Abstract
The discovery of modern medicine relies on the sustainable development of synthetic methodologies to meet the needs associated with drug molecular design. Heterocycles containing difluoromethyl groups are an emerging but scarcely investigated class of organofluoro molecules with potential applications in pharmaceutical, agricultural and material science. Herein, we developed an organophotocatalytic direct difluoromethylation of heterocycles using O2 as a green oxidant. The C–H oxidative difluoromethylation obviates the need for pre-functionalization of the substrates, metals and additives. The operationally straightforward method enriches the efficient synthesis of many difluoromethylated heterocycles in moderate to excellent yields. The direct difluoromethylation of pharmaceutical moleculars demonstrates the practicability of this methodology to late-stage drug development. Moreover, 2′-deoxy-5-difluoromethyluridine (F2TDR) exhibits promising activity against some cancer cell lines, indicating that the difluoromethylation methodology might provide assistance for drug discovery.
Subject terms: Photocatalysis, Synthetic chemistry methodology, Organic chemistry
Heterocycles containing difluoromethyl groups are molecules with potential application in pharmaceutical, agricultural and materials science. Here, the authors show an organophotocatalytic difluoromethylation of heterocycles using O2 as green oxidant and preliminarily study the products’ bioactivity.
Introduction
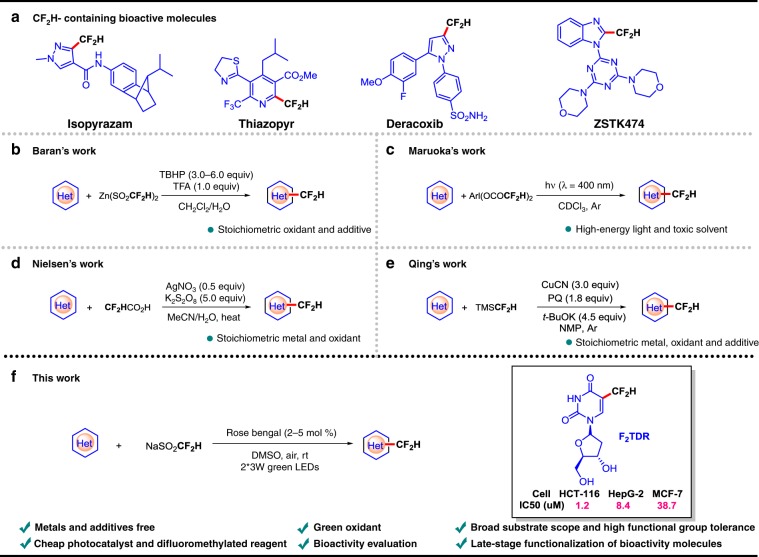
Organofluoro compounds are widely used in the fields of pharmaceutical, agricultural, and material science1–3. The introduction of fluorine atoms or fluorine-containing groups into the framework of organic molecules often changes the physicochemical properties or biological activities of compounds4–8, and has become an essential topic for chemists. During the past few decades, much attention has been focused on the synthesis of fluorinated9–12 and trifluoromethylated molecules13–19. On the other hand, the difluoromethyl is also a critical fluorinated functional group due to its use as a lipophilic hydrogen bond donor. In addition, CF2H group can be considered as the isostere of a thiol, a hydroxyl, and an amide, which brings out its potential value in drug development with novel scaffold20,21. However, unlike well-developed synthesis of fluorinated and trifluoromethylated compounds, the construction of difluoromethylated substances22–27, especially for difluoromethylated heterocycles, which widely exist in bioactive molecules (Fig. 1a), has been less explored. The traditional strategies for the synthesis of difluoromethylated heterocycles include deoxyfluorination of aldehydes28, difluorination of benzylic C−H bonds29,30, decarbonylation/decarboxylation difluoromethylation31,32, cycloaddition reactions24,33,34, and conversion of CF2R containing heterocycles precursors35,36. However, these above-mentioned methods need expensive and toxic fluorinating agents, harsh reaction conditions, and are limited in functional group compatibility. Recently, difluoromethylation of heteroaromatic compounds catalyzed by transition metals37, such as copper, palladium, nickel, has been developed. However, most of these approaches depend on preactivation of substrates (i.e., aryl halides38–42, aryl boronic acids43–45, arylzincs46, arenediazonium salts47).
Fig. 1. Construction of CF2H-containing heterocycles.
a Examples of CF2H- bioactive molecules. b TBHP promoted direct difluoromethylation of heterocycles. c High-energy light promoted direct difluoromethylation of heterocycles. d Ag and K2S2O8 co-catalyzed direct difluoromethylation of heterocycles. e Cu and PQ co-catalyzed direct difluoromethylation of heterocycles. f Organic photoredox catalysis triggered direct difluoromethylation of heterocycles.
The radical-based difluoromethylation method has been developed as an advantageous synthetic strategy for the construction of difluoromethylated heterocycles. The pioneering work was reported by Baran and co-workers (Fig. 1b), in which they developed a new difluoromethylated reagent Zn(SO2CF2H)2 (DFMS) that can effectively release CF2H radical in the presence of tert-butyl hydroperoxide (TBHP) as a stoichiometric oxidant48. Sakamoto et al. presented a photolytic direct C–H difluoromethylation of heterocycles using hypervalent iodine(III) reagents that contain difluoroacetoxy ligands (Fig. 1c)49. Shortly afterward, Tung et al. developed direct difluoromethylation of heterocycles using difluoroacetic acid as CF2H radical precursors via transition metal catalysis (Fig. 1d)50. In 2018, Zhu et al. reported a new strategy through the copper-mediated C−H oxidative difluoromethylation of heterocycles with TMSCF2H (Fig. 1e)51. It should be noted that these protocols of direct difluoromethylation of heterocycles need either expensive/toxic metal catalysts or external oxidants and strictly inert conditions, which narrow the functional group tolerance and limit the substrate scope. During the submission of this manuscript, Duan and Xia reported an efficient photocatalytic strategy for C–H perfluoroalkylation of quinoxalinones under aerobic oxidation conditions, however, the corresponding difluoromethylation has not been reported52. Therefore, it is highly desirable to develop new approaches to achieve direct C–H difluoromethylation of heterocycles, which can overcome the above-mentioned defects and avoid the safety problems with stoichiometric oxidant at a larger scale.
