Abstract
One of the major hurdles faced in tissue engineering is the inability to monitor and control the function of an engineered tissue following transplantation. Recent years have seen major developments in the field by integrating electronics within engineered tissues. Previously, the most common types of devices integrated into the body used to be pacemakers and deep brain stimulation electrodes that are stiff and non-compliant; the advent of ultra-thin and flexible electronics has brought forth a significant expansion of the field. Recent developments have enabled interfacing electronics onto, into, and within all tissues and organs with minimal adverse reactions. These have introduced the ability to engineer tissues with built-in electronics that allow for remote monitoring and regulation of tissue function. In this review, we discuss the development of technologies that allowed for the formation of tissue-electronics hybrids and give an overview of the existing examples of these hybrid “cyborg” tissues.
Subject Areas: Bioengineering, Biotechnology, Bioelectronics, Tissue Engineering
Graphical Abstract
Bioengineering; Biotechnology; Bioelectronics; Tissue Engineering
Introduction
The field of tissue engineering has developed over the years as an interface between the biological, chemical, and material sciences to tackle the increasing demand for replacement organs in the world (Dvir et al., 2011). Although certain tissues and organs in the body are capable of self-replenishment, other cell populations do not have the ability to proliferate and so there is a need for new types of therapies that can circumvent the need for organ donation. One possible solution to this problem is the injection of isolated cells grown in vitro to replace those lost due to injury or disease. These types of therapies have been explored for their potential to replace a specific cell population with either differentiated cells or stem cells that can differentiate in situ to a specific type of cell in the target tissue (Sanganalmath and Bolli, 2013). Alternatively, cell therapy can take advantage of certain types of cell populations' ability to secrete various factors that aid in tissue regeneration (Liu et al., 2018, Teixeira et al., 2013). Although there has been considerable progress in the understanding of the molecular mechanisms underlying this type of approach, one of the main issues with cell injection therapy is that it does not allow for the delivered cells to organize into a functional tissue prior to replacement; this is due to a lack of communication between the cells and their surroundings, as well as among themselves (Dvir et al., 2011).
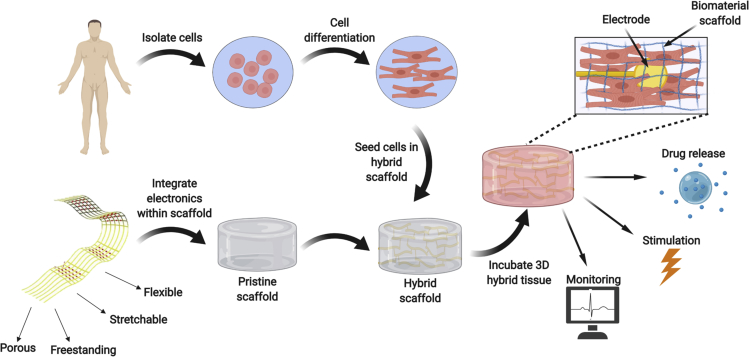
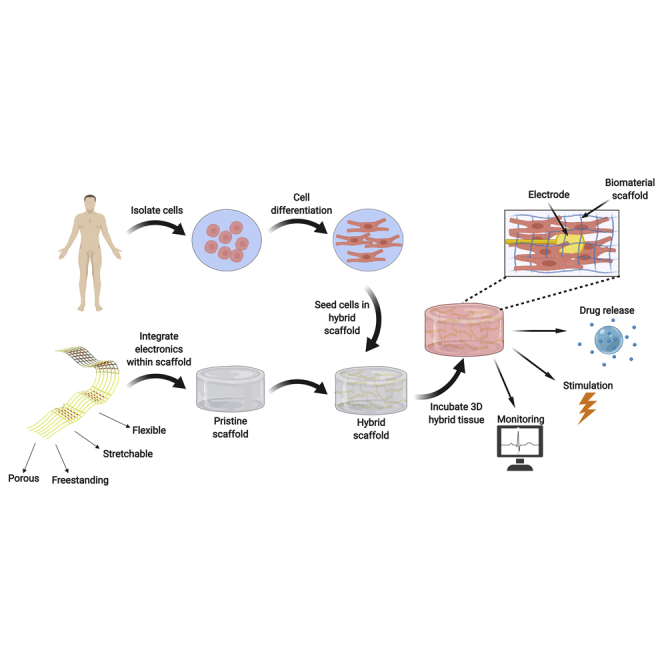
In vivo, tissue and organs are composed of cells residing with an extracellular matrix (ECM). This is a non-cellular environment composed of sugars and protein filaments, which serves both as a physical scaffolding for the cells in the tissue, but is also responsible for important biochemical and biomechanical cues that regulate cell migration, proliferation, differentiation, and general tissue homeostasis (Frantz et al., 2010). Rather than simply introducing cells into the diseased area to repopulate it, tissue engineering involves the seeding of cells in or onto three-dimensional (3D) biomaterials prior to transplantation. These materials serve as temporary scaffolds supporting the cells and promoting their reorganization into a functional tissue (Dvir et al., 2011, Langer and Vacanti, 1993, Vunjak-Novakovic et al., 2009, Fleischer et al., 2017). In recent years, significant progress has been made in the field of tissue engineering, demonstrating a better understanding of the impact of the topography of the biomaterial scaffold (Carlier and Alsberg, 2016), an improved ability to program stem cells into differentiated cells with native tissue-like properties (Ronaldson-Bouchard et al., 2018), and even the ability to recapitulate complex tissue structures using 3D printing (Noor et al., 2019, Grigoryan et al., 2019). However, one of the main challenges remaining in the field is the ability to monitor the outcome of the engineered tissue and its effect on the treated organ once it has been transplanted. In addition, once it has become possible to monitor the implanted tissue's function, a tool to intervene in the outcome of the therapy without the need for further surgical intervention or constant medical attention would be highly beneficial to the outcome of the treatment. In order to do so, there needs to be a technology to integrate electronics with engineered tissues to collect data from within the tissue and report on it in real time, as well as introduce certain stimuli that will lead to a response from the tissue (Figure 1).
Figure 1.
Integrating Electronics with Engineered Tissues
The electronics are integrated within 3D biomaterial scaffolds and then seeded with cells to form the 3D hybrid tissue. The electronics include sensing, stimulating, and controlled drug release elements for regulating tissue function.
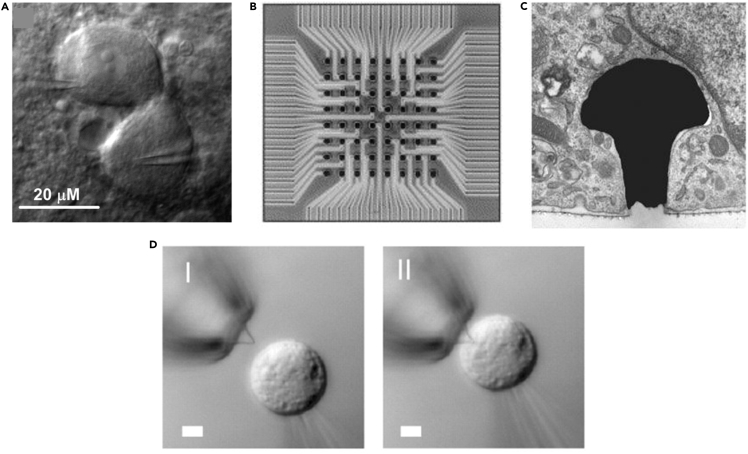
All cells and tissues communicate through secreted signals such as cytokines, growth factors, microRNAs, and extracellular vesicles (Raposo and Stoorvogel, 2013, Chen et al., 2012). However, certain types of tissues such as neural, muscular, and cardiac tissue also communicate among themselves through the propagation of electrical signals, specifically action potentials. An action potential arises due to depolarization of the cell membrane beyond a certain threshold due to the opening of certain ion channels. Once this threshold has been crossed, more ion channels are opened leading to an increased flux of ions through the cell membrane and an acute depolarization event takes place. An action potential leads to a sequence of events that results in the contraction of a muscle cell (Katz, 2010) or neurotransmitter release in a neuron (Bean, 2007). Over the years, tools have been developed that take advantage of the electrical activity of these types of tissues in order to study and control their function. For example, patch clamp technology has been widely used over the years to study the mechanisms underlying the electrical activity of electrogenic cells (Figure 2A) (Zhao et al., 2008), and multielectrode arrays based on metal electrodes have allowed to record and deliver extracellular signals from isolated tissues and organs without harming them (Figure 2B) (Feiner and Dvir, 2018). More recent advances have allowed recording from within cells using metal electrodes with unique morphologies (Figure 2C) (Shmoel et al., 2016) or silicon nanowired field-effect transistors (Figure 2D) (Tian et al., 2010, Duan et al., 2012, Zhao et al., 2019). Although these and other works have significantly increased our level of understanding and control over cellular and tissue function, a different type of approach needs to be taken to allow proper communication with and within tissues. These tissue-electronics interactions require a different type of interface in order to optimize the function of the electronics and most importantly not harm the tissue.
