Abstract
Background
Cough and sputum are highly prevalent in patients with chronic obstructive pulmonary disease (COPD). Pulmonary rehabilitation (PR) has shown to be effective in managing these symptoms. However, the interpretation of the magnitude of PR effects is hindered by the lack of minimal clinically important differences (MCIDs).
Purpose
This study established MCIDs for the Leicester cough questionnaire (LCQ) and the cough and sputum assessment questionnaire (CASA-Q), in patients with COPD after PR.
Patients and Methods
An observational prospective study was conducted in patients with COPD who participated in a 12-weeks community-based PR program. Anchor- (mean change, receiver operating characteristic curves and linear regression analysis) and distribution-based methods [0.5*standard deviation; standard error of measurement (SEM); 1.96*SEM; minimal detectable change and effect size] were used to compute the MCIDs. The anchors used were: i) patients and physiotherapists global rating of change scale, ii) COPD assessment test, iii) St. George’s respiratory questionnaire and iv) occurrence of an exacerbation during PR. Pooled MCIDs were computed using the arithmetic weighted mean (2/3 for anchor- and 1/3 for distribution-based methods).
Results
Forty-nine patients with COPD (81.6% male, 69.8±7.4years, FEV150.4±19.4%predicted) were used in the analysis. The pooled MCIDs were 1.3 for LCQ and for CASA-Q domains were: 10.6 - cough symptoms; 10.1 - cough impact; 9.5 - sputum symptoms and 7.8 - sputum impact.
Conclusion
The MCIDs found in this study are potential estimates to interpret PR effects on cough and sputum, and may contribute to guide interventions.
Keywords: COPD, symptoms, pulmonary rehabilitation, patient health questionnaire, measurement characteristics, statistics
Introduction
Chronic obstructive pulmonary disease (COPD) is a growing global health concern that poses major burden on individuals, as well as, on economics and social systems.1–3 Cough and sputum are present in approximately 60% of patients with COPD4–6 and have been recognized to affect significantly and negatively patients’ health-related quality of life (HRQoL).7,8 Nevertheless, these symptoms have been scarcely explored and underappreciated in COPD research.9–12
Pulmonary rehabilitation (PR) is a well-established non-pharmacological intervention to manage patients with COPD.3,13,14 However, to interpret the magnitude of the results achieved with PR on symptoms relief, it is important to understand the minimal clinically important differences (MCIDs) of patient-reported outcome measures (PROMs), ie, the smallest change in a measure score that is subjectively perceived as relevant to the patient.15–17 Having MCIDs for symptoms-related PROMs will: aid to guide interventions;18,19 enhance judgement about the clinical relevance and magnitude of the PR effect15 allow samples size calculations; and contribute for defining expected endpoints in clinical trials.16,20,21 Thus, establishing MCIDs is of paramount importance for several stakeholders, from health professionals and researchers to guideline developers and policymakers.
We estimated the MCID of PROMs that assess symptoms of cough and sputum, ie, the Leicester cough questionnaire (LCQ)22 and the cough and sputum assessment questionnaire (CASA-Q)23 in patients with COPD, following a PR program.
Materials and Methods
Study Design and Participants
An observational prospective study, part of a larger trial (3R: Revitalising pulmonary rehabilitation – NCT03799666 on ClinicalTrials.gov) was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee for Health of the Administração Regional de Saúde do Centro (Ref. 73/2016) and from the National Committee for Data Protection (no. 7295/2016). Prior to enrolment and data collection, a written description of the study was provided to every participant, who then signed an informed consent.
Patients were recruited via clinicians at Centro Hospitalar do Baixo Vouga and primary healthcare centers of the center region of Portugal during January 2019 and enrolled a community-based PR program. Patients were eligible if diagnosed with COPD,3 and clinically stable over the previous month, ie, no hospital admissions or exacerbations and no change in medication for the cardiorespiratory system. Exclusion criteria included the presence of other respiratory diseases or any clinical condition that precluded participants of being involved in a community-based PR program, ie, signs of cognitive impairment or presence of a significant cardiovascular, neurological or musculoskeletal disease.
Data Collection
Data were collected before (T0) and after 12 weeks of PR (T1). Sociodemographic (age, gender), anthropometric (height and weight to compute body mass index-BMI) and clinical data (smoking status, medication, number of exacerbations, hospitalizations or emergency admissions in the past year) were first obtained. The severity of comorbid diseases was recorded and scored according to Charlson Comorbidity Index (CCI): i) scores of 1–2; ii) scores of 3–4; and iii) scores ≥5.24 The modified British medical research council questionnaire (mMRC) was used to assess functional dyspnea,25 the COPD assessment test (CAT)26 to evaluate the impact of the disease and the St. George’s respiratory questionnaire (SGRQ) to assess HRQoL.27
The LCQ was used to evaluate cough-related quality of life. The LCQ is a 19-items scale organized in 3 domains (physical, psychological and social).22 Each domain has a score ranging from 1 to 7 and the total score varies from 3 to 21.22 Higher scores express a better cough-related quality of life and less impact of cough.22 The LCQ has shown to be a valid, reliable and responsive instrument, namely in COPD.22,28–30
The CASA-Q was used to assess cough and sputum symptoms, based on its reported frequency and severity, and impact on daily life activities.23 CASA-Q is a 20-item questionnaire containing 4 domains: cough symptoms, cough impact, sputum symptoms and sputum impact.23 All items are rescored and summed, achieving a score ranging from 0 to 100 for each domain, with higher scores indicating fewer symptoms and less cough and sputum impact.23 The CASA-Q has shown to be valid, reliable and responsive in patients with COPD.23,31,32
The global rating of change scale (GRC)33 was administered using an 11-point Likert scale ranging from −5 (much worse) to +5 (much better).33 Participants were asked to rate their perceived amount of change in cough and sputum after the PR program, compared to the initial assessment.