In the past decade, visible-light catalysis has attracted extensive attention, by which highly active radical species generated under mild conditions can be involved in various chemical bond formation53–59. Notably, organic dyes are cheaper and more reliable than expensive metal photoredox catalysts in many valuable reactions55,56,59. In this study, we focus our attention on developing an straightforward and practical method for difluoromethylation reactions using the inexpensive, commercially available, and user friendly Hu’s reagent sodium difluoromethane sulfonate (CF2HSO2Na) as the difluoromethyl radical precursor60 under mild conditions. This protocol combines organic photocatalysis with green air oxidant for the difluoromethylation of many categories of heterocycles. Notably, the difluoromethylation product 2′-deoxy-5-difluoromethyluridine (F2TDR) commendably inhibits the proliferation of cancer cells, such as HCT116, HepG-2, and MCF-7 (Fig. 1f).
Results
Investigation of the reaction conditions
We started our investigation by the model reaction of 1-methyl quinoxolin-2-one 1a with CF2HSO2Na 2 as a fluorine source and eosin Y as a photocatalyst in DMSO at room temperature under green LEDs irradiation. To our delight, the difluoromethylation product 3a was obtained in 64% yield (Table 1, entry 1). Then, different photocatalysts were examined (Table 1, entries 2–8), in which rose bengal (RB) was the best one, providing 3a in 72% yield in 12 h. After identifying the optimal photocatalyst, we found that DMSO was the best reaction media through solvent screening (Table 1, entries 2 and 9–16). The yield of product reduced obviously when the loading of the photocatalyst was further decreased to 1 mol% (Table 1, entry 17). Control experiments indicated that photocatalyst and green light source were both essential for the reaction efficiency (Table 1, entries 18 and 19).
Table 1.
Reaction optimizationa,b.
| Entry | Photocatalysts | Solvent | Time (h) | Yieldb/% |
|---|---|---|---|---|
| 1 | Eosin Y | DMSO | 15 | 64 |
| 2 | Rose bengal | DMSO | 12 | 72 |
| 3 | Acridinium salt | DMSO | 36 | 64 |
| 4 | DCA | DMSO | 15 | NR |
| 5 | MB | DMSO | 15 | Trace |
| 6 | Ensin B | DMSO | 24 | 61 |
| 7 | Ru(bpy)3Cl2·6H2O | DMSO | 36 | 62 |
| 8 | fac-Ir(ppy)3 | DMSO | 36 | 56 |
| 9 | Rose bengal | DMF | 12 | 10 |
| 10 | Rose bengal | MeCN | 12 | 20 |
| 11 | Rose bengal | MeOH | 12 | 51 |
| 12 | Rose bengal | CHCl3 | 12 | Trace |
| 13 | Rose bengal | Acetone | 12 | 55 |
| 14 | Rose bengal | Toluene | 12 | NR |
| 15 | Rose bengal | EtOAc | 12 | 15 |
| 16 | Rose bengal | 1,4-dioxane | 12 | 22 |
| 17c | Rose bengal | DMSO | 12 | 51 |
| 18 | – | DMSO | 12 | NR |
| 19d | Rose bengal | DMSO | 12 | NR |
 | ||||
NR no reaction.
aThe reactions were carried out with 1a (0.2 mmol), CF2HSO2Na 2 (0.4 mmol), photocatalyst (2 mol%) in 1 mL solvent under two 3 W green LEDs irradiation at room temperature.
bIsolated yield.
cThe photocatalyst loading was decreased to 1 mol%.
dIn the dark.
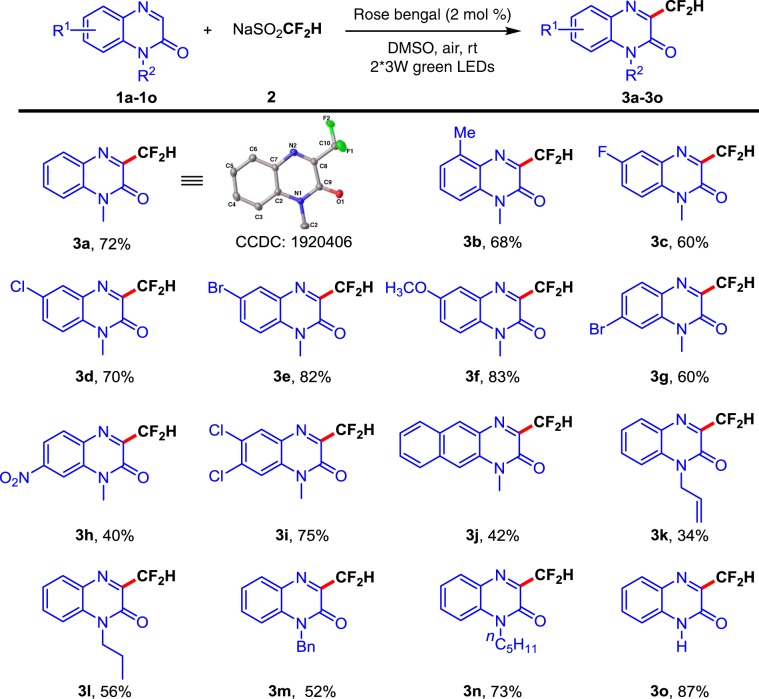
Scope of quinoxalin-2(1H)-ones on the phenyl ring
With the optimal reaction conditions in hand, the substrate scope of various quinoxalin-2(1H)-ones was then investigated. As exhibited in Fig. 2, the reactions worked well with a range of substituted quinoxalin-2(1H)-ones bearing either electron-donating or -withdrawing substituents (methyl, fluoro, chloro, bromo, methoxy, nitro, and naphthyl), giving the desired difluoromethylation products 3a–3j in moderate to good yields. To highlight the utility of this transformation, a variety of N-substituted quinoxalin-2(1H)-ones (1k−1n) were also tested. As a result, all the N-substituted quinoxalin-2(1H)-ones are compatible with the reaction, furnishing the expected products 3k–3n in 34–73% yields. Notably, N-unsubstituted quinoxalin-2(1H)-one also proceeded smoothly, delivering the desired product 3o in 87% yield.
Fig. 2. Substrate scope of quinoxalin-2(1H)-ones.