Figure 2.
Interrogating Cellular Electrical Activity
(A) Infrared-differential interference contrast microscopy image of a pair of electrically coupled neurons during dual whole-cell patch clamp. Scale bar, 20 μm.
(B) Multielectrode array containing 64 electrodes, 50 × 50 μm(Sanganalmath and Bolli, 2013) each. Scale bar, 150 μm.
(C) Electron micrograph of a mushroom-shaped electrode engulfed by a PC12 cell. Scale bar, 5 μm.
(D) Differential interference contrast microscopy images of an HL-1 cell and kinked nanowire probe as the cell approaches the probe (I), contacts, and internalizes it (II). Scale bar, 5 μm. Reproduced with permission from Tian et al., 2010, Palacios-Prado et al., 2013, Oka et al., 1999; and Hai et al., 2010.
Matching the Mechanical Properties of the Electronics to the Engineered Tissue
Although most tissues in the body, excluding bone and teeth, are relatively soft with elastic moduli ranging from 1 to 100 kPa, the properties of traditional silicon- and gallium-based electronics used in most day–-to-day devices are significantly stiffer with elastic moduli in the high GPa range (Kim et al., 2011). If proper integration of the electronics within tissues is to be achieved, this large gap in mechanical properties needs to be overcome. This, in general, has been the subject of ongoing focus in the field of tissue engineering, because the mechanical properties of the substrate on which cells are seeded can impact the behavior of individual cells as well as the success of their development into a functional tissue (Engler et al., 2004, Engler et al., 2006, Discher et al., 2005, Rehfeldt et al., 2007). As the elastic modulus varies from tissue to tissue, it is important to put an emphasis on developing scaffolds with tailored mechanical properties that allow for optimal tissue organization and function (Feiner and Dvir, 2018). The same approach needs to be applied to developing electronics that are meant to interface with tissues. Although silicon-electronics-based technology allows for a wide variety of components and facile fabrication technologies, this extreme mismatch in mechanical properties needs to be overcome. In order to do so, technologies for fabricating soft, flexible, and stretchable electronic devices have been devised.
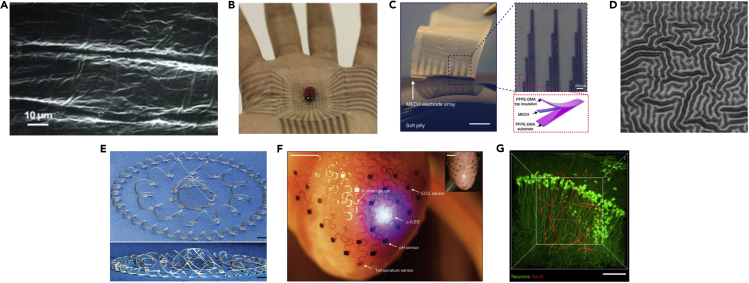
One of the first methods examined was the use of organic conductive polymers, such as polypyrrole (Wang et al., 2011), polyaniline (Blinova et al., 2008), and poly(3,4-ethylenedioxythiophene):poly(styrene sulfonic acid) (PEDOT:PSS) (Hansen et al., 2007) (Figure 3A). These materials are soft, biocompatible, and naturally conductive and so serve as appealing choices for flexible electronics. When compared with metals the resistivity of most commonly used metal conductors is ∼10−6 Ωcm, whereas that of organic polymers is ∼10−1 to 10−3 Ωcm (McCoul et al., 2016). However, despite the large differences in resistivity between the two material classes, organic polymers are often deposited on metal electrodes to improve their functionality in physiological settings. For example, PEDOT:PSS-coated platinum-iridium electrodes were shown to have superior recording and stimulating performance both in vitro and in vivo when compared with the bare metal electrode as exemplified by a much lower impedance, a higher charge injection, and a higher signal-to-noise ratio when recording from neurons (Venkatraman et al., 2011, Gerwig et al., 2012). Similarly, a much lower impedance was exhibited when platinum electrodes were coated with a layer of PPy (Lu et al., 2010). Another relatively simple method for fabricating soft, flexible electrodes is by depositing conducting elements onto or inside elastomers such as polydimethylsiloxane (PDMS) or polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (SEBS) (Park et al., 2016). Recent works by the Bao lab has demonstrated the development of intrinsically stretchable transistors and transistor arrays with skin-like mechanical properties using this approach (Figure 3B) (Xu et al., 2017, Wang et al., 2018). Additional work from this group has described a method for fabricating stretchable and flexible electronics based on conductive hydrogels that are also compatible with photolithography technology. This allows better control over the structure of the electronics and location of specific components within the devices. These types of hydrogel-based devices were shown to conform and wrap around the sciatic nerve of mice to perform neural stimulation (Figure 3C) (Liu et al., 2019).
Figure 3.
Flexible Electronics
(A) Scanning electron micrograph of a polypyrrole film, containing the dopant p-toluenesulfonate anion deposited on a gold layer over a PDMS substrate.
(B) A photograph of an intrinsically stretchable transistor array adhering and conforming to a human palm.
(C) A multielectrode array based on a hydrogel prepared by photolithography, showing good control over electrode placement and size. Scale bar, 2 mm and 200 μm (insert).
(D) Optical image of a 100-nm-thick gold layer deposited on a PDMS substrate after strain has been relieved from the substrate. Scale bar, 100 μm.
(E) SEM image of a complex 3D mesostructure formed from a 2D precursor that consists of closed-loop circular filamentary serpentines and radially oriented ribbons, selectively bonded to a biaxially stretched elastomer substrate. Scale bar, 400 μm.
(F) Image of a flexible device consisting of serpentine filaments integrated on a Langendorff-perfused rabbit heart. The white arrows highlight various function elements in this system. Scale bar, 6 mm.
(G) 3D interface between an ultra-flexible device with neuron-size features (red) and neurons (green). Scale bar, 100 μm. Reproduced with permission from Wang et al., 2011, Wang et al., 2018, Liu et al., 2019, Xu et al., 2014, Xu et al., 2015, Yang et al., 2019; and Lacour et al., 2003.
Another approach is based on varying the architecture of the underlying substrate supporting the conductor. Metal conductors can be deposited onto a pre-stretched elastomer and then by releasing the strain, buckling of the conductor together with the substrate leads to the formation of a surface morphology that allows stretching without cracking (Figure 3D) (Jones et al., 2004, Lacour et al., 2004). Alternatively, highly elastic devices can be designed by localized bonding of the device to a pre-stretched elastomer, which upon release of the strain leads to the formation of predesigned complex 3D architectures. This method allows for the integration of more diverse electronic components that can also be based on silicon (Figure 3E) (Xu et al., 2015).