Intervention
All participants completed a 12-weeks community-based PR program, with two exercise training sessions per week and one psychoeducational session every two weeks, in 6 primary healthcare centers and in the Lab3R-Respiratory Research and Rehabilitation laboratory of the School of Health Sciences, University of Aveiro. Further information regarding the intervention has been published elsewhere.34
Data Analysis
Statistical analysis was performed using IBM SPSS Statistics, version 24 (IBM Corporation, Armonk, NY, USA) and plots created using GraphPad Prism, version 7 (GraphPad, San Diego, CA, USA) and MetaXL 5.3 (EpiGear International, Queensland, Australia). The level of significance was set at 0.05. The analysis included only participants that adhered to at least 65% of PR sessions (ie, participated in at least 8 weeks of PR).3,35
Changes in PROMs from T0 to T1 were analyzed with paired t-test or Wilcoxon signed-rank tests, accordingly to data normality verified with the Kolmogorov–Smirnov test. Floor or ceiling effects (more than 15% of the patients scoring at the bottom or top)36 were checked. Outlier’s analysis was performed (ie, inspection of extreme points on the plotted graphs of the variables studied) and, when present, were excluded.37 MCIDs were calculated for the LCQ and CASA-Q.
Since a gold standard to determine the MCID has not been established, concurrent comparisons of different methods were performed, integrating both anchor-based and distribution-based approaches.19,38 The final MCID for each measure was pooled by calculating the arithmetic weighted mean with the MCID generated by each anchor- and distribution-based method, which were then introduced into the MetaXL39 to create the MCIDs'plots. Anchor-based methods were weighed more than distribution methods (ie, 2/3 against 1/3).19,21,38,40
Minimal Important Clinical Differences
Anchor-Based Methods
Four anchor-based approaches were applied: i) patients referencing – GRC; ii) physiotherapists referencing – GRC; iii) questionnaire referencing – CAT and SGRQ and iv) criterion referencing – occurrence of an exacerbation, as following described.
A change of 2 points or more in the patients’ GRC scale was considered clinically meaningful change.33 Thus, patients were categorized into two groups, those rating ≥2 and those rating <2 points in the GRC.
Physiotherapists that conducted the exercise sessions were asked to judge about patients’ change in cough and sputum using a GRC. A change ≥2 was used as the cut-off point for improvement.33 Physiotherapists answered the GRC questions prior to assessing patients.
Changes in the LCQ and CASA-Q scores were anchored against changes in the CAT total score and in the SGRQ total score. The MCIDs of CAT (2 points) and SGRQ (4 points) were used to discriminate between patients.41,42
Having had an exacerbation during PR.18
The presence of a significant and moderate association (≥0.3) between the change in the PROM and the anchor was a requirement to proceed with the MCID calculation.38 Correlations were assessed using Pearson’s or Spearman’s coefficients and scatter plots were generated.
To calculate the MCID according to patients, physiotherapists and questionnaire referencing, three methods were used: i) the mean change, ie, the absolute difference between the two means of the PROM score (T1 and T0), calculated for patients who achieved the MCID established for the anchor17,19 ii) receiver operating characteristic (ROC) curves [the area under the curve (AUC) of a ROC≥0.7 was considered adequate43–45 and the closest point to the left corner, where specificity (SP) and sensitivity (SN) are both optimized was considered the optimal cut-off point] and iii) linear regression analysis, with the anchor change score being used as an independent variable.19
As for the criterion referencing, the difference in the baseline score between patients who experienced an exacerbation and those who did not was considered the MCID.18,46 Independent t-tests or Mann–Whitney tests, depending on data normality, were used.
Distribution-Based Methods
To calculate the MCID, five distribution-based methods were used: i) 0.5 times standard deviation (SD);47 ii) standard error of measurement (SEM);16 iii) 1.96 times SEM;47 iv) minimal detectable change (MDC)47 and v) effect size (ES)48 (Table 1).
Table 1.
Distribution-Based Methods to Estimate the Minimal Clinically Important Difference
| Method | MCID Calculation |
|---|---|
| 0.5SD | 0.5*SDT0 |
| SEM | SDT0 √(1-r) |
| 1.96SEM | 1.96*(SDT0√(1-r)) |
| MDC | 1.96 x SEM x √2 |
| ES | (meanT1 – meanT0)/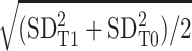
|
| ESNP | IzI/(√n) |
Notes: The test–retest reliability coefficients used were: LCQ ICC=0.92,28 CASA-Q - ICC cough symptoms =0.77, ICC cough impact =0.88, ICC sputum symptoms =0.80 and ICC sputum impact =0.82.23
Abbreviations: MCID, minimal clinically important difference; SEM, standard error measurement; SD, standard deviation; r, test–retest reliability coefficient; MDC, minimal detectable change; ES, effect size; ESNP, nonparametric effect sizes; T0, baseline; T1, after the pulmonary rehabilitation program; z, statistic test; n, number of total matched pairs.
ES were interpreted as small (≥0.2), medium (≥0.5) or large (≥0.8).48 ES greater than 0.2 were considered to be minimally clinically/subjectively important.48 After combining both anchor- and distribution-based methods and pooling the final MCID for each PROM, the corresponding percentage of change was calculated. Furthermore, we used the pooled MCID value to compute the matching ES,19 using this formula:
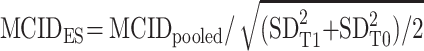 .
.
