Abstract
Noise-induced hearing loss generally induces loudness recruitment, but sometimes gives rise to hyperacusis, a debilitating condition in which moderate intensity sounds are perceived abnormally loud. In an attempt to develop an animal model of loudness hyperacusis, we exposed rats to a 16-20 kHz noise at 104 dB SPL for 12 weeks. Behavioral reaction time-intensity functions were used to assess loudness growth functions before, during and 2-months post-exposure. During the exposure, loudness recruitment (R) was present in the region of hearing loss (26 and 20 kHz), but subtle evidence of hyperacusis (H) started to emerge at the border of the hearing loss (16 kHz). Unexpectedly, robust evidence of hyperacusis appeared below and near the edge of the hearing loss (4 and 16 kHz) 2-months post-exposure. To identify the neural correlates of hyperacusis and test the central gain model of hyperacusis, we recorded population neural responses from the cochlea, auditory cortex and lateral amygdala 2-months post-exposure. Compared to controls, the neural output of the cochlea was greatly reduced in the noise group. Consistent with central gain models, the gross neural responses from the auditory cortex and amygdala were proportionately much larger than those from the cochlea. However, despite central amplification, the population responses in the auditory cortex and amygdala were still below the level needed to fully account for hyperacusis and/or recruitment. Having developed procedures that can consistently induce hyperacusis in rats, our results set the stage for future studies that seek to identify the neurobiological events that give rise to hyperacusis and to develop new therapies to treat this debilitating condition.
Keywords: Loudness recruitment, hyperacusis, central gain, noise exposure, auditory cortex, amygdala
Listeners with hearing loss often struggle to detect low-level sounds, but as the sound intensity rises above threshold, loudness initially grows at a faster than normal rate, but the growth rate slows with increasing intensity so that normal loudness is achieved at high intensities (Buus and Florentine, 2002). This phenomenon is referred to as loudness recruitment (Fowler, 1937a). However, in some hearing-impaired listeners, loudness continues to grow more rapidly than normal at suprathreshold intensities causing moderately intense sounds to be perceived as louder than normal, a debilitating condition known as hyperacusis or loudness intolerance (Tyler et al., 2014, Mohrle et al., 2019). Because the neural output of the cochlea is reduced when the cochlea is damaged, the neural correlates of hyperacusis are unlikely to originate in the cochlea (Heinz et al., 2005). Instead, a growing body of evidence suggests that loudness hyperacusis results from excessive neural gain in the central nervous system (Auerbach et al., 2014, Chen et al., 2015, Chambers et al., 2016, Jiang et al., 2017). Enhanced suprathreshold responses have been observed at multiple sites along the central auditory pathway following cochlear damage (Popelar et al., 1987, Salvi et al., 1990, Salvi, 1992, Boettcher and Salvi, 1993, Syka et al., 1994, Salvi et al., 2000b, Syka and Rybalko, 2000, Cai et al., 2008, Popelar et al., 2008). In general, the increase in sound-evoked neural hyperactivity is more pronounced at higher levels of the central auditory pathway, but the magnitude and time course of the changes depends on many factors (Salvi, 1992, Salvi et al., 2000a, Popelar et al., 2008, Auerbach et al., 2014, Chambers et al., 2016). In some frequency regions, suprathreshold responses in the central auditory pathway increase more rapidly than normal, but then saturate at normal levels, changes consistent with loudness recruitment. However, in other cases, sound-evoked responses are greater than normal at suprathreshold intensities (Salvi et al., 1990, Salvi, 1992, Salvi et al., 2016) possibly due to the loss of inhibition (Wang et al., 1996, Scholl and Wehr, 2008), consistent with hyperacusis. Neural hyperactivity in both the classical auditory pathway, such as the auditory cortex (AC), and non-classical auditory centers, such as the lateral amygdala (LA) has been suggested to relate to hyperacusis-like behavior in rats (Sun et al., 2012, Chen et al., 2014b).
While enhanced central gain is a potential mechanism to account for hyperacusis (Zeng, 2013, Auerbach et al., 2014), there is limited experimental evidence directly correlating the increase in neural response amplitude with behavioral measures of hyperacusis due to the difficulty of obtaining behavioral measures of loudness hyperacusis. To address this problem, we implemented a behavioral technique to assess loudness growth functions (Moody, 1973, Pfingst et al., 1975, Feitosa, 1981) by measuring a rat’s reaction time (RT) to tone bursts or noise bursts at various intensities (Chen et al., 2014b, Radziwon et al., 2017). RTs decreased with increasing intensity consistent with human studies (Marshall and Brandt, 1980) in which RT versus intensity (RT-I) functions were closely correlated with loudness growth. To determine if we could induce hyperacusis in an animal model, we treated the rats with sodium salicylate, an ototoxic drug that induces temporary hearing loss and tinnitus, a condition often linked to hyperacusis (Baguley, 2003, Chen et al., 2014b, Radziwon et al., 2017). Immediately after salicylate treatment, RTs became shorter than normal at high intensities, behavioral evidence of loudness hyperacusis. After drug washout, RTs recovered to normal values. Consistent with the central gain model, neural responses in salicylate-treated rats become larger than normal in the AC, medial geniculate body (MGB), and LA (Chen et al., 2013, Chen et al., 2014b, Chen et al., 2015). By performing chronic electrophysiological recordings from behaviorally-phenotyped animals, we demonstrated that salicylate-induced gain changes were closely correlated with behavioral changes to RT-I functions within individual animals (Auerbach et al., 2019). Thus, salicylate-induced hyperacusis was associated with enhanced central gain (Yang et al., 2007, Jiang et al., 2017, Radziwon et al., 2017, Auerbach et al., 2019).
Intense, short-duration noise exposures have been shown to cause loudness recruitment, but not hyperacusis (Moody, 1973, Pugh et al., 1979, May et al., 2009). However, it is unclear from the literature whether hyperacusis can be reliably induced by long-duration noise exposure that cause permanent hearing loss. To test this hypothesis, we exposed rats to a series of long-duration, high-frequency noises that induced varying amount of high-frequency hearing loss (Chen et al., 2014a). From these experiments, we identified a noise-exposure condition that induced loudness hyperacusis at low-frequencies where hearing thresholds were normal and loudness recruitment in the high-frequency region with hearing loss. Afterwards, we carried out electrophysiological measurements from the AC and LA of the noise-exposed rats, some with behavioral evidence of hyperacusis and loudness recruitment. Suprathreshold responses from AC and LA of the noise-exposed rats were proportionately much larger than predicted from the neural output of the cochlea, consistent with enhanced central gain; however, the response amplitudes and neurophysiological growth functions were insufficient to account for the behavioral measures of hyperacusis and loudness recruitment.
METHODS
Subjects:
Thirty Sprague–Dawley male rats were acquired from Charlese River Laboratories, Inc. and housed in the Laboratory Animal Facility (LAF) at the University at Buffalo and given free access to food and water. Ambient noise levels in the LAF were below 40 dB SPL (see dashed line in Fig. 1). The colony rooms were maintained at 22 °C with a 12-hour light-dark cycle. All procedures used in this project were approved by the Institutional Animal Care and Use Committee at the University at Buffalo and carried out in accordance with NIH guidelines.
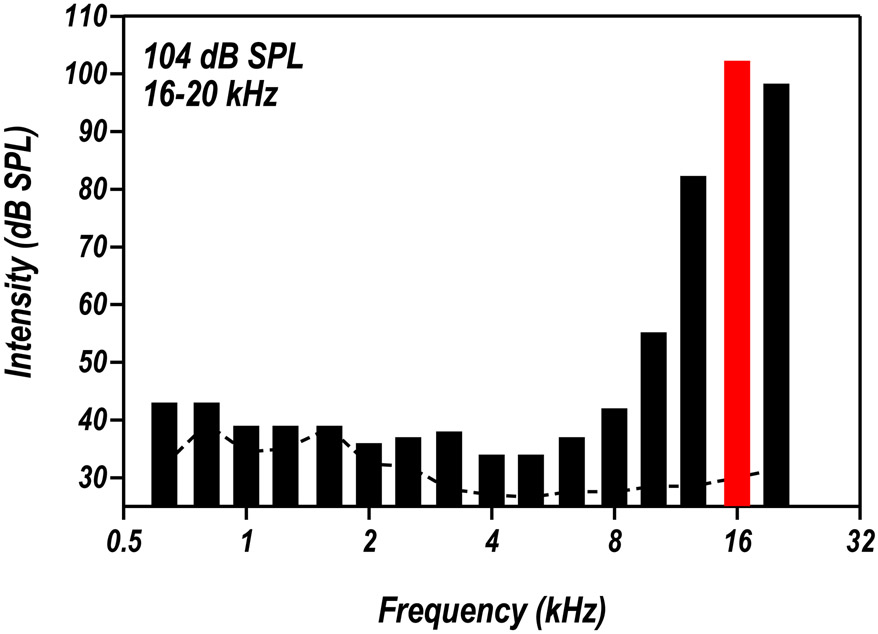
Figure 1:
Spectrum of the narrow-band (16-20 kHz) noise used. Each bar represent one-third octave band. The overall noise level is 104 dB SPL (±1.5 dB). The dashed line represents background noise in the colony room.
Noise exposure:
In the behavioral measurement of loudness perception, 4 rats were housed in a separate colony room and exposed to a narrowband noise (NBN, 16-20 kHz) at 104 dB SPL at an age of ~15 months after the animals were behaviorally trained. The rats were continuously exposed to the noise over a period of 12 weeks except for behaviorally training (approximately 1 hour per day). Four additional rats were noise-exposed in a similar manner for LA-response recording. The NBN was generated using a TDT RP2 Real time signal processor (TDT, Gainesville, FL), amplified, and delivered to a speaker (FT17H, Fostex) located 28 cm above the bottom of the acrylic cage (L = 48.2 cm, W = 25.4 cm, H = 20.3 cm) in which the rats were housed. Sound levels in the cage were measured at the height of the animal’s ear directly below the speaker using a sound level meter (Larson Davis System 824) and half-inch microphone (model 2540, Larson Davis). The sound level at various locations within the cage varied ~1-2 dB. Figure 1 shows the sound pressure level (SPL) measured in 1/3 octave bands; the overall level of the noise was 104 dB SPL (±1.5 dB). The control rats (11 in cochlear functional and histological examination, 8 in AC recording, and 3 in LA recording) were housed in identical colony rooms without noise exposure.
Behavioral measures of threshold:
Four rats were trained in a go/no-go operant conditioning paradigm to detect tone bursts in a sound attenuating chamber as described previously (Radziwon et al., 2017). The rats were food restricted and kept at approximately 85% of their free-feeding weight during the course of experiment. The tone bursts used for the study were 300 ms in duration (5 ms rise/fall time, cosine gated) and presented at frequencies of 4, 16, 20, and 26 kHz across a range of stimulus intensities. The range of stimulus intensities began at 30-90 dB SPL, presented in 10-dB steps, but was systematically lowered to obtain a threshold for each frequency. The tone bursts were presented according to the psychophysical Method of Constant Stimuli. Within each 10-trial block, seven predetermined target intensities were presented randomly along with 3 catch trials without sound stimulus. The target intensities were chosen so that only the lowest one or two intensities were estimated to be below threshold; whereas the remaining intensities were well above threshold. Sound pressure levels were calibrated using a Larson-Davis sound level meter (Larson-Davis System 824; Fast setting; Flat weighing) with a microphone (1/2” free field microphone, Larson-Davis model 2520) placed at the position where the animal’s head would be when its nose is inside the nose-poke hole (Radziwon et al., 2017).