Reaction conditions: 1 (0.2 mmol), CF2HSO2Na 2 (0.4 mmol), rose bengal (2 mol%) in 1 mL DMSO under two 3 W green LEDs irradiation at room temperature. Isolated yields based on 1.
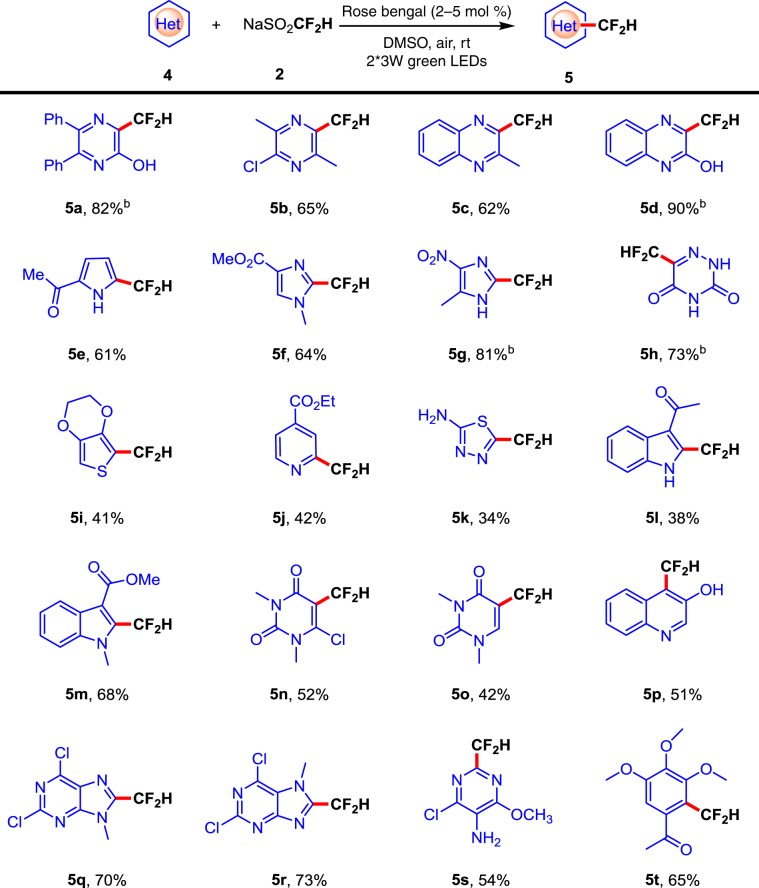
Scope of heteroaromatics
To further extend the scope of this methodology, some other heteroaromatic substrates were investigated (Fig. 3). A wide range of five- and six-membered difluoromethylated heteroarenes, such as pyrazines (5a and 5b), quinoxalines (5c and 5d), pyrrole (5e), imidazoles (5f and 5g), thiophene (5i), pyridines (5j), thiadiazole (5k), indoles (5l and 5m), RNA-based dimethyluracils (5n and 5o), quinoline (5p), purine derivatives (5q and 5r), and pyrimidine (5 s), provided final products with good yields. Several heteroarenes with potentially sensitive functional groups (OH, NH2, or CHO) (5a, 5k, and 6j) that are barely reported in the previous study48–51 are also compatible with this difluoromethylation strategy. Moreover, this direct C–H difluoromethylation method also suitable for some arenes (5t and 6j).
Fig. 3. Substrate scope of heteroaromatics.
Reaction conditions: 4 (0.1 mmol), CF2HSO2Na 2 (0.4 mmol), rose bengal (5 mol.%) in 1 mL DMSO under two 3 W green LEDs irradiation at room temperature. Isolated yields based on 4. bWith CF2HSO2Na 2 (0.4 mmol), rose bengal (2 mol%) in 1 mL DMSO.
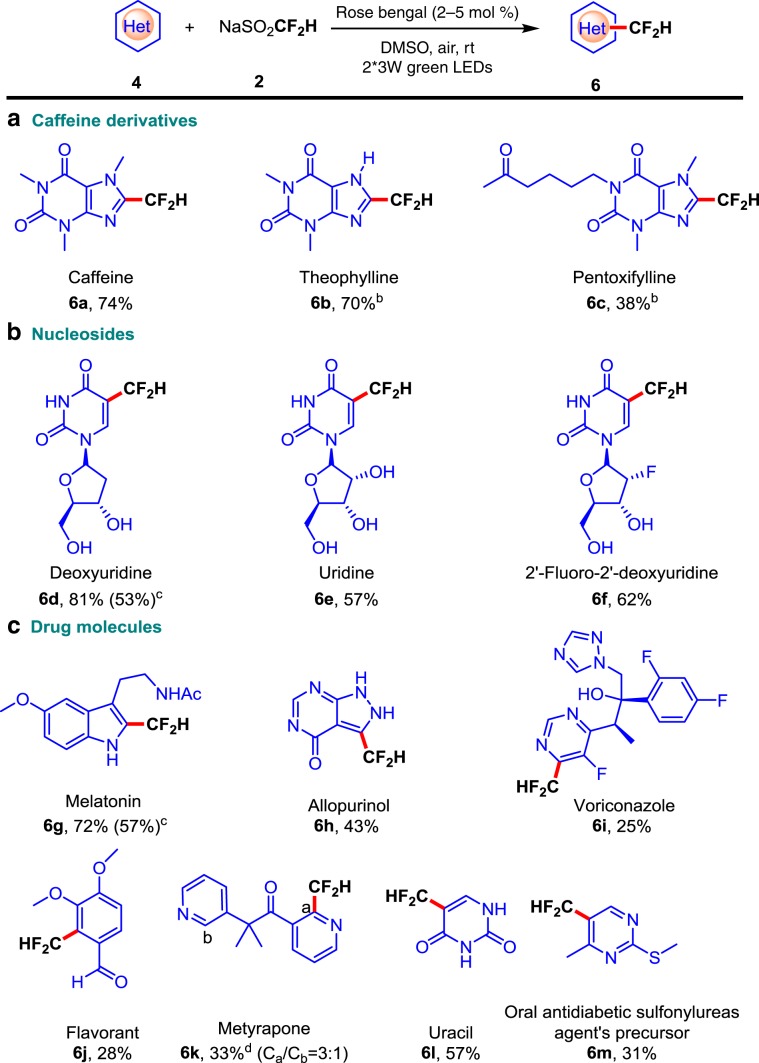
Scope of bioactive molecules
The methodology can also be applied to late-stage functionalization of complex nitrogen-containing bioactive molecules (Fig. 4). For example, the modification of caffeine and its derivatives delivers the desired difluoromethylated products 6a–6c in 38–74% yields. In addition, deoxyuridine, uridine, and 2′-fluoro-2′-deoxyuridine, which have free OH group as well as amide group, could tolerate this difluoromethylation reaction, leading to 6d–6f in 57–81% yields. Furthermore, melatonin, allopurinol, and uracil, which bear free secondary N–H groups, furnished the difluoromethylation reaction with moderate to good yields (6g, 6h, and 6l). Moreover, some other bioactive heteroarene substrates, such as voriconazole, flavorant, metyrapone, and sulfonylureas, can also proceed well with the reaction, giving the difluoromethylated products 6i, 6j, 6k, and 6m in acceptable yields.