Results
Sample Characterization
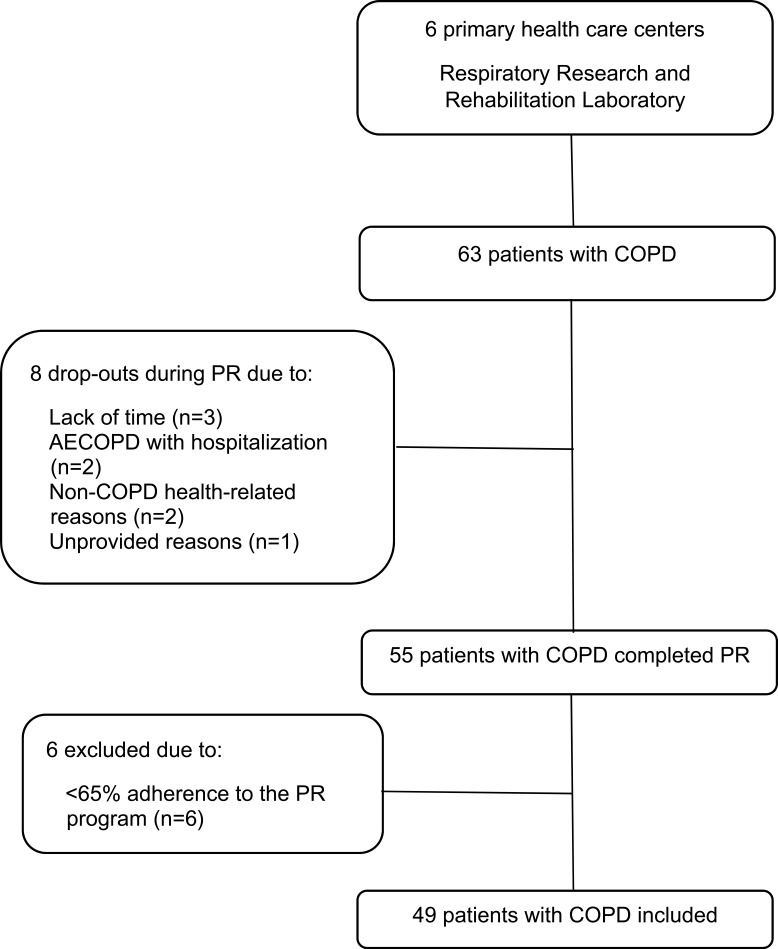
A flow diagram of the forty-nine patients included in the study is provided in Figure 1.
Figure 1.
Flow diagram of participants with chronic obstructive pulmonary disease included in the study.
Abbreviations: COPD, chronic obstructive pulmonary disease; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; PR, pulmonary rehabilitation.
At baseline, no differences were observed between the included patients and drop-outs (p>0.05). Patients’ characteristics are summarized in Table 2.
Table 2.
Sample Characterization (n=63)
| Characteristics | Patients Included (n=49) | Drop-Outs (n=14) | p-value |
|---|---|---|---|
| Age, years | 69.8±7.4 | 64.4±13.1 | 0.154 |
| Gender, male n (%) | 40 (81.6) | 9 (64.3) | 0.169 |
| BMI, kg/m2 | 26.4±4.9 | 27.7±5.4 | 0.410 |
| Smoking status, n (%) | 0.554 | ||
| Current | 8 (16.3) | 4 (28.6) | |
| Former | 31 (63.3) | 7 (50.0) | |
| Never | 10 (20.4) | 3 (21.4) | |
| Packs/year | 40.0 [26.0–70.0] | 35.0 [15.8–75.4] | 0.573 |
| Exacerbations/yeara | 1.0 [0.0–1.0] | 1.0 [0.0–3.0] | 0.142 |
| AECOPD hospitalisations,a n (%) | 4 (8.2) | 2 (14.3) | 0.292 |
| Duration of hospitalizations (days) | 9.3±4.0 | 10.5±9.5 | 0.923 |
| COPD-related emergencies,a n (%) | 16 (32.7) | 5 (35.7) | 0.463 |
| Lung function (post-bronchodilator) | |||
| FEV1, | 1.3±0.5 | 1.3±0.5 | 0.769 |
| FEV1, %predicted | 50.4±19.4 | 53.6±20.2 | 0.589 |
| FEV1/FVC, % | 49.9±13.5 | 54.6±11.8 | 0.247 |
| GOLD stages, n (%) | 0.996 | ||
| I | 6 (12.2) | 2 (14.3) | |
| II | 17 (34.7) | 5 (35.7) | |
| III | 22 (44.9) | 6 (42.9) | |
| IV | 4 (8.2) | 1 (7.1) | |
| GOLD groups, n (%) | 0.280 | ||
| A | 8 (16.3) | 3 (21.4) | |
| B | 32 (65.3) | 6 (42.9) | |
| C | 0 (0.0) | 0 (0.0) | |
| D | 9 (18.4) | 5 (35.7) | |
| CCI, n (%) | 0.781 | ||
| Mild | 5 (10.2) | 2 (14.3) | |
| Moderate | 26 (53.1) | 6 (42.9) | |
| Severe | 18 (36.7) | 6 (42.9) | |
| Medication, n (%) | |||
| Bronchodilators | |||
| SABA | 6 (12.2) | 1 (7.1) | 0.451 |
| SAMA | 3 (6.1) | 0 (0.0) | 0.292 |
| LABA | 7 (14.3) | 3 (21.4) | 0.745 |
| LAMA | 18 (36.7) | 7 (50.0) | 0.747 |
| LAMA/LABA combination | 14 (28.6) | 3 (21.4) | 0.568 |
| ICS | 10 (20.4) | 1 (7.1) | 0.206 |
| ICS/LABA combination | 20 (40.8) | 7 (50.0) | 0.580 |
| LTRA | 3 (6.1) | 2 (14.3) | 0.331 |
| Xanthines | 9 (18.4) | 3 (21.4) | 0.823 |
| Expectorants | 6 (12.2) | 1 (7.1) | 0.577 |
| Antibiotics | 1 (2.0) | 0 (0.0) | 0.540 |
| mMRC | 2 [1.0–3.0] | 2 [1.0–3.0] | 0.791 |
| CAT | 17.2±7.8 | 14.6±7.6 | 0.287 |
| SGRQ (Total score) | 45.6±20.4 | 40.0±20.3 | 0.341 |
| LCQ | 16.6±3.5 | 18.6±3.2 | 0.063 |
| CASA-Q | |||
| Cough symptoms | 66.7 [41.7; 83.3] | 87.5 [66.7; 91.7] | 0.068 |
| Cough impact | 71.9 [56.3; 93.8] | 89.1 [71.9; 100.0] | 0.132 |
| Sputum symptoms | 66.7 [50.0; 83.3] | 75.0 [58.3; 91.7] | 0.437 |
| Sputum impact | 79.2 [62.5; 95.8] | 87.5 [79.2; 100.0] | 0.117 |
Notes: Values are presented as mean±standard deviation or median [interquartile range], unless otherwise stated. aPast-year.