A rat began a trial by placing its nose in a nose-poke hole, which initiated a variable waiting interval ranging from 1 to 4s. During the waiting interval, the rat had to maintain its position in the nose-poke hole until it heard a tone burst or the trial was aborted. In the go condition, the target stimulus was the tone burst. If the rat detected this signal, it removed its nose from the nose-poke hole resulting in a food reward (45 mg dustless rodent grain pellets, Bio-Serv); a hit was recorded if the rat correctly responded to the tone within 2s. A miss was recorded if the rat failed to remove its nose from the nose-poke within the 2s response interval. Approximately 30% of all trials were catch trials where tone bursts were not presented. This constituted the no-go part of the procedure. If the rat removed its nose during a catch trial, a false alarm was recorded and the rat received a 4s timeout, during which the house light was turned off and the rat could not start another trial. However, if the rat continued to nose-poke, a correct rejection was recorded. No reinforcement was given for a correct rejection.
200 consecutive trials were used to calculate thresholds for each frequency resulting in 20 presentations per stimulus intensity (7 tone trials x 20 presentations per intensity =140 trials) along with 60 catch trials. Thresholds were calculated over at least two separate testing days. Therefore, at least 400 trials were used to obtain threshold measurements per frequency for each rat. If false alarm rates were greater than 20% during any testing session, the resulting data were excluded from analysis. However, high false alarm rates rarely occurred, thus ensuring that the animals were consistently under stimulus control (Klink et al., 2006). Mean hit and false alarm rates were used to calculate thresholds using signal detection theory with a set threshold criterion of d’=1.5 (Steckler, 2001). Thresholds were calculated for each rat by taking the z-scores for the number of “hits” for each stimulus intensity and correcting for response bias. A d’ of 1.5 was chosen as our threshold criterion because this is commonly used among animal researchers (Wagner et al., 2003, Klink et al., 2006, Radziwon et al., 2009) and it is a relatively conservative criterion. After baseline tone burst thresholds were collected, the rats were exposed to the 16-20 kHz NBN and tone burst thresholds were measured during and two months following the noise exposure. The permanent noise-induced threshold shifts were computed for each rats by finding the difference between the pre- vs. the during or 2-months post-exposure thresholds.
Behavioral measure of loudness growth:
The four rats used for threshold testing above were also tested on a go/no-go operant conditioning paradigm to detect tone bursts (300 ms duration, 5 ms rise/fall time, cosine gated) presented over a 30-90 dB SPL intensity range. We measured their RT-I functions to assess the growth of loudness, consistent with previous human and animal studies (Moody, 1970, Marshall and Brandt, 1980, Chen et al., 2014b, Radziwon et al., 2017). RT was measured from the onset of the tone burst to the time the rat removed its nose from the nose-poke hole. Only RTs for “hits”, when the rat correctly detected the stimulus, were included in our analysis. As in the above-mentioned threshold procedure, 200 consecutive trials were used to assess loudness growth for each frequency resulting in 20 presentations per stimulus intensity along with 60 catch trials. RTs were measured over three separate testing days. Therefore, 600 trials overall were used to obtain RT-I functions for each frequency. RTs for each frequency-intensity combination were averaged across trials within a given session, and then across sessions for a given animal to obtain averaged RT-I functions for each animal. The rats were tested before, during, and at approximately two months post-noise exposure. The four rats were approximately 20 months of age when they completed all the behavioral experiments; these four rats were then used for the final AC electrophysiological studies followed by measurement of the cochlear compound action potential (CAP) and histological examination.
AC:
Electrophysiological recordings were obtained from multiple regions of the AC including the primary AC (A1), anterior auditory field (AAF), and the ventral auditory field (VAF) for maximum coverage. The AC data from the four behaviorally-trained, noise-exposed rats (~2.5-months post-exposure) were compared to the data collected from eight age-matched control rats.
LA:
A second group of rats, of similar age as those used for the AC recordings, were used to obtain electrophysiological data from the lateral amygdala. Data were collected from four noise-exposed rats and three control rats matched for age. The noise-exposure parameters and post-exposure recovery time for LA were the same as for the AC.
AC and LA Electrophysiology:
Our procedures for recording from the AC and LA have been described previously (Chen et al., 2012, Chen et al., 2014b). Briefly, after behavioral measurements, the rats were initially anesthetized with ketamine (50 mg/kg, I.M.) and xylazine (6 mg/kg, I.M.) and maintained under a deep anesthesia by supplementary one third dose per hour during the recording. The dorsal surface of the skull was exposed and a small stainless steel screw was inserted into the right parietal bone. Then a head-bar holder was firmly attached to the head using dental cement in order to hold the rat’s head in the stereotaxic frame after the right ear bar was removed to allow for acoustic stimulation using a free-field loudspeaker. An incision was made on the dorsal surface of the skull over the appropriate region to gain access to the left AC and LA as described previously (Chen et al., 2012, Chen et al., 2013). The skull was carefully opened and the dura was removed from the surface of the cortex. Then a 16-channel linear silicon microelectrode array (A-1x16-10mm 100-177, NeuroNexus Technologies) was vertically inserted into the AC at several different positions (3.0-6.5 mm caudal from Bregma; ~6.6 mm laterally from the mid-line; 4.0-6.0 mm ventral to Bregma) or the LA (2.0-4.0 mm caudal to Bregma; ~5.6 mm lateral to the mid-line; 7.0-8.0 mm ventral to Bregma) using stereotaxic coordinates (Paxinos and Watson, 2005). Tone bursts (50 ms of duration, 1 ms of rise/fall time cosine gated) were generated by a TDT RX6-2 (~100 kHz sampling rate) and presented at a rate of 3/s for AC-recording and 2/s for LA-recording. The stimuli were delivered through a loudspeaker (FT28D, Fostex) located 10 cm in front of the right ear. Sound levels at the position of the ear were calibrated using a microphone preamplifier (Larson Davis, model 2221) equipped with a ¼" microphone (Larson Davis, model 2520).
The neural responses from the 16-channel electrode were digitized with a resolution of 40.96 μs using an RA16PA preamplifier and RX5 base station (Tucker-Davis Technologies System-3, Alachua, FL) using custom-written data acquisition and analysis software (MATLAB R2007b, MathWorks) as previously described (Chen et al., 2016). Spike discharges from multiunit clusters (MUC) were recorded in response to tone bursts (1.0, 1.5, 2.3, 3.5, 5.3, 8.0, 12.1, 18.3, 27.7, 42.0 kHz) presented from 0 to 100 dB SPL in steps of 10 dB (AC) or 20 dB (LA); 50 stimuli were presented in pseudo-random order at each frequency-intensity combination. Spike detection was performed online using a manually set voltage threshold (spike signal filtered 300–3500 Hz). Peristimulus time histograms (PSTH, 100 ms window, bin width 1 ms for AC; 300 ms window, bin width of 5 ms for LA) were constructed from the spike discharges from MUCs for each frequency-intensity combination. To provide a global perspective on the population neural responses from the AC and LA, a grand mean population PSTH was constructed by averaging together all the individual PSTHs at each frequency-intensity combination; data were averaged across all recording sites in all the animals in each group.
Compound action potential (CAP):
The CAP was recorded in the 4 noise-exposed rats after behavioral measurement and AC recording and the CAP-amplitudes were compared to the normal CAPs obtained in 8 normal control rats. The recording method is as previously described (Chen et al., 2010, Sheppard et al., 2017). Briefly, the right cochlea of the anesthetized rat was surgically opened to expose the round window. A piece of Teflon-coated silver wire (76 μm in diameter in bare, Cat# 785500, A-M Systems Inc.) was exposed at the end and formed into a ring (~200-300 μm in diameter). The ring electrode was placed on the round window membrane to record the CAP. A silver chloride electrode was placed in the neck muscles as a reference or ground electrode. Tone bursts (10 ms duration, 1 ms rise/fall time, cosine gated) at 2, 4, 6, 8, 12, 16, 20, 24, 30, 35 and 40 kHz were generated using a TDT RP2 Real time signal processor (TDT, Gainesville, FL). Stimulus amplitude was controlled through an attenuator (TDT PA5), fed to a power-amplifier and delivered to a transducer (ACO ½” 7013 microphone driven in reverse) located in a speculum-like housing; the opening of the speculum was directly coupled to the eardrum. The sound levels at the eardrum was calibrated indirectly by using a customized connector coupling the opening of the speculum to the ½" microphone (model 2540, Larson Davis). The speculum-microphone distance was similar as the speculum-eardrum distance.
The output of the round window electrode was filtered (0.1 Hz – 10 kHz) and amplified (1000x) with a DAM-50 preamplifier (WPI). The output of the amplifier was digitized (100 kHz sampling rate) and averaged 100 times with a TDT RP2.1 real time processor using custom data acquisition and analysis software (MATLAB 6.1). The CAP waveform was analyzed offline by setting a low-pass filter at a frequency 1 kHz below the stimulus frequency to remove the cochlear microphonic. CAP amplitude was quantified as the difference between the first negative wave (N1) and the positive peak before or after N1. CAP amplitudes at each frequency were plotted as a function of stimulus intensity and mean CAP input/output functions were constructed.
Cochlear hair cell loss:
Following the CAP recording, the cochleae were harvested for histological analysis as previously described (Chen et al., 2009). The deeply anesthetized animal was decapitated, the cochlea was rapidly removed, the round window, oval window and cochlear apex were opened to facilitate the slow perfusion of warm incubation solution (0.05% nitrotetrazolium blue chloride (cat#N6876, Sigma), 50 mM sodium succinate and 50 mM phosphate buffer) through the oval and round window. The cochleae were placed into the incubation solution for 1 h at 37 °C and then fixed in 10% buffered formalin for 2 days. Afterwards, the cochleae were decalcified in 7% EDTA (ethylenediaminetetraacetic acid) solution for 3-5 days. The entire length of the organ of Corti was then carefully dissected out as a flat surface preparation and examined over its entire length using a light microscope (DMBA300 Digital Microscope, Microscope World) to count the loss of both inner and outer hair cells (IHC and OHC). Based on lab norms, the percentages of missing OHCs and IHCs were plotted as a function of percent distance from the cochlear apex for each of the behaviorally trained rats (Chen et al., 2014a). Data from the four noise-exposed rats from the behavioral experiments (approximately 20 months of age) were used to construct mean (n=4, ±SEM) cochleograms (Fig. 5). For comparison, a mean cochleogram from three age-matched control rats was also constructed using Harris Hematoxylin staining as described previously (Ding, 2001, Baizer et al., 2015).
