessed. Many studies involved small sample sizes. All studies were published over the past 15 years, demonstrating that this approach remains relatively new in rehabilitation. However, our review of the 22 trials provides information about the current state of telerehabilitation research. We also identified 22 ongoing studies, which suggests that research in this area is increasing. The quality of the evidence was low for most outcomes suggesting that further research is very likely to have an important impact on our confidence in the estimate of effect. Some comparisons and outcomes provided moderate‐quality evidence which still suggests that more research is required to provide more definitive information.
It is important to note that we excluded many trials because the intervention did not meet our predefined definition of telerehabilitation. There are now many published trials that involve a number of different methods of technology use and communication, and this approach is more likely to be offered by health services in clinical practice. For example, Van den Berg 2016 involved a caregiver‐mediated ehealth programme and video consultations to deliver a rehabilitation programme designed to improve physical function and independence. We excluded this study from our review as, although it involved telerehabilitation, there was a higher number of home visits performed by the therapist than video consultations. Guidelines from the World Health Organization suggest that digital health and non‐digital health interventions are more likely to be packaged together than delivered individually. The World Health Organization stated that digital health interventions are not a substitute for healthcare systems and that this form of service provision has significant limitations (WHO 2019).
Several studies evaluated interventions involving specialised software and hardware programs (Carey 2007; Deng 2012; Huijgen 2008; Lin 2014; Llorens 2015; Meltzer 2018; Piron 2008; Piron 2009). Although these studies provided important information regarding the effects of novel technologies, these intervention programs are not readily accessible to clinicians. As technology develops, purpose‐designed telerehabilitation systems are including more sophisticated features, such as vital sign monitoring and integration with medical records, and it is likely that these features will become more prevalent over time. In contrast, many studies that involve interventions such as counselling, goal‐setting, and case management use simpler methods of communication (such as teleconferencing or videoconferencing using readily available software). It is evident that different therapy approaches require use of different telerehabilitation systems. We found a recent emergence of studies conducted in low‐resources settings that utilise mobile phone technology to offer rehabilitation services to large numbers of people at low cost. However, these studies were either excluded due to their design or have not yet been completed (Kamwesiga 2018; Sureshkumar 2018). Future information about the efficacy of this approach has the potential to have great impact in these settings.
This updated review highlighted the growth in studies that aim to provide enhanced support for people after hospital discharge to reduce depressive symptoms following stroke. These studies offer a mix of psychosocial support, coaching, goal‐setting, education, and case management, and as such, are considered complex intervention trials. To determine the benefit or otherwise of delivery of such interventions using telerehabilitation, further studies that hold the intervention constant and change mode of delivery between groups are required.
Several of the studies in this review were primarily designed to evaluate the delivery of common rehabilitation interventions to stroke survivors via telerehabilitation (Chen 2017; Chumbler 2012; Cramer 2019; Forducey 2012; Lin 2014; Llorens 2015; Meltzer 2018; Vauth 2016). More research is required to investigate whether telerehabilitation can be used as an alternative or as a supplement to conventional therapy that is delivered face‐to‐face. Furthermore, although telerehabilitation is purported to reduce the cost of administering an intervention, none of the studies included in this review reported on cost‐effectiveness. Randomised trials are now beginning to describe the costs of telerehabilitation and compare these costs to the more expensive in‐person model of service delivery. Studies are also examining effect on health service utilisation following intervention, although there is currently insufficient evidence upon which to draw conclusions.
In addition, the studies included in this review provided little information regarding usability of information and communication technologies that are used to deliver telerehabilitation. Most studies used simple telephone or videoconferencing equipment, and few examples were provided of more complex technologies such as wearable sensors or remote monitoring or combinations of technology. Indeed, it is widely acknowledged that mixed‐methods studies are essential in this field in order to evaluate acceptability (for health professionals and healthcare recipients) as well as usability (Greenhalgh 2012).
Participants in these studies tended to be aged in their 50s, 60s, or 70s, whereas the average age of stroke is one to two decades older. It is commonly assumed that older people are less confident in using new technologies and may prefer to participate in face‐to‐face therapy. Some studies excluded patients with cognitive impairment, which may limit the transferability of this approach. None of the studies reported on participants' level of confidence or familiarity with technologies. Other research has suggested that therapists need to use a series of steps to support technology use within rehabilitation, such as positive first experiences and ongoing support (Hamilton 2018). More information is needed regarding the support required to administer telerehabilitation: whether a caregiver is required to assist, how much technology support is required, and whether the person needs to have a certain infrastructure in place (such as a high‐speed Internet connection). Studies rarely reported on these factors or how investigators dealt with issues of privacy and protection of data.
The use of technology to facilitate communication may lead to miscommunication. For example, the healthcare professional may make errors in assessment of the patient, or the patient may misunderstand advice or instructions provided by the healthcare professional. We were unable to identify any information in the included trials regarding harms associated with telerehabilitation, although one study reported that non‐serious adverse events had occurred due to pain and fatigue.
Quality of the evidence
Many studies involved small sample sizes; larger, more adequately‐powered studies are required to provide more conclusive evidence. The reporting of many studies was not consistent with the CONSORT guidelines (Schulz 2010), nor the extension which applies to equivalence trials (Piaggio 2012), and it was unclear in many cases whether studies were at risk of bias because of poor reporting and lack of clarification from study authors. In particular, in some cases, we were unable to determine whether the outcome assessor was blinded to the intervention, or whether allocation was concealed. Selective outcome reporting was apparent in several studies.
Potential biases in the review process
Our search strategy was comprehensive and included searches of clinical trial registers and the grey literature. However, with the sheer volume of citations reviewed, it is possible that we missed studies. Although we contacted the authors of included and ongoing studies, not all study authors responded. Therefore, the methodology of some studies was unclear, and we were unable to obtain some data for analyses.
We did not prespecify the outcome measurement tools that we would accept within this review. Where there were multiple measures used to measure the same outcome in a study, we used the measure which was most commonly used amongst included studies. This method of outcome selection could be open to selection bias. For future updates of the review, we will establish outcome measurements tools that we will include, as well as establish a hierarchy of measures.
Agreements and disagreements with other studies or reviews
This review identified a greater number of randomised trials than were described in previous reviews (Appleby 2019; Chen 2015; Tchero 2018). However, our conclusions are similar: despite the theoretical advantages of telerehabilitation, evidence is currently insufficient to allow conclusions on its effects.
Authors' conclusions
Implications for practice.
The finding for low or moderate‐quality evidence suggests that further research could change our estimate of the effect. However, many services have introduced telerehabilitation services as a way of offering services within limited resources and improving access for people who live long distances from rehabilitation services. Our findings suggest that telerehabilitation may not be inferior to in‐person therapy and therefore appears to be a reasonable model of service delivery for people after stroke who require rehabilitation beyond the acute or subacute phase.
Implications for research.
The potential advantages of telerehabilitation are clear and have the potential to facilitate access to services (thereby improving equity) and reduce costs associated with providing rehabilitation programmes. Therefore, more research in the form of adequately‐powered high‐quality randomised controlled trials (RCTs) is urgently required. Researchers in this area should familiarise themselves with the ongoing studies identified within this review and should address the remaining gaps, which are substantial and are detailed below.
Although we have identified a growing body of pilot and feasibility studies, additional RCTs are required to determine the effectiveness of the intervention. Researchers should ensure that studies are adequately powered, are of high methodological quality, and are reported in compliance with CONSORT guidelines (Schulz 2010). For studies intended to determine equivalence, they should comply with the CONSORT extension statement for non‐inferiority and equivalence trials (Piaggio 2012).
Telerehabilitation offers great potential as a replacement for or, as an addition to, current therapies. In the first instance, it is important to understand whether differences have been identified in delivery of the same therapy programme in‐person or via information and communication technologies. Therefore, of interest to clinicians are studies that compare telerehabilitation versus conventional therapy; that is, treatment delivered face‐to‐face, or studies that provide telerehabilitation in addition to conventional therapy.
Evaluation of cost‐effectiveness should be prioritised and incorporated into future studies. Furthermore, the use of mixed‐methods research is incredibly valuable in this field in uncovering further information about the usability of telerehabilitation technologies, participant satisfaction with the intervention, and challenges associated with recruitment of participants.
It is currently unclear which patient groups are most likely to benefit from telerehabilitation; for example, whether people living in remote areas may benefit and whether people that require enhanced support or rehabilitation on discharge or those many years post‐stroke would benefit from a short‐term programme of rehabilitation.
It is also unclear which types of therapies are best suited to telerehabilitation. Health professionals may find it difficult to adapt their practice to provide services via information and communication technologies, particularly when 'hands‐on' assessment or treatment is typically involved. It may be that some therapies that do not typically involve 'hands‐on' assessment (e.g. speech therapy or counselling) are best suited to this method of delivery.
The studies in this review identified a wide range of outcome measures. It is worth noting that trials do not necessarily have to demonstrate that telerehabilitation services result in superior outcomes in contrast to face‐to‐face therapy but rather that they result in equivalent outcomes.
The use of telerehabilitation has only recently emerged and is likely to become increasingly viable as information and communication technologies become more sophisticated and user friendly. It is important that therapists consider how their practice may be adapted so that services can be delivered remotely.
What's new
| Date | Event | Description |
|---|---|---|
| 4 July 2019 | New citation required but conclusions have not changed | The conclusions of the review have not changed. |
| 4 July 2019 | New search has been performed | We updated the searches to June 2019. We have added 12 new studies bringing the total number of included studies to 22 (involving a total of 1937 participants). We have revised the review throughout. We have added new studies to the 'studies awaiting classification' list. |
Acknowledgements
The review authors thank Josh Cheyne (2019 version) and Brenda Thomas (2013 version) for their assistance in designing the search strategy and running the searches. We also thank Cochrane editors, Peter Langhorne and Alex Pollock, and peer reviewers, Coralie English, Berber Brouns, Aryelly Rodriguez, Annabel Dawson, and Paul Davies for their input into this review and the previous version.
We thank Andrea Turolla, Nancy Mayo, Greg Smith, Han Boter, Annie Rochette, Pamela Mitchell, and Nataša Bizovičar who generously provided additional details and analyses from their trials to assist us with preparation of the review.
Appendices
Appendix 1. CENTRAL search strategy
#1MeSH descriptor: [Cerebrovascular Disorders] this term only #2MeSH descriptor: [Basal Ganglia Cerebrovascular Disease] this term only #3MeSH descriptor: [Brain Ischemia] explode all trees #4MeSH descriptor: [Carotid Artery Diseases] explode all trees #5MeSH descriptor: [Intracranial Arterial Diseases] explode all trees #6MeSH descriptor: [Intracranial Embolism and Thrombosis] explode all trees #7MeSH descriptor: [Intracranial Hemorrhages] explode all trees #8MeSH descriptor: [Stroke] explode all trees #9MeSH descriptor: [Stroke, Lacunar] this term only #10MeSH descriptor: [Vasospasm, Intracranial] this term only #11MeSH descriptor: [Vertebral Artery Dissection] this term only #12(stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH):ti,ab,kw (Word variations have been searched) #13((brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebr* or mca* or anterior circulation or basilar artery or vertebral artery) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus* or hypoxi*)):ti,ab,kw (Word variations have been searched) #14((brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid) near/5 (h?emorrhag* or h?ematoma* or bleed*)):ti,ab,kw (Word variations have been searched) #15MeSH descriptor: [Hemiplegia] this term only #16MeSH descriptor: [Paresis] explode all trees #17MeSH descriptor: [Gait Disorders, Neurologic] explode all trees #18(hemipleg* or hemipar* or paresis or paraparesis or paretic):ti,ab,kw (Word variations have been searched) #19{or #1‐#18} #20MeSH descriptor: [Telemedicine] explode all trees #21MeSH descriptor: [Telemetry] this term only #22MeSH descriptor: [Videoconferencing] explode all trees #23MeSH descriptor: [Telecommunications] this term only #24MeSH descriptor: [Remote Consultation] this term only #25MeSH descriptor: [Remote Sensing Technology] this term only #26MeSH descriptor: [Telephone] explode all trees #27MeSH descriptor: [Electronic Mail] this term only #28MeSH descriptor: [Internet] explode all trees #29MeSH descriptor: [Text Messaging] this term only #30MeSH descriptor: [Computers] this term only #31MeSH descriptor: [Microcomputers] explode all trees #32MeSH descriptor: [Minicomputers] this term only #33MeSH descriptor: [Cell Phones] explode all trees #34(telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication* or videoconference$ or video‐conferenc* or videoconsultation or video‐consultation or telestroke or teleconference* or tele‐conference* or teleconsultation or tele‐consultation or telecare or ehealth or e‐health):ti,ab,kw (Word variations have been searched) #35(telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap*):ti,ab,kw (Word variations have been searched) #36((rehabilitation or therap* or treatment or communication or consultation) near/5 (telephone* or phone* or video* or internet* or computer* or sensor* or modem or webcam or website* or email)):ti,ab,kw (Word variations have been searched) #37((remote* or distance* or distant) near/5 (rehabilitation or therap* or treatment or physio* or occupational therap* or communication or consultation or care or specialist* or monitor* or virtual reality or virtual environment* or technolog*)):ti,ab,kw (Word variations have been searched) #38((cell* or smart* or mobile or android or internet or web) near/3 (comput* or device or app* or phone)):ti,ab,kw (Word variations have been searched) #39(smartphone or text‐messag* or (tablet near/3 (device* or comput*))):ti,ab,kw (Word variations have been searched) #40(tele near/3 (game* or game* or exergame* or virtual reality*)):ti,ab,kw (Word variations have been searched) #41(mhealth or m‐health or m health or mobile health):ti,ab,kw (Word variations have been searched) #42MeSH descriptor: [Fitness Trackers] this term only #43MeSH descriptor: [Accelerometry] explode all trees #44((physical or physiolog* or perform* or fit* or train* or activ* or endur* or exercise) near/3 (track* or monitor* or measur* or device* or app*)):ti,ab,kw (Word variations have been searched) #45((step* or walk*) near/3 (count* or meter* or daily)):ti,ab,kw (Word variations have been searched) #46(pedometer* or actigraph* or acceleromet*):ti,ab,kw (Word variations have been searched) #47{or #20‐#46} #48##19 and #47
