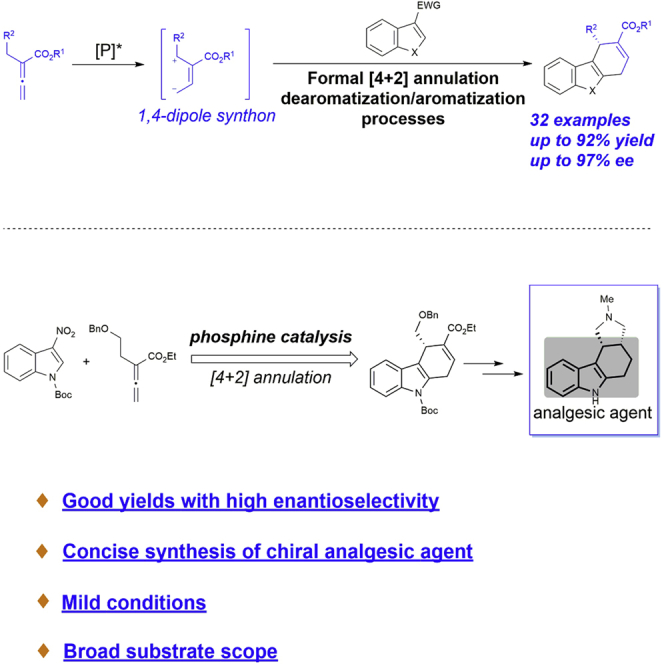
Summary
A highly efficient phosphine-catalyzed enantioselective [4 + 2] annulation of allenoates with 3-nitroindoles or 3-nitrobenzothiophenes has been developed. The protocol represents a unique dearomatization–aromatization process to access functionalized dihydrocarbazoles or dihydrodibenzothiophenes with high optical purity (up to 97% ee) under mild reaction conditions. The synthetic utility of the highly enantioselective [4 + 2] annulation enables a concise synthesis of analgesic agent.
Subject Areas: Catalysis, Organic Synthesis, Organic Reaction
Graphical Abstract
Highlights
-
•
High regio-, chemo-, and enantioselectivity
-
•
Broad substrate scope
-
•
Dearomatization/aromatization steps proceed under mild conditions
-
•
Concise synthesis of chiral analgesic agent
Catalysis; Organic Synthesis; Organic Reaction
Introduction
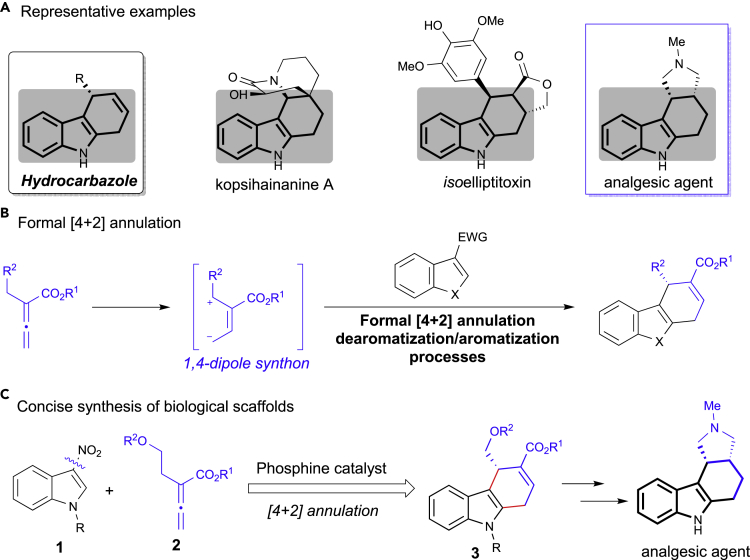
Fused polycyclic indoles are common structural motifs found in a vast array of natural and biologically active molecules (Saxton, 1996, Knölker and Reddy, 2002, Schmidt et al., 2012, Tan and Cheng, 2019), such as kopsihainanine A, isoelliptitoxin, and analgesic agents (Scheme 1A) (Madalengoitia and Macdonald, 1993, Carmosin et al., 2000, Chen et al., 2011). In this regard, the development of efficient methods for enantioselective construction of hydrocarbazole skeleton is still highly demanded (Sings et al., 2001, Lu et al., 2012, Zhou et al., 2015, Gu et al., 2016). The group of Jørgensen disclosed a novel [4 + 2] annulation by trienamine catalysis, thus obtaining dihydrocarbazoles in good yields and enantioselectivities (Li et al., 2016b). In this context, we hypothesized that the development of new methods through the enantioselective phosphine-catalyzed [4 + 2] dearomatization would provide practical and efficient approach to this class of enantioenriched heterocycles (Scheme 1B).
Scheme 1.
Phosphine-catalyzed [4 + 2] Dearomatization/Aromatization Reactions for the Formation of Enantioenriched Heterocycles
(A) Representative examples of chiral hydrocarbazole derivatives.
(B) Formal [4 + 2] annulation for the preparation of hydrocarbazole.
(C) Concise approach to the enantioselective synthesis of analgesic agent.
