Abstract
The own name is a salient stimulus, used by others to initiate social interaction. Typically developing infants orient towards the sound of their own name and exhibit enhanced event-related potentials (ERP) at 5 months. The lack of orientation to the own name is considered to be one of the earliest signs of autism spectrum disorder (ASD). In this study, we investigated ERPs to hearing the own name in infants at high and low risk for ASD, at 10 and 14 months. We hypothesized that low-risk infants would exhibit enhanced frontal ERP responses to their own name compared to an unfamiliar name, while high-risk infants were expected to show attenuation or absence of this difference in their ERP responses. In contrast to expectations, we did not find enhanced ERPs to own name in the low-risk group. However, the high-risk group exhibited attenuated frontal positive-going activity to their own name compared to an unfamiliar name and compared to the low-risk group, at the age of 14 months. These results suggest that infants at high risk for ASD start to process their own name differently shortly after one year of age, a period when frontal brain development is happening at a fast rate.
Keywords: Social development, Own name, Event-related potentials, Autism spectrum disorder, High-risk siblings, Infants
1. Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder emerging during early childhood. It reveals itself in two main domains: impairments in social-communicative behaviours and restricted interests together with repetitive behaviours (The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); American Psychiatric Association (APA), 2013). New-borns who have a sibling with ASD are considered to be at a higher risk (approximately 20 %) of developing ASD themselves (Ozonoff et al., 2011, 2014). Further, they may exhibit subclinical characteristics of ASD, described as the broader autism phenotype (BAP) (Pisula and Ziegart-Sadowska, 2015). Therefore, research in siblings of children with ASD is of high importance, particularly in terms of detecting the emergence of different BAP characteristics and early markers of ASD before the age of 3, aiming to contribute to the development of early prodromal interventions (Yirmiya and Charman, 2010).
Studies showed that already in the very first months of life, typically developing (TD) infants identify and attend more to socially relevant stimuli such as biological motion, faces, direct gaze, social sounds, infant-directed speech, compared to non-social stimuli (Cooper and Aslin, 1990; Falck-Ytter et al., 2011; Guy et al., 2018; Johnson et al., 1991; Key and Stone, 2012; Parise and Csibra, 2013; Vernetti et al., 2018). According to the directed attention (DA) model, with an attentional bias towards socially relevant information, TD infants can limit further processing of irrelevant and excessive information. Hence, despite of their limited capacity, they successfully process social information at very young ages (Reid and Striano, 2007). Supporting this model, two different social cues with different modalities were shown to share common neural mechanisms, namely, both infant-directed speech (vs adult-directed speech) and direct gaze (vs averted gaze) elicited a stronger mid-latency frontal positive activity in TD 5 month-olds, implying “a preferential treatment of ostensive signals” (Parise and Csibra, 2013, pg.7).
Among all, one’s own name is a particularly unique social cue, as a signal of directed speech, indicating a direct self-relevance (Csibra, 2010). Earlier studies showed that TD infants orient towards the sound of their own name (Mandel et al., 1995), can also recognize it in noisy environments (Newman, 2005) and even differentiate it from names with only a different first phoneme (Mandel-Emer and Jusczyk, 2003) by the age of 5 months. The own name may also play an important role in early language development through speech segmentation and selection. Accordingly, infants around 6 months of age listened longer to previously familiarized speech passages involving their own name (Mandel-Emer and Jusczyk, 2003) and were able to isolate and segment the new words following their own name (Bortfeld et al., 2005).
A limited number of studies investigated infants’ neural responses to hearing their own name. Two functional Near-Infrared-Spectroscopy (fNIRS) studies showed that at the age of 5 months, when infants’ own names were called in an infant directed way, left dorsal prefrontal cortex was activated (Grossmann et al., 2010) and at the age of 6 months, when the infants’ own names (compared to an unfamiliar name) were called by their mothers as compared to a female stranger, their dorsal-medial prefrontal cortex was activated and considered to function as an early precursor of self-referencing (Imafuku et al., 2014). To the best of our knowledge, the only established infant ERP research applying an own name paradigm was conducted by Parise et al. (2010). They tested the own name recognition in two different groups of TD 5 month-old infants, using either one or ten different unfamiliar names as control conditions. They found a significant difference in an early anterior positive activity (100−380 ms post-stimuli) with higher amplitudes in response to the own name only within the group having one unfamiliar name as the control condition. In addition, they found a negative enhancement (N200−600 ms post-stimuli) in parietal electrodes for the own name, which was only significant in the group with ten unfamiliar names used as the control condition (Parise et al., 2010).
In infants who are at high-risk (HR) for ASD, several studies indicated lack or diminished behavioural response and orientation to the own name, becoming most apparent by the age of 12 months (Nadig et al., 2007; Osterling et al., 2002; Zwaigenbaum et al., 2005). Furthermore, this diminished response to the own name in HR infants was predictive for a later diagnosis of ASD (Zwaigenbaum et al., 2005). However, to the best of our knowledge, there has been no study investigating the neural responses to the own name in HR infants.
In the current ERP study, we aimed to identify the neural correlates of hearing the own name in LR and HR infants longitudinally at the ages of 10 and 14 months, to ensure that possible differences are attributable to a social attentional bias rather than possible variances in infants recognition of their own name, as the lack of behavioural response to the own name in HR infants becomes clear from 12 months onwards. By choosing these two ages, we could investigate whether the expected differentiation of response to own name would be already visible in ERPs at the age of 10 months, even before the overt behavioural responses can be observed as from the age of 12 months. The choice of these two age groups also provided the possibility to monitor the changes that may come along with the accelerated brain development of language areas around one year of age, together with possible differences (e.g., delays) in HR infants’ language development. We expected enhanced ERPs to the own name in LR infants at both ages (Parise et al., 2010) and hypothesized this difference to be less pronounced or absent in HR infants and be observable at 14 months or even before, based on the previous behavioural studies (Nadig et al., 2007; Osterling et al., 2002; Zwaigenbaum et al., 2005). Further, we expected a correlation between the own name effect and language development scores, given the relevance and importance of recognizing the own name for speech segmentation and language development (Bortfeld et al., 2005; Mandel-Emer and Jusczyk, 2003).
2. Materials and methods
2.1. Participants
Data were collected from a total of 69 infants when there were 10 and 14 months old. This was part of an ongoing longitudinal study with contact moments at the ages of 5, 10, 14, 24 and 36 months. The low-risk group (LR) consisted of 35 younger siblings of children with no diagnosis or family history of ASD, the high-risk group (HR) consisted of 34 younger siblings of children with ASD. The final sample included data from 26 LR and 25 HR infants at both age points, after the exclusion of data from 18 infants (9 LR, 9 HR) due to high artefact levels (see further below for rejection criteria). No initial power analysis to determine the final sample size was conducted, since there had been no other study comparing ERPs to the own name in young infants between 10 and 14 months of age and between HR and LR groups. Characteristics of the two final samples, that provided valid data at both age points, are displayed in Table 1.
Table 1.
