Abstract
Serotonin (5-hydroxytryptamine; 5-HT) coordinates behavioral responses to stress through a variety of presynaptic and postsynaptic receptors distributed across functionally diverse neuronal networks in the central nervous system. Efferent 5-HT projections from the dorsal raphe nucleus (DRN) to the bed nucleus of the stria terminalis (BNST) are generally thought to enhance anxiety and aversive learning by activating 5-HT2C receptor (5-HT2CR) signaling in the BNST, although an opposing role for postsynaptic 5-HT1A receptors has recently been suggested. In the present study, we sought to delineate a role for postsynaptic 5-HT1A receptors in the BNST in aversive behaviors using a conditional knockdown of the 5-HT1A receptor. Both males and females were tested to dissect out sex-specific effects. We found that male mice have significantly reduced fear memory recall relative to female mice and inactivation of 5-HT1A receptor in the BNST increases contextual fear conditioning in male mice so that they resemble the females. This coincided with an increase in neuronal excitability in males, suggesting that 5-HT1A receptor deletion may enhance contextual fear recall by disinhibiting fear memory circuits in the BNST. Interestingly, 5-HT1A receptor knockdown did not significantly alter anxiety-like behavior in male or female mice, which is in agreement with previous findings that anxiety and fear are modulated by dissociable circuits in the BNST. Overall, these results suggest that BNST 5-HT1A receptors do not significantly alter behavior under basal conditions, but can act as a molecular brake that buffer against excessive activation of aversive circuits in more threatening contexts.
Keywords: Serotonin (5-HT), 5-HT1A receptor, bed nucleus of the stria terminalis (BNST), anxiety, contextual fear
1. INTRODUCTION
Serotonin (5-hydroxytryptamine; 5-HT) neurons originate in a cluster of nuclei in the midbrain and pons known as the raphe nuclei, where they orchestrate emotional responses to stress through a variety of pre- and postsynaptic receptors distributed across functionally diverse neuronal networks in the central nervous system1–4. The dorsal raphe nucleus (DRN) has reciprocal connections with the bed nucleus of the stria terminalis (BNST), a part of the extended amygdala that acts as a conduit between forebrain limbic structures and hypothalamic and brainstem regions5,6. The BNST is therefore ideally positioned to relay autonomic, sensory and stress-related information to emotional processing centers of the brain. Efferent projections from the DRN to the BNST are generally thought to enhance anxiety and aversive learning by activating 5-HT2C receptor signaling in the BNST7–10, although an opposing role for 5-HT at 5-HT1A receptors has recently been suggested11. Earlier studies also support a model in which 5-HT1A receptor signaling in the BNST is anxiolytic. In 2004, Levita et al. found that direct infusion of a non-selective 5-HT1 receptor agonist (of 5-carboxamidotryptamine or 5-CT) into the BNST attenuated acoustic startle response, while others have shown that cannabidiol reduces anxiety and contextual fear recall via activation of 5-HT1A receptors12–14. These diverging behavioral actions of 5-HT seem to mirror the physiological effects of 5-HT2C and 5-HT1A receptor activation on neuronal excitability, with 5-HT2C receptors having a net excitatory and 5-HT1A receptors having a net inhibitory postsynaptic effect15.
Converging lines of evidence point toward an anxiolytic role for 5-HT1A receptors in the BNST, but the precise contribution of presynaptic and postsynaptic 5-HT1A receptors to these behavioral outcomes is unclear. While activation of postsynaptic 5-HT1A receptors may articulate the anti-aversive effects of 5-HT, there is also the possibility that activation of presynaptic 5-HT1A heteroreceptors may exert an anxiolytic effect by inhibiting glutamate release from presynaptic terminals16–19, which would tend to reduce postsynaptic 5-HT2C receptor signaling. In the present study, we generated a conditioned knockout of the htr1a allele (htr1afl/fl) to delineate the role of postsynaptic 5-HT1A receptors in aversive behavior. Site-specific infusion of an adeno-associated viral vector (AAV-cre-GFP) into the BNST allowed for selective targeting of 5-HT1A receptor knockdown to postsynaptic neurons in order to isolate their contribution to aversive behaviors.
The BNST plays a key role in processing threat, but its precise contribution to unconditioned or diffuse threat (“anxiety”) and conditioned or discrete threat (fear) remains controversial20–23. In light of previous reports that 5-HT can reduce anxiety via 5-HT1A receptor activation in the BNST12,13,24, we interrogated the role of postsynaptic 5-HT1A receptor knockdown in multiple behavioral assays that explore conditioned responses threat and unconditioned anxiety. Our overarching hypothesis was that genetic knockdown of 5-HT1A receptors in the BNST would enhance anxiety and conditioned fear memory by increasing neuronal excitability in neural circuits that process anxiety and fear. The BNST is also a sexually dimorphic region that displays differentially responsiveness to stress25–28, so we compared male and female htr1afl/fl mice in this study. Earlier studies report that inactivation of the BNST can prevent impaired learning after stress in males but not in females29, suggesting that 5-HT1A receptor knockdown in the BNST may differentially impact aversive behaviors in male and female mice.
2. RESULTS AND DISCUSSION
2.1. Generation and validation of conditional 5-HT1A receptor knockout mouse line
The 5-HT1A receptor is widely distributed throughout the mammalian brain and is generally thought to have an anxiolytic function based on pharmacological and genetic knockout studies30–34. In the search for a neural substrate underpinning the anxiolytic effects of 5-HT1A receptors, many studies have pinpointed the extended amygdala and particularly the BNST as a potential candidate, although to date these studies have used pharmacological manipulations that do not discriminate between pre and postsynaptic effects12–14. The general consensus of these earlier studies is that 5-HT1AR agonists administered into the BNST are anxiolytic and reduce contextual fear recall, presumably by activating postsynaptic 5-HT1A receptors. We generated a conditional htr1afl/fl line with lox P sites flanking the sites flanking the htr1a open reading frame (ORF) to determine whether postsynaptic 5-HT1A receptors were critically involved in its putative anti-aversive actions in the BNST. We used a combination of fluorescence in situ hybridization and slice electrophysiology to verify that mice homozygous for the floxed htr1a allele exhibit functional deletion of 5-HT1A receptors upon Cre-mediated recombination with AAV-Cre-GFP. Our results indicate that 5-HT1A receptor mRNA was significantly reduced in htr1afl/fl:CreBNST mice relative to controls in the dBNST (t10=3.10, p<0.05) and vBNST (t10=3.36, p<0.01) (Figure 1A–D). Next, htr1afl/fl:CreBNST and htr1afl/fl:GFPBNST mice were injected with red fluorescent retrograde tracer beads into the ventral tegmental area (VTA) to target neuronal populations in the BNST that are known to express 5-HT1A receptors9. Recording from these VTA-projecting neurons in the presence of the selective 5-HT7 antagonist SB 269970, we found that bath application of the 5-HT1 agonist 5-CT hyperpolarized 5/11 neurons tested from control mice and 0/10 neurons from htr1afl/fl:CreBNST mice (Fisher’s exact test, odds ratio = 17.77, 95% CI (0.8360, 377.7), p<0.05) (Figure 1E–H). Together, these results indicate that htr1afl/fl mice can be used to genetically knockdown 5-HT1A receptors in the BNST.
Figure 1: Generation and validation of htr1afl/fl mice.
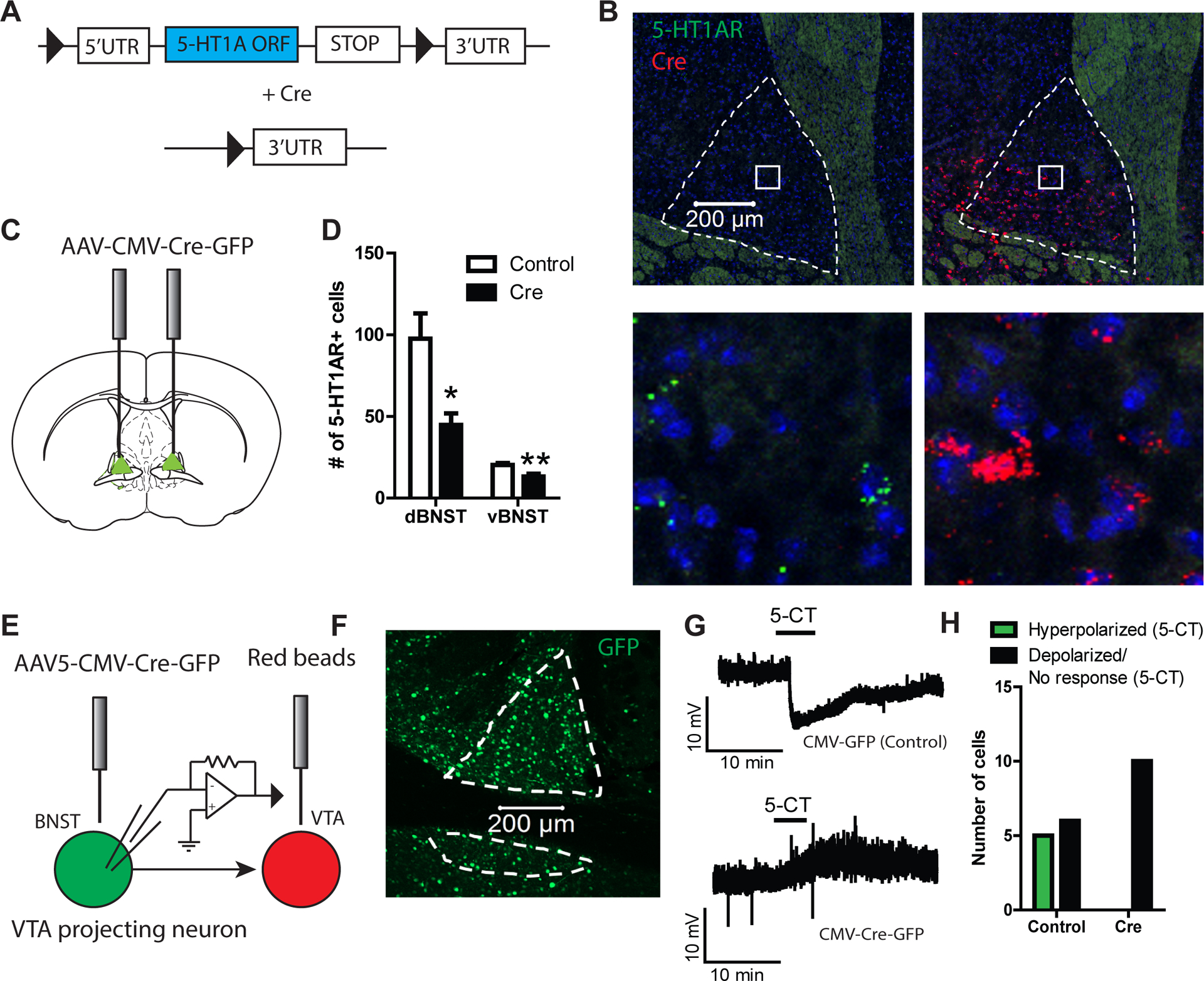
(A) Schematic of genetic targeting strategy. (B). Confocal images from htr1afl/fl::CreBNST mice using fluorescence in situ hybridization against the 5-HT1A receptor and Cre showing genetic ablation of 5-HT1A receptor. (C) Experimental configuration for microinjection of AAV-CMV-Cre-GFP into the BNST. (D) Histogram showing reduction in 5-HT1A expression in the BNST of htr1afl/fl::CreBNST mice (n=6 Control, n=6 Cre). (E) Experimental configuration of electrophysiological recordings in the BNST from VTA projecting neurons. (F) Representative confocal image of GFP stained BNST tissue from a htr1afl/fl::CreBNST mouse. (G) Representative traces showing the effect of 5-CT on resting membrane potential in VTA-projecting BNST neurons in brain slices from htr1afl/fl::CreBNST and htr1afl/fl::GFPBNST mice. (H) Histograms depicting the number of cells (n=11 Control, n=10 Cre) that responded to 5-HT with a depolarization (>2 mV), hyperpolarization (<−2 mV) or no response.
