Abstract
DNA lesions induce recruitment and accumulation of various repair factors, resulting in formation of discrete nuclear foci. Using superresolution fluorescence microscopy as well as live cell and quantitative imaging, we demonstrate that X-ray repair cross-complementing protein 1 (XRCC1), a key factor in single-strand break and base excision repair, is recruited into nuclear bodies formed in response to replication-related single-strand breaks. Intriguingly, these bodies are assembled immediately in the vicinity of these breaks and never fully colocalize with replication foci. They are structurally organized, containing canonical promyelocytic leukemia (PML) nuclear body protein SP100 concentrated in a peripheral layer, and XRCC1 in the center. They also contain other factors, including PML, poly(ADP-ribose) polymerase 1 (PARP1), ligase IIIα, and origin recognition complex subunit 5. The breast cancer 1 and -2 C terminus domains of XRCC1 are essential for formation of these repair foci. These results reveal that XRCC1-contaning foci constitute newly recognized PML-like nuclear bodies that accrete and locally deliver essential factors for repair of single-strand DNA breaks in replication regions.—Kordon, M. M., Szczurek, A., Berniak, K., Szelest, O., Solarczyk, K., Tworzydło, M., Wachsmann-Hogiu, S., Vaahtokari, A., Cremer, C., Pederson, T., Dobrucki, J. W. PML-like subnuclear bodies, containing XRCC1, juxtaposed to DNA replication-based single-strand breaks.
Keywords: DNA damage, PML nuclear bodies, superresolution microscopy
Induction of DNA damage results in recruitment and accumulation of various repair factors. It is tacitly assumed that the recruited repair proteins surround the damaged DNA regions. We have focused our attention on X-ray repair cross-complementing protein 1 (XRCC1). XRCC1 is commonly used as a molecular marker of single-strand DNA breaks (SSBs) because it is recruited rapidly to these lesions and forms distinct repair foci (1–5). Recruitment of XRCC1 to the sites of DNA damage is promoted by direct interactions with poly(ADP-ribose) (PAR) and the PAR polymerases PARP1 and PARP2 (6–8). It also coordinates and interacts with other factors involved in repair processes, such as polynucleotide kinase phosphatase (9), DNA polymerase β (6, 10), and DNA ligase IIIα (Lig IIIα) (11). XRCC1 contains 2 functionally important breast cancer (BRCA) 1 C terminus (BRCT) domains responsible for formation of XRCC1 dimers (12) and interactions with other proteins. BRCT2 domain is responsible for formation of XRCC1 dimers (12). It is known to interact with the BRCT domain of Lig IIIα (13, 14). It was demonstrated that interactions between BRCT2 domains of XRCC1 monomers were involved in XRCC1/Lig IIIα heterodimer formation (15). In turn, BRCT1 is thought to be essential for efficient repair of DNA damage caused by a methylating agent and postrepair replication reinitiation (16, 17). It interacts with the BRCT domain of PARP1 and with PARP2 (7, 8, 12, 18). The domain is required for recruitment of XRCC1 to the sites of DNA damage (19). It encompasses the phosphate binding pocket that is required for selective binding to PAR at low levels of ADP-ribosylation; this process is considered to be directly responsible for recruitment of XRCC1 molecules to the sites of DNA damage (20). It also promotes interactions with PARP1 and is essential for accumulation of XRCC1 at the sites of DNA damage induced using UVA laser light or hydrogen peroxide (21). It has been recently demonstrated that during S phase, XRCC1 is relocated to stalled replication forks, and both PARP1 and DNA-dependent protein kinase, catalytic subunit, are required in this process, while the presence of XRCC1 at the site of stalled replication forks is essential to ensure effective repair of small lesions and to restart the stalled forks (22). Interestingly, a higher number of XRCC1 foci in S phase than in G1 phase was reported (23).
We studied XRCC1 foci formed in a subpopulation of cells that develop a high number of SSBs occurring within active replication regions. Using quantitative and standard confocal laser scanning microscopy and superresolution imaging, we demonstrate that, surprisingly, these XRCC1 foci are always located in close vicinity to, rather than within or tightly wrapped around, replication sites containing the SSBs. Many of these repair foci contain SP100 nuclear antigen (SP100), a marker of promyelocytic leukemia (PML) nuclear bodies, the PML protein itself, PARP1, Lig IIIα, and another, newly described partner of XRCC1, a DNA replication licensing factor called origin recognition complex subunit 5 (ORC5). We propose that XRCC1 foci forming adjacent to replication-related SSBs are PML-like nuclear bodies that provide essential factors for the repair process. We also demonstrate that, while being responsible for various interactions, the two functional domains of XRCC1 are involved in accumulation of the protein and formation of a damage-induced nuclear body at the site of damage.
MATERIALS AND METHODS
Cell culture and treatment
Human HeLa cells and human primary skin fibroblasts (HSFs) were used. Cells were cultured in DMEM (MilliporeSigma, Burlington, MA, USA) supplemented with 10% fetal bovine serum (MilliporeSigma) and antibiotics in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The cells were cultured in T-25 flasks on plastic petri dishes (40-mm diameter) or in 12-well polystyrene plates (MilliporeSigma) and subcultured every 2 or 3 d using 0.25% trypsin solution. For imaging experiments, cells were seeded onto round coverslips (0.17 mm thick; 22 or 18 mm diameter; Menzel-Gläser, Braunschweig, Germany). In order to prepare samples for 3-dimensional (3D) structured illumination, microscopy cells were seeded onto square glass coverslips (18 × 18 mm, thickness 0.17 mm; Carl Zeiss, Jena, Germany). For immunofluorescent staining and detection of 5-ethynyl-2′-deoxyuridine (EdU) incorporation, cells were fixed with 3.7% formaldehyde for 15 min and permeabilized with 0.1% Triton X-100 (MilliporeSigma) in PBS for 10 min. Before imaging live cells, cell cultures were maintained on coverslips, submerged in DMEM/F-12 culture medium supplemented with 10% fetal bovine serum (in some long-term measurements, the duration of live cell imaging exceeded 48 h), no phenol red, and a content of sodium bicarbonate adjusted for equilibrium with air (pH 6.8–7.4). Fixed cells were imaged while maintained at room temperature in PBS. For 3D structured illumination microscopy imaging, coverslips with fixed cells were mounted on slides using ProLong Gold Antifade Mountant (Thermo Fisher Scientific, Waltham, MA, USA). For single-molecule localization imaging of monomeric red fluorescent protein (mRFP) and monomeric enhanced green fluorescent protein (mEGFP)-tagged proteins, cell samples were also embedded in ProLong Gold Antifade Mountant (Thermo Fisher Scientific).
Creation of site-specific XRCC1 amino acid variant constructs
Point mutations were introduced to the gene encoding XRCC1, which had been previously subcloned into the vector pmRFP-C1 using the Quik Change PCR technique. Phusion High-Fidelity DNA Polymerase and DpnI enzyme (Thermo Fisher Scientific) were used. Thus, 2 expression vectors encoding mutated proteins were produced: XRCC1Δ538−633 (the deletion mutant with no BRCT2 domain, where proline 537 was replaced with a stop codon) and XRCC1L360R (the XRCC1 mutant with an inactive, incorrectly folded BRCT1 domain). Leucine 360 was replaced with arginine as previously described (17, 24). Base changes were introduced with following primers: for XRCC1Δ538−633 (P537stop) forward primer: 5′-CCTGATCTGCCAGTCTAAGAGCTCCCAGATTTC-3′; and for XRCC1L360R (L360R) forward primer: 5′-GGACAGCACGCACAGAATCTGTGCCTTTG-3′. The underlined sections that consist of 3 nucleotides indicate the localizations of the introduced mutations, and are the aa sequences that replace the ones that are normally present in the sequence of XRCC1. The constructs were sequenced in order to verify the presence of the desired mutations and the absence of any additional changes that could occur due to PCR errors.
Transfection of cells
Cells (HeLa and HSF) grown for at least 24 h on coverslips, and those at about 60% to 80% confluence were transfected or cotransfected with vectors pmRFP-C1-XRCC1 (also with vectors pmRFP-C1-XRCC1Δ538−633 and pmRFP-C1-XRCC1L360R) and pcDNA3.1+-mEGFP-proliferating cell nuclear antigen (PCNA). We also cotransfected HeLa cells with vectors encoding fusion proteins: enhanced green fluorescent protein (EGFP)-XRCC1, EGFP-SP100, green fluorescent protein (GFP)-PARP1, EGFP–Lig IIIα or enhanced yellow fluorescent protein (EYFP)-ORC1, and EYFP-ORC5. Two different vectors encoding mRFP-XRCC1 were used as previously described (5). The construct used in preliminary experiments embraced the gene encoding XRCC1 containing 6 point mutations; it was lacking a stop codon, thus resulting in the presence of 23 additional amino acids linked to the protein. We confirmed, however, that the presence of these mutations did not influence the recruitment of XRCC1 to DNA lesions. Other vectors used in the major experiments contained the correct gene encoding XRCC1; it was ordered and synthesized by GenScript (Piscataway, NJ, USA) and subcloned into empty vectors pEGFP-C2 (Clontech Laboratories, Mountain View, CA, USA), and pmRFP-C1, obtained from Professor T. C. He (Molecular Oncology Laboratory, University of Chicago Medical Center, Chicago, IL, USA). Before transfection (FuGene HD Transfection Reagent; Promega, Madison, WI, USA), the standard culture medium was changed to Opti-MEM (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (MilliporeSigma). Transfection mixture (Opti-MEM without serum, transfection reagent, and vector DNA, all added in previously optimized proportions) was added directly to the culture medium. HSFs were transfected using reagents and techniques as previously described (25). Experiments were carried out 24 to 48 h after transfection. Vectors used in experiments were multiplicated in bacterial cells (Escherichia coli DH5α) that underwent standard heat-shock transformation. Isolation of vector DNA from bacteria was performed using a GenElute Plasmid Miniprep Kit (MilliporeSigma).