Phosphine catalysis has been recognized as a reliable tool for the development of unique transformations of allenoates, allowing for the discovery of novel asymmetric synthetic methodology (Lu et al., 2001, Methot and Roush, 2004, Ye et al., 2008, Cowen and Miller, 2009, Wei and Shi, 2010, Wei and Shi, 2017, Fan and Kwon, 2013, Wang et al., 2014, Wang et al., 2016, López and Mascareñas, 2014, Xie and Huang, 2015, Li and Zhang, 2016a, Li and Lu, 2017, Ni et al., 2018, Guo et al., 2018). Considerable research efforts have been devoted to the development of new methods for the phosphine-catalyzed enantioselective reactions. The use of phosphine catalysts has introduced a set of elementary steps that operate via discrete reactive species, allowing access to natural products and pharmaceuticals (Tran and Kwon, 2005, Andrews and Kwon, 2012, Han et al., 2012, Wang and Krische, 2003, Cai et al., 2016). One particularly versatile and reactive species is the phosphine-mediated 1,4-dipole generated upon addition of the phosphine catalyst to an allenoate substrate, thus providing a concise approach for accessing enantioselective annulations. Specially, Kwon group reported the result of their pioneering studies toward the development of a novel [4 + 2] annulation reaction of allenoates and N-tosylimines in the presence of phosphine catalyst (Zhu et al., 2003). Later, Fu group reported the phosphine-catalyzed highly enantioselective [4 + 2] annulation of N-tosylimines with allenoates (Wurz and Fu, 2005). Although great achievements have been made, concise syntheses of useful heterocycles involving phosphine-catalyzed [4 + 2] annulations in asymmetric versions were still rare (Tran and Kwon, 2007, Wang and Ye, 2010, Tran et al., 2011, Xiao et al., 2011, Baskar et al., 2011, Yu and Ma, 2012, Zhong et al., 2012, Takizawa et al., 2014, Yu et al., 2014, Liu et al., 2016, Wang and Guo, 2019a). Moreover, the development of an enantioselective phosphine-catalyzed [4 + 2] dearomatization reaction would provide an attractive and complementary approach for construction of privileged motifs, which will be of great value for the synthesis of bioactive molecules (Scheme 1C).
Enantioselective dearomatization reactions of heteroaromatic compounds are very powerful transformations because they provide direct access to a wide variety of chiral heterocycles (You, 2016, Roche and Porco, 2011, Zhuo et al., 2012, Zhuo et al., 2014, Zheng and You, 2016, Sun et al., 2016, Wu et al., 2016). In recent years, 3-nitroindole was demonstrated to be a good substrate for various dearomatization processes, and a number of enantioselective approaches have been reported (Awata and Arai, 2014, Zhao et al., 2015a, Zhao et al., 2015b, Zhao et al., 2018, Zhao et al., 2019, Gerten and Stanley, 2016, Trost et al., 2014, Cheng et al., 2018, Zhang et al., 2018, Sun et al., 2018, Yue et al., 2017, Yang et al., 2019). Importantly, Lu group (Li et al., 2019) and Zhang group (Wang et al., 2019b) independently reported the efficient phosphine-catalyzed enantioselective [3 + 2] annulation of 3-nitroindoles with allenoates to afford cyclopentaindoline products in high yields and excellent enantioselectivities. We envisaged that heteroaromatic systems bearing an electron-withdrawing group could react with phosphine-mediated zwitterionic intermediate in a process involving the [4 + 2] reaction to achieve the chiral dihydrocarbazole scaffold (Scheme 1C). With this objective in mind, a readily available 3-nitroindole derivative was selected as a model substrate to investigate the optimum reaction condition for the enantioselective [4 + 2] dearomatization reaction using a phosphine catalyst.
Results and Discussion
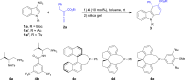
Based on our previous work on phosphine chemistry (Wang and Guo, 2019a), we initiated the study by investigating the reaction between 1a and 2a in the presence of the phosphine 4a (Table 1, entry 1). Initially, diverse chiral phosphine catalysts were examined (entries 1–5). However, the catalyst 4a to 4c did not work for this reaction (entries 1–3). To our great delight, the desired dihydrocarbozole3a could be obtained when the chiral phosphine 4d was employed (entry 4). After surveying an array of additives, we determined that silica gel can promote elimination of HNO2 for the aromatization process to afford the corresponding adduct in 92% yield with 94% ee (entry 4) (So and Mattson, 2012, Long et al., 2016). Other additives, such as Sc(OTf)3, Et3N, and SnCl2 led to byproducts (for further details, see Table S1 in the Supplemental Information). Furthermore, the ee values of the 3a decreased to 80% with low yield in the presence of 4e as catalyst (entry 5). Varying the solvents led to no improvement in the reaction, and toluene was proven to be the best choice (entries 4 vs 6–8). Further optimization studies revealed that the protection group of the 3-nitroindole was also sensitive to the reaction, and the variation of the N-substituent of the 3-nitroindole 1a′ or 1a’’ generated no product at all (entries 9 and 10) (Rivinoja et al., 2017, Suo et al., 2018).
Table 1.
Optimization of Reaction Conditions
 | |||||
|---|---|---|---|---|---|
| Entry | 1 | 4 | Solvent | Yield (%)a | ee (%)b |
| 1 | 1a | 4a | Toluene | nr | – |
| 2 | 1a | 4b | Toluene | nr | – |
| 3 | 1a | 4c | Toluene | nr | – |
| 4 | 1a | 4d | Toluene | 92 | 94 |
| 5 | 1a | 4e | Toluene | 26 | 80 |
| 6 | 1a | 4d | CH2Cl2 | 23 | 73 |
| 7 | 1a | 4d | THF | 73 | 84 |
| 8 | 1a | 4d | Dioxane | 57 | 90 |
| 9 | 1a′ | 4d | Toluene | nr | – |
| 10 | 1a’’ | 4d | Toluene | nr | – |
Unless indicated otherwise, the reaction were conducted with 1 (0.1 mmol), 2a (0.15 mmol), and 4 (0.01 mmol) in toluene (1.0 mL) at room temperature for 18 h. Then silica gel (200 mg) was added to the reaction mixture to complete elimination of HNO2. nr = no reaction.
Yield of isolated product.
Determined by HPLC analysis.
