Abstract
The diagnosis of osteoarthritis (OA) currently depends on the presence of pain and radiographic imaging findings, which generally do not present until later stages of the disease when the condition is difficult to treat. Therefore, earlier detection of OA pathology is needed for improved disease management. Ex vivo cartilage studies indicate that changes in the mechanical function of cartilage occur as degeneration progresses during OA. Thus, measurement of the in vivo cartilage mechanical response may serve as an earlier indicator of OA pathology. Though mechanical characterization is classically performed during loading, the unloading (recovery) response of cartilage may also enable determination of mechanical response. Therefore, the purpose of this study was to validate the use of the recovery response for mechanical characterization of cartilage in a controlled, ex vivo environment. To do so, confined compression creep and recovery tests were conducted on cartilage explants (N = 10), and the resulting mechanical properties from both the creep and recovery phases were compared. No statistically significant differences were found in the mechanical properties between the two phases, reinforcing the hypothesis that unloading (recovery) may be a good surrogate for loading.
Subject terms: Cartilage, Osteoarthritis
Introduction
Osteoarthritis (OA) is a degenerative disease of articular cartilage and affects over 27 million Americans1. OA is currently diagnosed via the presence of pain and radiographic features, such as osteophytes and joint space narrowing, from which cartilage loss is inferred2–4. Unfortunately, radiographic imaging techniques rely upon gross morphological changes to be present in the tissue, which may not occur until late in the disease2. Further, gross morphological changes visible on radiography do not always correlate with pain or functional impairment3. As an alternative, magnetic resonance imaging (MRI) has been used to assess OA5; however, radiography remains the traditional modality for clinical OA assessment and diagnosis2,3. Currently, treatment for end-stage OA is limited and includes pain management or joint replacement surgery to restore function and reduce pain3. While these treatment strategies ameliorate symptoms associated with the disease, they do not directly treat or reverse cartilage degeneration. Therefore, earlier detection of OA degeneration is needed for more effective disease management and treatment.
Prior to gross morphological changes, other changes occur in cartilage tissue during the progression of OA6. These include compositional changes, such as the loss of proteoglycan content7–9, the loss of collagen content and organization9,10, and changes in tissue hydration6,8. These changes alter the tissue’s response to mechanical load and thus its mechanical function8,9,11–14. Altered mechanical function may also contribute to further pathology and degeneration within the tissue11. As such, mechanical response may represent a biomarker of OA pathology. Because these compositional and mechanical changes potentially occur prior to the onset of pain or gross imaging findings, detection of these mechanical changes may lead to an earlier diagnosis of OA.
Cartilage mechanical assessment is classically performed in the ex vivo environment by excising explants of cartilage tissue, or by exposing the cartilage surface and indenting upon it15–18. As such, in vivo application of these techniques is limited, especially as diagnostic or prognostic tools. On the other hand, previous work has used MR imaging to measure in vivo cartilage response by quantifying changes in cartilage volume and thickness19–35. In these studies, an exercise activity is used to mechanically load the cartilage tissue, and MR images from before and after the activity are compared to quantify the resulting deformation. This technique is possible due to cartilage’s viscoelastic nature36,37. During loading, cartilage exhibits time-dependent behavior, which is primarily due to water flow out of the tissue37–39 but is also due in part to the intrinsic viscoelasticity of the extracellular matrix40–44. Because of this time-dependent behavior, MR images can be used to measure load-induced deformation, such as changes in cartilage thickness or volume19,20.
Furthermore, due to the viscoelastic nature of cartilage tissue, mechanical characterization of cartilage requires knowledge of the strain-time history. Therefore, cartilage’s mechanical response may be quantified in vivo using this MRI methodology by assessing deformation repeatedly after different intensities or doses of exercise. For example, prior work by our group45 varied the duration and intensity of walking to quantify how cartilage strain changed over time after walking. This was analogous to a creep test, where the load was applied and held for a set duration, and the resulting strain was measured across time during that duration. However, this paradigm—repeated applications of pre/post MR imaging and exercise—led to an expensive and time-intensive study.