Abbreviations: PR, pulmonary rehabilitation; BMI, body mass index; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; CCI, Charlson comorbidity index; SABA, short-acting beta-agonists; SAMA, short-acting muscarinic antagonist; LABA, long-acting beta-agonists; LAMA, long-acting muscarinic antagonist; ICS, inhaled corticosteroid; LRTA, leukotriene receptor antagonist; mMRC, modified medical research council questionnaire; CAT, COPD assessment test; SGRQ, St George’s Respiratory Questionnaire; LCQ, Leicester cough questionnaire; CASA-Q, Cough and Sputum Assessment Questionnaire.
At baseline, all PROMs were completed by the forty-nine participants, except for the CASA-Q. Data for CASA-Q was not possible to collect from eight participants, due to data collection commencement prior to obtaining the authorization to use the scale from the author. Additionally, one participant failed to complete the LCQ at the follow-up appointment. As missing data from CASA-Q and LCQ were completely unrelated to questionnaires scores, disease and symptoms severity, or patients’ adherence to PR (no statistical significant differences were present between the 8 patients with missing data on CASA-Q and the remaining 41 patients for GOLD stages/groups, adherence to PR, SGRQ and CAT scores), they were considered missing-completely-at-random.49,50 Thus, we chose to use the listwise deletion method to handle missing data, since this is the most frequently used method and is known for producing unbiased results.49,50 After the PR programme, significant improvements were found for CAT, SGRQ, LCQ and CASA-Q cough impact dimension. Baseline and post-PR scores can be found in Table S.1. Thirty-seven (75.5%) patients improved beyond the MCID of 2 points established for the CAT and 31 (63.3%) above the 4 points in the SGRQ. Only cough and sputum impact dimensions of CASA-Q demonstrated a ceiling effect, at T0 and T1. After the PR programs, 56.2% and 60.4% of the participants perceived improvements (GRC) in their cough (2.0, [0.0–3.0]) and sputum (2.0, [0.0–4.0]), respectively. Physiotherapists reported improvements in cough for 51% (2.0, [0.0–3.0]), and in sputum for 55.1% (2.0, [0.0–2.0]) of their patients.
Minimal Clinically Important Differences
After checking for outliers, three participants were excluded from the LCQ analysis. No differences were found between the baseline characteristics of the included patients and the outliers (p>0.05). No outliers were found in the CASA-Q analysis.
Resume tables of the correlation values between changes in the PROM and changes in the anchors (Table S.2) and the MCID achieved with the mean change method (Table S.3) can be found in Supplementary materials. It was not possible to use the criterion referencing method to compute the MCIDs since no significant differences were observed between patients who experienced an exacerbation and those who did not (Table S.4). In our sample, only mild to moderate exacerbations occurred.
Leicester Cough Questionnaire
Changes in the LCQ correlated significantly with changes in patients’ GRC for cough symptoms (r=0.340). No other correlations were found (Table S.2).
The MCID established for the LCQ using the mean change according to patients’ GRC was 1.4 (Table 3). It was not possible to use ROC statistics to compute the MCID, since the AUC generated was not significant. Using linear regression, the estimated MCID for the LCQ was 0.7 (Figure S.1).
Table 3.
Anchor and Distribution-Based Methods Used to Compute the Minimal Clinically Important Difference of Patient-Reported Outcome Measures Assessing Cough and Sputum
| LCQ | CASA-Q | ||||
|---|---|---|---|---|---|
| Cough Symptoms | Cough Impact | Sputum Symptoms | Sputum Impact | ||
| Mean change | |||||
| SGRQ | – | 9.3 (2.3 to 16.4) | – | 7.7 (−1.5 to 16.8) | 6.0 (−0.7 to 12.7) |
| CAT | – | 9.1 (3.9 to 14.4) | – | – | – |
| Patient’s GRC | 1.4 (0.7 to 2.2) | 9.9 (2.6 to 17.3) | 11.8 (3.7 to 19.8) | – | – |
| ROC | |||||
| SGRQ | – | 4.2 SN=68%; SP=75% |
– | 4.2 SN=80%; SP=60% |
– |
| CAT | – | 4.2 SN=61%; SP=80% |
– | – | – |
| Patient’s GRC | – | – | 4.7 SN=67%; SP=75% |
– | – |
| Linear regression | |||||
| SGRQ | – | 1.6 (−3.4 to 6.6) | – | – | 2.2 (−1.5 to 6.0) |
| CAT | – | – | – | – | – |
| Patient’s GRC | 0.7 (−0.9 to 2.4) | – | – | – | – |
| Distribution methods | |||||
| 0.5*SD | 1.7 | 11.5 | 11.2 | 11.4 | 10.3 |
| SEM | 1.0 | 11.0 | 7.8 | 10.2 | 8.7 |
| 1.96SEM | 1.9 | 21.6 | 15.2 | 20.0 | 17.1 |
| MDC | 2.6 | 30.5 | 21.5 | 28.2 | 24.2 |
| ES | 0.21 | 0.23 | 0.19 | 0.09 | 0.12 |
| Pooled MCID | 1.3 | 10.6 | 10.1 | 9.5 | 7.8 |
| % of change | 6.8 | 10.6 | 10.1 | 9.7 | 7.8 |
| MCID ES | 0.50 | 0.45 | 0.45 | 0.40 | 0.35 |
Notes: Values are presented as mean and 95% confidence intervals. % of change was computed within each scale range. The MCID ES are computed as the MCID value divided by the pooled SD.
Abbreviations: LCQ, Leicester cough questionnaire; CASA-Q, Cough and Sputum Assessment Questionnaire; GRC, global rating of change; CAT, COPD assessment test; SGRQ, St George’s Respiratory Questionnaire; ROC, receiver operating characteristic curves; SD, standard deviation; SEM, standard error measurement; MDC, minimal detectable change; ES, effect size; MCID, minimal clinically important difference; SN, sensitivity; SP, specificity.
