Abstract
Background:
The association of white matter hyperintensities (WMH) with age-related vascular and neurodegenerative pathologies remains incompletely understood.
Objective:
The objective of this work was to elucidate the neuropathologic correlates of WMH in a large community-based cohort of older adults.
Methods:
Cerebral hemispheres from 603 community-based older adults were imaged with MRI ex-vivo. All participants underwent annual clinical evaluation, cognitive assessment, and neuropathologic examination. WMH burden was assessed using a modified Fazekas rating scale. Multiple ordinal logistic regression was used to test the association of WMH burden with an array of age-related neuropathologies, adjusting for demographics. Mixed effects models of cognition controlling for neuropathologies and demographics were used to determine whether WMH burden contributes to cognitive decline beyond measured pathologies.
Results:
WMH burden in the whole group was associated with both vascular and Alzheimer’s pathologies: arteriolosclerosis (p<10−4), gross (p<10−4) and microscopic infarcts (p=0.04), and amyloid-β plaques (p=0.028). In non-demented participants (mild or no cognitive impairment) (N=332), WMH burden was related to gross infarcts (p=10−4) and arteriolosclerosis (p<10−4), but not to Alzheimer’s pathology. Similarly, in those with no cognitive impairment (N=178), WMH burden was related to gross infarcts (p=8×10−4) and arteriolosclerosis (p=0.014). WMH burden was associated with faster decline in perceptual speed in both the whole (p=0.038) and non-demented (p=0.006) groups.
Conclusion:
WMH burden has independent associations with vascular pathologies in older adults regardless of clinical status, and with Alzheimer’s pathology later in the progression of Alzheimer’s disease. Moreover, WMH burden may reflect additional tissue injury not captured with traditional neuropathologic indices.
Keywords: white matter hyperintensities, WMH, MRI, pathology, cognition
INTRODUCTION
White matter lesions appearing hyperintense in T2-weighted magnetic resonance imaging (MRI), typically referred to as white matter hyperintensities (WMH), are common in older adults [1]. WMH burden increases with age [2,3], has been associated with cardiovascular risk factors [4–12] and linked to cerebral small vessel disease [13]. WMH have been associated with worse cognitive and motor function, and higher risk of cognitive decline, mild cognitive impairment and Alzheimer’s dementia [14–22].
Although WMH have been studied extensively in the last three decades, the association of WMH burden with age-related neuropathologies remains inconclusive due to mixed findings from the few MRI-pathology studies conducted to date. A longitudinal study on 66 community-dwelling older adults showed an association of WMH accumulation over time with arteriolosclerosis, myelin pallor, and neurofibrillary tangles [23]. That study used longitudinal in-vivo MRI, with the last MRI occurring within 3 years of death, and tested the association of WMH accumulation with multiple pathologies in a single model. A study on 93 older adults with Alzheimer’s pathology and/or cerebrovascular disease, or none of the above, showed an association of WMH volume with infarcts and demyelination [24]. MRI was conducted 0.2-8.7 years prior to death, and multiple pathologies were considered in the same model of WMH volume. An investigation on 132 community-dwelling older adults showed an association of WMH with neurofibrillary tangles, neuritic plaques, and infarcts [25]. MRI was performed ex-vivo, and models of WMH considered each pathology separately. A study on 57 older adults, including cognitively normal controls, persons with advanced risk for cerebrovascular disease, and patients with Alzheimer’s dementia, showed a positive correlation of WMH volume with arteriolosclerosis, micro-infarcts, and cerebral hemorrhages [26]. The same study showed a negative correlation of WMH with neurofibrillary tangles. MRI was conducted on average 6 years prior to death, and models of WMH considered each pathology separately. A study on 50 community-dwelling older adults showed an association of WMH burden with Braak [27] and CERAD [28] scores [29]. Participants were imaged with MRI either in-vivo (9-25 months prior to death) or ex-vivo, or both, and WMH burden was modelled as a function of multiple pathologies. An investigation on 43 community-dwelling older adults showed an association of WMH burden with vascular integrity [30]. MRI was conducted 4-87 months prior to death in 23 persons and ex-vivo in 20 persons, and WMH burden was modelled as a function of multiple pathologies. A recent study on 60 older adults with and 22 without Alzheimer’s pathology showed associations of WMH volume with Alzheimer’s pathology, cerebral amyloid angiopathy (CAA), and infarcts [31]. MRI data were collected on average 3 years prior to death, and models of WMH volume considered each pathology separately. When CAA was controlled for in the model of Alzheimer’s pathology, statistical significance was lost. Although there are some similar findings in the above studies, there are also multiple discrepancies.