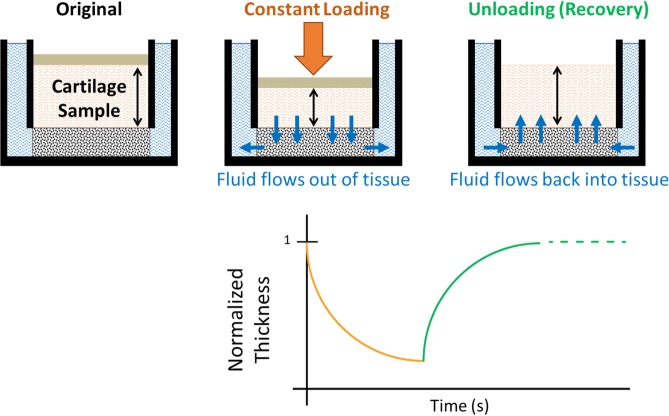
Alternatively, adapting this current in vivo MRI methodology45 to assess the reversal of cartilage strain during the recovery period after loading may lead to a more efficient study design, while still enabling assessment of the mechanical response (Fig. 1). As previously stated, water flows out of the tissue during loading and deformation accumulates in a time-dependent manner within the cartilage37–39. Conversely, upon the removal of load during the recovery period, water flows back into the tissue, causing the reversal of the deformation over time as the cartilage returns to its baseline state. If the unloading trajectory is similar to the loading trajectory, then measuring the strain history during recovery should allow for similar mechanical characterization as measuring the strain history during creep.
Figure 1.
Comparison of loading and unloading (recovery) response of cartilage.
Therefore, the purpose of this study was to measure and compare the mechanical response of healthy cartilage to loading (creep) and unloading (recovery). Specifically, the mechanical response was studied in a controlled ex vivo environment by conducting confined compression creep and recovery tests on porcine cartilage explants from the tibial plateau and femoral trochlea. Our goal was to compare the strain trajectories during loading and unloading to determine whether the recovery response may be used as a surrogate measure for the creep response when making mechanical assessments of healthy cartilage tissue in vivo. This represents a first step toward understanding whether measurements of recovery in OA cartilage may serve as earlier indicators of cartilage pathology.
Results
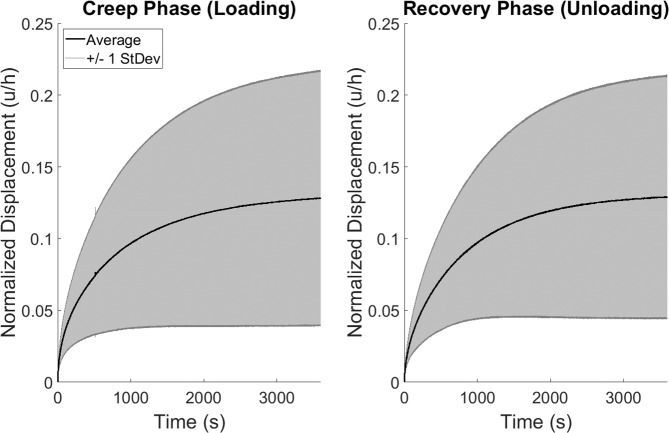
Overall, explants in this experiment experienced a mean strain of 12.8% ± 8.9% at creep equilibrium (end of the creep phase). Explants recovered to 99.0% ± 0.9% of their baseline thickness by the end of the recovery phase (baseline thickness: 0.75 ± 0.18 mm). Furthermore, no statistically significant differences were found in mechanical properties between the creep and recovery phases (Fig. 2). The mean aggregate moduli were 0.71 ± 0.50 and 0.68 ± 0.48 MPa (creep and recovery, respectively), and the mean characteristic times (Eq. 1c, a measure of how quickly equilibrium is reached) were 11.6 ± 5.2 and 12.6 ± 7.3 min, respectively. Cartilage location (tibial plateau vs femoral trochlea) had a statistically significant main effect on both of the mechanical properties (aggregate modulus and characteristic time) measured in this study.
Figure 2.
Creep and recovery deformation response corridors (mean ± 1 standard deviation) across all explants (N = 10). Mechanical properties (aggregate modulus and characteristic time) were not statistically significantly different between the creep and recovery phases.
Furthermore, statistically significant correlations were found between the aggregate moduli of each phase (Pearson r = 0.996), and between the characteristic times of each phase (Pearson r = 0.897) (Table 1). Additionally, the characteristic times of each phase were significantly correlated with the aggregate moduli of each phase, respectively (Table 1).
Table 1.
