Summary
Pneumonia accounted for 15% of the 6.3 million deaths among children younger than five years in 2013, a total of approximately 935,000 deaths worldwide. Routine vaccination against common childhood illnesses has been identified as one of the most cost-effective strategies to prevent death from pneumonia. Vaccine-preventable or potentially preventable diseases commonly linked with respiratory tract infections include Streptococcus pneumoniae, Haemophilus influenza type-b (Hib), pertussis, influenza, measles, and tuberculosis. Although here have been great strides in the development and administration of effective vaccines, the countries that carry the largest disease burdens still struggle to vaccinate their children and newer conjugated vaccines remain out of reach for many. The Global Vaccine Action Plan (GVAP) has identified priority areas for innovation in research in all aspects of immunisation development and delivery to ensure equitable access to vaccines for all.
Keywords: Vaccines, Pneumonia, Children, Developing countries
Background
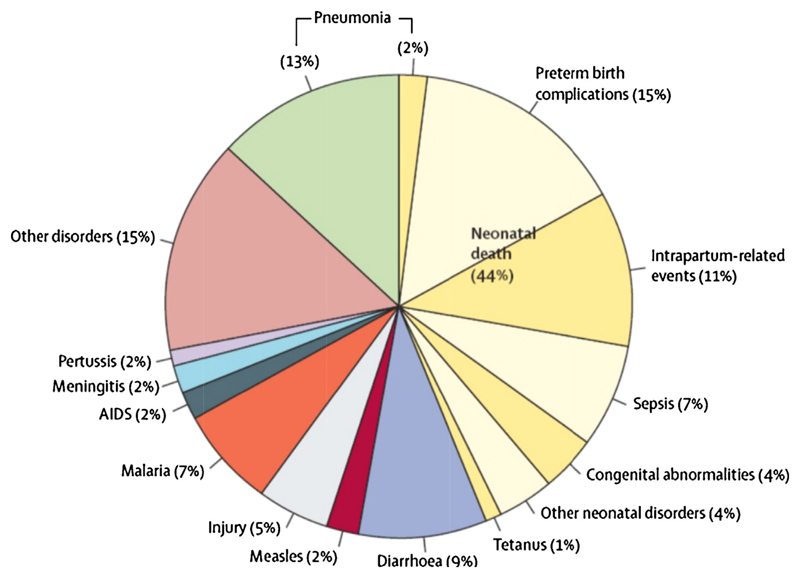
Globally, acute respiratory tract infections are the most common cause of both illness and mortality in children. In developing countries, there are approximately 0.22 episodes of pneumonia per child-year with 11.5% progressing to severe disease [1]. Pneumonia accounted for 15% of the 6.3 million deaths among children younger than five years in 2013, a total of approximately 935,000 deaths worldwide (Figure 1) [2,3]. This translates to a young child dying from an acute respiratory infection every 30 seconds, yet most of these deaths are preventable [3].
Figure 1. Causes of death in children under 5 years of age (2013).
‘Reproduced by kind permission of Elsevier from Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis’ [2]
Outside the neonatal period, pneumonia and diarrhoea account for approximately 40% of all deaths in young children (Figure 1), [2] which motivated the World Health Organisation (WHO) and the United Nations Children’s Fund (UNICEF) to develop the Integrated Global Action Plan for the Prevention and Control of Pneumonia and Diarrhoea (GAPPD) in 2013 [4]. GAPPD aims to ensure access to proven and appropriate preventive and treatment measures for pneumonia and diarrhoea, especially in resource-limited settings where most of these deaths occur [3,4].
Five simple interventions have been recommended to reduce severe pneumonia in childhood: i) exclusive breastfeeding for six months and continued breastfeeding complemented by nutritious solid foods up to age two years; ii) routine vaccination against pertussis, measles, Haemophilus influenzae type B (HiB) and pneumococcus; iii) safe drinking water, sanitation and hand washing facilities; iv) improved cooking stoves to reduce indoor air pollution; and v) effective treatment including amoxicillin dispersible tablets and oxygen [4]. We provide an overview of vaccine preventable respiratory infections, current implementation status and novel developments, with a specific focus on the developing world.