Characterization of participants.
| LR group (n = 26) | HR group (n = 25) | Group comparisons | |
|---|---|---|---|
| Age 10 m (M(SD)) | 10.11(0.37) | 10.32(0.48) | t(49) = -1.72, p = .091, d = -0.49 |
| Age 14 m (M(SD)) | 14.28(0.40) | 14.44(0.68) | t(49) = -0.98, p = .334, d = -0.28 |
| Sex ratio (male %) | 65.4 % | 60.0 % | χ2 (1,N = 51) = .158, p = .691 |
| Attrition rates | 25.7 % | 26.5 % | χ2 (1,N = 69) = .005, p = .943 |
| ELC 10 m (M(SD)) | 118.50(10.11) | 107.92(14.02) | t(49) = 3.05, p = .004,d = 0.87 |
| ELC 14 m (M(SD)) | 102.72(8.54)* | 94.24(13.64) | t(48) = 2.64, p = .011,d = 0.76 |
| RL 10 m (M(SD)) | 52.96(7.56) | 47.72(9.40) | t(49) = 2.20, p = .033,d = 0.62 |
| RL 14 m (M(SD)) | 41.92 (7.16)* | 40.12(9.68) | t(48) = 0.75, p = .458, d = 0.22 |
Note: LR group = low-risk group, HR group = high-risk group, ELC = Early Learning Composite and RL = receptive language scores were both measured via the Mullen Scales of Early Learning (MSEL, Mullen, 1995).
Subject number equals to 25 for the LR group.
All infants were born full-term (37+ gestational weeks) and no hearing or visual problems were reported by the parents. All of them were enrolled into a longitudinal study and were tested in the lab. The ethical approval was obtained from the appropriate ethical committee and all parents signed an informed consent.
2.1.1. Behavioural measures
The Mullen Scales of Early Learning (MSEL; Mullen, 1995) assesses the cognitive abilities of children from birth up to 68 months. In this study Early Learning Composite (ELC), receptive and expressive language scores were taken into account. The ELC was calculated based on the scores on four subscales: visual perception, receptive language, expressive language and fine motor abilities. In addition, infants’ social and communication abilities were evaluated through the parent questionnaire Vineland Screener (Scholte et al., 2008; van Duijn et al., 2009). These measures were taken into account for reporting the concurrent brain behaviour correlations.
2.2. Design and procedure
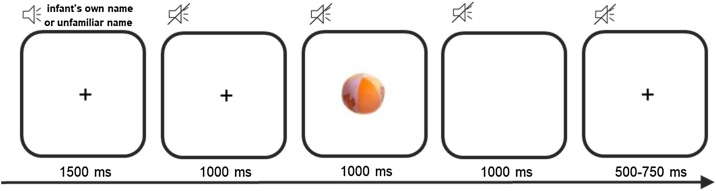
The ERP experiment consisted of 50 trials, with a minimum of 23 per condition. The design of the task was similar to the one used in 5-month-old infants by Parise et al. (2010). All trials had the same duration and structure (see Fig. 1). The trials were presented in two pseudo-randomized orders. In the middle of the experiment a short break was provided in which a cartoon was playing during five seconds. The design allows focusing separately on auditory and visual components. In this study only the auditory components are described, due to a limited amount of valid visual ERP data. Although the infants were often attentive to the auditory information during the testing, their visual attention to the screen was limited. The infants’ auditory and visual attention to the screen was determined via an offline coding procedure trial by trial. Whenever the auditory presentations were clearly audible (e.g., during the auditory presentation the infant was not vocalizing, crying, or clapping hands, and there was no other sound in the room coming from parents or experimenters), the trial was accepted as behaviourally valid to process the auditory components. With respect to visual behaviour, the trial was considered as invalid, if the infant did not look at the screen during the entire trial from hearing one name stimulus to hearing another one. Since for many children the total number of valid trials was less than 1/3 of the total number of trials per condition, the visual ERP data are not included in this paper.
Fig. 1.
Example of one trial.
At the contact times of both 10 and 14 months of age, the infants were administered a variety of cognitive, language, motor and social developmental measures as part of the longitudinal sibling protocol. Within this protocol they completed a battery of ERP tasks. The current task was always administered first, when the family arrived at the lab on the research day. Upon arrival at the EEG lab, the infants were seated on their parents’ lap at a distance of approximately 60 cm from the screen and the two speakers. The EEG-cap was prepared before the participants entered the room. After the procedure was explained to the parents and when the infants were acclimatized, the EEG-cap was fitted. Meanwhile a cartoon was playing to entertain the infants. Electrolytic conducting gel was inserted into each of the active electrodes after placement of the EEG-cap. A chinstrap and hairnet were used to hold the cap in place. During testing, the lights were dimmed and the experimenters sat behind a screen to avoid distraction. The parent was instructed to avoid interacting with the infant during testing unless the infant was clearly distressed. An attention grabber (a video of a moving object) was used when the participants were distracted or upset. The behaviour of the infants was followed online using a webcam. The duration of the testing was around 10–15 min (cap-placement excluded).
2.3. Stimuli
Auditory and visual stimuli were presented during the experiment. The auditory stimuli were sound presentations of the infant’s own name and an unfamiliar name, pronounced by a female voice using infant-directed intonation. All unfamiliar names were retrieved from a list of the most popular names in Flanders (http://www.kindengezin.be/toepassingen/populaire-voornamen.jsp). The personal name of the participant was paired with one of the names on the list. Both names were matched for gender and the number of syllables, while the first phoneme of the unfamiliar name was always different from the first phoneme of the personal name. Further, the unfamiliar name was always different from the personal names of the first-degree relatives of the participant. We asked parents for the correct pronunciation of the personal names if this was unclear. All names were recorded before the start of the experiment and were digitalized on 32bit/44.1 Hz with the program Audacity. They were presented with a mean sound pressure level (SPL) of 70 dB. There was no difference in mean duration between conditions (own name condition M(SD) = 869.25(116) ms and unfamiliar name condition M(SD) = 759.55(95.80) ms, t(50) = 0.65, p=.518, d = 0.09).
The visual stimuli were coloured photographs of toys. All visual stimuli had the same size (10.50 cm (h) x11.00 cm (w)) and were matched on different visual characteristics. For that purpose, twelve parents of young children rated 22 pictures of objects (toys) on luminosity of the picture, and on familiarity, attractiveness and complexity of the photographed objects on a 5-point Likert scale. Ten of these objects had previously been used by Parise et al. (2010). The ones previously used by Parise et al. (2010) and the new pictures did not differ for luminosity of the picture (F(1,17) = 2.14 p=.162), neither for attractiveness (F(1,17) = .044 p=.836), complexity (F(1,17) = .023, p=.881) and familiarity of the object (F(1,17) = 1.31, p=.268).
2.4. Electrophysiological recordings
Electrical brain activity was recorded with Brain Vision Recorder (Brain products, 2007) and registered with 32 active Ag/AgCl electrodes through an EEG amplifier (QuickAmp, Brain Products, GmbH, Munich, Germany) with a sample rate of 500 Hz. We used an EEG-cap (Easycap, Brain Products GmbH, Munich, Germany), in which all electrodes were embedded according to the international 10/20 method of electrode placement (Jasper, 1958) with an AFz ground electrode. A common average reference was used online. Horizontal and vertical eye movements were registered via the electrodes next to the eyes (F9 and F10) and through comparing the activity of electrode Fp2 (above the eye) with the average reference, respectively. The EEG was recorded with a 50 Hz notch filter. Further analyses were processed offline.
2.5. Data analysis
The analyses of the EEG data were processed offline via BrainVision Analyzer 2.1. Before the start of the ERP analyses, those trials where the auditory stimuli were not clearly perceptible due to sounds in the environment or vocalizations of the infants themselves, were excluded based on behavioural coding of the recorded videos of the ERP administration.