To address this long-standing debate on the neuropathologic correlates of WMH burden, it is important to first understand the causes of the discrepant findings across previous MRI-pathology investigations. First, the majority of studies were conducted on relatively small samples [24–26,29–32], often not in community cohorts [24,26,31], limiting statistical power and generalizability of findings. Second, models of WMH considered one pathology at a time [25,26,31] or only few pathologies, which is problematic due to the fact that mixed pathologies are common in the brain of older adults and the effects of comorbid pathologies may have not been controlled for [33]. Furthermore, the list of pathologies considered was not consistent across studies. Investigations based on in-vivo MRI often suffered by long intervals between in-vivo MRI and death [24,26,30,31], allowing additional pathology to develop after MRI data collection, thereby weakening the observed link between WMH and pathology. Finally, important differences may exist in the characteristics of persons included in previous MRI-pathology investigations. For example, some studies included community-dwelling individuals while others included clinical patients. Also, individuals imaged with MRI in-vivo [24,26,29–32] are typically less frail than those imaged ex-vivo [25,29] who are imaged independent of frailty level. All of the above may have contributed to the discrepant findings across MRI-pathology studies on the age-related neuropathologies associated with WMH burden.
The purpose of this study was to investigate the neuropathologic correlates of WMH burden by combining ex-vivo MRI and pathology (from autopsy) in a large community-based cohort of older adults. MRI was conducted ex-vivo to ensure that it captures brain characteristics at the same brain condition as pathologic examination and to allow imaging independent of frailty level. A comprehensive array of neuropathologies was considered in the same model of WMH burden, including gross and microscopic infarcts, atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy, amyloid-β plaques, neurofibrillary tangles, Lewy bodies, hippocampal sclerosis, and TDP-43 pathology. Detailed longitudinal cognitive assessments were also conducted, and the independent association of WMH burden with cognitive decline was tested after accounting for neuropathologies and demographics. Finally, WMH burden assessed ex-vivo was compared to that in-vivo in a segment of the cohort, and the association of an increase in WMH burden from in-vivo to ex-vivo with the time interval between in-vivo MRI and death was tested.
MATERIALS AND METHODS
Study population
Older adults participating in three longitudinal clinical-pathologic cohort studies of aging, the Rush University Memory and Aging Project (MAP), the Religious Orders Study (ROS) [34], and the Minority Aging Research Study (MARS) [35], were included in this work. All three studies were approved by the institutional review board of Rush University Medical Center, and participants provided written informed consent and signed an anatomical gift act. All participants underwent annual uniform structured clinical evaluation including cognitive function testing, medical history, and neurologic examination. At the time of these analyses 4079 MAP/ROS/MARS participants had completed the baseline clinical evaluation. Of these, 586 died and 99 withdrew from the study before the ex-vivo MRI sub-study began. Of the remaining 3394 persons, 992 died and 783 were autopsied. The first 620 consecutive participants with ex-vivo MRI and pathology data were considered in this work. Participants with structural brain abnormalities not typical of aging (e.g. tumors) or with ex-vivo MRI data that did not pass quality tests were excluded (N=17). Analyses were based on 603 eligible participants (Table 1).
Table 1.
Demographic and clinical characteristics by cognitive status proximate to death.