However, the most facile and diverse method for the fabrication of stretchable electronics is by varying their thickness and architecture. Meandering or “serpentine” architectures allow for the electronics to be stretched to high degrees of strain without incurring any stress, thus minimizing damage to the device, whereas reducing the thickness of the device directly reduces its stiffness (Kim et al., 2011, 2, Liu et al., 2018, Feiner et al., 2019, Xie et al., 2015, Fu et al., 2016, Zhou et al., 2017). These structures can be stretched as a spring until they are straight and can easily extend over several times of their relaxed length. The meandering shape method provides a simple, yet elegant way to retain electrical performance, while withstanding the mechanical and topographical constraints of the body. This approach has been used to integrate various types of sensors such as ECG and EMG sensors (Xu et al., 2014), heat sensors (Kim et al., 2011), strain gauges (Kim et al., 2011), photodetectors (Song et al., 2013), and metabolite sensors (Huang et al., 2014) into flexible and stretchable devices. Additional components such as LEDs (Kim et al., 2011), heating coils (Kim et al., 2011), wireless power coils (Kim et al., 2011), radiofrequency coils (Kim et al., 2011), and antennas (Kim et al., 2011) have also been described using this approach. These devices could be interfaced with smooth and complex topographies such as the heart epicardium, brain parenchyma, and blood vessel lumen (Kim et al., 2011, Xu et al., 2014, Kim et al., 2011, Huang et al., 2014, Webb et al., 2013, Norton et al., 2015, Bareket et al., 2016, Kim et al., 2012). Advanced devices designed using the serpentine geometry approach were developed by the Rogers lab to perform spatiotemporal measurements of several different parameters as well as stimulation across the entire epicardial surface. Due to their unique morphology, the devices were able to adapt to and withstand the motions of the beating heart without failing or causing significant damage to the organ (Figure 3F) (Xu et al., 2014). By minimizing the thickness of the device and the size of the structures within it, the Lieber group has described the development of devices with neuron scale structural features. The subcellular size scale of their features and consequent optimal mechanical properties allowed them to penetrate the brain parenchyma and interrogate individual neurons with minimal damage. In addition, the devices formed intimate contact with neural and glial networks within the brain (Figure 3G) (Fu et al., 2016, Yang et al., 2019). These advances in flexible electronics help overcome the issue of the mechanical mismatch between the electronics and the tissue they are meant to integrate with. Table 1 summarizes the different properties of these technologies for the fabrication of flexible electronics. The next step toward developing smart tissues and organs is to combine them within biomaterials for tissue engineering that allows for seamless integration.
Table 1.
Properties of Different Technologies for Fabricating Flexible Electronics
| Technology | Fabrication | Components | Young's Modulus | Resistivity/Conductivity | References |
|---|---|---|---|---|---|
| Stretchable conductive polymers | Electropolymerization | Various polymers such as polypyrrole, polyaniline. and poly(3,4-ethylenedioxythiophene) | Giga pascal range; however, thin layers allow for flexibility. | ~10−1-10−3 Ωcm | (McCoul et al., 2016, Valentová and Stejskal, 2010, Bloor et al., 1986, Qu et al., 2016) |
| Doping elastomers | Mixture of conducting or semiconducting elements within an elastomer such as polydimethylsiloxane (PDMS) or polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene (SEBS) and subsequent definition for example by photolithography | Elastomer and conductive or semi-conductive elements | Depending on the elastomer, its thickness, and the dopant. | Depends on the choice of dopant and its concentration. | (Park et al., 2016, Xu et al., 2017, Wang et al., 2018) |
| Conductive hydrogel-based electrodes by photolithography | Mixture of the ionic (4-(3-butyl-1-imidazolio)-1-butanesulfonic acid trifate) with the conductive polymer poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) and subsequent photolithography | A hydrogel of the conductive polymer poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) | ~30 kPa | ~0.02 Ωcm | (Liu et al., 2019) |
| Buckled metal conductors | Deposition on a pre-stretched elastomer and release of the strain | Metal conductor and elastomer substrate | Depending on the thickness of the elastomer substrate | −10−6 Ωcm depending on the choice of metal. | (Jones et al., 2004, Lacour et al., 2004) |
| Localized bonding to pre-stretched elastomer | Microfabrication using photolithography and thin film metal deposition and concomitant release and transfer to a pre-stretched elastomer with predefined bonding sites | Primarily polyimide or SU-8 and a metal conductor on top of an elastomer | Varies depending on thickness of the device ranging from kilo to giga pascals. | −10−6 Ωcm depending on the choice of conductor. | (Xu et al., 2015) |
| Stretchable architecture | Microfabrication using photolithography and thin film metal deposition | Substrate such as polyimide or SU-8 and a metal conductor | Varies depending on thickness of the device ranging from kilo to giga pascals. | −10−6 Ωcm depending on the choice of conductor. | (Feiner et al., 2019, Fu et al., 2016, Gonzalez et al., 2009, Kim et al., 2011, Xie et al., 2015, Zhou et al., 2017, 2) |
| Ultra-thin electronics | Microfabrication using photolithography and thin film metal deposition | Substrate such as polyimide or SU-8 and a metal conductor | Varies depending on thickness of the device ranging from kilo to giga pascals. | −10−6 Ωcm depending on the choice of conductor | (Fu et al., 2016, Yang et al., 2019) |
Tissue-Engineered Electronic Hybrids
Although advanced electronics have allowed for conformal integration on and into existing organs, they do not provide regeneration to the failing tissues. In order to do so, these novel electronics need to be integrated within biomaterial scaffolds that will allow cell seeding and function without interfering with tissue organization. This needs to be achieved while allowing proper regulation of tissue function from within.
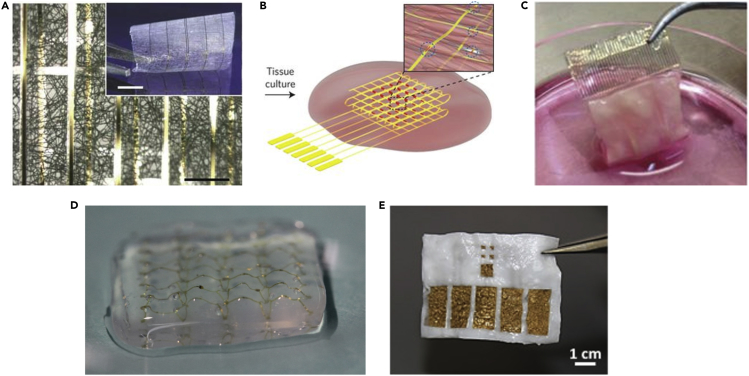
The first example of such a hybrid approach was described by Tian and colleagues. Here, lithography was used to fabricate a planar, thin, porous, and electronic mesh with integrated silicon nanowired field-effect transistors (nanoFETs). This device was then released from the silicon wafer into its freestanding form by eliminating an underlying sacrificial layer. The design of the mesh was highly porous and due to its low thickness of ∼2 μm was highly flexible and easy to manipulate in 3D. The nanoelectronic mesh was then integrated within a planar electrospun biomaterial fiber mat and used as a hybrid scaffold for seeded neurons and cardiomyocytes (Figure 4A). As the entire hybrid material is highly flexible, it could be rolled and folded after cell seeding into a thicker 3D engineered tissue. The work showed the formation of an engineered cardiac tissue, whereby the electronics had a minimal impact on its organization and demonstrated the capability of the scaffold to monitor the function of the tissue from within, in a spatiotemporal manner (Tian et al., 2012).
Figure 4.
Hybrid Tissues
(A) A bright-field optical micrograph of the folded hybrid scaffold, showing multilayered structures of PLGA integrated with the nanoelectronic mesh. The inset shows a photograph of the hybrid sheet before folding. Scale bars, 200 μm and 5 mm (inset).
(B) Schematic of a free-standing macroporous nanoelectronic scaffold with nanowire FET arrays and platinum stimulating electrodes integrated within a cardiac tissue.
(C) An image of a folded hybrid cardiac tissue with integrated gold electrodes after seven days of cultivation with cardiac cells.
(D) A freestanding degradable device fabricated by depositing gold electrodes on top of an electrospun fiber scaffold with a second scaffold serving as a passivation layer.
(E) Optical image of the freestanding 3D electronic scaffold integrated within an ECM hydrogel. Scale bar, 1 cm. Reproduced with permission from(Tian et al., 2012, Feiner et al., 2016, Feiner et al., 2018, Dai et al., 2016, Wang et al., 2020).