2.2. 5-HT1A receptor knockdown differentially modulates fear memory in male and female mice.
In light of previous studies pinpointing a role for 5-HT1A receptor signaling in the effects of cannabidiol on contextual fear memory14, we analyzed contextual fear recall in male and female mice htr1afl/fl:CreBNST mice. There was no significant interaction between Sex and Cre on percent freezing during fear learning, nor any significant main effects. However, there was a significant interaction between Sex and Cre on percent freezing during contextual fear recall (2-way ANOVA; F1,33=16.99, p<0.001). Bonferroni post-tests revealed that 5-HT1A receptor knockdown increased percent freezing in males (t33=4.045 p<0.001) but not in females (t33=1.755, ns) (Figure 2A–C)35. We also found that contextual freezing was significantly higher in females compared to males in the control groups (t33=3.498, p<0.01), suggesting that females may exhibit heightened contextual fear that is relatively insensitive to manipulations of 5-HT1A receptors. This led us to speculate that females may have lower basal levels of expression of 5-HT1A receptors in aversive memory circuits. However, our results indicate that 5-HT1A receptor protein levels in the BNST did not significantly differ by sex (t8=0.2431, ns) (Figure 3A–B). It is interesting to note that the BNST is a sexually dimorphic region in which males display twice as many neurons in the principal nucleus of the BNST as females36. It was also found that CRF mRNA is enhanced in male mice with diminished expression of GAD67 relative to female mice26,28. These anatomical and neurochemical differences highlight that BNST circuits are differentially organized in males and females, which may account for the divergent behavioral phenotypes observed here.
Figure 2: 5-HT1A receptor knockdown in the BNST enhances contextual fear memory in a sex-dependent manner.
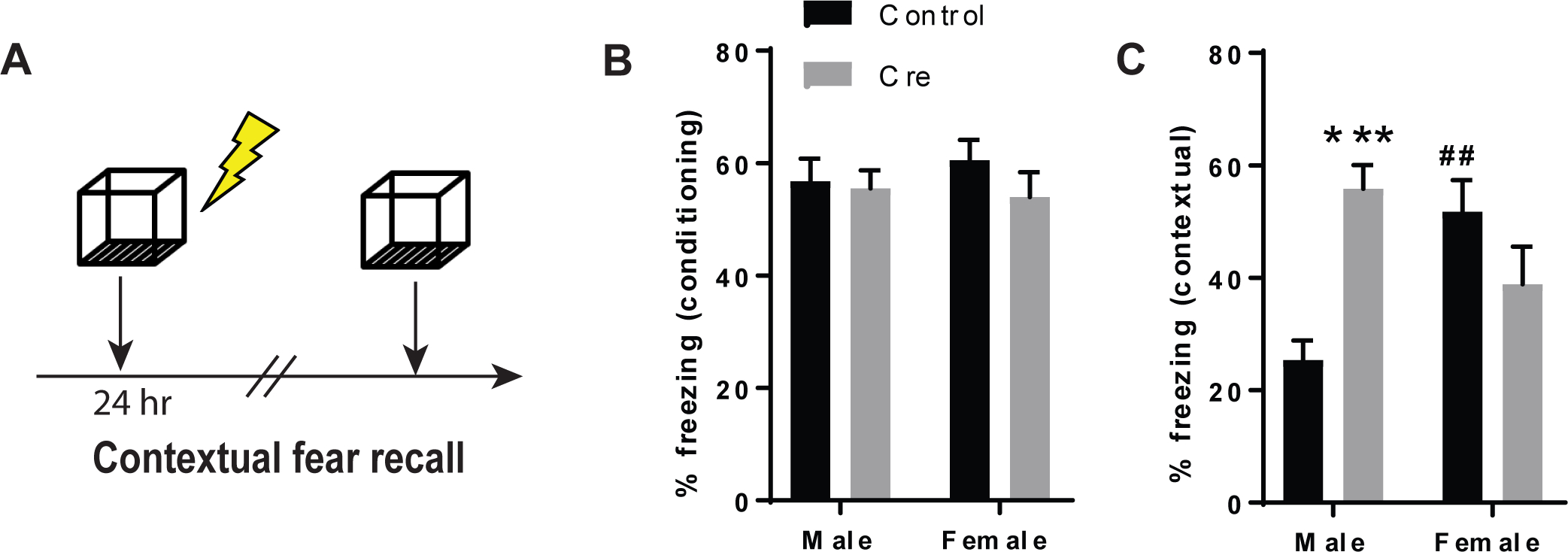
(A) Schematic of the experimental design for contextual fear conditioning.(B) Histogram depicting percent time freezing during fear conditioning and (C) contextual fear recall. N=9 Control males, N=9 Cre males, N=9 Control females and N=10 Cre females. *** denotes p<0.001 for Cre males relative to Control males and ## denotes p<0.01 for Control females relative to Control males.
Figure 3: 5-HT1A receptor protein levels in the BNST do not significantly differ between males and females.
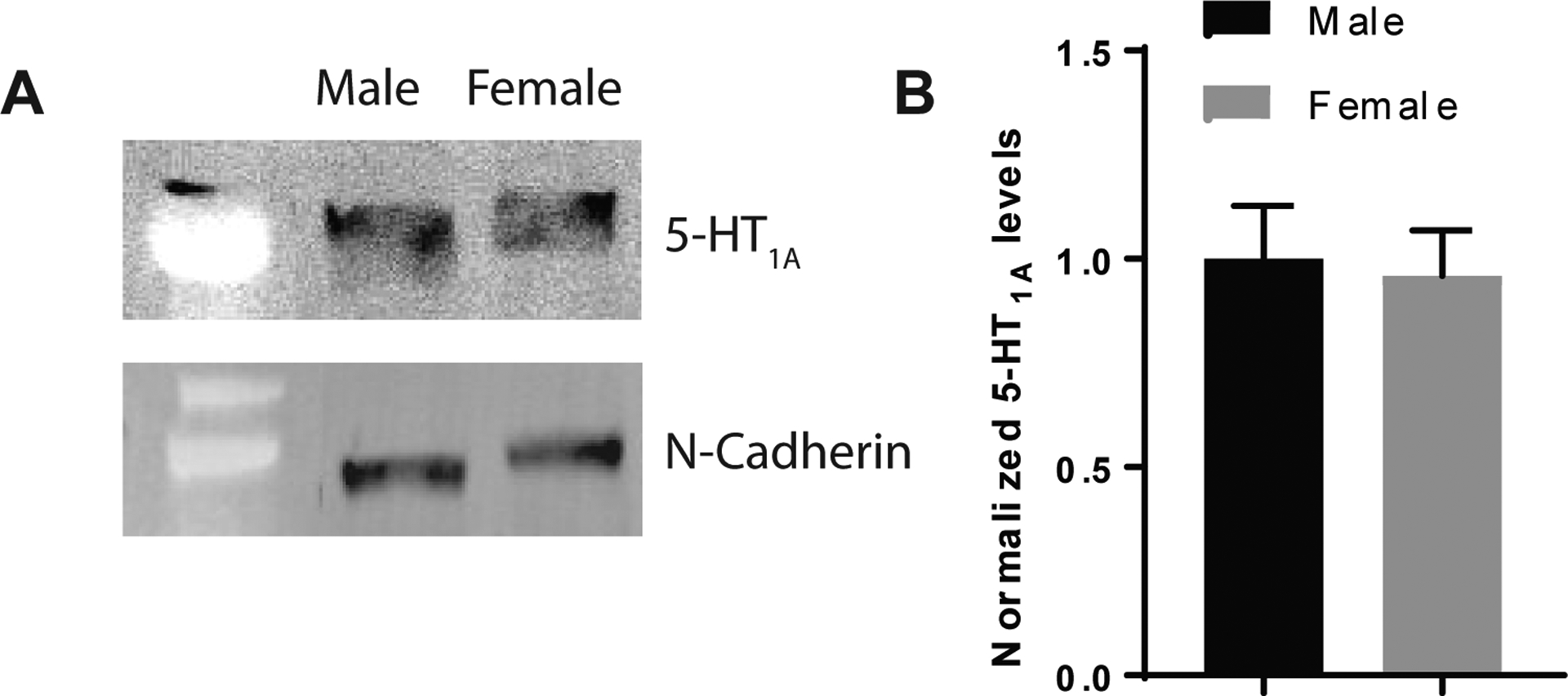
(A) 5-HT1A and N-Cadherin immunoblots from male and female BNST lysates. (B) Histograms showing normalized 5-HT1A protein levels in male and female mice indicating that 5-HT1A receptor levels in the BNST do not significantly differ by sex. N=5 Male and N=5 female lysate samples (each lysate sample was pooled from 4 mice).