Currently Available Vaccines
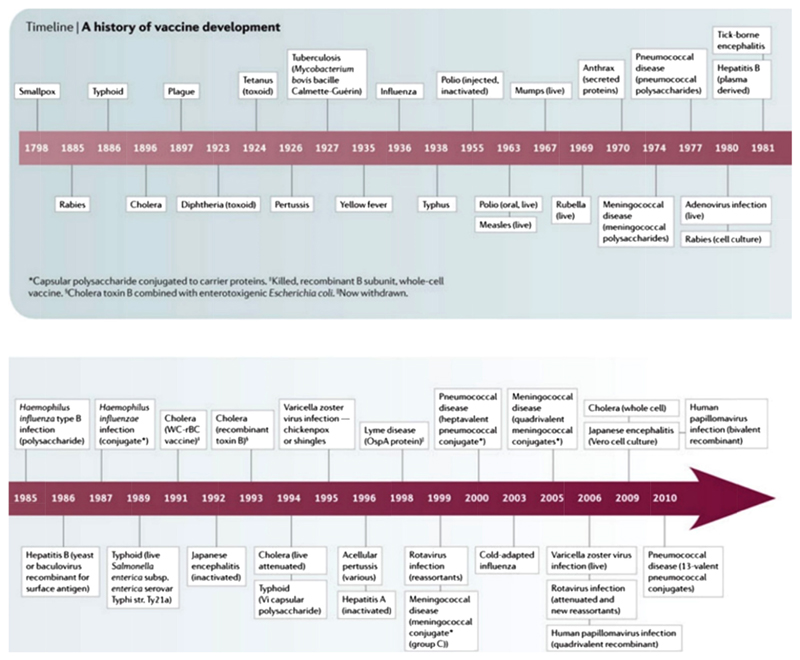
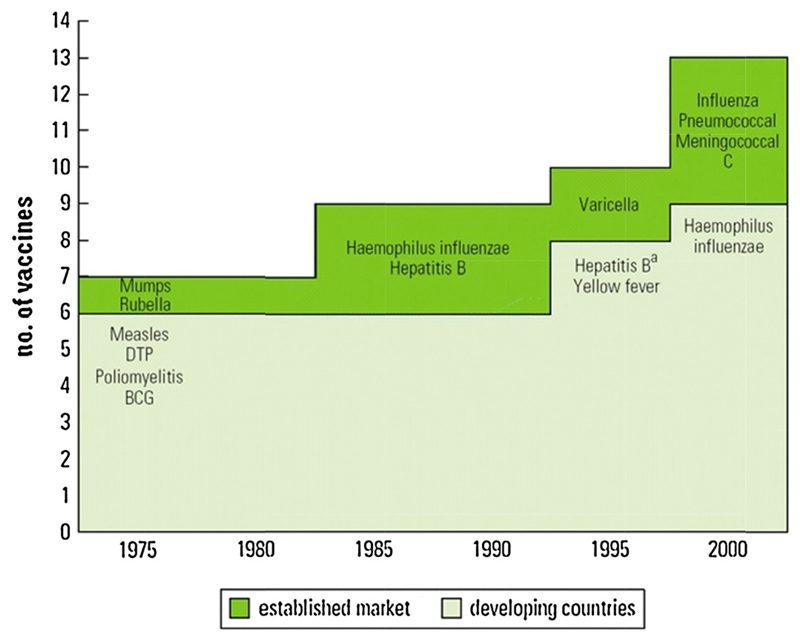
The first vaccine was developed against smallpox in 1796, but it was not widely used until the 20th century (Figure 2) [5]. Since then vaccination has proven to be one of the most cost-effective health interventions available, leading to the global eradication of small pox in 1980. It is also a key intervention to reduce the unacceptably high mortality associated with childhood pneumonia [6,7]. Uptake of vaccination against important childhood infections, especially in the developing world, increased greatly after the creation of the Expanded Programme of Immunisation (EPI) and the Global Alliance for Vaccination and Immunisation (GAVI) [8]. It is estimated that by 2013, GAVI-led immunisation efforts had averted six million deaths from vaccine preventable diseases [6]. However, progress beyond the core EPI set of vaccines has been relatively slow and the “Expanded Programme of Immunisation (EPI)” term has become a bit of a misnomer with the exclusion of key conjugate vaccines. The WHO estimates that 1.5 million children continue to die every year from vaccine-preventable diseases, accounting for 17% of all under-5 deaths [9]. Delayed introduction of conjugated vaccines against Haemophilus influenza type-b (Hib) and pneumococcus in developing countries is mostly attributed to high cost and lack of political will; resulting in widening vaccine schedule gaps in developed and developing countries (Figure 3) [10].
Figure 2. Timeline of vaccine development against major vaccine preventable diseases.
‘Adapted by permission from Macmillan Publishers Ltd: [NATURE REVIEWS] (The development of vaccines: how the past led to the future) [5], copyright 2011
Figure 3. Childhood vaccines included in routine vaccination schedules in Developing and Established-Market Countries.
‘Translated with permission of the WHO from State of the World’s Vaccines and Immunisation, 2002.’ [77]
The WHO lists 27 vaccine-preventable or potentially preventable diseases; [11] those commonly linked with respiratory tract infections include Streptococcus pneumoniae, Hib, pertussis, influenza, measles, and tuberculosis. Streptococcus pneumonia and Hib are considered to be the leading causes of child pneumonia deaths worldwide and universal use of conjugated Hib and pneumococcal vaccines should prevent approximately 1 million child deaths per year [12]. It has been argued that with the expected decline of bacterial causes of pneumonia due to increasing vaccine coverage, viral causes may be emerging as commoner causes of pneumonia in children. A study done in Pakistan found that of children admitted with WHO-defined severe pneumonia, up to 36% had at least a viral cause identified; human metapneumovirus was detected in 24 (14.2%), influenza A virus in 9 (5.3%) and respiratory syncytial virus (RSV) in 30 (17.8%) [13].
Pneumococcal disease
Pneumococcal disease is caused by the gram-positive encapsulated bacterium Streptococcus pneumonia; over 90 different strain types have been recognized [14]. Streptococcus pneumonia is a common coloniser of the nasopharynx, but clinical disease mainly presents as pneumonia, sinusitis, otitis media and invasive pneumococcal disease (IPD). IPD occurs when the infection spreads into the bloodstream or to a normally sterile site such as the brain, resulting in bacteraemia and/or bacterial meningitis that are associated with significant morbidity and mortality. Global estimates indicate that pneumococcal disease is responsible for approximately 500, 000 deaths in children annually [15].