Although the nanoFETs allow for sensitive recordings of extracellular potentials from within the engineered tissue, it was not until metal electrodes were integrated within these tissues that control over its function was demonstrated. Using either platinum or gold planar electrodes, it was possible to control the pace of cardiac tissue contraction, as well as the direction of conduction of the electrical signal throughout it (Feiner et al., 2016, Dai et al., 2016). Achieving both monitoring and regulation of tissue function is possible either through a combination of metal electrodes and nanoFETs (Figure 4B) (Dai et al., 2016) or simply by using the metal electrodes for both functions (Feiner et al., 2016, Feiner et al., 2019). This was demonstrated in a more recent work by our lab whereby planar gold electrodes were used as part of a flexible and porous electronic mesh integrated within electrospun fibers to create a hybrid microelectronic scaffold (Feiner et al., 2016). Due to the ability to inject current in sufficiently high levels through the metal electrodes, it was possible to both monitor and stimulate tissue function from within the engineered cardiac tissue (Figure 4C). An additional level of control through these devices was demonstrated by their ability to release either proteins or small molecules from electroactive polymers deposited onto the metal electrodes to provide a physiological effect within the engineered tissue. This work demonstrated biomolecule release through two types of polymers, allowing the delivery of both positive and negative molecules of varying sizes in a highly controlled manner, all this, without harming the function of both the released anti-inflammatory drug dexamethasone and the chemokine CXCL12. In a more recent work, an electronic mesh was developed which integrated within it serpentine design elements so as to make it not only flexible but also able to withstand mechanical strain up to two times its original length. Using this device it was shown that several different anti-inflammatory drugs could be released in parallel from electrochemically deposited layers of polypyrrole in a controlled manner (Feiner et al., 2019).
The devices described thus far are composed entirely of biocompatible materials that are FDA approved; however, they are not biodegradable, and so will remain in the patient's body following implantation for an indefinite lifetime unless extracted. Although this is not always detrimental, there are instances in which the presence of obsolete electronic devices within the tissue is unnecessary, and if it became commonplace, it could pose a risk. Many works over the years have described the fabrication of biodegradable electronics based on a variety of materials and platforms ranging from paper to nanoscale films of silicon (Russo et al., 2011, Hwang et al., 2012, Yin et al., 2014, Luo et al., 2014, Boutry et al., 2015). These allow for control over the lifetime of the electronics by modulating its thickness and components, which is pertinent for the type of tissue being regenerated. In order to develop a hybrid tissue based on biodegradable electronics, two recent examples have shown the utilization of electrospun fiber scaffolds as the substrate and dielectric of the electronic mesh. One example described the use of the synthetic polymer polyurethane (Lee et al., 2019), whereas the other used the natural protein albumin (Feiner et al., 2018). Both examples utilized biodegradable materials that can serve as a scaffold for tissue engineering as well, and when electrospun into fiber form can generate scaffolds that are also flexible and elastic. By evaporating metal electrodes through a shadow mask onto the electrospun fiber scaffolds, it was possible to create a mesh that serves both as the electronic component and as the scaffold for the engineered tissue (Figure 4D). When cardiomyocytes were seeded on these devices, they organized into a functional cardiac tissue, as they did on their pristine counterparts. Using the evaporated gold electrodes, it was possible to record extracellular potentials from within the engineered tissues, as well as stimulate it and release anti-inflammatory drugs in a controlled manner (Lee et al., 2019, Feiner et al., 2018). As proof of its biodegradable nature, the albumin fiber-based device was implanted subcutaneously in rats and was shown to degrade over several weeks (Feiner et al., 2018). Ideally, in the future, controlled degradation of the electronic component within the tissue could be achieved. This controlled degradation could be triggered by heating (Park et al., 2015) or illuminating (Phillips et al., 2017) the electronics.
These works brought forth the ability to control and monitor tissue function from within through the integration of electronics; however, they were based on planar electronic devices. One of the major concerns in the field of tissue engineering in general and in cardiac tissue engineering specifically, is the ability to engineer thick tissues (Fleischer et al., 2017, Fleischer et al., 2017). Although the devices described until now allow for an excellent level of control over tissue function, they are based on planar devices and are integrated within scaffolds that are mostly two dimensional. These devices are flexible enough to be rolled or folded and in such a manner to achieve a more 3D electrode distribution and allow for the formation of a thicker engineered tissue (Feiner et al., 2016, Feiner et al., 2018, Feiner et al., 2019, Tian et al., 2012, Dai et al., 2016). However, in order to integrate electronics in a controlled 3D pattern and combine them with biomaterial scaffolds that are thicker, a different type of fabrication approach is required. A possible approach toward achieving highly elastic devices is through localized bonding of the device to a pre-stretched elastomer, which upon release of the strain leads to the formation of predesigned complex architectures. This method was developed by the Rogers group and has been used to fabricate structures with increasing complexity in recent years (Xu et al., 2015, Yan et al., 2016, Fu et al., 2018). It was also shown that it is possible to integrate electronic components within these devices and even record extracellular potentials by seeding neurons onto them (Fu et al., 2018, Yan et al., 2017). In a recent work, we have shown that it is possible to fabricate highly porous 3D electronic scaffolds and integrate them within an ECM-based hydrogel scaffold for tissue engineering. Cardiomyocytes were mixed inside the liquid ECM-based hydrogel. As the hydrogel is liquid in room temperature, it could penetrate the porous structure of the device and surround all of its components without harming them to create a thoroughly integrated hybrid (Figure 4E). Due to the nature of the hydrogel, it was deposited in its liquid form with the isolated cardiac cells mixed inside of it. Once the hybrid was placed in a physiological temperature of 37°C, it solidified and allowed the seeded cells to form a confluent, functional engineered cardiac tissue. Using the underlying electronic device, it was possible to record extracellular potentials, release drugs, and stimulate tissue function in a real 3D spatiotemporal manner (Wang et al., 2019).
Future Outlook
The examples given here provide a glimpse into how the field of biomedical electronic implants has evolved from the days of stiff implantable pacemakers into micro- and nanoscale, thin electronic meshes with features reaching the size of single cells, and mechanical properties that have been reduced to those of the softest tissues. The rapid advances made in the field of flexible and stretchable electronics has allowed us to overcome the significant organic-inorganic barrier that usually exists between electronics and tissues and organs. The product of these advances has allowed to overcome the negative effect a foreign device can have on a tissue and its rejection from the body. Some of the technologies discussed here allow for devices that are so delicate that they can be injected through a syringe into the target tissue (Zhou et al., 2017, Yang et al., 2019, Hong et al., 2015, Schuhmann et al., 2017). These devices can contain within them electronic components ranging from simple planar electrodes to field-effect transistors (Xie et al., 2015, Tian et al., 2012), batteries (Dagdeviren et al., 2014, Xu et al., 2013, Kil et al., 2013), radiofrequency antennas (Fu et al., 2018), and more, thus making it possible to sense a wide range of physical, chemical, and biological variables and give a much improved picture of the internal environment being probed. Such features may be integrated in the future with technologies to engineer injectable tissues (Montgomery et al., 2017), providing controllable tissue regeneration with a minimally invasive approach. In addition to the progress made in the field of soft electronics, significant breakthroughs have been made in controlling the lifetime of the electronic components. There are now a variety of different approaches toward creating electronics with a limited lifetime. Materials and fabrication techniques now exist that allow for the development of various substrates, conductors, semiconductors, and dielectrics with transient properties. These can also be composed of thin and stretchable materials, so that the options in terms of device functionality and its lifetime are numerous (Feiner et al., 2018, Hwang et al., 2015).
The advances outlined here in terms of electronics go hand in hand with advances in the field of tissue engineering. One can imagine how all the electronics developed for delicate interrogation of the human body can also be implemented into the field of tissue engineering to create hybrid or “bionic” tissues. As we have described here, the examples of integrated electronics within engineered tissues are rather limited and contain within them a simple demonstration of what can be achieved when integrating electronics. Yet, simple as they are, they have demonstrated the ability to monitor the tissue from within, control its function, and even release into its environment a variety of molecules that can affect its development and function.