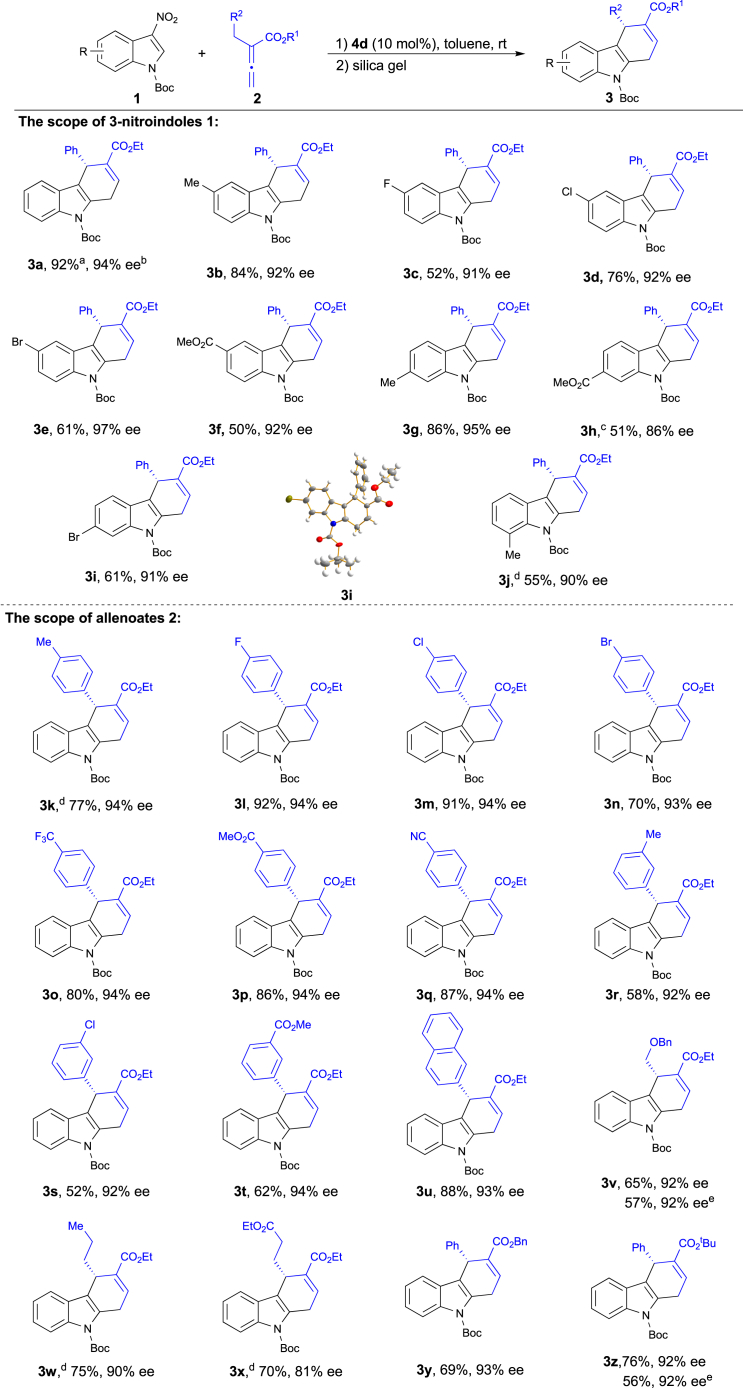
With the optimal reaction conditions in hand, we set out to explore the substrate scope of the procedure. As shown in Scheme 2, various electron-withdrawing or donating groups on the indole ring were well tolerated and resulted in excellent levels of enantioselectivities ranging from 86% to 97% ee (3a–3j). The extension of the protocol to the 3-nitroindole with a variety of substitution patterns at the 5-position was successful to afford corresponding adducts with excellent enantioselectivities (3b–3f). To our delight, substrates bearing substituents on different positions of the indole ring also facilitate the annulation with high yields and ee values (3b, 3g, and 3j). The absolute configuration of the enantiopure 3i, recrystallized from ethyl acetate and petroleum ether, was assigned by single-crystal X-ray diffraction analysis.
Scheme 2.
Substrate Scope of Enantioselective [4 + 2] Annulation
Unless indicated otherwise, the reactions were conducted with 1 (0.1 mmol), 2 (0.15 mmol), and catalyst 4d (0.01 mmol) in toluene at room temperature for 12–48 h. Then silica gel was added to the reaction mixture to complete elimination of HNO2.
aYield of the isolated product after purification by chromatography on silica gel.
bEnantiomeric excess determined by HPLC analysis.
cAromatization process was performed at 50°C.
d20 mol% of 4d.
eThe reaction was performed on 1 mmol scale.
The generality of the reaction with respect to the scope of the allenoates 2 was also investigated using 3-nitroindole 1a as the reaction partner under the optimized conditions. A diverse array of allenoates (2) with a variety of functional groups (methyl, fluoro, chloro, bromo, ester, trifluoromethyl, and cyano) performed well in this annulation reaction, and the corresponding products were isolated in good yields with high ee values (3k-3q). Remarkably, this method was compatible with alkyl allenoate, affording the desired products in good yields with good enantioselectivity (3v-3x). Additionally, all reactions with different esters attached to the allenoates proceeded smoothly, giving the corresponding products in good yields and excellent ee (3y and 3z). To test the synthetic utility of the current annulation, we performed the reaction on a 1 mmol scale with the formation of 3z in 56% yield and 92% ee.