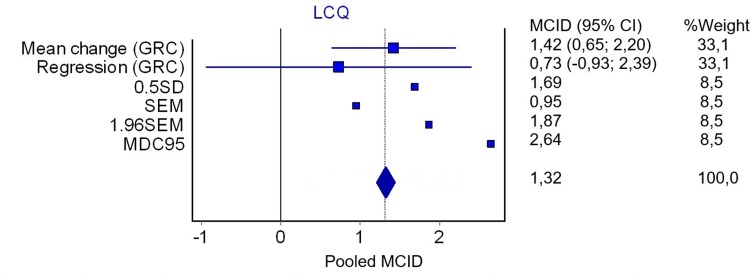
The distribution-based methods for the LCQ, and the overall MCID pooled statistics are presented in Table 3. The pooled MCID for the LCQ was 1.3 (Figure 2).
Figure 2.
Plot of the pooled MCID for the Leicester cough questionnaire. The plot represents the MCID estimates derived in this study, and where appropriate the estimates include the 95% confidence interval (n=45).
Abbreviations: LCQ, Leicester cough questionnaire; GRC, Global rating of change; SD, standard deviation; SEM, standard error measurement; MDC, minimal detectable change.
Cough and Sputum Assessment Questionnaire
Changes in CASA-Q cough symptoms domain correlated significantly with changes in SGRQ (s=−0.322), in CAT (r=−0.378) and with patients’ GRC for cough (s=0.317). Changes in CASA-Q cough impact domain correlated significantly with patients’ GRC for cough (s=0.464). Changes in CASA-Q sputum domains, both symptoms and impact, correlated significantly with changes in SGRQ (s=−0.398 and r=−0.407, respectively). No other correlations were found (Table S.2).
The MCID derived from the mean change methods were: i) 9.3, 9.1 and 9.9 for cough symptoms with SGRQ, CAT and patients’ GRC, respectively; ii) 11.8 for cough impact; iii) 7.7 for sputum symptoms, and iv) 6 for sputum impact (Table 3).
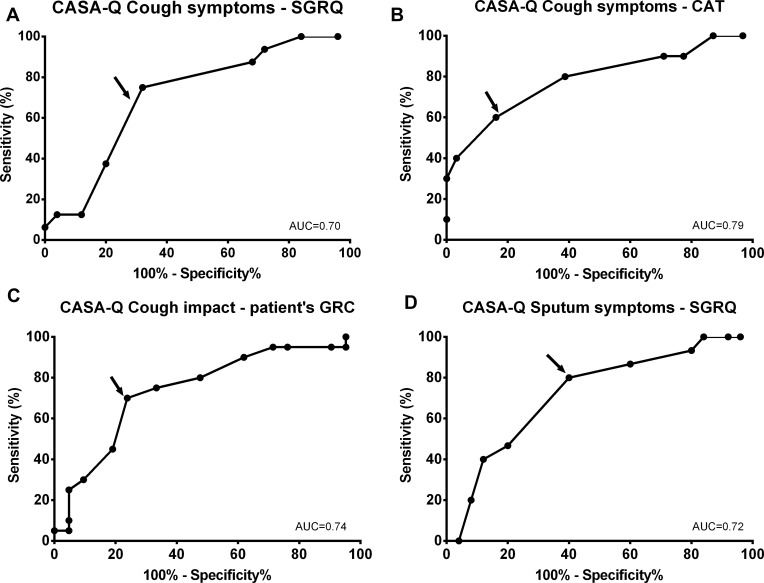
Using ROC statistics, the AUCs generated for CASA-Q cough symptoms domain showed adequate discrimination between those improving above and below the MCID for SGRQ (AUC=0.70; 95% CI 0.54 to 0.86; p=0.031) and for CAT (AUC=0.79; 95% CI 0.62 to 0.97; p=0.005) (Figure 3). The AUCs obtained for the CASA-Q cough impact (patient’s GRC: AUC=0.74; 95% CI 0.59 to 0.90; p=0.008) and sputum symptoms (SGRQ: AUC=0.72; 95% CI 0.56 to 0.88; p=0.019) were also able to distinguish between patients who improved from those who did not (Figure 3). The AUCs’ discrimination ability was not acceptable for CASA-Q sputum impact using SGRQ and for CASA-Q cough symptoms using patients’ GRC for cough as anchors (ie, AUC<0.7). According to the ROC analysis, the MCID found were 4.2 for both cough and sputum symptoms and 4.7 for cough impact.
Figure 3.
Receiver operating characteristic curves to discriminate between patients with COPD above and below the MCID established for the anchors for the CASA-Q domains (n=41) using the: (A) SGRQ for cough symptoms domain; (B) CAT for cough symptoms domain; (C) patients' global rating of change for cough impact; and (D) SGRQ for sputum symptoms.
Abbreviations: CASA-Q, cough and sputum assessment questionnaire; SGRQ, St. George Respiratory Questionnaire; CAT, COPD assessment test; GRC, Global rating of change; AUC, area under the curve.
Using linear regression, the estimated MCID for the cough symptoms domain was 1.6 and for sputum impact domain was 2.2 (Figure S.2).
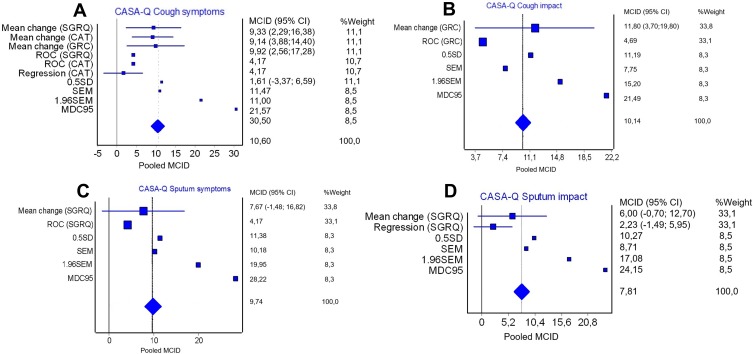
The distribution-based methods for the CASA-Q and the overall MCID pooled statistics are presented in Table 3. Pooled MCID for the CASA-Q subscales were 10.6 for cough symptoms; 10.1 for cough impact; 9.5 for sputum symptoms and 7.8 for sputum impact (Figure 4).