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebral small vessel diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or stroke, lacunar/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h? ematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ or exp Gait Disorders, Neurologic/ 6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw. 7. or/1‐6 8. telemedicine/ or telemetry/ or exp videoconferencing/ or telecommunications/ or computer communication networks/ or remote consultation/ or remote sensing technology/ or exp telephone/ or electronic mail/ or exp internet/ 9. computer/ or exp microcomputer/ or minicomputer/ or exp cell phone/ or mobile application/ 10. (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication$ or videoconference$ or video‐conferenc$ or videoconsultation or video‐consultation or telestroke or teleconference$ or tele‐conference$ or teleconsultation or tele‐consultation or telecare or ehealth or e‐health).tw. 11. (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap$).tw. 12. ((rehabilitation or therap$ or treatment or communication or consultation) adj5 (telephone$ or phone$ or video$ or internet$ or computer$ or sensor$ or modem or webcam or website$ or email)).tw. 13. ((remote$ or distance$ or distant) adj5 (rehabilitation or therap$ or treatment or physio$ or occupational therap$ or communication or consultation or care or specialist$ or monitor$ or virtual reality or virtual environment$ or technolog$)).tw. 14. ((cell$ or smart$ or mobile or android or internet or web) adj3 (comput$ or device or app$ or phone)).tw. 15. (smartphone or text‐messag$ or (tablet adj3 (device$ or comput$))).tw. 16. (mhealth or m‐health or m health or mobile health).tw. 17. activity tracker/ or exp accelerometry/ 18. ((physical or physiolog$ or perform$ or fit$ or train$ or activ$ or endur$ or exercise) adj3 (track$ or monitor$ or measur$ or device$ or app$)).tw. 19. ((step$ or walk$) adj3 (count$ or meter$ or daily)).tw. 20. (pedometer$ or actigraph$ or acceleromet$).tw. 21. or/8‐20 22. Randomized Controlled Trials as Topic/ 23. Random Allocation/ 24. Controlled Clinical Trials as Topic/ 25. control groups/ 26. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/ 27. double‐blind method/ 28. single‐blind method/ 29. Placebos/ 30. placebo effect/ 31. cross‐over studies/ 32. randomized controlled trial.pt. 33. controlled clinical trial.pt. 34. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt. 35. (random$ or RCT or RCTs).tw. 36. (controlled adj5 (trial$ or stud$)).tw. 37. (clinical$ adj5 trial$).tw. 38. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 39. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 40. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 41. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 42. (cross‐over or cross over or crossover).tw. 43. (placebo$ or sham).tw. 44. trial.ti. 45. (assign$ or allocat$).tw. 46. controls.tw. 47. or/22‐46 48. 7 and 21 and 47
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. exp hemiplegia/ or exp paresis/ or neurologic gait disorder/ 6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw. 7. or/1‐6 8. telehealth/ or exp telemedicine/ or telenursing/ or exp telemetry/ or telephone/ or telecommunication/ or teleconsultation/ or telephone interview/ or videoconferencing/ or videorecording/ 9. remote sensing/ or e‐mail/ or text messaging/ or internet/ or wireless communication/ or mobile application/ or exp mobile phone/ or tablet/ 10. computer/ or computer system/ or microcomputer/ or minicomputer/ or personal computer/ or personal digital assistant/ 11. (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication$ or videoconference$ or video‐conferenc$ or videoconsultation or video‐consultation or telestroke or teleconference$ or tele‐conference$ or teleconsultation or tele‐consultation or telecare or ehealth or e‐health).tw. 12. (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap$).tw. 13. ((rehabilitation or therap$ or treatment or communication or consultation) adj5 (telephone$ or phone$ or video$ or internet$ or computer$ or sensor$ or modem or webcam or website$ or email)).tw. 14. ((remote$ or distance$ or distant) adj5 (rehabilitation or therap$ or treatment or physio$ or occupational therap$ or communication or consultation or care or specialist$ or monitor$ or virtual reality or virtual environment$ or technolog$)).tw. 15. (smartphone or text‐messag$ or textmessag$ or sms or (tablet adj3 (device$ or comput$))).tw. 16. ((cell$ or smart$ or mobile or android or internet or web) adj3 (comput$ or device or app$ or phone)).tw. 17. (tele adj3 (game$ or game$ or exergame$ or virtual reality$)).tw. 18. (mhealth or m‐health or m health or mobile health).tw. 19. accelerometer/ or accelerometry/ or actimetry/ or pedometer/ 20. ((physical or physiolog$ or perform$ or fit$ or train$ or activ$ or endur$ or exercise) adj3 (track$ or monitor$ or measur$ or device$ or app$)).tw. 21. ((step$ or walk$) adj3 (count$ or meter$ or daily)).tw. 22. (pedometer$ or actigraph$ or acceleromet$).tw. 23. or/8‐22 24. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/ 25. Randomization/ 26. Controlled clinical trial/ or "controlled clinical trial (topic)"/ 27. control group/ or controlled study/ 28. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ 29. Crossover Procedure/ 30. Double Blind Procedure/ 31. Single Blind Procedure/ or triple blind procedure/ 32. placebo/ or placebo effect/ 33. (random$ or RCT or RCTs).tw. 34. (controlled adj5 (trial$ or stud$)).tw. 35. (clinical$ adj5 trial$).tw. 36. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 37. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 38. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 39. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 40. (cross‐over or cross over or crossover).tw. 41. (placebo$ or sham).tw. 42. trial.ti. 43. (assign$ or allocat$).tw. 44. controls.tw. 45. or/24‐44 46. 7 and 23 and 45
Appendix 4. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paraparesis or paretic).tw. 7. or/1‐6 8. telemedicine/ or telephone/ 9. telecommunications/ 10. computer systems/ or exp computers/ or internet/ or virtual reality/ 11. (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication$ or videoconference$ or video‐conferenc$ or videoconsultation or video‐consultation or telestroke or teleconference$ or tele‐conference$ or teleconsultation or tele‐consultation or telecare or ehealth or e‐health).tw. 12. (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap$).tw. 13. ((rehabilitation or therap$ or treatment or communication or consultation) adj5 (telephone$ or phone$ or video$ or internet$ or computer$ or sensor$ or modem or webcam or website$ or email)).tw. 14. ((remote$ or distance$ or distant) adj5 (rehabilitation or therap$ or treatment or physio$ or occupational therap$ or communication or consultation or care or specialist$ or monitor$ or virtual reality or virtual environment$ or technolog$)).tw. 15. ((cell$ or smart$ or mobile or android or internet or web) adj3 (comput$ or device or app$ or phone)).tw. 16. (smartphone or text‐messag$ or (tablet adj3 (device$ or comput$))).tw. 17. (tele adj3 (game$ or game$ or exergame$ or virtual reality$)).tw. 18. (mhealth or m‐health or m health or mobile health).tw. 19. ((physical or physiolog$ or perform$ or fit$ or train$ or activ$ or endur$ or exercise) adj3 (track$ or monitor$ or measur$ or device$ or app$)).tw. 20. ((step$ or walk$) adj3 (count$ or meter$ or daily)).tw. 21. (pedometer$ or actigraph$ or acceleromet$).tw. 22. or/8‐21 23. 7 and 22
Appendix 5. CINAHL search strategy
S1(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR ( (MH "Intracranial Embolism and Thrombosis") ) OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") OR (MH "Stroke Patients") OR (MH "Stroke Units") S2TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH) S3TI ( brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) S4AB ( brain* or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) N5 AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus*) S5TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S6AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) N5 AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S7(MH "Hemiplegia") or (MH "Gait Disorders, Neurologic+") S8TI ( (hemipleg* or hemipar* or paresis or paretic) ) OR AB ( (hemipleg* or hemipar* or paresis or paretic) ) S9S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10(MH "Telehealth") OR (MH "Telemedicine+") OR (MH "Telerehabilitation") OR (MH "Remote Consultation") OR (MH "Telenursing") OR (MH "Telemetry") S11(MH "Electronic Mail") OR (MH "Instant Messaging") OR (MH "Internet+") OR (MH "Teleconferencing") OR (MH "Telephone+") OR (MH "Videoconferencing+") OR (MH "Remote Consultation") OR (MH "Text Messaging") OR (MH "Microcomputers") OR (MH "Computers, Portable") OR (MH "Computers, Hand‐Held+") OR (MH "Minicomputers") OR (MH "Mobile Applications") S12TI ( (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication* or videoconference* or video‐conferenc* or videoconsultation or video‐consultation or telestroke or teleconference* or tele‐conference* or teleconsultation or tele‐consultation or telecare or ehealth or e‐health) ) OR AB ( (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication* or videoconference* or video‐conferenc* or videoconsultation or video‐consultation or telestroke or teleconference* or tele‐conference* or teleconsultation or tele‐consultation or telecare or ehealth or e‐health) ) S13TI ( (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap*) ) OR AB ( (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap*) ) S14TI ( ((rehabilitation or therap* or treatment or communication or consultation) N5 (telephone* or phone* or video* or internet* or computer* or sensor* or modem or webcam or website* or email)) ) OR AB ( ((rehabilitation or therap* or treatment or communication or consultation) N5 (telephone* or phone* or video* or internet* or computer* or sensor* or modem or webcam or website* or email)) ) S15TI ( ((remote* or distance* or distant) N5 (rehabilitation or therap* or treatment or physio* or occupational therap* or communication or consultation or care or specialist* or monitor* or virtual reality or virtual environment* or technolog*)) ) OR AB ( ((remote* or distance* or distant) N5 (rehabilitation or therap* or treatment or physio* or occupational therap* or communication or consultation or care or specialist* or monitor* or virtual reality or virtual environment* or technolog*)) ) S16TI ( ((cell* or smart* or mobile or android or internet or web) N3 (comput* or device or app* or phone)) ) OR AB ( ((cell* or smart* or mobile or android or internet or web) N3 (comput* or device or app* or phone)) ) S17TI ( (smartphone or text‐messag* or (tablet N3 (device* or comput*))) ) OR AB ( (smartphone or text‐messag* or (tablet N3 (device* or comput*))) ) S18TI ( (tele N3 (game* or game* or exergame* or virtual reality*)) ) OR AB ( (tele N3 (game* or game* or exergame* or virtual reality*)) ) S19TI ( (mhealth or m‐health or m health or mobile health) ) OR AB ( (mhealth or m‐health or m health or mobile health) ) S20(MH "Accelerometry+") S21TI ( ((physical or physiolog* or perform* or fit* or train* or activ* or endur* or exercise) N3 (track* or monitor* or measur* or device* or app*)) ) OR AB ( ((physical or physiolog* or perform* or fit* or train* or activ* or endur* or exercise) N3 (track* or monitor* or measur* or device* or app*)) ) S22TI ( (pedometer* or actigraph* or acceleromet*) ) OR AB ( (pedometer* or actigraph* or acceleromet*) ) S23S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 S24(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+") S25(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials") S26(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies") S27(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect") S28(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies") S29PT (clinical trial or randomized controlled trial) S30TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs) S31TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*)) S32TI (clinical* N5 trial*) or AB (clinical* N5 trial*) S33TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) S34S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S35S9 AND S23 AND S34
Appendix 6. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke$ or post stroke or poststroke or post‐stroke or apoplex$ or cerebral vasc$ or cerebrovasc$ or cva or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebr$ or mca$ or anterior circulation or basilar artery or vertebral artery) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. or/1‐6 8. telemedicine/ or teleconferencing/ or exp telemetry/ 9. internet/ or blog/ or online therapy/ or exp social media/ or exp websites/ or exp mobile devices/ or exp electronic communication/ or exp technology/ or text messaging/ 10. computer applications/ or computer assisted instruction/ or computer assisted therapy/ or computer simulation/ or electronic learning/ or computers/ or microcomputers/ or online therapy/ or virtual reality/ 11. (telemedicine or telemetry or telerehabilitation or tele‐rehabilitation or telerehab or telehealth or tele‐health or telehomecare or tele‐homecare or telecoaching or tele‐coaching or telecommunication$ or videoconference$ or video‐conferenc$ or videoconsultation or video‐consultation or telestroke or teleconference$ or tele‐conference$ or teleconsultation or tele‐consultation or telecare or ehealth or e‐health).tw. 12. (telespeech or tele‐speech or teleOT or tele‐OT or telepractice or teletherap$).tw. 13. ((rehabilitation or therap$ or treatment or communication or consultation) adj5 (telephone$ or phone$ or video$ or internet$ or computer$ or sensor$ or modem or webcam or website$ or email)).tw. 14. ((remote$ or distance$ or distant) adj5 (rehabilitation or therap$ or treatment or physio$ or occupational therap$ or communication or consultation or care or specialist$ or monitor$ or virtual reality or virtual environment$ or technolog$)).tw. 15. ((cell$ or smart$ or mobile or android or internet or web) adj3 (comput$ or device or app$ or phone)).tw. 16. (smartphone or text‐messag$ or (tablet adj3 (device$ or comput$))).tw. 17. (tele adj3 (game$ or game$ or exergame$ or virtual reality$)).tw. 18. (mhealth or m‐health or m health or mobile health).tw. 19. ((physical or physiolog$ or perform$ or fit$ or train$ or activ$ or endur$ or exercise) adj3 (track$ or monitor$ or measur$ or device$ or app$)).tw. 20. ((step$ or walk$) adj3 (count$ or meter$ or daily)).tw. 21. (pedometer$ or actigraph$ or acceleromet$).tw. 22. or/8‐21 23. clinical trials/ or treatment effectiveness evaluation/ or placebo/ 24. (random$ or RCT or RCTs).tw. 25. (controlled adj5 (trial$ or stud$)).tw. 26. (clinical$ adj5 trial$).tw. 27. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 28. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 29. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 30. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 31. (cross‐over or cross over or crossover).tw. 32. (placebo$ or sham).tw. 33. trial.ti. 34. (assign$ or allocat$).tw. 35. controls.tw. 36. or/23‐35 37. 7 and 22 and 36
Appendix 7. PsycBITE search strategy
keyword search: tele AND stroke
Method: Randomised controlled trials
Appendix 8. OT Seeker search strategy
search terms: tele AND stroke AND random (in any field)
Appendix 9. PEDRO search strategy
tele (in asbtract or title) AND clinical trial (method) AND neurology (subdiscipline)
Appendix 10. REHABDATA search strategy
tele AND stroke AND random (in abstract)
Appendix 11. Health Technology Assessment Database
tele AND stroke AND random (any field)
Appendix 12. Search of clinical trial registers
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) telerehabilitation OR telemedicine OR telehealth OR activity tracker | Interventional Studies | Brain Infarction OR Intracranial Hemorrhages OR Carotid Artery Diseases OR Brain Ischemia OR Cerebral Hemorrhage OR Cerebrovascular Disorders OR Stroke World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) stroke AND tele‐rehabilitation or telerehabilitation stroke AND telehealth stroke AND telemedicine
Appendix 13. ProQuest Dissertations and Theses Global search strategy
telerehabilitation OR telehealth OR telemedicine (abstract)
AND stroke (abstract)
AND trial OR random (abstract)
Data and analyses
Comparison 1. Telerehabilitation versus in‐person rehabilitation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.59 [‐5.50, 6.68] |
| 2 Balance | 3 | 106 | Mean Difference (IV, Random, 95% CI) | 0.48 [‐1.36, 2.32] |
| 3 Upper limb function | 3 | 170 | Mean Difference (IV, Random, 95% CI) | 1.23 [‐2.17, 4.64] |
| 4 Functional communication | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.52, 4.72] |
Comparison 2. Telerehabilitation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activities of daily living | 2 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.15, 0.15] |
| 2 Mobility | 1 | 190 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.12, 0.14] |
| 3 Health‐related quality of life | 3 | 569 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.14, 0.20] |
| 4 Depression | 6 | 1145 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.11] |
| 5 Upper limb function | 2 | 54 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.21, 0.87] |
2.2. Analysis.
Comparison 2 Telerehabilitation versus usual care, Outcome 2 Mobility.