2.3. Neuronal excitability in the BNST enhanced by 5-HT1A receptor knockdown after contextual fear
Previous reports found that cannabidiol attenuated c-fos expression in the BNST after contextual fear recall in a 5-HT1A receptor dependent manner37, supporting the idea that 5-HT1A receptors can inhibit the activity of aversive memory circuits in the BNST. Together with earlier studies indicating that 5-HT1A receptors have a net inhibitory effect on BNST neurons via activation of Gi-signaling pathways12,15, we predicted that 5-HT1A receptor knockdown would exacerbate the increase in neuronal excitability during contextual fear recall which may account for exaggerated freezing responses in male mice during re-exposure to these aversive contexts. In order to test this hypothesis, htr1afl/fl::CreBNST and htr1afl/fl::GFPBNST mice were submitted to fear conditioning followed by contextual fear recall. A second group of htr1afl/fl::CreBNST and htr1afl/fl::GFPBNST mice were exposed to the conditioning chamber on the first day without receiving footshocks as a control group. Three-way ANOVAs revealed that there was no significant interaction between Sex, Cre and Conditioning on resting membrane potential (RMP) (F1,65=0.342, ns), but there was a significant two-way interaction between Cre and Conditioning (F1,65=7.716, p<0.01) and significant main effects of Conditioning (F1,65=7.753, p<0.01) and Cre (F1,65=18.973, p<0.001) (Figure 4A, C). This suggests that the effect of Conditioning on RMP differs in Cre and Control mice, but this two-way interaction is not modified by Sex. Simple main effects of Cre reveal differences in both male and female conditioned but not in habituated mice, which was surprising given that activation 5-HT1A receptors has a hyperpolarizing effect on RMP in wild-type mice15. This indicates that the presence of 5-HT1A receptors may only exert an appreciable influence over resting membrane potential and neuronal excitability in the presence of an aversive stimulus, when 5-HT is released in the BNST. Given that these receptors are in relatively low abundance in the BNST, it may stand to reason that their absence would not significantly impact neuronal excitability in the resting state. It was also surprising that conditioning alone did not significantly alter excitability in the male or female control mice. This suggests that the 5-HT1A receptors may have a buffering effect on RMP after fear conditioning that is unmasked after 5-HT1A knockdown.
Figure 4: 5-HT1A receptor knockdown in the BNST enhances neuronal excitability in male mice after contextual fear recall.
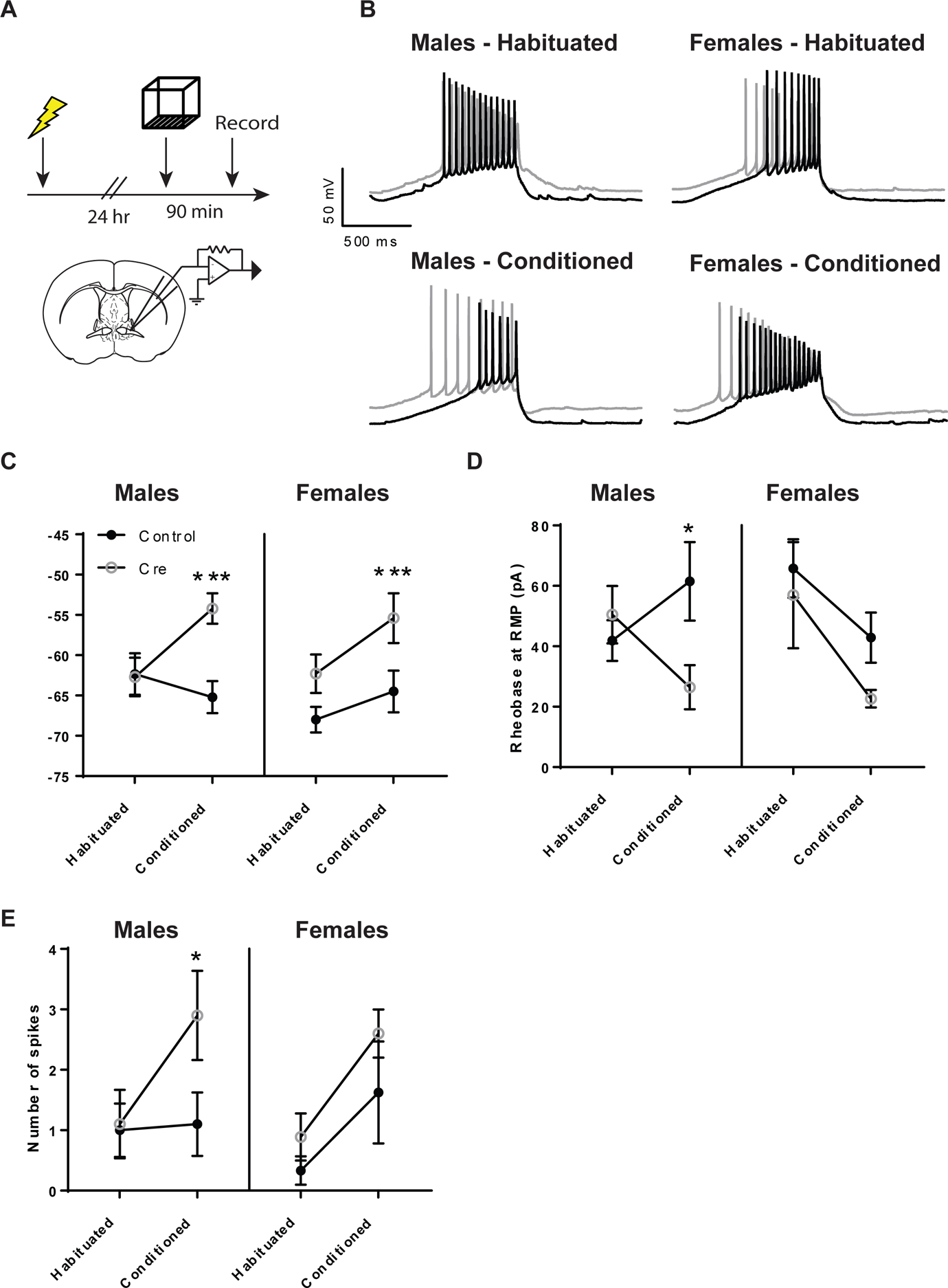
(A) Experimental configuration for contextual fear memory and BNST recordings. (B) Representative traces of rheobase recordings in the BNST (C) Histogram of resting membrane potential (D) Rheobase and (E) Current-induced spiking from habituated and conditioned mice. N= 9 cells from Habituated Control Males, N=10 cells from Habituated Cre Males, N=10 cells from Conditioned Control males, N=10 cells from Conditioned Cre Males, N=9 cells from Habituated Control females, N=10 cells from Habituated Cre females, N=8 cells from Conditioned Control females, N=5 cells from Conditioned Cre females. (*) denotes p<0.05 and (***) denotes p<0.001.
Next, we looked at two measures of neuronal excitability; rheobase and current induced spiking. Rheobase is defined as the minimum current amplitude required to reach the depolarization threshold for action potential firing and should decrease as the cell becomes more excitable. We found no significant 3-way interaction between Sex, Cre and Conditioning on rheobase (F1,63=0.990, ns) nor any significant two-way interactions or main effects. However, looking at simple main effects revealed a significant effect of Cre in conditioned male mice (F=5.546, p<0.05), which is consistent with our contextual fear data that 5-HT1A knockdown augments fear in male mice (Figure 4B, D). We then looked at current-induced spiking, which was defined as the number of spikes during a 30 pA current injection. Here we found no significant three-way interaction between Sex, Cre and Conditioning (F1,62=0.607, ns) nor any significant two-way interactions. We did observe significant main effects of Cre (F1,62 = 4,359, p<0.05) and Conditioning (F1,62=8.903, p<0.01) on the number of action potentials fired at 30 pA. We then looked at simple main effects and found a significant effect of Cre in conditioned males only (F=5.750, p<0.05), which is similar to what we observed for rheobase (Figure 4E).
2.4. Genetic knockdown of 5-HT1A receptors in the BNST does not alter anxiety-like behavior in the EPM, open field or novelty-suppressed feeding tests.
In order to assess the contribution of postsynaptic 5-HT1A receptors to anxiety-like behavior, male and female htr1afl/fl:CreBNST mice were submitted to the elevated plus maze (EPM), which is based on the tendency of the mouse to explore new environments and their conflicting tendency to avoid open spaces. More time spent in the open arms is taken as an inverse index of anxiety-like behavior38. Overall, there was no significant interaction between Sex and Cre on open arm time, probability, latency or distance moved in the EPM. However, there was a significant main effect of Sex on open arm probability in the EPM (F1,78=7.386, p<0.01). Posthoc comparisons revealed a significant difference between male and female Cre mice, with females exhibiting a decrease in open arm probability which is indicative of increased anxiety-like behavior (t=2.303, p<0.05) (Figure 5A–D).
Figure 5: 5-HT1A receptor knockdown in the BNST does not significantly alter anxiety-like or depressive-like behavior behavior in male and female mice.