Figure 4.
Plot of the pooled MCID for the cough and sputum assessment questionnaire (CASA-Q): (A) CASA-Q cough symptoms; (B) CASA-Q cough impact; (C) CASA-Q sputum symptoms and (D) CASA-Q sputum impact. The plots represent the MCID estimates derived in this study.
Abbreviations: CASA-Q, cough and sputum assessment questionnaire; SGRQ, St. George Respiratory Questionnaire; CAT, COPD assessment test; GRC, Global rating of change; ROC, receiver operating characteristic; SD, standard deviation; SEM, standard error measurement; MDC, minimal detectable change; MCID, minimal clinically important difference.
Discussion
This study estimated a pooled MCID of 1.3 for the LCQ. The pooled MCIDs established for CASA-Q domains were: 10.6 for cough symptoms; 10.1 for cough impact; 9.5 for sputum symptoms and 7.8 for sputum impact.
The pooled MCID found for LCQ matched previous estimates for patients with chronic cough, ie, 1.3 points.51 Nevertheless, higher MCIDs (from 2 to 3 points) have been suggested,52–54 using a GRC with a period recall of 6 months,52 increasing the recall risk of bias,19,46 and including patients with acute cough only.53,54 Higher levels of baseline severity (e.g., acute cough) usually lead to greater improvements,19,20,47 which result in larger MCIDs. Moreover, studies have involved pharmacological interventions only, whilst our study reports on PR. Since PR demands more from patients, expectations of benefits and improvements are often higher, producing larger effect sizes when compared to medication, thus, generating larger MCIDs.55
The pooled MCIDs for each CASA-Q domain were similar. Although CASA-Q emerged as a good tool to discriminate between patients above and below the anchor’s MCIDs, the ceiling effect observed was notorious; thus, its MCIDs should be interpreted with caution. Scores close to the end of the scale, limit the amount of potential change, affect responsiveness and consequently the establishment of the MCIDs.19 Presence of chronic cough was not an inclusion criterion of our study. This may explain the observed ceilings effects and why impact of cough and sputum in the HRQoL of our participants was comparable to other studies enrolling patients with COPD,31,56 but different (our sample scored better) from studies considering patients with chronic cough only.28,57,58
Although previous research has showed a relationship between CAT and cough and sputum,59,60 in our study an association was verified only with cough. However, association was explored with mean changes, while previous studies have focused on absolute scores.59,60 Nevertheless, correlations between changes in SGRQ and changes in CASA-Q cough and sputum dimensions were found (Table S.2). These findings further establish the impact of cough and sputum on HRQoL of patients with COPD, as previously demonstrated,7,59–64 and emphasise the urge for assessing and implementing tailored interventions to manage these symptoms.
It was not possible to use the physiotherapists’ GRC, probably due to the well-known lack of agreement on symptoms perception between patients with COPD and health professionals.65 Moreover, the non-significant differences in baseline symptoms between patients who experienced an AECOPD and those who did not, hindered the use of this variable in the anchor-based approach. In our sample, only mild to moderate exacerbations3 occurred and during PR, patients were closely monitored; therefore, exacerbations were promptly identified and tackled, thus enhancing a faster recovery.66 Exacerbations were managed as follows: patients were referred to their clinician, who adjusted their pharmacological therapy, and were instructed to follow the symptoms management strategies taught during educational sessions of PR (energy conservation techniques, postures to relieve dyspnoea and active cycle of breathing techniques).34 As soon as the contagious risk was controlled and patients felt capable they were encouraged to return to PR (around 7 to 15 days).67 When patients re-integrated the PR programme the training load was readjusted to their physical condition.
This study has some limitations that need to be acknowledged. First, the presence of ceiling effects in the CASA-Q might have biased the results. Secondly, our sample was mainly composed of patients with moderate to severe COPD. Therefore, the established MCIDs might not be generalizable to all patients and should be interpreted with caution in patients with mild or very severe COPD. Since MCIDs are influenced by disease severity,19,20,47 we recommend interpreting PROMs changes within the MCIDs ranges provided by the different methods and not limiting it to the absolute proposed value. Finally, this study is integrated in a larger trial,34 therefore, a specific sample size calculation to establish MCIDs was not performed, which might have underpowered this study for its goal. Nevertheless, similar samples sizes have been used to establish MCIDs in other studies,40,68,69 and the fact that all MCIDs fell within the recommended range of 6 to 10% change in the scale range, which corresponded to a desirable effect size of 0.2 to 0.5,19,38,48 strengthens the validity of our estimates. Moreover, to our best knowledge, this is the first study to provide MCIDs estimations for LCQ and CASA-Q in patients with COPD; thus, we believe that our MCIDs estimates can be useful for health professional and policy makers, ensuring they are used with caution and in accordance with each clinical context. To confirm our MCIDs estimates, future studies in this area with larger sample sizes are still required.
An important strength of our study is that MCIDs were computed through a combination of methodologies, including a wide range of anchor and distribution-based approaches. In addition, the pooled method selected allowed to attribute a higher weight to anchor than distribution-based methods, following the recommendations for establishing MCIDs.19,38 Standardization of community-based PR programs in terms of structure, intensity, frequency, duration and progression, as recommended,13 minimised the heterogeneity of intervention, assuring that the MCIDs proposed are valid and suitable for PR.
Conclusion
In summary, this study suggests that improvements of 1.3 in the LCQ, 10.6 in the cough symptoms, 10.1 in cough impact, 9.5 in sputum symptoms and 7.8 points in the sputum impact dimensions of CASA-Q are clinically relevant for patients with COPD, following a PR program. These estimates have the potential to be used to interpret clinical relevance, as thresholds for the intervention effectiveness and to inform future studies regarding sample calculation.
Data Sharing Statement
Data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Author Contributions
PR and CP were responsible for data acquisition. PR also performed formal data analysis and drafted the manuscript. AM and AO were responsible for the conception and design of the study. CV and LA provided a substantial contribution for data acquisition. AM was responsible for obtaining the funding and ensured project administration and resources. All authors critically revised the manuscript, ensured accuracy and integrity of the work, approved the final version to be published, and agree to be accountable for all aspects of the work.