2.5. Analysis.
Comparison 2 Telerehabilitation versus usual care, Outcome 5 Upper limb function.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bishop 2014.
| Methods | RCT | |
| Participants | Recruited from University Medical Centre in the USA Inclusion criteria: fully oriented; able to follow a 3‐step command; evidence of stroke on neuroimaging or hemiparetic. Caregivers were defined as family or friends living with survivors or within a 30‐minute driving distance and acting as the primary source of assistance for survivors. Exclusion criteria: < 35 years old; subarachnoid haemorrhage; psychosis; lack of a caregiver; admission from a nursing home; non‐English speaking Age, years: mean (SD) 70.1 (11.6) Gender: 35% men Time post‐stroke: not reported but conducted on discharge home from hospital |
|
| Interventions | Telerehabilitation intervention: Family Intervention Telephone Tracking (FITT) which focuses on 5 key areas: (1) family functioning, (2) mood, (3) neurocognitive functioning, (4) functional independence, and (5) physical health. Telephone contacts took place during the 6‐month transition period after discharge from an acute care setting, with the FITT intervention formally beginning when stroke survivors arrived home. FITT contacts occurred weekly for 6 weeks, biweekly for the next 2 months, and then monthly for 2 months, for a total of 13 calls to each individual (26 calls per dyad). Calls were made by clinicians who came from different professional backgrounds (medical practitioner, nurse, and family therapist) Control intervention: usual care |
|
| Outcomes | Timing of outcome assessment: baseline, 3 months, 6 months Measures: healthcare utilisation (direct report), Frenchay Activities Index; Geriatric Depression Scale; Family Assessment Device; Perceived Criticism Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used urn randomisation |
| Allocation concealment (selection bias) | Unclear risk | Detail not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details of dropouts not clearly reported |
| Selective reporting (reporting bias) | Unclear risk | No mention of protocol or trial registration |
| Other bias | Low risk | No other bias noted |
Bizovičar 2017.
| Methods | RCT | |
| Participants | Recruited from patients discharged from inpatient stroke rehabilitation in Slovenia Inclusion criteria: stroke, requiring help with ADLs (FIM score 40 to 80) Exclusion criteria: orthopaedic problems, other neurological diseases and severe health complications that would prevent participation Age: intervention group mean 70, control group mean 63 Gender: intervention group 60% men, control group 40% men Time post‐stroke: intervention group mean 8.2 months, control group mean 5.1 months |
|
| Interventions | Telerehabilitation: participants were taught how to use a computer tablet and access selected videos on a web portal. Training focused on posture and exercises for the neck, shoulders, torso, and upper limbs. The participant was asked to do exercises daily 3 months after discharge from the rehabilitation setting. Therapists interviewed the participant and relatives once a week during which they checked adherence to exercises, answered questions, monitored progress, and adjusted the content of the exercise programme, as required. Control intervention: classified as usual care. Were provided with oral and written instructions for similar exercises. The person was instructed to do the exercises of their choice and abilities 1 to 2 times per day. |
|
| Outcomes | Timing of outcome assessment: baseline and 3 months after randomisation Measures: joint flexibility, Modified Ashworth Scale, Visual Analogue Scale for Pain Assessment, Motor Assessment Scale, Wolf Motor Function Test, Fugl Meyer Assessment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unable to determine |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about whether or not there were withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration available |
| Other bias | Low risk | None noted |
Boter 2004.
| Methods | RCT | |
| Participants | Recruited from 12 hospitals in the Netherlands Inclusion criteria: Dutch speaking, ≥ 18 years of age, first admission for a stroke, hospitalisation within 72 hours after onset of symptoms, life expectancy > 1 year, independent from or partially dependent on discharge (Rankin grade 0 to 3), discharged home, residence within 40 kilometres of catchment areas served by hospitals Exclusion criteria: failure to meet above criteria Age, years: intervention group median (IQR) = 66 (52 to 76), control group median (IQR) = 63 (51 to 74) Gender: intervention group 49% men, control group 48% men Time post‐stroke: not reported |
|
| Interventions | Telerehabilitation intervention: 3 nurses initiated telephone contacts (1 to 4; 4 to 8; and 18 to 24 weeks after discharge) and visits to participants in their homes (10 to 14 weeks after discharge). Stroke nurses used a standardised checklist of risk factors for stroke, consequences of stroke and unmet needs for services. Nurses supported participants and caregivers according to their individual needs (e.g. by providing information or reassurance) or advised participants to contact their GP when further follow‐up was required. Written educational material was provided and discussed. Nurses aimed to support participants and caregivers in solving problems themselves or coping with them rather than solving problems for them. Control intervention: standard care |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention (6 months after discharge) Measures: Barthel Index, Rankin Grade, Satisfaction with Stroke Care questionnaire, SF‐36, Hospital Anxiety and Depression Scale, health‐service utilisation (GP), readmissions, therapy, activities of daily living care, rehabilitation, aids, secondary prevention drugs, caregiver questionnaires |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised programme |
| Allocation concealment (selection bias) | Low risk | Central telephone service used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Additional data collected at 6 months and not reported in the paper |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unable to identify further bias |
Carey 2007.
| Methods | RCT | |
| Participants | Recruited from the community via advertising in a local paper and local stroke support group meetings in the USA Inclusion criteria: more than 12 months post‐stroke, between 30 and 80 years old, satisfactory corrected vision to recognise the full tracking target and cursor movement, ≥ 90 degrees of passive extension‐flexion movement at the index finger metacarpophalangeal joint of the paretic hand (no contracture) and at least 10 degrees of active movement at this joint Exclusion criteria: unable to undergo fMRI, pregnancy, or claustrophobia Age, years: intervention group (Track) mean = 65.9 (SD 7.4), intervention group (Move) mean = 67.4 (SD 11.8) Gender: intervention group (Track) 90% men, intervention group (Move) 60% men Time post‐stroke: intervention group (Track) mean 42.5 months (SD 24.3), intervention group (Move) mean 35.6 months (SD 26.1) |
|
| Interventions | Both groups received telerehabilitation. The aim of the intervention was to practice finger and wrist movements. Training was completed on a laptop using customised tracking software without direct supervision by the therapist. Both groups performed 180 tracking trials per day for 10 days. Regular teleconferencing (mobile phone and Webcam operating over the Internet) occurred between therapist and participant. Telerehabilitation intervention (Track group): tracking software provided feedback and an accuracy score Telerehabilitation intervention (Move group): tracking software showed a sweeping cursor representing movement, however did not provide the target or response or an accuracy score |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention Measures: Box and Block test, Jebsen Taylor test, finger ROM, finger movement tracking test, fMRI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial loss of participants at follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Small sample size and considerable differences between groups in mean values on some outcome measures at baseline, although these differences were not statistically significant |
Chen 2017.
| Methods | RCT | |
| Participants | Recruited from a Hospital in Shanghai, China Inclusion criteria: aged 35 to 85 years old; first diagnosis was ischaemic or haemorrhagic stroke or recurrent stroke but without hemiplegia symptoms before; have a symptom of hemiplegia, left or right; 14 to 90 days from stroke onset; National Institute of Health Stroke Scale scores from 2 to 20 and mRS scores from 1 to 5; have not previously received any rehabilitation intervention since this stroke onset Exclusion criteria: Glasgow Coma Scale scores under 15, have been confirmed as having dementia based on Mini Mental State Examination assessment, with mental disorders and unable to cooperate with examination, treatment or follow‐up; disability not induced by stroke or disability induced by historical stroke; associated severe primary disease of heart, liver, kidney, or haematological system; cognitive disorder, history of psychosis, substance abuse, or alcoholism; skin infections in the areas of surface electrodes attached; metal implants in the body, including cardiac pacemaker, metal stent, or steel plate; in the gestation or lactation period or have a fertility plan; associated malignant tumour or severe progressive disease in any other system; have been recruited by any other clinical trial in the preceding 90 days; unable to complete the basic course of treatment, with poor treatment adherence or inability to follow‐up Age, years: intervention group mean 66.5 SD (12.1), control group mean 66.2 SD (12.3) Gender: 67% men Time post‐stroke: intervention group mean (SD) 25.0 (5.6) days, control group 26.9 (4.7) days |
|
| Interventions | After discharge, participants in both groups were given physical exercises and electromyography‐triggered neuromuscular stimulation (ETNS). Exercises were conducted for 1 hour, twice in a working day for 12 weeks (total = 60 sessions). ETNS was conducted by using a portable muscle electricity biofeedback instrument for 20 minutes, twice in a working day for 12 weeks, a total of 60 sessions. Telerehabilitation intervention: Individualised telerehabilitation physical exercise plan selected by treating therapists and provided as prescription within the telerehabilitation apparatus. Therapists explained and demonstrated exercises. After discharge, participants received rehabilitation via the telerehabilitation system; therapists supervised via live video and collected data remotely. Therapists were available for advice if needed. Carers kept training logs of training. Control intervention: received rehabilitation in the outpatient therapy department. Exercises and ETNS were the same but the therapy was provided face‐to‐face with therapists. |
|
| Outcomes | Timing of outcome assessment: baseline, 12, and 24 weeks after randomisation Measures: Modified Barthel Index; Berg Balance Scale; mRS; Caregiver Strain Index; Root Mean Square |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Held in opaque sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few withdrawals, even across groups, and reasons reported |
| Selective reporting (reporting bias) | Low risk | Trial registered and all outcomes reported |
| Other bias | Low risk | No other sources of bias noted |
Chumbler 2012.
| Methods | RCT | |
| Participants | Recruited from 3 Veterans Affairs Medical Centres in the USA Inclusion criteria: ischaemic or haemorrhagic stroke within the previous 24 months; participants aged 45 to 90 years, discharged to the community, not cognitively impaired (no more than 4 errors on the Short Portable Mental Status Questionnaire), able to follow a 3‐step command, discharge motor Functional Independence Measure score of 18 to 88, approval by participants and physician; signed medical media release form Exclusion criteria: failure to meet above criteria Age, years: intervention group: mean = 67.1 (SD 9.5), control group: mean = 67.7 (SD 10) Gender: intervention group: 96% men; control group: 100% men Time post‐stroke: intervention group median 26 days, control group median 74 days |
|
| Interventions | Telerehabilitation intervention: the purpose of the intervention was to improve the participant's functional mobility. Intervention included 3 tele‐visits, use of an in‐home messaging device (IHMD) and 5 telephone calls over a 3‐month period. The tele‐visits involved assessment of physical function, goal‐setting and demonstration of exercises; a research assistant used a camcorder to record the home environment and the participant completing tests of physical and functional performance that were later reviewed by the teletherapist. The therapist asked the participant questions via the IHMD and provided positive encouragement to maximise exercise adherence. Telephone calls were used to problem‐solve any barriers to exercise and to review and advance the exercise programmes. Control intervention: usual care |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention (3 months) and 6 months Measures: motor subscale of the Functional Independence Measure (telephone version), Late Life Function and Disability Instrument, stroke‐specific participant satisfaction with care questionnaire, Falls Self‐Efficacy Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Centralised computer program |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses completed. Small numbers of missing data, which were explained and balanced across groups |
| Selective reporting (reporting bias) | High risk | The publication does not present the results for all outcome measures listed in the study protocol. |
| Other bias | Unclear risk | Unable to identify further bias |
Cramer 2019.
| Methods | RCT | |
| Participants | Recruited from: 11 sites in the USA Inclusion criteria: age ≥ 18 years, stroke onset 4 to 36 weeks prior, arm motor Fugl‐Meyer score 22‐56 (out of 66) Exclusion criteria: major active coexistent neurological or psychiatric disease; severe depression, cognitive impairment (MoCA < 22), communication deficits interfering with participation, life expectancy < 6 months, non‐English speaking, unable to perform the 3 rehabilitation exercise test examples Age, years: intervention group mean age 62 (14), control group 60 (13) Gender: 73% men Time post‐stroke: intervention group mean 132 (65) days, control group 129 (59) days |
|
| Interventions | Participants in both groups were offered 36 sessions (18 supervised, 18 unsupervised) lasting for 70 minutes each over 6 to 8 weeks. All participants signed a behavioural contract that included a treatment goal and treatment was based on an upper extremity task‐specific training manual and accelerated skill acquisition programme. Telerehabilitation intervention: rehabilitation treatment sessions via an in‐home internet‐connected computer. The participant performed daily assigned home‐based telerehabilitation exercises and functional training (including use of games and input devices such as PlayStation Move Controller) and 5 minutes of stroke education, all guided by the telerehabilitation system. During half of the sessions, therapists initiated a video conference with the participant's telerehabilitation system to discuss progress, issues, and revise treatment plans as needed. Control intervention: same intensity, duration, and frequency of therapy and stroke education content but provided in clinic with therapist feedback based on observations on supervised days |
|
| Outcomes | Timing of outcome assessment: baseline, 30 days after randomisation Measures: Fugl‐Meyer Arm, Box and Block test, Stroke Impact Scale‐Hand Domain |
|
| Notes | NCT02360488 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule developed at the StrokeNet National Data Management Centre |
| Allocation concealment (selection bias) | Low risk | Web‐based central randomisation system |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of withdrawals and balanced across groups |
| Selective reporting (reporting bias) | Low risk | Trial registered |
| Other bias | Low risk | None noted |
Deng 2012.
| Methods | RCT | |
| Participants | Recruited from the community. Study conducted in the USA Inclusion criteria: post‐stroke duration of at least 5 months, at least 10 degrees of active dorsiflexion/plantar flexion at the paretic ankle, ability to understand the tasks, ability to ambulate 30 metres Exclusion criteria: indwelling devices incompatible with MRI Age, years: telerehabilitation (Track) group mean = 51.4 (SD 11.5), telerehabilitation (Move) group mean = 58 (SD 13.4) Gender: Track group 38% men; Move group 100% men Time post‐stroke: Track group median 66 months; Move group median 16.5 months |
|
| Interventions | Both groups received telerehabilitation. The aim of the intervention was to practice ankle movements. Training was completed on a laptop using customised tracking software without direct supervision by the therapist. Both groups performed 180 repetitions for 20 days. Regular teleconferencing using Skype occurred between the therapist and the participant, and the computer automatically emailed daily records to the laboratory computer to allow monitoring of performance. Telerehabilitation intervention (Track group): tracking software provided feedback and an accuracy score. Telerehabilitation intervention (Move group): tracking software showed a sweeping cursor representing the movement; however, did not provide the target or response or an accuracy score. |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention Measures: gait analysis, 10‐metre walk test, fMRI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronically‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not clearly reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition reported with reasons and similarities between groups |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | High risk | Small sample size |
Forducey 2012.
| Methods | RCT | |
| Participants | Recruitment details unclear. Study took place in the USA. Inclusion criteria: first time medical diagnosis of acute stroke, onset of stroke was at 6 or fewer months, Medicare or Blue Cross and Blue Shield insurance coverage, moderate deficits in the areas of self‐care, functional mobility, transfers as documented by the Functional Independence Measure, caregiver present to set up telehealth videophone device Exclusion criteria: aphasia or major depressive disorder, as measured by the Beck Depression Inventory II Age, years: mean age of all participants was 60 Gender: 55% men Time post‐stroke: not reported |
|
| Interventions | Telerehabilitation intervention: 12 treatment sessions (6 occupational therapy and 6 physiotherapy) were provided over approximately 6 weeks. Interventions included education, retraining of self‐care, functional mobility and posture, home modifications and therapy to improve function in impaired limbs. Communication between therapist and participant occurred via a desktop videophone using standard telephone lines Control intervention: included the same content; however, was delivered in‐person |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention Measures: Functional Independence Measure, SF‐12 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lack of detail in reporting the results |
| Selective reporting (reporting bias) | Unclear risk | Not able to access protocol |
| Other bias | High risk | Small sample size |
Huijgen 2008.