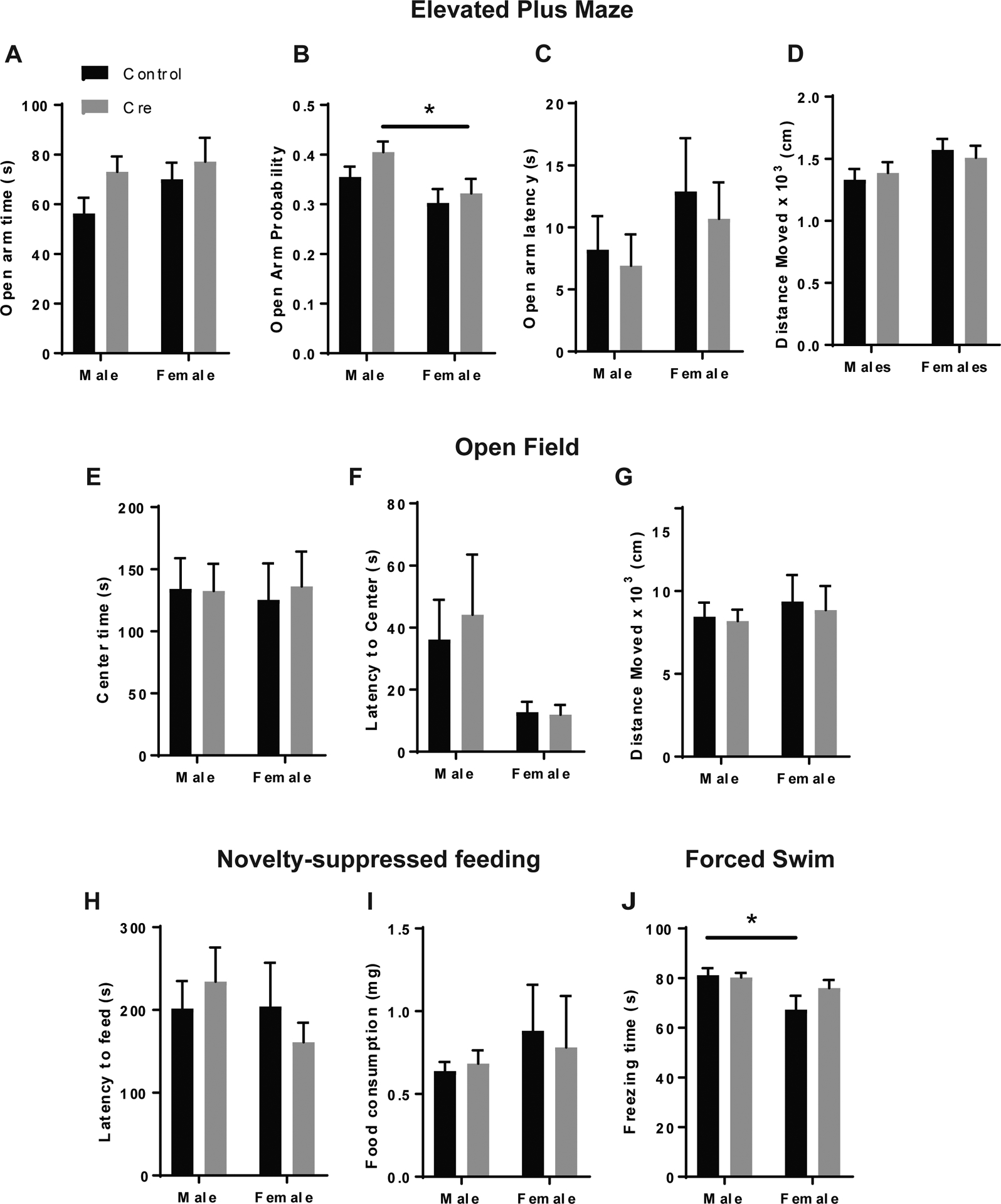
(A) Open arm time, (B) Probability, (C) Latency and (D) Distance moved in the EPM , (F) Latency to center and (G) Distance moved in the open field test. (H) Latency to feed and (I) post-test food consumption in the novelty-suppressed feeding test. (J) Percent immobility time in the forced swim test. For EPM: N=23 control males, N=21 Cre males, N=20 Control females, N=18 Cre females. For open field and novelty-suppressed feeding: N=16 Control males, N=16 Cre males, N=11 Control females, N=9 Cre females. For forced swim: N=10 Control males, N=12 Cre males, N=6 Control females and N=8 Cre females. (*) denotes p<0.05
We performed two additional assays to provide converging information on anxiety-like behavior: the open field test and the novelty suppressed feeding test.39. These tests both represent conflict assays between the natural tendency of the mouse to avoid open spaces and their conflicting drive to explore new environments (open field) and consume palatable food (novelty-suppressed feeding). Important differences between these two constructs should be considered, such as the fact that mice are food deprived for 24 hours before the novelty suppressed feeding test, which can be a stressor. Both assays also have potential confounds which should be considered, including differences in locomotor activity in the open field and satiety/hunger in the novelty-suppressed feeding test. We controlled for both of these variables by measuring distance moved in the open field and performing a 10-minute home cage feeding assay immediately following the novelty-suppressed feeding test.
In the open field test, there was no significant interaction between Sex and Cre on center latency or distance moved. Unlike the EPM, there were no main effects of Sex on any measure of anxiety-like behavior (Figure 5E–G). Likewise, in the novelty-suppressed feeding test, we saw no significant interaction between Sex and Cre on latency to feed nor any main effects of Sex or Cre. There were also no effects on food consumption in the post-test (Figure 5H–I). These wholly negative results of 5-HT1A receptor knockdown on anxiety-like behaviors were surprising in light of previous studies showing that 5-HT1A receptor agonists in the BNST have an anxiolytic effect12,13. Given that 5-HT1A receptor activation has a hyperpolarizing effect on BNST neurons15, we would expect 5-HT1A receptor knockdown to have an anxiogenic effect by disinhibiting anxiety circuits in the BNST. It is possible that the effects of 5-HT1A knockdown do not manifest under low stress conditions when 5-HT release in the BNST is relatively low. There is also the possibility that postsynaptic 5-HT1A receptors are expressed in both anxiogenic and anxiolytic circuits of the BNST and that their removal has a net neutral effect on anxiety-like behavior. We have previously shown that BNST neurons that project to the VTA and lateral hypothalamus (LH), two anxiolytic outputs of the BNST40,41, are hyperpolarized by 5-HT in the presence of a 5-HT2C receptor antagonist, which would indicate the presence of 5-HT1A receptors in this population9,12,15.
There is also evidence in the literature indicating that 5-HT1A receptor signaling can have an antidepressant effect42,43, so we decided to explore a potential role for 5-HT1A in the BNST in behavioral despair in the forced swim test. We did not observe a significant interaction between Sex and Cre on percent immobility in the forced swim test, but we did see a main effect of Sex suggesting that female mice exhibit elevated depressive-like behavior compared to males (F1,32=8.172, p<0.01) (Figure 5J). The absence of any effect of 5-HT1A knockdown in the BNST is, however, consistent with previous literature suggesting that the antidepressant effects of 5-HT1A modulation are mediated by prefrontal and hippocampal circuits44–46
2.5. 5-HT1A receptors in the BNST do not significantly modulate anxiety-like behavior after stress
In a previous study, we had found that stimulating 5-HT inputs to the BNST had a robust anxiogenic effect by activating 5-HT2C receptors in corticotropin releasing factor (CRF) neurons9. We also found that these neurons were hyperpolarized in the presence of 5-HT with pharmacological blockade of the 5-HT2C receptor, suggesting the presence of 5-HT1A receptors15. This led us to speculate whether stimulating 5-HT release in the BNST, which should activate 5-HT2C receptors in CRF neurons, would change the effect of 5-HT1A receptor knockdown from neutral to anxiogenic. Given that restraint stress can stimulate central CRF release, which in turn activates 5-HT neurons in the DRN that release 5-HT release in downstream circuits47,48, we decided to look at anxiety-like behavior under high stress conditions using two assays: the elevated zero maze (EZM) after restraint stress and the open field test under high light conditions. Previous reports indicate that restraint increases avoidance of the open arms in the elevated zero maze49, which is indicative of increased anxiety-like behavior. However, our results indicate that there are no discernable differences in anxiety-like behavior between male htr1afl/fl:CreBNST and control mice in the EZM after physical restraint (Figure 6A–D) or the open field under high light conditions (300 lux) (Figure 6E–G). This suggests that 5-HT1A receptors, which are present in low abundance in the BNST, may not have a discernible effect on anxiety-like behavior even under stressful conditions although they clearly do have a modulatory influence over aversive memory, specifically in males. One possible explanation is that 5-HT1A receptor signaling is upregulated in the BNST in response to footshock in order to buffer the system against excessive stimulation. This neural plasticity may require a consolidation period after the initial stimulus, so the effects of 5-HT1A receptor knockdown are apparent in the contextual fear recall test which occurs 24 hours after the conditioning session.
Figure 6: 5-HT1A receptor knockdown in the BNST does not alter anxiety-behavior in the the EZM after restraint stress or the open field in high light conditions.
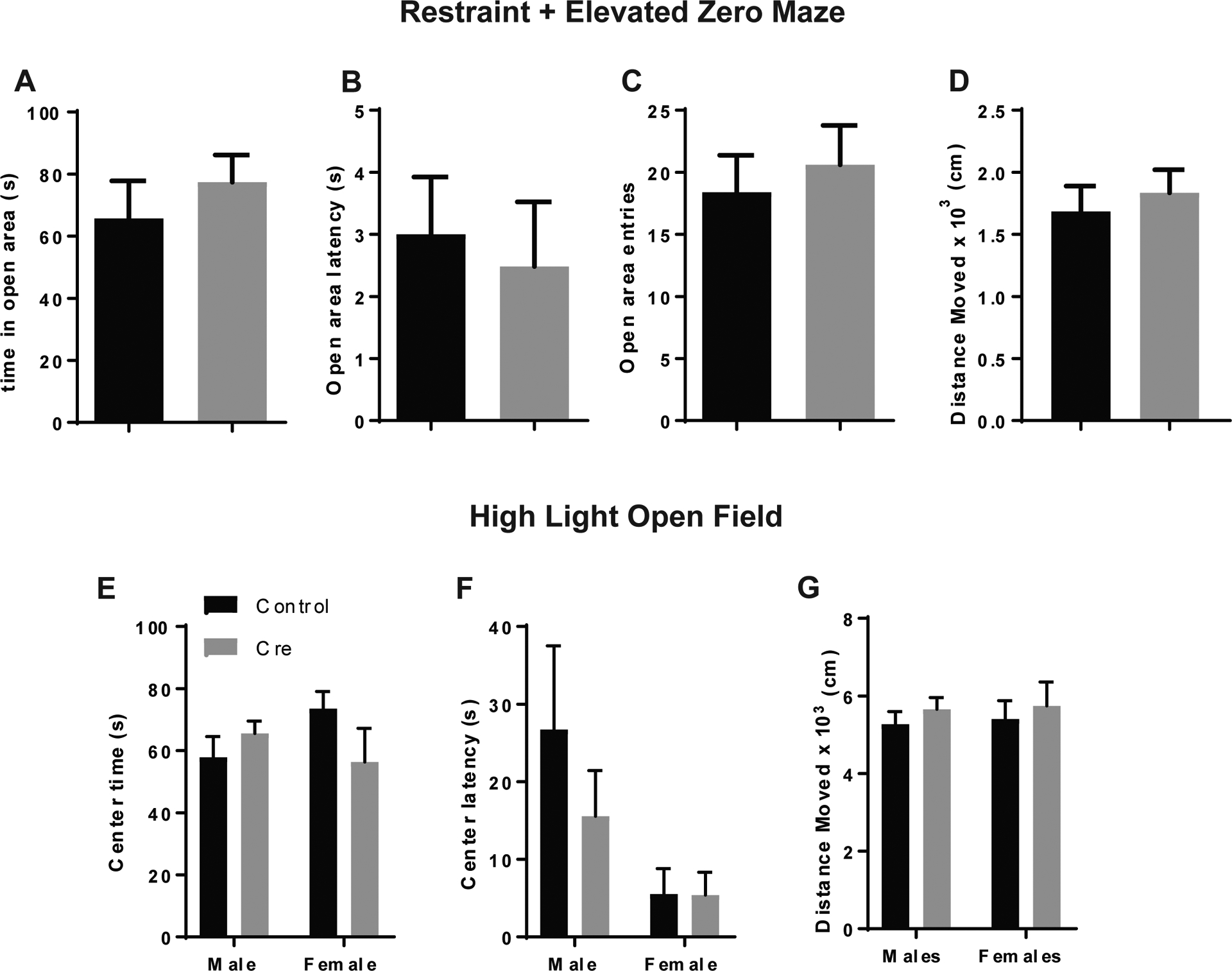
(A) Open area time, (B) Latency, (C) Entries and (D) Distance moved in the EZM after restraint stress. (E) Center time, (F) Center latency and (G) Distance moved in the high light (300 lux) open field. For restraint + EZM: N=5 Control males and N=5 Cre males. For high light open field: N=16 Control males, N=15 Cre males, N=8 Control females, N=7 Cre females.