The first pneumococcal vaccine was licensed in 1977. This polysaccharide vaccine protected against 14 different strains and expanded to protect against 23 strains in 1984 [16]. The vaccine was found to be effective in adults, but did not generate adequate protective immunity in children younger than two years of age [16]. An enhanced conjugate vaccine for young children (PCV-7) was licensed in 2000. The 7 strains included in this vaccine represented those causing the highest disease burden in the United States of America (USA) and other developed countries, with little consideration of common strains in resource-limited settings. Initial studies demonstrated that PCV-7 was highly effective, reducing IPD caused by vaccine serotypes by more than 90% in young children; with a median all serotype IPD reduction of 45%. [17] However, IPD caused by non-vaccine serotypes increased over time. Many countries have since replaced PCV-7 with higher valency vaccines (PCV-10 or PCV-13) to cover the most common emergent serotypes. A recent study reported vaccine effectiveness against IPD of 90% for the PCV-7 serotypes and 75% for the six additional serotypes included in PCV-13 [18]. Pneumococcal vaccine impact is measured by a decrease in the incidence of IPD and reduction in hospitalisation rather than a direct impact on under-5 mortality [7].
In 2007, WHO recommended the inclusion of pneumococcal vaccines in childhood immunisation programmes worldwide with the hope that international scale-up will reduce annual deaths in children under 5 years of age by 1.5 million in 2020 [6]. Several studies are already reporting declining incidence of childhood pneumonia with rising vaccine coverage even in resource-limited settings [16,19–22], but progress is frustratingly slow and the commitment of national governments to fund fully inclusive vaccination programs limited. Pneumococcal vaccine was introduced in 103 countries (including Pakistan, the Philippines and Uganda where the vaccine was partially introduced) by the end of 2013, up from 87 countries in 2012. However, global coverage was estimated at only 25% in 2013 [9].
It is important to not only ensure adequate vaccine uptake, but also the quality of these programmes. A recent publication from South Africa showed a high incidence of pneumonia in the first year of life despite good PCV coverage, emphasising the potential detrimental influence of human immune-deficiency virus (HIV) infection, malnutrition and the need for completion of the full primary series for optimal protection [23]. However, despite relatively high disease rates, the pneumonia case fatality rate in this cohort was quite low (1.4%), presumably due to reduced pneumonia severity from partial vaccine coverage and well-functioning primary care services [23].
Haemophilus influenzae type b (Hib)
Haemophilus influenzae type b is a gram negative bacterium transmitted by droplets. It is a common cause of meningitis, pneumonia, epiglottitis, septic arthritis, osteomyelitis and sepsis in unvaccinated children [24]. Hib is thought to be responsible for approximately 20% of acute lower respiratory infection deaths in the absence of immunisation and is the second most common cause of pneumonia in children [3]. In the absence of universal vaccination, Hib continues to cause more than 350 000 deaths and more than 8 million cases of severe disease annually [25].
Hib conjugate vaccine is available as a monovalent preparation or in combination as one of the routine childhood pentavalent vaccines. The vaccine is safe and efficacious if used appropriately; efficacy of two doses of Hib vaccine is reported as 94%, but only 38% for a single dose [26]. The revised EPI guidelines recommend three primary doses of HiB conjugate vaccine administered with the diphtheria, tetanus and pertussis vaccine, starting at 6 weeks of age, with 4–8 weeks between doses. The WHO reported coverage of 52% with three doses of Hib in 189 countries where it had been introduced by 2013 [9].
Studies from resource-limited settings demonstrate declining incidence of Hib disease, with greater effect seen with increased coverage and increase in number of years since introduction of the vaccine into national programmes [26–30]. Declining disease rates, in both vaccinated and unvaccinated children, may be explained by the indirect effect of Hib vaccination to reduce Hib nasal carriage within the general community [26]. Studies also report reduced all-cause radiologically confirmed pneumonia; in Vietnam the adjusted annual incidence of “all cause pneumonia” declined by 39% after introduction of Hib vaccine [31]. Cases of vaccine failure have been reported, which suggests that some non-type b Haemophilus influenzae strains are becoming more virulent, while there may also be a need for a booster Hib vaccine beyond infancy [32–35].
Pertussis
Pertussis (whooping cough) is a highly contagious bacterial disease caused by Bordetella pertussis and is typically associated with a paroxysmal cough followed by a characteristic whoop. Bronchopneumonia is the most common complication associated with high mortality [36]. WHO estimates that 16 million cases occur worldwide, mostly in developing countries, and that close to 200, 000 children die from the disease annually [12,36]. Two types of pertussis vaccines are available: whole-cell vaccines based on killed B. pertussis organisms, and acellular vaccines based on highly purified, selected components of the bacteria. The vaccines are usually offered in combination with diphtheria toxoid and tetanus toxoid (or additionally with poliomyelitis, hepatitis B and Haemophilus influenza type b). The whole-cell vaccines are effective and inexpensive but have been associated with adverse events related to local swelling, pain and high fever, which led to the development of more expensive acellular vaccines that are widely used in developed countries [10].
Pertussis vaccines have been associated with a greater than 90% reduction in pertussis incidence and mortality [12]. WHO estimates that global vaccination averts nearly 700, 000 deaths from pertussis annually [36,37]. There have been systematic reviews of pertussis vaccine effectiveness including a recent Cochrane review [38]; but most studies are old and were conducted in high income countries with limited data from resource-limited settings. A clinical trial from Senegal reported 96% vaccine efficacy for whole cell compared to 85% for the acellular pertussis vaccines respectively [39]. WHO recommends that the first dose of pertussis vaccine should be administered at six weeks of age and subsequent doses 4-8 weeks apart, with the last dose of the primary series delivered by 6 months and a booster during the second year of life. This should confer protection for at least six years, but waning immunity beyond adolescence is a major concern [37]. It has been estimated that more than 90% coverage with at least three doses of the vaccine is required to eliminate the risk of severe pertussis in infancy [37], although this is highly dependent on the duration of protection provided. In 2013, an estimated 84% (112 million) of infants worldwide were vaccinated with three doses of diphtheriatetanus-pertussis (DTP3) containing vaccine [9]. Despite good coverage rates, a resurgence of pertussis has been observed in several developed countries, also affecting infants too young to be vaccinated; this is possibly due to disease among the aged (grandfathers and mothers), inadequate coverage among certain pockets of the population and early waning of immunity with the use of acellular vaccines [7,40]. To mitigate this, some developed countries have developed a cocooning strategy with the vaccination of mothers and other contacts of newborns and infants in order to prevent the transmission of pertussis to infants who may not have completed their primary vaccination series [41].