| Methods | RCT | |
| Participants | Recruited from a rehabilitation service in the Netherlands Inclusion criteria: age > 18 years; established diagnosis of multiple sclerosis, stroke or traumatic brain injury; taking more than 25 seconds to perform the Nine‐Hole Peg Test, ability to move at least 1 peg in 180 seconds during the Nine‐Hole Peg Test, sufficient autonomous functioning, Internet connection or telephone line and reachable Internet provider, stable clinical status, living at home Exclusion criteria: disturbed upper limb function not related to multiple sclerosis, traumatic brain injury or stroke; serious cognitive and/or behavioural problems, major visual problems, communication problems, medical complications; other problems, possibly contraindicating autonomous exercise at home Age, years: telerehabilitation group mean = 69 (SD 8), control group mean = 71 (SD 7) Gender: telerehabilitation group 18% men, control group 80% men Time post‐stroke: telerehabilitation group mean 3 (SD 2) years, control group mean 1.8 (SD 0.8) years |
|
| Interventions | Telerehabilitation intervention: 1 month of usual care followed by approximately 4 training sessions with the Home Care Activity Device (HCAD) system in the hospital and intervention using the HCAD for 1 month. The system comprised a hospital‐based server and the portable unit installed at the participant's home. The portable unit consisted of 7 sensorised tools; a key, a light bulb, a book, a jar, writing, checkers and keyboard. The unit also had 2 webcams that allowed videoconferencing and recording. It was recommended that participants use the HCAD at least 5 days per week for 30 minutes. Control intervention: usual care and generic exercises prescribed by the physician |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention Measures: Barthel Index, participant satisfaction assessed using visual analogue scale, SF‐36, Action Research Arm Test, Nine‐Hole Peg Test, Wolf Motor Function Test, grip strength, Abilhand |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation scheme generated using 2:1 allocation ratio. Participants allocated to the study when the intervention was available |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts were reported and ITT analyses conducted |
| Selective reporting (reporting bias) | High risk | Some study data not reported in the published paper |
| Other bias | High risk | Small sample size Differences between groups at baseline |
Kirkness 2017.
| Methods | RCT | |
| Participants | Recruited from six hospitals in the USA Inclusion criteria: within 4 months of an ischaemic or haemorrhagic stroke (verified by CT or MRI) with clinical depression (≥ 11 on the Geriatric Depression Scale) Age, years: intervention (telephone) group mean 61.7, intervention group (in‐person) 58.5, control group 60.7 Gender: 50% men Time post‐stroke: not reported |
|
| Interventions | Telerehabilitation intervention: 'Living Well With Stroke 2 intervention': 1 in‐person orientation session with the psychosocial nurse practitioner therapist, either in their home or at the study offices. They received the participant manuals and discussed goals and expectations. Following the in‐person orientation session, each of the subsequent 6 sessions occurred by telephone. Topics were as follows: (1) introduction to behavioural therapy for depression after stroke, pleasant events; (2) scheduling pleasant events: problems and planning; (3) managing depression behaviours: problem‐solving techniques; (4) changing negative thoughts and behaviours; (5) problem‐solving in depth; (6) review of skills, generalisation and strategies for maintenance of skills. Session length ranged from 10 to 80 minutes, with the telephone sessions somewhat shorter than the in‐person ones (average 26 minutes versus 38 minutes). Participants in the intervention arms saw their primary care or stroke provider for stroke follow‐up care and were provided antidepressants as prescribed by their providers. In‐person intervention: same 'Living Well With Stroke 2' intervention but provided in‐person (usually in the participant's home) Control intervention: usual care |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention (8 weeks), 21 weeks, and 12 months Measures: Hamilton Rating Scale for Depression, Stroke Impact Scale and perceived recovery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised procedure using modified version of the minimisation method |
| Allocation concealment (selection bias) | Low risk | Managed online by research nurses and statistician |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of withdrawals; balanced across groups and explained clearly |
| Selective reporting (reporting bias) | Unclear risk | Trial registered ahead of time on clinical trial register. Published paper did not present results of Stroke Impact Scale or perceived recovery. |
| Other bias | Low risk | No other sources of bias noted |
Lin 2014.
| Methods | RCT | |
| Participants | Recruited from 3 long‐term care facilities (LTCFs) in Taiwan Inclusion criteria: history of cerebral vascular accident (including first and recurrent stroke) for more than 6 months; living in LTCFs for more than 3 months; having active movement of the proximal part of upper extremity in the hemiparetic side (Brunnstrom stage U/E ≥ 3); being able to sit for short periods without hand support for at least 30 seconds; having cognitive status screened using the Mini‐Cog test and being able to follow the instruction; and being able to communicate and follow a 3‐step command Exclusion criteria: having other neuromusculoskeletal condition and systemic diseases such as Parkinson's disease and uncontrolled heart disease; blindness and deafness; and having a psychiatric history Age, years: intervention group mean 74.6 (SE 2.3), control group 75.6 (SE 3.4) Gender: 71% men Time post‐stroke: not reported |
|
| Interventions | The treatment programme for both groups included 3 sessions of training per week for 4 weeks, with the duration of approximately 50 minutes for each session. The therapist instructed standing balance training from easy to difficult, depending on the severity and recovery of the participants. Telerehabilitation intervention: the tele‐balance training focused on 10 minutes of standing exercise according to 3D animation exercise videos and about 10 minutes of 3D interactive games with finger touching the touch screen in standing posture. 1 therapist conducted the telerehabilitation balance training at the therapist end to each facility for 1 month, separately. 1 volunteer or non‐medical person was assigned at the patient end for safety and assistance in telerehabilitation and conventional training. Control intervention: 2 post‐stroke participants attended the same session as the small therapy group. The therapist conducted conventional balance training programs following simple to complex principles. |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention Measures: Berg Balance Scale; Barthel Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer number generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear, details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one dropout and reason explained. Intention‐to‐treat analysis conducted |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported for all outcomes assessed in paper; however trial not registered and no protocol available |
| Other bias | Low risk | No other sources of bias noted |
Llorens 2015.
| Methods | RCT | |
| Participants | Recruited from outpatients of the neurorehabilitation unit of a large metropolitan hospital in Spain Inclusion criteria: age ≥ 40 and ≤ 75 years; chronicity > 6 months; Brunel Balance Assessment (BBA): section 3, levels 7 to 12; Mini Mental State Examination score > 23; and Internet access in their homes Exclusion criteria: individuals with severe aphasia (Mississippi Aphasia Screening Test cut‐off score < 45); individuals with hemispatial neglect; and individuals with ataxia or any other cerebellar symptom Age, years: intervention group mean 55.47 (SD 9.63), control group man 55.6 (7.29) Gender: intervention group 67% men, control group 47% men Time post‐stroke: intervention group mean 334 (60 days), control group 317 (49 days) |
|
| Interventions | All the participants underwent 20 x 45‐minute training sessions with the telerehabilitation system, conducted 3 times a week. The difficulty of the training was initially adjusted by PTA in an exploratory session. During the intervention, the difficulty of the task was adjusted either by the therapist or automatically by the system. The progress of all the participants was checked remotely once a week by PTA to detect possible issues and respond accordingly. In addition, PTB had a brief interview with participants of the experimental group each week to detect possible technical problems and to troubleshoot. On the remaining days (Tuesday and Thursday), both groups received conventional physical therapy in the clinic. The aim of the intervention was to improve balance. Telerehabilitation intervention: participants belonging to the experimental group trained in their homes Control intervention: participants belonging to the control group trained with the system in the clinic |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention (8 weeks), and follow‐up (12 weeks) Measures: cost, Berg Balance Scale, Performance Oriented Mobility Assessment‐Balance, Performance Oriented Mobility Assessment–Gait, Brunel Balance Assessment, system usability score, Intrinsic Motivation Inventory |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Held by external research in sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals and all participants included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | No mention of clinical trial registration or protocol |
| Other bias | Low risk | No other sources of bias noted |
Mayo 2008.
| Methods | RCT | |
| Participants | Recruited from 5 acute care hospitals in Canada Inclusion criteria: all persons returning home directly from the acute care hospital after a first or recurrent stroke with any of the following criteria indicating a specific need for healthcare supervision postdischarge (lives alone, mobility problem requiring assistive device, physical assistance or supervision, mild cognitive deficit, dysphagia, incontinence, social service consultation during acute hospitalisation, or need for postdischarge medical management for diabetes, congestive heart failure, ischaemic heart disease, arthritis, chronic obstructive pulmonary disease, atrial fibrillation, kidney disease, peripheral vascular disease) Exclusion criteria: people discharged to an inpatient rehabilitation facility or to long‐term care Age, years: telerehabilitation group = 70 (SD 14.5), control group = 72 (SD 12.95) Gender: telerehabilitation group 67% men, control group 55% men Time post‐stroke: telerehabilitation group 12 (SD 11.7 days), control group 13 (SD 15.7 days) |
|
| Interventions | Telerehabilitation intervention: received case management (defined as a 'collaborative process of assessment, planning, facilitation and advocacy for options and services to meet an individual's health needs through communication and available resources to promote quality cost‐effective outcomes'). Managed through home visits and telephone contacts for a period of 6 weeks. The nurse established contact with the GP and provided 24‐hour contact. Interventions included surveillance, information exchange, medication management, health system guidance, active listening, family support, teaching and risk identification. Control intervention: participant and family were instructed to make an appointment with their local GP. |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention, and 6‐month follow‐up Measures: reintegration to normal living index, Barthel Index, gait speed, Timed Up and Go test, SF‐36, EQ5D, Geriatric Depression Scale, health service utilisation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Reported that 'sealed envelopes' were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few instances of missing data. Balanced attrition across groups. ITT analyses conducted. Multiple imputation used for missing data |
| Selective reporting (reporting bias) | Unclear risk | Not able to access protocol |
| Other bias | Low risk | None apparent |
Meltzer 2018.
| Methods | RCT | |
| Participants | Recruited from the community in Canada Inclusion criteria: a history of unilateral stroke resulting in a communication disorder, occurring at least 6 months in the past; availability of a communication partner to participate in the treatment programme; ability to travel to the treatment site if not at home, and ability to hear instructions and operate an iPad tablet to perform homework exercises Exclusion criteria: dementia or other neurological disorder Age, years: intervention group 66.8 (11.2), control group 62.9 (11.6) Gender: 59% men intervention group, 69% men control group Time post‐stroke: not reported (at least 6 months post‐stroke) |
|
| Interventions | The study took place over 12 weeks for each participant, with an assessment in the first and last weeks and therapy during the intervening 10 weeks. Telerehabilitation intervention (Aphasia telerehab): remote therapy sessions were conducted via teleconferencing equipment and software. Participants possessing adequate equipment at home consulted the therapist using WebEx, a commercial teleconferencing program. Some clients visited the clinic to receive the telerehabilitation (provided in a separate room and contact with the therapist prohibited). During weeks 2‐11, the therapist conducted a 1‐hour weekly treatment session and TalkPath software was used for homework exercises. Control intervention (Aphasia in‐person): same therapy provided in‐person |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention (12 weeks) Measures: Western Aphasia Battery Revised Part 1, Cognitive Linguistic Quick Test, Communication Confidence Rating Scale for Aphasia, Communication Effectiveness Index |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessments were conducted by a therapist not involved in the study but not clear if they were blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting of recruitment and withdrawals had limited detail including balance between groups. |
| Selective reporting (reporting bias) | Unclear risk | Could not identify study protocol or trial registration |
| Other bias | Unclear risk | Groups were separated by diagnosis; however it was not clear whether this was factored into the randomisation process. |
Piron 2008.
| Methods | RCT | |
| Participants | Study took place in Italy Inclusion criteria: mild to intermediate arm motor impairment due to ischaemic stroke in the area of the middle cerebral artery; without cognitive problems that could interfere with comprehension Exclusion criteria: failure to meet above criteria Age, years: telerehabilitation group = 53 (SD 15) years, control group = 65 (SD 11) years Gender: telerehabilitation group 40% men, control group 60% men Time post‐stroke: telerehabilitation group 10 months (SD 3), control group 13 months (SD 2) |
|
| Interventions | Telerehabilitation intervention: the purpose of the intervention was to improve upper limb function using a virtual reality programme. Patient‐therapist interaction facilitated by a videoconferencing unit beside the telerehabilitation equipment. 1 computer was at the hospital and 1 at the participant's home Control intervention: virtual reality workstation with a 3D motion tracking system that recorded the participant's arm movements. The participant's movement was represented in the virtual environment. The therapist created a sequence of virtual tasks for the participant to complete with the affected arm. Participants could see their own trajectory and the ideal/desired trajectory. |
|
| Outcomes | Timing of outcome assessment: baseline and post‐intervention Measures: participant satisfaction questionnaire, Fugl‐Meyer Upper Extremity Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as 'simple randomisation' |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Not able to access protocol |
| Other bias | High risk | Small sample size |
Piron 2009.
| Methods | RCT | |
| Participants | Study took place in Italy Inclusion criteria: single ischaemic stroke in the middle cerebral artery region with mild to intermediate arm motor impairment (Fugl‐Meyer Upper Extremity Scale score 30 to 55) Exclusion criteria: clinical evidence of cognitive impairment, apraxia (< 62 points on the 'De Renzi' test), neglect or language disturbance interfering with verbal comprehension (> 40 errors on the Token test) Age, years: telerehabilitation group mean = 66 (SD 8), control group mean = 64 (SD 8) years Gender: 58% men Timing post‐stroke: intervention group mean (SD) 15 (7) months, control group 12 (4) months |
|
| Interventions | Telerehabilitation intervention: the virtual reality telerehabilitation programme used 1 computer workstation at the participant’s home and 1 at the rehabilitation hospital. The system used a 3D motion tracking system to record arm movements through a magnetic receiver into a virtual image. The participant moved a real object by following the trajectory of a virtual object displayed on the screen in accordance with the requested virtual task. 5 virtual tasks comprising simple arm movements were devised for training. Control intervention: specific exercises for the upper limb with progressive complexity. Started with control of isolated movements without postural control, then postural control including touching different targets and manipulating objects. Sessions were 60 minutes, 5 times per week for 4 weeks (20 hours total). | |
| Outcomes | Timing of outcome assessment: baseline, post‐intervention, and at 1 month Measures: Fugl‐Meyer Upper Extremity Scale, Abilhand Scale, modified Ashworth Scale |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal correspondence with study authors reported the use of a simple computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque sequentially numbered envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | No other outcomes collected |
| Other bias | Low risk | None apparent |
Rochette 2013.
| Methods | RCT | |
| Participants | Recruited from 11 acute care hospitals located in urban and rural areas across 4 Canadian provinces Inclusion criteria: all adults who sustained a first mild stroke defined as a score > 8.5/11.5 on the Canadian Neurological Scale or a mRS between 0 and 2 on admission and who were discharged home within 3 weeks of the index event were invited. They needed to have telephone access, ability to understand basic instructions and express basic needs, and ability to communicate in English or French. Exclusion criteria: individuals with moderate or severe cognitive deficits (based on clinical judgement) and those who experienced another stroke before baseline measures were completed Age, years: intervention group mean 61.7 (SD 12.7), control group mean 63.2 (12.4) Gender: intervention group 65% men, control group 56% men Time post‐stroke: intervention group mean 6.5 days, control group 5.2 days |
|
| Interventions | Telerehabilitation intervention: WE CALL participants received a multimodal (telephone, Internet, and paper) support intervention. Telephone interactions focused on any new or ongoing issues, as well as 6 key areas, including family functioning and individualised risk factors. Call frequency was weekly for the first 2 months, biweekly during the third month, and monthly for the past 3 months. Additional written information on stroke management was provided as needed (by regular mail, email, or Internet). Control intervention: YOU CALL participants were provided with the name and phone number of a trained healthcare professional who was not involved in providing the WE CALL intervention, whom they were free to contact should they feel the need. The health professional was instructed to answer the participant's queries on those topics initiated by the participant but not to probe further on other potential issues. |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention (6 months), 12 months Measures: unplanned use of health services (calendar), Quality of Life Index, EQ5D, Beck Depression Inventory, Assessment of Life Habits |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐managed stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with external research managing randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of people were not able to be reached. |
| Selective reporting (reporting bias) | Low risk | Protocol published and all outcomes reported |
| Other bias | Low risk | No other sources of bias noted |
Saal 2015.