| Characteristics | NCIa | MCIb | Dementia | Combined |
|---|---|---|---|---|
| N | 178 | 154 | 271 | 603 |
| Age at death, years (SD) | 88 (7) | 90 (6) | 90 (7) | 90 (7) |
| Male, n (%) | 59 (33) | 46 (30) | 82 (30) | 187 (31) |
| Education, years (SD) | 16 (4) | 16 (4) | 16 (3) | 16 (4) |
| Median time between last clinical evaluation and death, years | 0.76 | 0.77 | 0.85 | 0.81 |
| Global cognition scorec, mean (SD) | 0.1 (0.5) | −0.5 (0.5) | −2.1 (1.0) | −1.0 (1.2) |
| Episodic memory scorec, mean (SD) | 0.3 (0.6) | −0.6 (0.9) | −2.2 (1.1) | −1.0 (1.4) |
| Semantic memory scorec, mean (SD) | 0.0 (0.7) | −0.5 (0.7) | −2.6 (1.7) | −1.3 (1.7) |
| Working memory scorec, mean (SD) | 0.0 (0.7) | −0.3 (0.7) | −1.6 (1.1) | −0.8 (1.1) |
| Perceptual speed scorec, mean (SD) | −0.3 (0.8) | −0.9 (0.9) | −2.1 (0.9) | −1.2 (1.2) |
| Visuospatial ability scorec, mean (SD) | 0.2 (0.7) | −0.3 (0.8) | −1.2 (1.2) | −0.6 (1.1) |
| Mini-mental State Examinationc (MMSE), mean (SD) | 27.8 (1.7) | 25.5 (2.9) | 11.9 (8.4) | 20.1 (9.5) |
| Heart disease, n (%) | 52 (29) | 31 (20) | 43 (16) | 126 (21) |
| Hypertension, n (%) | 133 (75) | 104 (68) | 181 (67) | 418 (69) |
| Diabetes, n (%) | 44 (25) | 32 (21) | 53 (20) | 129 (21) |
| Smoking, n (%) | 67 (38) | 50 (32) | 87 (32) | 204 (34) |
| Postmortem interval to fixation, hrs (SD) | 9.0 (6.6) | 9.4 (6.8) | 8.1 (5.6) | 8.8 (6.3) |
| Scanner for ex-vivo MRI | ||||
| - 3T GE Signa, n (%) | 28 (16) | 23 (15) | 53 (20) | 104 (17) |
| - 3T Siemens Trio, n (%) | 31 (17) | 20 (13) | 45 (17) | 96 (16) |
| - 3T Philips Achieva, n (%) | 90 (51) | 85 (55) | 140 (52) | 315 (52) |
| - 3T Siemens Verio, n (%) | 29 (16) | 26 (17) | 33 (12) | 88 (15) |
NCI: No cognitive impairment
MCI: Mild cognitive impairment
Proximate to death
Assessment of cognitive function and clinical diagnosis
Cognition was assessed annually with a battery of 21 cognitive tests (19 in common between MAP, ROS and MARS). Of those, the Mini-Mental State Examination was used only for descriptive purposes and the Complex Ideational Material was used only for diagnostic purposes. The remaining 17 tests were used to assess performance in five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability [34]. Raw scores on individual tests were converted to z-scores and were averaged within each cognitive domain, as well as over all tests, to yield composite scores for each domain as well as for global cognition [36].
Clinical diagnosis of dementia followed accepted and validated criteria [37]. Participants who had cognitive impairment but did not meet the criteria for dementia were classified as having mild cognitive impairment (MCI) [38,39]. Persons without dementia or MCI were categorized as no cognitive impairment (NCI). At the time of death, all available clinical data were reviewed by a neurologist and a summary final diagnostic opinion was provided blinded to all postmortem data. The final diagnostic opinion was used to define three groups of participants, namely the whole group (including demented, MCI, and NCI participants), the subgroup of non-demented participants (including MCI and NCI), and the NCI subgroup, in descending order of average cognitive impairment.
Brain hemisphere preparation
At autopsy, a technician removed the brain and divided the cerebrum into left and right hemispheres. If a cerebral hemisphere had more visible pathology, it was immersed in phosphate-buffered 4% formaldehyde solution and refrigerated at 4°C within 30 minutes of removal from the skull, while the contralateral hemisphere was frozen and stored. If, however, there was no visible difference in the amount of pathology between the two hemispheres, the decision to refrigerate or freeze and store was made randomly. The refrigerated hemisphere was allowed to return to room temperature prior to ex-vivo MRI and was imaged while immersed in formaldehyde solution with its medial aspect facing the bottom of the container. Gross examination was performed within 2 weeks after ex-vivo MRI, followed by histopathologic diagnostic examination by a board-certified neuropathologist [40].