Pearson Correlation Coefficients Between Outcome Variables.
| Aggregate Modulus | Characteristic Time | ||||
|---|---|---|---|---|---|
| Creep | Recovery | Creep Time | Recovery | ||
| Aggregate Modulus | Creep | r = 1.000 |
r = 0.996, (p < 0.0001) |
r = −0.851, (p < 0.0018) |
r = −0.720, (p < 0.0188) |
| Recovery | r = 1.000 |
r = −0.843, (p < 0.0022) |
r = −0.718, (p < 0.0194) |
||
| Characteristic Time | Creep | r = 1.000 |
r = 0.897, (p < 0.0004) |
||
| Recovery | r = 1.000 | ||||
Lastly, the mean average residual between creep and recovery phases in this experiment represented an error of 5.4% ± 3.3% of the final (60 minute) creep strain, indicating a high degree of similarity between the creep and recovery responses in the confined compression environment. Indeed, a calculation of the pairwise differences between creep and recovery at each point in time (Fig. 3) illustrates that the mean pairwise difference remained low at all times.
Figure 3.
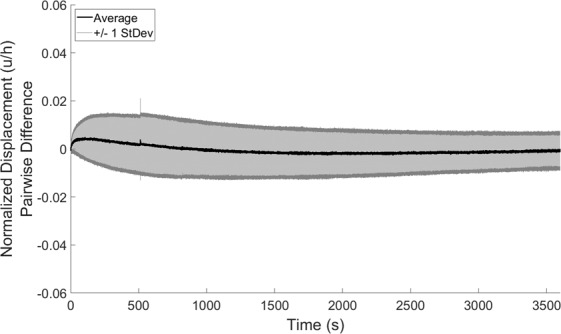
Pairwise differences in deformation response (creep minus recovery) at each point in time (mean ± 1 standard deviation) across all explants (N = 10).
Discussion
The mechanical response of cartilage changes as the tissue progresses from a healthy to a degenerated state, such as during OA pathology6–10,46. Therefore, measurement of the tissue’s mechanical response may serve as a useful biomarker of cartilage disease. Because traditional methods for the characterization of mechanical response are invasive when applied to cartilage tissue, they are not well suited for in vivo applications. Therefore, the development of a method to noninvasively assess the cartilage mechanical response in vivo is needed, which may be accomplished using MRI45. To this end, the current study evaluated the use of the recovery strain trajectory as a surrogate for the creep strain trajectory in healthy cartilage in a controlled, ex vivo environment, in order to validate the assessment of healthy cartilage recovery for quantifying in vivo mechanical properties.
Importantly, this study did not find statistically significant differences in either the aggregate modulus or the characteristic time between the creep and recovery phases. This finding motivates the use of the recovery phase as a surrogate for the creep phase, especially for measurement of bulk properties or response such as the total (engineering) strain within the tissue, as measured in the current study. This is useful as classically, mechanical properties are assessed during the loading response, but the unloading (or recovery) response is more accessible in vivo using MRI techniques. Therefore, understanding whether the recovery response reflects the loading response ex vivo is crucial for validating this in vivo approach. Moreover, the mean characteristic times measured in this study, 11.6 ± 5.2 min for creep and 12.6 ± 7.3 min for recovery, are on the same order as those measured in vivo. Specifically, in a prior study45, the mean in vivo creep trajectory of healthy human tibial cartilage had a characteristic time of 17.2 minutes. While future exploration of cartilage recovery in vivo is needed to determine whether the in vivo creep response may be represented by the in vivo recovery response, the findings of the current study support this idea.
Additionally, the current study found that the average residual, defined as the expected error between the creep and recovery response at any point in time, was low: it represented 5.4% ± 3.3% of the final (60 minute) creep strain. However, the average residual was not zero. Further, larger pairwise differences were seen at early time points (0–800 seconds) than at later time points (Fig. 3). These observations are consistent with prior literature, which also did not find the creep and recovery phases to be perfectly reversible39,47–50. These differences may be due in part to the extracellular matrix’s intrinsic viscoelasticity under compression41,44. However, it may be that these slight differences are due to strain-dependent permeability51, in which the tissue’s permeability decreases as the strain in the tissue increases. A decrease in permeability indicates more resistance to fluid flow, making it more difficult for fluid to enter or leave the cartilage matrix. Therefore, at early times in the creep response, there is little strain within the tissue and the permeability is near its maximum, making it easier for fluid to flow out of the tissue and strain to accumulate in response to the sustained load. Conversely, at early times in the recovery response, there is a large amount of strain in the tissue (the strain is near its maximum) and the permeability is lower, making it more difficult for fluid to flow back into the tissue and for strain to dissipate. This is supported by our observations of the pairwise differences (Fig. 3), which we defined as the creep response minus the recovery response at each point in time. The positive values at early times (0–800 seconds) indicate that the creep deformation was typically larger than the recovery deformation at these early times, which is consistent with a larger relative permeability at this point in creep than in recovery. In the current study, the permeability was assumed to be constant (Eq. 1, see Methods section). In the presence of strain-dependent permeability, a slower recovery is expected due to a large amount of tissue compaction and minimal permeability at early times, indicating that longer times are needed to reach equilibrium in the recovery phase. Indeed, this was also observed in the current study, as the characteristic recovery time was typically longer than the characteristic creep time (11.6 ± 5.2 min for creep and 12.6 ± 7.3 min for recovery), though these differences were not significant. Future studies investigating incorporation of a strain-dependent permeability term when modeling the creep and recovery deformation would be beneficial to test this hypothesis. Nonetheless, the observed pairwise differences are small, especially at later times throughout the deformation ( > 800 seconds), leading to a small average residual and nonsignificant differences in the creep and recovery characteristic times.