| Methods | RCT | |
| Participants | Recruited from 2 acute hospitals in Germany Inclusion criteria: age ≥ 18 years, ischaemic or haemorrhagic stroke for the first time (confirmed by imaging), main residency in the Federal States of Saxony‐Anhalt, Saxony, or Thuringia, and able to speak German Exclusion criteria: previous ischaemic or haemorrhagic stroke, alcoholism, National Institute of Health Stroke Scale (NIHSS) score > 25, and homelessness Age, years: intervention group mean 68.1 (SD 12.6), control group 68.4 (12.7) Gender: intervention group 34% men, control group 38% men Time post‐stroke: not reported but participants recruited from an acute hospital |
|
| Interventions | Telerehabilitation intervention: in‐depth assessment and stroke support service provided by a nurse and physiotherapist. The stroke support service comprised stroke outreach support, educational sessions, and written patient information and was directed to both the patient and the next of kin. The stroke outreach support included home visits and telephone contacts and was individually tailored based on an agreement between the stroke support organiser and the patient and carer. The number of contacts between stroke support organiser and patient/carer was: 12.31% of contacts face‐to‐face and 61% via telephone; the remaining were written communications per email and normal post, or patient educational sessions. Control intervention: usual care |
|
| Outcomes | Timing of outcome assessment: pre (prior to discharge from acute care), baseline (4 weeks after discharge prior to randomisation), and post (12 months after randomisation) Measures: Stroke Impact Scale (physical function domain), WHOQOL‐BREF, Geriatric Depression Scale, Symptom Checklist 90 Revised, health service use |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed and stapled envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals across both groups but more in the usual care group |
| Selective reporting (reporting bias) | Low risk | Registration as clinical trial performed in advance. All outcomes reported |
| Other bias | Low risk | No other sources of bias noted |
Smith 2012.
| Methods | RCT | |
| Participants | Study took place in the USA Inclusion criteria: female caregiver providing care at home to husband after a stroke; either stroke survivor or caregiver scored 5 or greater on the PHQ‐9 (at least mild depression), neither stroke survivor nor caregiver were medically unstable or terminally ill and both were cognitively able to participate Exclusion criteria: failure to meet above criteria Age, years: telerehabilitation group mean = 59.9 (SD 8.2), control group mean = 59.1 (SD 13.6) Gender: 100% men Time since onset of stroke: details not reported |
|
| Interventions | Telerehabilitation intervention: consisted of 5 components designed to support the caregiver and provide caregiver with knowledge, resources and skills to assist him or her in reducing 'personal distress' and providing optimal emotional care to the stroke survivor. The 5 components included:
Intervention took place over 11 weeks. Control group: had access to the Resource Room only |
|
| Outcomes | Timing of outcome assessment: baseline, post‐intervention and at 1 month Measures: CES‐D, PHQ‐9, parts of the Mastery Scale, 10‐item self‐esteem scale, parts of the MOS Social Support Survey, ratings of treatment credibility, reported effort and perceived benefit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated design |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses conducted. Few dropouts, all accounted for and balanced across groups |
| Selective reporting (reporting bias) | High risk | Additional outcomes assessed that were not reported in the paper |
| Other bias | Unclear risk | No other sources of bias identified |
Vauth 2016.
| Methods | RCT | |
| Participants | Recruited from Bayreuth, Germany Inclusion criteria: > 12 months post‐stroke, aphasia, lesion on language dominant hemisphere Exclusion criteria: cognitive deficit, perceptual disorders, other motor deficits Age: mean 56 years, range 18 to 76 Gender: not reported Time post‐stroke: more than 12 months as per inclusion criteria but detail not reported |
|
| Interventions | Telerehabilitation: therapy was delivered via a screen and the person and therapist were in separate rooms. 1 screen displayed the therapy material and the other displayed the people communicating. Control group: same form of therapy but delivered in‐person Dose: 3 times per week (60 minutes each) for 8 weeks. Assessment pre and post‐intervention lasting 5 to 8 hours |
|
| Outcomes | Timing of outcome assessment: baseline, 8 weeks Measures: series of aphasia‐related measures and conversation analysis |
|
| Notes | Article published in German and translated. Contacted the study author for more details but they did not respond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details not reported |
| Allocation concealment (selection bias) | Unclear risk | Details not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Details not reported |
| Selective reporting (reporting bias) | Unclear risk | Details not reported |
| Other bias | Unclear risk | Unclear |
Wan 2016.
| Methods | RCT | |
| Participants | Recruited from neurology departments of 2 major general hospitals in Guangzhou, China Inclusion criteria: age above 35 years, hospitalisation within 1 month from the onset of ischaemic stroke as diagnosed by neuroimaging (CT or MRI) based on Chinese Neuroscience Society criteria, previous independence in daily activities, score of 0 to 3 on the mRS at discharge and upon returning home following discharge, and ability to communicate and provide informed consent Exclusion criteria: a history of cardio‐embolic infarction, Wernicke's aphasia, cognitive impairment, a history of severe liver or kidney disease, and any known malignancy or other neurological diseases Age, years: intervention group mean (SD) 59.07 (12.36), control group 60.24 (12.57) Gender: intervention group 75% men, control group 68% men Time post‐stroke: not reported |
|
| Interventions | Telerehabilitation intervention: structured guideline‐based, goal‐setting programme for secondary prevention of ischaemic stroke. The telephone follow‐up sessions were conducted by stroke nurses and consisted of goal‐setting advice focused on selected areas. Participants set measurable behavioural goals and developed action plans. Participants received the same stroke education as the control group with an additional 3 telephone follow‐up calls at 1 week, and at 1 and 3 months after discharge, each lasting 15 to 20 minutes, to promote self‐management techniques and maintenance of behavioural improvements. Control intervention: usual care and education |
|
| Outcomes | Timing of outcome assessment: baseline, 3 months, 6 months Measures: Chinese version of the Health Promoting Lifestyle Profile II; mRS score |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal in both groups and reporting of details unclear |
| Selective reporting (reporting bias) | Low risk | Registered with clinical trial registry and outcomes reported |
| Other bias | Low risk | No other sources of bias noted |
ADL: activities of daily living BBA: Brunel Balance Assessment CES: Center for Epidemiologic Studies CT: computerised tomography EQ5D: Euroqol 5 Dimensions ETNS: Electromyography triggered neuromuscular stimulation FIM: Functional Independence Measure fMRI: functional magnetic resonance imaging FITT: Family Interventon Telephone Tracking GP: general practitioner HCAD: Home Care Activity Device IHMD: In home messaging device IQR: interquartile range ITT: intention‐to‐treat LTCF: Long term care facility MoCA: Montreal Cognitive Assessment MOS: Medical Outcomes Study MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institute of Health Stroke Scale PHQ‐9: Patient Health Questionnaire 9 PTA: physical therapist A PTB: physical therapist B RCT: randomised controlled trial ROM: range of movement SD: standard deviation SE: standard error SF‐12: Short Form 12 SF‐36: Short Form 36 U/E: Upper Extremity WebEx: (communications platform) WHOQOL‐BREF: World Health Organisation Quality of Life ‐ BREF tool
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adie 2010 | Included participants with TIA |
| Bergquist 2012 | Included participants with diagnoses other than stroke |
| Burton 2005 | Intervention did not match our definition of telerehabilitation |
| Eames 2013 | Intervention did not match definition of telerehabilitation ‐ little remote contact once discharged home |
| Eide 2012 | Included participants with diagnoses other than stroke and intervention did not meet our criteria |
| Emmerson 2017 | Intervention did not involve telerehabilitation |
| Gillham 2010 | Included participants with TIA |
| Graven 2016 | Did not meet definition of telerehabilitation |
| Held 2018 | Did not meet definition of telerehabilitation |
| Hodson 2018 | Mixed methods study underway in order to test feasibility of intervention and inform future RCT |
| Hoffman 2010 | Intervention did not match our definition of telerehabilitation |
| Huijbregts 2010 | Not an RCT |
| Jackson 2010 | Intervention did not match our definition of telerehabilitation |
| Joubert 2006 | Intervention did not match our definition of telerehabilitation |
| Joubert 2009 | Intervention did not match our definition of telerehabilitation |
| Kamwesiga 2018 | Not randomised ‐ pre/post design |
| Kerry 2010 | Intervention did not match our definition of telerehabilitation |
| Kim 2013 | Included population with TIA or stroke |
| Krpic 2013 | Intervention did not meet our definition of telerehabilitation |
| Linder 2015 | Both groups received tele‐consultations; the difference between groups was the use of a robotic vs conventional home exercises |
| Mclaughlin 2010 | Not an RCT |
| Nijenhuis 2017 | Intervention did not meet our definition of telerehabilitation |
| Palmer 2011 | Intervention did not match our definition of telerehabilitation |
| Palmer 2014 | Intervention did not match definition of telerehabilitation |
| Redzuan 2012 | Intervention did not match our definition of telerehabilitation |
| Reeves 2017 | Intervention did not meet our definition of telerehabilitation |
| Song 2010 | Intervention did not match our definition of telerehabilitation |
| Van den Berg 2016 | Intervention group received more home visits than telerehabilitation consultations |
| Zucconi 2012 | Intervention did not match our definition of telerehabilitation |
RCT: randomised controlled trial TIA: transient ischaemic attack
Characteristics of studies awaiting assessment [ordered by study ID]
Dawson 2017.
| Methods | RCT |
| Participants | Adults with stroke (n = 15) |
| Interventions | Telerehabilitation intervention: delivery of Cognitive Orientation to Occupational Performance approach (CO‐OP) via telerehabilitation. Dose of 16 hours over 10 weeks. CO‐OP involves having clients set meaningful everyday‐life goals and guiding them to discover contextually and personally relevant ways to improve performance on those goals. Control intervention: waiting‐list control group |
| Outcomes | Measures: Canadian Occupational Performance Measure |
| Notes | Abstract published in International Journal of Stroke (2017) detailing progress to date |
NCT01655264.
| Methods | RCT |
| Participants | Adults with stroke, aged between 18 and 80 years, and living at home eligible to participate. Participants needed to be 2 to 72 months post‐stroke, and no longer receiving rehabilitation as in or outpatient. They should have moderate impairment of the affected upper extremity determined by range of motion. |
| Interventions | Each participant receives 12 x 45‐60‐minute sessions over 4 weeks while seated. Telerehabilitation intervention: Gertner Tele‐Motion‐Rehabilitation System treatment of comparable duration and intensity to those in the conventional treatment group with remote online monitoring by the therapist. Treatment feedback given in the form of knowledge of results (game scores) and knowledge of performance (feedback of compensatory movements made while using the upper extremity) to enhance motor learning. The software generates a report which will include the duration and type of exercises performed by the participant. The Gertner TMR system is implemented via Microsoft's Kinect 3‐D camera‐based gesture recognition technology. Using the patient's natural hand and body movements to control all activity within customised computer games. The system runs off a standard desktop computer and is displayed on a large television screen. Control intervention: the control group receives self‐training exercises that are based on conventional therapy using principles of motor control and includes training of upper extremity movements in order to achieve better use of the affected arm in ADL. |
| Outcomes | Shoulder and elbow range of motion (measured with goniometer), Chedoke Arm and Hand Activity Inventory, Motor Activity Log, Functional Reach Test, Lawton's IADL, Fugl‐Meyer Motor Assessment, Visual Analogue Scale, Functional Independence Measure, Stroke Impact Scale |
| Notes |
Poulsen 2016.
| Methods | Randomised controlled (crossover) trial |
| Participants | Adults after stroke |
| Interventions | Telerehabilitation intervention: web‐based rehabilitation programme 'Move it to Improve it' (Mitii) Control intervention: waiting‐list control |
| Outcomes | Outcomes assessed at baseline, 16 and 32 weeks and included: Modified Rankin Sacle, Barthel Index, physical assessment (NIHSS, motor assessment scale), cognitive tests and general well‐being (WHO‐5) |
| Notes | Abstract published but further details of the trial not yet available |
Shaughnessy 2012.
| Methods | RCT |
| Participants | Stroke survivors discharged from rehabilitation |
| Interventions | Telerehabilitation intervention: education, home‐based exercise programme and telephone contacts weekly for 12 weeks Control intervention: education and surveillance |
| Outcomes | Outcomes assessed at baseline, 12 and 24 weeks Outcomes assessed: visual analogue quality of life scale, step activity profiles and self‐efficacy for falls |
| Notes |
Uswatte 2013.
| Methods | RCT |
| Participants | Inclusion criteria: stroke survivors more than 1 year after stroke with the ability to open fingers on affected side, raise wrist, transfer and stand independently for 2 minutes |
| Interventions | Telerehabilitation intervention: constraint‐induced therapy (automated, remotely administered) Control intervention: constraint‐induced therapy |
| Outcomes | Outcomes assessed at 2 weeks, 6 months, 12 months Outcomes assessed: Motor Activity Log, Wolf Motor Function Test |
| Notes |
Vloothuis 2019.
| Methods | RCT |
| Participants | People with stroke and their caregiver |
| Interventions | Telerehabilitation: CARE4STROKE caregiver‐mediated programme with ehealth support Control intervention: usual care |
| Outcomes | Primary outcome: mobility domain of the Stroke Impact Scale |
| Notes |
ADL: activities of daily living CO‐OP: Cognitive Orientation to Occupational Performance IADL: Instrumental activities of daily living Mitii: Move it to Improve it NIHSS: National Institutes of Health Stroke Scale RCT: randomised controlled trial WHO‐5: World Health Organisation 5 Well Being Index
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617000168358.
| Trial name or title | A telehealth transfer package to improve post‐stroke rehabilitation outcomes |
| Methods | Randomised controlled trial (RCT) |
| Participants | People after stroke receiving an outpatient or day patient rehabilitation programme will be recruited |
| Interventions | Telerehabilitation group: individual (1‐on‐1) face‐to‐face sessions 1 x per week after the patient's formal therapy visit and via individual (1‐on‐1) telehealth sessions 4 x per week. The intervention package includes: a behavioural contract where the participant and applicant will decide which activities the participant will complete with their more‐affected hand. This will be reviewed each day by the therapist and participant during their session and amended accordingly; a daily motor activity log; a daily activity diary, a daily schedule of home practice prescribed by the treating therapists and a list of optional motor‐function specific supplementary activities the participant can complete at their leisure. The telehealth component will be delivered via Skype on the patient's usual household computer. Control group: the control group will receive their usual 8‐week outpatient occupational programme. They will not receive the additional telehealth transfer package. |
| Outcomes | Primary outcome: Fugl Meyer Assessment |
| Starting date | 2016 |
| Contact information | A/Prof Steven Faux: sfaux@stvincents.com.au |
| Notes | ACTRN12617000168358 |
ACTRN12618001519246.
| Trial name or title | Inspiring Virtual Enabled Resources following Vascular Events (iVERVE) |
| Methods | RCT |
| Participants | People after stroke recruited from the Australian Stroke Survivor Clinical Registry |
| Interventions | Structured and comprehensive patient‐centred goal‐setting conducted over the phone in survivors of stroke with SMS support messages |
| Outcomes | Goal attainment |
| Starting date | 2017 |
| Contact information | Professor Dominic Cadilhac: dominique.cadilhac@monash.edu |
| Notes |
Chaparro 2018.