Immunofluorescent staining and DNA staining
After permeabilization, the fixed cells were rinsed with PBS and then treated with a 5% solution of bovine serum albumin (BSA; MilliporeSigma) in PBS overnight, and in 3% normal goat serum (NGS; Thermo Fisher Scientific) in PBS for 1 h to block nonspecific antibody binding. The samples were subsequently incubated in Tris-buffered saline (TBS)/1% BSA/0.2% Tween 20 solution for 30 min and then incubated for 1 h with a primary mouse monoclonal anti-XRCC1 antibody (ab 1838; Abcam, Cambridge, United Kingdom) or primary rabbit pAb to XRCC1 (phospho S518 + T519 + T523) (ab 84417; Abcam) diluted in 5% BSA. The samples were rinsed with TBS/0.2% Tween 20 solution twice, then with PBS, and incubated for 1 h with CyTM5-conjugated donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) or Alexa Fluor 633–conjugated goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific) diluted in either 5% BSA or 3% NGS, respectively, for 1 h, and rinsed again in PBS. In order to detect histone γH2A.X, PML protein, and SP100, samples were blocked with 5% BSA, incubated with primary mouse monoclonal anti–phospho-histone H2A.X (Ser139) antibody (MilliporeSigma), rabbit monoclonal (EPR16792) antibody to PML protein (ab179466; Abcam), or goat pAb to SP100 (N20) (sc-16328; Santa Cruz Biotechnology, Dallas, TX, USA) diluted in 5% BSA. Secondary antibodies were as follows: CyTM5-conjugated donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories), Alexa Fluor 488–conjugated goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific), or Alexa Fluor 546–conjugated donkey anti-goat IgG (H+L) (Thermo Fisher Scientific) diluted in 3% NGS or in 5% BSA. In order to detect PAR, fixed and permeabilized samples were rinsed with TBS. A blocking solution (TBS/1% BSA/0.2% Tween 20) was subsequently applied for 30 min; the samples were then rinsed with TBS/0.2% Tween 20 and incubated for 1 h with primary mouse mAb directed against PAR (ALX-804-220-R100, clone 10H; Enzo Life Sciences, Farmingdale, NY, USA) diluted in TBS/1% BSA/0.2% Tween 20. They were rinsed with TBS/0.2% Tween 20, incubated for 2 h with Alexa Fluor 488–conjugated goat anti-mouse IgG (H+L) (Thermo Fisher Scientific), and rinsed again with TBS/0.2% Tween 20. DNA in fixed and permeabilized cells was stained with DAPI (1 μM, 30 min, room temperature) (MilliporeSigma).
Proximity ligation assay
The protein–protein interactions and complexes formed between XRCC1 and PCNA were detected using reagents for in situ proximity ligation assay (PLA; Duolink PLA; Olink Bioscience, Uppsala, Sweden; MilliporeSigma). The reactions were carried out according to the manufacturer’s instructions, with some minor modifications. After fixation and permeabilization, cells were blocked with 5% BSA overnight, then incubated with primary antibodies diluted in 5% BSA for 1 h and subsequently washed in PBS overnight. All the following procedures were performed according to the manufacturer’s instructions. Primary antibodies were mouse monoclonal anti-XRCC1 antibody (ab 1838; Abcam) and rabbit monoclonal anti-PCNA antibody (EPR3821) (ab 92552; Abcam). Secondary antibodies conjugated with oligonucleotides were Duolink In Situ PLA Probe Anti-Mouse MINUS, Affinity Purified Donkey Anti-Mouse IgG (H+L), and Duolink In Situ PLA Probe Anti-Rabbit Plus, Affinity Purified Donkey Anti-Rabbit IgG (H+L). Samples were washed using Duolink In Situ Wash Buffers. The fluorescence signal was generated using either Duolink In Situ Detection Reagents Red or Duolink In Situ Detection Reagents Green. The preparations were mounted with ProLong Gold Antifade Mountant (Thermo Fisher Scientific).
Detection of DNA replication
We detected regions of newly replicated DNA using a method of incorporating a nucleoside analog of thymidine, EdU (30 min), and staining it fluorescently by means of a “click” reaction according to the manufacturer’s instructions (Click-iT EdU Alexa Fluor 488 Imaging Kit; Thermo Fisher Scientific). DNA synthesis was measured by detecting the incorporated EdU. This technique was used to detect nascent DNA both in cells expressing mRFP-XRCC1 and wild-type cells used for immunofluorescence (IF).
Detection of DNA damage
DNA damage was detected using a modified version of TUNEL assay (data not shown) enabling amplification of immunofluorescent signal of bromodeoxyuridine staining.
Microscopy and image analysis
Confocal microscopy
Images were recorded using a Leica TCS SP5 II confocal laser-scanning microscope (Leica, Wetzlar, Germany), equipped with a stage microincubator for live cell imaging and a 63× HCX PL APO CS NA 1.4 oil immersion lens. The following instrumental parameters were used: excitation 405 nm (pulsed laser, Chaser UV), 488 nm (Ar), 561 nm (HeNe), 594 nm (HeNe), and 633 nm (HeNe), emission detection bands 498–540 nm for Alexa Fluor 488 (Click-iT EdU) and EGFP (mEGFP-PCNA and EGFP-SP100 fusion proteins), 600–700 nm or 590–620 nm (in the case of triple staining in combination with Alexa Fluor 488 and CyTM5) for mRFP (mRFP-XRCC1 fusion protein), and 660–800 nm for CyTM5 (immunofluorescent detection of histone γH2A.X). Signals in different channels were detected with photomultipliers (gain 800–990 V, offset −0.1%). Coverslips with cells were mounted in a custom-made steel holder, and the samples were placed on a microscope stage. Sequential fluorescence live cell confocal imaging was performed to image endogenous XRCC1 foci in time and analyze their dynamics. Image stacks (1–1.5 μm thick) were recorded in time, with time interval ranging from 18 s to 4 min. Images presented in Supplemental Fig. S2 (Supplemental Movies S3–S6, available at http://helios.wbbib.uj.edu.pl/publikacje) are sequences of maximal projections of deconvolved and aligned images recorded using live cell 3D confocal imaging. The stacks were 1 μm (Aa, Ba, b) or 1.5 μm (Ab, c, Bc) thick, and each stack was registered with either 4 min (Aa, Ba, b) or 18 s (Ab, c, Bc) long intervals. Cells presented in Supplemental Fig. S1 (Supplemental Movies S1 and S2, available at http://helios.wbbib.uj.edu.pl/publikacje) were recorded sequentially for many hours. Images (single focal planes) were acquired every 30 min. Additionally, quantitative analysis of deconvolved 3D stack images was carried out. Images were analyzed by ImageJ software (26). Some images (see Figs. 1, 3, and 4 and Supplemental Figs. S2, S4–S6) were deconvolved using SVI 3D Huygens Deconvolution and Analysis Software (Scientific Volume Imaging, Hilversum, The Netherlands). Tracking and analyses of foci trajectories were also performed using a module of the Huygens software; the movement of the whole nucleus was taken into account.
Figure 1 .
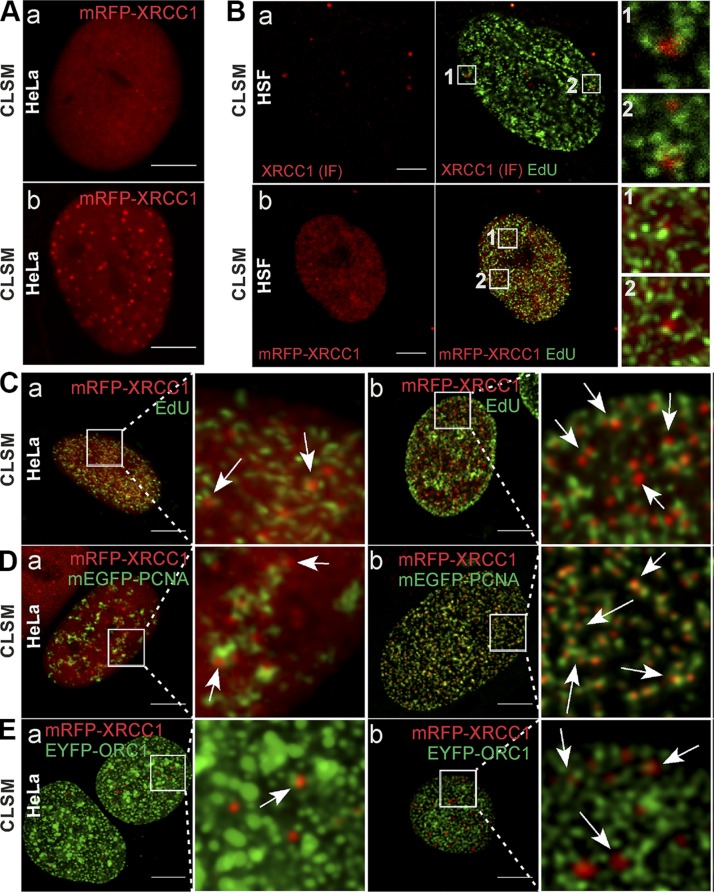
Association between XRCC1 foci and replication sites in HeLa and HSF cells. A) Examples of HeLa nuclei containing very few (a) or large numbers of XRCC1 foci (mRFP-XRCC1, red) (b) in live cells. B) XRCC1 foci detected by IF (a, red) or imaging of mRFP-XRCC1 (b, red), and replication sites (EdU, green) demonstrating their close spatial association (enlarged fragments of images) in nuclei of normal HSFs. C) XRCC1 foci [low (a) or high (b) number] and neighboring replication sites (arrows) in cells expressing mRFP-XRCC1; regions of active DNA replication visualized via EdU (green). D) XRCC1 foci [low (a) or high (b) number] and neighboring replication sites (arrows) in cells expressing mRFP-XRCC1; replication regions visualized via mEGFP-PCNA (green). E) XRCC1 foci [low (a) or high (b) number] and neighboring replication sites (arrows) in cells expressing mRFP-XRCC1; regions of early replication visualized via EYFP-ORC1 (green; note that EYFP fluorescent signal is represented by green color instead of yellow in all images in order to avoid confusion with colocalization analyses, where overlap of green and red is represented by yellow color). Microscopy images were acquired using confocal laser scanning microscopy. Scale bars, 5 μm; ROI, 5 × 5 μm.