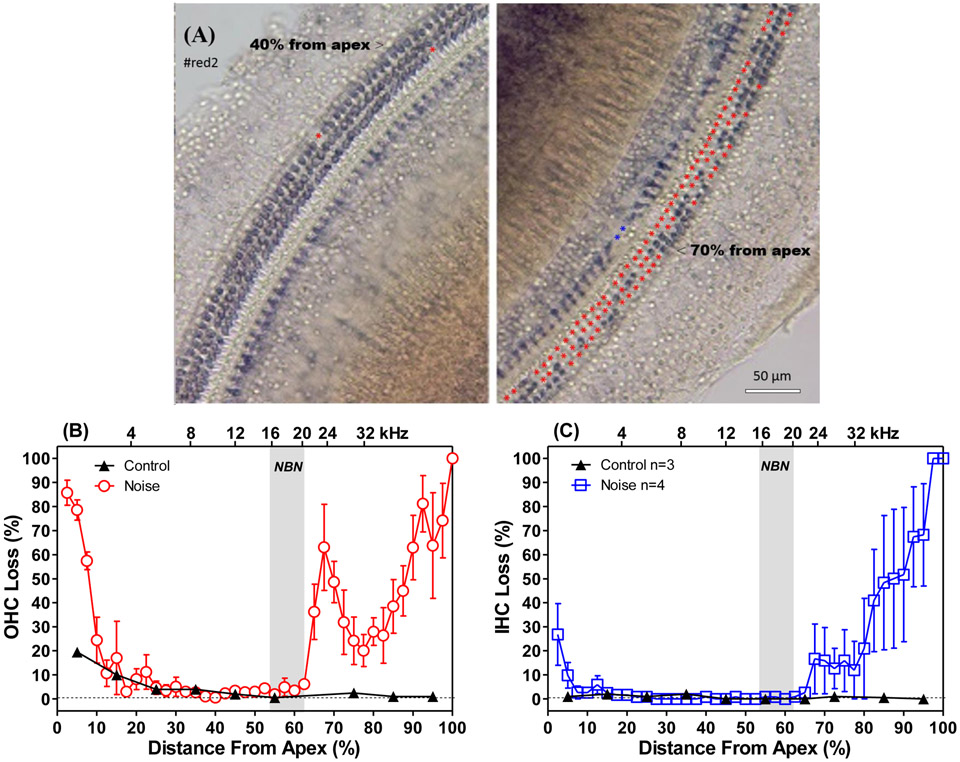
Figure 5:
Noise-induced hair cell loss. (A) Representative microscopy images of the cochlea surface preparation at the location of ~40% from the apex below the noise band (left) and the location of ~70% from the apex in the noise trauma area; The red and blue asterisks mark missing OHCs and IHCs respectively. (B) Mean (±SEM) cochleograms showing OHC loss; and (C) IHC loss in the rats used for the behavioral study (n=4) and age-matched controls (n=3). Shaded area shows 16-20 kHz noise-exposure.
Statistical analysis:
GraphPad Prism (version 5) was used for the graphical presentation and statistical analyses of the behavioral and physiological changes as described below. For behavioral audiograms, a two-way repeated measures ANOVA comparing effects of time (before, during, or post-noise exposure) and sound frequency (4,16,20,26 kHz), with Bonferroni post-hoc analysis corrected for multiple comparisons, was conducted to determine any significant threshold shifts. For RT testing, a two-way repeated measures ANOVA comparing effects of time (before, during, or post-noise exposure) and sound intensity, with Bonferroni post-hoc analysis corrected for multiple comparisons, was conducted to determine significant changes to RT-I functions for each frequency. For electrophysiological studies, two-way ANOVA for condition (control vs. noise-exposed) and intensity, with Bonferroni post-hoc analysis corrected for multiple comparisons, was conducted to determine significant differences in evoked response functions for each frequency.
RESULTS
Noise-induced hearing loss:
Behavioral thresholds were obtained from four rats; thresholds were measured before, during, and approximately 2 months after the noise exposure. The mean pre-exposure thresholds were 10.1 dB SPL (2.9 dB standard error of the mean, SEM), 10.8 dB SPL (2.6 dB), 2.1 dB SPL (0.8 dB) and 2.2 dB SPL (2.5 dB) at 4, 16, 20, and 26 kHz respectively, values similar to those reported previously (Gourevitch and Hack, 1966). The magnitude of the threshold shifts resulting from the 16-20 kHz band of noise presented at 104 dB SPL were determined by computing the difference between each rat’s pre-exposure threshold at 4, 16, 20, and 26 kHz with those measured during the exposure and 2 months post-exposure. The mean threshold shifts (Figure 2, n=4, ±SEM) for frequencies within the noise band (20 and 26 kHz) were approximately 70 dB during the exposure, whereas those measured 2 months post-exposure were 60 dB. At 16 kHz, the threshold shift was approximately 33 dB during and 2 months after the exposure. There was no threshold shift during or 2 months after the noise exposure at 4 kHz, two-octaves below the noise exposure. Two-way repeated-measures ANOVA between threshold shifts during-noise (red wide bars) and post-exposure (blue narrow bars) showed a significant main effect of noise condition (F=18.6; Df=1,12; ηp2 = 0.59; **p=0.001) and the Bonferroni posttests showed significant difference at 20 and 26 kHz (see the asterisks), indicating post-noise recovery. These results are consistent with previous studies showing that the maximum hearing loss occurs approximately a half-octave above the noise exposure (Salvi et al., 1983).
Figure 2:
Mean (±SEM, n=4) threshold shifts measured during the noise exposure (red wide bar) and 2-months post-exposure (blue narrow bar). Thresholds at 20 and 26 kHz wer elevated approximately 70 dB during the noise exposure and about 60 dB 2-months post-exposure, differing from each other significantly (** p<0.01; *** p<0.001). No threshold shift occurred at 4 kHz, 2-ocataves below the exposure. Gray area shows location of the 16-20 kHz noise-exposure. The pre-noise thresholds are presented on the top.
Noise-Induced Loudness Recruitment and Loudness Hyperacusis:
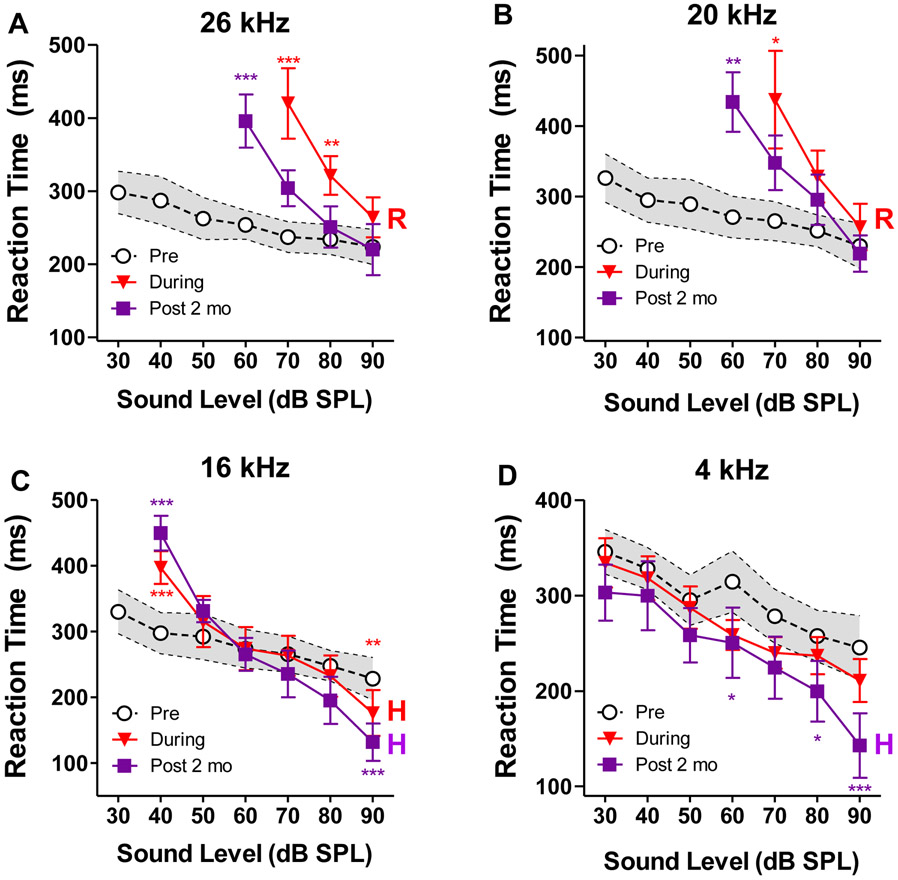
Figure 3A shows the mean (n=4) RT-I functions at 26 kHz before the noise exposure (black open circles ±SEM), during the noise exposure (red filled inverted triangles, ±SEM) and 2 months post-exposure (purple filled squares, ±SEM). The pre-exposure RTs decreased from 298 ms at 30 dB SPL to 226 ms at 90 dB SPL. RTs measured during the noise exposure could only be measured at high intensities due to the hearing loss. The mean RTs declined from 420 ms at 70 dB to 220 ms at 90 dB SPL. Two way repeated measures ANOVA between pre-exposure (black open circles) and during-noise (red filled inverted triangles) RT-I functions in the intensity range of 70-90 dB SPL showed a significant main effect of noise condition (F=49.7; Df=1,12; ηp2 = 0.61; ***p<0.0001) and intensity (F=4.20; Df=2,12; ηp2 = 0.41; *p=0.0414), as well as an interaction between the two (F=8.13; Df=2,12; ηp2 = 0.34; **p=0.0059). Bonferroni posttests showed significant difference at 70 and 80 dB SPL (see asterisks). The 26 kHz RT-I function was recruitment-like (R), i.e., steeper slope and nearly normal RTs at 90 dB SPL. The 26 kHz RT-I functions measured 2 months post-exposure was measured over a slightly greater range of intensities than during the exposure because of a slight post-exposure recovery of threshold (Fig. 2). Two way repeated measures ANOVA between pre-exposure (black open circles) and post-noise (purple filled squares) RTs in the intensity range of 60-90 dB SPL showed a significant main effect of noise condition (F=19.8; Df=1,16; ηp2 = 0.28; ***p = 0.0004) and intensity (F=4.01; Df=3,12; ηp2 = 0.43; *p=0.0263), as well as an interaction between the two (F=6.68; Df=3,12; ηp2 = 0.28; **p=0.0039). Bonferroni posttests showed significant difference at 60 dB SPL (see asterisks). While the post-exposure 26 kHz RT-I function had the same recruitment-like slope as the function taken during the exposure, there was more substantial recovery of RTs at higher intensities (e.g. 80-90 dB). It is possible that if we had measured RT at higher stimulus intensities we would have uncovered a hyperacusis-like response for high-intensity 26 kHz tones.