Since a synchronization problem was detected between the stimulus presentation software (E-prime) and Brainvision Recorder, the delays of the time locked name stimuli found in the ERP files were corrected offline in Brainvision Analyzer. Since the loading of the auditory files caused the largest delays, these were corrected with an extra step for editing markers per trial. The total duration from a name presentation to the start of an object presentation remained 2500 ms. This interval was therefore used as a reference to re-adjust the markers (see above task design & Fig. 1). During this step, minor delays between the start of the audio recording files and the actual start of the speech, when present, were also manually corrected in the markers. The EEG signal was filtered with a 1 Hz (12 dB/oct) high-pass filter and a 20 Hz (12 dB/oct) low-pass filter so as to remove the slow drifts and muscle artefacts commonly seen in infancy and so as to increase the signal-to-noise ratio. The decision on the ultimate band-pass filter (1−20 Hz) was done after the initial data collection, allowing an inspection on the nature of the data, yet before the completion of data collection. During the EEG analysis, the filters were applied as the first step of data editing to minimize data distortion. The data were re-referenced to the average and segmented into epochs of 1200 ms (200 ms baseline duration before the stimulus onset and 1000 ms from the stimulus onset). Segments containing eye movements, blinks and/or other artefacts (e.g., due to movement) were manually removed based on visual inspection of the data. A high number of artefacts, mainly due to (head) movement, was detected. Yet the proportion of 26.08 % of excluded infants is within the normal range expected for infant ERP studies (Hoehl and Wahl, 2012). The remaining data were baseline corrected using mean voltage during the 200 ms pre-stimulus baseline period.
The infants were included in the dataset when they had artefact free data for 40 % of the presented trials. When all the channels had more than 30 % artefacts after filtering, re-referencing and interpolations, the data were excluded from further analyses (based on raw data inspection; with maximal allowed voltage step: 50 μV/ms, maximal allowed difference of values in intervals: 200 μV within 200 ms, lowest allowed activity in intervals: 0.5 μV within 100 ms). For the included data the individual channels with too many artefacts were interpolated using spherical spline interpolation. The mean percentages of the interpolated channels were less than the suggested rate of 10 % (Farroni et al., 2004; Hoehl and Wahl, 2012; Macchi Cassia et al., 2006). In total, 23 trials per condition (own name condition, unfamiliar name condition) underwent semi-automatic artefact rejection. Average waveforms were calculated with minimum 10 artefact-free trials per condition for each subject, at both time points. The final artefact-free trial numbers per condition per group were listed in Table 2. Multiple comparisons revealed no significant differences of trial numbers, neither within groups comparing two conditions nor between the groups per condition. The grand average waveforms were calculated by averaging the subject average waveforms, including in total 814 trials for the own name condition and 809 trials for the unfamiliar name condition at the age of 10 months and total 857 trials for the own name condition and 856 trials for the unfamiliar name condition at the age of 14 months.
Table 2.
Trial numbers included in the final analyses.
| Final trial numbers | LR group (n = 26) | HR group (n = 25) | Between group comparisons |
|---|---|---|---|
| Own name at 10 m (M(SD)) | 15.58(3.87) | 16.36(3.24) | t(49) = -0.78, p = .438 |
| Unfamiliar name at 10 m (M(SD)) | 15.69(3.48) | 16.04(3.26) | t(49) = -0.37, p = .715 |
| Within group comparisons at 10M | t(25) = -0.25, p = .805 | t(24) = 0.67, p = .507 | |
| Own name at 14 m (M(SD)) | 16.73(3.40) | 16.88(3.79) | t(49) = -0.15, p = .883 |
| Unfamiliar name at (M(SD)) | 16.00(2.99) | 17.6(3.49) | t(49) = -1.76, p = .085 |
| Within group comparisons at 14M | t(25) = 1.77, p = .089 | t(24) = -1.80, p = .083 |
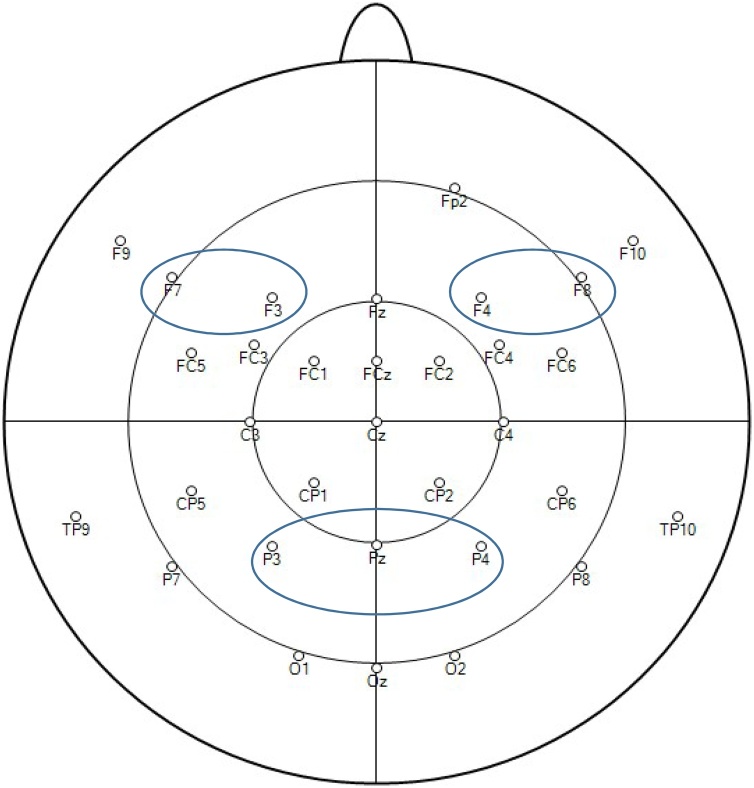
A time-window of 200−600 ms was selected both for the frontal areas (left frontal electrodes: F3, F7 and right frontal electrodes: F4, F8) and the parietal area (P3, Pz, P4) (Fig. 2), based on the previous infant ERP study (Parise et al., 2010) and based on the visual inspection of the grand averages of our ERP data together with the topography plot across all infants and conditions per age group (Fig. 3). Frontal ERPs were evaluated by averaging activity at the electrodes in each hemisphere (F3 and F7 for left hemisphere and F4 and F8 for the right hemisphere). This hemisphere specific investigation of the frontal components allowed for the exploration of hemisphere specific neural activity differences in the language processing of young infants who are at high and low risk for developing ASD (Finch et al., 2017; Seery et al., 2013; Tager-Flusberg, 2016). For the frontal area, 2 × 2 × 2 × 2 mixed analyses of variance (ANOVA) with group (HR vs LR) as between-subject factor and with condition (own name vs unfamiliar name), age (10 m vs 14 m) and hemisphere (left vs right) as within-subject factors were calculated for the time-window selected. For the parietal area, 2 × 2 × 2 mixed ANOVAs with group (HR vs LR) as between-subject factor and with condition (own name vs unfamiliar name) and age (10 m vs 14 m) as within-subject factors were calculated for the selected time window. Further follow-up and exploratory analyses were executed via Bayesian Statistics for related and independent samples. For each of those tests, a Bayes Factor (BF), which shows a natural ratio that compares the likelihoods of the null and alternative hypotheses, were also reported. When the comparisons revealed a significant result (p < 0.05) a BF indicating the likelihood ratio of the alternative hypothesis over the null hypothesis was reported as BF10. Otherwise, the likelihood ratio of null hypothesis over the alternative hypothesis was reported as BF01. This way, only BF values equal or above 1 were presented, aiming to simplify the interpretations. Based on Raftery’s classification, BF values between 1–3 were considered as indicating a weak evidence, between 3–20 a positive evidence, between 20–150 strong and >150 very strong evidence in either direction (Kass and Raftery, 1995; Raftery, 1995). Initial Application of a Bonferroni correction for multiple comparisons was considered to control for Type I errors in the results. However, due to the nature of the current study, where a small sample size might lead to low statistical power, applying a Bonferroni correction would increase the risk of not being able to detect the potential group differences. Therefore, we present the results without a Bonferroni correction, aiming to prevent the risk of inflated Type II error rate (Nakagawa, 2004; Perneger, 1998). The article reports all measures, manipulations, and exclusions in this study.
