Abstract
White matter hyperintensities (WMH), a marker of small vessel cerebrovascular disease, increase risk for mild cognitive impairment (MCI). Less is known about whether regional WMH distinguish MCI subtypes and predict decline in everyday functioning. 618 Alzheimer’s Disease Neuroimaging Initiative participants (301 cognitively normal [CN]; 232 amnestic MCI [aMCI]; 85 nonamnestic MCI [naMCI]) underwent neuropsychological testing, MRI, and assessment of everyday functioning. aMCI participants showed greater temporal (p=.002) and occipital WMH (p=.030) relative to CN whereas naMCI participants had greater frontal (p=.045), temporal (p=.003), parietal (p=.018), and occipital (p<.001) WMH compared to CN. Relative to those with aMCI, individuals with naMCI showed greater occipital WMH (p=.013). Greater WMH in temporal (p=.001) and occipital regions (p=.006) was associated with faster decline in everyday functioning across the sample. Temporal lobe WMH were disproportionately associated with accelerated functional decline among naMCI (p=.045). Regional WMH volumes vary across cognitive groups and predict functional decline. Cerebrovascular markers may help identify individuals at risk for decline and distinguish subtypes of cognitive impairment.
Keywords: Mild cognitive impairment, MCI subtypes, White matter hyperintensity, Cerebrovascular disease, Neuropsychology, Daily functioning
1. Introduction
Although mild cognitive impairment (MCI) is often recognized as representing a prodromal stage of Alzheimer’s disease (AD), it has become clear that MCI is a heterogeneous disorder with distinct clinical subtypes (e.g., amnestic versus nonamnestic) that may differ in underlying pathology (Petersen and Morris, 2005). Distinguishing MCI subtypes may have implications for detection of MCI, treatment selection, prognosis, and selection for clinical trials. Notably, many previous studies designed to identify biological markers of MCI focused on amnestic MCI (aMCI), gray matter changes, and measures of amyloid and tau whereas less research has focused on nonamnestic MCI (naMCI) and cerebrovascular alterations, the latter which are prominent in MCI (Bangen et al., 2018; Brickman et al., 2012; Schneider et al., 2009).
White matter hyperintensities (WMH), visualized as increased signal on T2-weighted magnetic resonance imaging (MRI), are a very common finding on neuroimaging among older adults. WMH are associated with small vessel cerebrovascular disease and may reflect demyelination and axonal loss due to ischemia or neuronal loss and microglial and endothelial activation (Wardlaw et al., 2015). Although WMH were once thought to reflect benign changes in the underlying tissue in “normal” aging, it is now clear that they are associated with cognitive impairment and substantially increase risk of stroke and dementia. In a recent study of individuals with autosomal dominant genetic mutations for AD, increased WMH volume, particularly in posterior regions, was observed several years prior to estimated symptom onset suggesting that WMH are an important feature of AD among younger to middle-aged individuals generally thought to be devoid of non-AD pathologies (Lee et al., 2016). It is also now clear that WMH volume predicts risk and progression of clinical symptoms in MCI (Bangen et al., 2018; Brickman et al., 2012; Brickman et al., 2015; Silbert et al., 2012; Tosto et al., 2014). However, to date, no longitudinal studies have examined whether lobar WMH volume is associated with changes in everyday functioning over time among older adults at risk for AD.
Despite strong evidence of heterogeneity in MCI and AD together with the important role of WMH in AD, few studies have examined WMH in MCI clinical subtypes. Findings from existing studies have been mixed. Some have shown that naMCI subgroups have greater white matter lesion burden relative to aMCI subgroups (Delano-Wood et al., 2009), whereas others have found that WMH are associated with amnestic but not naMCI (Luchsinger et al., 2009). Other studies provided evidence for a threshold effect whereby mild small-vessel cerebrovascular disease showed poorer episodic memory compared with working memory, moderate small-vessel cerebrovascular disease displayed equal impairment on episodic memory and working memory, and those with the most severe small-vessel cerebrovascular disease showed poorer working memory relative to episodic memory (Libon et al., 2008). Another study found that volume of WMH distinguished among MCI subtypes only when combined with data related to gray matter atrophy (van de Pol et al., 2009).
We used a well-characterized sample of older adults without dementia from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) in order to investigate the associations between regionally-distributed WMH, MCI clinical subtypes, and informant-reported everyday functioning. We hypothesized that (1) greater WMH volume, particularly in posterior regions, would be associated with MCI, and (2) the temporal lobe may be disproportionally affected in aMCI versus those with normal cognition whereas the frontal, parietal, and occipital lobe may be disproportionally affected in naMCI relative to those with normal cognition. We also expected that greater WMH volume would be related to functional decline over time, particularly among participants with MCI.
2. Material and methods
2.1. The ADNI dataset
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD).
2.2. Participants
All participants included in ADNI were between the ages of 55 and 90 years old, had completed at least 6 years of education, were Spanish or English speakers, had Geriatric Depression Scale scores <6 (possible score range is 0-15) (Sheikh and Yesavage, 1986), had modified Hachinski Ischemic Scale (HIS) scores ≤4, and were free of any significant neurological disease or systemic illness. Only participants without dementia at baseline and who underwent neuropsychological testing, assessment of everyday functioning with the Functional Assessment Questionnaire (FAQ), and magnetic resonance imaging (MRI) anatomical scans with lobar WMH volume data available were included in the present analyses (n = 618).
Participants were diagnostically classified as aMCI (n=230), naMCI (n=85), or cognitively normal (n=303) for this study using an actuarial neuropsychological diagnostic method (Bondi et al., 2014; Jak et al., 2009) applied to each participant’s baseline neuropsychological assessment data. Six neuropsychological measures from ADNI were chosen because they are routinely used in assessing early cognitive changes in AD, they were administered to all ADNI participants, they assess multiple cognitive domains, and have been used by several previously published studies applying these same actuarial neuropsychological criteria to diagnose MCI in ADNI (Bangen et al., 2016; Bondi et al., 2014; Edmonds et al., 2015). The six measures included: Animal Fluency total score and 30-item Boston Naming Test (BNT) total score in the language domain; Trail Making Test, Parts A and B times to completion in the speed/executive functioning domain; and Rey Auditory Verbal Learning Test (AVLT) 30-minute delayed free recall (number of words recalled) and AVLT recognition (number of words correctly recognized minus false positive errors) in the episodic memory domain. Of note, none of these cognitive measures were used in making the initial ADNI diagnostic classification. Each cognitive measure was converted to an age-, education- and sex-corrected z-score based on a sample of cognitively normal ADNI participants who remained normal throughout their participation in the study.