In this study, statistically significant differences in mechanical properties between cartilage from the tibial plateau and femoral trochlea were found, which is consistent with prior literature indicating that regional differences exist in mechanical properties (for example, between femoral and tibial cartilage)47,48,52–54. Porcine tibial and femoral cartilage was found to have aggregate moduli during creep of 1.17 ± 0.48 and 0.40 ± 0.18 MPa, respectively. Likewise, the creep characteristic times were 7.4 ± 3.2 and 14.4 ± 4.4 min, respectively. Further, these mechanical property values—both for the modulus and characteristic time—are consistent with literature values of ex vivo cartilage mechanical properties measured via confined compression9,14,54–56. Finally, upon reaching recovery equilibrium (end of the recovery phase), explants recovered to within 99.0% ± 0.9% of their baseline thickness, confirming previous reports of thickness recovery upon load removal in ex vivo creep experiments39,47–49. Future work is needed to expand on these results by testing degenerated or OA cartilage, to understand whether pathology affects the loading response in the same manner as it affects the unloading response.
Overall, measuring the recovery response may be a useful surrogate for the creep response, especially in the in vivo context of measuring cartilage mechanical function where the recovery phase is more accessible via MRI than the loading phase. This study investigated recovery of healthy cartilage in the controlled ex vivo environment of confined compression, and did not find statistically significant differences in cartilage mechanical properties (aggregate modulus and characteristic time) between the creep and recovery phases. These results help validate the use of the recovery response to measure in vivo mechanical properties, with the eventual goal of leveraging mechanical changes as prognostic or diagnostic indicators of cartilage degeneration. The findings of this study motivate the investigation of creep and recovery in degenerated cartilage to further validate whether the recovery response is indicative of the creep response in OA, and whether mechanical changes may represent earlier indicators of cartilage degeneration than pain or radiographic findings.
Materials and Methods
Mechanical testing
Ex vivo confined compression creep and recovery experiments were carried out using full-thickness cartilage explants (N = 10) from porcine femurs and tibiae. Skeletally mature porcine knee joints were obtained intact from already deceased animals from a local abattoir, so Institutional Animal Care and Use Committee (IACUC) approval was not required. Joints were dissected to expose the articular cartilage surfaces of the femur and tibia, and 5 mm diameter cartilage explants were harvested from the medial and lateral tibial plateaus and medial and lateral femoral trochlea of the joints. Explants were harvested from visually healthy regions of cartilage, identified as areas with a Collins grade of 057. After harvest, explants were promptly wrapped in PBS-soaked gauze and stored at −20 °C until testing.