Figure 3 .
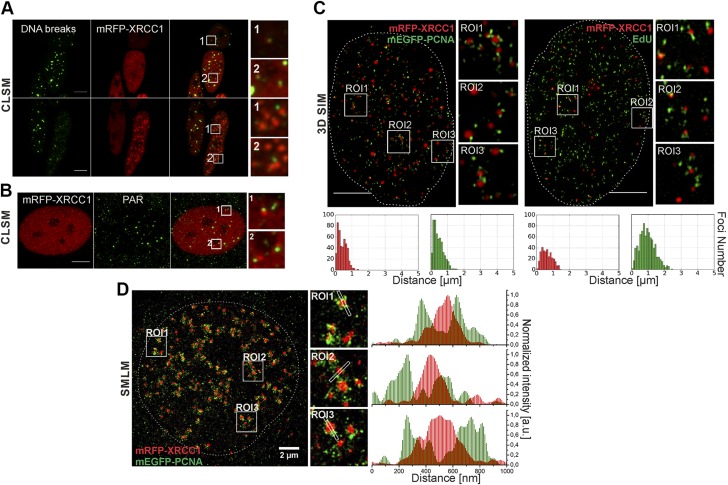
Direct detection of free DNA ends (DNA breaks), XRCC1 foci, and replication sites, and superresolution imaging of positions of XRCC1 foci and replication sites. A) Localization of DNA breaks (improved TUNEL assay, green) outside or within periphery of XRCC1 foci (mRFP-XRCC1, red). B) Localization of PAR chains (IF, green) detected always outside or within periphery of XRCC1 foci (mRFP-XRCC1, red). C) XRCC1 (mRFP-XRCC1, red) and replication (mEGFP-PCNA or EdU) in superresolution images (3D SIM). XRCC1 regions appear as tightly packed foci or nuclear bodies. Images and histograms of distances between barycenters of XRCC1 foci and small replication regions in these images confirm spatial association between XRCC1 foci and sites of active replication. Only some sites of active replication develop SSB and have adjacent XRCC1 focus (Supplemental Fig. S3B). Two maxima in these histograms indicate that there are two subpopulations of XRCC1 foci and two subpopulations of replication foci. XRCC1 foci of one subpopulation are located very close to sites of active replication (presumably groups of replication forks). Foci of other group have no adjacent replication region; these foci most likely represent stress bodies formed afar of sites of DNA damage (5). Only some sites of active replication develop SSB and have adjacent XRCC1 focus. Supplemental Figure 3B shows more examples of histograms corresponding to other 3D SIM superresolution images of XRCC1 foci and replication sites. D) Superresolution single-molecule localization image shows nucleus expressing mRFP-XRCC1 and mEGFP-PCNA. Replication sites consist of numerous small objects, while XRCC1 foci appear as larger solid structures. XRCC1 foci are adjacent to but do not overlap regions of replicating DNA (fluorescence profiles). Scale bars, 5 μm. ROI: 3.5 × 3.5 μm (A); 3.2 × 3.2 μm (B); 2.7 × 2.7 μm (C, D).
Figure 4 .
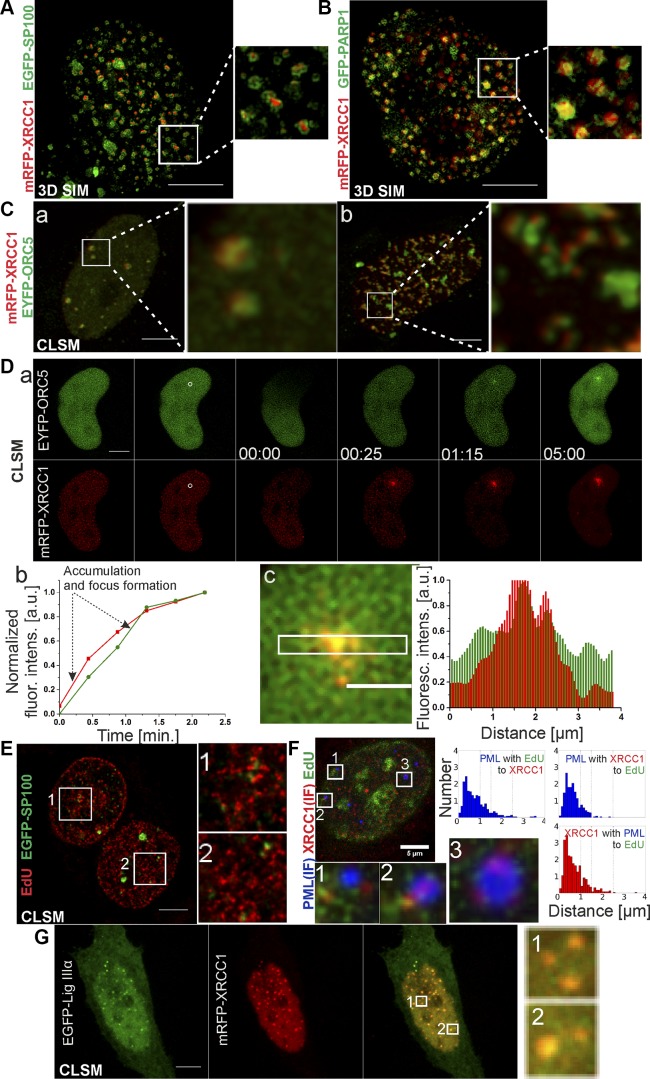
Components and architecture of nuclear foci formed by XRCC1, imaged by 3D SIM and fluorescence confocal microscopy. A) XRCC1 (mRFP-XRCC1, red) foci contain SP100 (EGFP-SP100, green). XRCC1 foci are tightly associated with SP100. 3D SIM images (equatorial slice, maximum projection) show that SP100 forms shells of spherical nuclear bodies, wrapped around subcompartments containing XRCC1. B) XRCC1 (mRFP-XRCC1, red) foci contain PARP1 (GFP-PARP1, green). PARP1 foci are generally smaller than foci of XRCC1. Their diameter ranges between 100 and 400 nm. 3D SIM images show that usually few small PARP1 foci are closely adjacent to single XRCC1 focus most likely belonging to same nuclear structure. C) Confocal imaging demonstrates that XRCC1 (mRFP-XRCC1, red) foci colocalize (a) or are adjacent to (b) ORC5 (EYFP-ORC5, green) foci, marking early replication origins. D) ORC5 (EYFP-ORC5, green) is recruited to local DNA damage ∼30 s after XRCC1 (red) which is presented in the sequential confocal images (a). Accumulation of these 2 proteins in time is illustrated (b). Mean fluorescence intensity values measured in the area of accumulation of the proteins were normalized to the values of the mean fluorescence intensity measured in the undamaged area of the nucleus. The accumulation of proteins and foci formation are represented by the increased steepness of the curves. Fluorescence intensity profiles additionally confirm accumulation of the proteins in the same area (c), colocalization of XRCC1 and ORC5 foci is represented by an overlap of the green and red fluorescent signals which results in the yellow color in the enlarged part of the merged image (c). E) SP100 (EGFP-SP100) foci (green) are closely adjacent to replication sites (EdU, red), similar to previously described XRCC1 foci. F) Often XRCC1 (mRFP-XRCC1, red) is found within PML bodies (IF, blue). Histograms of distances between centers of foci containing both PML and XRCC1 to replication foci (EdU, green) demonstrate that when PML body is located in close vicinity of replication region, it always comprises XRCC1 component. G) Confocal imaging shows that nuclear bodies rich in mRFP-XRCC1 (red) colocalize with EGFP-Lig IIIα foci (green); overlapping green and red fluorescent signals result in conspicuous yellow color, clearly visible in enlarged areas of merged image, thus confirming presence of Lig IIIα in XRCC1-containing PML nuclear bodies. Scale bars, 5 μm (A–C, Da, E–G), 2 μm (Dc); ROI: 3.4 × 3.4 μm (A, B); 3.6 × 3.6 μm (Ca); 4.25 × 4.25 μm (Cb); 5.3 × 5.3 μm (E); 2.1 × 2.1 μm (F); 2.5 × 2.5 μm (G).