Measles
Measles virus belongs to the Paramyxoviridae family. Measles is a highly contagious viral disease and remains a leading killer among vaccine-preventable diseases. It is responsible for approximately 44% of the 1.7 million vaccine-preventable deaths among children annually [42]. In 2013, there were 145, 700 measles deaths globally; mostly among children under 5 years of age [43]. Bacterial pneumonia is a common complication of measles, occurring in 2-27% of children in community-based studies and in 16-77% of hospitalised children [12,44]. Pneumonia is estimated to contribute to 56-86% of all deaths attributed to measles, and the most commonly identified causative organism is Streptococcus pneumoniae [12,42,44].
Measles vaccine, in use since the 1960s, is safe, inexpensive and effective in preventing disease in young children (1-5 years of age) [45,46]. Measles vaccination coverage has been used as a marker of access to child health services and is thought to be one of the most effective public health strategies in mitigating child deaths [43,46]. It has been estimated that from 2000 to 2010, the annual measles incidence aggregated across all countries fell 66%, from 4·6 to 1·6 cases per 1000 population [47]. During the same period, global measles vaccine coverage increased from 72% to 85%, the reported number of measles cases declined 62% from 853 480 (140 per million total population) to 327 305 and measles mortality decreased 74%, from approximately 535 300 deaths to 139 300 [47]. The WHO estimates that between 2000-2013 measles vaccination prevented approximately 15·6 million deaths globally [43]. Increased measles vaccine coverage has made a key contribution to reduced under-5 mortality and achieving millennium development goal number 4 [6]. However, frequent disease outbreaks among unvaccinated pockets of the population remain a serious concern, due to measles exceptional infectiousness and high morbidity.
Influenza
Influenza viruses belong to the Orthomyxoviridae family. There are three types of seasonal influenza viruses that cause epidemics: A, B and C, with C occurring less frequently [48]. Some animal influenza strains e.g. avian (H5N1 & H7N9) and swine (H1&H3) strains have caused serious infections in human beings, but so far limited person-to-person transmission restricted pandemic potential [49]. In the very young, as well as in the elderly or immune-compromised, influenza infection can lead to severe pneumonia and death [48]. The WHO estimates that flu epidemics cause 3-5 million cases of severe illness and 250 000-500 000 deaths annually [48].
The most effective way to prevent common influenza is seasonal vaccination. Changes in circulating virus subtypes (due to antigenic shift and antigenic drift) require frequent reformulation of influenza vaccines. The WHO Global Influenza Surveillance and Response System monitors and provides recommendations for choice of vaccines for each season [48]. The currently circulating subtypes are: influenza A (H1N1); A (H3N2); the influenza B Victoria and Yamagata lineages. Influenza C cause milder sporadic infections and are not included in seasonal influenza vaccines [50]. There are trivalent and newer quadrivalent vaccines available as well, as a live attenuated influenza vaccine (LAIV) administered as a nasal spray. Studies suggest that LAIV is easy and safe to administer and offers good protection; it is recommended in some developed countries for use in children aged 2-8yr [51,52].
The WHO recommends annual seasonal vaccination of all risk groups. A review of influenza vaccines used in middle and high-income countries found pooled efficacy of 72-81% [53]. In the US influenza vaccination was associated with a 75% reduction in life-threatening illness with admission to a paediatric intensive care unit [54]. Use of the flu vaccine in children with mild persistent asthma has been associated with reduced episodes of acute respiratory tract illnesses and asthma exacerbations in Asia [55]. However, there is paucity of data from developing countries on the effect of influenza vaccine due to poor vaccine uptake and inadequate surveillance systems [53,56].
Respiratory Syncytial Virus (RSV)
Respiratory syncytial virus (RSV) belongs to the Paramyxoviridae family, and is transmitted by contact with nasal or oral secretions from infected persons [57]. It occurs seasonally in temperate countries while in tropical countries the virus is detectible year round [57]. RSV is thought to be the leading cause of pneumonia in children especially infants younger than 1yr of age [57,58]. Primary RSV infection increases the risk of secondary bacterial pneumonia, and co-infection is thought to occur in up to a third of pneumonia cases in children in developing countries [59]. A review of the burden of RSV found that there were more than 30 million episodes of RSV-associated respiratory tract infections in children in 2005 [58,60]. This is likely an underestimate especially for developing countries due to lack of proper surveillance and reporting. Mortality was estimated at close to 200, 000 deaths with 99% of these RSV-associated deaths occurring in developing countries [58,60].
There is currently no licensed vaccine for RSV, but there are over 50 vaccine candidates in development, majority in pre-clinical stages and four in clinical stage [61] The first 6 months of life are associated with the highest burden of severe RSV disease [60]. This group however is difficult to target for vaccines because of their immunological immaturity and varying levels of transferred maternal antibodies interfering with the immunogenicity of live vaccines [58]. There are additional challenges RSV vaccine development for children in developing countries due to high rate of HIV infection, malnutrition and other competing public health priorities. A maternal immunisation strategy is considered a viable strategy to protect young infants. One modelling study found that administration of an RSV vaccine to pregnant women able to boost natural maternal antibodies to 8 months of protection would reduce RSV infant incidence by over 30% [62]. The other priority strategy for developing countries is active paediatric immunisation to target to older infants and young children after maternal protection has waned off [61].