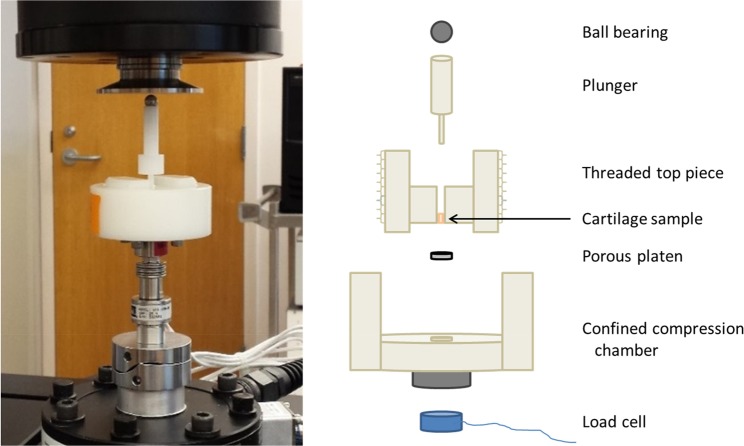
Explants were thawed at room temperature for 30 minutes prior to mechanical testing (as a result, explants experienced only one freeze-thaw cycle in this experiment). After thawing, 3 mm diameter cylindrical plugs were cut from the original 5 mm diameter explants and loaded into a confined compression chamber filled with PBS for mechanical testing (Fig. 4). The diameter of the cylindrical chamber within which the explants were loaded was 3 mm, resulting in a very tight fit between the explant and the lateral walls of the confined compression chamber. Further, a stainless steel porous platen (McMaster-Carr, Douglasville, GA) with porosity = 0.95 comprised the bottom of the confined compression chamber, allowing fluid to flow out of and into the tissue during creep and recovery, respectively.
Figure 4.
Confined compression fixturing.
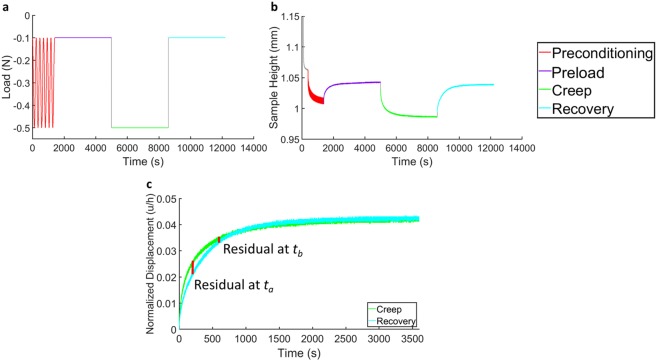
Creep and recovery tests were performed in load control on an MTS Acumen 3 materials test system (MTS Systems Corporation, Eden Prairie, MN). The measured force and displacement data were recorded at a sampling rate of 50 Hz. The test battery included four steps: 1) cyclic preconditioning (peak-to-peak sinusoid of −0.1 N to −0.5 N at 0.10 Hz, 100 cycles), to ensure explants reached a repeatable steady-state level of hydration throughout the depth of the tissue58; 2) a preload (−0.1 N, 60 min), to allow explants to equilibrate to a baseline thickness under a small compressive load39,47; 3) a creep load (−0.5 N, 60 min), to measure the strain response during creep; 4) a recovery load (−0.1 N, 60 min), to measure the strain response during recovery (Fig. 5a). Notably, the preload and recovery levels (−0.1 N) were the same to ensure that the change in force occurring between steps 2 and 3 was equal in magnitude and opposite in direction to that occurring between steps 3 and 4. The preload, creep, and recovery loads were each applied at a rate of 0.05 N/s. Further, the magnitude of the change in force (0.4 N) during creep and recovery was chosen to result in less than 20% strain in the tissue at equilibrium37,39,49. Similarly, the preconditioning cycled between the same creep and recovery loads (−0.1 N and −0.5 N), and consisted of a 0.10 Hz sinusoid for 100 cycles as this rate and number have been shown to be sufficient for the tissue to reach a dynamic equilibrium in which no further ratcheting strain occurs per cycle58. Finally, the preload, creep load, and recovery load were each held for 60 minutes to allow the explants to reach equilibrium in each phase. When the recovery load was initiated, there was no evidence that the indenter pulled away from the explant. Specifically, negative force readings (indicating compression) were maintained throughout the testing.
Figure 5.
Confined compression test battery. (a) Applied load protocol. (b) Measured data from a representative explant. (c) Illustration of residuals from a representative explant for assessment of similarity of creep and recovery curves via calculation of the average residual across time.
Data analysis
After testing, the data trace from each explant was checked to ensure that explants reached equilibrium. Equilibrium was defined as a change in explant height less than 0.6 microns over the final 60 seconds of creep, corresponding to a change in explant height less than 0.010 microns per second at the end of the creep phase9,49. All explants included in the analysis met this equilibrium criterion.