Fig. 4. Substrate scope of bioactive molecules.
Reaction conditions: 4 (0.1 mmol), CF2HSO2Na 2 (0.4 mmol), rose bengal (5 mol%) in 1 mL DMSO under two 3 W green LEDs irradiation at room temperature. Isolated yields based on 4. bWith CF2HSO2Na 2 (0.4 mmol), rose bengal (2 mol%) in 1 mL DMSO. cLarge scale with 2 mmol heteroarenes. dThe minor regioisomeric position is labeled with the respective carbon atom number.
Site-selectivity study
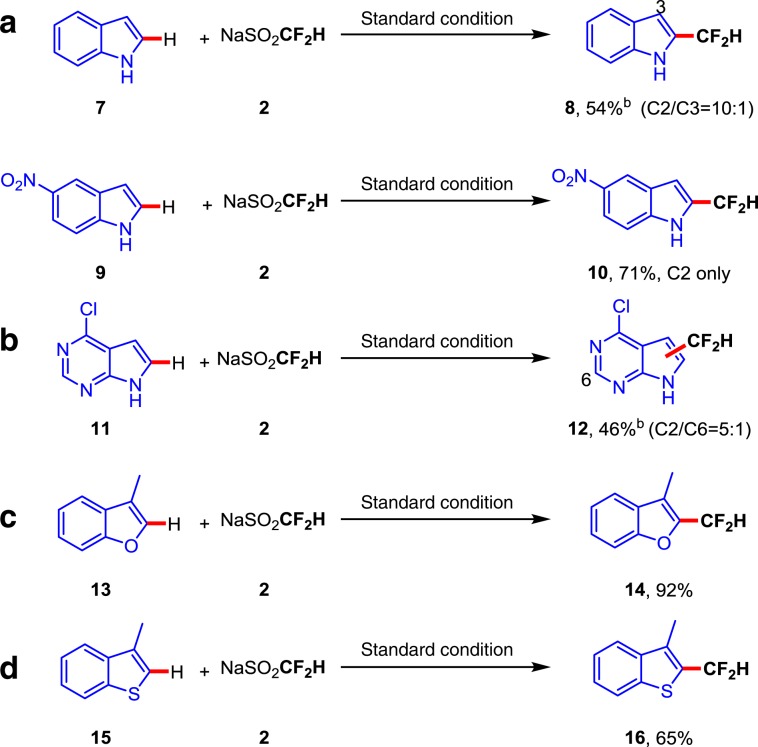
The substrate scope results of Figs. 2–4 show that most of the difluoromethylation occurs on the C2–H bond adjacent to the heteroatom. Some heterocycle substrates bearing more than one radical attacked carbon center were also examined (Fig. 5). As a result, the unsubstituted indole substrate 7 gave the major product at the C2 position in 54% yield with 10:1 regioselectivity. The 5-nitro-substituted indole substrate 9 can obtain a single regioselective difluoromethylation product 10 with 71% yield. When 4-chloro-7H-pyrrolo[2,3-d] pyrimidine was used as substrate, the reaction furnish the mixture of product 12 (C2/C6 = 5:1) in 46% yield. Notably, other electron-rich heteroarenes (benzofuran 13 and thianaphthene 15), which have not been investigated as substrates in previous reported radical difluoromethylation reactions48–50, also exhibited good reaction efficiency, producing the difluoromethylation products 14 and 16 in 92% and 65% yield, respectively. Unfortunately, other types of heteroarenes, including phenanthroline, 1,3,5-triazine, and thiazone, are not ideal substrates in this difluoromethylation reaction (Supplementary Fig. 2).
Fig. 5. Site-selectivity study.
a Regioselectivity for indoles. b Regioselectivity for 4-chloro-7H-pyrrolo[2,3-d] pyrimidine. c Regioselectivity for benzofuran. d Regioselectivity for thianaphthene. Reaction conditions: heterocycles (0.1 mmol), CF2HSO2Na 2 (0.4 mmol), rose bengal (5 mol%) in 1 mL DMSO under two 3 W green LEDs irradiation at room temperature. Isolated yields based on heterocycles. bThe minor regioisomeric position is labeled with the respective carbon atom number.