2.6. 5-HT1A receptor knockdown in the BNST does not significant alter innate responses to visual threat in the looming disc assay.
The BNST receives retinal inputs and has extensive projections to the midbrain nuclei that process visual stimuli, suggesting that 5-HT1A receptors may contribute innate visual defense responses50–52. Another recent study found that innate defensive responses to looming visual stimuli are mediated by parvalbumin-projecting neurons in the superior colliculus of the mouse, which project to neurons in the parabigeminal nucleus and the lateral posterior thalamic nucleus52, all of which project to the amygdala and may also have direct or indirect connections with the BNST53–55. To interrogate a potential role for the BNST in visual threat processing, we examined behavior in the looming disc assay. We found a non-significant trend toward a reduction in maximum escape velocity in male htr1Afl/fl::CreBNST mice(t13.67 (Welch-corrected)=1.85, ns) (Figure 7A–H), but no significant differences in escape latency or freezing time. These data indicate that 5-HT1A knockdown in the BNST does not significantly impair visual function or threat response in male mice.
Figure 7: 5-HT1A receptor knockdown in the BNST does not alter visual threat processing in the looming disc assay.
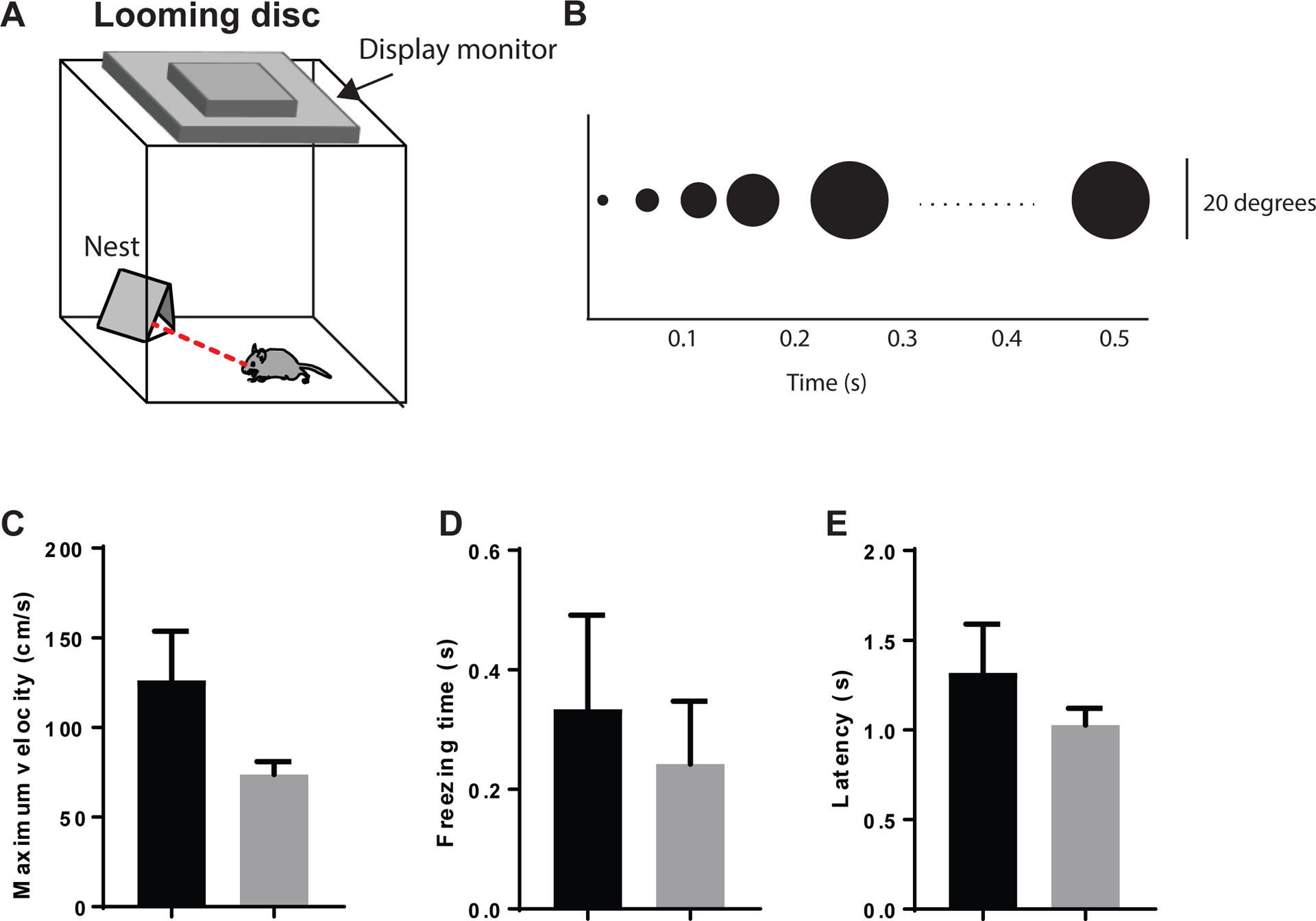
(A) Pictorial representation of the looming disc assay. (B) Time course of expanding disc on the display monitor above the looming disc arena. (C) Histogram of maximum velocity, (D) Freezing time, and (E) Escape latency after the looming disc was triggered. N=13 Control males and N=12 Cre males.
3. CONCLUSIONS
Experimental manipulations that increase neuronal activity in the BNST tend to enhance anxiety56–58, although there may be differential contributions of specific subnuclei and subpopulations that have led to conflicting reports40,59. There is also accumulating evidence from neuroimaging studies that the BNST is involved in the representation of anxiety states in humans with generalized anxiety disorder60,61. Given that 5-HT1A receptor activation has been reported to hyperpolarize (inhibit) neurons in the BNST, we hypothesized that 5-HT1A deletion would potentiate anxiety-like behavior. Contrary to our expectations, our results indicate that 5-HT1A receptor knockdown in the BNST does not significantly alter anxiety-like behavior, suggesting that 5-HT1A receptors may not have an appreciable influence over BNST circuits under basal conditions due to their low abundance. On the other hand, 5-HT1A knockdown does enhance contextual fear recall after fear conditioning in males, which is in agreement with previous reports that cannabidiol can reduce contextual fear by activating 5-HT1A receptors in the BNST14. This discrepancy between anxiety and fear suggests that high arousal states may induce plasticity in 5-HT1A receptor signaling that enables this receptor to act as a molecular brake that buffers against excessive stimulation. The observation that neuronal excitability is slightly lower in male control mice after fear conditioning but increases in htr1Afl/fl::CreBNST mice lends further support to the idea that 5-HT1A acts as a molecular switch that can be turned on under conditions of high arousal.
It is interesting that 5-HT1A knockdown does not appear to have any appreciable effects on contextual fear memory in female mice, especially given that 5-HT1A receptor levels do not significantly differ between males and females. However, it could be argued that the ability to buffer against excessive arousal states may be more adaptive for males from an evolutionary standpoint, especially since males tend to spend more time engaging in high-risk foraging and aggressive behaviors compared to females. This view is supported by the observation that males have significantly reduced contextual fear recall compared to females. Another possibility is that innate differences in fear memory consolidation plays a role in the significant discrepancy between males and females in contextual fear recall. Another possibility is that females have increased sensitivity to the unconditioned stimulus due to their smaller size leading to an exaggerated stress response compared to males, although the fact that males and females exhibit the same level of freezing during conditioning would seem to argue against this. Additional testing at a range of shock intensities to determine whether 5-HT1A knockdown can enhance fear memory in females would be required to rule out this possibility.
4. METHODS
4.1. Animals
Htr1afl/fl mice were backcrossed for several generations to C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) before using in experiments. All procedures were conducted in accordance with the National Institutes of Health guidelines for animal research and with the approval of the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill and the Vanderbilt University Medical Center. All animals were group housed on a 12 h light cycle (lights on at 7 a.m.) with ad libitum access to rodent chow and water, unless described otherwise.
Generation of Htr1afl/fl mice.
Htr1afl/fl mice were generated using homologous recombination in ES cells, the unique exon (exon 1) was targeted to introduce loxP sites around the gene ORF. A loxP site was introduced in the middle of the 5’UTR, while a neomycin resistance gene cassette flanked by Frt and loxP sites was introduced in the 3’ UTR. Upon Flp recombination (using Flpe mice), the neomycin-resistance gene cassette was eliminated. Reduction from 3 loxP to 2 loxP was confirmed using PCR. Elimination of exon was also confirmed by crossing the 3 loxP mouse with EIIa-CRE mice and obtaining a 1 loxP mouse.
4.2. Viruses
All AAV viruses were produced by the Gene Therapy Center Vector Core at the University of North Carolina at Chapel Hill and had titres of > 1012 genome copies per ml. Mice were injected with AAV-CMW-Cre-GFP or AAV-CMW-GFP as a control.
4.3. Drugs
5-Carboxamidotryptamine maleate (5-CT), a 5-HT1 agonist, was kept in stock solution in 10 mM at −20°C. The day of use it was diluted in (aCSF) to a working solution of 10 μM. SB 269970, a potent and selective 5-HT7 antagonist, was first dissolved in DMSO at 100 mM and stored at −20°C, then diluted in aCSF to a working concentration of 1 μM.