Ex-vivo MRI data acquisition and pre-processing
Ex-vivo MRI data were collected on 3 Tesla scanners approximately 30 days postmortem using 2D fluid-attenuated inversion recovery (FLAIR) and 2D multi-echo spin-echo (ME-SE) sequences (Table 2). Due to the long duration of this study and scanner upgrades, four MRI scanners were used for data collection (Table 2). From the ME-SE data, only T2-weighted images collected at echo times between 49.5-55 ms were used in the analysis to maintain consistency in image contrast across scanners. All data were collected sagittally and were converted to the axial plane. The data from different participants were intensity-normalized for standardization.
Table 2.
Ex-vivo MRI protocols.
| 3T GE Signa | 3T Siemens Trio | 3T Philips Achieva | 3T Siemens Verio | ||
|---|---|---|---|---|---|
| FLAIRa | Acquired voxel size (mm3) | 1.25 × 1.25 × 1.5 | 0.63 × 1.2 × 1.5 | 1.25 × 1.25 × 1.5 | 0.9 × 0.9 × 2 |
| TEc (ms) | 40 | 103 | 125 | 199 | |
| TRd (ms) | 5000 | 5370 | 8000 | 6520 | |
| TIe (ms) | 1200 | 2350 | 1800 | 1550 | |
| Repetitions | 2 | 1 | 1 | 2 | |
| Scan time (min) | 7.3 | 4.9 | 8.8 | 4.6 | |
| ME-SEb | Acquired voxel size (mm3) | 0.63 × 0.63 × 1.5 | 0.63 × 0.63 × 1.5 | 0.62 × 0.62 × 1.5 | 0.63 × 0.63 × 1.5 |
| TEc (ms) | 13, 52 | 11, 33, 55 | 16.5, 33, 49.5, 66, 82.5 | 22, 33, 55 | |
| TRd (ms) | 3600 | 3600 | 4055 | 3750 | |
| Repetitions | 6 | 4 | 2 | 4 | |
| Scan time (min) | 31 | 30.3 | 35 | 32 |
FLAIR: fluid-attenuated inversion recovery
ME-SE: multi-echo spin-echo
TE: echo-time
TR: repetition time
TI: inversion time
WMH burden rating
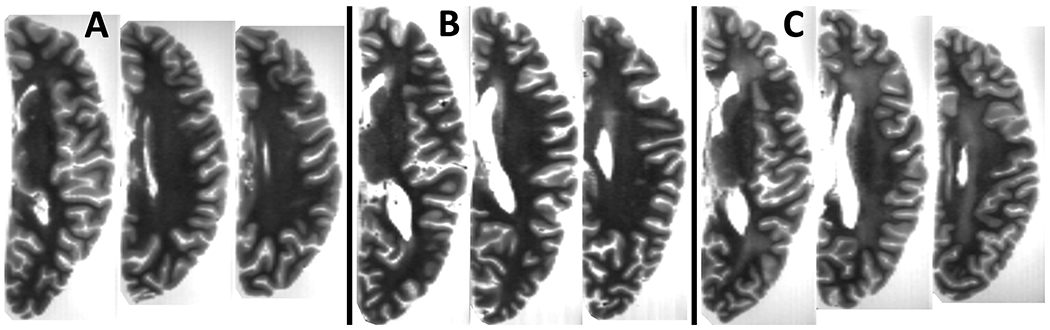
One rater was trained by an expert to rate WMH burden in all participants based on ex-vivo FLAIR and ME-SE images, using a modification of the original Fazekas approach. The rater first rated WMH in periventricular and deep white matter, separately, according to the original four-level Fazekas scale (0, 1, 2, 3) [41]. The whole brain WMH burden was then defined as the maximum of the periventricular and deep white matter ratings. Finally, ratings of 0 and 1 were combined into one level because distinguishing these two levels from each other was complex due to incomplete cancellation of formaldehyde solution signals. This resulted into a WMH burden scale with three levels. Examples of cases with different WMH ratings are shown in Figure 1. Intraclass correlation (ICC) was used to assess the intra-rater reliability as well as the agreement between the rater and the expert. For intra-rater reliability assessment, the rater repeated the rating in 50 participants. To assess agreement between the rater and the expert, the expert rated WMH burden in 32 participants. Both the rater and expert were blinded to all other clinical and pathology data.
Figure 1. Examples of ex-vivo WMH burden.