Synthetic applications
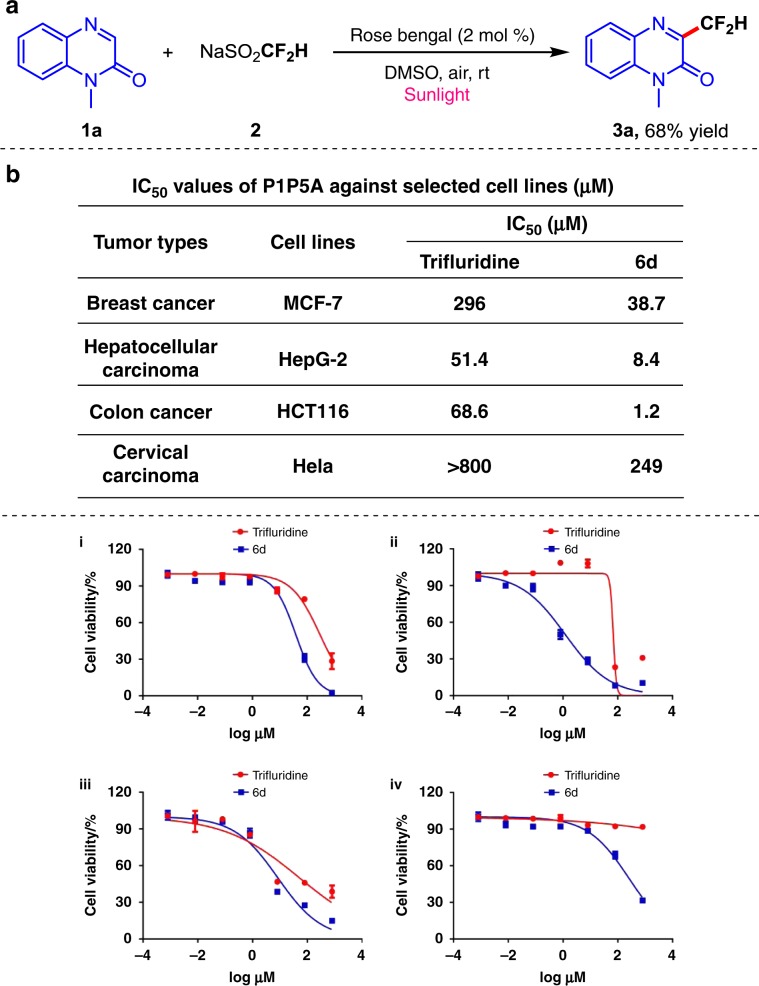
To evaluate the synthetic potential of this methodology, sunlight-driven experiment was performed. When the reaction was conducted under sunlight irradiation, the desired product 3a was obtained in 68% yield (Fig. 6a). Furthermore, a slight reduction of yields with large scale preparation of 6d and 6g also prove the practicability of this difluoromethylation strategy (Fig. 4, yields in parentheses for 6d and 6g). We further explored the potential application of the synthesized difluoromethylated product in medicinal chemistry. The F2TDR 6d has a similar structure to the trifluridine, which has been approved by FDA for the treatment of adult patients with metastatic colorectal cancer (for details, see https://www.drugbank.ca/drugs/DB00432). Therefore, we selected four tumor cell lines to evaluate the inhibitory activities of 6d, and made the comparison of the result with trifluridine. As shown in Fig. 6b, 6d higher tumor cell inhibitory capability than the trifluridine with relatively low IC50 values. Notably, the IC50 values of 6d against HCT116 and HepG-2 cells reach low micromolar level, which are about 57- and 6-fold lower than that of trifluridine, respectively. The improvement of antitumor activity indicates the practicality of this difluoromethylation methodology and the potential in the field of discovery of active drug molecules.
Fig. 6. Synthetic applications.
a The reactions were carried out with 4 (0.1 mmol), CF2HSO2Na 2 (0.2 mmol), rose bengal (2 mol%) in 1 mL DMSO under sunlight irradiation at room temperature. b Cytotoxicity of compounds. i MCF-7 (human breast adenocarcinoma cells), ii HepG-2 (human liver hepatocellular carcinoma cells), iii HCT116 (human colorectal cancer cells), and iv Hela (human cervical carcinoma cells) were treated with various concentrations of each compound for 72 h. Cell death was then measured by using a CCK-8 assays (n = 5, mean ± SD).
Mechanistic investigations
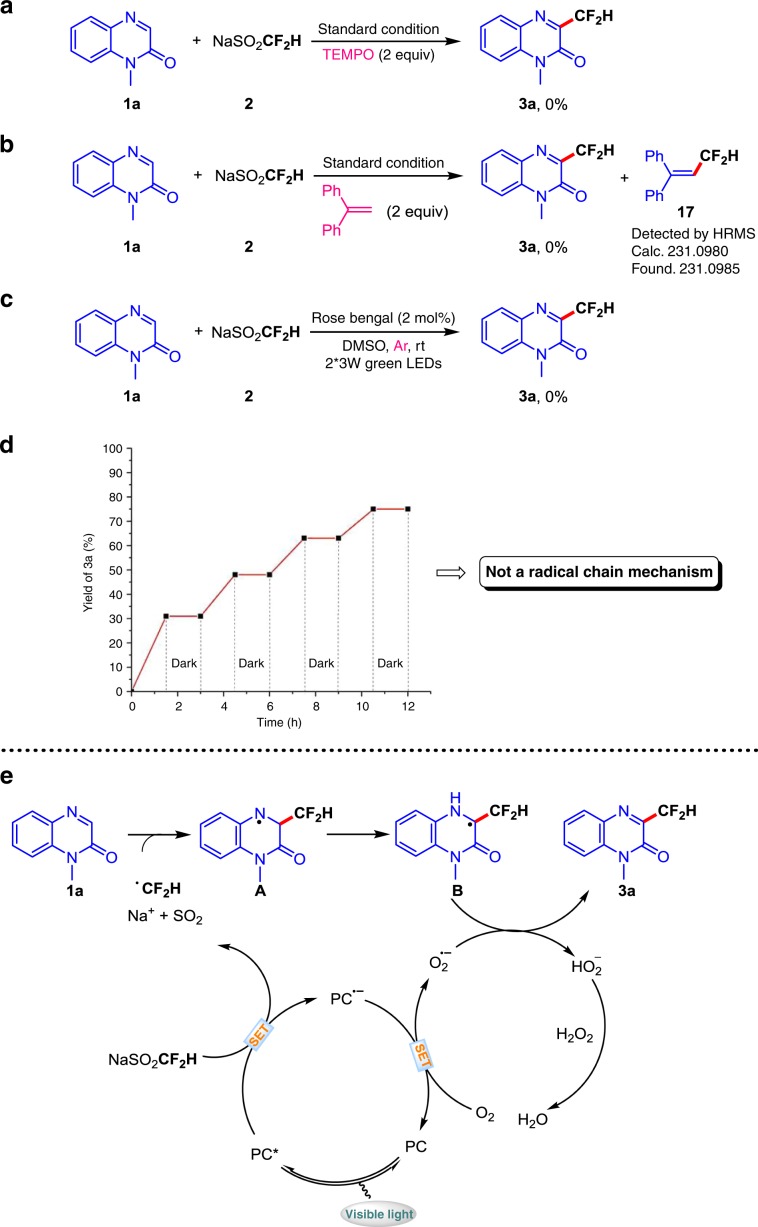
To gain insights into the current studied reaction, control experiments were conducted. When a radical scavenger, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or 1,1-diphenylethylene (Fig. 7a, b) was existing in the mixture containing 1a and CF2HSO2Na, the reaction was completely suppressed, while the radical intermediate was detected by ESI-HRMS (Supplementary Fig. 5), indicating the existence of CF2H radical. When the reaction was carried out under inert atmosphere (Fig. 7c), the formation of 3a was completely inhibited, revealing that oxygen is crucial for the reaction. In situ 1H NMR experiment demonstrated that hydrogen peroxide (H2O2) does not form after irradiation of the reaction mixture in DMSO-d6 for 12 h under the optimized reaction condition. In addition, the observed water peak (H2O) growth indicating that the oxygen was eventually converted to H2O rather than H2O2 (Supplementary Figs. 6 and 7). The generated H2O2 could participate in the catalytic cycle and ultimately converted to H2O as the byproduct61. These experimental results illustrated an available radical pathway. Moreover, we also conducted the light/dark experiment. As shown in Fig. 7, the desired product 3a formed only under continuous irradiation, which ruled out the possibility of a radical chain propagation. On the basis of our experimental observations and previous studies59, a possible mechanism was proposed (Fig. 7e). Upon absorption of visible light, the photocatalyst RB is excited into RB* (Ered = 0.99 V vs SCE) and a single electron is transferred from CF2HSO2Na62 (E = 0.59 V vs SCE) to RB*, which affords CF2H radical and generates an RB•− radical anion. The photoredox cycle is completed by the molecular oxygen oxidation of RB•−, giving RB and O2•−. After that, addition of CF2H radical to 1a occurs, leading to intermediate A, which undergoes a 1,2-H shift to generate carbon radical intermediate B. The intermediate B loses a hydrogen atom to O2•− to furnish the desired product 3a.