Participants were considered to have MCI if any one of the following criteria were met: 1) they had an impaired score, defined as >1 SD below the age-, education-, and sex-corrected normative mean, on both measures within at least one cognitive domain (i.e., memory, language, or speed/executive function) or 2) they had one impaired score, defined as >1 SD below the age-, education-, and sex-corrected corrected normative mean, in each of the three cognitive domains sampled. If neither of these criteria were met, the participant was classified as cognitively normal. Participants classified as MCI were further categorized as aMCI if memory was impaired based on the above criteria and naMCI if memory was intact but non-memory domains (i.e., speed/executive function and/or language) were impaired.
Participants underwent everyday functioning assessment (i.e., informant-rated FAQ) at ADNI’s baseline assessment as well as at annual follow-up visits (i.e., 12-, 24-, 36-, and 48-months). Of the 618 participants in the sample for the present study, all 618 participants had complete data at baseline; 560 had complete data at the 12-month follow-up visit; 498 had complete data at the 24-month follow-up visit; 438 had complete data at the 36-month follow-up visit; and 266 participants (155 cognitively normal, 80 aMCI, 31 naMCI) had complete data at the 48-month follow-up visit. A smaller subset of the sample had FAQ follow-up at 60 months and beyond. Given the reduction in available data at 60 months of follow-up and some MCI subtype groups having fewer than 20 participants with FAQ data at that time point, our primary analyses focused on follow-up to 48 months. This study was approved by the Institutional Review Boards of all participating institutions. Informed written consent was obtained from all participants at each site.
2.3. Assessment of Everyday Functioning
Everyday functioning was operationalized as total score on the FAQ, a standardized assessment of instrumental activities of daily living (IADL). As previously described (Bangen et al., 2019; Thomas et al., 2017), the FAQ was completed by each participant’s study partner at baseline, 6-month follow-up, and then annually thereafter. The study partner rated the participant’s performance over the previous 4 weeks on 10 categories of daily activities including: (1) writing checks, paying bills, or balancing a checkbook; (2) assembling tax records, business affairs, or other papers; (3) shopping alone for clothes, household necessities, or groceries; (4) playing a game of skill such as bridge or chess or working on a hobby; (5) making coffee or tea; (6) preparing a balanced meal; (7) keeping track of current events; (8) paying attention to and understanding a TV program, book, or magazine; (9) remembering appointments, family occasions, holidays, medications; and (10) traveling out of the neighborhood. Each of the 10 items was rated on a 4-point scale, with higher scores indicating greater dependence (dependent = 3; requires assistance = 2; has difficulty but does by self = 1; normal = 0). The FAQ total score was calculated as the sum of the 10 individual activity scores (range = 0-30).
2.4. Image Acquisition
A detailed description of ADNI MR imaging data acquisition can be found online (www.loni.usc.edu). Briefly, a standardized protocol for MR image acquisition was implemented across ADNI sites and platforms. The protocol was validated across MR platforms (Jack et al., 2008). All data was acquired on 1.5 T systems. 3D T1-weighted magnetization prepared rapid gradient echo sequences (MP-RAGE) were acquired in the sagittal orientation for morphometric analyses. A proton density/T2-weighted fast spin echo (FSE) sequence was acquired in the axial orientation for pathology detection including white matter hyperintensity quantification. All imaging sites included in the ADNI study were required to pass rigorous scanner validation tests and phantom-based monitoring (Jack et al., 2008).
2.5. Image Analysis
A detailed description of ADNI MR imaging data acquisition and processing can be found online (www.loni.usc.edu). Briefly, T1-weighted structural scans collected at baseline were motion corrected and segmented and parcellated using an analysis pipeline based on FreeSurfer and customized Matlab code (Holland et al., 2009). Brain volume was normalized by dividing absolute whole brain volume by the estimated intracranial volume. Normalized brain volume was used as a covariate in longitudinal analyses.
WMH volumes were obtained from ADNI, WMH were detected on coregistered T1-, T2-, and PD-weighted images using an automated method that has been previously described in detail (Carmichael et al., 2010; Schwarz et al., 2009). Briefly, the T1-weighted image was stripped of nonbrain tissues and nonlinearly aligned to a minimum deformation template (Kochunov et al., 2001; Rueckert et al., 1999). The T2- and PD-weighted images were stripped of nonbrain tissues and warped to the space of the minimum deformation template image based on the T1 alignment and warping parameters. WMH were detected in minimum deformation template space at each voxel based on image intensities of the PD, T1, and T2 images combined with a spatial prior (i.e., the prior probability of WMH occurring at a given voxel) and a contextual prior (i.e., the conditional probability of WMH occurring at a given voxel based on the presence of WMH at neighboring voxels). WMH volumes quantified with the above method agreed strongly with WMH volumes estimated based on fluid attenuated inversion recovery (FLAIR) MRI scans in a diverse sample of older adults including individuals with normal cognition, MCI, and dementia (Schwarz et al., 2009). An a priori lobar atlas was used to obtain regional WMH volumes of the frontal, temporal, parietal, and occipital lobes. Development of this atlas have been previously described (DeCarli et al., 2005). Voxels labeled as WMH were summed and multiplied by voxel dimensions to obtain volumes and reported in units of cm3. Brain infarctions were identified on the T2/PD images through qualitative review by an expert specially trained in the detection of MRI infarcts.
2.6. Statistical Analyses
The distribution of WMH volumes was positively skewed and natural log transformation was used to improve distribution normality. Baseline demographic and clinical characteristics by cognitive status (i.e., aMCI, naMCI, normal cognition) were examined using analysis of variance (ANOVA) and chi-square tests. Post-hoc pairwise comparisons were performed if the omnibus test was significant. A 3 (cognitive group) × 4 (lobe) repeated measures analysis of covariance (ANCOVA) compared groups on regional WMH adjusting for age and sex. Region/lobe was treated as a within-subjects factor to determine whether the pattern of regional differences across groups was significant. Mauchly’s test was used to assess deviations from sphericity and, if significant, the Huynh-Feldt correction was applied to analyses. Post-hoc pairwise comparisons were conducted for significant omnibus tests.