The field of tissue engineering tries to recapitulate the natural components of the body so they can serve as viable replacements. Although there have been significant improvements and developments in the field, each type of tissue under research comes with its own set of challenges that need to be overcome in order for it to qualify for clinical use. The concept of integrating electronics within all types of engineered tissues entails within its various prospects for improving tissue development and function. For example, the ability to monitor the amount of force generated from an engineered muscle tissue can indicate on its state of development. These data can be combined with a tissue-wide view of the conduction of the action potentials throughout it and the secretion and concentration of various factors to understand the underlying factors contributing to a successful or unsuccessful development. Add to that the ability to stimulate the tissue from various locations and in a controlled manner, a feat that has been shown to be crucial to the development of cardiac tissue (Ronaldson-Bouchard et al., 2018, Radisic et al., 2004). This type of approach can be implemented systematically to better understand the in vitro development of tissues, as well as to understand responses to various drugs and their combination (Esch et al., 2015).
Other, more advanced components have yet to be realized in tissue engineering. For example, nanoFETs and carbon nanotubes have been demonstrated as useful tools not only in the monitoring of tissue electrical function but also as sensors for a variety of secreted extracellular and intracellular molecules such as DNA, lipids, cytokines, and more (Budhathoki-Uprety et al., 2017, Harvey et al., 2017, Patolsky et al., 2006, Zheng et al., 2005, Heller et al, nd). These could be used to understand the developmental state of the tissue and to test what conditions take us closer or farther from the goal of creating a functional replacement tissue. Piezoelectric components could be integrated within tissues that generate force, such as striated, smooth, or cardiac muscle. Advances in battery technology would allow to store the energy harvested using piezoelectricity to power active components such as data transmitters or actuators (Dagdeviren et al., 2014, Xu et al., 2013, Kil et al., 2013). With recent advances made in the field of optoelectronics, and conditional on genetic engineering being approved for use in replacement tissues, it would be possible to control not only electrogenic tissues such as nerve and muscle but ultimately any type of cell (Kim et al., 2013, Kim et al., 2016, Park et al., 2015).
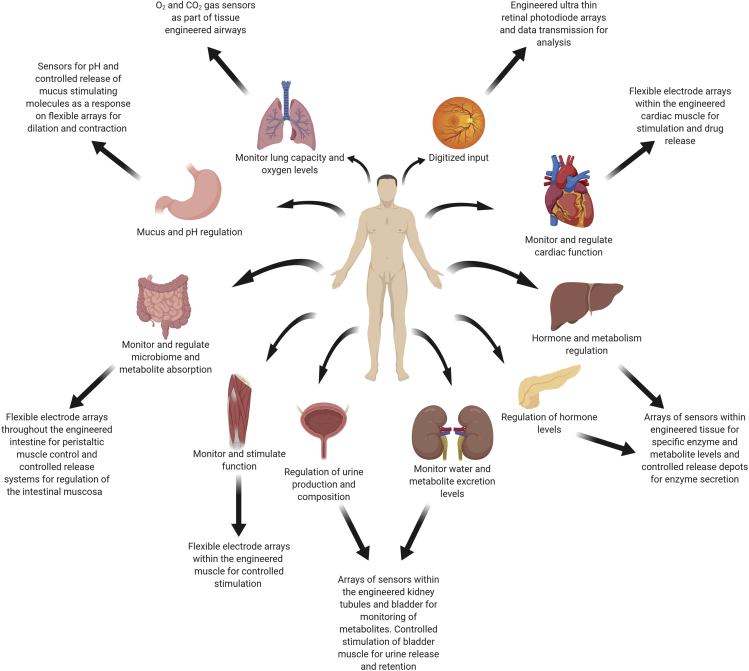
The work done thus far in the field has demonstrated the integration of electronics within engineered tissues that were mostly composed of a single type of cell population, specifically, cardiac or neural tissue (Feiner et al., 2016, Feiner et al., 2018, Feiner et al., 2019, Tian et al., 2012, Dai et al., 2016). Although it is understandable that preliminary works will focus on relatively non-complex models, there is no reason why this hybrid approach to tissue replacement cannot be implemented in the field of whole organ engineering. Significant progress has been made in recent years in the development of entire organs for replacement (Khademhosseini and Langer, 2016). These are mostly based on either using decellularized organs as scaffolds or utilizing 3D printing to create replacement organs. Based on these and the developments in the field of 3D printing of tissues and electronics (Noor et al., 2019, Valentine et al., 2017) it is easy to imagine the emergence of engineered organs with built-in electronics. Figure 5 depicts some ideas for the future of engineered tissues and organs with integrated electronics and a general description of how they could be implemented. One possible example could be the fabrication of replacement hearts with built-in electrical pacemakers that could take over in case of arrhythmia or failure and in case of cardiac arrest could be used as a defibrillator. These would of course be combined with a network or electrodes that can give a detailed report on the state of the organ. Bionic livers and pancreases could be developed and these could report on the levels of secreted enzymes and whether or not they are in balance with the level of metabolites they sense. Bionic intestine replacements can sense whether the amount of nutrients present within them and their absorption is sufficient or even report on the microbiome population. Engineered kidneys could potentially report on the level of metabolites within the blood and whether or not they can handle their excretion. Engineered retinas could allow restoration of vision and digitization of the recorded data for various purposes (Prévot et al., 2019). For each of these engineered tissues or organs, the lifetime of the integrated electronics could also be controlled. Ideally, the electronics could be eliminated once organ function has been restored and there is no need for constant monitoring or intervention. Alternatively, if the presence of the electronics is not detrimental to tissue function, they could remain within the tissue indefinitely to improve the quality of life of the patient. The development of these types of replacements would not only help diminish the scarcity in donor organs but using their abilities would reduce the need for frequent follow ups and procedures.
Figure 5.
Engineering of Bionic Tissues
The integration of electronic devices capable of monitoring and actuation into engineered tissues opens up the field of hybrid tissues and organ engineering. Hybrid tissues and organs would allow monitoring of tissue development and function, as well as correcting its performance following implantation. Using this technology, monitoring and treating patients online from afar would replace frequent and unnecessary hospital visits. The possibility of elective surgery using engineered bionic tissues would enhance the organ's natural abilities. Described are a few possible future concepts for engineered tissues and organs with integrated electronics and how they would improve function.
Although these bionic organs may seem a lifetime away from implementation, recent progress in all related fields and their combination makes them more and more likely. There are still many challenges that need to be overcome before these types of hybrid tissues can be realized in a clinical setting. These can be divided into the challenges facing the field of tissue engineering and the challenges regarding the integration of electronics within tissues. In the field of tissue engineering, one of the main challenges is the choice of cell source. Cells for tissue engineering can be sourced either from adult stem cells or from embryonic or induced pluripotent stem cells. Although adult stem cells are more differentiated and thus could prove a better choice for a specific tissue, in some cases such as cardiac or neural tissue, obtaining adult stem cells is not a simple feet. Other stem cell sources such as induced pluripotent or embryonic stem cells are an attractive choice due to their availability; however, issues of differentiation and safety require further investigation before clinical application. Another important challenge in the field of tissue engineering is the problem of oxygen supply for thicker tissues. A well-developed vascular system should be part of any engineered functional tissue or organ. Although major progress has been made in this regard, the engineering of thick vascularized tissues still remains a major hurdle before clinical application. In regard to the electronic components, it still remains to be seen what is the best method to integrate it within the engineered tissue and whether it should remain there for long periods of time or not. If not, what is the ideal timescale for its function and how do we precisely control it, and if so, what is the best method to supply it with energy and communicate with it for long periods of time. To this end, thin, flexible, miniature devices have been described that employ wireless technology for both data transmittance and energy harvesting for optogenetics, drug delivery, and monitoring of parameters such as heat and pressure (Luo et al., 2014, Park et al., 2015, Kim et al., 2016, Huang et al., 2014, Han et al., 2018, Koo et al., 2018). Other equally important issues that need to be dealt with are those of ethical concern. Is it enough to create a replacement organ or should we improve its function at the same time? Should organ replacement be elective or only used in extreme cases? All of these questions and challenges would need to be dealt with before such a technology could be introduced into the clinic.