Tuberculosis
Mycobacterium tuberculosis (M. tuberculosis), the aetiological agent of tuberculosis (TB), is a leading cause of human disease and death, particularly in developing countries. According to WHO estimates, 80 000 children die from TB each year worldwide with over half a million new cases annually [63]. Bacille Calmette-Guérin (BCG) developed in 1921 from a strain of attenuated live Mycobacterium bovis is the only available vaccine against tuberculosis [64]. Given to newborns, it provides protection against severe forms of paediatric TB with 60-80% protective efficacy [65]. BCG also offers some protection against M. tuberculosis infection, with a risk reduction of 19-27% [65], however, it offers no protection against adult-type disease which is essential to improve epidemic control. A more effective TB vaccine is essential to achieve the ambitious target of global TB elimination by 2050. Results from initial trials have been disappointing, but current efforts are exploring multiple potential applications.
There are around 20 new TB vaccine candidates in various stages of research and development [66]. The various strategies being explored include: i) prevention of infection (pre-exposure vaccines); ii) prevention of disease after exposure (post-exposure vaccines); iii) prevention of recurrence after treatment [64,66]. The candidate vaccines are being designed to be primers that are efficacious for a longer period of time, preventing TB infection as well as disease in infants who have not been infected with M. tuberculosis. Additional candidates are in research for booster vaccines delivered during adolescence to prevent infection and/or progression to active disease for those who are latently infected, as BCG immunity wanes [64]. Host-directed therapies are being developed that could be beneficial for drug resistant tuberculosis by shortening the duration of treatment, restricting inflammatory damage and reducing risk of reinfection [66].
Indirect Vaccine Effects and Economic Impact
Herd immunity occurs when vaccination of a critical number of a population provides protection for non-immune, unvaccinated individuals, with less circulation of the disease and reduced risk of transmission between infected and susceptible subjects [7]. A good example of this is seen from the Gambia where Hib vaccine coverage of less than 70% resulted in dramatic reduction of incidence of meningitis in infants from 200 per 100, 000 to zero in the fifth year after introduction of the vaccine [26]. Other examples of herd immunity are seen in the cocooning strategy for pertussis where young unvaccinated infants are protected once their mothers and other close contacts have been vaccinated and conversely in the case of pneumococcal vaccine where older contacts of vaccinated children are protected from invasive disease [14,41].
Vaccination also has wider pathogen selection consequences and non-specific effects such as: i) serotype replacement; ii) prevention of antibiotic resistance; iii) protection against unrelated pathogens. Serotype replacement with an increase in the incidence of disease caused by non-vaccine serotypes has been observed with pneumococcal vaccines [67]. A meta-analysis of four randomised control trials of PCV vaccination in Sub-Saharan Africa found that nasopharyngeal carriage of vaccine type serotypes was reduced by PCV vaccination while carriage of non-vaccine type serotypes was higher among vaccinated children [68]. Immunisation programmes for pneumococcal disease have also been associated with reduction in antibiotic utilisation, presumably due to less severe disease. Vaccination may therefore reduce the selection of antibiotic resistant bacteria by decreasing antimicrobial exposure within the community which is a wider benefit beyond the target pathogen [7,69]. A South African study illustrated greater than 80% reduction in prevalence of invasive disease caused by antibiotic-resistant pneumococcal serotypes among children younger than 2 years of age after PCV introduction [21].
Live vaccines may increase protection against non-target pathogens through immune priming, for instance, tuberculosis and measles vaccines have been associated with a substantial reduction in overall child mortality, which cannot be explained solely by prevention of the target disease [70–73]. Conversely, inactivated vaccines such as diphtheria-pertussis-tetanus, inactivated polio vaccine and hepatitis B vaccine have been associated with increased morbidity and mortality from other infections [71,72]. Influenza vaccines have been associated with a reduction in acute otitis media in children as well as reductions in the use of antibiotics to treat secondary bacterial infections [74].
Economic impact of vaccines
A review on economic benefits of vaccines found that ecological externalities and productivity gains due to reduced disease risk were an indirect effect of vaccines [75]. Developing countries often have to grapple with competing priorities for limited resources and may fail to appreciate the full economic benefit of a well-functioning vaccination program. Factors contributing to limited uptake of new vaccines include: poor recognition of disease burden and costs; low political commitment; cost of the vaccines relative to national income; civil unrest; and weak health service delivery systems [10].
Commonly reported economic benefits of immunisation include: i) health effects due to morbidity and mortality averted; ii) health treatment costs saved; and short term productivity losses averted due to being unwell or caring for someone who is unwell [75]. There are also broader economic benefits benefitting the wider community. These include: i) long term productivity gains due to reduced cognitive or educational deficits caused by to childhood illness; ii) ecological externalities like herd immunity and reduced antibiotic resistance; iii) equity due to more equal distribution of health outcomes; iv) improved financial stability of health care programmes due to synergies created by vaccination programmes; v) macroeconomic impact due to improved gross domestic product [75].
Conclusion
The Global Vaccine Action Plan (GVAP) was approved by the World Health Assembly in May 2012 to prevent millions of deaths through more equitable access to vaccines [76]. GVAP identified the need for innovation and research across all aspects of immunisation development and delivery, as well as key research priorities (summarised in Table 1).