Funding
This work was funded by Fundo Europeu de Desenvolvimento Regional (FEDER) - Comissão Diretiva do Programa Operacional Regional do Centro, by Fundação para a Ciência e Tecnologia - FCT (SAICT-POL/23926/2016, PTDC/DTP-PIC/2284/2014, PTDC/SAU-SER/28806/2017, UIDB/04501/2020) and by Programa Operacional Competitividade e Internacionalização (COMPETE), through COMPETE 2020 (POCI-01-0145-FEDER-016701, POCI-01-0145-FEDER-007628, POCI-01-0145-FEDER-028806).
Disclosure
The authors report no financial, or non-financial, conflicts of interest in this work.
References
- 1.Forum of International Respiratory Societies. The Global Impact of Respiratory Disease. 2nd ed. Sheffield: European Respiratory Society; 2017. [Google Scholar]
- 2.López‐Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23. doi: 10.1111/resp.12660 [DOI] [PubMed] [Google Scholar]
- 3.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2019 Report). 2019.
- 4.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD – a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi: 10.1183/09031936.00051110 [DOI] [PubMed] [Google Scholar]
- 5.Miravitlles M, Worth H, Cataluña JJS, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15(1):122. doi: 10.1186/s12931-014-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crooks MG, Brown T, Morice AH. Is cough important in acute exacerbations of COPD? Respir Physiol Neurobiol. 2018;257:30–35. doi: 10.1016/j.resp.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res. 2017;18(1):67. doi: 10.1186/s12931-017-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Satia I, Badri H, Lahousse L, Usmani OS, Spanevello A. Airways diseases: asthma, COPD and chronic cough highlights from the European Respiratory Society Annual Congress 2018. J Thorac Dis. 2018;10(Suppl 25):S2992. doi: 10.21037/jtd.2018.08.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crooks MG, Hayman Y, Innes A, Williamson J, Wright CE, Morice AH. Objective measurement of cough frequency during COPD exacerbation convalescence. Lung. 2016;194(1):117–120. doi: 10.1007/s00408-015-9782-y [DOI] [PubMed] [Google Scholar]
- 10.Smith J, Woodcock A. Cough and its importance in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(3):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Buul AR, Kasteleyn MJ, Chavannes NH, Taube C. Morning symptoms in COPD: a treatable yet often overlooked factor. Expert Rev Respir Med. 2017;11(4):311–322. doi: 10.1080/17476348.2017.1305894 [DOI] [PubMed] [Google Scholar]
- 12.Calverley PM. Cough in chronic obstructive pulmonary disease: is it important and what are the effects of treatment? Cough. 2013;9(1):17. doi: 10.1186/1745-9974-9-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634ST [DOI] [PubMed] [Google Scholar]
- 14.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;23(2):CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston BC, Ebrahim S, Carrasco-Labra A, et al. Minimally important difference estimates and methods: a protocol. BMJ Open. 2015;5(10):e007953. doi: 10.1136/bmjopen-2015-007953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371 [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6 [DOI] [PubMed] [Google Scholar]
- 18.Jones P. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi: 10.1183/09031936.02.00063702 [DOI] [PubMed] [Google Scholar]
- 19.Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. J Clin Epidemiol. 2017;82:128–136. doi: 10.1016/j.jclinepi.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 20.Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID). J Man Manip Ther. 2012;20(3):160–166. doi: 10.1179/2042618612Y.0000000001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alma H, de Jong C, Tsiligianni I, Sanderman R, Kocks J, van der Molen T. Clinically relevant differences in COPD health status: systematic review and triangulation. Eur Respir J. 2018;52(3):1800412. doi: 10.1183/13993003.00412-2018 [DOI] [PubMed] [Google Scholar]
- 22.Birring S, Prudon B, Carr A, Singh S, Morgan M, Pavord I. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–343. doi: 10.1136/thorax.58.4.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crawford B, Monz B, Hohlfeld J, et al. Development and validation of a cough and sputum assessment questionnaire. Respir Med. 2008;102(11):1545–1555. doi: 10.1016/j.rmed.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 25.Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 27.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. Am Rev Respir Dis. 1992;145(6):1321–1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 28.Berkhof FF, Boom LN, Ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. The validity and precision of the Leicester Cough Questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes. 2012;10(1):4. doi: 10.1186/1477-7525-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faruqi S, Thompson R, Wright C, Sheedy W, Morice AH. Quantifying chronic cough: objective versus subjective measurements. Respirology. 2011;16(2):314–320. doi: 10.1111/res.2011.16.issue-2 [DOI] [PubMed] [Google Scholar]
- 30.Kelsall A, Decalmer S, Webster D, et al. How to quantify coughing: correlations with quality of life in chronic cough. Eur Respir J. 2008;32(1):175–179. doi: 10.1183/09031936.00101307 [DOI] [PubMed] [Google Scholar]
- 31.Deslee G, Burgel P-R, Escamilla R, et al. Impact of current cough on health-related quality of life in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2091. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monz BU, Sachs P, McDonald J, Crawford B, Nivens MC, Tetzlaff K. Responsiveness of the cough and sputum assessment questionnaire in exacerbations of COPD and chronic bronchitis. Respir Med. 2010;104(4):534–541. doi: 10.1016/j.rmed.2009.10.026 [DOI] [PubMed] [Google Scholar]
- 33.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–170. doi: 10.1179/jmt.2009.17.3.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marques A, Jácome C, Rebelo P, et al. Improving access to community-based pulmonary rehabilitation: 3R protocol for real-world settings with cost-benefit analysis. BMC Public Health. 2019;19:676. doi: 10.1186/s12889-019-7045-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alison JA, McKeough ZJ, Johnston K, et al. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology. 2017;22(4):800–819. doi: 10.1111/resp.2017.22.issue-4 [DOI] [PubMed] [Google Scholar]