Acknowledgments
R.F. thanks the Clore Scholarship program, Marian Gertner Institute for Medical Nanosystems Fellowship and the Argentinian friends of Tel Aviv University. T.D. acknowledges support from the European Research Council (ERC) Starting Grant 637943, the Slezak Foundation, and the Israel Science Foundation (700/13).
Author Contributions
Both authors contributed equally to the preparation of this manuscript.
References
- Bareket L., Inzelberg L., Rand D., David-Pur M., Rabinovich D., Brandes B., Hanein Y. Temporary-tattoo for long-term high fidelity biopotential recordings. Sci. Rep. 2016;6:25727. doi: 10.1038/srep25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Blinova N.V., Stejskal J., Trchová M., Prokeš J. Control of polyaniline conductivity and contact angles by partial protonation. Polym.Int. 2008;57:66–69. [Google Scholar]
- Bloor D., Hercliffe R., Galiotis C., Young R. Springer; 1986. Integration of Fundamental Polymer Science and Technology; pp. 630–633. [Google Scholar]
- Boutry C.M., Nguyen A., Lawal Q.O., Chortos A., Rondeau-Gagné S., Bao Z.A. Sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015;27:6954–6961. doi: 10.1002/adma.201502535. [DOI] [PubMed] [Google Scholar]
- Budhathoki-Uprety J., Langenbacher R.E., Jena P.V., Roxbury D., Heller D.A. A carbon nanotube optical sensor reports nuclear entry via a noncanonical pathway. ACS nano. 2017;11:3875–3882. doi: 10.1021/acsnano.7b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier A., Alsberg E. Harnessing topographical cues for tissue engineering. Tissue Eng. A. 2016;22:995–996. doi: 10.1089/ten.TEA.2016.0188. [DOI] [PubMed] [Google Scholar]
- Chen X., Liang H., Zhang J., Zen K., Zhang C.Y. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–132. doi: 10.1016/j.tcb.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Dagdeviren C., Yang B.D., Su Y., Tran P.L., Joe P., Anderson E., Xia J., Doraiswamy V., Dehdashti B., Feng X. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl. Acad. Sci. U S A. 2014;111:1927–1932. doi: 10.1073/pnas.1317233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Zhou W., Gao T., Liu J., Lieber C.M. Three-dimensional mapping and regulation of action potential propagation in nanoelectronics-innervated tissues. Nat. Nanotechnol. 2016;11:776–782. doi: 10.1038/nnano.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher D.E., Janmey P., Wang Y.-l. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Duan X., Gao R., Xie P., Cohen-Karni T., Qing Q., Choe H.S., Tian B., Jiang X., Lieber C.M. Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor. Nat. Nanotechnol. 2012;7:174–179. doi: 10.1038/nnano.2011.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T., Timko B.P., Kohane D.S., Langer R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A.J., Griffin M.A., Sen S., Bönnemann C.G., Sweeney H.L., Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Esch E.W., Bahinski A., Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015;14:248. doi: 10.1038/nrd4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiner R., Dvir T. Tissue–electronics interfaces: from implantable devices to engineered tissues. Nat. Rev. Mater. 2018;3:17076. [Google Scholar]
- Feiner R., Engel L., Fleischer S., Malki M., Gal I., Shapira A., Shacham-Diamand Y., Dvir T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016;15:679–685. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiner R., Fleischer S., Shapira A., Kalish O., Dvir T. Multifunctional degradable electronic scaffolds for cardiac tissue engineering. J. Control. Release. 2018;281:189–195. doi: 10.1016/j.jconrel.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiner R., Wertheim L., Gazit D., Kalish O., Mishal G., Shapira A., Dvir T. A stretchable and flexible cardiac tissue–electronics hybrid enabling multiple drug release, sensing, and stimulation. Small. 2019;15:e1805526. doi: 10.1002/smll.201805526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Feiner R., Dvir T. Cardiac tissue engineering: from matrix design to the engineering of bionic hearts. Regen.Med. 2017;12:275–284. doi: 10.2217/rme-2016-0150. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Shapira A., Feiner R., Dvir T. Modular assembly of thick multifunctional cardiac patches. Proc. Natl. Acad. Sci. U S A. 2017;114:1898–1903. doi: 10.1073/pnas.1615728114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz C., Stewart K.M., Weaver V.M. The extracellular matrix at a glance. J. Cell Sci. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T.M., Hong G., Zhou T., Schuhmann T.G., Viveros R.D., Lieber C.M. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods. 2016;13:875–882. doi: 10.1038/nmeth.3969. [DOI] [PubMed] [Google Scholar]
- Fu H., Nan K., Bai W., Huang W., Bai K., Lu L., Zhou C., Liu Y., Liu F., Wang J. Morphable 3D mesostructures and microelectronic devices by multistable buckling mechanics. Nat. Mater. 2018;17:268–276. doi: 10.1038/s41563-017-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig R., Fuchsberger K., Schroeppel B., Link G.S., Heusel G., Kraushaar U., Schuhmann W., Stett A., Stelzle M. PEDOT–CNT composite microelectrodes for recording and electrostimulation applications: fabrication, morphology, and electrical properties. Front. Neuroeng. 2012;5:8. doi: 10.3389/fneng.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez M., Axisa F., Bulcke M.V., Brosteaux D., Vandevelde B., Vanfleteren J. 2007 International Conference on Thermal, Mechanical and Multi-Physics Simulation Experiments in Microelectronics and Micro-Systems. EuroSime 2007. IEEE; 2007. Design of metal interconnects for stretchable electronic circuits using finite element analysis. pp. 1–6. [Google Scholar]
- Gonzalez M., Axisa F., Bossuyt F., Hsu Y.Y., Vandevelde B., Vanfleteren J. Design and performance of metal conductors for stretchable electronic circuits. Circ. World. 2009;35:22–29. [Google Scholar]
- Grigoryan B., Paulsen S.J., Corbett D.C., Sazer D.W., Fortin C.L., Zaita A.J., Greenfield P.T., Calafat N.J., Gounley J.P., Ta A.H. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai A., Shappir J., Spira M.E. In-cell recordings by extracellular microelectrodes. Nat. Methods. 2010;7:200–202. doi: 10.1038/nmeth.1420. [DOI] [PubMed] [Google Scholar]
- Han S., Kim J., Won S.M., Ma Y., Kang D., Xie Z., Lee K.T., Chung H.U., Banks A., Min S. Battery-free, wireless sensors for full-body pressure and temperature mapping. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aan4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T.S., West K., Hassager O., Larsen N.B. Highly stretchable and conductive polymer material made from poly (3, 4-ethylenedioxythiophene) and polyurethane elastomers. Adv. Funct. Mater. 2007;17:3069–3073. [Google Scholar]
- Harvey J.D., Jena P.V., Baker H.A., Zerze G.H., Williams R.M., Galassi T.V., Roxbury D., Mittal J., Heller D.A. A carbon nanotube reporter of microRNA hybridization events in vivo. Nat. Biomed. Eng. 2017;1:0041. doi: 10.1038/s41551-017-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller, D.A. et al. (n.d.)inMeeting Abstracts.1935 (The Electrochemical Society).