Table 1. Research needs and future directions.
|
Routine vaccination against common childhood illnesses is one of the most cost-effective strategies to prevent death from pneumonia, the leading cause of death in young children. Although there have been great strides in the development and administration of effective vaccines globally, the countries that carry the greatest burden of pneumonia and other infectious diseases still struggle with vaccinating their children. Gaps in immunisation coverage results from many health system problems and low levels of vaccine research in the developing world, which can be addressed by securing the necessary political will and focussing on key priorities identified by the Global Vaccine Action Plan [76].
Educational Aims.
To describe the burden of disease caused by pneumonia and review efforts to reduce this
To identify discrepancies in vaccine availability in the developed and developing worlds
To review key pulmonary pathogens and assess the evidence base for vaccine prevention
To explore indirect effects and economic impact of vaccines
To identify priorities for pneumonia research in the developing world
Role of Funding Source
None
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare
References
- [1].Rudan I, O’Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of global health. 2013;3(1) doi: 10.7189/jogh.03.010401. 010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organisation. Pneumonia Fact Sheet. [accessed 11th May 2015 2015];2014 Nov; http://www.who.int/mediacentre/factsheets/fs331/en/
- [4].World Health Organisation/The United Nations Children’s Fund. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD) Geneva: World Health Organisation; 2013. [Google Scholar]
- [5].Plotkin SA, Plotkin SL. The development of vaccines: how the past led to the future. Nature reviews Microbiology. 2011;9(12):889–93. doi: 10.1038/nrmicro2668. [DOI] [PubMed] [Google Scholar]
- [6].Bustreo F, Okwo-Bele JM, Kamara L. World Health Organisation perspectives on the contribution of the Global Alliance for Vaccines and Immunization on reducing child mortality. Archives of disease in childhood. 2015;100(Suppl 1):S34–7. doi: 10.1136/archdischild-2013-305693. [DOI] [PubMed] [Google Scholar]
- [7].Lernout T, Theeten H, Leuridan E, Van Damme P. Do vaccines save lives? Yes they do! Acta medica portuguesa. 2014;27(2):160–2. [PubMed] [Google Scholar]
- [8].Greenwood B. The contribution of vaccination to global health: past, present and future. Philosophical transactions of the Royal Society of London Series B Biological sciences. 2014;369(1645) doi: 10.1098/rstb.2013.0433. 20130433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].World Health Organisation. Global Immunisation Data. 2014 [Google Scholar]
- [10].Miller MA, S J. Vaccine-Preventable Diseases. In: Jamison DT, F R, Makgoba MW, et al., editors. Disease and Mortality in Sub-Saharan Africa. 2nd edition. Washington (DC): World Bank; 2006. [Google Scholar]
- [11].World Health Organisation. Vaccines and diseases. [accessed 16th April 2015];2015 http://www.who.int/immunization/diseases/en/
- [12].Madhi SA, Levine OS, Hajjeh R, Mansoor OD, Cherian T. Vaccines to prevent pneumonia and improve child survival. Bulletin of the World Health Organization. 2008;86(5):365–72. doi: 10.2471/BLT.07.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ali A, Khowaja AR, Bashir MZ, Aziz F, Mustafa S, Zaidi A. Role of human metapneumovirus, influenza A virus and respiratory syncytial virus in causing WHO-defined severe pneumonia in children in a developing country. PloS one. 2013;8(9):e74756. doi: 10.1371/journal.pone.0074756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organisation. Pneumococcal vaccines WHO position paper 2012. 2012 [Google Scholar]
- [15].World Health Organisation. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. [accessed 10th April 2015];2008 http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/
- [16].Lucero MG, Dulalia VE, Nillos LT, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. The Cochrane database of systematic reviews. 2009;(4) doi: 10.1002/14651858.CD004977.pub2. Cd004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Myint TT, Madhava H, Balmer P, et al. The impact of 7-valent pneumococcal conjugate vaccine on invasive pneumococcal disease: a literature review. Advances in therapy. 2013;30(2):127–51. doi: 10.1007/s12325-013-0007-6. [DOI] [PubMed] [Google Scholar]
- [18].Andrews NJ, W P, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis. 2014;14(9):839–46. doi: 10.1016/S1473-3099(14)70822-9. [DOI] [PubMed] [Google Scholar]
- [19].Abrao WM, Mello LM, Silva AS, Nunes AA. Impact of the antipneumococcal conjugate vaccine on the occurrence of infectious respiratory diseases and hospitalization rates in children. Revista da Sociedade Brasileira de Medicina Tropical. 2015;48(1):44–9. doi: 10.1590/0037-8682-0007-2015. [DOI] [PubMed] [Google Scholar]
- [20].Conklin L, Loo JD, Kirk J, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children. The Pediatric infectious disease journal. 2014;33(Suppl 2):S109–18. doi: 10.1097/INF.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].von Gottberg A, de Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. The New England journal of medicine. 2014;371(20):1889–99. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- [22].Hammitt LL, Ojal J, Bashraheil M, et al. Immunogenicity, impact on carriage and reactogenicity of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in Kenyan children aged 1-4 years: a randomized controlled trial. PloS one. 2014;9(1):e85459. doi: 10.1371/journal.pone.0085459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: the Drakenstein Child Health Study. The Lancet Global health. 2015;3(2):e95–103. doi: 10.1016/S2214-109X(14)70360-2. [DOI] [PubMed] [Google Scholar]