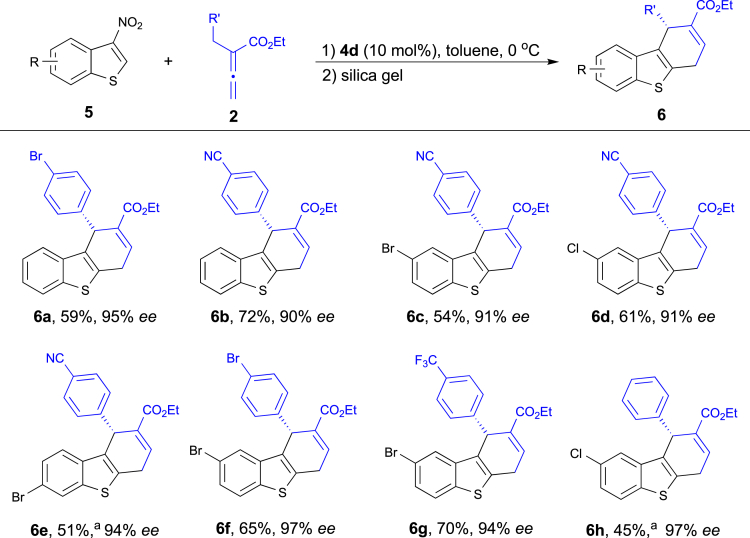
Encouraged by the excellent results with various 3-nitroindoles, we then investigated the [4 + 2] annulation reaction with a range of 3-nitrobenzothiophenes (5). Remarkably, process where the 3-nitrobenzothiophene as a reactive partner for asymmetric annulation has been much less studied (Tran and Kwon, 2007, Wang and Ye, 2010, Tran et al., 2011, Xiao et al., 2011, Baskar et al., 2011, Yu and Ma, 2012, Zhong et al., 2012, Takizawa et al., 2014, Yu et al., 2014, Liu et al., 2016, Wang and Guo, 2019a, Cheng et al., 2000, Cheng et al., 2017, Suo et al., 2018, Yue et al., 2018, Chen et al., 2019). Using phosphine 4d in toluene at 0°C, we were able to access dihydrodibenzothiophene products 6 (Scheme 3). Under the optimized reaction condition (for further details, see Table S2 in the Supplemental Information), a broad range of allenoates 2 and 3-nitrobenzothiophenes 5 were investigated. Allenoates with different substituents on the aromatic ring underwent this catalytic transformation smoothly in good yields with excellent ee (6a and 6b). Furthermore, various substitutions of 3-nitrobenzothiophenes 5 at the aromatic ring had little impact on the reactions (6c–6h, 91%–97% ee).
Scheme 3.
Enantioselective [4 + 2] Annulation of 3-Nitrobenzothiophene 5
Unless indicated otherwise, the reactions was conducted with 5 (0.1 mmol), 2 (0.15 mmol), and catalyst 4d (0.01 mmol) in toluene (1.0 mL) at 0°C for 48–60 h. Then silica gel was added to the reaction mixture to complete elimination of HNO2. Yield of the isolated product after purification by chromatography on silica gel. Enantiomeric excess determined by HPLC analysis.
a0.02 mmol of 4d was used.
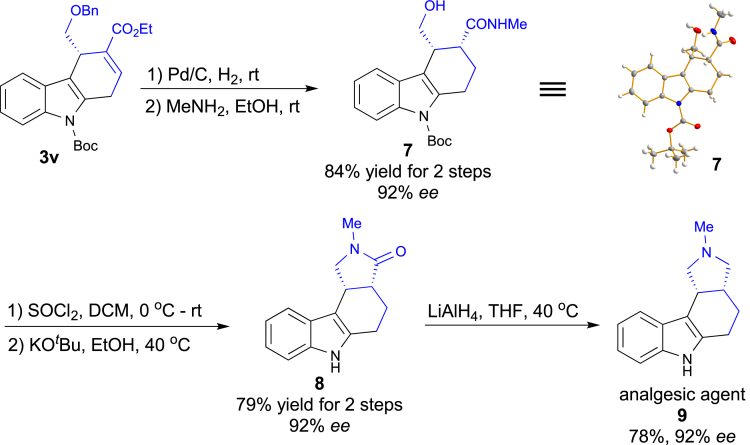
To highlight the synthetic potential of the present method, the dihydrocabazole 3v, which was obtained from the enantioselective [4 + 2] annulation, can be easily converted into analgesic agent 9 (Scheme 4). In 2000, Carmosin and co-workers obtained the racemic analgesic agent 9 via the Diels-Alder reaction, and the optical product was obtained by using preparative chromatography (Carmosin et al., 2000). Taking advantage of our current phosphine-catalyzed enantioselective [4 + 2] reaction, we can easily obtain the analgesic agent 9 with excellent enantioselectivity. Hydrogenation of 3v in the presence of a catalytic amount of Pd/C, followed by amidation with MeNH2 gave rise to the desired amide 7 in 84% yield over two steps. The configuration of compound 7 was assigned by X-ray analysis. The subsequent chlorination of alcohol, deprotection of the N-Boc group and cyclization furnished 8 in good yield. Finally, the amide 8 was reduced to generate the corresponding analgesic agent 9 in 78% yield and 92% ee.
Scheme 4.
Enantioselective Synthesis of Analgesic Agent 9
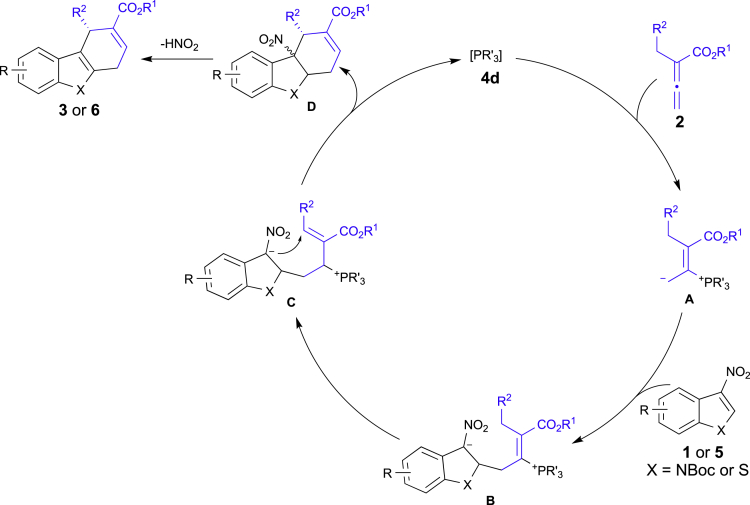
The proposed catalytic cycle for the enantioselective [4 + 2] annulation is illustrated in Figure 1. The addition of phosphine catalyst 4d to the allenoate 2 gives the intermediate A, which could react with the 3-nitroindole 1 or 3-nitrobenzothiophenes 5 to form the intermediate B. Following migration and intramolecular conjugate addition give rise to the intermediate D and regenerate the phosphine 4d. This species D then undergoes elimination of HNO2 through the aromatization process to furnish the final dihydrocarbzole 3 or dihydrodibenzothiophene 6.