- Hong G., Fu T.M., Zhou T., Schuhmann T.G., Huang J., Lieber C.M. Syringe injectable electronics: precise targeted delivery with quantitative input/output connectivity. Nano Lett. 2015;15:6979–6984. doi: 10.1021/acs.nanolett.5b02987. [DOI] [PubMed] [Google Scholar]
- Huang X., Liu Y., Chen K., Shin W.J., Lu C.J., Kong G.W., Patnaik D., Lee S.H., Cortes J.F., Rogers J.A. Stretchable, wireless sensors and functional substrates for epidermal characterization of sweat. Small. 2014;10:3083–3090. doi: 10.1002/smll.201400483. [DOI] [PubMed] [Google Scholar]
- Hwang S.W., Tao H., Kim D.H., Cheng H., Song J.K., Rill E., Brenckle M.A., Panilaitis B., Won S.M., Kim Y.S. A physically transient form of silicon electronics. Science. 2012;337:1640–1644. doi: 10.1126/science.1226325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.W., Lee C.H., Cheng H., Jeong J.W., Kang S.K., Kim J.H., Shin J., Yang J., Liu Z., Ameer G.A. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett. 2015;15:2801–2808. doi: 10.1021/nl503997m. [DOI] [PubMed] [Google Scholar]
- Jones J., Lacour S.P., Wagner S., Suo Z. Stretchable wavy metal interconnects. J. Vac. Sci. Technol.A Vac. Surf. Films. 2004;22:1723–1725. [Google Scholar]
- Katz A.M. Lippincott Williams & Wilkins; 2010. Physiology of the Heart. [Google Scholar]
- Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11:1775. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- Kil E.H., Choi K.H., Ha H.J., Xu S., Rogers J.A., Kim M.R., Lee Y.G., Kim K.M., Cho K.Y., Lee S.Y. Imprintable, bendable, and shape-conformable polymer electrolytes for versatile-shaped lithium-ion batteries. Adv. Mater. 2013;25:1395–1400. doi: 10.1002/adma.201204182. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Lu N., Ma R., Kim Y.S., Kim R.H., Wang S., Wu J., Won S.M., Tao H., Islam A. Epidermal electronics. Science. 2011;333:838–843. doi: 10.1126/science.1206157. [DOI] [PubMed] [Google Scholar]
- Kim D.H., Lu N., Ghaffari R., Kim Y.S., Lee S.P., Xu L., Wu J., Kim R.H., Song J., Liu Z. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 2011;10:316–323. doi: 10.1038/nmat2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.H., Ghaffari R., Lu N., Wang S., Lee S.P., Keum H., D'Angelo R., Klinker L., Su Y., Lu C. Electronic sensor and actuator webs for large-area complex geometry cardiac mapping and therapy. Proc. Natl. Acad. Sci. U S A. 2012;109:19910–19915. doi: 10.1073/pnas.1205923109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.I., McCall J.G., Jung Y.H., Huang X., Siuda E.R., Li Y., Song J., Song Y.M., Pao H.A., Kim R.H. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Salvatore G.A., Araki H., Chiarelli A.M., Xie Z., Banks A., Sheng X., Liu Y., Lee J.W., Jang K.I. Battery-free, stretchable optoelectronic systems for wireless optical characterization of the skin. Sci. Adv. 2016;2:e1600418. doi: 10.1126/sciadv.1600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J., MacEwan M.R., Kang S.K., Won S.M., Stephen M., Gamble P., Xie Z., Yan Y., Chen Y.Y., Shin J. Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy. Nat. Med. 2018;24:1830. doi: 10.1038/s41591-018-0196-2. [DOI] [PubMed] [Google Scholar]
- Lacour S.P., Wagner S., Huang Z., Suo Z. Stretchable gold conductors on elastomeric substrates. Appl. Phys. Lett. 2003;82:2404–2406. [Google Scholar]
- Lacour S.P., Jones J., Suo Z., Wagner S. Design and performance of thin metal film interconnects for skin-like electronic circuits. IEEE Electron. Device Lett. 2004;25:179–181. [Google Scholar]
- Langer R., Vacanti J.P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Lee S., Sasaki D., Kim D., Mori M., Yokota T., Lee H., Park S., Fukuda K., Sekino M., Matsuura K. Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nat. Nanotechnol. 2019;14:156–160. doi: 10.1038/s41565-018-0331-8. [DOI] [PubMed] [Google Scholar]
- Liu B., Lee B.W., Nakanishi K., Villasante A., Williamson R., Metz J., Kim J., Kanai M., Bi L., Brown K. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2018;2:293. doi: 10.1038/s41551-018-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu J., Chen S., Lei T., Kim Y., Niu S., Wang H., Wang X., Foudeh A.M., Tok J.B., Bao Z. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 2019;3:58–68. doi: 10.1038/s41551-018-0335-6. [DOI] [PubMed] [Google Scholar]
- Lu Y., Li T., Zhao X., Li M., Cao Y., Yang H., Duan Y.Y. Electrodeposited polypyrrole/carbon nanotubes composite films electrodes for neural interfaces. Biomaterials. 2010;31:5169–5181. doi: 10.1016/j.biomaterials.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Luo M., Martinez A.W., Song C., Herrault F., Allen M.G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromech. Syst. 2014;23:4–13. [Google Scholar]
- McCoul D., Hu W., Gao M., Mehta V., Pei Q. Recent advances in stretchable and transparent electronic materials. Adv. Electron. Mater. 2016;2:1500407. [Google Scholar]
- Montgomery M., Ahadian S., Davenport Huyer L., Lo Rito M., Civitarese R.A., Vanderlaan R.D., Wu J., Reis L.A., Momen A., Akbari S. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017;16:1038–1046. doi: 10.1038/nmat4956. [DOI] [PubMed] [Google Scholar]
- Noor N., Shapira A., Edri R., Gal I., Wertheim L., Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019;6:1900344. doi: 10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton J.J., Lee D.S., Lee J.W., Lee W., Kwon O., Won P., Jung S.Y., Cheng H., Jeong J.W., Akce A. Soft, curved electrode systems capable of integration on the auricle as a persistent brain-computer interface. Proc. Natl. Acad. Sci. U S A. 2015;112:3920–3925. doi: 10.1073/pnas.1424875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka H., Shimono K., Ogawa R., Sugihara H., Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J. Neurosci. Methods. 1999;93:61–67. doi: 10.1016/s0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Palacios-Prado N., Hoge G., Marandykina A., Rimkute L., Chapuis S., Paulauskas N., Skeberdis V.A., O'Brien J., Pereda A.E., Bennett M.V., Bukauskas F.F. Intracellular magnesium-dependent modulation of gap junction channels formed by neuronal connexin36. J. Neurosci. 2013;33:4741–4753. doi: 10.1523/JNEUROSCI.2825-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.W., Kang S.K., Hernandez H.L., Kaitz J.A., Wie D.S., Shin J., Lee O.P., Sottos N.R., Moore J.S., Rogers J.A., White S.R. Thermally triggered degradation of transient electronic devices. Adv. Mater. 2015;27:3783–3788. doi: 10.1002/adma.201501180. [DOI] [PubMed] [Google Scholar]
- Park S.I., Brenner D.S., Shin G., Morgan C.D., Copits B.A., Chung H.U., Pullen M.Y., Noh K.N., Davidson S., Oh S.J. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat. Biotechnol. 2015;33:1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Choi S., Janardhan A.H., Lee S.Y., Raut S., Soares J., Shin K., Yang S., Lee C., Kang K.W. Electromechanical cardioplasty using a wrapped elasto-conductive epicardial mesh. Sci. Transl. Med. 2016;8:344ra386. doi: 10.1126/scitranslmed.aad8568. [DOI] [PubMed] [Google Scholar]
- Patolsky F., Zheng G., Lieber C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006;1:1711–1724. doi: 10.1038/nprot.2006.227. [DOI] [PubMed] [Google Scholar]
- Phillips O., Schwartz J.M., Engler A., Gourdin G., Kohl P.A. IEEE; 2017. IEEE 67th Electronic Components and Technology Conference (ECTC) pp. 772–779. [Google Scholar]
- Prévot P.H., Gehere K., Arcizet F., Akolkar H., Khoei M.A., Blaize K., Oubari O., Daye P., Lanoë M., Valet M. Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates. Nat. Biomed. Eng. 2019 doi: 10.1038/s41551-019-0484-2. [DOI] [PubMed] [Google Scholar]