- [24].World Health Organisation. Haemophilus influenzae type B Fact Sheet. [accessed 15th April 2015]; http://www.who.int/topics/haemophilus_influenzae/en/
- [25].Watt JP, Wolfson LJ, O’Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374(9693):903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- [26].Adegbola RA, Secka O, Lahai G, et al. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366(9480):144–50. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- [27].Cowgill KD, Ndiritu M, Nyiro J, et al. Effectiveness of Haemophilus influenzae type b Conjugate vaccine introduction into routine childhood immunization in Kenya. Jama. 2006;296(6):671–8. doi: 10.1001/jama.296.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khowaja AR, Mohiuddin S, Cohen AL, et al. Effectiveness of Haemophilus influenzae type b conjugate vaccine on radiologically-confirmed pneumonia in young children in Pakistan. The Journal of pediatrics. 2013;163(1 Suppl):S79–S85.e1. doi: 10.1016/j.jpeds.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sigauque B, Vubil D, Sozinho A, et al. Haemophilus influenzae type b disease among children in rural Mozambique: impact of vaccine introduction. The Journal of pediatrics. 2013;163(1 Suppl):S19–24. doi: 10.1016/j.jpeds.2013.03.026. [DOI] [PubMed] [Google Scholar]
- [30].de Andrade AL, de Andrade JG, Martelli CM, et al. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: a case-control study in Brazil. International journal of epidemiology. 2004;33(1):173–81. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- [31].Flasche S, Takahashi K, Vu DT, et al. Early indication for a reduced burden of radiologically confirmed pneumonia in children following the introduction of routine vaccination against Haemophilus influenzae type b in Nha Trang, Vietnam. Vaccine. 2014;32(51):6963–70. doi: 10.1016/j.vaccine.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].von Gottberg A, Cohen C, Whitelaw A, et al. Invasive disease due to Haemophilus influenzae serotype b ten years after routine vaccination, South Africa, 2003–2009. Vaccine. 2012;30(3):565–71. doi: 10.1016/j.vaccine.2011.11.066. [DOI] [PubMed] [Google Scholar]
- [33].Mangtani P, Mulholland K, Madhi SA, Edmond K, O’Loughlin R, Hajjeh R. Haemophilus influenzae type b disease in HIV-infected children: a review of the disease epidemiology and effectiveness of Hib conjugate vaccines. Vaccine. 2010;28(7):1677–83. doi: 10.1016/j.vaccine.2009.12.011. [DOI] [PubMed] [Google Scholar]
- [34].Scheifele DW, Halperin SA, Rubin E, et al. Safety and immunogenicity of a pentavalent combination vaccine (diphtheria, tetanus, acellular pertussis, polio, and haemophilus influenzae type B conjugate) when administered as a fourth dose at 15 to 18 months of age. Human vaccines. 2005;1(5):180–6. doi: 10.4161/hv.1.5.2079. [DOI] [PubMed] [Google Scholar]
- [35].Ladhani SN. Two decades of experience with the Haemophilus influenzae serotype b conjugate vaccine in the United Kingdom. Clinical therapeutics. 2012;34(2):385–99. doi: 10.1016/j.clinthera.2011.11.027. [DOI] [PubMed] [Google Scholar]
- [36].World Health Organisation. Pertussis 7th April 2015. [accessed 13/05/2015 2015];2015 http://www.who.int/immunization/diseases/pertussis/en/
- [37].World Health Organisation. Pertussis vaccines: WHO position paper. 2010 [Google Scholar]
- [38].Zhang L, Prietsch SO, Axelsson I, Halperin SA. Acellular vaccines for preventing whooping cough in children. The Cochrane database of systematic reviews. 2014;9 doi: 10.1002/14651858.CD001478.pub6. Cd001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Simondon F, Preziosi MP, Yam A, et al. A randomized double-blind trial comparing a two-component acellular to a whole-cell pertussis vaccine in Senegal. Vaccine. 1997;15(15):1606–12. doi: 10.1016/s0264-410x(97)00100-x. [DOI] [PubMed] [Google Scholar]
- [40].World Health Organisation. Revised guidance on the choice of pertussis vaccines. 2014 Jul; [PubMed] [Google Scholar]
- [41].Lim GH, Deeks SL, Crowcroft NS. A cocoon immunisation strategy against pertussis for infants: does it make sense for Ontario? Euro surveillance: bulletin Europeen sur les maladies transmissibles European communicable disease bulletin. 2014;19(5) doi: 10.2807/1560-7917.es2014.19.5.20688. [DOI] [PubMed] [Google Scholar]
- [42].Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. The Cochrane database of systematic reviews. 2013;8 doi: 10.1002/14651858.CD001477.pub4. Cd001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].World Health Organisation. Measles Fact Sheet. 2015 [Google Scholar]
- [44].Duke T, Mgone CS. Measles: not just another viral exanthem. Lancet. 2003;361(9359):763–73. doi: 10.1016/S0140-6736(03)12661-X. [DOI] [PubMed] [Google Scholar]
- [45].Bhutta ZA, Das JK, Walker N, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: what works and at what cost? Lancet. 2013;381(9875):1417–29. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- [46].World Health Organisation. Global measles and rubella strategic plan: 2012-2020. 2012 [Google Scholar]
- [47].Simons E, Ferrari M, Fricks J, et al. Assessment of the 2010 global measles mortality reduction goal: results from a model of surveillance data. Lancet. 2012;379(9832):2173–8. doi: 10.1016/S0140-6736(12)60522-4. [DOI] [PubMed] [Google Scholar]
- [48].World Health Organisation. Influenza (Seasonal) Fact Sheet. [accessed 25th May 2015 2015];2014 Mar; http://www.who.int/mediacentre/factsheets/fs211/en/
- [49].World Health Organisation. Avian Influenza Fact Sheet. [accessed 25th May 2015];2014 Mar; http://www.who.int/mediacentre/factsheets/avian_influenza/en/
- [50].World Health Organisation. Influenza update. [accessed 25th May 2015];2015 May 2; http://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/
- [51].Centers for Disease Control and Prevention. CDC Statement on LAIV Effectiveness and Vaccination of Children. [accessed 25th May 2015];2014 Novemebr; http://www.cdc.gov/flu/news/nasal-spray-effectiveness.htm.