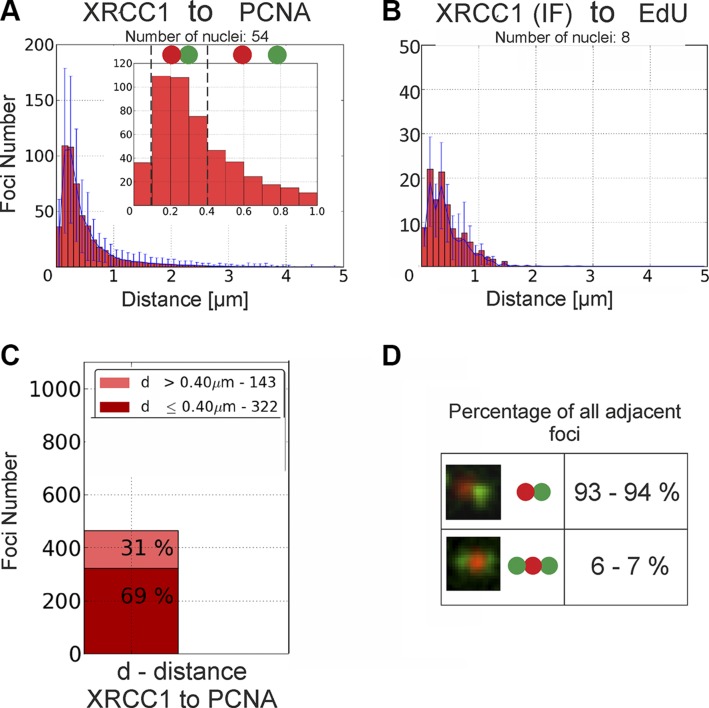
Histograms of distances between objects in microscopy images—for instance, XRCC1 foci and replication regions—were constructed as previously described (27). Taking the size of XRCC1 and replication foci into consideration (300–400 nm in diameter), we categorized the objects as adjacent when the distance between their centers was 400 nm or smaller. The analysis was modified in the case of histograms presented in Fig. 4F. In this particular case, the algorithm analyzed the localization of 3 different entities: immunofluorescently labeled PML-containing nuclear bodies, immunofluorescently labeled XRCC1 foci, and replication regions (EdU). The PML nuclear bodies and XRCC1 foci were scored as colocalizing when the distance between their centers of mass was <1 µm. One micrometer was the criterion used in the classification of objects as the nearest neighboring ones. This value resulted from the size of PML nuclear bodies recorded in the confocal images. First the algorithm fished out the spatially associated pairs of objects (one of which was always a PML nuclear body), and it subsequently measured and analyzed their distance toward their nearest neighbor of the third type.
Structured illumination microscopy
Images of subdiffraction resolution were obtained using two 3D structured illumination microscopes: the microscope based on a prototype of the commercial model Deltavision OMX V2.0 (Applied Precision, Issaquah, WA, USA) and OMX V3 (API; GE Healthcare, Chicago, IL, USA) superresolution microscope platform based on structured illumination. The fluorophores were excited with 405, 488, 561, and 592 nm laser lines, as applicable, and the emitted fluorescence was filtered using properly assorted filters. In order to project the grating’s image plane onto the sample, two types of objectives were used: ×60/1.4 oil objective and ×100/1.4 oil objective (Olympus, Tokyo, Japan) and 1.514 refractive index immersion oil. Each channel was imaged by a dedicated Cascade II 512 EM-CCD camera (Photometrics, Tucson, AZ, USA), which used 50, 70, or 100 ms exposures, 1 preamp gain, and variable electron-multiplying gain. Acquisition was controlled by the OMXN controller software (Applied Precision), while reconstructions and alignments were made with the OMX-specific SoftWoRx 4.5.0 software package (Applied Precision). Each channel required 5 exposures for a given angle and 3 different angular grating positions; thus, in total there were 15 exposures per optical slice. In order to obtain 3D images, each sample was translated vertically in steps of 125 nm. The images were analyzed by ImageJ software or SVI 3D Huygens Deconvolution and Analysis Software.
Single-molecule localization microscopy
Localization microscopy of fluorescent proteins (28, 29) was performed on a microscopy setup described by Szczurek et al. (30). Fluorescent proteins are known to faintly photoswitch and are susceptible to rapid photobleaching. In this case, cells were relatively bright—that is, fluorophore abundance did not constitute an issue. High-intensity light illumination was applied to provoke blinking of mRFP and mEGFP: 1.5 kW/cm2 561 nm and 1.5 kW/cm2 491 nm illumination, respectively. This was followed by an acquisition of 8000 frames in the 585–636 nm detection channel (mRFP) and 5000 frames in the 500–550 nm detection channel (mEGFP). For data visualization, each localized signal was assigned to a joint localization map and blurred with 20 nm Gaussian kernel. Chromatic shift between 2 detection channels above the coverslip was corrected by applying manually extracted shift vector between 2 reconstructed images of microtubule structures immunofluorescently labeled with 2 respective fluorophores simultaneously (Alexa Fluor 488 and Alexa Fluor 647).
DNA damage induction
Local DNA damage was induced with focused visible laser light in cells maintained under optimal growth conditions on the stage of Leica TCS SP5 II confocal microscope, as previously described (4, 5). Regions of interest (ROI) (2.5 × 2.5 μm or 1 × 6.25 μm, or bleached point) were illuminated with laser line 488 nm (83 μW delivered to the sample) by either scanning ROI several times (6, 10, or 7 scans in Fig. 5A, Cc, and De, respectively) or by a parking laser beam for 5 s (Fig. 4D). ROI were scanned at a resolution of 512 × 512 pixels and at a scanning speed of 200 lines per second.
Figure 5 .
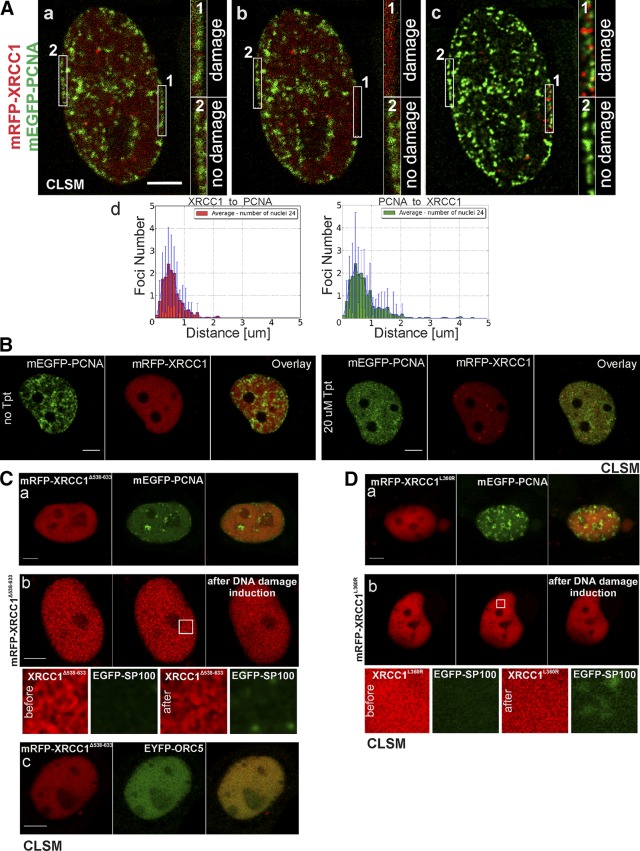
Formation of XRCC1 foci in response to local DNA damage induced by focused light (A) or topoisomerase inhibitor topotecan (20 µM) (B), and nuclear localization of XRCC1 mutants. A) Image of cell nucleus in middle S phase (a–c) before induction of local DNA damage (a), immediately after damage [1] (b), and 4 min after damage (deconvolved maximum intensity projections of 3D stacks) (c), showing distinct XRCC1 (mRFP-XRCC1) foci in damaged area [1]. No repair foci are present in intact control region [2] (labeled “no damage”). Foci are always adjacent to replication sites (mEGFP-PCNA) and they never overlap, as shown by histograms representing damaged ROI (d); ROI: 6.25 × 1 μm. B) XRCC1 (mRFP-XRCC1) and PCNA (mEGFP-PCNA) before and 1.5 h after exposure to topotecan. Topotecan inhibits replication, leads to disappearance of replication sites, and induces DNA breaks. XRCC1 repair foci are formed after dismantling of replication foci (bottom). They are likely formed in response to SSBs induced by topotecan interfering with replication or transcription. C) Images of fluorescently (mRFP) tagged XRCC1 with deleted BRCT2 domain (XRCC1Δ538−633) (red); in replicating cell coexpressing mEGFP-PCNA (green), no XRCC1Δ538−633 foci can be detected in close vicinity of replicating DNA or anywhere in nucleu (a); XRCC1Δ538−633 is not recruited to repair foci containing SP100 (green) formed in response to light-induced DNA damage (30 min after damage induction) (ROI, 2.5 × 2.5 μm) (b); impaired mechanism of XRCC1Δ538−633 foci formation leads to absence of ORC5 foci (green) in nuclei (c). D) Images of fluorescently (mRFP) tagged XRCC1 with mutated BRCT1 (XRCC1L360R) (red); (a) XRCC1L360R does not form typical repair foci located adjacent to replication sites (tagged with mEGFP-PCNA, green); (b) XRCC1L360R is not recruited to repair foci containing SP100 (green) formed in response to local DNA damage (ROI, 2.5 × 2.5 μm) (10 min after damage induction). Scale bars, 5 μm.
In order to induce single-strand DNA breaks in the position of replication forks, topotecan was added to growth medium, in which cells were maintained on the stage of Leica TCS SP5 II confocal microscope, to a final concentration of 20 μM. Sequential 3D imaging was performed in order to observe the effects in time.
The presented results were partly obtained for the cells expressing fluorescently labeled XRCC1. However, it is important to emphasize that the level of endogenous protein was significantly decreased in the cells expressing the fusion protein (data not shown). Moreover, all the experiments were performed using cells characterized by low levels of expression of the fusion protein.