Figure 3:
Mean (±SEM, n=4) reaction time-intensity (RT-I) functions used to assess loudness growth at (A) 26 kHz, (B) 20 kHz, (C) 16 kHz and (D) 4 kHz . Pre: Pre-exposure data showing the mean (dashed line) and 95% confidence interval (shaded area); During: Mean (±SEM) RT-I functions obtained during the noise exposure; Post 2 Mo: Mean (±SEM) RT-I functions obtained 2-months post-exposure. During the noise-exposure and 2-months post-exposure, the RT-I functions at 26 and 20 kHz were classified as predominantly recruitment-like (R). The RT-I functions at 16 and 4 kHz 2-months post-exposure were classified as hyperacusis-like (H) because the RTs were significantly shorter than the controls at high stimulation levels. * p<0.05; ** p<0.01; *** p<0.001.
The RT-I functions measured at 20 kHz (Figure 3B) during the noise exposure was also recruitment like (R), i.e., steeper slope, but essentially normal RT at 90 dB SPL. Two way repeated measures ANOVA between the during-noise RTs (red filled inverted triangles) and the pre-exposure RTs (black open circles) in the intensity range of 70-90 dB SPL showed a significant main effect of noise condition (F=13.3; Df=1,12; ηp2 = 0.40; **p = 0.0034) but not intensity (F=3.12; Df=2,12; ηp2 = 0.38; p=0.0555), and no statistically significant interaction between the two (F=2.85; Df=2,12; ηp2 = 0.22; p=0.0969). Bonferroni posttests showed significant difference at 70 dB SPL (see asterisks). As with the 26 kHz RT-I functions, there was similar recruitment-like (R) slopes for 20 kHz RT-I functions at 2-months post-exposure but greater recovery of RTs at high intensities. Two way repeated measures ANOVA between the post-noise RTs (purple filled squares) and the pre-exposure RTs (black open circles) in the intensity range of 60-90 dB SPL showed a significant main effect of noise condition (F=11.1; Df=1,16; ηp2 = 0.37; **p = 0.0043) and intensity (F=5.60; Df=3,12; ηp2 = 0.51; **p=0.0081), but the interaction between the two did not quite reach significance (F=3.03; Df=3,12; ηp2 = 0.33; p=0.0599). Bonferroni posttests showed significant difference at 60 dB SPL (see asterisks).
Because the noise-exposure caused less hearing loss at 16 kHz, the RT-I functions could be measured over a greater range of intensities during and after the exposure. The RTs at 40 dB SPL were significantly longer than normal during and after the exposure while those at 50 dB SPL were largely unchanged (Fig. 3C). During the noise-exposure, the mean RTs from 60-80 dB were within the 95% confidence interval, but at 90 dB SPL, the mean RT was below 95% confidence interval. The two way ANOVA between the during-noise RTs (red filled inverted triangles) and the pre-exposure RTs (black open circles) in the intensity range of 40-90 dB SPL did not show a significant main effect of noise condition (F=3.0; Df=1,24; ηp2 = 0.07; p=0.097), but there was a significant effect of intensity (F=2.81; Df=5,24; ηp2 = 0.37; *p=0.0389) and a highly significant interaction between noise condition and intensity (F=16.4; Df = 5,24; ηp2 = 0.13; ***p < 0.0001). Bonferroni posttests showed significant difference at 40 and 90 dB SPL (see asterisks) reflecting reduced hearing and subtle evidence of hyperacusis (H) respectively. At 2-months post-exposure, the 16 kHz RTs were much longer than normal at 40 dB SPL, but because the slope of the function was extremely steep, the RT at 50 dB SPL had recovered to normal. Interestingly, with further increases in intensity, the RTs fell well below pre-noise levels, features consistent with hyperacusis (H). The shorter than normal RTs at high intensities are difficult to attribute to a generalized RT shortening due to a practice effect because the 16 kHz RTs measured at low intensities were longer than normal. Two way ANOVA between the post-noise RTs (purple filled squares) and the pre-exposure RTs (black open circles) in the intensity range of 40-90 dB SPL did not show a significant main effect of noise condition (F=0.004; Df=1,24; ηp2 < 0.0001; p=0.95), but there was once again a significant effect of intensity (F=6.86; Df=5,24; ηp2 = 0.59; ***p=0.0004) and a highly significant interaction between noise condition and intensity (F=13.0; Df = 5,24; ηp2 = 0.37; ***p < 0.0001). Bonferroni posttests showed significant difference at 40 and 90 dB SPL (see asterisks) reflecting reduced hearing and hyperacusis respectively.
Since the threshold at 4 kHz was unaffected by the 16-20 kHz exposure, the RT-I functions could be measured over the full 30-90 dB range before, during and after the noise exposure (Figure 3D). During the noise exposure, 4 kHz RTs remain relatively stable compared to pre-noise RT-I functions. However, at 2-months post-exposure, RTs became significantly shorter than pre-noise levels, particularly at higher intensities. Two way ANOVA between the post-noise RTs (purple filled squares) and the pre-exposure RTs (black open circles) in the intensity range of 30-90 dB SPL showed a significant main effect of noise condition (F=25.6; Df=1,28; ηp2 = 0.07; ***p<0.0001) and intensity (F=3.31; Df=6,28; ηp2 = 0.42; *p = 0.0137) but no significant interaction (F=1.04; Df=6,28; ηp2 = 0.05; p = 0.4246). Bonferroni posttests showed significant difference at 60, 80 and 90 dB SPL (see asterisks). The post-exposure shortening of high-intensity RTs suggests that the 4 kHz sounds had become louder than normal (i.e., hyperacusis, H).
To summarize, during the noise exposure, RT-I functions at 20 and 26 kHz were recruitment-like, the 16 kHz RT-I was also recruitment-like with subtle evidence of hyperacusis at 90 dB SPL where the RT was shorter than normal, the 4 kHz function was unchanged. At 2-months post-exposure, the RT-I functions at 20 and 26 kHz were predominantly recruitment-like. Importantly, the 16 kHz RT-I functions showed evidence of hyperacusis at higher intensities while at 4 kHz there was evidence of hyperacusis from even lower stimulation levels. Thus, during the exposure, the first subtle hint of hyperacusis emerged at 16 kHz at 90 dB SPL, but by 2-months post-exposure, the hyperacusis had become more pronounced at 4 and 16 kHz. Taken together, these results are suggestive of a low-frequency hyperacusis spectral profile.
Decreased Cochlear Output:
To determine the extent to which the noise exposure altered the neural output of the cochlea, CAP input/output functions were measured in the four behaviorally-trained rats approximately 3-months post-exposure (~20 months of age). The CAP measurements were obtained from these four rats immediately after completing electrophysiological recordings from their AC (see below). The CAP data from the four behaviorally trained, noise-exposed rats were compared to 8 normal controls. In the Noise group, CAP amplitudes at 24, 20, and 16 kHz were far below the 95% confidence interval of the Control group (Figure 4A-C). The I/O functions in the Noise group were also shifted to the right of Controls approximately 50 dB and the slopes of the functions at 20 and 24 kHz were shallower than normal. CAP amplitudes were also reduced compared to the Control group at 12, 8 and 4 kHz, but the reductions were less than those at high frequencies. The rightward shifts of the I/O functions at 12, 8 and 4 kHz were roughly 15-20 dB, much less than at higher frequencies (Figure 4D-F). Two way ANOVA between the noise-group (red filled circles) and the control group (black open circles) showed significant main effects of noise condition (F=167.6, 240.5, 238.4, 237.6, 379.3, and 610.3 for 4, 8, 12, 16, 20 and 24 kHz respectively; Df=1,170; ***p<0.0001) and the Bonferroni posttests showed significant difference at multiple sound levels (see the asterisks).
Figure 4:
Mean compound action potential (CAP) amplitudes greatly reduced in the noise- exposed group (red filled circles, ±SEM, n=4) 3 months post-exposure compared to the controls (black open circles, ±95% confidence interval, n=8). (A) 24 kHz, (B) 20 kHz, (C) 16 kHz, (D) 12 kHz, (E) 8 kHz and (F) 4 kHz.
Noise-induced hair cell loss:
To quantify the hair cell loss resulting from the noise exposure, the cochleae from the 4 behaviorally trained rats and 3 age-matched, untrained control rats were dissected out (Fig. 5A) and the cochleograms were prepared (Fig. 5B and C). Figure 5A presents example of cochlea surface preparation of a noise-exposed and behavioral trained rat (#red2) showing rarely loss of hair cells in the region 40% from the apex (left) and great outer hair cell loss (red asterisks) in the region 70% from the apex (right). In the noise-exposed group, the mean OHC losses (±SEM) in the high-frequency, basal third of the cochlea ranged from 30-90%. The mean OHC loss corresponding to the 24 kHz region was ~60%. Substantial OHC loss was evident in the extreme apex, but the loss quickly dropped to less than 10% at the 15-65% locations (Fig. 5B). IHC losses in the Noise group decreased from 100% at the extreme base to 20% at the 32 kHz cochlear location. There was also 25% IHC loss in the extreme apex. In the Controls, mean (n=3) OHC losses were negligible except in the apex where the average loss was approximately 20% in the apical 10% of the cochlea. There was little IHC loss in the Control (Figure 5C) consistent with previous reports (Keithley and Feldman, 1982).
Noise-Induced Changes in AC versus Cochlea:
It is clear that major disparities exist between the behavioral measures of loudness growth and neural output of the cochlea. In the region of high-frequency hearing loss, post-exposure CAP amplitudes were reduced by ~90% at 80 dB SPL (Fig. 4A-B). Despite this massive neural deficit in the cochlea measured after the behavioral measurement, behavioral measures of loudness were normal at 80-90 dB SPL because of loudness recruitment (see the purple filled squares in Fig. 3A-B). At 4 and 16 kHz, post-exposure CAP amplitudes were reduced ~65% at 80 dB SPL (Fig. 4C, F); nevertheless, these sounds were perceived as louder than normal due to steepening of the loudness growth functions (see the purple filled squares in Fig. 3C-D). It has been suggested that enhanced central gain could boost these weak cochlear signals to normal levels leading to recruitment or over-amplification of neural responses, resulting in hyperacusis (Gu et al., 2010, Diehl and Schaette, 2015, Auerbach et al., 2019). To test this hypothesis, we measured the gross neural output from major AC subdivisions (A1, AAF, VAF) in response to tone-bursts (50 ms) presented over a broad range of frequencies and intensities. AC recordings were obtained from the 4 behaviorally-trained, noise-exposed rats and eight age-matched controls. To gain a global perspective, a PSTH was constructed from each MUC at each frequency-intensity combination. All of the PSTHs for a specific frequency-intensity combination were then averaged together from all the animals in each experimental group to form a grand mean population PSTH for each frequency-intensity combination (Chen et al., 2016). The population PSTHs show the average discharge rates from all MUCs in the Noise group (n=330 units, 4 animals) and Control group (n=813 units, 8 animals) as function of time following stimulus onset (see Fig. 6A-H).