Next, the biphasic creep solution (Eq. 1)37 was fit to the measured creep and recovery deformation responses (Fig. 5b,c) separately to calculate the tissue’s characteristic time (τ0) and aggregate modulus (HA) during both the creep and recovery phases. The characteristic time (Eq. 1c) represents the time constant of the exponential term of Eq. 1a when n = 0, corresponding to the first term in the summation55. The fit procedure was performed in MATLAB (version R2018a, Mathworks, Natick, MA) using the nonlinear least-squares curve-fitting algorithm lsqcurvefit. Baseline thickness was defined as the mean explant thickness over the final five minutes of the preload phase (step 2 of the test battery, described above). Similarity between the creep and recovery response was assessed via the average residual, defined as the mean absolute error between the creep and recovery strain curves across time for a given explant, expressed as a percent of the final (60 minute) creep strain (Fig. 5c). Therefore, the average residual represents the expected error at any point in time between the creep and recovery curves for a given explant. Likewise, the mean average residual across multiple explants represents the mean expected error between the creep and recovery phases. Statistical analyses were performed in SAS (version 9.4, SAS Institute, Cary, NC) with p < 0.05 indicating significance. Outcome variables assessed were the aggregate modulus and characteristic time from each phase (creep and recovery). One-way repeated-measures analyses of variance (ANOVA) were performed to test for differences in outcome variables between the creep and recovery phases. Cartilage location (tibial plateau vs femoral trochlea) was included as a factor to examine differences in mechanical properties across location. Further, Pearson correlations were calculated between outcome variables. Data are summarized using the mean ± one standard deviation unless otherwise indicated.
| 1a |
| 1b |
| 1c |
where u = surface displacement, h = baseline thickness, σ0 = applied stress,
HA = aggregate modulus, τ = time constant, τ0 = characteristic time, and
k = permeability.
Acknowledgements
The authors would like to gratefully acknowledge Lia Meirose for her helpful discussions concerning this study. This study was supported by the NIH grants AR065527, AR074800, and AR075399.
Author contributions
H.C. contributed to the study design, data collection, data analysis, data interpretation, and writing of this manuscript. L.D. contributed to the study design, data analysis, data interpretation, and writing of this manuscript.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis & Rheumatism. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Litwic, A., Edwards, M. H., Dennison, E. M. & Cooper, C. Epidemiology and burden of osteoarthritis. British medical bulletin, 105(1), 185–199 (2013). [DOI] [PMC free article] [PubMed]
- 3.Glyn-Jones S, et al. Osteoarthritis. The Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 4.Kellgren J, Lawrence J. Radiological assessment of osteo-arthrosis. Annals of the Rheumatic Diseases. 1957;16:494. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peterfy C, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis and Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz H, Richter W. Osteoarthritis: cellular and molecular changes in degenerating cartilage. Progress in Histochemistry and Cytochemistry. 2006;40:135–163. doi: 10.1016/j.proghi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Thompson R, Oegema T. Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979;61:407–416. doi: 10.2106/00004623-197961030-00017. [DOI] [PubMed] [Google Scholar]
- 8.Rivers P, et al. Osteoarthritic changes in the biochemical composition of thumb carpometacarpal joint cartilage and correlation with biomechanical properties. Journal of Hand Surgery. 2000;25:889–898. doi: 10.1053/jhsu.2000.16358. [DOI] [PubMed] [Google Scholar]
- 9.Hatcher CC, et al. Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. Journal of Biomechanics. 2017;55:18–26. doi: 10.1016/j.jbiomech.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollander A, et al. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. Journal of Clinical Investigation. 1995;96:2859. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilak F. Biomechanical factors in osteoarthritis. Best Practice & Research Clinical Rheumatology. 2011;25:815–823. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grenier S, Bhargava MM, Torzilli PA. An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. Journal of Biomechanics. 2014;47:645–652. doi: 10.1016/j.jbiomech.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knecht S, Vanwanseele B, Stüssi E. A review on the mechanical quality of articular cartilage–implications for the diagnosis of osteoarthritis. Clinical biomechanics. 2006;21:999–1012. doi: 10.1016/j.clinbiomech.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Collins, A. T. et al. Selective enzymatic digestion of proteoglycans and collagens alters cartilage T1rho and T2 relaxation times. Annals of biomedical engineering, 1–12 (2018). [DOI] [PMC free article] [PubMed]
- 15.Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annual Review of Biomedical Engineering. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 16.Mansour, J. M. Biomechanics of cartilage. Kinesiology: the mechanics and pathomechanics of human movement, 66–79 (2003).
- 17.Mow, V. C. & Huiskes, R. Basic Orthopaedic Biomechanics & Mechano-biology. (Lippincott Williams & Wilkins, 2005).