Fig. 7. Mechanistic investigations.
a Investigation on the effect of TEMPO. b Radical trapping with 1,1-diphenylethylene. c Investigation on the effect of oxygen. d Light/dark experiment. e Proposed reaction mechanism.
Discussion
In summary, we have achieved a visible-light triggered direct C–H difluoromethylation of heterocycles by using commercially available and inexpensive sodium difluoromethane sulfonate as CF2H radical source. The process is under mild conditions using O2 as a green oxidant and without using metal additive. Furthermore, we also use this highly efficient methodology for direct difluoromethylation of some nitrogen-containing biological and pharmaceutical active molecules. In addition, the bioactivity evaluation of a representative difluoromethylation product 2′-deoxy-5-difluoromethyluridine (6d) exhibited promising activity against cancer cell lines. We expect this simple protocol to be of broad utility for the development of new drugs. Further synthetic applications and bioactivity tests are ongoing.
Methods
Procedure for difluoromethylation of quinoxalin-2(1H)-ones
To a 10 mL Schlenk tube equipped with a magnetic stir bar added quinoxalin-2(1H)-ones 1 (0.2 mmol), CF2HSO2Na 2 (0.4 mmol), and RB (0.004 mmol, 2 mol.%) in DMSO (1.0 mL). Then the mixture was stirred and irradiated by two 3 W green LEDs at room temperature for 12 h. The residue was added water (10 mL) and extracted with ethyl acetate (5 mL × 3). The combined organic phase was dried over Na2SO4. The resulting crude residue was purified via column chromatography on silica gel to afford desired products.
Procedure for difluoromethylation of other heterocycles
To a 10 mL Schlenk tube equipped with a magnetic stir bar added heteroarenes 4 (0.1 mmol), CF2HSO2Na 2 (0.4 mmol), and RB (0.002–0.005 mmol, 2–5 mol.%) in DMSO (1.0 mL). Then the mixture was stirred and irradiated by two 3 W green LEDs at room temperature for 24 h. The residue was added water (10 mL) and extracted with ethyl acetate (5 mL × 3). The combined organic phase was dried over Na2SO4. The resulting crude residue was purified via column chromatography on silica gel to afford desired products.
Supplementary information
Acknowledgements
The project was supported by NSFC (21971120, 21933008, and 81573354). We thanks Prof. Bin Chen and Dr Xu-Zhe Wang at the Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, for helpful discussions.
Author contributions
X.L. and W.Z. conceived and designed the experiments. W.Z. and X.-X.X. expanded the substrate scope, performed the synthetic application, and characterized all the products. J.Y.C performed the bioactive tests. C.Y. gave some helpful suggestions for the reaction. Y.-L.P. synthesized some substrates. Q.B.M. and X.L. directed the investigations. W.Z., Q.B.M., X.L. and J.-P.C. wrote the manuscript.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the author upon reasonable request. The X-ray crystallographic coordinates for structures of 3a reported in this article have been deposited at the Cambridge Crystallographic Data Center as CCDC 1920406. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
This work is dedicated to the 100th anniversary of Nankai University.
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qingbin Meng, Email: nankaimqb@sina.com.
Xin Li, Email: xin_li@nankai.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-14494-8.
References
- 1.Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications 2nd edn (Wiley-VCH, Weinheim, 2013).
- 2.Hagmann WK. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu M, Hiyama T. Modern synthetic methods for fluorine-substituted target molecules. Angew. Chem. Int. Ed. 2005;44:214–231. doi: 10.1002/anie.200460441. [DOI] [PubMed] [Google Scholar]
- 4.Chambers, R. D. Fluorine in Organic Chemistry (Blackwell, Oxford, 2004).