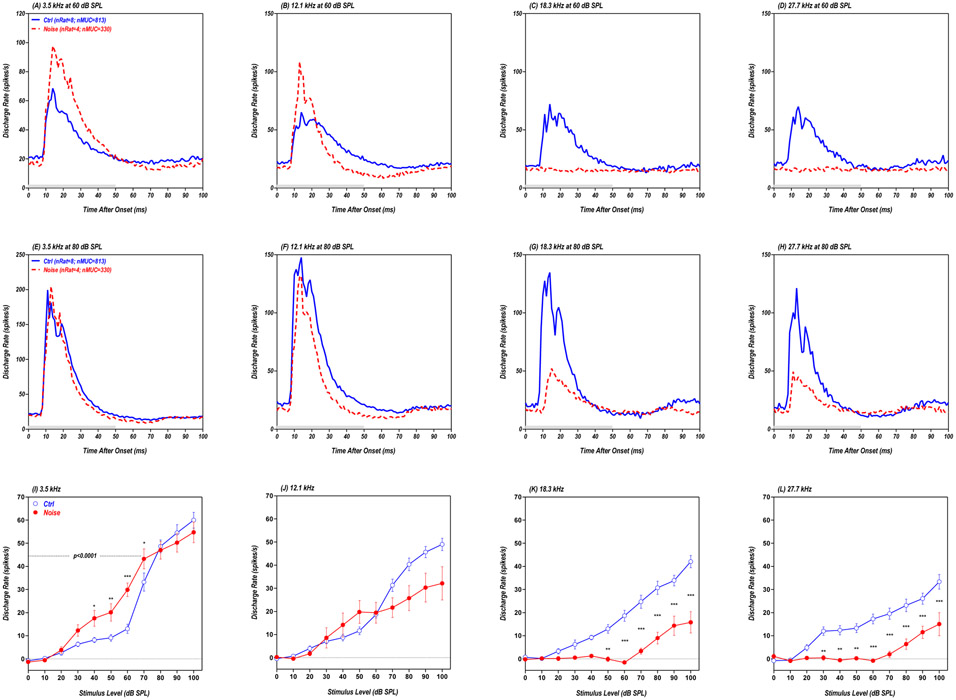
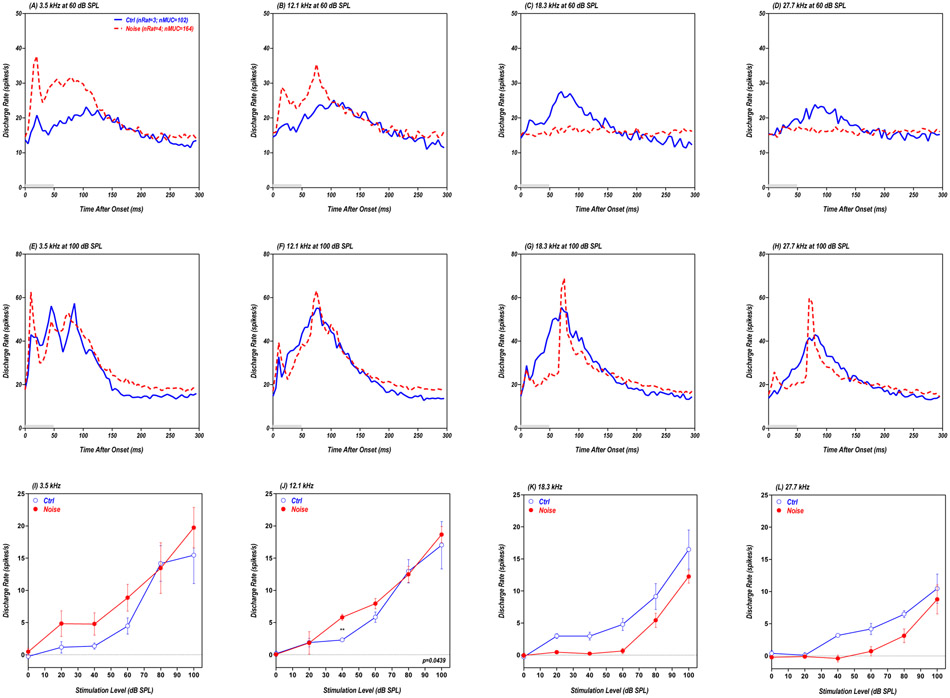
Figure 6:
(A-H) Grand mean population PSTHs (bin width: 1 ms) from AC showing the average discharge rate of 330 MUCs from four noise-exposed rats (red dashed line) and 813 MUCs from eight control rats (blue solid line). Data shown for 50 ms tone bursts (gray bar) presented at 3.5, 12.1, 18.3 or 27.7 kHz at 60 dB SPL (A-D, top row) or 80 dB SPL (E-H, middle row). (I-L) Mean (±SEM) population driven discharge rates vs. intensity shown for 3.5, 12.1, 18.3 and 27.7 kHz; mean values calculated over 50 ms time window (5-55ms window) of individual PSTHs. * p<0.05; ** p<0.01; *** p<0.001.
The AC population PSTHs from the Noise group obtained at 3.5 kHz and 60 dB SPL (red dashed line in Fig. 6A) exhibited clear evidence of sound-evoked hyperactivity compared to the Control group (blue solid line in Fig. 6A). For the 12.1 kHz stimulus presented at 60 dB SPL, the population PSTH from the Noise group (red dashed line in Fig. 6B) exhibited a more robust onset response, but activity declined more rapidly than that from the Control group (blue solid line in Fig. 6B). At higher frequencies, sound-evoked responses from the Noise group were weak or largely absent at 18.3 and 27.7 kHz (Fig. 6C-D) due to the reduced cochlear outputs (Fig. 4A-C). At 80 dB SPL, the AC population PSTHs elicited at 3.5 kHz were nearly identical for the Noise group and Control group (Fig. 6E) and slightly smaller at 12.1 kHz (Fig. 6F). However, the population PSTHs from the Noise group were much smaller than those from the Control group at 18.3 and 27.7 kHz (i.e., hypoactive). To quantify these results, the driven discharge rates were computed from 5-55 ms from the PSTHs for each MUC in the Noise group (n=330) and Control group (n=813); the mean driven discharge rate was then plotted as a function of intensity for each group. At 3.5 kHz, the mean driven discharge rates in the Noise group were consistently higher than in the Control group from 30 to 70 dB SPL (Fig. 6I); however, the rate-level functions largely overlapped at the higher intensities. Thus AC hyperactivity was only seen in the Noise group at intermediate intensities. Two way ANOVA between the noise group (n = 4; red filled circles) and the control group (n =8; blue open circles) in the intensity range of 0-70 dB SPL showed a significant main effect of noise condition (F=35.2; Df=1,80; p<0.0001) and the Bonferroni posttests showed significant difference at 70, 60, 50, and 40 dB SPL (see the asterisks). At 12.1 kHz, mean discharge rates in the Noise group were slightly higher than the Control group from 40-50 dB, but substantially less at higher intensities (Fig. 6J). Two way ANOVA between the noise group (n = 4; red filled circles) and the control group (n = 8; blue open circles) in the intensity range of 0-70 dB SPL did not show a significant main effect of noise condition (F=0.148; Df=1,80; p=0.7017). At 18.3 and 27.7 kHz, the driven discharge rates were much less in the Noise group than in Controls at suprathreshold levels (Fig. 6K-L). Two way ANOVA between the noise group (n = 4; red filled circles) and the control group (n = 8; blue open circles) showed a significant main effect of noise condition (F=168.2 and 132.6 for 18.3 and 27.7 kHz respectively; Df=1,110; p<0.0001) and the Bonferroni posttests showed significant differences at multiple sound levels (see the asterisks).
Noise-Induced Changes in LA:
Because the amygdala has been implicated in loudness perception and hyperacusis (Moller and Rollins, 2002, Levitin et al., 2003, Moller, 2007), we exposed a second group of rats to the same 16-20 kHz noise and looked for evidence of sound-evoked hyperactivity in the LA, a region that becomes hyperactive during salicylate-induced hyperacusis (Chen et al., 2012, Chen et al., 2015). PSTHs were constructed for each MUC in the Noise group (n=164, 4 rats) and Control group (n=102, 3 rats). These data were then averaged to generate a grand mean population PSTH for each frequency-intensity combination (Chen et al., 2016).
Figure 7A shows LA population PSTHs in response to a 60 dB SPL, 3.5 kHz tone burst. Consistent with previous results, the population PSTH to 50-ms tone burst had an early onset peak followed by a prolonged response lasting 150-200 ms (Chen et al., 2012, Chen et al., 2016). Both the onset and sustained portions of the population PSTH were substantially larger in the Noise group (red dashed line) compared to the Control group (blue solid line). PSTH results similar to this were seen in the Noise group with the 12.1 kHz, 60 dB SPL stimulus (Fig. 6B). In the Control group, the population PSTHs evoked by 18.3 and 27.7 kHz tone bursts presented at 60 dB SPL showed a gradual buildup of activity with a peak around 80 ms followed by a gradual decay (Fig. 7C-D). In contrast, the 60 dB SPL stimuli failed to evoke a driven response in the Noise group presumably due to the loss of cochlear output. When the intensity was increased to 100 dB SPL, robust sound-driven responses were observed in both the Noise group and Control group across stimulus frequency. The LA population PSTHs in response to 3.5 kHz were characterized by a series of peaks riding on broad plateau lasting roughly 130 ms (Fig. 7E). The first peak was noticeably larger and the duration of the response slightly longer in the Noise group (re dashed line) compared to the Control group (blue solid line). The LA population PSTHs in the Control group evoked by 12.1, 18.3 and 27.7 kHz were characterized by a gradual buildup to a peak around 80 ms followed by a gradual decay resulting in a broad response. At 12.1 kHz, the LA population PSTH in the Noise group was similar to the Control group (Fig. 7F). However, at 18.3 and 27.7 kHz, the PSTHs in the Noise group (red dashed lines) were qualitatively different from the Controls. The Noise group PSTHs were characterized by a small but clearly defined onset response followed by a narrower but larger peak than the control around 80 ms (Fig. 7G-H).
Figure 7:
(A-H) Grand mean population PSTHs (bin width: 5 ms) from LA showing the average discharge rate for 164 MUCs from four noise-exposed rats (red dashed line) and 102 MUCs from three control rats (blue solid line). Data shown for 50 ms tone bursts (gray bar) presented at 3.5, 12.1 kHz, 18.3 and 27.7 kHz at 60 dB SPL (A-D, top row) or 100 dB SPL (E-H, middle row). (I-L) Mean (±SEM) population driven discharge rate vs. intensity shown for 3.5, 12.1, 18.3 and 27.7 kHz; mean values calculated over 200 ms time window (0-200ms) from individual PSTHs. ** p<0.01.