Example axial slices of three hemispheres with ex-vivo WMH burden of (A) 1, (B) 2, and (C) 3.
Neuropathologic evaluation
After each cerebral hemisphere was immersion fixed and imaged with MRI ex-vivo, it was sectioned into 1 cm thick coronal slabs. The slabs were macroscopically evaluated, and then selected tissue blocks were dissected, embedded in paraffin, cut into sections, and mounted on glass slides. Each case was reviewed by a board-certified neuropathologist blinded to all clinical data, age, ex-vivo MRI data, and WMH burden ratings. The procedures for neuropathologic evaluation are well-established and a detailed description can be found in [40]. In brief, for each hemisphere, a composite score of amyloid-β plaques and a composite score of neurofibrillary tangles were generated based on counts in five brain regions (more details in [42]). TDP-43 pathology was rated on four levels: no TDP-43 inclusions; inclusions in amygdala only; inclusions in amygdala as well as entorhinal cortex or hippocampus CA1; and inclusions in amygdala, entorhinal cortex or hippocampus CA1, and neocortex [43]. Hippocampal sclerosis was defined as severe neuronal loss and gliosis in the hippocampus and was rated as present or absent [44]. Lewy bodies were detected in six regions and were rated as present or absent [45,46]. Gross infarcts were scored as none, one, or more than one. Microscopic infarcts were detected in a minimum of nine regions and were also scored as none, one, or more than one [47]. Assessment of atherosclerosis was based on the number and extent of vascular involvement at the circle of Willis and was rated as none, mild, moderate, and severe. Assessment of arteriolosclerosis was based on the severity of wall thickening and luminal occlusion of the small arterioles in one section of anterior basal ganglia and was rated as none, mild, moderate, and severe. Cerebral amyloid angiopathy was assessed in four regions and was rated as none, mild, moderate, and severe [48].
Statistical analysis
One-way analysis of variance (ANOVA) and chi-squared tests were used to identify significant differences in demographic, clinical, imaging, and other experimental variables across groups of participants. Pearson’s and Spearman’s correlations and Wilcoxon rank sum tests were used to investigate relationships of WMH burden with demographic and clinical variables.
Multiple ordinal logistic regression was used to investigate the association of WMH burden assessed ex-vivo with amyloid-β plaques score, neurofibrillary tangles score, TDP-43 level, hippocampal sclerosis presence, Lewy bodies presence, gross and microscopic infarcts severity, atherosclerosis severity, arteriolosclerosis severity and cerebral amyloid angiopathy severity, while controlling for age at death, sex, years of education, and duration between death and immersion of the brain hemisphere in formaldehyde solution. The WMH burden was the dependent variable and all other variables were considered as independent variables in the same ordinal logistic regression model. Associations of WMH burden with neuropathologies were considered significant at p<0.05. Variance inflation factors were calculated for all independent variables in the model to assess potential collinearity among variables. The above analysis was conducted in the whole group of 603 participants and was repeated in the subgroup of non-demented participants, as well as in the subgroup with no cognitive impairment, in descending order of average cognitive impairment.
A linear mixed-effects model was used to investigate the independent association of WMH burden measured ex-vivo with the rate of decline in global cognition above and beyond what was explained by neuropathologies and demographics. The composite score of global cognition was the longitudinal dependent variable. The independent variables were the WMH burden, all the neuropathologies, demographics and covariates listed in the previous paragraph, as well as the interaction of each one of these variables with the time before death. Associations of WMH burden with the rate of decline in global cognition were considered significant when the interaction term of WMH burden with time before death was statistically significant (p<0.05). The same analysis was repeated for each of the five cognitive domains.
WMH burden in-vivo and ex-vivo
WMH burden was assessed both in-vivo and ex-vivo in the first 79 participants with available in-vivo MRI data (in addition to all other data described above) (Table 3). The cerebral hemisphere imaged ex-vivo was segmented in the in-vivo FLAIR images and the rest of the brain was masked out. In-vivo WMH burden was then rated in the segmented FLAIR images of the same cerebral hemisphere as that imaged ex-vivo following the same approach as that described above. A 2-way frequency table of WMH burden assessed in-vivo and ex-vivo was generated. Logistic regression was used to investigate if an increase in WMH burden from in-vivo to ex-vivo was associated with a longer antemortem interval (AMI) between in-vivo MRI and death. This analysis excluded participants with in-vivo WMH burden of 3 because this was the maximum rating and a higher rating ex-vivo was not possible, regardless of AMI.