- 5.Filler R, Kobayashi Y, Yagupolskii LM. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. New York: Elsevier; 1993. [Google Scholar]
- 6.Mgller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007). [DOI] [PubMed]
- 7.Purser S, Moore PR, Swallow S, Gouverneur V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008;37:320–330. doi: 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]
- 8.Cametti M, Crousse B, Metrangolo P, Milani R, Resnati G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 2012;41:31–42. doi: 10.1039/C1CS15084G. [DOI] [PubMed] [Google Scholar]
- 9.Champagne PA, Desroches J, Hamel J-D, Vandamme M, Paquin JF. Monofluorination of organic compounds: 10 years of innovation. Chem. Rev. 2015;115:9073–9174. doi: 10.1021/cr500706a. [DOI] [PubMed] [Google Scholar]
- 10.Campbell MG, Ritter T. Modern carbon–fluorine bond forming reactions for aryl fluoride synthesis. Chem. Rev. 2015;115:612–633. doi: 10.1021/cr500366b. [DOI] [PubMed] [Google Scholar]
- 11.Furuya T, Kamlet AS, Ritter T. Catalysis for fluorination and trifluoromethylation. Nature. 2011;473:470–477. doi: 10.1038/nature10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang T, Neumann CN, Ritter T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 2013;52:8214–8264. doi: 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]
- 13.Charpentier J, Frîh N, Togni A. Electrophilic trifluoromethylation by use of hypervalent iodine reagents. Chem. Rev. 2015;115:650–682. doi: 10.1021/cr500223h. [DOI] [PubMed] [Google Scholar]
- 14.Merino E, Nevado C. Addition of CF3 across unsaturated moieties: a powerful functionalization tool. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egami H, Sodeoka M. Trifluoromethylation of alkenes with concomitant introduction of additional functional groups. Angew. Chem. Int. Ed. 2014;53:8294–8308. doi: 10.1002/anie.201309260. [DOI] [PubMed] [Google Scholar]
- 16.Chu L, Qing FL. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl) trimethylsilane as a nucleophilic CF3 source. Acc. Chem. Res. 2014;47:1513–1522. doi: 10.1021/ar4003202. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Wu T, Phipps RJ, Toste FD. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 2015;115:826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso C, Mart.nez de Marigorta E, Rubiales G, Palacios F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 2015;115:1847–1935. doi: 10.1021/cr500368h. [DOI] [PubMed] [Google Scholar]
- 19.Ni C, Hu M, Hu J. Good partnership between sulfur and fluorine: sulfur-based fluorination and fluoroalkylation reagents for organic synthesis. Chem. Rev. 2015;115:765–825. doi: 10.1021/cr5002386. [DOI] [PubMed] [Google Scholar]
- 20.Studer AA. “Renaissance” in radical trifluoromethylation. Angew. Chem. Int. Ed. 2012;51:8950–8958. doi: 10.1002/anie.201202624. [DOI] [PubMed] [Google Scholar]
- 21.Sessler CD, et al. CF2H, a hydrogen bond donor. J. Am. Chem. Soc. 2017;139:9325–9332. doi: 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang X-J, Dolbier WR. Efficient Cu-catalyzed atom transfer radical addition reactions of fluoroalkylsulfonyl chlorides with electron-deficient alkenes induced by visible light. Angew. Chem. Int. Ed. 2015;54:4246–4249. doi: 10.1002/anie.201412199. [DOI] [PubMed] [Google Scholar]
- 23.Lin Q-Y, Xu X-H, Zhang K, Qing F-L. Visible-light-induced hydrodifluoromethylation of alkenes with a bromodifluoromethylphosphonium bromide. Angew. Chem. Int. Ed. 2016;55:1479–1483. doi: 10.1002/anie.201509282. [DOI] [PubMed] [Google Scholar]
- 24.Rong J, et al. Radical fluoroalkylation of isocyanides with fluorinated sulfones by visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2016;55:2743–2747. doi: 10.1002/anie.201510533. [DOI] [PubMed] [Google Scholar]
- 25.Noto N, Koike T, Akita M. Metal-free di- and tri-fluoromethylation of alkenes realized by visible-light-induced perylene photoredox catalysis. Chem. Sci. 2017;8:6375–6379. doi: 10.1039/C7SC01703K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiong P, Xu H-H, Song J, Xu H-C. Electrochemical difluoromethylarylation of alkynes. J. Am. Chem. Soc. 2018;140:2460–2464. doi: 10.1021/jacs.8b00391. [DOI] [PubMed] [Google Scholar]
- 27.Meyer CF, Hell SM, Misale A, Trabanco AA, Gouverneur V. Hydrodifluoromethylation of alkenes with difluoroacetic acid. Angew. Chem. Int. Ed. 2019;58:8829–8833. doi: 10.1002/anie.201903801. [DOI] [PubMed] [Google Scholar]
- 28.Dishington A, et al. Synthesis of functionalized cyanopyrazoles via magnesium bases. Org. Lett. 2014;16:6120–6123. doi: 10.1021/ol502977a. [DOI] [PubMed] [Google Scholar]
- 29.Xia JB, Zhu C, Chen C. Visible light-promoted metal-free C–H activation: diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc. 2013;135:17494–17500. doi: 10.1021/ja410815u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu P, Guo S, Wang L, Tang P. Silver-catalyzed oxidative activation of benzylic C-H bonds for the synthesis of difluoromethylated arenes. Angew. Chem. Int. Ed. 2014;53:5955–5958. doi: 10.1002/anie.201400225. [DOI] [PubMed] [Google Scholar]
- 31.Pan F, Boursalian, G. B. Ritter T. Palladium-catalyzed decarbonylative difluoromethylation of acid chlorides at room temperature. Angew. Chem. Int. Ed. 2018;57:16871–16876. doi: 10.1002/anie.201811139. [DOI] [PubMed] [Google Scholar]
- 32.Zeng XJ, et al. Copper-catalyzed decarboxylative difluoromethylation. J. Am. Chem. Soc. 2019;141:11398–11403. doi: 10.1021/jacs.9b05363. [DOI] [PubMed] [Google Scholar]
- 33.Mykhailiuk PK. In situ generation of difluoromethyl diazomethane for [3+2] cycloadditions with alkynes. Angew. Chem. Int. Ed. 2015;54:6558–6561. doi: 10.1002/anie.201501529. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Xu W, Yu ZX, Wang J. Ruthenium-catalyzed formal dehydrative [4 + 2] cycloaddition of enamides and alkynes for the synthesis of highly substituted pyridines: reaction development and mechanistic study. J. Am. Chem. Soc. 2015;137:9489–9496. doi: 10.1021/jacs.5b06400. [DOI] [PubMed] [Google Scholar]