To characterize the overall changes in neural activity, the LA driven discharge rates were computed over the first 200 ms of the PSTH for each MUC in the Noise group (n=164) and Control group (n=102). The mean driven discharge rates for each group were then plotted as a function of intensity. At 3.5 kHz, the mean driven discharge rates in the Noise group were consistently higher than those in the Control group except at 80 dB SPL (Fig. 7I). Two way ANOVA between the noise group (n = 4; red filled circles) and the control group (n =3; blue open circles) in the intensity range of 0-60 dB SPL showed a significant main effect of noise condition (F=8.2; Df=1,20; p<0.01). However, the firing rates in the Noise group evoked by 12.1 kHz were only higher than Controls at 40 dB SPL. Two way ANOVA between the noise group (n = 4; red filled circles) and the control group (n =3; blue open circles) in the intensity range of 0-60 dB SPL showed a significant main effect of noise condition (F=4.6; Df=1,20; p<0.05). In contrast, the driven discharge rates at 18.3 and 27.7 kHz were noticeably less in the Noise group than in Controls.
DISCUSSION
Delayed Onset and Spectral Profile of Hyperacusis:
RT-I functions have been used for many years to study normal and abnormal growth of loudness in humans and animals (Stebbins, 1966, Pfingst et al., 1975, Dooling et al., 1978, Marshall and Brandt, 1980, Lauer et al., 2007, May et al., 2009, Radziwon et al., 2017). Intense noise exposures consistently induce loudness recruitment, but we are unaware of any studies that have shown that intense, long-duration noise exposures evoke hyperacusis. To determine if hyperacusis could be induced by long duration noise exposures, we measured RT-I functions before, during and after exposing rats to a 104 dB SPL, 16-20 kHz noise. During the 12-week noise exposure, we only found evidence of loudness recruitment in the region of maximum hearing loss, 20 and 26 kHz. At the border of the hearing loss, 16 kHz, the RT-I function was mostly recruitment-like, but at 90 dB SPL, the RTs were shorter than normal suggestive of hyperacusis at high intensities. RT-I functions were unchanged at 4 kHz, below the hearing loss, consistent with previous reports (Moody, 1973).
Unexpectedly, robust signs of hyperacusis emerged 2-months post-exposure. At 4 as well as 16 kHz, RTs were consistently shorter than normal at high stimulation levels. Thus, hyperacusis emerged at 4 kHz where thresholds were normal and at the edge of the hearing loss (16 kHz) where thresholds were elevated ~30 dB. Thus, one of our major new findings is that prolonged exposure to intense high-frequency noise leads to hyperacusis. Interestingly, hyperacusis-like behavioral changes were most evident at lower frequencies where hearing loss was minimal and began to emerge at low intensities around 60 dB SPL. It is unclear why hyperacusis became more pronounced and widespread at 2-months post-exposure, but its delayed onset at the lower frequencies and intensities may reflect homeostatic changes that lead to extensive modifications of neural circuits in the central auditory pathway.
While this is the first study to our knowledge to demonstrate persistent low frequency hyperacusis as a result of high-frequency noise exposure, our findings are reminiscent of the acoustic startle response results reported extensively by Ison and Willott in a mouse model of hereditary high-frequency hearing loss (Willott, 1984, Willott et al., 1994, Willott and Carlson, 1995, Carlson and Willott, 1996, Ison and Allen, 2003, Ison et al., 2007). Those studies found that as age-related high-frequency hearing loss progressed in their mouse model (C57BL/6J), low to mid-frequency tones provided greater inhibition of the acoustic startle reflex as assessed in prepulse inhibition (PPI) experiments, likely reflecting tonotopic reorganization in the inferior colliculus and auditory cortex as a result of basal cochlear pathology (Carlson and Willott, 1996). These studies, in addition to our current findings, suggest that aging and intense noise exposure may share common pathophysiological mechanisms in the development of hyperacusis, particularly for low frequency sounds.
Loudness Recruitment:
Sensorineural hearing loss induced by intense noise, ototoxic drugs and aging is generally accompanied by loudness recruitment (Fowler, 1937b, Moody, 1973, Feitosa, 1981, Knight and Margolis, 1984). In animal studies of noise-induced hearing loss, loudness recruitment was restricted to frequencies where hearing loss had developed. Moreover, in cases of unilateral hearing loss, recruitment was only present in the noise-exposed ear suggesting separate loudness processing channels for each ear (Moody, 1973). Our behavioral data are generally consistent with previous human and animal studies. During the noise exposure, we observed clear evidence of loudness recruitment at 20 and 26 kHz (Fig. 3A-B), where hearing loss would be greatest, but no change in loudness growth at 4 kHz, below the region of hearing loss.
Hyperacusis:
Loudness hyperacusis, a condition in which sound are perceived as louder than normal, is associated with a number of clinical disorders including hearing loss (Banerjee et al., 2005, Geisser et al., 2008, Zarchi et al., 2015, Paulin et al., 2016). Some studies have found that hyperacusis is linked to hearing loss based on the fact that loudness discomfort levels (LDLs) are significantly lower among hyperacusis patients with mild high-frequency hearing loss than those with normal hearing (Sheldrake et al., 2015). On the other hand, many patients with a primary complaint of hyperacusis have clinically normal hearing (Sheldrake et al., 2015). This suggests that hearing loss is not a necessary condition to cause loudness intolerance problems.
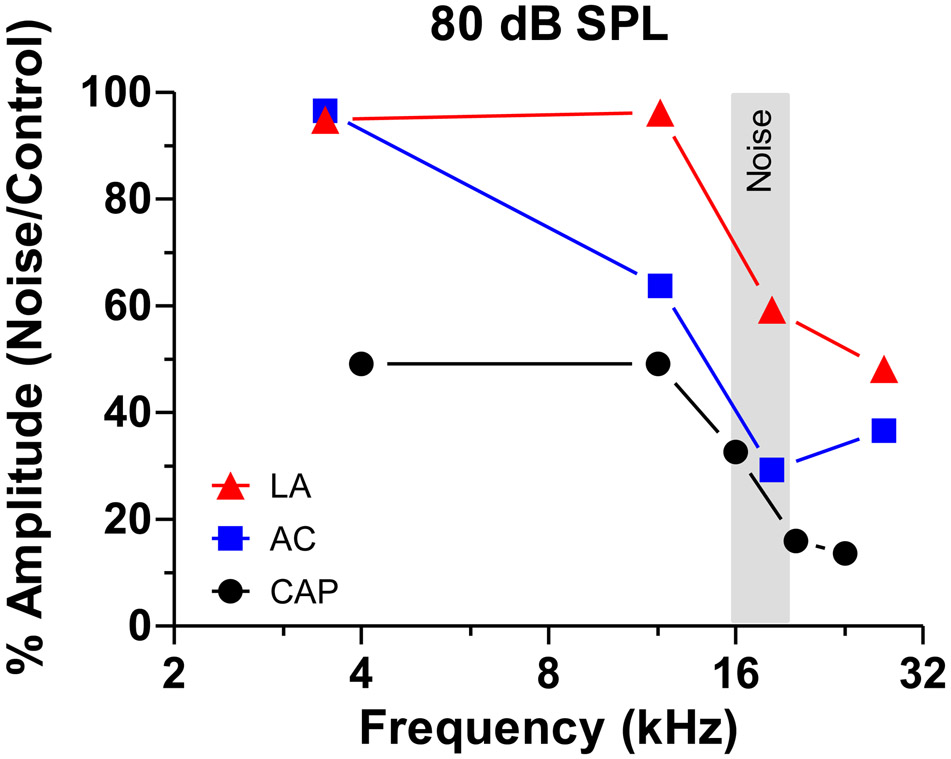
Some have reported that LDLs are decreased over a broad frequency range after ear plugging (Formby et al., 2003) and in hyperacusis patients (Sheldrake et al., 2015). This has led to speculation that hyperacusis arises from a global, frequency-independent increase in central auditory gain (Gu et al., 2010). Our results based on detailed pre- and post-exposure loudness growth measurement provide some relevant insights. We observed strong evidence for hyperacusis at 4 kHz at intensities from 60-90 dB SPL. Because hearing thresholds were normal at this frequency, this would support the view that hyperacusis can occur at frequencies with an absence of hearing loss. Hyperacusis was also present at 16 kHz where thresholds were elevated 30 dB, and potentially for high intensities at 20 and 26 kHz where thresholds were elevated 60 dB. Based on these results, one could argue that hyperacusis is associated with a generalized increase in central gain; however, our results suggest that the gain is greater at the lower frequencies where there is little or no hearing loss. This interpretation is partially supported by the electrophysiological data showing greater gain enhancement at the low frequencies in subjects with high-frequency hearing loss. At the lower frequencies, our suprathreshold neural responses were much larger in the AC and LA than in the cochlea (Fig. 8). Our electrophysiological data, however, fall far short of accounting for the post-exposure changes in loudness growth functions. There was strong evidence for hyperacusis at 4 and 16 kHz at 80 dB SPL, yet the gross neural output of the AC and LA in the Noise group was either equal to or below the values in the Control group (Fig. 8). While the neural responses from the AC and LA of the Noise group were proportionately much larger than those from the cochlea, they were still only 30-60% of the values seen in the normal Control group at the high frequencies (Fig. 8). It has to be pointed out that LA-response in the noise-group showed higher peak than the controls at high frequencies at 100 dB SPL (Fig. 7G,H) as well as at 80 dB SPL (data not shown) in spite of the lower mean discharge rate (Fig. 7K,L). Altogether, these results suggest that in cases of severe high-frequency hearing loss, there is insufficient amplification along the auditory pathway to fully compensate for the cochlear deficits as previously noted (Qiu et al., 2000, Salvi et al., 2016).
Figure 8:
Relative amplitude (%) of Noise group and Control group as function of frequency. Data shown for CAP, AC and LA responses collected at 80 dB SPL. Response amplitudes in the LA and AC are proportionately much larger than for the CAP, indicated of central gain enhancement.
Sound Deprivation vs. Enrichment:
Bilateral conductive hearing loss, a form of sensory deprivation, can induce a modest (~7 dB) increase in loudness following ear plug removal (Formby et al., 2003). Unlike sound deprivation caused by unilateral noise exposure (Moody, 1973), monaural ear plugging not only caused a modest loudness increase in the plugged ear, but also the unplugged, contralateral ear (Munro et al., 2014), results suggesting some form of binaural crosstalk (Scharf and Fishken, 1970). In the ear plugging studies, the changes in loudness enhancement extended equally to lower frequencies where plugging provided less sound attenuation, results interpreted as evidence for broadband amplification possibly due to a reduction in lateral inhibition (Wang et al., 1996, Milbrandt et al., 2000, Salvi et al., 2000b).
Sound enrichment, a widely used treatment for tinnitus and hyperacusis (Formby, 2002), has the opposite effect of deprivation. Two-weeks of continuous sound stimulation, which raised thresholds 25-35 dB in the 24 kHz region, resulted in a ~6 dB increased LDLs after discontinuing stimulation (Formby et al., 2003). LDLs also increased at both low and high frequencies, a result interpreted as evidence for a generalized increase in centrally gain. Recent electrophysiological studies in which cats underwent prolonged, low-level sound exposure revealed suppression of sound-evoked neural response in the AC largely confined to frequencies within the noise band, but enhanced sound-evoked response near the edges of the noise band (Pienkowski et al., 2011). Similar experiments in rats revealed a frequency-specific enhancement of sound-evoked activity in the inferior colliculus; however, the enhancement, which was level dependent, mainly occurred at moderate intensities (Sheppard et al., 2017, Sheppard et al., 2018).