RESULTS
Association between XRCC1 foci and replication sites: proximity without overlap
Recruitment of XRCC1 to DNA lesions is conspicuous because of a high local concentration of the recruited protein. Among HeLa cells and HSF in culture, there is a small population of cells that contain very high numbers (over 10, or even a few hundred per a single cell nucleus) of XRCC1-containg foci (Fig. 1A). These cells constitute ∼5% of an entire cell population and show signs of spontaneous massive replication-related DNA damage that leads to rapid recruitment of XRCC1 (Fig. 1 and Supplemental Fig. S1A, B; the process of XRCC1 recruitment and foci formation is shown in Supplemental Movies S1 and S2, available at http://helios.wbbib.uj.edu.pl/publikacje). We noticed that the recruited XRCC1 formed distinct nuclear foci, and most of these foci were adjacent to replication sites (detected with EdU, PCNA, or ORC1) (Fig. 1). As would be expected for objects bound to chromatin, these XRCC1 foci showed only limited mobility (∼0.5 µm within 7–15 min; Supplemental Fig. S2Aa, b, c, and Supplemental Movies S3 and S4, available at http://helios.wbbib.uj.edu.pl/publikacje). Only a few XRCC1 foci were moving in one direction over long distances (several micrometers) rather than in a Brownian fashion (∼2 µm within 30 min; Supplemental Fig. S2Ba, b, and Supplemental Movies S5 and S6, available at http://helios.wbbib.uj.edu.pl/publikacje). In some cases the direction of their movement was visibly correlated with the position of nearby replication sites. For instance, the XRCC1 focus shown in Supplemental Fig. S2Ba and S2Bc moved directly from one replication region to another (Supplemental Movies S5 and S6).
We next carried out quantitative analysis of the numbers of adjacent foci, and the distances separating them (27) in order to objectively judge the tendency of these foci to reside near the sites of replicating DNA. In accordance with the general visual impressions, we found that the distances separating XRCC1 foci and replication sites were in the range of 100 to 400 nm (i.e., close but not overlapping) (Fig. 2 and Supplemental Fig. S3A). Sixty-nine percent of all repair foci were localized in close vicinity of replication regions (Fig. 2C), suggesting some spatial and functional association. We conclude that indeed formation of XRCC1 foci had a clear preference for the regions of replicating DNA. Interestingly, ∼6 to 7% of all XRCC1 foci had not just one but two distinct neighboring replication regions, regardless of the substage of S phase (Fig. 2D), possibly representing the reactivated replication machinery near the site of the damage. These observations raise several intriguing questions regarding the functional significance of the association of XRCC1 foci with replication-related DNA damage sites, the molecular structure of the XRCC1 foci, and the reason behind recruitment of a high number of XRCC1 molecules within one focus.
Figure 2 .
Quantitative analyses of spatial positions of XRCC1 foci relative to replication sites in cells exhibiting high number of XRCC1 foci [live (A), IF (B)], showing their close association but little overlap. A) Histogram of distances measured from barycenters of mRFP-XRCC1 repair foci to barycenters of nearest replication sites (mEGFP-PCNA). Inset shows enlarged fragment of histogram and presents results obtained for range of distances between 0 and 1 µm. Low number of cases where distance between neighboring foci is smaller than 100 nm suggests occurrence of nearly no or very little overlap between XRCC1 foci and replication sites; see also analysis of all substages of S phase in Supplemental Fig. S3A. B) Histogram of distances measured from barycenters of immunofluorescently labeled XRCC1 foci (IF) to replication foci (EdU). C) Percentage of XRCC1 foci located close to or far from of nearest replication site. D) Percentage of all XRCC1 foci (analyzed in A and B) classified as adjacent to replication regions that have either only one or two neighboring replication regions in close vicinity.
Imaging of the XRCC1 foci adjacent to replication sites
A close proximity, without an overlap, of XRCC1 foci with replication sites does not agree with the generally held view, which assumes that the recruited repair factors form a focus surrounding the lesion (in this case, centers of repair foci should represent the positions of DNA lesions). Therefore, to determine if the DNA breaks were surrounded by the repair proteins, forming a focus, we studied the exact positions of DNA lesions using improved TUNEL as well as PAR staining (Fig. 3A, B and Supplemental Fig. S4B, D. DNA breaks (single and double strand; Supplemental Fig. S5A), while positioned within replication sites, were consistently detected adjacent to or in the peripheral regions of XRCC1 repair foci, but not in their centers. Standard confocal laser scanning microscopy images do not have the spatial resolution required to show the individual replication forks or their groups embraced by one replication site; nor can this method of imaging be expected to demonstrate the internal structure of the replication-associated XRCC1 foci. Therefore, we resorted to two superresolution (subdiffraction) imaging techniques: 3D structured illumination microscopy (3D SIM) and single-molecule localization microscopy (SMLM). The 3D SIM images (Fig. 3C) showed the replicating regions, consisting of several local maxima or individual spots likely representing groups of active replication forks (PCNA) or stretches of nascent DNA (EdU), and usually just one adjacent prominent XRCC1 focus. Thus, superresolution 3D SIM images confirmed that one XRCC1 focus was usually associated with a replication site, which embraced a number of actively replicating subregions. Quantitative analyses of distances between centers of XRCC1 foci and regions embracing groups of replication sites were performed to confirm the findings based on confocal imaging. Indeed, we found that repair foci were always adjacent to, but never overlapped, regions rich in replication machinery (Fig. 3C and Supplemental Fig. S3B).
In superresolution single-molecule localization images, the individual regions of replicating DNA were also resolved into many foci representing groups or even individual closely spaced active replication forks (Fig. 3D). In agreement with 3D SIM imaging, single-molecule localization images demonstrated that XRCC1 foci were in fact clusters of tightly packed XRCC1 molecules, indicating that they were components of large nuclear structures positioned near replication sites. The overall size of these XRCC1 clusters was similar to the size observed in confocal images (i.e., 300–400 nm in diameter), thus indicating their real size. The large size of XRCC1 foci, resembling nuclear bodies, may explain why they were found in interchromatin compartment, but never in the areas of high DNA density (Supplemental Fig. S5B).
SP100, PML, PARP1, and ORC5 are components of damage-induced XRCC1 foci
To identify the type of a replication-related XRCC1-containing nuclear body, we searched for other proteins associated with this structure. We applied PLA to look for a previously described partner of XRCC1, PCNA (31), but did not detect XRCC1-PCNA complexes inside the foci. Interestingly, a number of XRCC1-PCNA complexes were found in close proximity to replication sites (Supplemental Fig. S4C).
Superresolution 3D SIM imaging demonstrated the presence of SP100, a protein known to be involved in the formation of PML nuclear bodies (32–35) in the XRCC1 foci (Fig. 4A and Supplemental Fig. S6A). The 3D SIM superresolution images revealed the internal architecture of the repair foci, which was not revealed by standard confocal images. SP100 forms a characteristic protein shell that wraps around a central core containing XRCC1 (Fig. 4A). This observation agrees with the description of 3D organization of SP100-containing nuclear bodies, which was revealed by 4Pi superresolution microscopy (35) and other microscopy techniques (36). We also demonstrate that the investigated SP100-containing nuclear bodies are always localized in close vicinity to but outside regions of active DNA replication (Fig. 4E). Moreover, we note that a subgroup of PML bodies directly detected by immunofluorescent staining of PML protein was shown to overlap some of the XRCC1-containing foci (Fig. 4F and Supplemental Fig. S6B), thus underscoring a similarity between these foci and PML bodies. We also found that PARP1, a known factor in SSB repair (37–39), was a component of XRCC1 foci (Fig. 4B). In an attempt to detect early replication origins and their localization in relation to XRCC1 repair foci, we labeled the ORC1 and another component of origin recognition complex, ORC5. To our surprise, in some cases ORC5 was also present within the nuclear bodies containing XRCC1 (Fig. 4Ca). We also identified ORC5 in foci formed near experimentally induced local DNA damage (Fig. 4D). In cases where ORC5 formed conspicuous foci positioned in a manner resembling well-known replication patterns of PCNA (40), XRCC1 foci were located in close vicinity of the regions rich in ORC5 (Fig. 4Cb). All these observations reinforce a notion that the foci containing XRCC1 are PML-like nuclear bodies and are juxtaposed with, but do not surround, the DNA lesions.
XRCC1 protein is known to interact and form complexes with another repair protein factor that is involved in SSB repair, Lig IIIα (11, 13–15, 41). Here we demonstrate, by investigating nuclear localization of EGFP-Lig IIIα in live HeLa cells, that the endogenous nuclear bodies that contain mRFP-XRCC1 are also rich in Lig IIIα (Fig. 4G). However, direct detection of XRCC1-Lig IIIα complexes using PLA did not confirm their presence inside these heterogeneous nuclear structures (data not shown).
Another question arises as to the exact location of posttranslationally modified repair factors in relation to the XRCC1 foci (Supplemental Fig. S4A, B). We found both unmodified XRCC1 molecules and the ones containing the sites phosphorylated by CK2 kinase (casein kinase II) (Supplemental Fig. S4A) within the foci. Interestingly, however, PAR polymers were never found within XRCC1 foci, yet they were often detected in localizations adjacent to the foci (Fig. 3B and Supplemental Fig. S4B), in agreement with our notion of the lesions and the foci being spatially separate.
Explicit connectivity of novel XRCC1 foci to single-strand break repair
To explore the hypothesis that the XRCC1 foci are formed adjacent to DNA lesions induced in replication sites rather than surrounding them, we next induced DNA damage in an elongated region embracing the nuclear envelope in a mid–S-phase cell. In this area, active replication sites mark heterochromatin. Almost all XRCC1 foci were formed just outside of the replication sites (Fig. 5A). Although the damage was distributed randomly, nuclear bodies were always formed immediately next to the region of heterochromatin undergoing replication. In another experiment, where DNA lesions were induced by the topoisomerase inhibitor topotecan (which leads to dismantling of replication foci), formation of XRCC1 foci was dependent on the induction of DNA damage but not on the presence of replication foci (Fig. 5B).