- [52].Ambrose CS, Wu X, Knuf M, Wutzler P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: a meta-analysis of 8 randomized controlled studies. Vaccine. 2012;30(5):886–92. doi: 10.1016/j.vaccine.2011.11.104. [DOI] [PubMed] [Google Scholar]
- [53].Breteler JK, Tam JS, Jit M, Ket JC, De Boer MR. Efficacy and effectiveness of seasonal and pandemic A (H1N1) 2009 influenza vaccines in low and middle income countries: a systematic review and meta-analysis. Vaccine. 2013;31(45):5168–77. doi: 10.1016/j.vaccine.2013.08.056. [DOI] [PubMed] [Google Scholar]
- [54].Ferdinands JM, Olsho LEW, Agan AA, et al. Effectiveness of Influenza Vaccine Against Life-threatening RT-PCR-confirmed Influenza Illness in US Children, 2010-2012. Journal of Infectious Diseases. 2014;210(5):674–83. doi: 10.1093/infdis/jiu185. [DOI] [PubMed] [Google Scholar]
- [55].Jaiwong C, Ngamphaiboon J. Effects of inactivated influenza vaccine on respiratory illnesses and asthma-related events in children with mild persistent asthma in Asia. Asian Pacific journal of allergy and immunology/launched by the Allergy and Immunology Society of Thailand. 2015;33(1):3–7. doi: 10.12932/AP0511.33.2.2015. [DOI] [PubMed] [Google Scholar]
- [56].Ntshoe GM, McAnerney JM, Tempia S, et al. Influenza epidemiology and vaccine effectiveness among patients with influenza-like illness, viral watch sentinel sites, South Africa, 2005-2009. PloS one. 2014;9(4):e94681. doi: 10.1371/journal.pone.0094681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].World Health Organisation. Status of Vaccine Research and Development of Vaccines for RSV. [accessed 15th June 2015];2014 http://who.int/immunization/research/meetings_workshops/WHO_PDVAC_RSV.pdf.
- [58].Nair H, Verma VR, Theodoratou E, et al. An evaluation of the emerging interventions against Respiratory Syncytial Virus (RSV)-associated acute lower respiratory infections in children. BMC public health. 2011;11(Suppl 3):S30. doi: 10.1186/1471-2458-11-S3-S30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008;86(5):408–16. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–55. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].World Health Organisation. WHO Consulatation on Respiratory Syncytial Virus (RSV) vaccine development. 2015 Mar 23rd-24th; [Google Scholar]
- [62].Poletti P, Merler S, Ajelli M, et al. Evaluating vaccination strategies for reducing infant respiratory syncytial virus infection in low-income settings. BMC medicine. 2015;13:49. doi: 10.1186/s12916-015-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].World Health Organisation. Tuberculosis Fact Sheet. [accessed 6th April 2015];2015 Mar; http://www.who.int/mediacentre/factsheets/fs104/en/
- [64].World Health Organisation. Tuberculosis vaccine development. [accessed 28th May 2015];2015 May 12th; http://www.who.int/immunization/research/development/tuberculosis/en/
- [65].Roy A, Eisenhut M, Harris RJ, et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis. BMJ (Clinical research ed) 2014;349:g4643. doi: 10.1136/bmj.g4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kaufmann SH, Lange C, Rao M, et al. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. The Lancet Respiratory medicine. 2014;2(4):301–20. doi: 10.1016/S2213-2600(14)70033-5. [DOI] [PubMed] [Google Scholar]
- [67].Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. International journal of antimicrobial agents. 2008;32(3):199–206. doi: 10.1016/j.ijantimicag.2008.01.021. [DOI] [PubMed] [Google Scholar]
- [68].Usuf E, Bottomley C, Adegbola RA, Hall A. Pneumococcal carriage in sub-Saharan Africa–a systematic review. PloS one. 2014;9(1):e85001. doi: 10.1371/journal.pone.0085001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bulletin of the World Health Organization. 2008;86(2):140–6. doi: 10.2471/BLT.07.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends in immunology. 2013;34(9):431–9. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- [71].Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for non-targeted infections. JAMA. 2014;311(8):826–35. doi: 10.1001/jama.2014.470. [DOI] [PubMed] [Google Scholar]
- [72].Shann F. The non-specific effects of vaccines. Archives of disease in childhood. 2010;95(9):662–7. doi: 10.1136/adc.2009.157537. [DOI] [PubMed] [Google Scholar]
- [73].Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nature immunology. 2014;15(10):895–9. doi: 10.1038/ni.2961. [DOI] [PubMed] [Google Scholar]
- [74].Norhayati MN, Ho JJ, Azman MY. Influenza vaccines for preventing acute otitis media in infants and children. The Cochrane database of systematic reviews. 2015;3 doi: 10.1002/14651858.CD010089.pub2. Cd010089. [DOI] [PubMed] [Google Scholar]
- [75].Deogaonkar R, Hutubessy R, van der Putten I, Evers S, Jit M. Systematic review of studies evaluating the broader economic impact of vaccination in low and middle income countries. BMC public health. 2012;12:878. doi: 10.1186/1471-2458-12-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].World Health Organisation. Global Vaccine Action Plan 2011-2020. Geneva: WHO Press; 2013. [Google Scholar]
- [77].World Health Organisation. State of the World’s Vaccines and Immunisation. Geneva: 2002. [Google Scholar]