Fig. 2.
The electrodes chosen for the analyses showing the parietal, right and left frontal areas of interests.
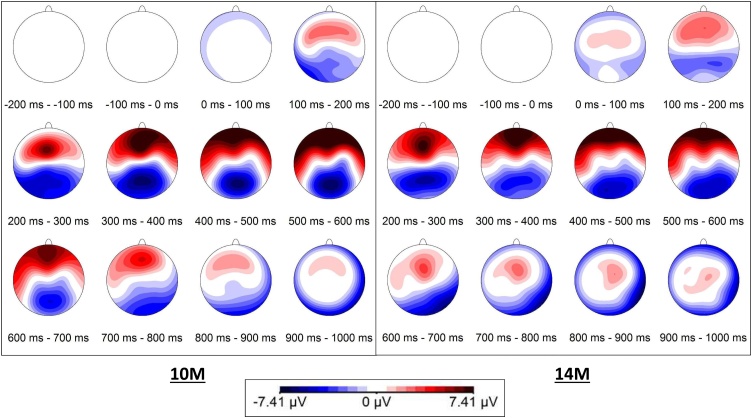
Fig. 3.
Scalp topographies across groups and conditions, per age group (10M = 10 months, 14M = 14 months).
The topography plot of all infants, independent of group and condition, revealed a frontal positivity and parietal negativity in the time-window of 200−600 ms, as shown in Fig. 3.
3. Results
3.1. Frontal areas
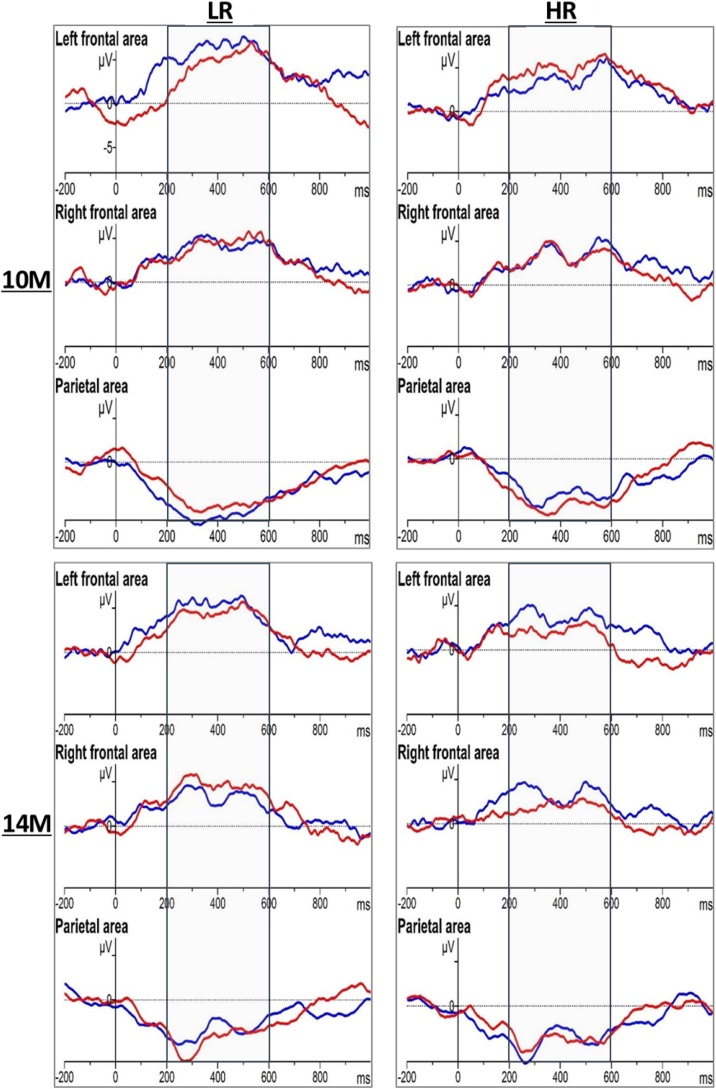
There was a significant main effect of group (F(1,49) = 4.56, p=.038, ηp2 = 0.085) showing overall increased positive neural activity in the LR group (M=4.65, SE = 0.44) compared to the HR group (M=3.31, SE = 0.45). There was also a trend for a main effect of hemisphere (F(1,49) = 3.90, p=.054, ηp2 = 0.074) with increased positive activity in the left frontal area (left frontal area (M=4.40, SE = 0.42) vs right frontal area (M=3.56, SE = 0.34)). In addition, the main effects were partly qualified by two marginally significant three-way interactions between hemisphere, condition and group (F(1,49) = 3.58, p=.065, ηp2 = 0.068) and between age, condition and group (F(1,49) = 3.00, p=.089, ηp2 = 0.058). All other main effects and interactions were non-significant, all F ≤2.78, all p ≥ .102 (Fig. 4).
Fig. 4.
Grand average waveforms for own name (red), unfamiliar name (blue), in the left frontal area (averaged signal from F3 and F7), in the right frontal area (averaged signal from F4 and F8) and parietal area (averaged signal from P3, Pz, and P4) (LR = low-risk, HR = high-risk) (10M = 10 months, 14M = 14 months).
To clarify the three-way interaction between hemisphere, condition and group, condition*group interactions were followed up using the averaged values from the two age groups, in the left and right frontal areas separately. The repeated measures ANOVA showed no significant main effect or interaction F ≤3.58, all p ≥ .064.