Linear mixed effects models analyzed longitudinal rate of change in FAQ score over the 4-year interval as a function of baseline WMH volume. FAQ data from 5 timepoints including baseline, 12-, 24-, 36-, and 48-months follow-up visits was used in analyses. The covariates listed above as well as baseline normalized brain volume were included as fixed effects, as were all simple effects, two-way, and three-way interactions between baseline WMH volume, cognitive group, and visit. The visit variable represents time (in months) from baseline with 0 months representing the baseline visit. Individual differences in intercept and slope were modeled as random effects. Full information maximum likelihood estimation was used to allow for all available data to be included (Singer and Willett, 2003; Woodard, 2017), which has been demonstrated to be less biased than list-wise deletion (Schafer & Graham, 2002). All continuous variables in the model were standardized to have a mean of zero and a standard deviation of one using z-score transformations. The naMCI group served as the reference group and analyses were run a second time with the cognitively normal group serving as the reference group to allow for all pairwise comparisons. Each of the 4 lobes and total WMH volume were examined in separate models. See Supplemental Material for the equation for the longitudinal model.
To address potential inflation of type I error resulting from multiple comparisons, we applied the Benjamini-Hochberg procedure (Benjamini and Hochberg, 1995) to the cross-sectional post-hoc pairwise comparisons of regional WMH volume as well as the two-way group × visit and three-way group × WMH × visit interactions from the longitudinal models. In all cases we corrected for three pairwise comparisons (CN vs aMCI, CN vs naMCI, and aMCI vs naMCI). We assessed results when the false discovery rate (FDR) was controlled at 0.05 and 0.10.
In order to assess potential selective attrition, ANOVA and chi-square tests were performed to examine whether demographic or clinical characteristics differed between participants in the overall analytic sample who completed the month 48 visit (n=266) and those who were missing data at month 48 (n=352). All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 25 (SPSS IBM, New York, USA) and figures were created using the ggplot2 package in R (https://cran.r-project.org/web/packages/ggplot2/index.html).
3. Results
3.1. Participant characteristics
Descriptive data for demographic and clinical characteristics of the sample are presented in Table 1. In comparison to both the cognitively normal and naMCI groups, the aMCI group was significantly younger. In addition, the naMCI group completed fewer years of education relative to the cognitively normal and aMCI groups. Both the amnestic and naMCI groups had a higher proportion APOE ε4 carriers relative to the cognitively normal group. In addition, the aMCI group had higher proportion of APOE ε4 carriers relative to the naMCI group. There were no significant differences across groups in terms of sex distribution or vascular risk burden (i.e., pulse pressure or modified HIS score).
Table 1.
Baseline demographics and clinical characteristics by cognitive status
| Normal Cognition n = 301 |
Amnestic MCI n = 232 |
Nonamnestic MCI n = 85 |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F or χ2 | p | |
| Age, years | 75.97 | 6.16 | 73.78 | 6.97 | 77.07 | 6.44 | 11.03 | <0.001 |
| Education, years | 15.98 | 2.85 | 15.88 | 2.95 | 14.67 | 3.29 | 6.81 | 0.001 |
| Sex (% female) | 42.2% | — | 39.2% | — | 32.9% | — | 2.42 | 0.298 |
| APOE ε4 carrier (%)* | 30.9% | — | 59.5% | — | 44.7% | — | 43.61 | <0.001 |
| Pulse Pressure | 59.51 | 15.00 | 59.97 | 16.31 | 63.27 | 16.27 | 1.95 | 0.143 |
| HIS | 0.58 | 0.66 | 0.62 | 0.76 | 0.62 | 0.67 | 0.22 | 0.804 |
| FAQ | 1.12 | 2.73 | 4.09 | 4.65 | 3.04 | 4.20 | 41.84 | <0.001 |
| Animal Fluency | −0.07 | 0.98 | −0.98 | 0.89 | −1.15 | 0.81 | 84.34 | <0.001 |
| Boston Naming Test | −0.12 | 1.14 | −1.40 | 1.82 | −2.20 | 1.99 | 79.01 | <0.001 |
| Trails A | 0.04 | 0.89 | −0.84 | 1.95 | −2.21 | 2.53 | 66.81 | <0.001 |
| Trails B | 0.06 | 0.89 | −1.27 | 1.94 | −2.02 | 2.04 | 83.33 | <0.001 |
| AVLT Delayed Recall | −0.08 | 0.94 | −1.75 | 0.45 | −0.61 | 0.88 | 296.83 | <0.001 |
| AVLT Recognition | 0.07 | 0.87 | −2.37 | 1.15 | −0.50 | 1.15 | 384.76 | <0.001 |
| Total Brain Volume** | 0.68 | 0.03 | 0.67 | 0.03 | 0.67 | 0.03 | 13.47 | <0.001 |
| Brain Infarct(s) (% yes)*** | 7.6% | 9.1% | 7.1% | 0.49 | 0.782 | |||
| WMH volume, cm3**** | ||||||||
| Total | 0.76 | 2.55 | 0.85 | 2.33 | 1.11 | 3.20 | 3.59 | 0.028 |
| Frontal | 0.25 | 1.17 | 0.28 | 1.01 | 0.38 | 1.82 | ||
| Temporal | 0.08 | 0.19 | 0.10 | 0.22 | 0.13 | 0.22 | Group: 6.72 | 0.001 |
| Parietal | 0.27 | 0.89 | 0.30 | 0.86 | 0.38 | 0.98 | Group | |
| Occipital | 0.15 | 0.31 | 0.17 | 0.31 | 0.28 | 0.38 | × region: 2.78 | 0.015 |
Results from analysis of variance (ANOVAs) for continuous variables and chi-square tests for dichotomous variables. Data are summarized as mean (standard deviation), unless otherwise indicated. Significant group differences (p < 0.05) appear in bold font.
Abbreviations: MCI = mild cognitive impairment; SD = standard deviation; APOE = apolipoprotein E; HIS = Hachinski Ischemic Scale; FAQ = Functional Assessment Questionnaire; AVLT = Rey Auditory Verbal Learning Test; WMH = white matter hyperintensity; cm = centimeter
APOE ε4+ = at least one APOE ε4 allele
Total brain volume was normalized by dividing whole brain volume by total intracranial volume.