4.4. Stereotaxic surgery
All surgeries were conducted using aseptic technique. Adult htr1ARfl/fl mice were used in this study. Before the surgery, mice were deeply anesthetized with isoflurane and then placed into a stereotactic frame (Kopf instruments). An incision was made down the midline of the scalp and a craniotomy was performed above the target region. Mice were injected with 400 nl of AAV-CMW-Cre-GFP or AAV-CMW-GFP into the BNST (± 1.00, 0.30, − 4.35 in mm, midline, Bregma, dorsal surface). Red IX retrograde tracer beads (Lumafluor) were microinjected into the VTA (− 0.30, − 2.90, − 4.60) to fluorescently label VTA-projecting BNST neurons during ex vivo slice electrophysiology recordings. Viruses and fluorescent tracers were microinjected using a Neuros Hamilton syringe at a rate of 100 nl min−1. Following surgery, all mice were returned to group housing. Mice were allowed to recover for at least 3 weeks before being used for behavioral studies. After all experiments, each mouse was perfused and validated for injection efficiency and viral expression.
4.5. Behavioral assays
All behavioral assays were conducted in accordance with previously published procedures from using conditions that were demonstrated to detect differences in anxiety-like and affective behaviors9,43,50,52. The order in which the tests were conducted was as follows: 1) EPM, 2) open field, 3) NSF, 5) forced swim, 6) fear conditioning. Animals were given at least 48 hours to recover after the EPM and open field tests and at least 7 days after the NSF and forced swim tests before further behavioral testing was conducted. A subset of animals was tested in the following order: 1) EPM, 2) looming disc, 3) restraint plus EZM and 4) fear conditioning.
Elevated plus maze.
This test was conducted according to previously published results which were found to detect anxiogenic effects of fluoxetine in mice9. Mice were placed in the center of an elevated plus maze, which consisted of one open arm (35 cm × 5 cm) crossed at a right angle by an enclosed arms (35 cm × 5 cm) with walls that are 15 cm in height. The entire arena is raised at a height of 60 cm from the floor. The mice were allowed to freely explore during a 5 min session. Light levels in the open arms were at ~ 25 lux. Time spent in the open arm, latency to the open arm, open arm probability, and total distance moved were measured.
Open field (Low and High-Light).
Mice were placed into the corner of a white Plexiglas open field arena (50 × 50 × 50 cm) and allowed to freely explore for 30 min. Time spent in the center, latency to the center, the number of center entries, and the total distance moved were measured. The center of the open field was defined as the central 25% of the arena. The open field test took place either under high light conditions (300 lux) or low light (25 lux) conditions.
Novelty-suppressed feeding.
Forty-eight hours before testing, mice were provided with access to a single piece of Froot Loops cereal (Kellogg’s) in their home cage. Twenty-four hours before testing, home cage chow was removed. Mice were weighed both before food deprivation and before testing to assess body weight loss. Water remained available ad libitum. Beginning at least one hour before testing, mice were transferred to a new clean cage so that they were singly housed for the testing session. During the testing session mice were placed into a brightly lit arena (50 × 50 × 50 cm) that contained a single Froot Loop on top of a piece of circular filter paper. Mice were monitored by a live observer and the latency for the mouse to begin eating the pellet was measured. Following the initiation of feeding, mice were removed from the arena and placed back into their home cages. Mice were then provided with 10 min of access to a pre-weighed amount of Froot Loops for a post-test feeding session. After this 10 min post-test, the remaining Froot Loops were weighed, and mice were returned to ad libitum home cage chow. Mice were returned to group housing at the end of this session.
Restraint + Elevated zero maze.
Mice were placed inside of a 50 ml conical tube with air holes throughout the body of the tube which was taped down to a lab bench for 30 minutes. Immediately afterwards, they were placed in the elevated zero maze (EZM) for behavioral testing. The EZM has a diameter of 60 cm with a 5 cm wide circular corridor that has 16 cm high walls on opposite sides. Time spent in the open areas of the maze is taken as an inverse measure of anxiety-like behavior.
Forced swim test.
Forced swim procedures were carried out as described previously43. Briefly, mice were placed in a clear, plexiglass container that was filled with 24°C to a height of 15 cm for 6 min. Immobility during the last 4 minutes was hand-scored using Ethovision XT 10 and used as a measure of behavioral despair. Animals exhibiting behavioral despair will spend a higher percentage of their time in an immobile state62.
Contextual Fear conditioning.
A two-day protocol was used to assess contextual fear recall. On the first day, mice were placed into a fear conditioning chamber (Med Associates) containing a grid floor. Prior to the session, the chamber was cleaned with a scented paper towel (19.5% ethanol, 79.5% H2O, 1% vanilla). After a 3 min baseline period during which mice were allowed to explore freely, mice were exposed to a 30 s tone (3 kHz, 80 dB) that co-terminated with a 2 s scrambled foot shock (0.6 mA). A total of 5 tone-shock pairings were delivered with a random inter-tone interval (ITI) of 60–120 s. Following delivery of the last foot shock, mice remained in the conditioning chamber for a 2-min consolidation period. The following day, mice were returned to the same training chamber for 10 min and allowed to freely explore. Freezing behavior was scored at every 5 second interval and averaged for the first 3 minutes. For each session, cameras were mounted overhead for recording freezing behavior, which was later hand-scored every 5s by a trained observer blinded to experimental treatment. Freezing was defined as a lack of movement except as required for respiration.
Looming disc.
The looming disc assay was performed as described previously.50,52 Mice were placed inside of an open top plexiglass box with white matte flooring (40 × 40 × 30 cm) and a dark shelter nest in far left corner. The nest was in the shape of a triangular prism (20 × 12 cm). An LED monitor with a gray screen was placed on top of the arena, which would display an expanding black disc when triggered. The floor and three walls were covered with a matte coating to prevent reflections of the stimulus. Infrared LEDs were placed around the outer edges of the area for video recording. Htr1afl/fl mice aged 8–12 weeks were group-housed and maintained on a regular light/dark cycle. On the day of looming disc test, each mouse was allowed to freely explore the looming box for 10 min. At the end of the habituation phase, an experimenter would trigger the looming disc when the mouse was at the center of the arena. Flight latency (ms) and velocity to escape into the nest were measured by trained observer blinded to the experimental conditions.
4.6. Whole-cell patch clamp electrophysiology
Mice were decapitated under isoflurane anesthesia and brains were rapidly removed and placed in an ice-cold solution of sucrose-artificial cerebrospinal fluid (aCSF) [(in mM): 194 sucrose, 20 NaCl, 4.4 KCl, 2 CaCl2, 1 MgCl2, 1.2 NaH2PO4, 10 glucose, and 26 NaHCO3 saturated with 95% O 2/5% CO2. Brains were sectioned at 0.07 (mm/s) on a Leica 1200S vibratome to obtain 300 μm coronal slices of the BNST, which were incubated in a heated holding chamber containing normal, oxygenated aCSF [(in mM):124 NaCl, 4.4 KCl, 2 CaCl2,1.2 MgSO4,1 NaH2PO4,10.0 glucose, and 26.0 NaHCO3] maintained at 30 ± 1°C for at least 1 h before recording. Slices were transferred to a recording chamber (Warner Instruments) submerged in normal, oxygenated aCSF maintained at 28–30°C delivered at a flow rate of 2 ml/min. Neurons of the BNST were visualized using infrared-differential interference contrast (DIC) video-enhanced microscopy (Olympus). Slices were bathed in normal aCSF for at least 30 min before performing whole-cell patch clamp experiments. Borosilicate electrodes pulled with a Flaming-Brown micropipette puller (Sutter Instruments) with a pipette resistance between 3 and 6 MΩ filled with internal solution [(in mM): 135 KCl-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 ATP, and 0.4 GTP, pH 7.35, 290 mOsmol] were used to patch cells in the BNST. Signals were acquired using a Multiclamp 700B amplifier and analyzed with Clampfit 10.3 software (Molecular Devices, Sunnyvale, CA, USA). All experiments were conducted in current clamp mode with the holding current set to zero. For excitability experiments, a current ramp protocol was used to determine the current required to induce firing (rheobase) and the voltage at first spike. The number of spikes generated by discrete current steps was also determined using a 10 pA current step protocol from 0 to 150 pA (VeI plot). In a subset of experiments, the 5HT1a-R agonist 5-CT was bath applied for 10 min before rheobase and VI plots were performed. The 5-HT7 antagonist SB267970 was included in the bath to isolate the contribution of 5-HT1A receptors.
4.7. Double Fluorescence In situ hybridization (FISH)
Mice were anesthetized using isoflurane, rapidly decapitated, and brains were rapidly extracted. Immediately after removal, the brains were placed on a square of aluminum foil on dry ice to freeze. Brains were then placed in a − 80°C freezer for no more than 1 week before slicing. Slices of the BNST (12 μm) were made on a Leica CM3050S cryostat (Germany) and placed directly on coverslips. FISH was performed using the Affymetrix ViewRNA 2-Plex Tissue Assay Kit with custom probes for 5-HT1A and Cre designed by Affymetrix (Santa Clara, CA). Slides were coverslipped with Southern Biotech DAPI Fluoromount-G (Birmingham, AL). 3 × 5 tiled z stack (15 optical sections comprising 14 μ m total) images of the entire 12 μ m slice were obtained on a Zeiss 780 confocal microscope for assessment of 5-HT1A and Cre expression. All images were preprocessed with stitching and maximum intensity projection. An image of the BNST from 3 mice in each condition was hand counted for each study using the cell counter plugin in FIJI (ImageJ).
4.8. Immunohistochemistry and microscopy
All mice used for behavioral and anatomical tracing experiments were stained for GFP to verify that viral spread of AAV-Cre-GFP was confined to the BNST. Mice were anesthetized with Avertin and transcardially perfused with 30 ml of 0.01 M PBS followed by 30 ml of 4% paraformaldehyde (PFA) in PBS. Brains were extracted and stored in 4% PFA for 24 h at 4 °C before being rinsed twice with and stored in PBS. 45 μm slices were obtained on a Leica VT100S and stored in 50/50 PBS/Glycerol at 4 °C. For the immunohistochemistry, slices were first washed in PBS, then incubated in 0.5% Triton/PBS. Slices were washed in PBS again followed by a 1 hour blocking step in 1% BSA, 10% Normal Donkey Serum in 0.1% Triton-X-100 followed by a 24 h incubation at 4 °C in chicken anti-GFP (1:500; Santa-Cruz) in blocking buffer. The following day, slices were washed in PBS and incubated in Alexafluor 488 Donkey anti-chicken (1:500; Jackson ImmunoResearch), then washed again in PBS. Slices were then mounted on glass slides, coverslipped with Vectashield with Dapi, and imaged using a Zeiss AXIO Zoom V16 microscope with ZEN pro 2012 software.