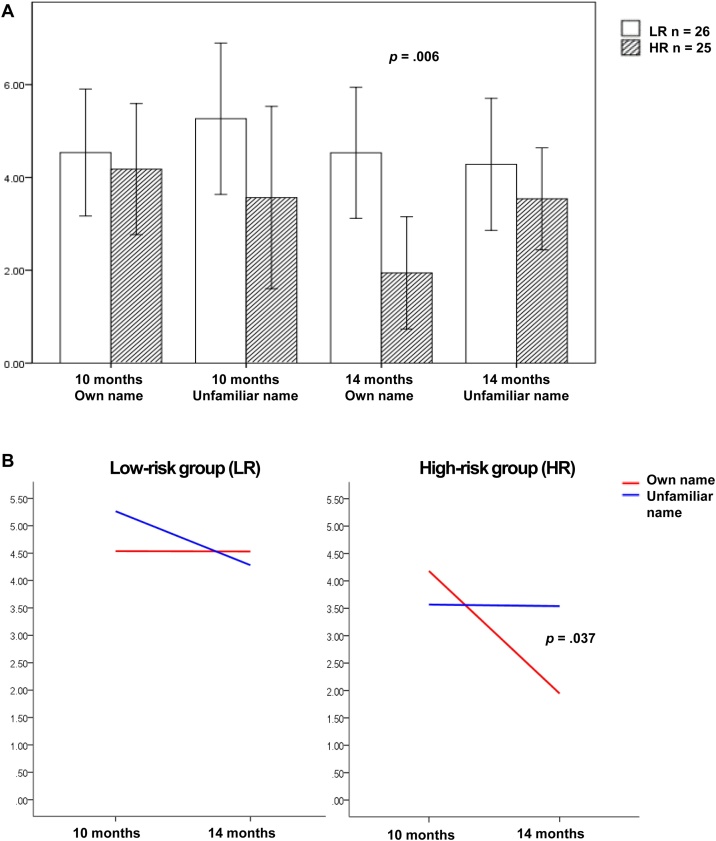
To clarify the three-way interaction between age, condition and group, age*group interactions were followed up in the entire frontal area using the averaged values from hemispheres, separately for the own name and the unfamiliar name conditions. For the own name condition the repeated measures ANOVA revealed a significant main effect of age (F(1,49) = 4.13, p=.048, ηp2 = 0.078), qualified by a significant interaction between age and group (F(1,49) = 4.09, p=.049, ηp2 = 0.077). Following up on this interaction, via Bayesian statistics for independent samples, revealed that the groups did not differ in their neural responses to the unfamiliar name condition, at either of the age points (difference at 10 months (M=-1.70, SE = 1.23), t(49) = 1.38, p = .175, d = 0.39, BF01 = 2.06, difference at 14 months (M=-0.74, SE = 0.88), t(49)=-0.85, p = .402, d=-0.24, BF01 = 3.47). This was also true for the own name condition at the age of 10 months (difference (M=-0.36, SE = 0.96), t(49)=-0.37, p = .710, d = 0.11, BF01 = 4.49). However, the groups significantly differed in their responses to the own name at the age of 14 months with reduced positive-going activity in the HR group compared to the LR group (difference(M=-2.59, SE = 0.91), t(49)=-2.86, p=.006, d = 0.82, BF10 = 6.7 indicating that the H1 is approximately 6.7 times more likely than the H0) (Fig. 5a). In addition, further exploration of the data via Bayesian statistics for related samples at the age of 14 months revealed a reduced neural activity to hearing the own name (M = 1.94, SD = 2.93) compared to the unfamiliar name (M = 3.54, SD = 2.67) (t(24)=-2.20 p = .037, d=-0.46, BF10 = 1.3 indicating weak evidence in favour of the H1) in the HR group only. There was no significant condition difference within the LR group at this age (own name (M = 4.53, SD = 3.50) vs unfamiliar name (M = 4.28, SD = 3.52), t(25) = 0.283 p = .779, d = 0.07, BF01 = 6.37 indicating positive evidence in favour of the H0). There was no significant condition difference at the age of 10 months for either of the groups (HR group, own name (M = 4.18, SD = 3.42) vs unfamiliar name (M = 3.57, SD = 4.76), t(24) = 0.663 p = .514, d = 0.15, BF01 = 5.26 indicating positive evidence in favour of the H0; LR group, own name (M = 4.54, SD = 3.38) vs unfamiliar name (M = 5.26, SD = 4.04), t(25)=-0.818 p = .421, d=-0.19, BF01 = 4.80 indicating positive evidence in favour of the H0). Furthermore, plotting these differences, potential developmental trajectory differences between groups were observed (Fig. 5b). In order to explore the true nature of these age related changes, the difference scores of the ERPs (calculated per infant by subtracting the values at 10 months from the values at 14 months) were compared between the groups, per condition. The Bayesian statistics for independent samples indicated a significant difference between the developmental trajectories of the two groups for the own name (LR group (M=-0.01, SD = 4.4) vs HR group (M=-2.24, SD = 3.4), t(49)= -2.02, p=.049, d=-0.57, BF10 = 1.2), yet indicating weak evidence in favour of the H1. 14 out of 26 (54 %) LR infants’ ERPs showed a reduction in amplitude from 10 months to 14 months of age (Z=-0.11, p=.909), whereas this was true for 19 out of 25 (76 %) HR infants ERPs to their own name (Z=-2.89, p=.004). Groups did not differ in their developmental trajectories for the unfamiliar name condition (LR group (M=-0.98, SD = 5.8) vs HR group (M=-0.03, SD = 5.1), t(49) = 0.62, p=.538, d = 0.18, BF01 = 4.03 indicating positive evidence in favour of H0), with 11 out of 26 LR infants (Z=-0.01, p=.990) and 11 out of 25 HR (Z=-0.07, p=.946) infants showing a reduction of ERP values to the unfamiliar name condition from 10 to 14 months.
Fig. 5.
Mean values of averaged ERPs across left and right frontal areas A. Between group comparisons per condition per age B. Within group comparisons of conditions per age.
Lastly, planned correlational analyses gave the following significant results. The difference scores (own name minus unfamiliar name) of frontal responses at the age of 14 months significantly correlated with the receptive language scores (MSEL; Mullen, 1995) measured at the age of 10 months r(49) = .351, p = .012. Likewise, the frontal positive responses to the own name at the age of 14 months significantly correlated with the receptive language scores r(49) = .417, p = .002 and the Early Learning Composite r(49) = .348, p = .012, both measured at the age of 10 months (MSEL; Mullen, 1995). In addition, frontal ERPs to the infants’ own name at 14 months were significantly correlated with the communication scores r(42) = .312, p = .044 but not social scores r(42) = .211, p = .179 of the Vineland screener at 14 months.
3.2. Parietal area
The repeated measures ANOVA revealed only a trend for a main effect of age (F(1,49) = 3.76, p=.058, ηp2 = 0.071) with higher values of negative activity at the age of 10 months (M=-5.02, SD = 0.54) compared to 14 months of age (M=-3.91, SD = 0.45). All other main effects and interactions were not significant, all F ≤2.44, all p ≥ .125.
4. Discussion
In this ERP study, we investigated infants’ neural responses to hearing their own name versus an unfamiliar name longitudinally at two age points shortly before and after their first birthday, namely at the age of 10 and 14 months. We investigated the neural responses in frontal and parietal central areas within a time-window of 200−600 ms post stimuli, based on the common topographic activity observed in both age groups. We expected an enhanced neural activity in response to the own name versus an unfamiliar name in LR infants, based on the previous ERP study using the own name paradigm in 5 month-old infants (Parise et al., 2010). In the HR group, we hypothesized attenuation or absence of this difference, based on earlier behavioural studies with siblings (Nadig et al., 2007; Osterling et al., 2002; Zwaigenbaum et al., 2005).