Roles of BRCT1 and BRCT2 domains of XRCC1 in foci formation
In order to obtain insight into the mechanism of formation of repair foci and the role played by XRCC1 in the repair process, we investigated the behavior of mutated XRCC1, devoid of BRCT2 domain, or with an inactivated BRCT1 domain. The XRCC1 deletion mutant with no BRCT2 domain (XRCC1∆538−633) was localized in the nucleus. However, regardless of the level of expression or the stage of the cell cycle, the mutated protein did not form characteristic foci of the type that are always formed by a wild-type XRCC1 (Fig. 5Ca). The absence of foci with XRCC1∆538−633 could be interpreted as a consequence of a general failure to form PML-like bodies at these sites. However, this was not the case because we found PML bodies in cells expressing the mutant by detecting foci of SP100 (data not shown). The truncated protein was not recruited to the nuclear bodies; nor did it form foci in response to local DNA damage. However, nuclear repair bodies containing SP100 were formed after DNA damage induction (Fig. 5Cb). It is therefore likely that the removal of BRCT2 abolished the process of recruitment of XRCC1 to constitutive PML nuclear bodies, but not the endogenous ability of a cell to form these bodies. In accordance with this hypothesis, no XRCC1∆538−633 foci were seen accompanying replication, although replication sites were readily detectable (Fig. 5Ca). In cells expressing EYFP-ORC5 and mRFP- XRCC1∆538−633, no foci containing these proteins could be detected (Fig. 5Cc). Aggregation of XRCC1, which was usually seen in cells entering apoptosis, was absent in cells expressing XRCC1∆538−633 (data not shown).
XRCC1 mutant with inactive BRCT1 domain (XRCC1L360R) was imported into nuclei and detected in both nonreplicating and replicating cells, but it did not form small foci of the type seen for the wild type or the wild-type protein tagged with a fluorescent protein (Fig. 5Da). XRCC1L360R was not recruited to local DNA damage, although nuclear bodies containing SP100 were formed in the damaged region (Fig. 5Db).
DISCUSSION
XRCC1 foci recruited to SSBs at replication sites form juxtaposed PML-like bodies
Over the years, when proteins have been identified at certain nuclear sites by immunostaining or fluorescent protein expression, there has been a consensus of functional relevance because the spatial concordance is high. There is often supporting biochemical evidence for the functional roles of the sighted proteins. However, in the present study, we came upon a different situation, and to the extent it is different, we find it of particular interest. The quantitative confocal microscopy and superresolution images of XRCC1 foci and replication sites presented here demonstrate that numerous XRCC1 molecules accumulate in the regions immediately near (rather than coincidently) the endogenous DNA lesion induced in replicating DNA, and they form a large aggregate. If the recruited XRCC1 molecules were directly involved in postreplication DNA repair, then their images should coincide with the images of replication sites. However, the centers of most of these foci are separated by a relatively large distance, ranging between 100 and 400 nm. Moreover, the number of the recruited XRCC1 molecules [estimated in (42)] exceeds by far the estimated number of the lesions. This is in agreement with the previously reported recruitment of many molecules of repair factors to a local (possibly individual) DNA damage site (4, 5, 43).
Our superresolution images demonstrate that XRCC1 is not simply recruited to the site of a SSB as a featureless cloud. Instead, distinct foci containing XRCC1 are formed and exhibit a complex architecture and various degrees of mobility. We note that binding of XRCC1 molecules to PAR chains, known to be attached to the repair protein factors and nucleic acids directly at the location of the DNA lesion, takes place outside of the XRCC1 repair foci, frequently in the regions adjacent to these structures. The formation of the topotecan-induced XRCC1 foci after dismantling of replication factories indicates that it is the DNA lesion, not a replication focus, which is required for the assembly of XRCC1 body.
The XRCC1-containing nuclear bodies are the regions of accumulation of both the unmodified variants and the CK2 phosphorylated molecules of XRCC1. Phosphorylation of this type is required for stabilization of the protein, the stimulation of the interactions and complex formation with other repair factors, and efficiency of repair of SSBs (44). Additionally, it has been shown that it reduces affinity of XRCC1 to DNA (45). The presence of CK2 phosphorylated and unmodified protein inside the XRCC1 foci suggests that there may be an exchange of molecules between these bodies and the sites of DNA damage. Thus, the XRCC1 foci could play a role of molecular reservoirs responsible for storage, delivery, and exchange of the repair factors, in unmodified and modified forms, prepared for functional complex formation at the sites of DNA lesions.
The fact that the XRCC1 foci we imaged were also located close to ORC5 or ORC1 foci is also in agreement with our notion of a spatial correlation between XRCC1 foci and regions of replication. Surprisingly, however, in response to DNA damage, ORC5 was also detected inside the preformed nuclear bodies containing XRCC1. ORC5 is known to be involved in transcription silencing in yeast and is also regarded to be involved in heterochromatin remodeling (46–51); therefore, its presence, along with XRCC1 in eukaryotic cells, could imply that the structure, which we originally detected as an XRCC1 focus, was in fact a multifunctional aggregate of the many proteins responsible for various DNA damage-response functions, including local inhibition of transcription, which we also observed (data not shown). We note that some classes of PML nuclear bodies have been reported to contain and deliver repair factors to the sites of DNA lesions (52–56). The nuclear bodies we describe here resemble PML bodies in terms of their dynamics as well. Similar to previous observations (57), most showed saltatory movements in the vicinity of the DNA lesions; only some performed movements over longer distance.
The fact that we detected XRCC1 foci adjacent to, but not overlapping with, the replicating heterochromatin at the nuclear envelope may have been due to an inability of these nuclear bodies to penetrate into heterochromatin blocks. It is also possible that the repair factors do not have to penetrate into such heterochromatin blocks because the stretch of DNA that contains a SSB is moved toward the periphery of a heterochromatic region to facilitate interaction with repair factors concentrated in the newly formed nuclear body. These observations, combined with the fact that SSBs detected by improved TUNEL were located at the edge of XRCC1-containing bodies, lead us to propose a model of spatial arrangement of the replication-related DNA lesion and the repair focus.
How might PML-like bodies assemble at DNA lesions in replication sites?
Removal of the BRCT2 domain abolished 3 key features of XRCC1: formation of the typical nuclear XRCC1 foci in intact cells, recruitment and accumulation of this protein at the sites of endogenous DNA damage induced within replicating DNA, and accumulation at local DNA lesions (4, 5). A lack of BRCT2 domain could also be a reason for a loss of an ability to interact with ORC5 and a hampering of the recruitment of ORC5 to the nuclear bodies. The process of BRCT2-driven formation of XRCC1 dimers (12) and interactions between XRCC1 molecules with other repair factors (13–15) may be crucial for accumulation of the repair protein in the form of a nuclear body positioned in the vicinity of the damage in replication sites.
BRCT1 is thought to be essential for postrepair replication reinitiation (16, 17). Deactivation of BRCT1 domain abolished an ability of XRCC1 to accumulate at the sites of endogenous or experimentally induced DNA damage. Because BRCT1, which contains a PAR-binding pocket, exhibits affinity to PAR and PARP1 (7, 8, 12, 18, 20, 21), we suggest that XRCC1 with deactivated BRCT1 domain is devoid of the ability to interact with PCNA; it is thus unable to form properly localized nuclear structures and cannot be recruited to the nuclear bodies localized at the sites of DNA damage. Therefore, we suggest that undisturbed binding of XRCC1 molecules to PAR and PARP1 is required for recruiting XRCC1 to nuclear bodies formed in response to DNA damage. All these data, taken together, lead to a conclusion that both domains, BRCT1 and BRCT2, are involved in the process of accumulation and in the mechanism of incorporation of XRCC1 into nuclear bodies in response to endogenous DNA damage occurring in replication sites as well as locally induced DNA damage.
XRCC1 response to replication-related SSBs: model of spatial organization of process of repair protein accumulation
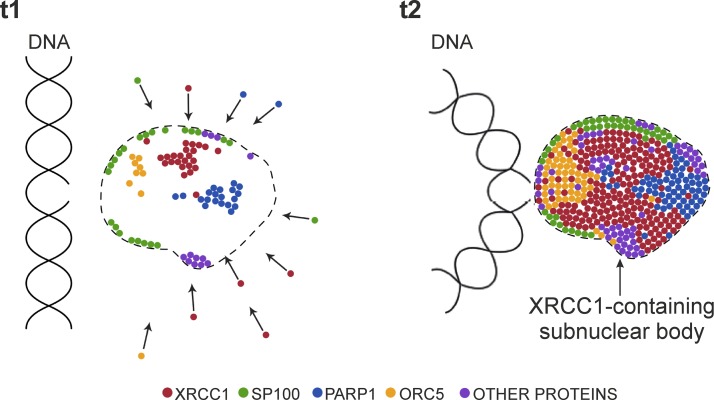
Superresolution and live cell imaging demonstrate that a large number of XRCC1 molecules gather near the sites of DNA damage and form a nuclear body that is located adjacent to a replication site containing the damaged DNA. We hypothesize that these repair foci may constitute SP100-rich PML-like nuclear bodies. We postulate that these bodies, formed in response to endogenous or induced DNA SSBs, are multifunctional entities that behave like mobile molecular reservoirs responsible for delivery of various protein factors or preassembled protein complexes involved in DNA damage repair (Fig. 6). A single nuclear body containing XRCC1 that is formed in response to a SSB can decay after completion of the repair processes or migrate toward another closely located DNA lesion.
Figure 6 .