Table 3.
In-vivo MRI protocol used on 79 of the participants.
| 1.5T GE Signa | 3T Siemens Trio | 3T Philips Achieva | ||
|---|---|---|---|---|
| Participants | 58 | 18 | 3 | |
| FLAIRa | Acquired voxel size (mm3) | 0.94 × 1.07 × 3 | 0.86 × 0.86 × 4 | 0.86 × 1.09 × 4 |
| TEb (ms) | 120 | 150 | 90 | |
| TRc (ms) | 8000 | 9000 | 9000 | |
| TId (ms) | 2000 | 2490 | 2500 | |
| Acceleration factor | 1 | 2 | 1.6 | |
| Scan time (min) | 4.0 | 2.7 | 6.3 | |
FLAIR: fluid-attenuated inversion recovery
TE: echo-time
TR: repetition time
TI: inversion time
RESULTS
Demographic, clinical and neuropathologic characteristics, and WMH burden
Demographic and clinical characteristics of the participants are presented in Table 1. Among the 603 participants, 178 had no cognitive impairment (NCI), 154 had mild cognitive impairment (MCI), and 271 had dementia at the last evaluation, which was completed 0.81 years (median) prior to death. The group with dementia was on average 2 years older at the time of death (ANOVA, p=0.009) and had higher frequency of heart disease (chi-squared test, p=0.01) than the NCI group. No other differences across groups of participants with NCI, MCI and dementia were observed for sex, years of education, history of hypertension, diabetes, smoking, hemisphere side, postmortem interval to fixation, or scanner used for ex-vivo MRI.
Neuropathologic characteristics for different levels of WMH burden measured ex-vivo are presented in Table 4. Intra-rater reliability for WMH burden rating was excellent (ICC=0.75, p<10−50) and agreement of the rater with the expert was good (ICC=0.64, p=3×10−5). WMH burden in the whole group was correlated with age (Pearson’s r=0.09, p=0.03) and was higher in women (Wilcoxon rank sum z=3.53, p=0.0004). WMH burden was higher in participants with dementia than those with NCI (chi-squared test, p<10−4). WMH burden was not associated with years of education, history of heart disease, hypertension, diabetes, smoking, hemisphere side, or postmortem interval to fixation. No differences in WMH burden were observed across scanners used for ex-vivo MRI.
Table 4.
Neuropathologic findings for different levels of WMH burden.
| Characteristics | aWMH burden = 1 | aWMH burden = 2 | aWMH burden = 3 | Total |