- 18.Guo, X. E., Lu, H. H., Likhitpanichkul, M. & Mow, V. C. In Frontiers in Biomedical Engineering 37–60 (Springer, 2003).
- 19.Eckstein F, et al. Effect of physical exercise on cartilage volume and thickness in vivo: MR imaging study. Radiology. 1998;207:243–248. doi: 10.1148/radiology.207.1.9530322. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Tieschky M, Faber S, Englmeier K-H, Reiser M. Functional analysis of articular cartilage deformation, recovery, and fluid flow following dynamic exercise in vivo. Anatomy and Embryology. 1999;200:419–424. doi: 10.1007/s004290050291. [DOI] [PubMed] [Google Scholar]
- 21.Hudelmaier M, et al. Age-related changes in the morphology and deformational behavior of knee joint cartilage. Arthritis & Rheumatology. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::AID-ART436>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Eckstein F, et al. In vivo cartilage deformation after different types of activity and its dependence on physical training status. Annals of the Rheumatic Diseases. 2005;64:291–295. doi: 10.1136/ard.2004.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Ginckel A, Roosen P, Almqvist K, Verstraete K, Witvrouw E. Effects of in vivo exercise on ankle cartilage deformation and recovery in healthy volunteers: an experimental study. Osteoarthritis and Cartilage. 2011;19:1123–1131. doi: 10.1016/j.joca.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Volume changes in the menisci and articular cartilage of runners. The American Journal of Sports Medicine. 2006;34:832–836. doi: 10.1177/0363546505282622. [DOI] [PubMed] [Google Scholar]
- 25.Kessler MA, Glaser C, Tittel S, Reiser M, Imhoff AB. Recovery of the menisci and articular cartilage of runners after cessation of exercise Additional aspects of in vivo investigation based on 3-dimensional magnetic resonance imaging. The American Journal of Sports Medicine. 2008;36:966–970. doi: 10.1177/0363546507313093. [DOI] [PubMed] [Google Scholar]
- 26.Boocock M, McNair P, Cicuttini F, Stuart A, Sinclair T. The short-term effects of running on the deformation of knee articular cartilage and its relationship to biomechanical loads at the knee. Osteoarthritis and Cartilage. 2009;17:883–890. doi: 10.1016/j.joca.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Mosher TJ, Liu Y, Torok CM. Functional cartilage MRI T2 mapping: evaluating the effect of age and training on knee cartilage response to running. Osteoarthritis and cartilage. 2010;18:358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Ginckel A, Verdonk P, Victor J, Witvrouw E. Cartilage status in relation to return to sports after anterior cruciate ligament reconstruction. The American Journal of Sports Medicine. 2013;41:550–559. doi: 10.1177/0363546512473568. [DOI] [PubMed] [Google Scholar]
- 29.Coleman JL, et al. Diurnal variations in articular cartilage thickness and strain in the human knee. Journal of Biomechanics. 2013;46:541–547. doi: 10.1016/j.jbiomech.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widmyer MR, et al. High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis & Rheumatism. 2013;65:2615–2622. doi: 10.1002/art.38062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutter EG, et al. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. The American Journal of Sports Medicine. 2015;43:370–376. doi: 10.1177/0363546514559821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lad NK, et al. Effect of normal gait on in vivo tibiofemoral cartilage strains. Journal of Biomechanics. 2016;49:2870–2876. doi: 10.1016/j.jbiomech.2016.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cher, W. L. et al. An analysis of changes in in vivo cartilage thickness of the healthy ankle following dynamic activity. Journal of Biomechanics (2016). [DOI] [PMC free article] [PubMed]
- 34.Collins AT, et al. Obesity alters the in vivo mechanical response and biochemical properties of cartilage as measured by MRI. Arthritis Research & Therapy. 2018;20:232. doi: 10.1186/s13075-018-1727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutter, E. G. et al. Effects of anterior cruciate ligament deficiency on tibiofemoral cartilage thickness and strains in response to hopping. The American Journal of Sports Medicine, 0363546518802225 (2019). [DOI] [PMC free article] [PubMed]
- 36.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mow VC, Kuei S, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. Journal of Biomechanical Engineering. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 38.Mak A, Lai W, Mow V. Biphasic indentation of articular cartilage—I. Theoretical analysis. Journal of Biomechanics. 1987;20:703–714. doi: 10.1016/0021-9290(87)90036-4. [DOI] [PubMed] [Google Scholar]