| Trial name or title | Home‐based physical activity incentive and education programme in subacute phase of stroke recovery (Ticaa’dom) |
| Methods | RCT |
| Participants | People in the subacute phase after stroke |
| Interventions | Telerehabilitation: home‐based physical activity incentive and education programme (Ticaa’dom). The intervention group will follow the programme over 6 months: their physical activity will be monitored with an accelerometer during the day at home while they record their subjective perception of physical activity on a chart; they will observe a weekly telephone call and a home visit every 3 weeks. Control: usual care |
| Outcomes | Primary outcome: 6‐Minute Walk Test. Secondary outcomes will include measurements of lower limb strength, independence level, body composition, cardiac analysis, fatigue and depression state. |
| Starting date | 2013 |
| Contact information | David Chaparro: david.chaparro@etu.unilim.fr |
| Notes |
Chen 2018.
| Trial name or title | Effectiveness and neural mechanisms of home‐based telerehabilitation in patients with stroke based on fMRI and DTI |
| Methods | RCT |
| Participants | People after stroke |
| Interventions | Telerehabilitation vs conventional rehabilitation |
| Outcomes | Fugl Meyer Assessment |
| Starting date | Unknown |
| Contact information | Chuancheng Ren: rccfns17@sina.com |
| Notes |
ChiCTR‐IOR‐15006763.
| Trial name or title | Effectiveness, safety and cost efficiency of telerehabilitation for stroke patients in hospital and home |
| Methods | RCT |
| Participants | People after stroke with unilateral motor deficits |
| Interventions | Telerehabilitation compared with other models of service delivery (further detail not reported) |
| Outcomes | Primary outcome: Fugl Meyer outcome assessment |
| Starting date | 2015 |
| Contact information | Yun Qu: quyben@163.com |
| Notes | Listed on Chinese Clinical Trial Registry |
Gauthier 2017.
| Trial name or title | VIdeo Game Rehabilitation for OUtpatient Stroke (VIGoROUS) |
| Methods | RCT |
| Participants | People with chronic hemiparesis following stroke |
| Interventions | The researchers will test different forms of constraint‐induced (CI) movement therapy: (1) traditional clinic‐based CI therapy, (2) therapist‐as‐consultant video game CI therapy, (3) therapist‐as‐consultant video game CI therapy with additional therapist contact via telerehabilitation/video consultation, and (4) standard upper extremity rehabilitation |
| Outcomes | Primary outcome: Wolf Motor Function Test |
| Starting date | Unknown |
| Contact information | A/Prof Lynne Gauthier: lynne.gauthier@osumc.edu |
| Notes |
Koh 2015.
| Trial name or title | Singapore Tele‐technology Aided Rehabilitation in Stroke (STARS) trial |
| Methods | RCT |
| Participants | People with recent stroke |
| Interventions | Telerehabilitation: exercise 5 days‐a‐week using an iPad‐based system that allows recording of daily exercise with video and sensor data and weekly videoconferencing with teletherapists after data review Control: usual care |
| Outcomes | Primary outcome: Jette Late Life Functional and Disability Instrument |
| Starting date | 2015 |
| Contact information | Gerald_Koh@nuhs.edu.sg |
| Notes |
NCT01350453.
| Trial name or title | Development and pilot evaluation of a Web‐supported programme of constraint‐induced therapy following stroke (LifeCIT) |
| Methods | RCT |
| Participants | Stroke patients |
| Interventions | Intervention group: participants will be asked to aim to wear the mitt for 9 hours a day for 5 days/week, including 4 to 6 hours of structured activities per day: 2 x 30 to 60‐minute sessions of Web‐based activities and 3 to 4 hours of practicing everyday activities Control group: usual care |
| Outcomes | Motor Activity Log, Wolf Motor Function Test, Fugl‐Meyer Upper Extremity Scale, Stroke Impact Scale, Canadian Occupational Performance Measure, EQ5D, service utilisation |
| Starting date | May 2011 |
| Contact information | Claire Meagher: cm3v08@soton.ac.uk |
| Notes |
NCT02615132.
| Trial name or title | TeleRehab for stroke patients using mobile technology |
| Methods | RCT |
| Participants |
|
| Interventions | The study SLP will instruct the patient to use the iPad apps as an intervention for at least 1 hour per day, until they are admitted to outpatient SLP services or for a maximum of 8 weeks, whichever comes first. Throughout the telemedicine treatment phase, participants' progress will be monitored remotely by a study SLP through Apps/Skype/Facetime/Telephone consultation on a weekly basis Control group: usual care |
| Outcomes | Feasibility |
| Starting date | 2015 |
| Contact information | Karen Mallet: kmallet@toh.on.ca |
| Notes | Completed recruitment and currently writing up |
NCT02665052.
| Trial name or title | Translating intensive arm rehabilitation in stroke to a telerehabilitation format (TeleBATRAC) |
| Methods | RCT |
| Participants | People > 6 months after stroke with moderate to severe arm impairment (Fugl Meyer score 19 to 50) |
| Interventions | Telerehabilitation: home‐based training will consist of 45 minutes of high‐intensity bilateral reaching and rest periods using the Bilateral Arm Training with Rhythmic Auditory Cuing (BATRAC device) followed by 15 minutes of video‐guided transition to task training. These videos will be linked from the Veterans Affairs MyHealtheVet site to study specific Youtube videos of the study therapist demonstrating the exercise. Asynchronous communication between the therapist and participant will be completed using the MyHealtheVet secure messaging system Control group: clinic‐based approach of same therapy approach |
| Outcomes | Primary outcome: Wolf Motor Function Test |
| Starting date | 2016 |
| Contact information | Susan Conroy: susan.conroy@va.gov |
| Notes |
NCT03228264.
| Trial name or title | A trial investigating telerehabilitation as an add‐on to face‐to‐face speech and language therapy in post‐stroke aphasia |
| Methods | RCT |
| Participants | People after stroke with aphasia |
| Interventions | High teleSLT frequency intervention in which the experimental group trains for 96 minutes per day using a tablet computer delivering speech and language exercises Low teleSLT frequency intervention in which the control group trains for 24 minutes per day |
| Outcomes | Primary outcome: Amsterdam‐Nijmegen Everyday Language Test |
| Starting date | 2017 |
| Contact information | Professor René Müri: rene.mueri@insel.ch |
| Notes |
NCT03484182.
| Trial name or title | Efficacy of an interactive web‐based home therapy program after stroke |
| Methods | RCT |
| Participants | Patients after stroke who are discharged from outpatient rehabilitation and have impaired upper limb function |
| Interventions | Web‐based home exercise programme vs standard home exercise programme |
| Outcomes | Primary outcome: Fugl Meyer Upper Extremity |
| Starting date | 2018 |
| Contact information | A/Professor Sandy McCombe Waller |
| Notes |
NCT03531567.
| Trial name or title | Game‐based home exercise programs in chronic stroke: a feasibility study |
| Methods | RCT |
| Participants | People within 6 months of having a stroke |
| Interventions | Virtual reality Mystic Isle game vs standard home exercise programme |
| Outcomes | Primary outcome: Canadian Occupational Performance Measure |
| Starting date | 2018 |
| Contact information | A/Professor Rachel Proffitt |
| Notes |
NCT03759106.
| Trial name or title | Optimising a home‐based virtual reality exercise programme for chronic stroke patients: a telerehabilitation approach |
| Methods | RCT |
| Participants | People with stroke who are no longer receiving rehabilitation services and have upper limb impairment |
| Interventions | Telerehabilitation (8‐week home‐based virtual reality and telerehabilitation system) versus usual care |
| Outcomes | Primary outcome: Fugl Meyer‐upper extremity |
| Starting date | 2018 |
| Contact information | A/Professor Dahlia Kairy |
| Notes |
Nguyen 2011.
| Trial name or title | Pharmacist telephone interventions improve adherence to stroke preventative medications and reduce stroke risk factors: an RCT |
| Methods | RCT |
| Participants | Stroke patients |
| Interventions | Intervention group: received telephone follow‐up calls at 3 months and 6 months from time of randomisation Telephone follow‐up call included evaluation of medication adherence based on pharmacy refill history, as well as continuing stroke education and reassessment of stroke prevention goals with the participant. Recommendations for medication therapy and relevant clinical studies or laboratories were communicated to the primary care provider and/or stroke provider when appropriate. Control group: usual care |
| Outcomes | Adherence to medication, achievement of stroke prevention goals |
| Starting date | Unknown |
| Contact information | Unavailable |
| Notes |
Ora 2018.
| Trial name or title | Telerehabilitation for aphasia |
| Methods | RCT |
| Participants | People with aphasia post‐stroke |
| Interventions | Telerehabilitation: standard speech and language therapy and additional 5 hours of telerehabilitation per week over 4 weeks through video conference focusing on spoken language and word naming Usual care: standard speech and language therapy |
| Outcomes | Primary outcome: naming ability 3 months after intervention |
| Starting date | Unclear |
| Contact information | Hege Prag Øra: hege.ora@sunnaas.no |
| Notes |
Rodgers 2015.
| Trial name or title | Evaluating an extended rehabilitation service for stroke patients: study protocol for a randomised controlled trial |
| Methods | RCT |
| Participants | Participants are adults who have experienced a new stroke (and carer if appropriate), discharged from hospital under the care of an early supported discharge (ESD) team |
| Interventions | The intervention group receives an extended stroke rehabilitation service provided for 18 months following completion of ESD. Control group receives usual care. |
| Outcomes | The primary outcome is extended activities of daily living (Nottingham Extended Activities of Daily Living Scale) at 24 months post‐randomisation. Secondary outcomes (at 12 and 24 months post‐randomisation) are health status, quality of life, mood and experience of services for patients, and quality of life, experience of services, and carer stress for carers. Resource use and adverse events are also collected. |
| Starting date | |
| Contact information | Helen Rodgers: Helen.Rodgers@newcastle.ac.uk |
| Notes | ISRCTN45203373 |
Sakakibara 2017.
| Trial name or title | A telehealth intervention to promote healthy lifestyles after stroke: the Stroke Coach protocol |
| Methods | RCT |
| Participants | Participants will be recruited from acute, rehabilitation, and outpatient stroke units. Individuals will be included for study if they: are within 1 year following a confirmed stroke (ischaemic or haemorrhagic, diagnosis either by computerised tomography scan or magnetic resonance imaging); 50 years of age; have a modified Rankin Scale (mRS) 9 score varying from 1 to 4; live in the community and have phone access; and are able to communicate in English |
| Interventions | The Stroke Coach is a patient‐centred telehealth self management intervention to improve lifestyle behaviours after stroke that was developed using an Intervention Mapping process. |
| Outcomes | Primary outcome: Lifestyle Profile II questionnaire |
| Starting date | Not known |
| Contact information | Janice Eng: janice.eng@ubc.ca |
| Notes |
Saywell 2017.
| Trial name or title | Telerehabilitation to improve outcomes for people with stroke (ACTIV) |
| Methods | RCT |
| Participants | People will be eligible for inclusion if they have had a first ever hemispheric stroke of haemorrhagic or ischaemic origin; are over the age of 20 years; have been discharged from inpatient, outpatient and community physiotherapy services to live in their own home (participants involved in other forms of therapy such as occupational therapy, Tai Chi, or community exercise programmes will not be excluded); have medical clearance from their General Practitioner to participate in a low to moderate‐level activity programme; score at least 3 on a telephone cognitive screening questionnaire; have a limitation in physical function of leg, arm, or both |
| Interventions | The Augmented Community Telerehabilitation Intervention (ACTIV) is a 6‐month standardised programme delivered in the participant’s home, focusing on two functional categories: ‘staying upright’ and ‘using your arm’. |
| Outcomes | The primary outcome measure is the physical function subcomponent of the SIS 3.0. |
| Starting date | Unclear |
| Contact information | Nicola Saywell: nsaywell@aut.ac.nz |
| Notes | Note that abstract was presented at the 26th European Stroke Conference in Germany (2017) detailing recruitment of 95 participants and preliminary analysis but full results are not yet available. |
Sheehy 2018.
| Trial name or title | Home‐based virtual reality training after stroke: preliminary data of a telerehabilitation feasibility randomised controlled trial |
| Methods | RCT |
| Participants | People after stroke |
| Interventions | Home‐based virtual reality therapy |
| Outcomes | Feasibility |
| Starting date | 2018 |
| Contact information | Unknown |
| Notes |
Sureshkumar 2018.
| Trial name or title | 'Care for Stroke' intervention in India: a smart phone‐enabled, carer‐supported, educational intervention for management of disabilities following stroke |
| Methods | RCT |
| Participants | People after stroke |
| Interventions | Telerehabilitation: the 'Care for Stroke' intervention will be delivered through a smart phone and it will include information about stroke and the ways to manage post‐stroke disabilities. Control: standard stroke rehabilitation |
| Outcomes | Primary outcome: modified Rankin Scale |
| Starting date | Unknown |
| Contact information | K Sureshkumar: suresh.kumar@iiphh.org |
| Notes |
Tousignant 2014.
| Trial name or title | Tai Chi‐based exercise programme provided via telerehabilitation compared to home visits |
| Methods | RCT |
| Participants | People after stroke who have been discharged home without requiring intensive rehabilitation |
| Interventions | Teletreatment: personalised Tai Chi‐based exercise programme conducted by a trained physiotherapist (8 weeks) Home visits: same as above but delivered by an interventionist in the home |
| Outcomes | Community Balance and Mobility Scale |
| Starting date | 2013 |
| Contact information | Professor Michel Tousignant: michel.tousignant@usherbrooke.ca |
| Notes | NCT01848080 |
ACTIV: augmented community telerehabilitation intervention CI: constraint induced EQ5D: Euroqol 5 Dimensions ESD: early supported discharge IVERVE: Inspiring virtual enabling resources following vascular events LifeCIT: Life Constraint Induced Therapy mRS: Modified Rankin Scale RCT: randomised controlled trial SIS: stroke impact scale SLP: speech and language pathology SMS: short message service STARS: Singapore tele‐technology aided rehabilitation in stroke TeleBATRAC: Translating intensive arm rehabilitation in stroke to a telerehabilitation format VIGoROUS: Video game rehabilitation for outpatient stroke vs: versus
Differences between protocol and review
In this updated review (2019), we added two additional secondary outcomes: balance and depression. Several of the included studies involved interventions that aimed to improve balance or reduce depressive symptoms after discharge and so we felt it was important to report on the efficacy for these outcomes.
Contributions of authors
Kate E Laver is the guarantor of the review. Contributions included co‐ordinating the review, drafting the protocol, developing the search strategy, searching for trials, obtaining copies of the trials, selecting which trials to include, extracting data from the trials, entering data, carrying out the analysis, interpreting the analysis, and drafting the final review.
Zoe Adey‐Wakeling was involved in selecting which trials to include, extracting data from trials, interpreting the analysis, and drafting the final review.
Maria Crotty was involved in drafting the protocol, selecting which trials to include (arbiter), interpreting the analysis, and drafting the final review.
Natasha A Lannin was involved in drafting the protocol, carrying out the analysis, interpreting the analysis, and drafting the final review.
Stacey George was involved in drafting the protocol, selecting which trials to include (arbiter), interpreting the analysis, and drafting the final review.
Catherine Sherrington was involved in drafting the protocol, guiding and interpreting the analysis, and drafting the final review.