Figure 1.
Proposed Mechanism
In summary, we have developed simple and efficient synthetic routes to highly enriched hydrocarbozoles through chiral phosphine-catalyzed [4 + 2] annulation utilizing 3-nitroindole and allenoates as starting materials. This phosphine-catalyzed enantioselective [4 + 2] annulation procedure involving tandem dearomatization and aromatization steps proceeds under mild conditions. This reaction displays a broad substrate scope with respect to the substituents. Additionally, the obtained dihydrocarbozole could be efficient transformed to an analgesic agent containing polycyclic indole frameworks.
Limitations of the Study
The synthesis of the products needs two steps in one pot. No product was formed with the initial addition of silica gel.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors acknowledge financial support from the National Natural Science Foundation of China (grant no. 21702198), the Anhui Provincial Natural Science Foundation (grant no. 1808085MB30), "1000-Youth Talents Plan," and the Fundamental Research Funds for the Central Universities (WK2340000090).
Author Contributions
H.W. carried out the experimental and data analysis work. Q.H. and M.W. prepared some starting materials. C.G. designed the reaction and directed the project. The paper was written by C.G. with assistance of H.W., Q.H., and M.W.
Declaration of Interests
The authors declare no conflict of interest.
Published: February 21, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100840.
Data and Code Availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession numbers CCDC 1938371 (3i) and CCDC 1955757 (7). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/.
Supplemental Information
References
- Andrews I.P., Kwon O. Enantioselective total synthesis of (+)-ibophyllidine via an asymmetric phosphine-catalyzed [3 + 2] annulation. Chem. Sci. 2012;3:2510–2514. doi: 10.1039/C2SC20468A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awata A., Arai T. Pybidine/Copper catalyst: asymmetric exo’-selective [3+2] cycloaddition using imino ester and electrophilic indole. Angew. Chem. Int. Ed. 2014;53:10462–10465. doi: 10.1002/anie.201405223. [DOI] [PubMed] [Google Scholar]
- Baskar B., Dakas P.-Y., Kumar K. Natural product biosynthesis inspired concise and stereoselective synthesis of benzopyrones and related scaffolds. Org. Lett. 2011;13:1988–1991. doi: 10.1021/ol200389p. [DOI] [PubMed] [Google Scholar]
- Cai L., Zhang K., Kwon O. Catalytic asymmetric total synthesis of (−)-actinophyllic acid. J. Am. Chem. Soc. 2016;138:3298–3301. doi: 10.1021/jacs.6b00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmosin, R.J., Carson, J.R., and Pitis, P.M.. (2000). Octahydropyrrolo-[3,4-C]-carbazoles useful as analgesic agents. US Patent No. 6063803.
- Chen J., Chen J.-J., Yao X., Gao K. Kopsihainanines A and B, two unusual alkaloids from kopsiahainanensis. Org. Biomol. Chem. 2011;9:5334–5336. doi: 10.1039/c1ob05724c. [DOI] [PubMed] [Google Scholar]
- Chen X.-M., Lei C.-W., Yue D.-F., Zhao J.-Q., Wang Z.-H., Zhang X.-M., Xu X.-Y., Yuan W.-C. Organocatalytic asymmetric dearomatization of 3-nitroindoles and 3-nitrobenzothiophenes via thiol-triggered diastereo- and enantioselective double michael addition reaction. Org. Lett. 2019;21:5452–5456. doi: 10.1021/acs.orglett.9b01688. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Zhang F., Cai Y., Guo Y.-L., You S.-L. Stereodivergent synthesis of tetrahydrofuroindoles through Pd-catalyzed asymmetric dearomative formal [3+2] cycloaddition. Angew.Chem. Int. Ed. 2018;57:2134–2138. doi: 10.1002/anie.201711873. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Zhang H.-J., Yue W.-J., You S.-L. One pot preparation of bicyclopentenones from propargylmalonates (and propargylsulfonamides) and allylic acetates by a tandem action of catalysts. J. Am. Chem. Soc. 2000;122:10220–10221. [Google Scholar]
- Cheng Q., Zhang H.-J., Yue W.-J., You S.-L. Palladium-catalyzed highly stereoselectivedearomative [3 + 2] cycloaddition of nitrobenzofurans. Chem. 2017;3:428–436. [Google Scholar]
- Cowen B.J., Miller S.J. Enantioselective catalysis and complexity generation from allenoates. Chem. Soc. Rev. 2009;38:3102–3116. doi: 10.1039/b816700c. [DOI] [PubMed] [Google Scholar]
- Fan Y.C., Kwon O. Advances in nucleophilic phosphine catalysis of alkenes, allenes, alkynes, and MBHADs. Chem. Commun. (Camb.) 2013;49:11588–11619. doi: 10.1039/c3cc47368f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerten A.L., Stanley L.M. Enantioselectivedearomative [3 + 2] cycloadditions of indoles with azomethineylides derived from alanine imino esters. Org. Chem. Front. 2016;3:339–343. [Google Scholar]