First, in contrast to the expectations, LR infants did not show a difference between the two conditions. In other words there was no enhancement of neural activity for the own name compared to the presented unfamiliar name within the frontal and parietal areas and during the specified 200−600 ms time-window, at either of the age points. It is very unlikely that at the ages of 10 and 14 months the LR infants were not able to differentiate their own name from an unfamiliar name, as several other studies indicated differentiated behavioural response to the own name even at younger ages (Bortfeld et al., 2005.; Mandel-Emer and Jusczyk, 2003; Mandel et al., 1995; Nadig et al., 2007; Newman, 2005; Yirmiya et al., 2006). In TD 5 month-old infants Parise and colleagues (2010) reported no enhanced parietal response to the own name when compared to only one unfamiliar name within this time window, while the enhanced response was only apparent when compared to ten different unfamiliar names. However, comparing the own name with several other names is confounded since the illustrated effect could rather be related to the different number of repetitions and/or the variation of the unfamiliar names. Taking this possibility into account, a later study investigating ERPs to the own name in older children (aged between 4–12 years) included two separate control conditions, one repeated unfamiliar name and multiple non-repeated unfamiliar names, aiming to control for the confounds of the multiple unfamiliar names used against the own name. In this study, Key et al. (2016) did not observe a differentiated neural response in the frontal area for the own name versus one unfamiliar name in typically developing young children. Nevertheless, it is important to emphasize that the similar outcome of this study with ours could be confounding considering the important differences between the two studies (e.g., design, age groups, selected ERP component) and also given the age difference, it may reflect a completely different underlying mechanism. On the other hand, in 5 month-old infants, the significant neural difference between the own name and one unfamiliar name was established in the frontal area in an earlier time-window than ours and attributed to phonological discrimination of the own name from the unfamiliar name (Parise et al., 2010). The unexpected result found in our study based on this earlier study could easily be related to technical and procedural differences as well as to the difference in the choice of evaluated time-windows. However, it is more likely that the lack of enhancement for the own name in our sample may imply a different underlying function compared to that in 5 month-old infants. It is also possible that the suggested acoustic phonological discrimination may not be apparent anymore in older infants. One possibility is that, the similar response to the own and one unfamiliar name in our study may be attributed to two competing saliency levels: the social bias for the own name and the novelty of the presented unfamiliar name. Given the accelerated language development around one year of age, the unfamiliar name might have caught the attention of these young infants by being a novel word. When compared to a novel auditory stimulus, a regular familiar stimulus is expected to show attenuated neural response, either due to habituation and/or lack of preference (Sokolov et al., 2002). Furthermore, this novelty response is expected to occur at a more mature state only after the complete recognition and processing of familiar stimuli (DePaolis et al., 2016). In line with this, it was shown in the neural components of 7 and 10 month-old infants that familiarization of unfamiliar words by repetition attenuated the frontal positive activity in a time window similar to ours (200−500 ms post-stimuli) (Kooijman et al., 2013, 2005). In addition, a lack of preference for familiar words compared to rare (novel) words was observed in 11 month-old infants’ neural responses (Thierry et al., 2003). Interestingly, similar to the enhanced positive neural responses to the own name in 5 month-old infants, studies at earlier ages also showed an enhancement for familiar speech compared to unfamiliar speech (Sheehan and Mills, 2008; Zinke et al., 2018). Therefore, as seen in older infants, attenuated neural activity for the familiar stimuli could have been observed in our study for the own name, at both 10 and 14 months of age. However, earlier studies showed the saliency of the own name compared to any typical familiar stimulus. Therefore, with the assumption of the unfamiliar name being a novel stimulus and the own name acting as a special social cue, neither of the two stimuli might have induced an enhancement over the other one, revealing no significant difference in the neural activity of LR infants in our study. Further research involving an additional familiar name condition may clarify the possible competing saliency levels of the one unfamiliar name and the own name in TD infants at these ages. One would then expect an enhanced neural response to both the own name and the unfamiliar name, but not to the familiar name.
Further to the above point, taking into account a potential effect of learning i.e. memory processing on the enhancement of ERP responses, previous studies showed increased ERP responses with repeated exposure to novel unfamiliar names (Hirata et al., 2011; Tacikowski et al., 2011). This might well explain the young infants’ ERP responses to the repeated unfamiliar name condition and could further support the competing saliency level of this control condition against the own name effect. Unfortunately, given the low number of trial numbers included in the final analyses in our sample, it was not possible to check for this effect by splitting the data and comparing potential differences in the early and late trials. Future studies with a higher number of trials could control for this possibility.
Second, the HR infants’ neural responses revealed an attenuated frontal response to their own name compared to the unfamiliar name at the age of 14 months. Likewise, exploratory group comparisons for the own name also revealed an attenuation of the frontal positive activity in the HR infants’ ERPs compared to the LR infants’. Following the suggestion of competing saliency levels in LR infants’ neural responses, one might conclude that the own name may act like a regular familiar sound but not a special social cue for the HR group at the age of 14 months. This is in line with the behavioural studies showing reduction in response to the own name from 1 year of age in HR siblings. In addition, the reduced ERPs to the own name may be related to the earlier potential delay in language development in this group, as implied by the group differences and correlations with the receptive language scores obtained at the age of 10 months. However, it is important to emphasize that these ERPs were not correlated with language scores concurrently obtained at the age of 14 months. Yet, one might expect that the attribution of self-relevance may be related with perceived language around one year of age and a delay with its perceived meaning could later interfere with social interest to the own name. This suggestion is partly reflected in the significant correlation between the frontal neural responses and communication scores obtained at the age of 14 months. A follow-up on this sample would be interesting to see whether the effect is driven by those infants later receiving a diagnosis of ASD, or whether it should solely be attributed to the delays in their language development. Additionally, for a better understanding of the mechanism behind these ERP responses at these ages, it would be important to reveal the activity in specific neural associates, such as dorsal-medial prefrontal cortex and temporo-parietal junction which were suggested to function as an early precursor of self-referencing before (Holeckova et al., 2008; Imafuku et al., 2014; Nijhof et al., 2018; Perrin et al., 2005).
It is not clear how this positive-going neural activity in the frontal area should be classified in these age groups. One could argue that it might be considered as an early precursor of adult P3a or novelty P3, since the adult P3a is mostly pronounced at the frontal electrodes with a peak around 280 ms post-stimuli and it is induced by engagement of attention and attention to/processing of novel stimuli (Comerchero and Polich, 1999; Friedman et al., 2001). Whereas the well-established negative Nc component is considered to be the precursor of the novelty P3 in the visual domain in infancy (Courchesne et al., 1981), similar to the frontal ERPs in our study, positive frontal neural activity to novel sounds in infants has also been associated earlier with the adult novelty P3 (Kushnerenko et al., 2002; Marshall et al., 2009). However, it is also important to emphasize that the novelty P3 is mostly pronounced with oddball paradigms, different from equally presented stimuli as in our design. Alternatively, this positive-going neural activity may also be interpreted as the more mature version of the early anterior positive neural activity that was observed in 5-month-old infants (Parise et al., 2010).
Finally, the planned analyses of the negative-going activity in the parietal area indicated neither a particular group nor a condition specific difference. The lack of condition and group differences in our findings is in line with the previous study showing a significant difference only when the neural responses to the own name were compared with the ten unfamiliar names control condition as discussed before (Parise et al., 2010). On the other hand, together with a trend towards a main effect of age in the young infants’ ERPs in the parietal area, it is possible to observe a more specifically differentiated morphology of neural activity at the age of 14 months compared to the activity at the age of 10 months, through the visual inspection of the ERPs in this area. Therefore, future investigations of age specific components might yield more meaningful results in this area, which might then be related with components illustrated at the childhood (4–12 years old) in the study of Key et al. (2016), revealing an enhanced parietal P300 activity for the own name when compared to both one repeated and multiple non-repeated unfamiliar names (Key et al., 2016).