- 39.Mow VC, Gibbs M, Lai WM, Zhu W, Athanasiou KA. Biphasic indentation of articular cartilage—II. A numerical algorithm and an experimental study. Journal of Biomechanics. 1989;22:853–861. doi: 10.1016/0021-9290(89)90069-9. [DOI] [PubMed] [Google Scholar]
- 40.Mak A. The apparent viscoelastic behavior of articular cartilage—the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. Journal of biomechanical engineering. 1986;108:123–130. doi: 10.1115/1.3138591. [DOI] [PubMed] [Google Scholar]
- 41.Setton LA, Zhu W, Mow VC. The biphasic poroviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. Journal of Biomechanics. 1993;26:581–592. doi: 10.1016/0021-9290(93)90019-B. [DOI] [PubMed] [Google Scholar]
- 42.Suh J-K, Bai S. Finite element formulation of biphasic poroviscoelastic model for articular cartilage. Journal of biomechanical engineering. 1998;120:195–201. doi: 10.1115/1.2798302. [DOI] [PubMed] [Google Scholar]
- 43.DiSilvestro MR, Zhu Q, Wong M, Jurvelin JS, Suh J-KF. Biphasic poroviscoelastic simulation of the unconfined compression of articular cartilage: I—simultaneous prediction of reaction force and lateral displacement. Journal of biomechanical engineering. 2001;123:191–197. doi: 10.1115/1.1351890. [DOI] [PubMed] [Google Scholar]
- 44.DiSilvestro MR, Suh J-KF. A cross-validation of the biphasic poroviscoelastic model of articular cartilage in unconfined compression, indentation, and confined compression. Journal of biomechanics. 2001;34:519–525. doi: 10.1016/S0021-9290(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 45.Paranjape CS, et al. A New Stress Test for Knee Joint Cartilage. Scientific Reports. 2019;9:2283. doi: 10.1038/s41598-018-38104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Setton L, Mow V, Müller F, Pita J, Howell D. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. Journal of Orthopaedic Research. 1994;12:451–463. doi: 10.1002/jor.1100120402. [DOI] [PubMed] [Google Scholar]
- 47.Athanasiou K, Rosenwasser M, Buckwalter J, Malinin T, Mow V. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. Journal of Orthopaedic Research. 1991;9:330–340. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 48.Athanasiou K, Agarwal A, Dzida F. Comparative study of the intrinsic mechanical properties of the human acetabular and femoral head cartilage. Journal of Orthopaedic Research. 1994;12:340–349. doi: 10.1002/jor.1100120306. [DOI] [PubMed] [Google Scholar]
- 49.Ateshian G, Warden W, Kim J, Grelsamer R, Mow V. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. Journal of Biomechanics. 1997;30:1157–1164. doi: 10.1016/S0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- 50.Stolberg-Stolberg J, et al. Analysis of Cartilage Creep Recovery Using a Highly Dynamic Closed-loop Test System. Journal of Bionic Engineering. 2018;15:1057–1066. doi: 10.1007/s42235-018-0093-x. [DOI] [Google Scholar]
- 51.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. Journal of Biomechanics. 1984;17:377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 52.Athanasiou K, Niederauer G, Schenck R. Biomechanical topography of human ankle cartilage. Annals of biomedical engineering. 1995;23:697–704. doi: 10.1007/BF02584467. [DOI] [PubMed] [Google Scholar]
- 53.Froimson MI, Ratcliffe A, Gardner TR, Mow VC. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis and Cartilage. 1997;5:377–386. doi: 10.1016/S1063-4584(97)80042-8. [DOI] [PubMed] [Google Scholar]
- 54.Treppo S, et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. Journal of Orthopaedic Research. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 55.Armstrong C, Mow V. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88–94. doi: 10.2106/00004623-198264010-00013. [DOI] [PubMed] [Google Scholar]
- 56.Jurvelin J, Buschmann M, Hunziker E. Mechanical anisotropy of the human knee articular cartilage in compression. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2003;217:215–219. doi: 10.1243/095441103765212712. [DOI] [PubMed] [Google Scholar]
- 57.Collins D, McElligott T. Sulphate (35SO4) uptake by chondrocytes in relation to histological changes in osteo-arthritic human articular cartilage. Annals of the rheumatic diseases. 1960;19:318. doi: 10.1136/ard.19.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gao L-L, et al. Ratcheting behavior of articular cartilage under cyclic unconfined compression. Materials Science and Engineering: C. 2015;57:371–377. doi: 10.1016/j.msec.2015.07.061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.