Brain infarct(s) present versus absent on MRI.
Note that descriptive statistics for the regional WMH volumes are absolute volume in centimeters cubed (cm3) although analyses were performed on log transformed values. Models are adjusted for age and sex.
As expected, the amnestic and naMCI groups had lower performance relative to the cognitively normal group on each of the six individual cognitive measures. In addition, the naMCI group had lower scores on the BNT and Trails A and B relative to the aMCI group, whereas the aMCI performed more poorly than the naMCI group on memory recall and recognition. Also as expected, the amnestic and nonamnestic groups had higher FAQ scores at baseline relative to the cognitively normal group. In addition, the aMCI had significantly higher FAQ scores relative to the naMCI group. Both the amnestic and naMCI had significantly reduced total brain volume relative to the cognitively normal group although the two MCI groups did not differ.
3.2. Attrition
Attrition from baseline to 48 months differed across the cognitive groups (χ2= 16.032, p < .001). Pairwise comparisons showed that attrition was disproportionately higher in those with aMCI and naMCI relative to the cognitively normal group (χ2= 14.218, p < .001 and χ2= 5.735, p = .017) but did not differ between the amnestic and naMCI subgroups (χ2= .077, p = .781). Attrition did not differ based on age (F = .110, p = .740), sex (χ2= .415, p = .519), or APOE e4 carrier status (χ2= .208, p = .648).
3.3. Cross-sectional Associations of Regional WMH Volume and MCI
Adjusting for age and sex, there was a main effect of cognitive group on total WMH volume (F4, 613=3.59, p = .028). Post-hoc pairwise comparison tests showed that the naMCI group had higher total WMH volume relative to the cognitively normal group (p = .011) although there were no differences across the other groups (aMCI versus cognitively normal: p = .114; amnestic versus naMCI: p = .179). The statistical significance for these post-hoc pairwise comparisons was retained using a 0.05 FDR.
For the repeated measures ANCOVA model, Mauchly’s test of sphericity was significant (p < .05) and, therefore, the Huynh-Feldt correction was applied. Again, there was a main effect of group (F(2, 613) = 6.72, p = .001). Adjusting for age and sex, there was a significant lobe × group interaction (F(5.175, 1586.116) = 2.78, p = .015). The effects indicated that mean WMH volume varied across groups and the magnitude of group differences varied across the lobes.
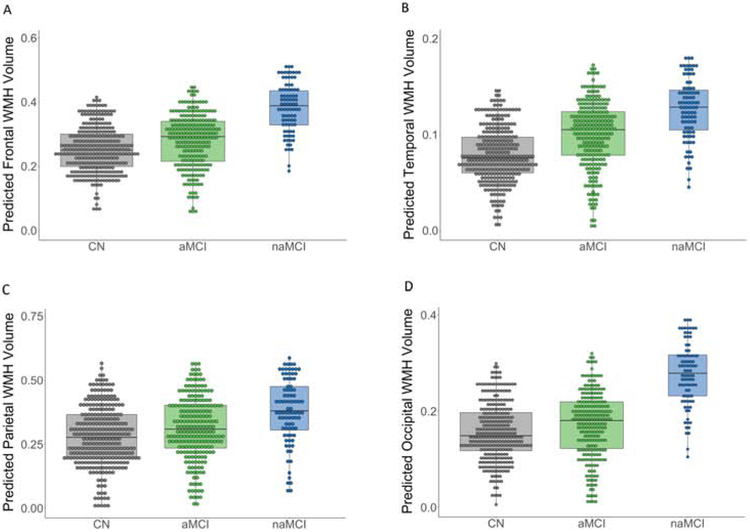
To decompose the interaction, we conducted post-hoc univariate pairwise comparison tests for each lobe. These analyses showed that, adjusting for age and sex: (1) the aMCI group had greater WMH volume relative to the cognitively normal group in the temporal (F(1, 529) = 9.26, p = .002) and occipital lobes (F(1, 529) = 4.71, p = .030); (2) the naMCI group had greater WMH volume compared to the cognitively normal group in the frontal (F(1, 382) = 4.03, p = .045), temporal (F(1, 382) = 9.24, p = .003), parietal (F(1, 382) = 5.69, p = .018), and occipital lobes (F(1, 382) = 16.07, p < .001); and (3) the naMCI group showed significantly greater occipital WMH volume relative to the aMCI group (F(1, 313) = 6.18, p = .013). None of the other post-hoc pairwise comparisons were statistically significant (all p-values ≥ .050). Statistical significance of these post-hoc pairwise comparisons were retained using a 0.10 FDR. With the exception of the difference between the naMCI and CN groups in the frontal lobe, significance of these results was also retained using a 0.05 FDR. See Figure 1.
Figure 1.
Dot-box plots demonstrating regional white matter hyperintensity (WMH) volume for mild cognitive impairment (MCI) and cognitively normal groups. Plots illustrate the predicted WMH volume values from regression models, adjusting for age and sex, for frontal (panel A), temporal (panel B), parietal (panel C), and occipital (panel D) lobes. As described in the results section, univariate post-hoc tests for each lobe revealed that, adjusting for age and sex: (1) the amnestic MCI had significantly greater temporal (p = .002) and occipital WMH volume (p = .030) relative to the cognitively normal group; (2) the nonamnestic MCI had significantly greater WMH volume compared to the cognitively normal group in the frontal (p = .045), temporal (p = .003), parietal (p = .020), and occipital lobes (p < .001); and (3) the nonamnestic MCI showed significantly greater occipital WMH volume relative to the amnestic MCI group (p = .013).
3.4. Longitudinal Prediction of Daily Functioning by Baseline WMH
Linear mixed effects models, adjusting for baseline age, sex, education, baseline normalized total brain volume, and cognitive group (aMCI, naMCI, or normal cognition), examined whether baseline WMH volume predicted longitudinal change in FAQ score across the 48-month follow-up period. Table 2 shows all parameter estimates for the primary models for which the naMCI group served as the reference group. Analyses were re-run with the cognitively normal group (rather than the naMCI group) serving as the reference group (see Supplemental Table 1 for parameter estimates for these models).