The own name versus an unfamiliar name comparison of neural activity in the entire frontal area raises the hypothesis that HR infants may process the sound of their own name as a typical familiar auditory stimulus rather than a highly salient special ostensive cue. However, it is important to emphasize that particularly the within group comparison of the conditions in the HR infants’ neural responses did not exhibit a high power. This could be partly explained by the heterogeneity of the HR groups: the infants later developing ASD may be the only ones showing differentiated neural responses to their own name, also compared to those who develop typically within the HR group. Therefore, this kind of a differentiated effect possibly arising only from a few infants’ ERPs might have resulted in low statistical power in our study. On the other hand, taken together with the unexpected findings in the LR group, this low power difference could also be an indication of false positive findings. Therefore, the replication of these results is crucial to be able to draw concrete conclusions and population-related generalizations. A further limitation of our study is that we did not control for the effect that might be caused simply by the familiarity of the own name stimulus. Rather than the expected establishment of the self-relevance of the own name, the familiarity of this stimulus itself may have an effect on the results of the investigated age groups. Therefore, future infant studies may consider including a familiar name (such as in the study of Key et al. (2016) showing the ERPs to own name in children and in the study of Nijhof et al. (2018) comparing the ERPs to own name in typical adults and adults with ASD), or a general word familiar to infants (e.g., baby) in the paradigm, in order to explore the different responses of HR and LR groups to the own name, independently of this possible confound of familiarity. Furthermore, using a design that allows to investigate behavioural orientation to the names together with ERPs could provide clearer answers to the studied research questions. Such kind of a design, for example when applied longitudinally, may help to reveal the initiation of the attribution of self-relevance to the own name in young infants. However, it is also important to address a limitation of experimental research in young infants: their short attention span and the increased mobility in these ages result in increased artefact and attrition rates in ERP data collection, as in our study, which further lead to low sample size and statistical power. Therefore, complicated designs with long experimental durations are not applicable in these age groups.
Finally, it is important to note that the established differences in the frontal areas can be associated with rather general social, cognitive or language-related developmental problems and are as such not necessarily a candidate neural marker for ASD. Further follow-up of our sample through the assignment of a research diagnosis at 36 months will allow us to determine whether the atypicalities found in the neural responses of HR infants can mainly be related to infants who are later diagnosed with ASD or whether they represent a rather general characteristic of the HR group.
5. Conclusion
This is the first study investigating neural responses in (10 and 14-month-old) LR and HR infants to the very important and unique social cue which introduces periods of communication: “the own name”. The results suggest that a novelty preference over the own name may only be present in HR infants at the age of 14 months and that their neural responses to the own name may be reduced compared to the LR infants. The occurrence of this modification of frontal neural responses in HR infants concurrently with a reduction in their communication skills may imply a modification in their initial attentional bias to social information.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgements
We thank all the parents and infants who participated in this research.
This research was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (No 642996, 2015; Brainview), the Support Fund Marguerite-Marie Delacroix, the Research Foundation Flanders and the Ghent University Special Research Fund (BOF13/PDO/027).
References
- Bortfeld H., Morgan J.L., Golinkoff R.M., Rathbun K. 2005. Mommy and Me Familiar Names Help Launch Babies Into Speech-Stream Segmentation. n.d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerchero M.D., Polich J. P3a and P3b from typical auditory and visual stimuli. Clin. Neurophysiol. 1999;110:24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Cooper R.P., Aslin R.N. 1990. Preference for Infant-direeted Speech in the First Month After Birth Cooper and Aslin; pp. 1584–1595. [PubMed] [Google Scholar]
- Courchesne E., Ganz L., Norcia A.M. Event-related brain potentials to human faces in infants. Child Dev. 1981;52:804–811. [PubMed] [Google Scholar]
- Csibra G. Recognizing communicative intentions in infancy. Mind Lang. 2010;25:141–168. [Google Scholar]
- DePaolis R.A., Keren-Portnoy T., Vihman M. Making sense of infant familiarity and novelty responses to words at lexical onset. Front. Psychol. 2016 doi: 10.3389/fpsyg.2016.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falck-Ytter T., Bakker M., von Hofsten C. Human infants orient to biological motion rather than audiovisual synchrony. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Farroni T., Johnson M.H., Csibra G. Mechanisms of eye gaze perception during infancy. J. Cogn. Neurosci. 2004;16:1320–1326. doi: 10.1162/0898929042304787. [DOI] [PubMed] [Google Scholar]
- Finch K.H., Seery A.M., Talbott M.R., Nelson C.A., Tager-flusberg H. Lateralization of ERPs to speech and handedness in the early development of Autism Spectrum disorder. J. Neurodev. Disord. 2017:1–14. doi: 10.1186/s11689-017-9185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D., Cycowicz Y.M., Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neurosci. Biobehav. Rev. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Grossmann T., Parise E., Friederici A.D. The detection of communicative signals directed at the self in infant prefrontal cortex. Front. Hum. Neurosci. 2010;4:201. doi: 10.3389/fnhum.2010.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy M.W., Richards J.E., Tonnsen B.L., Roberts J.E. Neural correlates of face processing in etiologically-distinct 12-month-old infants at high-risk of autism spectrum disorder. Dev. Cogn. Neurosci. 2018;29:61–71. doi: 10.1016/j.dcn.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata S., Matsuda G., Ueno A., Fuwa K., Sugama K., Kusunoki K., Fukushima H., Hiraki K., Tomonaga M., Hasegawa T. Event-related potentials in response to subjects’ own names. Commun. Integr. Biol. 2011;4:321–323. doi: 10.4161/cib.4.3.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Wahl S. Recording infant ERP data for cognitive research. Dev. Neuropsychol. 2012;37:187–209. doi: 10.1080/87565641.2011.627958. [DOI] [PubMed] [Google Scholar]
- Holeckova I., Fischer C., Morlet D., Delpuech C., Costes N., Mauguière F. Subject’s own name as a novel in a MMN design: a combined ERP and PET study. Brain Res. 2008;1189:152–165. doi: 10.1016/j.brainres.2007.10.091. [DOI] [PubMed] [Google Scholar]
- Imafuku M., Hakuno Y., Uchida-Ota M., Yamamoto Jichi, Minagawa Y. “Mom called me!” Behavioral and prefrontal responses of infants to self-names spoken by their mothers. Neuroimage. 2014;103:476–484. doi: 10.1016/j.neuroimage.2014.08.034. [DOI] [PubMed] [Google Scholar]
- Jasper H. The ten twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Johnson M.H., Dziurawiec S., Ellis H., Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- Kass R.E., Raftery A.E. Bayes factors. J. Am. Stat. Assoc. 1995;90:773–795. [Google Scholar]