All review authors will be responsible for updating the review.
Declarations of interest
Kate E Laver: none known Zoe Adey‐Wakeling: none known Maria Crotty: none known Natasha A Lannin: none known Stacey George: none known Catherine Sherrington: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bishop 2014 {published data only}
- Bishop D, Miller I, Weiner D, Guilmette T, Mukand J, Feldmann E, et al. Family Intervention: Telephone Tracking (FITT): a pilot stroke outcome study. Topics in Stroke Rehabilitation 2014;21 Suppl 1:S63‐S74. [DOI] [PubMed] [Google Scholar]
Bizovičar 2017 {published data only}
- Bizovičar N, Rudolf M, Javh M, Goljar N, Rudel D, Obržan D, et al. Effects of home exercise assisted by written and video instructions in patients after stroke [Učinki vadbe na domu ob pomoči vaj v pisni in video obliki pri bolnikih po možganski kapi]. Rehabilitacija 2016;3:26‐32. [Google Scholar]
Boter 2004 {published data only}
- Boter H, HESTIA Study Group. Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke 2004;35:2867‐72. [DOI] [PubMed] [Google Scholar]
Carey 2007 {published data only}
- Carey J, Durfee W, Bhatt E, Nagpal A, Weinstein S, Anderson K, et al. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabilitation and Neural Repair 2007;21(3):216‐32. [DOI] [PubMed] [Google Scholar]
Chen 2017 {published data only}
- Chen J, Jin W, Dong WS, Jin Y, Qiao FL, Zhou YF, et al. Effects of home‐based telesupervising rehabilitation on physical function for stroke survivors with hemiplegia. American Journal of Physical Medicine and Rehabilitation 2017;96(3):152‐60. [DOI] [PubMed] [Google Scholar]
Chumbler 2012 {published data only}
- Chumbler N, Quigley P, Li X, Morey M, Rose D, Sanford J, et al. Effects of telerehabilitation on physical function and disability for stroke patients. Stroke 2012;43:2168‐74. [DOI] [PubMed] [Google Scholar]
Cramer 2019 {published data only}
- Cramer S, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. Telerehabilitation in the home versus therapy in‐clinic for patients with stroke. European Stroke Journal 2018;3, 1 Suppl:590‐1. [Google Scholar]
- Cramer SC, Dodakian L, Le V, See J, Augsburger R, McKenzie A, et al. Efficacy of home‐based telerehabilitation vs in‐clinic therapy for adults after stroke: a randomized clinical trial. JAMA Neurology 2019;76(9):1079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deng 2012 {published data only}
- Deng H, Durfee W, Nuckley D, Rheude B, Severson A, Skluzacek K, et al. Complex versus simple ankle movement training in stroke using telerehabilitation: a randomized controlled trial. Physical Therapy 2012;92(2):197‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forducey 2012 {published data only}
- Forducey P, Glueckauf R, Bergquist T, Maheu M, Yutsis M. Telehealth for persons with severe functional disabilities and their caregivers: facilitating self‐care management in the home setting. Psychological Services 2012;9(2):144‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huijgen 2008 {published data only}
- Huijgen B, Vollenbroek‐Hutton M, Zampolini M, Opisso E, Bernabeu M, Nieuwenhoven J, et al. Feasibility of a home based telerehabilitation system compared to usual care: arm/hand function in patients with stroke, traumatic brain injury and multiple sclerosis. Journal of Telemedicine and Telecare 2008;14:249‐56. [DOI] [PubMed] [Google Scholar]
Kirkness 2017 {published data only}
- Kirkness C, Cain K, Becker K, Tirschwell D, Buzaitis A, Weisman P, et al. Randomized trial of telephone versus in‐person delivery of a brief psychosocial intervention in post‐stroke depression. BMC Research Notes 2017;10:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2014 {published data only}
- Lin K, Chen C, Chen Y, Huang W, Lai J, Yu S, et al. Bidirectional and multi‐user telerehabilitation system: clinical effect on balance, functional activity, and satisfaction in patients with chronic stroke living in long‐term care facilities. Sensors 2014;14:12451‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Llorens 2015 {published data only}
- Llorens R, Noe E, Colomer C, Alcaniz M. Effectiveness, usability and cost benefit of a virtual‐reality based telerehabilitation program for balance recovery after stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2015;96:418‐25. [DOI] [PubMed] [Google Scholar]
Mayo 2008 {published data only}
- Mayo N, Nadeau L, Ahmed S, White C, Grad R, Huang A, et al. Bridging the gap: the effectiveness of teaming a stroke coordinator with patient's personal physician on the outcome of stroke. Age and Ageing 2008;37:32‐8. [DOI] [PubMed] [Google Scholar]
Meltzer 2018 {published data only}
- Meltzer J, Baird A, Steele R, Harvey S. Computer‐based treatment of poststroke language disorders: a non‐inferiority study of telerehabilitation compared to in‐person service delivery. Aphasiology 2018;32(3):290‐311. [Google Scholar]
Piron 2008 {published data only}
- Piron L, Turolla A, Tonin P, Piccione F, Lain L, Dam M. Satisfaction with care in post‐stroke patients undergoing a telerehabilitation programme at home. Journal of Telemedicine and Telecare 2008;14:257‐60. [DOI] [PubMed] [Google Scholar]
Piron 2009 {published data only}
- Piron L, Turolla A, Agostini M, Zucconi C, Cortese F, Zampolini M, et al. Exercises for paretic upper limb after stroke: a combined virtual‐reality and telemedicine approach. Journal of Rehabilitation Medicine 2009;41:1016‐20. [DOI] [PubMed] [Google Scholar]
Rochette 2013 {published data only}
- Rochette A, Korner‐Bitensky N, Bishop D, Teasell R, White CL, Bravo G, et al. The YOU CALL–WE CALL randomized clinical trial: impact of a multimodal support intervention after a mild stroke. Circulation: Cardiovascular Quality and Outcomes 2013;6(6):674‐9. [DOI] [PubMed] [Google Scholar]
Saal 2015 {published data only}
- Saal S, Becker C, Lorenz S, Schubert M, Kuss O, Stang A, et al. Effect of a stroke support service in Germany: a randomized trial. Topics in Stroke Rehabilitation 2015;22(6):429‐36. [DOI] [PubMed] [Google Scholar]
Smith 2012 {published data only}
- Smith G, Egbert N, Palmieri P, Dellman‐Jenkins M, Nanna K. Reducing depression in stroke survivors and their informal caregivers: a randomized clinical trial of a web‐based intervention. Rehabilitation Psychology 2012;57(3):196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vauth 2016 {published data only}
- Vauth F, Richter J, Scibor M, Keidel M. Tele online therapy in patients with aphasia after stroke [Telesprachtehrapie (synchrotel) beia aphasie nach schlaganfall]. Nervenheilkunde 2016;35(3):119‐24. [Google Scholar]
Wan 2016 {published data only}
- Wan LH, Zhang XP, Mo MM, Xiong XN, Ou CL, You LM, et al. Effectiveness of goal‐setting telephone follow‐up on health behaviors of patients with ischemic stroke: a randomized controlled trial. Journal of Stroke and Cerebrovascular Diseases 2016;25(9):2259‐70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adie 2010 {published data only}
- Adie K, James M. Does telephone follow‐up improve blood pressure after minor stroke or TIA?. Age and Ageing 2010;39:598‐603. [DOI] [PubMed] [Google Scholar]
Bergquist 2012 {published data only}
- Bergquist T, Yutsis M. The effect of cognitive rehabilitation delivered via instant messaging on functional independence in persons with ABI. Brain Injury 2012;26(4‐5):700‐1. [Google Scholar]
Burton 2005 {published data only}
- Burton C, Gibbon B. Expanding the role of the stroke nurse: a pragmatic clinical trial. Journal of Advanced Nursing 2005;52(6):640‐50. [DOI] [PubMed] [Google Scholar]
Eames 2013 {published data only}
- Eames S, Hoffman T, Worrall L, Read S, Wong A. Randomised controlled trial of an education and support package for stroke patients and their carers. BMJ Open 2013;3:e002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eide 2012 {published data only}
- Eide L, Schanke A. Examining computerized working memory training as a supplement to cognitive rehabilitation for patients with an acquired brain injury. Brain Injury 2012;26(4‐5):749‐50. [Google Scholar]
Emmerson 2017 {published data only}
- Emmerson KB, Harding KE, Taylor NF. Home exercise programmes supported by video and automated reminders compared with standard paper‐based home exercise programmes in patients with stroke: a randomized controlled trial. Clinical Rehabilitation 2017;31(8):1068‐77. [DOI] [PubMed] [Google Scholar]
Gillham 2010 {published data only}
- Gillham S, Endacott R. Impact of enhanced secondary prevention on health behaviour in patients following minor stroke and transient ischaemic attack: a randomized controlled trial. Clinical Rehabilitation 2010;24(9):822‐30. [DOI] [PubMed] [Google Scholar]
Graven 2016 {published data only}
- Graven C, Brock K, Hill KD, Cotton S, Joubert L. First year after stroke: an integrated approach focusing on participation goals aiming to reduce depressive symptoms. Stroke 2016;47(11):2820‐7. [DOI] [PubMed] [Google Scholar]
Held 2018 {published data only}
- Held JP, Luft AR, Veerbeek JM. Encouragement‐induced real‐world upper limb use after stroke by a tracking and feedback device: a study protocol for a multi‐center, assessor‐blinded, randomized controlled trial. Frontiers in Neurology 2018;25(9):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodson 2018 {published data only}
- Hodson T, Cornwell P, Gustafsson L. Meeting the needs of the mild stroke population through targeted interventions: a pilot study. European Stroke Journal 2018;3 Suppl 1:516. [Google Scholar]
Hoffman 2010 {published data only}
- Hoffman T, Worrall L, Eames S. Measuring outcomes in people who have had a stroke and their carers: can the telephone be used?. Topics in Stroke Rehabilitation 2010;17(2):119‐27. [DOI] [PubMed] [Google Scholar]
Huijbregts 2010 {published data only}
- Huijbregts M, Cameron J, Taylor D, McEwen S, Kagan A, Streiner D. Videoconference delivery of a stroke self‐management program: a mixed methods waiting list randomized controlled trial. Stroke 2010;41:e357. [Google Scholar]
Jackson 2010 {published data only}
- Jackson D, Elsom S, Joubert L, Joubert J. An exploration of the role of the nursing coordinator in a telemedicine based stroke secondary prevention model. International Journal of Stroke 2010;5(1):1. [Google Scholar]
Joubert 2006 {published data only}
- Joubert J, Reid C, Joubert L, Barton D, Ruth D, Jackson D, et al. Risk factor management and depression post stroke: the value of an integrated model of care. Journal of Clinical Neuroscience 2006;13:84‐90. [DOI] [PubMed] [Google Scholar]
Joubert 2009 {published data only}
- Joubert J, Reid C, Barton D, Cumming T, McLean A, Joubert L, et al. Integrated care improves risk‐factor modification after stroke: initial results of the Integrated Care for the Reduction of Secondary Stroke model. Journal of Neurology, Neurosurgery and Psychiatry 2009;80:279‐84. [DOI] [PubMed] [Google Scholar]
Kamwesiga 2018 {published data only}
- Kamwesiga H, Eriksson G, Tham K, Fors U, Ndiwalana A, Koch L, et al. A feasibility study of a mobile phone supported family‐centred ADL intervention, F@ce, after stroke in Uganda. Globalization and Health 2018;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kerry 2010 {published data only}
- Kerry S, Cloud G, Markus H, Khong T, Oakeshott P. Does self monitoring improve blood pressure control in hypertensive stroke patients—first results of a randomised trial. International Journal of Stroke 2010;5 (Suppl 3):6. [Google Scholar]
Kim 2013 {published data only}
- Kim H, Oksoo K. The lifestyle modification coaching program for secondary stroke prevention. Journal of the Korean Academy of Nursing 2013;43(3):331‐3. [DOI] [PubMed] [Google Scholar]
Krpic 2013 {published data only}
- Krpic A, Savanovic A, Cikajlo I. Telerehabilitation: remote multimedia‐supported assistance and mobile monitoring of balance training outcomes can facilitate the clinical staff’s effort. International Journal of Rehabilitation Research 2013;36(2):162‐71. [DOI] [PubMed] [Google Scholar]
Linder 2015 {published data only}
- Linder SM, Rosenfeldt AB, Bay RC, Sahu K, Wolf SL, Alberts JL. Improving quality of life and depression after stroke through telerehabilitation. American Journal of Occupational Therapy 2015;69(2):no pagination. [DOI: 10.5014/ajot.2015.014498] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mclaughlin 2010 {published data only}
- Mclaughlin M, Nam Y, Sanders S, Yeh S, Chang C, Kennedy B, et al. Virtual environments for stroke recovery: pilot clinical trials for user‐centric patient/clinician distribution platform with tele‐rehabilitation application using haptic devices. International Journal of Stroke 2010;5:66. [Google Scholar]
Nijenhuis 2017 {published data only}
- Nijenhuis SM, Prange‐Lasonder GB, Stienen AH, Rietman JS, Buurke JH. Effects of training with a passive hand orthosis and games at home in chronic stroke: a pilot randomised controlled trial. Clinical Rehabilitation 2017;31(2):207‐16. [DOI] [PubMed] [Google Scholar]
Palmer 2011 {published data only}
- Palmer R, Enderby P, Mortley J, Cooper C, Dixon S, Julious S, et al. Cost effectiveness of aphasia computer therapy compared with usual stimulation for people with long standing aphasia (CACTUS). Results of a pilot study. International Journal of Stroke 2011;5:4. [Google Scholar]
Palmer 2014 {published data only}
- Palmer R. Clinical and cost effectiveness of aphasia computer therapy compared with usual stimulation or attention control long term post stroke (CACTUS). Health Technology Assessment 2020 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Redzuan 2012 {published data only}
- Redzuan N, Engkasan J, Mazlan M, Abdullah S. Effectiveness of a video‐based therapy program at home after acute stroke: a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2012;93:2177‐83. [DOI] [PubMed] [Google Scholar]
Reeves 2017 {published data only}
- Reeves MJ, Hughes AK, Woodward AT, Freddolino PP, Coursaris CK, Swierenga SJ, et al. Improving transitions in acute stroke patients discharged to home: the Michigan stroke transitions trial. BMC Neurology 2017;17(1):115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2010 {published data only}
- Song C, Seo S, Lee G. Video game based exercise for upper extremity function rehabilitation of chronic stroke patients. Results from a randomized controlled single blind trial. International Journal of Stroke 2010;5:304. [Google Scholar]
Van den Berg 2016 {published data only}
- Berg M, Crotty M, Liu E, Killington M, Kwakkel G, Wegen E. Early supported discharge by caregiver‐mediated exercises and e‐Health support after stroke: a proof of concept trial. Stroke 2016;47:1885‐92. [DOI] [PubMed] [Google Scholar]
Zucconi 2012 {published data only}
- Zucconi C, Valt V, Agostini M, Turolla A, Tonin P, Piron L. Assessment of a virtual teacher feedback for the recovery of the upper limb after stroke. Neurorehabilitation and Neural Repair 2012;26:4. [Google Scholar]
References to studies awaiting assessment
Dawson 2017 {published data only}
- Dawson D, Bar Y, McEwen S, Skidmore E, Nalder E, Anderson N, et al. Enhancing participation in everyday life for people with stroke via telerehabilitation: a randomized controlled trial. International Journal of Stroke 2017;12 Suppl 4:87. [Google Scholar]
NCT01655264 {published data only}
- NCT01655264. Evaluation of the Gertner Tele‐Motion‐Rehabilitation system for stroke rehabilitation. clinicaltrials.gov/ct2/show/NCT01655264 (first received 1 August 2012).