Central Gain is Insufficient to Account for either Hyperacusis of Loudness Recruitment:
In previous studies of salicylate-induced hyperacusis (Chen et al., 2015, Jiang et al., 2017, Auerbach et al., 2019), we found clear evidence of sound-evoked hyperactivity in the AC and LA that was correlated with our RT-I behavioral measure of hyperacusis (Radziwon et al., 2017). Therefore, we predicted that noise-induced hyperacusis would be associated with sound-evoked hyperactivity in the AC and LA. The CAP, which reflects the gross neural output of the cochlea, was greatly reduced at suprathreshold levels, substantially more so at high frequencies than lows (Fig. 8). The hypoactive output of the cochlea was counteracted by varying degrees of enhanced central gain in the population PSTHs from the AC and LA. At 4 kHz where there was robust behavioral evidence of hyperacusis from 60-90 dB SPL (Fig.3D), the gross neural output of the AC at 60-70 dB SPL was greater in the Noise group than in the Controls (Fig. 6I), consistent with our working hypothesis that the AC should be hyperactive. However, at 90-100 dB, the gross neural responses from Noise group was slightly less than in the Controls. In this intensity range, the AC hypoactivity in the Noise group contradicts our working hypothesis. The results from the LA showed further compensation, but the responses were still insufficient to account for the behavioral measures of loudness recruitment and hyperacusis (Fig. 7I, 8).
The discrepancies between our behavioral hyperacusis data and predicted changes in neural activity in the AC and LA were much greater at higher frequencies. There was clear evidence of hyperacusis at 16 kHz from 70-90 dB SPL (Fig. 3C). However, the neural output of the AC at the surrounding frequencies (12.1, 18.3 kHz) was substantially less in the Noise group than in Controls (Fig. 6J-K, 8). These hypoactive responses cannot account for the hyperacusis-like behavior at 16 kHz. In the LA, the suprathreshold neural responses at 12.1 kHz were similar in both the Noise and Control group (Fig. 7J), whereas at 18.3 kHz, the mean neural responses in the Noise group were smaller than in Controls (Fig. 7K), although there was an enhance peak at ~75 ms at 100 dB SPL. The absence of sound-evoked hyperactivity at these frequencies is at odds with 16 kHz hyperacusis. At 20 and 26 kHz, where there was clear evidence of loudness recruitment, the neural responses at 18.3 and 27.7 kHz were smaller (hypoactive) in both the AC (Fig. 6K-L) and LA (Fig. 7K-L). It is also possible that the change in LA responses shape (see Fig. 7g and H) could contribute to loudness recruitment at these frequencies (see Fig. 3A and B). Thus, with the exception of 4 kHz at moderate intensities, we find that the amount of central gain enhancement in AC and LA of the Noise group is insufficient to boost the gross neural output of these regions above those in Controls. Because the neural responses from the AC and LA of the Noise group are generally hypoactive compared to Controls, these measures of central gain fail to account for our RT-I measures of hyperacusis and loudness recruitment.
Considerations and Limitations:
We used RT-I functions to assess the growth of loudness and test for noise-induced loudness recruitment and hyperacusis as has been done in previous human and animal studies (Moody, 1970, Marshall and Brandt, 1980, Chen et al., 2014b, Radziwon et al., 2017). Because our post-exposure RTs were shorter than pre-exposure values, we argued here that the rats were experiencing hyperacusis. It could be argued that the shorter RTs observed 2-months post-exposure are simply the results of greater training on the task. However, we do not see a simple and consistent downshift in RTs. For high frequencies, the RTs were much longer than normal for lower level stimuli while at 16 kHz, the RTs were longer than normal for low level stimuli, unchanged at intermediate intensities and shorter than normal at high intensities. If our observed changes in RT were simply a matter of training, then we would expect a consistent downshift in RT regardless of frequency and intensity. Furthermore, in earlier studies of salicylate-induced ototoxicity and hyperacusis, we observed RT-downshifts 2-3-hours post-treatment, but with salicylate washout, RTs returned to normal (Chen et al., 2017, Radziwon et al., 2017). The salicylate-induced RT-downshifts and return to normal occurred over months of RT-I testing confirming the stability of the baseline RT-I measurements. Finally, to independently verify that shorter RTs were assessing noise-induced hyperacusis, we developed an active sound avoidance paradigm (ASAP) and used to assess a rat’s aversion to sounds of different intensity (Manohar et al., 2017). After the rats were exposed to the same noise used in the current study and tested 2-months later, they showed a much greater aversion to low-frequency sounds consistent with the current study. Altogether, these results support our contention that prolonged exposure to the 104 dB, 16-20 kHz noise induces hyperacusis.
There were several imitations to our electrophysiological studies. First, all our electrophysiological experiments were carried out on anesthetized rats; this makes it difficult to directly compare the neurophysiological results to data obtained from behaving animals performing the RT-I task. Second, we compared the electrophysiological data from the AC of the behaviorally-trained rats to untrained Control rats. Because behavioral training could conceivably alter neural responses in the AC, it would have been better to obtain our electrophysiological data for the Control group from a group of rats with the same amount of behavioral training. A more challenging optimization of the current study would involve chronic electrophysiological recording and behavioral measurement from the same animal pre- and post-exposure. Finally, the tone duration used in the physiological study was 50 ms with 1 ms rise/fall time, but the tone duration used in the behavior study was 300 ms with 5 ms rise/fall time. Therefore, the rats in the behavioral study perceived loudness based on long-duration stimulation. However, the physiological data mainly reflect the phasic responses.
SYNOPSIS:
Our results show for the first time that is possible to induce noise-induced hyperacusis by exposing rats to intense, high-frequency narrow band noise for 12-weeks. The most robust hyperacusis occurred 2-months post-exposure at the low-frequencies and near the border of the high-frequency hearing loss. The gross neural output from the cochlea was greatly reduced in the noise-exposed rats. By the time the neural signals reached the AC and LA in the Noise group, they had been substantially amplified relative to the cochlea. However, it appears that the magnitude of central gain enhancement in the Noise group is not enough to account for the observed perceptual changes, in contrast to earlier studies of salicylate-induced hyperacusis.
Acknowledgments
Funding: This work was supported by NIH grants to BA (F32DC015160) and RS (R01DC014693)
Abbreviations:
- AAF
anterior auditory field
- AC
auditory cortex
- CAP
compound action potential
- dB
deciBel
- IHC
inner hair cell
- I/O
input/output
- LA
lateral amygdala
- MGB
medial geniculate body
- MUC
multiunit clusters
- NBN
narrowband noise
- OHC
outer hair cell
- PSTH
peristimulus time histogram
- RT
reaction time
- RT-I
reaction time-intensity
- SEM
standard error of the mean
- SPL
sound pressure level
- VAF
ventral auditory field
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
REFERENCES
- Auerbach BD, Radziwon K, Salvi R (2019) Testing the Central Gain Model: Loudness Growth Correlates with Central Auditory Gain Enhancement in a Rodent Model of Hyperacusis. Neuroscience 407:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach BD, Rodrigues PV, Salvi RJ (2014) Central gain control in tinnitus and hyperacusis. Frontiers in neurology 5:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguley DM (2003) Hyperacusis. Journal of the Royal Society of Medicine 96:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Js Baizer, Wong KM, Manohar S, Hayes SH, Ding D, Dingman R, Salvi RJ (2015) Effects of acoustic trauma on the auditory system of the rat: The role of microglia. Neuroscience 303:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Whyte A, Atlas MD (2005) Superior canal dehiscence: review of a new condition. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 30:9–15. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Salvi RJ (1993) Functional changes in the ventral cochlear nucleus following acute acoustic overstimulation. The Journal of the Acoustical Society of America 94:2123–2134. [DOI] [PubMed] [Google Scholar]
- Buus S, Florentine M (2002) Growth of loudness in listeners with cochlear hearing losses: recruitment reconsidered. Journal of the Association for Research in Otolaryngology : JARO 3:120–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Ma WL, Young ED (2008) Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. Journal of the Association for Research in Otolaryngology : JARO 10:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S, Willott JF (1996) The behavioral salience of tones as indicated by prepulse inhibition of the startle response: relationship to hearing loss and central neural plasticity in C57BL/6J mice. Hearing research 99:168–175. [DOI] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB (2016) Central Gain Restores Auditory Processing following Near-Complete Cochlear Denervation. Neuron 89:867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Decker B, Krishnan Muthaiah VP, Sheppard A, Salvi R (2014a) Prolonged noise exposure-induced auditory threshold shifts in rats. Hearing research 317:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hearing research 265:63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D (2009) Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hearing research 248:39–47. [DOI] [PubMed] [Google Scholar]
- Chen GD, Manohar S, Salvi R (2012) Amygdala hyperactivity and tonotopic shift after salicylate exposure. Brain research 1485:63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Radziwon KE, Kashanian N, Manohar S, Salvi R (2014b) Salicylate-induced auditory perceptual disorders and plastic changes in nonclassical auditory centers in rats. Neural plasticity 2014:658741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Sheppard A, Salvi R (2016) Noise trauma induced plastic changes in brain regions outside the classical auditory pathway. Neuroscience 315:228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Stolzberg D, Lobarinas E, Sun W, Ding D, Salvi R (2013) Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hearing research 295:100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chen GD, Auerbach BD, Manohar S, Radziwon K, Salvi R (2017) Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress. Hearing research 349:208–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, Jiao Y, Zang FC, Radziwon K, Chen GD, Sun W, Krishnan Muthaiah VP, Salvi R, Teng GJ (2015) Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife 4:e06576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl PU, Schaette R (2015) Abnormal Auditory Gain in Hyperacusis: Investigation with a Computational Model. Frontiers in neurology 6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D, McFadden S, Salvi R (2001) Cochlear hair cell densities and inner-ear staining techniques Willott James F Handbook of Mouse Auditory Research CRS Press; Florida: 189–204. [Google Scholar]
- Dooling RJ, Zoloth SR, Baylis JR (1978) Auditory sensitivity, equal loudness, temporal resolving power, and vocalizations in the house finch (Carpodacus mexicanus). Journal of comparative and physiological psychology 92:867–876. [DOI] [PubMed] [Google Scholar]
- Feitosa AG, Moody DB, Stebbins WC (1981) Loudness recruitment in the dihydrostreptomycin-treated patas monkey. The Journal of the Acoustical Society of America 70:s27. [Google Scholar]