- Gu B.-Q., Zhang H., Su R.-H., Deng W.-P. Organocatalytic asymmetric synthesis of dihydrocarbazoles via a formal [4+2] cycloaddition of in situ generated o-quinodimethanes with enals. Tetrahedron. 2016;72:6595–6602. [Google Scholar]
- Guo H., Fan Y.C., Sun Z., Wu Y., Kwon O. Phosphine organocatalysis. Chem. Rev. 2018;118:10049–10293. doi: 10.1021/acs.chemrev.8b00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Zhong F., Wang Y., Lu Y. Versatile enantioselective [3+2] cyclization between imines and allenoates catalyzed by dipeptide-based phosphines. Angew. Chem. Int. Ed. 2012;51:767–770. doi: 10.1002/anie.201106672. [DOI] [PubMed] [Google Scholar]
- Knölker H.-J., Reddy K.R. Isolation and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2002;102:4303–4427. doi: 10.1021/cr020059j. [DOI] [PubMed] [Google Scholar]
- Li H., Lu Y. Enantioselective construction of all-carbon quaternary stereogenic centers by using phosphine catalysis. Asian J. Org. Chem. 2017;6:1130–1145. [Google Scholar]
- Li K., Gonçalves T.P., Huang K.-W., Lu Y. Dearomatization of 3-nitroindoles by a phosphine-catalyzed enantioselective [3+2] annulation reaction. Angew.Chem. Int. Ed. 2019;58:5427–5431. doi: 10.1002/anie.201900248. [DOI] [PubMed] [Google Scholar]
- Li W., Zhang J. Recent developments in the synthesis and utilization of chiral β-aminophosphine derivatives as catalysts or ligands. Chem. Soc. Rev. 2016;45:1657–1677. doi: 10.1039/c5cs00469a. [DOI] [PubMed] [Google Scholar]
- Li Y., Tur F., Nielsen R.P., Jiang H., Jensen F., Jørgensen K.A. Enantioselective formal [4+2] cycloadditions to 3-nitroindoles by trienamine catalysis: synthesis of chiral dihydrocarbazoles. Angew. Chem. Int. Ed. 2016;55:1020–1024. doi: 10.1002/anie.201509693. [DOI] [PubMed] [Google Scholar]
- Liu H., Liu Y., Yuan C., Wang G.-P., Zhu S.-F., Wu Y., Wang B., Sun Z., Xiao Y., Zhou Q.-L., Guo H. Enantioselective synthesis of spirobarbiturate-cyclohexenes through phosphine-catalyzed asymmetric [4 + 2] annulation of barbiturate-derived alkenes with allenoates. Org. Lett. 2016;18:1302–1305. doi: 10.1021/acs.orglett.6b00239. [DOI] [PubMed] [Google Scholar]
- Long L., Sun W., Yang D., Li G., Wang R. Additive effects on asymmetric catalysis. Chem. Rev. 2016;116:4006–4123. doi: 10.1021/acs.chemrev.5b00676. [DOI] [PubMed] [Google Scholar]
- López F., Mascareñas J.L. [4+2] and [4+3] catalytic cycloadditions of allenes. Chem. Soc. Rev. 2014;43:2904–2915. doi: 10.1039/c4cs00024b. [DOI] [PubMed] [Google Scholar]
- Lu L.-Q., Chen J.-R., Xiao W.-J. Development of cascade reactions for the concise construction of diverse heterocyclic architectures. Acc. Chem. Res. 2012;45:1278–1293. doi: 10.1021/ar200338s. [DOI] [PubMed] [Google Scholar]
- Lu X., Zhang C., Xu Z. Reactions of electron-deficient alkynes and allenes under phosphine catalysis. Acc. Chem. Res. 2001;34:535–544. doi: 10.1021/ar000253x. [DOI] [PubMed] [Google Scholar]
- Madalengoitia J.S., Macdonald T.L. Synthesis of tetrahydrofurocarbazolones via intramolecular Diels-Alder reactions. Tetrahedron Lett. 1993;34:6237–6240. [Google Scholar]
- Methot J.L., Roush W.R. Nucleophilic phosphine organocatalysis. Adv. Synth. Catal. 2004;346:1035–1050. [Google Scholar]
- Ni H., Chan W.-L., Lu Y. Phosphine-catalyzed asymmetric organic reactions. Chem. Rev. 2018;118:9344–9411. doi: 10.1021/acs.chemrev.8b00261. [DOI] [PubMed] [Google Scholar]
- Rivinoja D.J., Gee Y.S., Gardiner M.G., Ryan J.H., Hyland C.J.T. Thediastereoselective synthesis of pyrroloindolines by Pd-catalyzed dearomativecycloaddition of 1-tosyl-2-vinylaziridine to 3-nitroindoles. ACS Catal. 2017;7:1053–1056. [Google Scholar]
- Roche S.P., Porco J.A., Jr. Dearomatization strategies in the synthesis of complex natural products. Angew.Chem. Int. Ed. 2011;50:4068–4093. doi: 10.1002/anie.201006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton J.E. Recent progress in the chemistry of the monoterpenoidindole alkaloids Recent progress in the chemistry of the monoterpenoidindole alkaloids. Nat. Prod. Rep. 1996;14:559–590. [Google Scholar]
- Schmidt A.W., Reddy K.R., Knölker H.-J. Occurrence, biogenesis, and synthesis of biologically active carbazole alkaloids. Chem. Rev. 2012;112:3193–3328. doi: 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- Sings H.L., Harris G.H., Dombrowski A.W. Dihydrocarbazole alkaloids from aspergillustubingensis. J. Nat. Prod. 2001;64:836–838. doi: 10.1021/np000613p. [DOI] [PubMed] [Google Scholar]
- So S.S., Mattson A.E. Urea activation of α-nitrodiazoesters: an organocatalytic approach to N−H insertion reactions. J. Am. Chem. Soc. 2012;134:8798–8801. doi: 10.1021/ja3031054. [DOI] [PubMed] [Google Scholar]