Poulsen 2016 {published data only}
- Poulsen MB, Badawey J, Anhøj M, Rostrup E, Ellemann K, Larsson BW, et al. Early web‐based tele‐rehabilitation in stroke patients: a randomised controlled pilot study. European Journal of Neurology 2016;23:460. [Google Scholar]
Shaughnessy 2012 {published data only}
- Shaughnessy M, Stookey A. Reshaping exercise habits and beliefs (REHAB): a randomized trial of home‐based exercise in sub‐acute stroke. Stroke 2012;43(2):Abstract NS 11. [Google Scholar]
Uswatte 2013 {published data only}
- Uswatte G, Taub E, Lum P, Brennan D, Barman J, Gilmone B, et al. Telerehabilitation versus outpatient delivery of constraint induced movement therapy: update on a randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(1):e27‐e28. [Google Scholar]
Vloothuis 2019 {published data only}
- Vloothuis J, Mulder M, Nijland R, Goedhart Q, Konijnenbelt M, Mulder H, et al. Caregiver‐mediated exercises with e‐health support for early supported discharge after stroke (CARE4STROKE): a randomized controlled trial. PLOS One 2019;14(4):e0214241. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617000168358 {published data only}
- ACTRN12617000168358. A telehealth transfer package to improve post stroke rehabilitation outcomes. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370120 (first received 1 February 2017).
ACTRN12618001519246 {published data only}
- ACTRN12618001519246. Inspiring Virtual Enabled Resources following Vascular Events (iVERVE) pilot randomised controlled trial in chronic stroke to determine the feasibility and acceptability of e‐health support after stroke. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375230 (first received 11 September 2018).
Chaparro 2018 {published data only}
- Chaparro D, Daviet JC, Borel B, Kammoun B, Salle JY, Tchalla A, et al. Home‐based physical activity incentive and education program in subacute phase of stroke recovery (Ticaa’dom): study protocol for a randomized controlled trial. Trials 2018;19(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2018 {published data only}
- Chen J, Liu M, Sun D, Jin Y, Wang T, Ren C. Effectiveness and neural mechanisms of home‐based telerehabilitation in patients with stroke based on fMRI and DTI. Medicine 2018;97(3):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
ChiCTR‐IOR‐15006763 {published data only}
- ChiCTR‐IOR‐15006763. Effectiveness, safety and cost efficiency of telerehabilitation for stroke patients in hospital and home. chictr.org.cn/showprojen.aspx?proj=11437 (first received 15 July 2015).
Gauthier 2017 {published data only}
- Gauthier L, Kane C, Borstad A, Strahl N, Uswatte G, Taub E, et al. VIdeo Game Rehabilitation for OUtpatient Stroke (VIGoROUS): protocol for a multicenter comparative effectiveness trial of in home gamified constraint‐induced movement therapy for rehabilitation of chronic upper extremity hemiparesis. BMC Neurology 2017;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koh 2015 {published data only}
- Koh G, Yen S, Tay A, Cheong A, Ng Y, Silva D, et al. Singapore Tele‐technology Aided Rehabilitation in Stroke (STARS) trial: protocol of a randomized clinical trial on tele‐rehabilitation for stroke patients. BMC Neurology 2015;15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01350453 {published data only}
- NCT01350453. Development and pilot evaluation of a web‐supported programme of constraint induced movement therapy following stroke (LifeCIT). clinicaltrials.gov/ct2/show/NCT01350453 (first received 9 May 2011).
NCT02615132 {published data only}
- NCT02615132. TeleRehab for stroke patients using mobile technology. clinicaltrials.gov/ct2/show/NCT02615132 (first received 26 November 2016).
NCT02665052 {published data only}
- NCT02665052. Translating intensive arm rehabilitation in stroke to a telerehabilitation format (TeleBATRAC). clinicaltrials.gov/ct2/show/record/NCT02665052 (first received 27 January 2016).
NCT03228264 {published data only}
- NCT03228264. A trial investigating telerehabilitation as an add‐on to face‐to‐face speech and language therapy in post‐stroke aphasia [A randomized controlled, evaluator‐blinded, multi‐center trial investigating telerehabilitation as an add‐on to face‐to‐face speech and language therapy in post‐stroke aphasia]. clinicaltrials.gov/ct2/show/record/NCT03228264 (first received 24 July 2017).
NCT03484182 {published data only}
- NCT03484182. Efficacy of an interactive web‐based home therapy program after stroke (STRONG) [Efficacy of an interactive web‐based home therapy program in the recovery of arm and hand function following stroke: a randomized trial]. clinicaltrials.gov/ct2/show/record/NCT03484182 (first received 30 March 2018).
NCT03531567 {published data only}
- NCT03531567. Game‐based home exercise programs in chronic stroke: a feasibility study. clinicaltrials.gov/ct2/show/NCT03531567 (first received 21 May 2018).
NCT03759106 {published data only}
- NCT03759106. Optimizing a home‐based virtual reality exercise program for chronic stroke patients: a telerehabilitation approach. clinicaltrials.gov/ct2/show/NCT03759106 (first received 29 November 2018).
Nguyen 2011 {published data only}
- Nguyen V, Poon J, Tokuda L, Sayers J, Wallis R, Dergalust S. Pharmacist telephone interventions improve adherence to stroke preventative medications and reduce stroke risk factors: a randomized controlled trial. Stroke 2011;42(3):e244. [Google Scholar]
Ora 2018 {published data only}
- Øra H, Kirmess M, Brady M, Winsnes I, Hansen S, Becker F. Telerehabilitation for aphasia ‐ protocol of a pragmatic, exploratory, pilot, randomized controlled trial. Trials 2018;19(208):no pagination. [DOI: 10.1186/s13063-018-2588-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodgers 2015 {published data only}
- Rodgers H, Shaw L, Cant R, Drummond A, Ford GA, Forster A, et al. Evaluating an extended rehabilitation service for stroke patients (EXTRAS): study protocol for a randomised controlled trial. Trials 2015;16(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakakibara 2017 {published data only}
- Sakakibara B, Lear S, Barr S, Benavente O, Goldsmith C, Silverberg N, et al. Development of a chronic disease management program for stroke survivors using intervention mapping: the stroke coach. Archives of Physical Medicine and Rehabilitation 2017;98:1195‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saywell 2017 {published data only}
- Saywell N, Vandal A, Taylor D. Augmented community telerehabilitation intervention to improve outcomes for people with stroke AKTIV ‐ a randomised controlled trial. Cerebrovascular Diseases 2017;43 Suppl 1:166. [Google Scholar]
Sheehy 2018 {published data only}
- Sheehy L, Chapman I, Sviestrup H, Yang C, Bilodeau M, Finestone H. Home‐based virtual reality training after stroke: preliminary data of a telerehabilitation feasibility randomized controlled trial. International Journal of Stroke 2018;13 Suppl 2:207. [Google Scholar]
Sureshkumar 2018 {published data only}
- Sureshkumar K, Murthy G, Kuper H. Protocol for a randomised controlled trial to evaluate the effectiveness of the 'Care for Stroke' intervention in India: a smartphone‐enabled, carer supported educational intervention for management of disabilities following stroke. BMJ Open 2018;8:e020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tousignant 2014 {published data only}
- Tousignant M, Corriveau H, Kairy D, Berg K, Dubois M, Gosselin S, et al. Tai Chi‐based exercise program provided via telerehabilitation compared to home visits in a post‐stroke population who have returned home without intensive rehabilitation: study protocol for a randomized, non‐inferiority clinical trial. Trials 2014;15(42):no pagination. [DOI: 10.1186/1745-6215-15-42] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
American Telemedicine Association 2010
- American Telemedicine Association. A blueprint for telerehabilitation guidelines. Telemedicine and e‐Health 2011;17:662‐5. [DOI] [PubMed] [Google Scholar]
Appleby 2019
- Appleby E, Gill S, Hayes L, Walker T, Walsh M, Kumar S. Effectiveness of telerehabilitation in the management of adults with stroke: A systematic review. PloS One 2019;14(11):doi: 10.1371/journal.pone.0225150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blacquiere 2017
- Blacquiere D, Lindsay MP, Foley N, Taralson C, Alcock S, Balg C, et al. Canadian stroke best practice recommendations: telestroke best practice guidelines update 2017. International Journal of Stroke 2017;12(8):886‐95. [DOI] [PubMed] [Google Scholar]
Brennan 2009
- Brennan D, Mawson S, Brownsell S. Telerehabilitation: enabling the remote delivery of healthcare, rehabilitation and self management. In: Gaggioli A editor(s). Advanced Technologies in Rehabilitation. Amsterdam: IOS Press, 2009:231‐48. [PubMed] [Google Scholar]
Brochard 2010
- Brochard S, Robertson J, Medee B, Remy‐Neris O. What's new in new technologies for upper extremity rehabilitation?. Current Opinion in Neurology 2010;23:683‐7. [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen J, Jin W, Zhang X, Xu W, Liu X, Ren C. Telerehabilitation approaches for stroke patients: systematic review and meta‐analysis of randomized controlled trials. Journal of Stroke and Cerebrovascular Diseases 2015;24(12):2660‐8. [DOI] [PubMed] [Google Scholar]
Crichton 2016
- Crichton SL, Bray BD, McKevitt C, Rudd AG, Wolfe CD. Patient outcomes up to 15 years after stroke: survival, disability, quality of life, cognition and mental health. Journal of Neurology, Neurosurgery and Psychiatry 2016;87(10):1091‐8. [DOI] [PubMed] [Google Scholar]
Feigin 2017
- Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circulation Research 2017;120:439‐48. [DOI] [PubMed] [Google Scholar]
Galea 2019
- Galea M. Telemedicine in rehabilitation. Physical Medicine and Rehabilitation Clinics of North America 2019;30(2):473‐83. [DOI] [PubMed] [Google Scholar]
Greenhalgh 2012
- Greenhalgh T, Procter R, Wherton J, Sugarhood P, Shaw S. The organising vision for telehealth and telecare: discourse analysis. BMJ Open 2012;2(4):e001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist G, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 2018
- Hamilton C, McCluskey A, Hassett L, Killington M, Lovarini M. Patient and therapist experiences of using affordable feedback‐based technology in rehabilitation: a qualitative study nested in a randomized controlled trial. Clinical Rehabilitation 2018;32(9):1258‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.handbook.cochrane.org.
Hill 2018
- Hill AJ, Breslin HM. Asynchronous telepractice in aphasia rehabilitation: outcomes from a pilot study. Aphasiology 2018;32:90‐2. [Google Scholar]
Kalra 2007
- Kalra L, Langhorne P. Facilitating recovery: evidence for organised stroke care. Journal of Rehabilitation Medicine 2007;39:97‐102. [DOI] [PubMed] [Google Scholar]
Kwakkel 2004
- Kwakkel G, Kollen B, LIndeman E. Understanding the pattern of functional recovery after stroke: facts and theories. Restorative Neurology and Neuroscience 2004;22:281‐99. [PubMed] [Google Scholar]
Langhorne 2011
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693‐702. [DOI] [PubMed] [Google Scholar]
National Institutes of Health 2014
- National Institute of Neurological Disorders and Stroke. Post stroke rehabilitation fact sheet 2014. ninds.nih.gov/Disorders/Patient‐Caregiver‐Education/Fact‐Sheets/Post‐Stroke‐Rehabilitation‐Fact‐Sheet.
Ninnis 2019
- Ninnis K, Berg M, Lannin N, George S, Laver K. Information and communication technology use within occupational therapy home assessments: a scoping review. British Journal of Occupational Therapy 2019;82(3):141‐52. [Google Scholar]
Piaggio 2012
- Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG, CONSORT Group. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA 2012;308(24):2594‐604. [DOI] [PubMed] [Google Scholar]
Pollock 2014
- Pollock A, Baer G, Campbell P, Choo PL, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD001920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richardson 2017
- Richardson BR, Truter P, Blumke R, Russell TG. Physiotherapy assessment and diagnosis of musculoskeletal disorders of the knee via telerehabilitation. Journal of Telemedicine and Telecare 2017;23(1):88‐95. [DOI] [PubMed] [Google Scholar]
Rogante 2010
- Rogante M, Grigioni M, Cordella D, Giacomozzi C. Ten years of telerehabilitation: a literature overview of technologies and clinical applications. NeuroRehabilitation 2010;27:287‐304. [DOI] [PubMed] [Google Scholar]
Russell 2009
- Russell T. Telerehabilitation: a coming of age. Australian Journal of Physiotherapy 2009;55:5‐6. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz K, Altman D, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Standing 2018
- Standing C, Standing S, McDermott ML, Gururajan R, Kiani Mavi R. The paradoxes of telehealth: a review of the literature 2000–2015. Systems Research and Behavioral Science 2018;35(1):90‐101. [Google Scholar]
Stroke Foundation 2017
- Stroke Foundation 2017. National Stroke Audit‐ Acute Services Report 2017. Melbourne, Australia 2017.
Tchero 2018
- Tchero H, Tabue Teguo M, Lannuzel A, Rusch E. Telerehabilitation for stroke survivors: Systematic review and meta‐analysis. Journal of Medical Internet Research 2018;20(10):doi: 10.2196/10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theodoros 2008
- Theodoros D, Russell T. Telerehabilitation: current perspectives. Studies in Health Technology and Informatics 2008;131:191‐209. [PubMed] [Google Scholar]
Thrift 2017
- Thrift AG, Thayabaranathan T, Howard G, Howard VJ, Rothwell PM, Feigin VL, et al. Global stroke statistics. International Journal of Stroke 2017;12(1):13‐32. [DOI] [PubMed] [Google Scholar]
Ullberg 2016
- Ullberg T, Zia E, Petersson J, Norrving B. Perceived unmet rehabilitation needs 1 year after stroke: an observational study from the Swedish stroke register. Stroke 2016;47(2):539‐41. [DOI] [PubMed] [Google Scholar]
Van Egmond 2018
- Egmond MA, Schaaf M, Vredeveld T, Vollenbroek‐Hutten MM, Berge Henegouwen MI, Klinkenbijl JH, et al. Effectiveness of physiotherapy with telerehabilitation in surgical patients: a systematic review and meta‐analysis. Physiotherapy 2018;104(3):277‐98. [DOI] [PubMed] [Google Scholar]
WHO 1989
- World Health Organization Task Force on Stroke and Other Cerebrovascular Disorders. Recommendations on stroke prevention, diagnosis, and therapy: report of the WHO Task Force on stroke and other cerebrovascular disorders. Stroke 1989;20:1407‐31. [DOI] [PubMed] [Google Scholar]
WHO 2010
- World Health Organization. International classification of functioning, disability and health. who.int/classifications/icf/en/ (accessed prior to 12 January 2020).
WHO 2019
- World Health Organisation. WHO Guideline: recommendations on digital interventions for health system strengthening. who.int/publications‐detail/who‐guideline‐recommendations‐on‐digital‐interventions‐for‐health‐system‐strengthening (accessed prior to 12 January 2020).
Winters 2002
- Winters J. Telerehabilitation research: emerging opportunities. Annual Review of Biomedical Engineering 2002;4:287‐320. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Laver 2012
- Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD010255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laver 2013
- Laver KE, Schoene D, Crotty M, George S, Lannin NA, Sherrington C. Telerehabilitation services for stroke. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD010255.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