- Formby C, Gold SL (2002) Modification of loudness discomfort level: evidence for adaptive chronic auditory gain and its clinical relevance In: Seminars in Hearing, vol 23, pp 021–034: Copyright© 2002 by Thieme Medical Publishers, Inc, 333 Seventh Avenue, New York, NY 10001, USA. [Google Scholar]
- Formby C, Sherlock LP, Gold SL (2003) Adaptive plasticity of loudness induced by chronic attenuation and enhancement of the acoustic background. The Journal of the Acoustical Society of America 114:55–58. [DOI] [PubMed] [Google Scholar]
- Fowler EP (1937a) The diagnosis of diseases of the neural mechanism of hearing by the aid of sounds well above threshold. Laryngoscope (StLouis) 47:289–300. [Google Scholar]
- Fowler EP (1937b) The diagnosis of diseases of the neural mechanisms of hearing by the aid of sounds well above threshold. Am Otol Soc 27:207–219. [Google Scholar]
- Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH (2008) A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. The journal of pain : official journal of the American Pain Society 9:417–422. [DOI] [PubMed] [Google Scholar]
- Gourevitch G, Hack MH (1966) Audibility in the rat. Journal of comparative and physiological psychology 62:289–291. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR (2010) Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. Journal of neurophysiology 104:3361–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz MG, Issa Jb, Young ED (2005) Auditory-nerve rate responses are inconsistent with common hypotheses for the neural correlates of loudness recruitment. Journal of the Association for Research in Otolaryngology : JARO 6:91–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD (2003) Low-frequency tone pips elicit exaggerated startle reflexes in C57BL/6J mice with hearing loss. Journal of the Association for Research in Otolaryngology : JARO 4:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O'Neill WE (2007) Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. Journal of the Association for Research in Otolaryngology : JARO 8:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Luo B, Manohar S, Chen GD, Salvi R (2017) Plastic changes along auditory pathway during salicylate-induced ototoxicity: Hyperactivity and CF shifts. Hearing research 347:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML (1982) Hair cell counts in an age-graded series of rat cochleas. Hearing research 8:249–262. [DOI] [PubMed] [Google Scholar]
- Klink KB, Bendig G, Klump GM (2006) Operant methods for mouse psychoacoustics. Behavior research methods 38:1–7. [DOI] [PubMed] [Google Scholar]
- Knight KK, Margolis RH (1984) Magnitude estimation of loudness. II: Loudness perception in presbycusic listeners. Journal of speech and hearing research 27:28–32. [DOI] [PubMed] [Google Scholar]
- Lauer AM, Dooling RJ, Leek MR, Poling K (2007) Detection and discrimination of simple and complex sounds by hearing-impaired Belgian Waterslager canaries. The Journal of the Acoustical Society of America 122:3615–3627. [DOI] [PubMed] [Google Scholar]
- Levitin DJ, Menon V, Schmitt JE, Eliez S, White CD, Glover GH, Kadis J, Korenberg JR, Bellugi U, Reiss AL (2003) Neural correlates of auditory perception in Williams syndrome: an fMRI study. NeuroImage 18:74–82. [DOI] [PubMed] [Google Scholar]
- Manohar S, Spoth J, Radziwon K, Auerbach BD, Salvi R (2017) Noise-induced hearing loss induces loudness intolerance in a rat Active Sound Avoidance Paradigm (ASAP). Hearing research 353:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Brandt JF (1980) The relationship between loudness and reaction time in normal hearing listeners. Acta oto-laryngologica 90:244–249. [DOI] [PubMed] [Google Scholar]
- May BJ, Little N, Saylor S (2009) Loudness perception in the domestic cat: reaction time estimates of equal loudness contours and recruitment effects. Journal of the Association for Research in Otolaryngology : JARO 10:295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM (2000) GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hearing research 147:251–260. [DOI] [PubMed] [Google Scholar]
- Mohrle D, Hofmeier B, Amend M, Wolpert S, Ni K, Bing D, Klose U, Pichler B, Knipper M, Ruttiger L (2019) Enhanced Central Neural Gain Compensates Acoustic Trauma-induced Cochlear Impairment, but Unlikely Correlates with Tinnitus and Hyperacusis. Neuroscience 407:146–169. [DOI] [PubMed] [Google Scholar]
- Moller AR (2007) Neurophysiologic Abnormaliteis in Autism. Nova Science Publishers. [Google Scholar]
- Moller AR, Rollins PR (2002) The non-classical auditory pathways are involved in hearing in children but not in adults. Neuroscience letters 319:41–44. [DOI] [PubMed] [Google Scholar]
- Moody DB (1970) Reaction time as an index of sensory function in animals . In: Animal Pscyhophysics: The Design and Conduct of Sensory Experiments(Stebbins WC, ed) New York: Appleton-Century-Crofts:277–301. [Google Scholar]
- Moody DB (1973) Behavioral studies of noise-induced hearing loss in primates: loudness recruitment. Advances in oto-rhino-laryngology 20:82–101. [DOI] [PubMed] [Google Scholar]
- Munro KJ, Turtle C, Schaette R (2014) Plasticity and modified loudness following short-term unilateral deprivation: evidence of multiple gain mechanisms within the auditory system. The Journal of the Acoustical Society of America 135:315–322. [DOI] [PubMed] [Google Scholar]
- Paulin J, Andersson L, Nordin S (2016) Characteristics of hyperacusis in the general population. Noise & health 18:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Fifth Edition. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Hienz R, Kimm J, Miller J (1975) Reaction-time procedure for measurement of hearing. I. Suprathreshold functions. The Journal of the Acoustical Society of America 57:421–430. [DOI] [PubMed] [Google Scholar]
- Pienkowski M, Munguia R, Eggermont JJ (2011) Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hearing research 277:117–126. [DOI] [PubMed] [Google Scholar]
- Popelar J, Grecova J, Rybalko N, Syka J (2008) Comparison of noise-induced changes of auditory brainstem and middle latency response amplitudes in rats. Hearing research 245:82–91. [DOI] [PubMed] [Google Scholar]
- Popelar J, Syka J, Berndt H (1987) Effect of noise on auditory evoked responses in awake guinea pigs. Hearing research 26:239–247. [DOI] [PubMed] [Google Scholar]
- Pugh JE Jr., Moody DB, Anderson DJ (1979) Electrocochleography and experimentally induced loudness recruitment. Archives of oto-rhino-laryngology 224:241–255. [DOI] [PubMed] [Google Scholar]
- Qiu C, Salvi R, Ding D, Burkard R (2000) Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hearing research 139:153–171. [DOI] [PubMed] [Google Scholar]
- Radziwon K, Holfoth D, Lindner J, Kaier-Green Z, Bowler R, Urban M, Salvi R (2017) Salicylate-induced hyperacusis in rats: Dose- and frequency-dependent effects. Hearing research 350:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziwon KE, June KM, Stolzberg DJ, Xu-Friedman MA, Salvi RJ, Dent ML (2009) Behaviorally measured audiograms and gap detection thresholds in CBA/CaJ mice. Journal of comparative physiology A, Neuroethology, sensory, neural, and behavioral physiology 195:961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi R, Ding D, Wang J, Jiang HY (2000a) Effects of selective inner hair cell loss on distortion product otoacoustic emissions, cochlear function and auditory evoked potentials. Noise & health 6:9–25. [PubMed] [Google Scholar]
- Salvi R, Powers N, Saunders S, Boettcher F, Clock A (1992) Enhancement of evoked response amplitude and single unit activity after noise exposure In: Noise-Induced Hearing Loss(Dancer A, et al. , eds), pp 156–171 St Louis: Mosby-Year Book, Inc. [Google Scholar]
- Salvi R, Sun W, Ding D, Chen GD, Lobarinas E, Wang J, Radziwon K, Auerbach BD (2016) Inner Hair Cell Loss Disrupts Hearing and Cochlear Function Leading to Sensory Deprivation and Enhanced Central Auditory Gain. Frontiers in neuroscience 10:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi RJ, Henderson D, Hamernik R, Ahroon WA (1983) Neural correlates of sensorineural hearing loss. Ear and hearing 4:115–129. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N (1990) Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hearing research 50:245–257. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D (2000b) Auditory plasticity and hyperactivity following cochlear damage. Hearing research 147:261–274. [DOI] [PubMed] [Google Scholar]
- Scharf B, Fishken D (1970) Binaural summation of loudness: reconsidered. Journal of experimental psychology 86:374–379. [DOI] [PubMed] [Google Scholar]
- Scholl B, Wehr M (2008) Disruption of balanced cortical excitation and inhibition by acoustic trauma. Journal of neurophysiology 100:646–656. [DOI] [PubMed] [Google Scholar]
- Sheldrake J, Diehl PU, Schaette R (2015) Audiometric characteristics of hyperacusis patients. Frontiers in neurology 6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard A, Liu X, Ding D, Salvi R (2018) Auditory central gain compensates for changes in cochlear output after prolonged low-level noise exposure. Neuroscience letters 687:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Chen GD, Manohar S, Ding D, Hu BH, Sun W, Zhao J, Salvi R (2017) Prolonged low-level noise-induced plasticity in the peripheral and central auditory system of rats. Neuroscience 359:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins WC (1966) Auditory reaction time and the derivation of equal loudness contours for the monkey. Journal of the experimental analysis of behavior 9:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckler T (2001) Using signal detection methods for analysis of operant performance in mice. Behavioural brain research 125:237–248. [DOI] [PubMed] [Google Scholar]
- Sun W, Deng A, Jayaram A, Gibson B (2012) Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain research 1485:108–116. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N (2000) Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hearing research 139:59–68. [DOI] [PubMed] [Google Scholar]
- Syka J, Rybalko N, Popelar J (1994) Enhancement of the auditory cortex evoked responses in awake guinea pigs after noise exposure. Hearing research 78:158–168. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC (2014) A review of hyperacusis and future directions: part I. Definitions and manifestations. American journal of audiology 23:402–419. [DOI] [PubMed] [Google Scholar]
- Wagner E, Klump GM, Hamann I (2003) Gap detection in Mongolian gerbils (Meriones unguiculatus). Hearing research 176:11–16. [DOI] [PubMed] [Google Scholar]
- Wang J, Salvi RJ, Powers N (1996) Plasticity of response properties of inferior colliculus neurons following acute cochlear damage. Journal of neurophysiology 75:171–183. [DOI] [PubMed] [Google Scholar]
- Willott JF (1984) Changes in frequency representation in the auditory system of mice with age-related hearing impairment. Brain research 309:159–162. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S (1995) Modification of the acoustic startle response in hearing-impaired C57BL/6J mice: prepulse augmentation and prolongation of prepulse inhibition. Behavioral neuroscience 109:396–403. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S, Chen H (1994) Prepulse inhibition of the startle response in mice: relationship to hearing loss and auditory system plasticity. Behavioral neuroscience 108:703–713. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W (2007) Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hearing research 226:244–253. [DOI] [PubMed] [Google Scholar]
- Zarchi O, Avni C, Attias J, Frisch A, Carmel M, Michaelovsky E, Green T, Weizman A, Gothelf D (2015) Hyperactive auditory processing in Williams syndrome: Evidence from auditory evoked potentials. Psychophysiology 52:782–789. [DOI] [PubMed] [Google Scholar]
- Zeng FG (2013) An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hearing research 295:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]