Table 2.
Estimates for the full longitudinal model of the association of regional WMH volume and functional abilities
| Frontal | Parietal | Temporal | Occipital | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | S.E. | p | b | S.E. | p | b | S.E. | p | b | S.E. | p | b | S.E. | p | |
| Intercept | 6.472 | .590 | <.001 | 6.471 | .594 | <.001 | 6.218 | .600 | <.001 | 6.082 | .622 | <.001 | 6.352 | .600 | <.001 |
| Age | −.833 | .241 | .001 | −.812 | .243 | .001 | −.802 | .239 | .001 | −.809 | .239 | .001 | −.840 | .241 | .001 |
| Education | −.238 | .208 | .252 | −.234 | .208 | .261 | −.225 | .207 | .279 | −.227 | .207 | .272 | −.228 | .207 | .272 |
| Sex | .024 | .423 | .955 | .016 | .423 | .970 | .034 | .422 | .937 | .042 | .422 | .922 | .031 | .422 | .941 |
| Brain volume | −1.998 | .243 | <.001 | −2.001 | .243 | <.001 | −1.961 | .250 | <.001 | −1.951 | .250 | <.001 | −1.978 | .245 | <.001 |
| Visit | 2.852 | .299 | <.001 | 2.842 | .300 | <.001 | 2.626 | .298 | <.001 | 2.548 | .311 | <.001 | 2.754 | .303 | <.001 |
| Group main effect | |||||||||||||||
| aMCI | 1.857 | .671 | .006 | 1.868 | .675 | .006 | 2.110 | .678 | .002 | 2.264 | .697 | .001 | 1.978 | .680 | .004 |
| CN | −3.709 | .648 | <.001 | −3.715 | .652 | <.001 | −3.421 | .655 | <.001 | −3.292 | .674 | <.001 | −3.572 | .657 | <.001 |
| WMH | −.034 | .639 | .958 | −.000 | .633 | 1.000 | 1.106 | .624 | .077 | 1.004 | .587 | .088 | .548 | .662 | .408 |
| Group × Visit | |||||||||||||||
| aMCI × Visit | .647 | .349 | .064 | .651 | .349 | .063 | .872 | .346 | .012 | .974 | .357 | .007 | .755 | .352 | .032 |
| CN × Visit | −2.070 | .334 | <.001 | −2.061 | .334 | <.001 | −1.799 | .332 | <.001 | −1.729 | .343 | <.001 | −1.963 | .336 | <.001 |
| WMH × Visit | .038 | .340 | .910 | .099 | .333 | .767 | 1.041 | .322 | .001 | .828 | .300 | .006 | .479 | .351 | .174 |
| Group × WMH | |||||||||||||||
| aMCI × WMH | .589 | .703 | .402 | .415 | .701 | .554 | −.965 | .702 | .170 | −.871 | .671 | .194 | −.196 | .726 | .787 |
| CN × WMH | .134 | .697 | .847 | −.028 | .691 | .968 | −.867 | .677 | .201 | −.681 | .645 | .291 | −.316 | .718 | .660 |
| Group × WMH × Visit | |||||||||||||||
| aMCI × WMH × Visit | −.006 | .377 | .988 | −.082 | .372 | .825 | −.737 | .367 | .045 | −.381 | .348 | .274 | −.342 | .388 | .378 |
| CN × WMH × Visit | .206 | .372 | .580 | .107 | .364 | .770 | −.681 | .351 | .053 | −.446 | .333 | .181 | −.172 | .381 | .652 |
WMH = white matter hyperintensity; S.E. = standard error of the estimate; aMCI = amnestic mild cognitive impairment; CN = cognitively normal. All continuous variables in the model were standardized to have a mean of zero and a standard deviation of one using z-score transformations. Nonamnestic MCI are the reference group. Significant effects (p < 0.05) appear in bold font.
The two-way group × visit interactions showed that both the aMCI and naMCI groups had a faster rate of functional decline (i.e., endorsed more functional difficulty resulting in higher FAQ score) than the cognitively normal group across all models; the aMCI group showed a faster rate of functional decline than the naMCI group for the models that included total WMH volume as well as WMH volume for the temporal and occipital regions. Statistical significance of these results was retained using a 0.05 FDR.
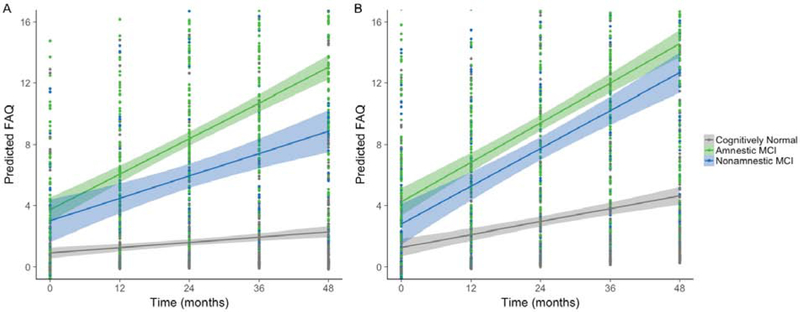
The two-way WMH × visit interactions showed that, across the entire sample, greater WMH in temporal and occipital regions was associated with faster decline in everyday functioning across time (p = .001 and .006, respectively). These effects remained significant using an FDR of 0.05. For temporal WMH, the WMH × visit interaction was moderated by group such that greater WMH disproportionally accelerated functional decline for naMCI when compared to the aMCI group (p = .045) and at a trend level when compared to the cognitively normal group (p = .053); see Figure 2. Statistical significance of the three-way interaction was maintained using a 0.10 FDR but not when FDR was limited to 0.05.
Figure 2.
Fitted plots displaying model predicted Functional Activities Questionnaire (FAQ) values over the 4-year interval, controlling for age, sex, education, and brain volume. For visual comparison, the graphs display results for low white matter hyperintensity (WMH) volume (depicted in panel A) and high WMH volume (depicted in panel B) which were determined by a median split of the values in the analytic sample. The graph shows results for temporal WMH. Higher FAQ scores indicate greater functional difficulty. Shaded regions represent the 95% confidence interval for the regression line.