Simplified hypothetical model of recruitment of XRCC1 and other proteins toward SSB. Formation of nuclear body containing XRCC1 and other proteins involved in repair, movement of DNA stretch containing lesion toward nuclear body, and transfer of repair factors and preassembled repair complexes toward damage.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank J. Drukała for providing primary cultures of human fibroblasts, M. Dziedzicka-Wasylewska for making the molecular biology facilities in her laboratory available, and M. Rak and Z. Madeja (all from Jagiellonian University) for their support in transfection procedures of human fibroblasts (all from Jagiellonian University). The authors also thank B. Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) for providing the vectors encoding EYFP-ORC1 and EYFP-ORC5, V. Schreiber (Institut de Recherche de l’Ecole de Biotechnologie de Strasbourg, Illkirch, France) for providing the vector encoding GFP-PARP1, and M. Szklarczyk (freelance graphic designer) for help with computer graphics. The Faculty of Biochemistry, Biophysics, and Biotechnology, Jagiellonian University is a partner within the Leading National Research Centre (KNOW) supported by the Polish Ministry of Science and Higher Education. This work was supported by Polish National Science Centre (NCN) (2013/09/N/NZ3/00203 to M.M.K., 2013/11/B/NZ3/00189 to J.W.D., and 2017/27/B/NZ3/01065 to J.W.D.). M.M.K. was a recipient of a Society–Environment–Technologies (SET) doctoral studentship from Jagiellonian University and a doctoral studentship awarded by the National Science Centre (DEC-2015/16/T/NZ3/00157). T.P. was partially supported by U.S. National Institutes of Health, National Institute on Drug Abuse Grant U01 DA-040588 with P. Kaufman (University of Massachusetts Medical School). M.M.K., K.S., and J.W.D. declare a potential conflict of interest associated with an invention (patent application pending) relating to a method of ultrasensitive detection of single- and double-stranded DNA breaks. The remaining authors declare no conflicts of interest.
Glossary
- 3D
3 dimensional
- 3D SIM
3D structured illumination microscopy
- BRCA1/2
breast cancer 1/2
- BRCT1/2
breast cancer 1/2 C terminus
- BSA
bovine serum albumin
- EdU
5-ethynyl-2′-deoxyuridine
- EGFP
enhanced green fluorescent protein
- EYFP
enhanced yellow fluorescent protein
- GFP
green fluorescent protein
- HSF
human skin fibroblast
- IF
immunofluorescence
- Lig IIIα
ligase IIIα
- mEGFP
monomeric enhanced green fluorescent protein
- mRFP
monomeric red fluorescent protein
- ORC1/5
origin recognition complex subunit 1/5
- PAR
poly(ADP-ribose)
- PARP
poly(ADP-ribose) polymerase
- PCNA
proliferating cell nuclear antigen
- PLA
proximity ligation assay
- PML
promyelocytic leukemia
- RFP
red fluorescent protein
- ROI
regions of interest
- SMLM
single-molecule localization microscopy
- SP100
SP100 nuclear antigen
- SSB
single-strand break
- TBS
Tris-buffered saline
- XRCC1
X-ray repair cross-complementing protein 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. M. Kordon, A. Szczurek, and J. W. Dobrucki conceived the original idea; T. Pederson contributed to the concept of the project; M. M. Kordon executed all experiments except single-molecule localization imaging; A. Szczurek performed single-molecule localization imaging, performed some confocal imaging of cell nuclei, and assisted in the quantitative analysis of images; M. M. Kordon and K. Berniak performed the analysis of distances between foci; O. Szelest and K. Solarczyk performed experiments presented in Fig. 5A; M. Tworzydło helped with site-directed mutagenesis of XRCC1 sequences in vectors; S. Wachsmann-Hogiu and A. Vaahtokari helped with SIM experiments; C. Cremer coordinated SMLM experiments; and M. M. Kordon, T. Pederson, and J. W. Dobrucki wrote the manuscript.
REFERENCES
- 1.Lan, L., Nakajima, S., Oohata, Y., Takao, M., Okano, S., Masutani, M., Wilson, S. H., Yasui, A. (2004) In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl. Acad. Sci. USA 101, 13738–13743 10.1073/pnas.0406048101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortusewicz, O., Leonhardt, H. (2007) XRCC1 and PCNA are loading platforms with distinct kinetic properties and different capacities to respond to multiple DNA lesions. BMC Mol. Biol. 8, 81 10.1186/1471-2199-8-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei, L., Nakajima, S., Hsieh, C. L., Kanno, S., Masutani, M., Levine, A. S., Yasui, A., Lan, L. (2013) Damage response of XRCC1 at sites of DNA single strand breaks is regulated by phosphorylation and ubiquitylation after degradation of poly(ADP-ribose). J. Cell Sci. 126, 4414–4423 10.1242/jcs.128272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solarczyk, K. J., Zarębski, M., Dobrucki, J. W. (2012) Inducing local DNA damage by visible light to study chromatin repair. DNA Repair (Amst.) 11, 996–1002 10.1016/j.dnarep.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 5.Solarczyk, K. J., Kordon, M., Berniak, K., Dobrucki, J. W. (2016) Two stages of XRCC1 recruitment and two classes of XRCC1 foci formed in response to low level DNA damage induced by visible light, or stress triggered by heat shock. DNA Repair (Amst.) 37, 12–21 10.1016/j.dnarep.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 6.Caldecott, K. W., Aoufouchi, S., Johnson, P., Shall, S. (1996) XRCC1 polypeptide interacts with DNA polymerase beta and possibly poly(ADP-ribose) polymerase, and DNA ligase III is a novel molecular “nick-sensor” in vitro. Nucleic Acids Res. 24, 4387–4394 10.1093/nar/24.22.4387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pleschke, J. M., Kleczkowska, H. E., Strohm, M., Althaus, F. R. (2000) Poly(ADP-ribose) binds to specific domains in DNA damage checkpoint proteins. J. Biol. Chem. 275, 40974–40980 10.1074/jbc.M006520200 [DOI] [PubMed] [Google Scholar]
- 8.Schreiber, V., Amé, J. C., Dollé, P., Schultz, I., Rinaldi, B., Fraulob, V., Ménissier-de Murcia, J., de Murcia, G. (2002) Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J. Biol. Chem. 277, 23028–23036 10.1074/jbc.M202390200 [DOI] [PubMed] [Google Scholar]
- 9.Whitehouse, C. J., Taylor, R. M., Thistlethwaite, A., Zhang, H., Karimi-Busheri, F., Lasko, D. D., Weinfeld, M., Caldecott, K. W. (2001) XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell 104, 107–117 10.1016/S0092-8674(01)00195-7 [DOI] [PubMed] [Google Scholar]
- 10.Kubota, Y., Nash, R. A., Klungland, A., Schär, P., Barnes, D. E., Lindahl, T. (1996) Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 15, 6662–6670 [PMC free article] [PubMed] [Google Scholar]
- 11.Caldecott, K. W., Tucker, J. D., Stanker, L. H., Thompson, L. H. (1995) Characterization of the XRCC1-DNA ligase III complex in vitro and its absence from mutant hamster cells. Nucleic Acids Res. 23, 4836–4843 10.1093/nar/23.23.4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beernink, P. T., Hwang, M., Ramirez, M., Murphy, M. B., Doyle, S. A., Thelen, M. P. (2005) Specificity of protein interactions mediated by BRCT domains of the XRCC1 DNA repair protein. J. Biol. Chem. 280, 30206–30213 10.1074/jbc.M502155200 [DOI] [PubMed] [Google Scholar]
- 13.Nash, R. A., Caldecott, K. W., Barnes, D. E., Lindahl, T. (1997) XRCC1 protein interacts with one of two distinct forms of DNA ligase III. Biochemistry 36, 5207–5211 10.1021/bi962281m [DOI] [PubMed] [Google Scholar]
- 14.Taylor, R. M., Wickstead, B., Cronin, S., Caldecott, K. W. (1998) Role of a BRCT domain in the interaction of DNA ligase III-alpha with the DNA repair protein XRCC1. Curr. Biol. 8, 877–880 10.1016/S0960-9822(07)00350-8 [DOI] [PubMed] [Google Scholar]
- 15.Cuneo, M. J., Gabel, S. A., Krahn, J. M., Ricker, M. A., London, R. E. (2011) The structural basis for partitioning of the XRCC1/DNA ligase III-α BRCT-mediated dimer complexes. Nucleic Acids Res. 39, 7816–7827 10.1093/nar/gkr419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor, R. M., Thistlethwaite, A., Caldecott, K. W. (2002) Central role for the XRCC1 BRCT I domain in mammalian DNA single-strand break repair. Mol. Cell. Biol. 22, 2556–2563 10.1128/MCB.22.8.2556-2563.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubota, Y., Horiuchi, S. (2003) Independent roles of XRCC1’s two BRCT motifs in recovery from methylation damage. DNA Repair (Amst.) 2, 407–415 10.1016/S1568-7864(02)00242-2 [DOI] [PubMed] [Google Scholar]