- 36.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res. 1995;4(4):293–307. doi: 10.1007/BF01593882 [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal R, Ranganathan P. Common pitfalls in statistical analysis: the use of correlation techniques. Perspect Clin Res. 2016;7(4):187. doi: 10.4103/2229-3485.192046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 39.Doi SA, Thalib L. A quality-effects model for meta-analysis. Epidemiology. 2008;19(1):94–100. doi: 10.1097/EDE.0b013e31815c24e7 [DOI] [PubMed] [Google Scholar]
- 40.Oliveira A, Machado A, Marques A. Minimal important and detectable differences of respiratory measures in outpatients with AECOPD. COPD. 2018;1–10. [DOI] [PubMed] [Google Scholar]
- 41.Jones PW. St. George’s respiratory questionnaire: MCID. COPD. 2005;2(1):75–79. doi: 10.1081/COPD-200050513 [DOI] [PubMed] [Google Scholar]
- 42.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med. 2014;2(3):195–203. doi: 10.1016/S2213-2600(14)70001-3 [DOI] [PubMed] [Google Scholar]
- 43.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 44.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159(6):1638–1645. doi: 10.1016/j.surg.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 45.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–1316. doi: 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- 46.Alma H, De Jong C, Jelusic D, et al. Health status instruments for patients with COPD in pulmonary rehabilitation: defining a minimal clinically important difference. NPJ Prim Care Respir Med. 2016;26:16041. doi: 10.1038/npjpcrm.2016.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copay AG, Subach BR, Glassman SD, Polly DW, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008 [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. A power primer. Psychol Bull. 1992;112(1):155. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 49.Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64(5):402. doi: 10.4097/kjae.2013.64.5.402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells BJ, Chagin KM, Nowacki AS, Kattan MW. Strategies for handling missing data in electronic health record derived data. EGEMS. 2013;1:3. doi: 10.13063/2327-9214.1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raj A, Pavord D, Birring S. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? Pharmacol Ther Cough. 2009;311–320. [DOI] [PubMed] [Google Scholar]
- 52.Brokkaar L, Uil SM, Van Den Berg JWK. Minimal Clinically Important Difference (MCID) of the Dutch version of the Leicester Cough Questionnaire and baseline predictors of reaching the MCID after six months. Chest. 2007;132(4):468B. doi: 10.1378/chest.132.4_MeetingAbstracts.468b [DOI] [Google Scholar]
- 53.Lee KK, Matos S, Evans DH, White P, Pavord ID, Birring SS. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 2013;187(9):991–997. doi: 10.1164/rccm.201209-1686OC [DOI] [PubMed] [Google Scholar]
- 54.Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough. 2011;7(1):4. doi: 10.1186/1745-9974-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Houchen-Wolloff L, Evans RA. Unravelling the mystery of the ‘minimum important difference’ using practical outcome measures in chronic respiratory disease. Chron Respir Dis. 2019;16:1479973118816491. doi: 10.1177/1479973118816491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arikan H, Savci S, Calik-Kutukcu E, et al. The relationship between cough-specific quality of life and abdominal muscle endurance, fatigue, and depression in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polley L, Yaman N, Heaney L, et al. Impact of cough across different chronic respiratory diseases: comparison of two cough-specific health-related quality of life questionnaires. Chest. 2008;134(2):295–302. doi: 10.1378/chest.07-0141 [DOI] [PubMed] [Google Scholar]
- 58.Reychler G, Schinckus M, Fremault A, Liistro G, Pieters T. Validation of the French version of the Leicester Cough Questionnaire in chronic obstructive pulmonary disease. Chron Respir Dis. 2015;12(4):313–319. doi: 10.1177/1479972315602618 [DOI] [PubMed] [Google Scholar]
- 59.Koo H-K, Park S-W, Park J-W, et al. Chronic cough as a novel phenotype of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2018;13:1793. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones PW, Brusselle G, Dal Negro RW, et al. Patient-centred assessment of COPD in primary care: experience from a cross-sectional study of health-related quality of life in Europe. Prim Care Respir J. 2012;21(3):329. doi: 10.4104/pcrj.2012.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee H, Jhun BW, Cho J, et al. Different impacts of respiratory symptoms and comorbidities on COPD-specific health-related quality of life by COPD severity. Int J Chron Obstruct Pulmon Dis. 2017;12:3301. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monteagudo M, Rodríguez-Blanco T, Llagostera M, et al. Factors associated with changes in quality of life of COPD patients: a prospective study in primary care. Respir Med. 2013;107(10):1589–1597. doi: 10.1016/j.rmed.2013.05.009 [DOI] [PubMed] [Google Scholar]
- 63.Miravitlles M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir Med. 2011;105(8):1118–1128. doi: 10.1016/j.rmed.2011.02.003 [DOI] [PubMed] [Google Scholar]
- 64.Stephenson JJ, Cai Q, Mocarski M, Tan H, Doshi JA, Sullivan SD. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:577. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miravitlles M, Ferrer J, Baró E, Lleonart M, Galera J. Differences between physician and patient in the perception of symptoms and their severity in COPD. Respir Med. 2013;107(12):1977–1985. doi: 10.1016/j.rmed.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 66.Oliveira A, Munhá J, Bugalho A, Guimarães M, Reis G, Marques A. Identification and assessment of COPD exacerbations. Pulmonology. 2018;24(1):42–47. doi: 10.1016/j.rppnen.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 67.Oliveira A, Afreixo V, Marques A. Enhancing our understanding of the time course of acute exacerbations of COPD managed on an outpatient basis. Int J Chron Obstruct Pulmon Dis. 2018;13:3759. doi: 10.2147/COPD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beauchamp MK, Harrison SL, Goldstein RS, Brooks D. Interpretability of change scores in measures of balance in people with COPD. Chest. 2016;149(3):696–703. doi: 10.1378/chest.15-0717 [DOI] [PubMed] [Google Scholar]
- 69.Hanson LC, Taylor NF, McBurney H. Interpreting meaningful change in the distance walked in the 10-metre ISWT in cardiac rehabilitation. Heart Lung Circ. 2018. [DOI] [PubMed] [Google Scholar]