- Qu J., Ouyang L., Kuo C.-C., Martin D.C. Stiffness, strength and adhesion characterization of electrochemically deposited conjugated polymer films. Acta Biomater. 2016;31:114–121. doi: 10.1016/j.actbio.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M., Park H., Shing H., Consi T., Schoen F.J., Langer R., Freed L.E., Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl. Acad. Sci. U S A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeldt F., Engler A.J., Eckhardt A., Ahmed F., Discher D.E. Cell responses to the mechanochemical microenvironment—implications for regenerative medicine and drug delivery. Adv. Drug Deliv. Rev. 2007;59:1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K., Ma S.P., Yeager K., Chen T., Song L., Sirabella D., Morikawa K., Teles D., Yazawa M., Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A., Ahn B.Y., Adams J.J., Duoss E.B., Bernhard J.T., Lewis J.A. Pen-on-paper flexible electronics. Adv. Mater. 2011;23:3426–3430. doi: 10.1002/adma.201101328. [DOI] [PubMed] [Google Scholar]
- Sanganalmath S.K., Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhmann T.G., Yao J., Hong G., Fu T.-M., Lieber C.M. Syringe-injectable electronics with a plug-and-play input/output interface. Nano Lett. 2017;17:5836–5842. doi: 10.1021/acs.nanolett.7b03081. [DOI] [PubMed] [Google Scholar]
- Shmoel N., Rabieh N., Ojovan S.M., Erez H., Maydan E., Spira M.E. Multisite electrophysiological recordings by self-assembled loose-patch-like junctions between cultured hippocampal neurons and mushroom-shaped microelectrodes. Sci. Rep. 2016;6:27110. doi: 10.1038/srep27110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y.M., Xie Y., Malyarchuk V., Xiao J., Jung I., Choi K.J., Liu Z., Park H., Lu C., Kim R.H. Digital cameras with designs inspired by the arthropod eye. Nature. 2013;497:95–99. doi: 10.1038/nature12083. [DOI] [PubMed] [Google Scholar]
- Teixeira F.G., Carvalho M.M., Sousa N., Salgado A.J. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol. Life Sci. 2013;70:3871–3882. doi: 10.1007/s00018-013-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Cohen-Karni T., Qing Q., Duan X., Xie P., Lieber C.M. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science. 2010;329:830–834. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B., Liu J., Dvir T., Jin L., Tsui J.H., Qing Q., Suo Z., Langer R., Kohane D.S., Lieber C.M. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine A.D., Busbee T.A., Boley J.W., Raney J.R., Chortos A., Kotikian A., Berrigan J.D., Durstock M.F., Lewis J.A. Hybrid 3D printing of soft electronics. Adv. Mater. 2017;29:1703817. doi: 10.1002/adma.201703817. [DOI] [PubMed] [Google Scholar]
- Valentová H., Stejskal J. Mechanical properties of polyaniline. Synth.Met. 2010;160:832–834. [Google Scholar]
- Venkatraman S., Hendricks J., King Z.A., Sereno A.J., Richardson-Burns S., Martin D., Carmena J.M. In vitro and in vivo evaluation of PEDOT microelectrodes for neural stimulation and recording. IEEE Trans. Neural Syst. Rehabil.Eng. 2011;19:307–316. doi: 10.1109/TNSRE.2011.2109399. [DOI] [PubMed] [Google Scholar]
- Vunjak-Novakovic G., Tandon N., Godier A., Maidhof R., Marsano A., Martens T.P., Radisic M. Challenges in cardiac tissue engineering. Tissue Eng. B Rev. 2009;16:169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Zheng W., Yue Z., Too C.O., Wallace G.G. Buckled, stretchable polypyrrole electrodes for battery applications. Adv. Mater. 2011;23:3580–3584. doi: 10.1002/adma.201101067. [DOI] [PubMed] [Google Scholar]
- Wang S., Xu J., Wang W., Wang G.N., Rastak R., Molina-Lopez F., Chung J.W., Niu S., Feig V.R., Lopez J. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature. 2018;555:83–88. doi: 10.1038/nature25494. [DOI] [PubMed] [Google Scholar]
- Wang X., Feiner R., Luan H., Zhang Q., Zhao S., Zhang Y., Sun R., Wang H., Guo X., Oved H. Three-dimensional electronic scaffolds for monitoring and regulation of multifunctional hybrid tissues. Extreme Mech. Lett. 2020 [Google Scholar]
- Webb R.C., Bonifas A.P., Behnaz A., Zhang Y., Yu K.J., Cheng H., Shi M., Bian Z., Liu Z., Kim Y.S. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013;12:938–944. doi: 10.1038/nmat3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C., Liu J., Fu T.M., Dai X., Zhou W., Lieber C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015;14:1286–1292. doi: 10.1038/nmat4427. [DOI] [PubMed] [Google Scholar]
- Xu S., Zhang Y., Cho J., Lee J., Huang X., Jia L., Fan J.A., Su Y., Su J., Zhang H. Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems. Nat. Commun. 2013;4:1543. doi: 10.1038/ncomms2553. [DOI] [PubMed] [Google Scholar]
- Xu L., Gutbrod S.R., Bonifa A.P., Su Y., Sulkin M.S., Lu N., Chung H.J., Jang K.I., Liu Z., Ying M. 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat. Commun. 2014;5:3329. doi: 10.1038/ncomms4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Yan Z., Jang K.I., Huang W., Fu H., Kim J., Wei Z., Flavin M., McCracken J., Wang R. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science. 2015;347:154–159. doi: 10.1126/science.1260960. [DOI] [PubMed] [Google Scholar]
- Xu J., Wang S., Wang G.N., Zhu C., Luo S., Jin L., Gu X., Chen S., Feig V.R., To J.W. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science. 2017;355:59–64. doi: 10.1126/science.aah4496. [DOI] [PubMed] [Google Scholar]
- Yan Z., Zhang F., Wang J., Liu F., Guo X., Nan K., Lin Q., Gao M., Xiao D., Shi Y. Controlled mechanical buckling for origami-inspired construction of 3D microstructures in advanced materials. Adv. Funct. Mater. 2016;26:2629–2639. doi: 10.1002/adfm.201504901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Han M., Shi Y., Badea A., Yang Y., Kulkarni A., Hanson E., Kandel M.E., Wen X., Zhang F. Three-dimensional mesostructures as high-temperature growth templates, electronic cellular scaffolds, and self-propelled microrobots. Proc. Natl. Acad. Sci. U S A. 2017;114:E9455–E9464. doi: 10.1073/pnas.1713805114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Zhou T., Zwang T.J., Hong G., Zhao Y., Viveros R.D., Fu T.M., Gao T., Lieber C.M. Bioinspired neuron-like electronics. Nat. Mater. 2019;18:510–517. doi: 10.1038/s41563-019-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Huang X., Xu H., Zhang Y., Lam J., Cheng J., Rogers J.A. Materials, designs, and operational characteristics for fully biodegradable primary batteries. Adv. Mater. 2014;26:3879–3884. doi: 10.1002/adma.201306304. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Inayat S., Dikin D.A., Singer J.H., Ruoff R.S., Troy J.B. Patch clamp technique: review of the current state of the art and potential contributions from nanoengineering. Proc. Inst. Mech. Eng. N J. Nanomater.Nanoeng.Nanosyst. 2008;222:1–11. [Google Scholar]
- Zhao Y., You S.S., Zhang A., Lee J.H., Huang J., Lieber C.M. Scalable ultrasmall three-dimensional nanowire transistor probes for intracellular recording. Nat. Nanotechnol. 2019;14:783–790. doi: 10.1038/s41565-019-0478-y. [DOI] [PubMed] [Google Scholar]
- Zheng G., Patolsky F., Cui Y., Wang W.U., Lieber C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005;23:1294. doi: 10.1038/nbt1138. [DOI] [PubMed] [Google Scholar]
- Zhou T., Hong G., Fu T.M., Yang X., Schuhmann T.G., Viveros R.D., Lieber C.M. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. U S A. 2017;114:5894–5899. doi: 10.1073/pnas.1705509114. [DOI] [PMC free article] [PubMed] [Google Scholar]