4.9. Western blots
Twenty male and twenty female C57BL6/J were euthanized with isoflurane and immediately decapitated. The brain was extracted and sliced into 1mm sections. Punches from four mice were taken from the BNST and pooled in a tube on dry ice, then stored at −80°C. Tissue was lysed using approximately 20ul of RIPA lysis buffer (150mM NaCl, 1.0% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50mM Tris-HCl pH 8.0) per milligram of tissue that included a 1:100 dilution of a protease inhibitor (Sigma Aldrich P8340) and a phosphatase inhibitor (Sigma Aldrich P5726). After adding the lysis buffer, the samples were sonicated and incubated on ice for 30 min. Protein concentrations were determined using the BioRad DC protein assay kit (Cat #500–0116).4X Laemmli buffer (4% SDS, 10% 2-mercaptoethanol, 20% glycerol, 0.004% bromophenol blue) was heated and added to each sample. Precision Plus Protein Dual Color Standard (Bio-Rad #1610374) and 20ug of each sample were added to 12% Bio-Rad Mini-PROTEAN TGX Stain-Free gels (Cat #456–8046) and ran in a vertical electrophoresis chamber. The gels were transferred onto low fluorescent PVDF membranes (Bio-Rad #162–0260) using the Bio-Rad Transblot Turbo semidry transfer system. After transfer, the membranes were blocked with 5% non-fat dry milk in TBST for 1 hr at room temperature with agitation. After 5 × 5 minute washes with TBST, the membranes were incubated in 5% BSA/TBST with 1:500 of N-Cadherin produced in rabbit (GeneTex GTX127345) and 1:500 of HTR1A produced in rabbit (ThermoFisher Scientific PA5–28090) for 48 hours at 4°C with agitation. The membranes were washed 5 × 5 min with TBST then incubated in 5% milk/TBST with 1:1000 IRDye 800CW donkey anti-rabbit (LI-COR 925–32213) for 2 hrs at room temperature with agitation. The membranes were washed 5 more times with TBST then imaged on the Azure BioSystems Sapphire Biomolecular Imager. Band quantification was done using AzureSpot 2.0 analysis software.
4.10. Data and Statistical Analysis.
Data are presented as mean ± SEM. A two-way analysis of variance (ANOVA) or a Student’s t-test was used to analyze behavioral and immunohistochemical data. Some of the sample groups were too small to detect normality (< 8 samples) but parametric tests were used because nonparametric tests lack sufficient power to detect differences in small samples (Graphpad Statistics Guide – http://www.graphpad.com). The standard error of the mean is indicated by error bars for each group of data. Differences were considered significant at P values below 0.05. All data were analyzed with GraphPad Prism software.
ACKNOWLEDGEMENT
This work was supported by NIH grant K99A024215 to C.A.M and NIH grant R01AA019454 to T.L.K.
Abbreviations:
- BNST
Bed nucleus of the stria terminalis
- DRN
dorsal raphe nucleus
- EPM
elevated plus maz
- EZM
elevated zero maze
- OF
open field
- NSF
novelty-suppressed feeding test
5. REFERENCES
- (1).Hornung J-P The Human Raphe Nuclei and the Serotonergic System. J. Chem. Neuroanat 2003, 26 (4), 331–343. [DOI] [PubMed] [Google Scholar]
- (2).Gaspar P; Lillesaar C Probing the Diversity of Serotonin Neurons. Philos. Trans. R. Soc. Lond. B. Biol. Sci 2012, 367 (1601), 2382–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Marcinkiewcz CA Serotonergic Systems in the Pathophysiology of Ethanol Dependence: Relevance to Clinical Alcoholism. ACS Chem. Neurosci 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Marcinkiewcz CA; Lowery-Gionta EG; Kash TL Serotonin’s Complex Role in Alcoholism: Implications for Treatment and Future Research. Alcohol. Clin. Exp. Res 2016, 40 (6), 1192–1201. [DOI] [PubMed] [Google Scholar]
- (5).Kash TL The Role of Biogenic Amine Signaling in the Bed Nucleus of the Stria Terminals in Alcohol Abuse. Alcohol 2012, 46 (4), 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Lebow MA; Chen A Overshadowed by the Amygdala: The Bed Nucleus of the Stria Terminalis Emerges as Key to Psychiatric Disorders. Mol. Psychiatry 2016, 21 (4), 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Ravinder S; Burghardt NS; Brodsky R; Bauer EP; Chattarji S A Role for the Extended Amygdala in the Fear-Enhancing Effects of Acute Selective Serotonin Reuptake Inhibitor Treatment. Transl. Psychiatry 2013, 3 (1), e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Marcinkiewcz CA; Dorrier CE; Lopez AJ; Kash TL Ethanol Induced Adaptations in 5-HT2C Receptor Signaling in the Bed Nucleus of the Stria Terminalis: Implications for Anxiety during Ethanol Withdrawal. Neuropharmacology 2014, 89C, 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Marcinkiewcz CA; Mazzone CM; D’Agostino G; Halladay LR; Hardaway JA; DiBerto JF; Navarro M; Burnham N; Cristiano C; Dorrier CE; et al. Serotonin Engages an Anxiety and Fear-Promoting Circuit in the Extended Amygdala. Nature 2016, 537 (7618), 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Pelrine E; Pasik SD; Bayat L; Goldschmiedt D; Bauer EP 5-HT2C Receptors in the BNST Are Necessary for the Enhancement of Fear Learning by Selective Serotonin Reuptake Inhibitors. Neurobiol. Learn. Mem 2016, 136, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Garcia-Garcia AL; Canetta S; Stujenske JM; Burghardt NS; Ansorge MS; Dranovsky A; Leonardo ED Serotonin Inputs to the Dorsal BNST Modulate Anxiety in a 5-HT1A Receptor-Dependent Manner. Mol. Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Levita L; Hammack SE; Mania I; Li X-Y; Davis M; Rainnie DG 5-Hydroxytryptamine1A-like Receptor Activation in the Bed Nucleus of the Stria Terminalis: Electrophysiological and Behavioral Studies. Neuroscience 2004, 128 (3), 583–596. [DOI] [PubMed] [Google Scholar]
- (13).Gomes FV; Resstel LBM; Guimarães FS The Anxiolytic-like Effects of Cannabidiol Injected into the Bed Nucleus of the Stria Terminalis Are Mediated by 5-HT1A Receptors. Psychopharmacology (Berl). 2011, 213 (2–3), 465–473. [DOI] [PubMed] [Google Scholar]
- (14).Gomes FV; Reis DG; Alves FHF; Corrêa FM; Guimarães FS; Resstel LBM Cannabidiol Injected into the Bed Nucleus of the Stria Terminalis Reduces the Expression of Contextual Fear Conditioning via 5-HT1A Receptors. J. Psychopharmacol 2012, 26 (1), 104–113. [DOI] [PubMed] [Google Scholar]
- (15).Guo J-D; Hammack SE; Hazra R; Levita L; Rainnie DG Bi-Directional Modulation of Bed Nucleus of Stria Terminalis Neurons by 5-HT: Molecular Expression and Functional Properties of Excitatory 5-HT Receptor Subtypes. Neuroscience 2009, 164 (4), 1776–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Azmitia EC; Gannon PJ; Kheck NM; Whitaker-Azmitia PM Cellular Localization of the 5-HT1A Receptor in Primate Brain Neurons and Glial Cells. Neuropsychopharmacology 1996, 14 (1), 35–46. [DOI] [PubMed] [Google Scholar]
- (17).Mengod G; Vilaró MT; Raurich A; López-Giménez JF; Cortés R; Palacios JM 5-HT Receptors in Mammalian Brain: Receptor Autoradiography and in Situ Hybridization Studies of New Ligands and Newly Identified Receptors. Histochem. J 1996, 28 (11), 747–758. [DOI] [PubMed] [Google Scholar]
- (18).Huot P; Johnston TH; Fox SH; Newman-Tancredi A; Brotchie JM The Highly-Selective 5-HT(1A) Agonist F15599 Reduces L-DOPA-Induced Dyskinesia without Compromising Anti-Parkinsonian Benefits in the MPTP-Lesioned Macaque. Neuropharmacology 2015, 97, 306–311. [DOI] [PubMed] [Google Scholar]
- (19).Meadows SM; Chambers NE; Conti MM; Bossert SC; Tasber C; Sheena E; Varney M; Newman-Tancredi A; Bishop C Characterizing the Differential Roles of Striatal 5-HT1A Auto- and Hetero-Receptors in the Reduction of l-DOPA-Induced Dyskinesia. Exp. Neurol 2017, 292, 168–178. [DOI] [PubMed] [Google Scholar]
- (20).Davis M; Walker DL; Lee Y Amygdala and Bed Nucleus of the Stria Terminalis: Differential Roles in Fear and Anxiety Measured with the Acoustic Startle Reflex. Philos. Trans. R. Soc. Lond. B. Biol. Sci 1997, 352 (1362), 1675–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Walker DL; Miles L; Davis M Selective Participation of the Bed Nucleus of the Stria Terminalis and CRF in Sustained Anxiety-like versus Phasic Fear-like Responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33 (8), 1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sink KS; Walker DL; Freeman SM; Flandreau EI; Ressler KJ; Davis M Effects of Continuously Enhanced Corticotropin Releasing Factor Expression within the Bed Nucleus of the Stria Terminalis on Conditioned and Unconditioned Anxiety. Mol. Psychiatry 2013, 18 (3), 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Gungor NZ; Paré D Functional Heterogeneity in the Bed Nucleus of the Stria Terminalis. J. Neurosci 2016, 36 (31), 8038–8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Garcia-Garcia AL; Canetta S; Stujenske JM; Burghardt NS; Ansorge MS; Dranovsky A; Leonardo ED Serotonin Inputs to the Dorsal BNST Modulate Anxiety in a 5-HT1A Receptor-Dependent Manner. Mol. Psychiatry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).del Abril A; Segovia S; Guillamón A The Bed Nucleus of the Stria Terminalis in the Rat: Regional Sex Differences Controlled by Gonadal Steroids Early after Birth. Brain Res. 1987, 429 (2), 295–300. [DOI] [PubMed] [Google Scholar]