- 35.Gui JH, et al. C–H methylation of heteroarenes inspired by radical SAM methyl transferase. J. Am. Chem. Soc. 2014;136:4853–4856. doi: 10.1021/ja5007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi H, et al. Synthesis of 18F-difluoromethylarenes from aryl (pseudo) halides. Angew. Chem. Int. Ed. 2016;55:10786–10790. doi: 10.1002/anie.201604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rong J, Ni C, Hu J. Metal-catalyzed direct difluoromethylation reactions. Asian J. Org. Chem. 2017;6:139–152. doi: 10.1002/ajoc.201600509. [DOI] [Google Scholar]
- 38.Fier PS, Hartwig JF. Copper-mediated difluoromethylation of aryl and vinyl iodides. J. Am. Chem. Soc. 2012;134:5524–5527. doi: 10.1021/ja301013h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu Y, Leng XB, Shen Q. Cooperative dual palladium/silver catalyst for direct difluoromethylation of aryl bromides and iodides. Nat. Commun. 2014;5:5405–5412. doi: 10.1038/ncomms6405. [DOI] [PubMed] [Google Scholar]
- 40.Xu LD, Vicic A. Direct difluoromethylation of aryl halides via base metal catalysis at room temperature. J. Am. Chem. Soc. 2016;138:2536–2539. doi: 10.1021/jacs.6b00053. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, et al. Difluoromethylation of (hetero) aryl chlorides with chlorodifluoromethane catalyzed by nickel. Nat. Commun. 2018;9:1170–1180. doi: 10.1038/s41467-018-03532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bacauanu V, et al. Metallaphotoredox difluoromethylation of aryl bromides. Angew. Chem. Int. Ed. 2018;57:12543–12548. doi: 10.1002/anie.201807629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng Z, Min QQ, Zhang X. Access to difluoromethylated arenes by Pd-catalyzed reaction of arylboronic acids with bromodifluoroacetate. Org. Lett. 2016;18:44–47. doi: 10.1021/acs.orglett.5b03206. [DOI] [PubMed] [Google Scholar]
- 44.Feng Z, Min Q-Q, Fu XP, An L, Zhang X. Chlorodifluoromethane-triggered formation of difluoromethylated arenes catalysed by palladium. Nat. Chem. 2017;9:918–923. doi: 10.1038/nchem.2746. [DOI] [PubMed] [Google Scholar]
- 45.Hori K, Motohashi H, Saito D, Mikami K. Precatalyst effects on Pd-catalyzed cross-coupling difluoromethylation of aryl boronic acids. ACS Catal. 2019;9:417–421. doi: 10.1021/acscatal.8b03892. [DOI] [Google Scholar]
- 46.Miao W, et al. Iron-catalyzed difluoromethylation of arylzincs with difluoromethyl 2-pyridyl sulfone. J. Am. Chem. Soc. 2018;140:880–883. doi: 10.1021/jacs.7b11976. [DOI] [PubMed] [Google Scholar]
- 47.Matheis C, Jouvin K, Goossen L. Sandmeyer difluoromethylation of (hetero-) arenediazonium salts. Org. Lett. 2014;16:5984–5987. doi: 10.1021/ol5030037. [DOI] [PubMed] [Google Scholar]
- 48.Fujiwara Y, et al. A new reagent for direct difluoromethylation. J. Am. Chem. Soc. 2012;134:1494–1497. doi: 10.1021/ja211422g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakamoto R, Kashiwagi H, Maruoka K. The direct C–H difluoromethylation of heteroarenes based on the photolysis of hypervalent iodine(III) reagents that contain difluoroacetoxy ligands. Org. Lett. 2017;19:5126–5129. doi: 10.1021/acs.orglett.7b02416. [DOI] [PubMed] [Google Scholar]
- 50.Tung TT, Christensen SB, Nielsen J. Difluoroacetic acid as a new reagent for direct C-H difluoromethylation of heteroaromatic compounds. Chemistry. 2017;23:18125–18128. doi: 10.1002/chem.201704261. [DOI] [PubMed] [Google Scholar]
- 51.Zhu S, Liu Y, Li H, Xu X, Qing F. Direct and regioselective C-H oxidative difluoromethylation of heteroarenes. J. Am. Chem. Soc. 2018;140:11613–11617. doi: 10.1021/jacs.8b08135. [DOI] [PubMed] [Google Scholar]
- 52.Wei Z, et al. Visible light-induced photocatalytic C-H perfluoroalkylation of quinoxalinones under aerobic oxidation condition. Adv. Synth. Catal. 2019;361:5490–5498. doi: 10.1002/adsc.201900885. [DOI] [Google Scholar]
- 53.Narayanam JMR, Stephenson CRJ. Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011;40:102–113. doi: 10.1039/B913880N. [DOI] [PubMed] [Google Scholar]
- 54.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hari DP, König B. Synthetic applications of eosin Y in photoredox catalysis. Chem. Commun. 2014;50:6688–6699. doi: 10.1039/C4CC00751D. [DOI] [PubMed] [Google Scholar]
- 56.König NA, Nicewicz DA. Organic photoredox catalysis. Chem. Rev. 2016;116:10075–10166. doi: 10.1021/acs.chemrev.6b00057. [DOI] [PubMed] [Google Scholar]
- 57.Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma S, Sharma A. Recent advances in photocatalytic manipulations of Rose Bengal in organic synthesis. Org. Biomol. Chem. 2019;17:4384–4405. doi: 10.1039/C9OB00092E. [DOI] [PubMed] [Google Scholar]
- 60.He Z, Tan P, Ni C, Hu J. Fluoroalkylative aryl migration of conjugated N-arylsulfonylated amides using easily accessible sodium Di- and monofluoroalkanesulfinates. Org. Lett. 2015;17:1838–1841. doi: 10.1021/acs.orglett.5b00308. [DOI] [PubMed] [Google Scholar]
- 61.Liu WQ, et al. Visible light promoted synthesis of indoles by single photosensitizer under aerobic conditions. Org. Lett. 2017;19:3251–3254. doi: 10.1021/acs.orglett.7b01367. [DOI] [PubMed] [Google Scholar]
- 62.Zou ZL, et al. Electrochemically promoted fluoroalkylation-distal functionalization of unactivated alkenes. Org. Lett. 2019;21:1857–1862. doi: 10.1021/acs.orglett.9b00444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the author upon reasonable request. The X-ray crystallographic coordinates for structures of 3a reported in this article have been deposited at the Cambridge Crystallographic Data Center as CCDC 1920406. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.