- Sun M., Zhu Z.-Q., Gu L., Wan X., Mei G.-J., Shi F. Catalytic asymmetric dearomative [3 + 2] cycloaddition of electron-deficient indoles with all-carbon 1,3-dipoles. J. Org. Chem. 2018;83:2341–2348. doi: 10.1021/acs.joc.7b03259. [DOI] [PubMed] [Google Scholar]
- Sun W., Li G., Hong L., Wang R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 2016;14:2164–2176. doi: 10.1039/c5ob02526e. [DOI] [PubMed] [Google Scholar]
- Suo J.-J., Liu W., Du J., Ding C.-H., Hou X.-L. Diastereo- and enantioselective Palladium-catalyzed dearomative [3+2] cycloaddition of 3-nitroindoles. Chem. Asian J. 2018;13:959–963. doi: 10.1002/asia.201800133. [DOI] [PubMed] [Google Scholar]
- Takizawa S., Arteaga F.A., Yoshida Y., Suzuki M., Sasai H. Enantioselectiveorganocatalyzed formal [4+2] cycloaddition of ketimines with allenoates: easy access to a tetrahydropyridineframework with a chiral tetrasubstitutedstereogenic carbon center. Asian J. Org. Chem. 2014;3:412–415. [Google Scholar]
- Tan F., Cheng H.-G. Catalytic asymmetric synthesis of tetrahydrocarbazoles. Chem. Commun. (Camb.) 2019;55:6151–6164. doi: 10.1039/c9cc02486g. [DOI] [PubMed] [Google Scholar]
- Tran Y.S., Kwon O. An application of the phosphine-catalyzed [4 + 2] annulation in indole alkaloid synthesis: formal syntheses of (±)-alstonerine and (±)-macroline. Org. Lett. 2005;7:4289–4291. doi: 10.1021/ol051799s. [DOI] [PubMed] [Google Scholar]
- Tran Y.S., Kwon O. Phosphine-catalyzed [4 + 2] annulation: synthesis of cyclohexenes. J. Am. Chem. Soc. 2007;129:12632–12633. doi: 10.1021/ja0752181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran Y.S., Martin T.J., Kwon O. Phosphine-catalyzed [4+2] Annulations of 2-alkylallenoates and oefins: synthesis of multisubstituted cyclohexenes. Chem. Asian J. 2011;6:2101–2106. doi: 10.1002/asia.201100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B.M., Ehmke V., O’Keefe B.M., Bringley D.A. Palladium-catalyzed dearomativetrimethylenemethanecycloaddition reactions. J. Am. Chem. Soc. 2014;136:8213–8216. doi: 10.1021/ja5044825. [DOI] [PubMed] [Google Scholar]
- Wang H., Guo C. Enantioselectiveγ-addition of pyrazole and imidazole heterocycles to allenoates catalyzed by chiral phosphine. Angew.Chem. Int. Ed. 2019;58:2854–2858. doi: 10.1002/anie.201813381. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhang J., Tu Y., Zhang J. Phosphine-catalyzed enantioselectivedearomative [3+2] cycloaddition of 3-nitroindoles and 2-nitrobenzofurans. Angew.Chem. Int. Ed. 2019;58:5422–5426. doi: 10.1002/anie.201900036. [DOI] [PubMed] [Google Scholar]
- Wang J.-C., Krische M.J. Intramolecularorganocatalytic [3+2] dipolar cycloaddition: stereospecific cycloaddition and the total synthesis of (±)-hirsutene. Angew.Chem. Int. Ed. 2003;42:5855–5857. doi: 10.1002/anie.200352218. [DOI] [PubMed] [Google Scholar]
- Wang T., Ye S. Diastereoselective synthesis of 6-trifluoromethyl-5,6-dihydropyrans via phosphine-catalyzed [4 + 2] annulation of α-benzylallenoates with ketones. Org. Lett. 2010;12:4168–4171. doi: 10.1021/ol101762z. [DOI] [PubMed] [Google Scholar]
- Wang T., Han X., Zhong F., Yao W., Lu Y. Amino acid-derived bifunctionalphosphines for enantioselective transformations. Acc. Chem. Res. 2016;49:1369–1378. doi: 10.1021/acs.accounts.6b00163. [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu X., Kwon O. Phosphine catalysis of allenes with electrophiles. Chem. Soc. Rev. 2014;43:2927–2940. doi: 10.1039/c4cs00054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Shi M. Multifunctional chiral phosphine organocatalysts in catalytic asymmetric Morita-Baylis-Hillman and related reactions. Acc. Chem. Res. 2010;43:1005–1018. doi: 10.1021/ar900271g. [DOI] [PubMed] [Google Scholar]
- Wei Y., Shi M. Lu’s [3 + 2] cycloaddition of allenes with electrophiles: discovery, development and synthetic application. Org. Chem. Front. 2017;4:1876–1890. [Google Scholar]
- Wu W.-T., Zhang L., You S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 2016;45:1570–1580. doi: 10.1039/c5cs00356c. [DOI] [PubMed] [Google Scholar]
- Wurz R.P., Fu G.C. Catalytic asymmetric synthesis of piperidine derivatives through the [4 + 2] annulation of imines with allenes. J. Am. Chem. Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d. [DOI] [PubMed] [Google Scholar]
- Xiao H., Chai Z., Wang H.-F., Wang X.-W., Cao D.-D., Liu W., Lu Y.-P., Yang Y.-Q., Zhao G. Bifunctional N-acyl-aminophosphine-catalyzed asymmetric [4+2] cycloadditions of allenoates and imines. Chem. Eur. J. 2011;17:10562–10565. doi: 10.1002/chem.201100850. [DOI] [PubMed] [Google Scholar]
- Xie P., Huang Y. Morita–Baylis–Hillman adduct derivatives (MBHADs): versatile reactivity in Lewis base-promoted annulation. Org. Biomol. Chem. 2015;13:8578–8595. doi: 10.1039/c5ob00865d. [DOI] [PubMed] [Google Scholar]