- Key A.P., Jones D., Peters S.U. Response to own name in children: ERP study of auditory social information processing. Biol. Psychol. 2016 doi: 10.1016/j.biopsycho.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key A.P.F., Stone W.L. Processing of novel and familiar faces in infants at average and high risk for autism. Dev. Cogn. Neurosci. 2012;2:244–255. doi: 10.1016/j.dcn.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman V., Hagoort P., Cutler A. Electrophysiological evidence for prelinguistic infants’ word recognition in continuous speech. Cogn. Brain Res. 2005;24:109–116. doi: 10.1016/j.cogbrainres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Kooijman V., Junge C., Johnson E.K., Hagoort P., Cutler A. Predictive brain signals of linguistic development. Front. Psychol. 2013;4:25. doi: 10.3389/fpsyg.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnerenko E., Ceponiene R., Balan P., Fellman V., Huotilaine M., Näätäne R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- Macchi Cassia V., Kuefner D., Westerlund A., Nelson C.A. A behavioural and ERP investigation of 3-month-olds’ face preferences. Neuropsychologia. 2006;44:2113–2125. doi: 10.1016/j.neuropsychologia.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel-Emer D., Jusczyk P.W. In: What’s in a Name?: How Infants Respond to Some Familiar Sound Patterns. Hust D., Seidl A., Hollich G., Johnson E., Jusczyk A., editors. 2003. [Google Scholar]
- Mandel D.R., Jusczyk P.W., Pisoni D.B. Infants’ recognition of the sound patterns of their own names. Psychol. Sci. 1995;6:314–317. doi: 10.1111/j.1467-9280.1995.tb00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Reeb B.C., Fox N.A. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev. Sci. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadig A.S., Ozonoff S., Young G.S., Rozga A., Sigman M., Rogers S.J. A prospective study of response to name in infants at risk for autism. Arch. Pediatr. Adolesc. Med. 2007;161:378–383. doi: 10.1001/archpedi.161.4.378. [DOI] [PubMed] [Google Scholar]
- Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 2004;15:1044–1045. [Google Scholar]
- Newman R.S. The cocktail party effect in infants revisited: listening to one’s name in noise. Dev. Psychol. 2005;41:352–362. doi: 10.1037/0012-1649.41.2.352. [DOI] [PubMed] [Google Scholar]
- Nijhof A.D., Dhar M., Goris J., Brass M., Wiersema J.R. Atypical neural responding to hearing one’s own name in adults with ASD. J. Abnorm. Psychol. 2018 doi: 10.1037/abn0000329. [DOI] [PubMed] [Google Scholar]
- Osterling J.A., Dawson G., Munson J.A. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev. Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Ozonoff S., Young G.S., Belding A., Hill M., Hill A., Hutman T., Johnson S., Miller M., Rogers S.J., Schwichtenberg A.J., Steinfeld M., Iosif A.-M. The broader autism phenotype in infancy: when does it emerge? J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S., Young G.S., Carter A., Messinger D., Yirmiya N., Zwaigenbaum L., Bryson S., Carver L.J., Constantino J.N., Dobkins K., Hutman T., Iverson J.M., Landa R., Rogers S.J., Sigman M., Stone W.L. Recurrence risk for autism spectrum disorders : a baby siblings research consortium study. Pediatrics. 2011;128:e1–e8. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise E., Csibra G. Neural responses to multimodal ostensive signals in 5-month-old infants. PLoS One. 2013;8:4–11. doi: 10.1371/journal.pone.0072360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise E., Friederici A.D., Striano T. “Did you call me?” 5-month-old infants own name guides their attention. PLoS One. 2010;5 doi: 10.1371/journal.pone.0014208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perneger T.V. 1998. What’s Wrong With Bonferroni Adjustments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F., Maquet P., Peigneux P., Ruby P., Degueldre C., Balteau E., Del Fiore G., Moonen G., Luxen A., Laureys S. Neural mechanisms involved in the detection of our first name: a combined ERPs and PET study. Neuropsychologia. 2005;43:12–19. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pisula E., Ziegart-Sadowska K. Broader autism phenotype in siblings of children with asd—a review. Int. J. Mol. Sci. 2015 doi: 10.3390/ijms160613217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery A.E. Bayesian model selection in social research STOR. Sociol. Methodol. 1995 [Google Scholar]
- Reid V.M., Striano T. The directed attention model of infant social cognition. Eur. J. Dev. Psychol. 2007;4:100–110. [Google Scholar]
- Scholte E., van Duijn G., Dijkxhoorn Y., Noens I., van Berckelaer-Onnes I. PITS; Leiden (Nederland): 2008. Vineland Screener 0-6 jaar. Handleiding. [Google Scholar]
- Seery A.M., Vogel-Farley V., Tager-Flusberg H., Nelson C.A. Atypical lateralization of ERP response to native and non-native speech in infants at risk for autism spectrum disorder. Dev. Cogn. Neurosci. 2013;5:10–24. doi: 10.1016/j.dcn.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan E.A., Mills D.L. The effects of early word learning on brain development. In: Friederici A.D., Thierry G., editors. Vol. 5. John Ben; Amsterdam, Netherlands: 2008. pp. 161–190. (Early Language Development: Bridging Brain and Behaviour). Trends in language acquisition research: [Google Scholar]
- Sokolov E.N., Nezlina N.I., Polyanskii V.B., Evtikhin D.V. The orientating reflex: the “targeting reaction” and “searchlight of attention.”. Neurosci. Behav. Physiol. 2002;32:347–362. doi: 10.1023/a:1015820025297. [DOI] [PubMed] [Google Scholar]
- Tacikowski P., Jednoróg K., Marchewka A., Nowicka A. How multiple repetitions influence the processing of self-, famous and unknown names and faces: an ERP study. Int. J. Psychophysiol. 2011 doi: 10.1016/j.ijpsycho.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H. Risk factors associated with language in autism Spectrum disorder: clues to underlying mechanisms. J. Speech Lang. Hear. Res. 2016;59:143–154. doi: 10.1044/2015_JSLHR-L-15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry G., Vihman M., Roberts M. Familiar words capture the attention of 11-month-olds in less than 250 ms. Neuroreport. 2003;14:2307–2310. doi: 10.1097/00001756-200312190-00004. [DOI] [PubMed] [Google Scholar]
- van Duijn G., Dijkxhoorn Y., Noens I., Scholte E., van Berckelaer-Onnes I. Vineland Screener 0-12 years research version (NL). Constructing a screening instrument to assess adaptive behaviour. Int. J. Methods Psychiatr. Res. 2009;18:110–117. doi: 10.1002/mpr.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernetti A., Ganea N., Tucker L., Charman T., Johnson M.H., Senju A. Infant neural sensitivity to eye gaze depends on early experience of gaze communication. Dev. Cogn. Neurosci. 2018;34:1–6. doi: 10.1016/j.dcn.2018.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya N., Charman T. The prodrome of autism: early behavioral and biological signs, regression, peri- and post-natal development and genetics. J. Child Psychol. Psychiatry Allied Discip. 2010;51:432–458. doi: 10.1111/j.1469-7610.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- Yirmiya N., Gamliel I., Pilowsky T., Feldman R., Baron-Cohen S., Sigman M. The development of siblings of children with autism at 4 and 14 months: social engagement, communication, and cognition. J. Child Psychol. Psychiatry Allied Discip. 2006;47:511–523. doi: 10.1111/j.1469-7610.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Zinke K., Thöne L., Bolinger E.M., Born J. Dissociating long and short-term memory in three-month-old infants using the mismatch response to voice stimuli. Front. Psychol. 2018;9:31. doi: 10.3389/fpsyg.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L., Bryson S., Rogers T., Roberts W., Brian J., Szatmari P. Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]