3.5. Sensitivity Analyses Excluding Participants with Brain Infarcts
In secondary analyses, we excluded individuals with brain infarcts observed on MRI (n = 51). All results remained qualitatively and statistically similar. That is, the repeated measures ANOVA showed an interaction at baseline. Univariate tests for each lobe showed the same pattern of results when the entire sample was included. Multilevel modeling analyzing longitudinal rate of change in FAQ score as a function of baseline WMH showed that findings remained qualitatively and statistically similar. That is, baseline temporal and occipital WMH volume predicted functional decline (p’s < .001). For temporal WMH, these effects were moderated by group such that greater WMH disproportionally accelerated functional decline for naMCI when compared to the aMCI group (p < .05).
3.6. False Discovery Rate
Statistical significance of all results described above was retained using a 0.10 FDR. The two findings for which statistical significance was not maintained under 0.05 FDR are indicated as such in the text above.
4. Discussion
In this large sample of older adults, we found that those with amnestic and naMCI showed greater WMH volume relative to cognitively normal older adults and that the pattern of group differences varied across regions. Specifically, the aMCI group showed elevated temporal and occipital WMH volume relative to the cognitively normal group whereas the naMCI group showed elevated WMH volume across frontal, parietal, temporal and occipital regions, suggesting more widespread WMH accumulation. In addition, the naMCI participants showed greater occipital WMH relative to the aMCI. Findings of this study also provide evidence that regional WMH volume predicts changes in informant-reported everyday functioning—even in cognitively normal individuals. To our knowledge, this is the first examination of lobar WMH volume as a longitudinal predictor of functional decline in MCI. Our findings add to a growing body of research highlighting the role of cerebrovascular changes, and suggest that WMH in particular play an important role in individuals at risk for dementia (Brickman et al., 2012; Lee et al., 2016; Tosto et al., 2014). Our study extends previous work by demonstrating associations between regional WMH volume in MCI subtypes and longitudinal changes in everyday functioning. These findings also highlight that regional measures of WMH may be more sensitive than total global WMH in discriminating among those at increased risk of developing AD.
The pattern of baseline regional WMH accumulation varied for the MCI subtypes. The aMCI group showed a memory deficit and significantly higher temporal and occipital WMH volume relative to the cognitively normal group. The naMCI group—which showed impairment in non-memory domains (i.e., language and/or executive functioning)—also showed more widespread WMH accumulation across frontal and parietal regions in addition to temporal and occipital regions. This pattern of regional WMH differences between amnestic and nonamnestic subtypes of MCI parallels the distinct cortical atrophy patterns that have been observed in MCI subgroups which correspond well to the heterogenous cognitive profiles observed in MCI (Edmonds et al., 2016). Of note, our models adjusted for whole brain volume normalized by intracranial volume, suggesting that the associations of WMH volume are independent of gray matter atrophy.
Although WMH are common in normal aging (DeCarli et al., 2005) and in non-AD forms of cognitive impairment (Gorelick et al., 2011), growing evidence suggests an important role in AD (Lee et al., 2016). While it has been proposed that amnestic and naMCI are linked to different etiologies (Petersen and Morris, 2005), it is clear that aMCI is not always a precursor to AD and cerebrovascular disease is often associated with memory deficits. Based on the current evidence, it appears that WMH play a role in both clinical subtypes. Notably, WMH themselves are heterogeneous and have been linked pathologically to demyelination, axonal loss due to ischemia or neuronal death, cerebral amyloid angiopathy, and microglia and endothelial activation (Wardlaw et al., 2015) (Fernando et al., 2004; Lee et al., 2016).
A recent study of individuals with autosomal-dominant genetic mutations for AD found that WMH volume is elevated among mutation carriers approximately six years before estimated symptom onset. When regional WMH were examined, parietal and occipital WMH was elevated among mutation carriers around 22 years before estimated symptom onset. Notably, posterior WMH volume increased in mutation carriers around the same time that CSF ptau181 and amyloid changes occur (Lee et al., 2016). In line with these findings, we also found that posterior WMH differentiated aMCI and naMCI groups from cognitively normal older adults and was associated with functional decline across the entire sample.
Although the ADNI sample is selected to have relatively low vascular risk burden, we performed secondary analyses excluding those with MRI defined brain infarct(s) and found quantitatively and statistically similar results, suggesting that WMH have an influence on cognition independent of infarcts, although we cannot rule out the possible presence of microinfarcts. Nonetheless, our findings support the notion that even relatively mild cerebrovascular changes play a role in the expression of MCI and the evolution of decline in everyday functioning.
We found that baseline WMH volume predicted increasing functional difficulty, although it should be noted that the sample was, on average, still functionally independent at the 48-month visit. A score of 6 or higher on the FAQ has been suggested to best indicate significant functional difficulties and shown to best discriminate between MCI and very mild AD (Teng et al., 2010). Although the mean decline in everyday functioning observed in our study did not reach the level of dependence in IADLs, increased functional difficulty is an important risk factor for future functional disability and cognitive decline (Farias et al., 2017; Nowrangi et al., 2016), and even modest functional decline may lead to increased frustration with everyday tasks. Our findings provide support for greater regional WMH as a risk factor for decline in daily functioning among nondemented older adults including those with normal cognition. Notably, the aMCI group showed more functional difficulties at baseline and at follow-up relative to the other groups. Although we would not expect clinically significant functional difficulties in cognitively normal individuals, some individuals in this group were experiencing subtle functional changes that are predicted by baseline WMH volume.
We observed an interaction whereby temporal WMH volume disproportionally predicted functional decline among the naMCI group. It is possible that progression (or increased functional difficulty) in naMCI is driven more by temporal white matter changes relative to other pathologies (e.g., tau). There is evidence that executive functioning is an important predictor of everyday functioning among older adults (McAlister et al., 2016) and accounts for more of the variance in everyday functioning compared to memory, attention, and visuospatial domains (Royall et al., 2007). In our own work we have shown that the combination of both memory and executive function difficulties seems to accelerate functional decline (Thomas et al., 2017). It is possible that, among the naMCI who already have executive functioning impairment at baseline, the increased temporal WMH may lead to future memory declines. Thus, perhaps the addition of new onset memory difficulties over time caused by greater baseline temporal WMH volume, in combination with existing executive dysfunction, resulted in a faster rate of decline in everyday functioning. In addition, compared to the naMCI group, a larger portion of aMCI in our sample were impaired in multiple cognitive domains versus a single cognitive domain. That is, in the aMCI group, 43% of individuals were impaired in multiple domains whereas, in the naMCI group, 14% were impaired in multiple domains. Given the small number of participants in the multidomain naMCI group, we did not have adequate statistical power to perform analyses for all four MCI clinical subtypes (single and multidomain within aMCI and naMCI each). Longitudinal studies examining the spatial patterns of regional WMH accumulation and cognitive progression of the subtypes over time will be necessary to examine these possibilities.