- Yang X.-H., Li J.-P., Wang D.-C., Xie M.-S., Qu G.-R., Guo H.-M. Enantioselectivedearomative [3+2] cycloaddition of 2-nitrobenzofurans with aldehyde-derived Morita–Baylis–Hillman carbonates. Chem. Commun. (Camb.) 2019;55:9144–9147. doi: 10.1039/c9cc04542b. [DOI] [PubMed] [Google Scholar]
- Ye L.-W., Zhou J., Tang Y. Phosphine-triggered synthesis of functionalized cyclic compounds. Chem. Soc. Rev. 2008;37:1140–1152. doi: 10.1039/b717758e. [DOI] [PubMed] [Google Scholar]
- You S.-L. WileyVCH; 2016. Asymmetric Dearomatization Reactions. [Google Scholar]
- Yu H., Zhang L., Li Z., Liu H., Wang B., Xiao Y., Guo H. Phosphine-catalyzed [4+2] cycloaddition of sulfamate-derived cyclic imines with allenoates: synthesis of sulfamate-fused tetrahydropyridines. Tetrahedron. 2014;70:340–348. [Google Scholar]
- Yu S., Ma S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 2012;51:3074–3112. doi: 10.1002/anie.201101460. [DOI] [PubMed] [Google Scholar]
- Yue D.-F., Zhao J.-Q., Chen X.-Z., Zhou Y., Zhang X.-M., Xu X.-Y., Yuan W.-C. Multiple hydrogen-bonding bifunctionalthiourea-catalyzed asymmetric dearomative [4 + 2] annulation of 3-nitroindoles: highly enantioselective access to hydrocarbazole skeletons. Org. Lett. 2017;19:4508–4511. doi: 10.1021/acs.orglett.7b02068. [DOI] [PubMed] [Google Scholar]
- Yue D.-F., Zhao J.-Q., Chen Y.-Z., Zhang X.-M., Xu X.-Y., Yuan W.-C. Zinc-catalyzed enantioselectivedearomative [3+2] cycloaddition reaction of 3-nitrobenzothiophenes and 3-nitrothieno[2,3-b]yridine with 3-isothiocyanato oxindoles. Adv. Synth. Catal. 2018;360:1420–1425. [Google Scholar]
- Zhang J.-Q., Tong F., Sun B.-B., Fan W.-T., Chen J.-B., Hu D., Wang X.-W. Pd-Catalyzed Asymmetric dearomativecycloaddition for construction of optically active pyrroloindoline and cyclopentaindoline derivatives: access to 3a-aminopyrroloindolines. J. Org. Chem. 2018;83:2882–2891. doi: 10.1021/acs.joc.8b00046. [DOI] [PubMed] [Google Scholar]
- Zhao J.-Q., Wu Z.-J., Zhou M.-Q., Xu X.-Y., Zhang X.-M., Yuan W.-C. Zn-catalyzed diastereo- and enantioselective cascade reaction of 3-isothiocyanato oxindoles and 3-nitroindoles: stereocontrolled syntheses of polycyclic spirooxindoles. Org. Lett. 2015;17:5020–5023. doi: 10.1021/acs.orglett.5b02489. [DOI] [PubMed] [Google Scholar]
- Zhao J.-Q., Zhou M.-Q., Wu Z.-J., Wang Z.-H., Yue D.-F., Xu X.-Y., Zhang X.-M., Yuan W.-C. Asymmetric michael/cyclization cascade reaction of 3-isothiocyanato oxindoles and 3-nitroindoles with amino-thiocarbamate catalysts: enantioselective synthesis of polycyclic spirooxindoles. Org. Lett. 2015;17:2238–2241. doi: 10.1021/acs.orglett.5b00850. [DOI] [PubMed] [Google Scholar]
- Zhao J.-Q., Zhou X.-J., Zhou Y., Xu X.-Y., Zhang X.-M., Yuan W.-C. Diastereo- and enantioselectivedearomative [3 + 2] cycloaddition reaction of 2-nitrobenzofurans with 3-isothiocyanato oxindoles. Org. Lett. 2018;20:909–912. doi: 10.1021/acs.orglett.7b03667. [DOI] [PubMed] [Google Scholar]
- Zhao J.-Q., Yang L., You Y., Wang Z.-H., Xie K.-X., Zhang X.-M., Xu X.-Y., Yuan W.-C. Phosphine-catalyzed dearomative (3 + 2) annulation of 2-nitrobenzofurans and nitrobenzothiophenes with allenoates. Org. Biomol. Chem. 2019;17:5294–5304. doi: 10.1039/c9ob00775j. [DOI] [PubMed] [Google Scholar]
- Zheng C., You S.-L. Catalytic asymmetric dearomatization by transition-metal catalysis: a method for transformations of aromatic compounds. Chem. 2016;1:830–857. [Google Scholar]
- Zhong F., Han X., Wang Y., Lu Y. Highlyenantioselective [4 + 2] annulations catalyzed by amino acid-based phosphines: synthesis of functionalized cyclohexenes and 3-spirocyclohexene-2-oxindoles. Chem. Sci. 2012;3:1231–1234. [Google Scholar]
- Zhou L., Xu B., Zhang J. Metal-Free dehydrogenative Diels–Alder reactions of 2-methyl-3-alkylindoles with dienophiles: rapid access to tetrahydrocarbazoles, carbazoles, and heteroacenes. Angew.Chem. Int. Ed. 2015;54:9092–9096. doi: 10.1002/anie.201503549. [DOI] [PubMed] [Google Scholar]
- Zhu X.-F., Lan J., Kwon O. An expedient phosphine-catalyzed [4 + 2] annulation: synthesis of highly functionalized tetrahydropyridines. J. Am. Chem. Soc. 2003;125:4716–4717. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]
- Zhuo C.-X., Zhang W., You S.-L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 2012;51:12662–12686. doi: 10.1002/anie.201204822. [DOI] [PubMed] [Google Scholar]
- Zhuo C.-X., Zheng C., You S.-L. Transition-metal-catalyzed asymmetric allylicdearomatization reactions. Acc. Chem. Res. 2014;47:2558–2573. doi: 10.1021/ar500167f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Center (CCDC) under accession numbers CCDC 1938371 (3i) and CCDC 1955757 (7). Copies of the data can be obtained free of charge from www.ccdc.cam.ac.uk/structures/.