- (26).Allen LS; Gorski RA Sex Difference in the Bed Nucleus of the Stria Terminalis of the Human Brain. J. Comp. Neurol 1990, 302 (4), 697–706. [DOI] [PubMed] [Google Scholar]
- (27).Han TM; De Vries GJ Organizational Effects of Testosterone, Estradiol, and Dihydrotestosterone on Vasopressin MRNA Expression in the Bed Nucleus of the Stria Terminalis. J. Neurobiol 2003, 54 (3), 502–510. [DOI] [PubMed] [Google Scholar]
- (28).Janitzky K; Peine A; Kröber A; Yanagawa Y; Schwegler H; Roskoden T Increased CRF MRNA Expression in the Sexually Dimorphic BNST of Male but Not Female GAD67 Mice and TMT Predator Odor Stress Effects upon Spatial Memory Retrieval. Behav. Brain Res. 2014, 272, 141–149. [DOI] [PubMed] [Google Scholar]
- (29).Bangasser DA; Shors TJ The Bed Nucleus of the Stria Terminalis Modulates Learning after Stress in Masculinized but Not Cycling Females. J. Neurosci 2008, 28 (25), 6383–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Rickels K; Weisman K; Norstad N; Singer M; Stoltz D; Brown A; Danton J Buspirone and Diazepam in Anxiety: A Controlled Study. J. Clin. Psychiatry 1982, 43 (12 Pt 2), 81–86. [PubMed] [Google Scholar]
- (31).Heisler LK; Chu HM; Brennan TJ; Danao JA; Bajwa P; Parsons LH; Tecott LH Elevated Anxiety and Antidepressant-like Responses in Serotonin 5-HT1A Receptor Mutant Mice. Proc. Natl. Acad. Sci. U. S. A 1998, 95 (25), 15049–15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Parks CL; Robinson PS; Sibille E; Shenk T; Toth M Increased Anxiety of Mice Lacking the Serotonin1A Receptor. Proc. Natl. Acad. Sci. U. S. A 1998, 95 (18), 10734–10739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Ramboz S; Oosting R; Amara DA; Kung HF; Blier P; Mendelsohn M; Mann JJ; Brunner D; Hen R Serotonin Receptor 1A Knockout: An Animal Model of Anxiety-Related Disorder. Proc. Natl. Acad. Sci. U. S. A 1998, 95 (24), 14476–14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Zhuang X; Gross C; Santarelli L; Compan V; Trillat AC; Hen R Altered Emotional States in Knockout Mice Lacking 5-HT1A or 5-HT1B Receptors. Neuropsychopharmacology 1999, 21 (2 Suppl), 52S–60S. [DOI] [PubMed] [Google Scholar]
- (35).Haufler D; Nagy FZ; Pare D Neuronal Correlates of Fear Conditioning in the Bed Nucleus of the Stria Terminalis. Learn. Mem 2013, 20 (11), 633–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hisasue S; Seney ML; Immerman E; Forger NG Control of Cell Number in the Bed Nucleus of the Stria Terminalis of Mice: Role of Testosterone Metabolites and Estrogen Receptor Subtypes. J. Sex. Med 2010, 7 (4 Pt 1), 1401–1409. [DOI] [PubMed] [Google Scholar]
- (37).Lemos JI; Resstel LB; Guimarães FS Involvement of the Prelimbic Prefrontal Cortex on Cannabidiol-Induced Attenuation of Contextual Conditioned Fear in Rats. Behav. Brain Res. 2010, 207 (1), 105–111. [DOI] [PubMed] [Google Scholar]
- (38).Walf AA; Frye CA The Use of the Elevated plus Maze as an Assay of Anxiety-Related Behavior in Rodents. Nat. Protoc 2007, 2 (2), 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bailey KR; Crawley JN Anxiety-Related Behaviors in Mice; 2009. [PubMed] [Google Scholar]
- (40).Jennings JH; Sparta DR; Stamatakis AM; Ung RL; Pleil KE; Kash TL; Stuber GD Distinct Extended Amygdala Circuits for Divergent Motivational States. Nature 2013, 496 (7444), 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Jennings JH; Rizzi G; Stamatakis AM; Ung RL; Stuber GD The Inhibitory Circuit Architecture of the Lateral Hypothalamus Orchestrates Feeding. Science 2013, 341 (6153), 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Wieland S; Lucki I Antidepressant-like Activity of 5-HT1A Agonists Measured with the Forced Swim Test. Psychopharmacology (Berl). 1990, 101 (4), 497–504. [DOI] [PubMed] [Google Scholar]
- (43).Günther L; Rothe J; Rex A; Voigt J-P; Millan MJ; Fink H; Bert B 5-HT(1A)-Receptor over-Expressing Mice: Genotype and Sex Dependent Responses to Antidepressants in the Forced Swim-Test. Neuropharmacology 2011, 61 (3), 433–441. [DOI] [PubMed] [Google Scholar]
- (44).Millón C; Flores-Burgess A; Narváez M; Borroto-Escuela DO; Santín L; Gago B; Narváez JA; Fuxe K; Díaz-Cabiale Z Galanin (1–15) Enhances the Antidepressant Effects of the 5-HT1A Receptor Agonist 8-OH-DPAT: Involvement of the Raphe-Hippocampal 5-HT Neuron System. Brain Struct. Funct 2016, 221 (9), 4491–4504. [DOI] [PubMed] [Google Scholar]
- (45).Takahashi K; Nakagawasai O; Nemoto W; Odaira T; Sakuma W; Tan-No K Antidepressant-like Effect of Aripiprazole via 5-HT1A, D1, and D2 Receptors in the Prefrontal Cortex of Olfactory Bulbectomized Mice. J. Pharmacol. Sci 2018, 137 (3), 241–247. [DOI] [PubMed] [Google Scholar]
- (46).Wang G-L; Wang Y-P; Zheng J-Y; Zhang L-X Monoaminergic and Aminoacidergic Receptors Are Involved in the Antidepressant-like Effect of Ginsenoside Rb1 in Mouse Hippocampus (CA3) and Prefrontal Cortex. Brain Res. 2018, 1699, 44–53. [DOI] [PubMed] [Google Scholar]
- (47).Lowry CA; Rodda JE; Lightman SL; Ingram CD Corticotropin-Releasing Factor Increases In Vitro Firing Rates of Serotonergic Neurons in the Rat Dorsal Raphe Nucleus : Evidence for Activation of a Topographically Organized Mesolimbocortical Serotonergic System. 2000, 20 (20), 7728–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Daniel SE; Rainnie DG Stress Modulation of Opposing Circuits in the Bed Nucleus of the Stria Terminalis. Neuropsychopharmacology 2016, 41 (1), 103–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Braun AA; Skelton MR; Vorhees CV; Williams MT Comparison of the Elevated plus and Elevated Zero Mazes in Treated and Untreated Male Sprague-Dawley Rats: Effects of Anxiolytic and Anxiogenic Agents. Pharmacol. Biochem. Behav 2011, 97 (3), 406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Yilmaz M; Meister M Rapid Innate Defensive Responses of Mice to Looming Visual Stimuli. Curr. Biol 2013, 23 (20), 2011–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Morin LP; Studholme KM Retinofugal Projections in the Mouse. J. Comp. Neurol 2014, 522 (16), 3733–3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Shang C; Chen Z; Liu A; Li Y; Zhang J; Qu B; Yan F; Zhang Y; Liu W; Liu Z; et al. Divergent Midbrain Circuits Orchestrate Escape and Freezing Responses to Looming Stimuli in Mice. Nat. Commun 2018, 9 (1), 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Doron NN; Ledoux JE Cells in the Posterior Thalamus Project to Both Amygdala and Temporal Cortex: A Quantitative Retrograde Double-Labeling Study in the Rat. J. Comp. Neurol 2000, 425 (2), 257–274. [PubMed] [Google Scholar]
- (54).Usunoff KG; Itzev DE; Rolfs A; Schmitt O; Wree A Brain Stem Afferent Connections of the Amygdala in the Rat with Special References to a Projection from the Parabigeminal Nucleus: A Fluorescent Retrograde Tracing Study. Anat. Embryol. (Berl) 2006, 211 (5), 475–496. [DOI] [PubMed] [Google Scholar]
- (55).Rafal RD; Koller K; Bultitude JH; Mullins P; Ward R; Mitchell AS; Bell AH Connectivity between the Superior Colliculus and the Amygdala in Humans and Macaque Monkeys: Virtual Dissection with Probabilistic DTI Tractography. J. Neurophysiol 2015, 114 (3), 1947–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Gafford GM; Guo J; Flandreau EI; Hazra R; Rainnie DG; Ressler KJ Cell-Type Specific Deletion of GABA(A)A1 in Corticotropin-Releasing Factor-Containing Neurons Enhances Anxiety and Disrupts Fear Extinction. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (40), 16330–16335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Sink KS; Chung A; Ressler KJ; Davis M; Walker DL Anxiogenic Effects of CGRP within the BNST May Be Mediated by CRF Acting at BNST CRFR1 Receptors. Behav. Brain Res 2013, 243, 286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Mazzone CM; Pati D; Michaelides M; DiBerto J; Fox JH; Tipton G; Anderson C; Duffy K; McKlveen JM; Hardaway JA; et al. Acute Engagement of Gq-Mediated Signaling in the Bed Nucleus of the Stria Terminalis Induces Anxiety-like Behavior. Mol. Psychiatry 2018, 23 (1), 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Kim S-Y; Adhikari A; Lee SY; Marshel JH; Kim CK; Mallory CS; Lo M; Pak S; Mattis J; Lim BK; et al. Diverging Neural Pathways Assemble a Behavioural State from Separable Features in Anxiety. Nature 2013, 496 (7444), 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Buff C; Brinkmann L; Bruchmann M; Becker MPI; Tupak S; Herrmann MJ; Straube T Activity Alterations in the Bed Nucleus of the Stria Terminalis and Amygdala during Threat Anticipation in Generalized Anxiety Disorder. Soc. Cogn. Affect. Neurosci 2017, 12 (11), 1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Brinkmann L; Buff C; Feldker K; Neumeister P; Heitmann CY; Hofmann D; Bruchmann M; Herrmann MJ; Straube T Inter-Individual Differences in Trait Anxiety Shape the Functional Connectivity between the Bed Nucleus of the Stria Terminalis and the Amygdala during Brief Threat Processing. Neuroimage 2018, 166, 110–116. [DOI] [PubMed] [Google Scholar]
- (62).Marcinkiewcz CA; Devine DP Modulation of OCT3 Expression by Stress, and Antidepressant-like Activity of Decynium-22 in an Animal Model of Depression. Pharmacol. Biochem. Behav 2015, 131, 33–41. [DOI] [PubMed] [Google Scholar]