- 18.Masson, M., Niedergang, C., Schreiber, V., Muller, S., Menissier-de Murcia, J., de Murcia, G. (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell. Biol. 18, 3563–3571 10.1128/MCB.18.6.3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanssen-Bauer, A., Solvang-Garten, K., Gilljam, K. M., Torseth, K., Wilson D. M. III, Akbari, M., Otterlei, M. (2012) The region of XRCC1 which harbours the three most common nonsynonymous polymorphic variants, is essential for the scaffolding function of XRCC1. DNA Repair (Amst.) 11, 357–366 10.1016/j.dnarep.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Khamisy, S. F., Masutani, M., Suzuki, H., Caldecott, K. W. (2003) A requirement for PARP-1 for the assembly or stability of XRCC1 nuclear foci at sites of oxidative DNA damage. Nucleic Acids Res. 31, 5526–5533 10.1093/nar/gkg761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslin, C., Hornyak, P., Ridley, A., Rulten, S. L., Hanzlikova, H., Oliver, A. W., Caldecott, K. W. (2015) The XRCC1 phosphate-binding pocket binds poly(ADP-ribose) and is required for XRCC1 function. Nucleic Acids Res. 43, 6934–6944 10.1093/nar/gkv623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying, S., Chen, Z., Medhurst, A. L., Neal, J. A., Bao, Z., Mortusewicz, O., McGouran, J., Song, X., Shen, H., Hamdy, F. C., Kessler, B. M., Meek, K., Helleday, T. (2016) DNA-PKcs and PARP1 bind to unresected stalled DNA replication forks where they recruit XRCC1 to mediate repair. Cancer Res. 76, 1078–1088 10.1158/0008-5472.CAN-15-0608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor, R. M., Moore, D. J., Whitehouse, J., Johnson, P., Caldecott, K. W. (2000) A cell cycle–specific requirement for the XRCC1 BRCT II domain during mammalian DNA strand break repair. Mol. Cell. Biol. 20, 735–740 10.1128/MCB.20.2.735-740.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang, X., Moréra, S., Bates, P. A., Whitehead, P. C., Coffer, A. I., Hainbucher, K., Nash, R. A., Sternberg, M. J., Lindahl, T., Freemont, P. S. (1998) Structure of an XRCC1 BRCT domain: a new protein–protein interaction module. EMBO J. 17, 6404–6411 10.1093/emboj/17.21.6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madeja, Z., Rak, M., Wybieralska, E., Rózański, I., Masnyk, M., Chmielewski, M., Łysek, R., Chojnacki, T., Jankowski, W., Ciepichal, E., Swiezewska, E., Tekle, M., Dallner, G. (2007) New cationic polyprenyl derivative proposed as a lipofecting agent. Acta Biochim. Pol. 54, 873–876 [PubMed] [Google Scholar]
- 26.Abràmoff, M. D., Magalhães, P. J., Ram, S. J. (2004) Image processing with ImageJ. Biophoton. Int. 11, 36–41 [Google Scholar]
- 27.Berniak, K., Rybak, P., Bernas, T., Zarębski, M., Biela, E., Zhao, H., Darzynkiewicz, Z., Dobrucki, J. W. (2013) Relationship between DNA damage response, initiated by camptothecin or oxidative stress, and DNA replication, analyzed by quantitative 3D image analysis. Cytometry A 83, 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jusuk, I., Vietz, C., Raab, M., Dammeyer, T., Tinnefeld, P. (2015) Super-resolution imaging conditions for enhanced yellow fluorescent protein (eYFP) demonstrated on DNA origami nanorulers. Sci. Rep. 5 14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang, Y., Máté, G., Müller, P., Hillebrandt, S., Krufczik, M., Bach, M., Kaufmann, R., Hausmann, M., Heermann, D. W. (2015) Radiation induced chromatin conformation changes analysed by fluorescent localization microscopy, statistical physics, and graph theory. PLoS One 10, e0128555 10.1371/journal.pone.0128555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczurek, A. T., Prakash, K., Lee, H.-K., Zurek-Biesiada, D. J., Best, G., Hagmann, M., Dobrucki, J. W., Cremer, C., Birk, U. (2014) Single molecule localization microscopy of the distribution of chromatin using Hoechst and DAPI fluorescent probes. Nucleus 5, 331–340 10.4161/nucl.29564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan, J., Otterlei, M., Wong, H. K., Tomkinson, A. E., Wilson D. M. III (2004) XRCC1 co-localizes and physically interacts with PCNA. Nucleic Acids Res. 32, 2193–2201 10.1093/nar/gkh556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koken, M. H., Puvion-Dutilleul, F., Guillemin, M. C., Viron, A., Linares-Cruz, G., Stuurman, N., de Jong, L., Szostecki, C., Calvo, F., Chomienne, C. (1994) The t(15;17) translocation alters a nuclear body in a retinoic acid–reversible fashion. EMBO J. 13, 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sternsdorf, T., Grötzinger, T., Jensen, K., Will, H. (1997) Nuclear dots: actors on many stages. Immunobiology 198, 307–331 10.1016/S0171-2985(97)80051-4 [DOI] [PubMed] [Google Scholar]
- 34.Hodges, M., Tissot, C., Howe, K., Grimwade, D., Freemont, P. S. (1998) Structure, organization, and dynamics of promyelocytic leukemia protein nuclear bodies. Am. J. Hum. Genet. 63, 297–304 10.1086/301991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang, M., Jegou, T., Chung, I., Richter, K., Münch, S., Udvarhelyi, A., Cremer, C., Hemmerich, P., Engelhardt, J., Hell, S. W., Rippe, K. (2010) Three-dimensional organization of promyelocytic leukemia nuclear bodies. J. Cell Sci. 123, 392–400 10.1242/jcs.053496 [DOI] [PubMed] [Google Scholar]
- 36.Hoischen, C., Monajembashi, S., Weisshart, K., Hemmerich, P. (2018) Multimodal light microscopy approaches to reveal structural and functional properties of promyelocytic leukemia nuclear bodies. Front. Oncol. 8, 125 10.3389/fonc.2018.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber, A., Bai, P., de Murcia, J. M., de Murcia, G. (2004) PARP-1, PARP-2 and ATM in the DNA damage response: functional synergy in mouse development. DNA Repair (Amst.) 3, 1103–1108 10.1016/j.dnarep.2004.06.002 [DOI] [PubMed] [Google Scholar]
- 38.Almeida, K. H., Sobol, R. W. (2007) A unified view of base excision repair: lesion-dependent protein complexes regulated by post-translational modification. DNA Repair (Amst.) 6, 695–711 10.1016/j.dnarep.2007.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godon, C., Cordelières, F. P., Biard, D., Giocanti, N., Mégnin-Chanet, F., Hall, J., Favaudon, V. (2008) PARP inhibition versus PARP-1 silencing: different outcomes in terms of single-strand break repair and radiation susceptibility. Nucleic Acids Res. 36, 4454–4464 10.1093/nar/gkn403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonhardt, H., Rahn, H. P., Weinzierl, P., Sporbert, A., Cremer, T., Zink, D., Cardoso, M. C. (2000) Dynamics of DNA replication factories in living cells. J. Cell Biol. 149, 271–280 10.1083/jcb.149.2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caldecott, K. W., McKeown, C. K., Tucker, J. D., Ljungquist, S., Thompson, L. H. (1994) An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Mol. Cell. Biol. 14, 68–76 10.1128/MCB.14.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verdaasdonk, J. S., Lawrimore, J., Bloom, K. (2014) Determining absolute protein numbers by quantitative fluorescence microscopy. Methods Cell Biol. 123, 347–365 10.1016/B978-0-12-420138-5.00019-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misteli, T., Soutoglou, E. (2009) The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat. Rev. Mol. Cell Biol. 10, 243–254 10.1038/nrm2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parsons, J. L., Dianova, I. I., Finch, D., Tait, P. S., Ström, C. E., Helleday, T., Dianov, G. L. (2010) XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA Repair (Amst.) 9, 835–841 10.1016/j.dnarep.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 45.Ström, C. E., Mortusewicz, O., Finch, D., Parsons, J. L., Lagerqvist, A., Johansson, F., Schultz, N., Erixon, K., Dianov, G. L., Helleday, T. (2011) CK2 phosphorylation of XRCC1 facilitates dissociation from DNA and single-strand break formation during base excision repair. DNA Repair (Amst.) 10, 961–969 10.1016/j.dnarep.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 46.Dillin, A., Rine, J. (1997) Separable functions of ORC5 in replication initiation and silencing in Saccharomyces cerevisiae. Genetics 147, 1053–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox, C. A., Loo, S., Dillin, A., Rine, J. (1995) The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev. 9, 911–924 10.1101/gad.9.8.911 [DOI] [PubMed] [Google Scholar]
- 48.Shore, D. (2001) Transcriptional silencing: replication redux. Curr. Biol. 11, R816–R819 10.1016/S0960-9822(01)00493-6 [DOI] [PubMed] [Google Scholar]
- 49.Suter, B., Tong, A., Chang, M., Yu, L., Brown, G. W., Boone, C., Rine, J. (2004) The origin recognition complex links replication, sister chromatid cohesion and transcriptional silencing in Saccharomyces cerevisiae. Genetics 167, 579–591 10.1534/genetics.103.024851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou, Y., Bi, X. (2008) Positive roles of SAS2 in DNA replication and transcriptional silencing in yeast. Nucleic Acids Res. 36, 5189–5200 10.1093/nar/gkn465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozaydin, B., Rine, J. (2010) Expanded roles of the origin recognition complex in the architecture and function of silenced chromatin in Saccharomyces cerevisiae. Mol. Cell. Biol. 30, 626–639 10.1128/MCB.00614-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bischof, O., Kim, S. H., Irving, J., Beresten, S., Ellis, N. A., Campisi, J. (2001) Regulation and localization of the Bloom syndrome protein in response to DNA damage. J. Cell Biol. 153, 367–380 10.1083/jcb.153.2.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carbone, R., Pearson, M., Minucci, S., Pelicci, P. G. (2002) PML NBs associate with the hMre11 complex and p53 at sites of irradiation induced DNA damage. Oncogene 21, 1633–1640 10.1038/sj.onc.1205227 [DOI] [PubMed] [Google Scholar]
- 54.Seker, H., Rubbi, C., Linke, S. P., Bowman, E. D., Garfield, S., Hansen, L., Borden, K. L., Milner, J., Harris, C. C. (2003) UV-C–induced DNA damage leads to p53-dependent nuclear trafficking of PML. Oncogene 22, 1620–1628 10.1038/sj.onc.1206140 [DOI] [PubMed] [Google Scholar]
- 55.Xu, Z. X., Timanova-Atanasova, A., Zhao, R. X., Chang, K. S. (2003) PML colocalizes with and stabilizes the DNA damage response protein TopBP1. Mol. Cell. Biol. 23, 4247–4256 10.1128/MCB.23.12.4247-4256.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conlan, L. A., McNees, C. J., Heierhorst, J. (2004) Proteasome-dependent dispersal of PML nuclear bodies in response to alkylating DNA damage. Oncogene 23, 307–310 10.1038/sj.onc.1207119 [DOI] [PubMed] [Google Scholar]
- 57.Muratani, M., Gerlich, D., Janicki, S. M., Gebhard, M., Eils, R., Spector, D. L. (2002) Metabolic-energy–dependent movement of PML bodies within the mammalian cell nucleus. Nat. Cell Biol. 4, 106–110 10.1038/ncb740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.