Strengths of this study include a large, well-characterized sample of older adults enrolled in a national study on aging and Alzheimer’s disease, assessment of neuropsychological functioning and IADLs, analysis of multimodal MRI data, quantification of lobar WMH, and the longitudinal design. In addition, we used comprehensive neuropsychological criteria for the identification of MCI subtypes. We have previously shown that MCI diagnosed via our actuarial neuropsychological method, which diagnoses MCI based on multiple objective neuropsychological measures assessing a range of cognitive abilities, results in greater diagnostic stability (Jak et al., 2009) and stronger relationships among cognition, biomarkers, and progression to AD (Bondi et al., 2014; Clark et al., 2013; Edmonds et al., 2016). We have also shown that conventional diagnostic methods for MCI—based on subjective memory complaints, cognitive screening measures, and a single memory test (Petersen, 2004; Petersen et al., 2010)—are susceptible to false-positive diagnostic errors. For instance, our work has shown that, in ADNI, over one-third of MCI participants were better classified as “cognitively normal” due to normal cognitive functioning, normal AD biomarkers, and low progression rates to AD (Bangen et al., 2016; Bondi et al., 2014; Clark et al., 2013; Edmonds et al., 2015).
Limitations of our study include that a 48 month follow-up may be a relatively short period of time to see changes in brain structure and everyday functioning among cognitively normal individuals in particular. Higher attrition rates were observed for amnestic and naMCI participants relative to cognitively normal individuals although the two MCI subtypes did not differ from each another. Nonetheless, this pattern of attrition may limit our understanding of the association of WMH and trajectories of decline in higher risk individuals. Furthermore, the participants in ADNI were relatively homogeneous and tended to be well-educated and Caucasian. Replication of our study findings in more heterogeneous samples that may better reflect the general population is warranted.
In closing, we found that regional WMH volumes discriminate among cognitively normal older adults and meaningful MCI subgroups and regional measures of WMH may be more sensitive than total global WMH in identifying those at increased risk of developing AD and predicting functional decline. Taken together with previous work showing that WMH increases AD risk (Brickman et al., 2012; Silbert et al., 2012), relates to heterogeneity among MCI subgroups (Delano-Wood et al., 2009; Luchsinger et al., 2009), predicts cognitive decline (Carmichael et al., 2010; Tosto et al., 2014), and is more reliably associated with neurodegeneration than measures of Aβ (Guzman et al., 2013), our findings suggest that regional WMH volume may be a useful alternative or addition to traditional biomarkers in identifying individuals at risk for decline. Future research is needed to further elucidate the link between small-vessel cerebrovascular disease and MCI, particularly given evidence that cerebrovascular changes may precede, initiate, and exacerbate neurodegeneration (Bell et al., 2012; Zlokovic, 2011). Importantly, given that many of the risk factors for WMH have been identified and may be modified through lifestyle and pharmacological interventions, our findings suggest that reducing risk burden through treatment of vascular factors is a promising approach and may be more relevant for particular MCI subgroups.
Supplementary Material
MCI subtypes are associated with greater baseline regional WMH compared to controls
Nonamnestic MCI show greater baseline occipital WMH relative to amnestic MCI
Posterior WMH volume predicts functional decline across the entire sample
Temporal WMH are disproportionately associated with decline among nonamnestic MCI
Cerebrovascular markers may help identify individuals at risk for functional decline
Acknowledgements
Sources of funding for this project include VA Clinical Science Research & Development (Career Development Award-2 1IK2CX000938 to KJB and 1IK2CX001415 to E.C.E.); Alzheimer’s Association (AARF-17-528918 to K.R.T., AARG-18-566254 to K.J.B., and AARG-17-500358 to E.C.E.); grants from the National Institutes of Health (National Institute on Aging R01 AG049810 and K24 AG026431 to M.W.B. and San Diego State University Advancing Diversity in Aging Research Program [R25AG043364]); and the Dana Foundation (to K.J.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Bondi serves as a consultant for Novartis and Eisai and receives royalties from Oxford University Press. The other authors report no disclosures. Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bangen KJ, Clark AL, Werhane M, Edmonds EC, Nation DA, Evangelista N, Libon DJ, Bondi MW, Delano-Wood L, 2016. Cortical Amyloid Burden Differences Across Empirically-Derived Mild Cognitive Impairment Subtypes and Interaction with APOE varepsilon4 Genotype. Journal of Alzheimer's disease : JAD 52(3), 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Preis SR, Delano-Wood L, Wolf PA, Libon DJ, Bondi MW, Au R, DeCarli C, Brickman AM, 2018. Baseline White Matter Hyperintensities and Hippocampal Volume are Associated With Conversion From Normal Cognition to Mild Cognitive Impairment in the Framingham Offspring Study. Alzheimer Dis. Assoc. Disord 32(1), 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Weigand AJ, Thomas KR, Delano-Wood L, Clark LR, Eppig J, Werhane ML, Edmonds EC, Bondi MW, 2019. Cognitive dispersion is a sensitive marker for early neurodegenerative changes and functional decline in nondemented older adults. Neuropsychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, Zlokovic BV, 2012. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485(7399), 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Statistical Methodology) 57, 289–300. [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP, 2014. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer's disease : JAD 42(1), 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R, 2012. Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Arch. Neurol, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman AM, Zahodne LB, Guzman VA, Narkhede A, Meier IB, Griffith EY, Provenzano FA, Schupf N, Manly JJ, Stern Y, Luchsinger JA, Mayeux R, 2015. Reconsidering harbingers of dementia: progression of parietal lobe white matter hyperintensities predicts Alzheimer's disease incidence. Neurobiol. Aging 36(1), 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR Jr., Weiner M, DeCarli C, 2010. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch. Neurol 67(11), 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Jak AJ, Au R, Salmon DP, Bondi MW, 2013. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J. Int. Neuropsychol. Soc. 19(6), 635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA, 2005. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol. Aging 26(4), 491–510. [DOI] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Sacco J, Abeles N, Jak AJ, Libon DJ, Bozoki A, 2009. Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated white matter lesion pathology. J. Int. Neuropsychol. Soc. 15(6), 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Libon DJ, Au R, Galasko D, Salmon DP, Bondi MW, 2015. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer's & dementia : the journal of the Alzheimer's Association 11(4), 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, McDonald CR, 2016. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology 87(20), 2108–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Lau K, Harvey D, Denny KG, Barba C, Mefford AN, 2017. Early Functional Limitations in Cognitively Normal Older Adults Predict Diagnostic Conversion to Mild Cognitive Impairment. J. Am. Geriatr. Soc 65(6), 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MS, O'Brien JT, Perry RH, English P, Forster G, McMeekin W, Slade JY, Golkhar A, Matthews FE, Barber R, Kalaria RN, Ince PG, 2004. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol. Appl. Neurobiol 30(4), 385–395. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S, American Heart Association Stroke Council, C.o.E., Radiology, P.C.o.C.N.C.o.C., Intervention, Surgery, C.o.C., Anesthesia, 2011. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 42(9), 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman VA, Carmichael OT, Schwarz C, Tosto G, Zimmerman ME, Brickman AM, 2013. White matter hyperintensities and amyloid are independently associated with entorhinal cortex volume among individuals with mild cognitive impairment. Alzheimer's & dementia : the journal of the Alzheimer's Association 9(5 Suppl), S124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Dale AM, 2009. Subregional neuroanatomical change as a biomarker for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A 106(49), 20954–20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, Borowski A, Britson PJ, J LW, Ward C, Dale AM, Felmlee JP, Gunter JL, Hill DL, Killiany R, Schuff N, Fox-Bosetti S, Lin C, Studholme C, DeCarli CS, Krueger G, Ward HA, Metzger GJ, Scott KT, Mallozzi R, Blezek D, Levy J, Debbins JP, Fleisher AS, Albert M, Green R, Bartzokis G, Glover G, Mugler J, Weiner MW, 2008. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27(4), 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC, 2009. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry 17(5), 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Woods R, Mazziotta J, Hardies J, Fox P, 2001. Regional spatial normalization: toward an optimal target. J. Comput. Assist. Tomogr 25(5), 805–816. [DOI] [PubMed] [Google Scholar]
- Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, Marcus DS, Fagan AM, Goate A, Fox NC, Cairns NJ, Holtzman DM, Buckles V, Ghetti B, McDade E, Martins RN, Saykin AJ, Masters CL, Ringman JM, Ryan NS, Forster S, Laske C, Schofield PR, Sperling RA, Salloway S, Correia S, Jack C Jr., Weiner M, Bateman RJ, Morris JC, Mayeux R, Brickman AM, 2016. White matter hyperintensities are a core feature of Alzheimer's disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol 79(6), 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, Price CC, Giovannetti T, Swenson R, Bettcher BM, Heilman KM, Pennisi A, 2008. Linking MRI hyperintensities with patterns of neuropsychological impairment: evidence for a threshold effect. Stroke 39(3), 806–813. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Brickman AM, Reitz C, Cho SJ, Schupf N, Manly JJ, Tang MX, Small SA, Mayeux R, DeCarli C, Brown TR, 2009. Subclinical cerebrovascular disease in mild cognitive impairment. Neurology 73(6), 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlister C, Schmitter-Edgecombe M, Lamb R, 2016. Examination of Variables That May Affect the Relationship Between Cognition and Functional Status in Individuals with Mild Cognitive Impairment: A Meta-Analysis. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 31(2), 123–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrangi MA, Rosenberg PB, Leoutsakos JS, 2016. Subtle changes in daily functioning predict conversion from normal to mild cognitive impairment or dementia: an analysis of the NACC database. Int. Psychogeriatr 28(12), 2009–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, 2004. Mild cognitive impairment as a diagnostic entity. J. Intern. Med 256(3), 183–194. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR Jr., Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, Weiner MW, 2010. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74(3), 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Morris JC, 2005. Mild cognitive impairment as a clinical entity and treatment target. Arch. Neurol 62(7), 1160–1163; discussion 1167. [DOI] [PubMed] [Google Scholar]
- Royall DR, Lauterbach EC, Kaufer D, Malloy P, Coburn KL, Black KJ, 2007. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J. Neuropsychiatry Clin. Neurosci 19(3), 249–265. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ, 1999. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans. Med. Imaging 18(8), 712–721. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA, 2009. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol 66(2), 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz C, Fletcher E, DeCarli C, Carmichael O, 2009. Fully-automated white matter hyperintensity detection with anatomical prior knowledge and without FLAIR. Information processing in medical imaging : proceedings of the … conference 21, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA, 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version, Clinical Gerontology: a Guide to Assessment and Intervention. The Haworth Press, New York, NY, pp. 165–173. [Google Scholar]
- Silbert LC, Dodge HH, Perkins LG, Sherbakov L, Lahna D, Erten-Lyons D, Woltjer R, Shinto L, Kaye JA, 2012. Trajectory of white matter hyperintensity burden preceding mild cognitive impairment. Neurology 79(8), 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J, Willett J, 2003. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press, New York, NY. [Google Scholar]
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH, 2010. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Dis. Assoc. Disord 24(4), 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Edmonds EC, Delano-Wood L, Bondi MW, 2017. Longitudinal Trajectories of Informant-Reported Daily Functioning in Empirically Defined Subtypes of Mild Cognitive Impairment. J. Int. Neuropsychol. Soc 23(6), 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosto G, Zimmerman ME, Carmichael OT, Brickman AM, 2014. Predicting aggressive decline in mild cognitive impairment: the importance of white matter hyperintensities. JAMA neurology 71(7), 872–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Valdes Hernandez MC, Munoz-Maniega S, 2015. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. Journal of the American Heart Association 4(6), 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard JL, 2017. A quarter century of advances in the statistical analysis of longitudinal neuropsychological data. Neuropsychology 31(8), 1020–1035. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV, 2011. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nature reviews. Neuroscience 12(12), 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.