|---|---|---|---|---|
| N | 105 | 212 | 286 | 603 |
| High or intermediate NIA Reagan (Likelihood of AD), n (%) | 53 (50) | 140 (66) | 210 (73) | 403 (67) |
| Amyloid-β plaques, mean (SD) | 0.65 (0.64) | 0.75 (0.61) | 0.90 (0.67) | 0.80 (0.65) |
| Neurofibrillary tangles, mean (SD) | 4.9 (6.4) | 6.4 (7.7) | 8.0 (7.9) | 6.9 (7.7) |
| Lewy bodies, n (%) | 22 (21) | 51 (24) | 75 (26) | 148 (25) |
| Hippocampal sclerosis, n (%) | 12 (11) | 23 (11) | 42 (15) | 77 (13) |
| TDP43 | ||||
| - bStage 3, n (%) | 13 (12) | 31 (14) | 53 (19) | 97 (16) |
| - cStage 2, n (%) | 22 (21) | 47 (22) | 60 (21) | 129 (21) |
| - dStage 1, n (%) | 17 (16) | 31 (14) | 56 (20) | 104 (17) |
| Gross infarcts, n (%) | 30 (29) | 61 (29) | 156 (55) | 247 (41) |
| Microscopic infarcts, n (%) | 26 (25) | 67 (32) | 119 (42) | 212 (35) |
| Atherosclerosis | ||||
| - Severe, n (%) | 2 (2) | 11 (5) | 33 (11) | 46 (8) |
| - Moderate, n (%) | 18 (17) | 41 (20) | 64 (22) | 123 (20) |
| - Mild, n (%) | 49 (47) | 109 (52) | 141 (49) | 299 (50) |
| Arteriolosclerosis | ||||
| - Severe, n (%) | 2 (2) | 8 (4) | 30 (10) | 40 (7) |
| - Moderate, n (%) | 11 (10) | 37 (18) | 80 (28) | 128 (21) |
| - Mild, n (%) | 41 (39) | 98 (46) | 122 (43) | 261 (43) |
| Cerebral amyloid angiopathy | ||||
| - Severe, n (%) | 8 (8) | 11 (5) | 45 (16) | 64 (11) |
| - Moderate, n (%) | 24 (23) | 48 (23) | 68 (24) | 140 (23) |
| - Mild, n (%) | 39 (37) | 111 (53) | 114 (40) | 264 (44) |
Assessed ex-vivo
TDP-43 Stage 3: Inclusions in amygdala, entorhinal cortex or hippocampus CA1, and neocortex
TDP-43 Stage 2: Inclusions in amygdala and entorhinal cortex or hippocampus CA1
TDP-43 Stage 1: Inclusions in amygdala only
Association of WMH burden with neuropathologies
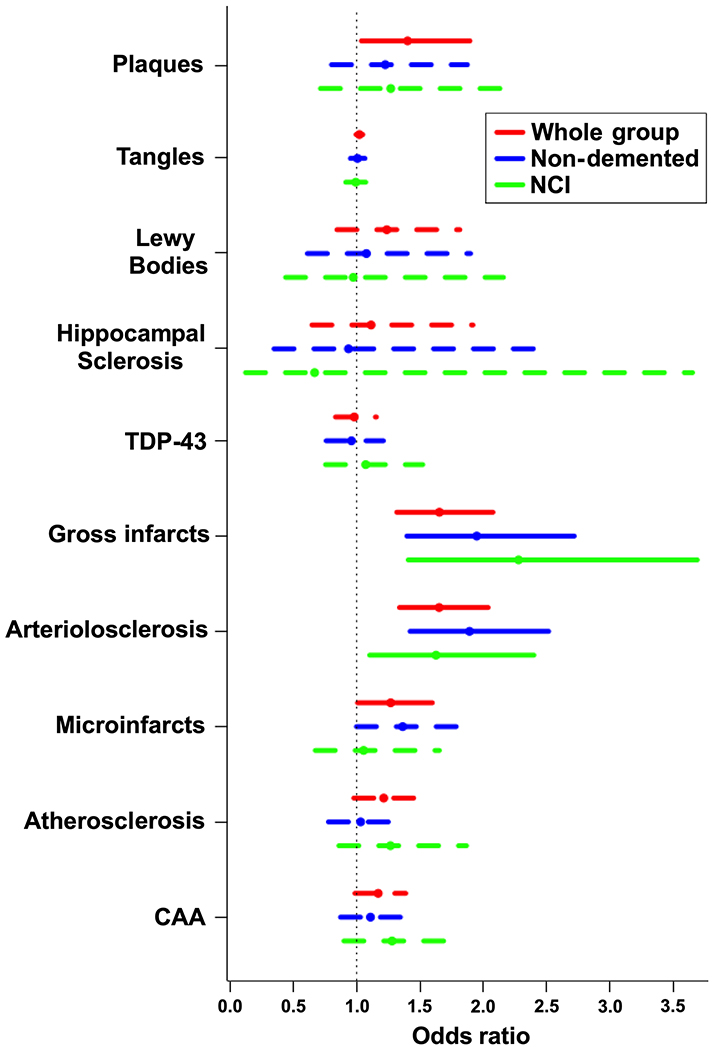
Multiple ordinal logistic regression including all neuropathologies in the same model showed that when considering the whole group of 603 participants, both neurodegenerative and vascular pathologies were associated with greater WMH burden assessed ex-vivo: amyloid-β plaques (odds ratio (OR)=1.40, 95% confidence interval (CI)=[1.04,1.90], p=0.028), arteriolosclerosis (OR=1.65, 95% CI=[1.34,2.04], p<10−4), gross infarcts (OR=1.65, 95% CI=[1.32,2.08], p<10−4), and microscopic infarcts (OR=1.27, 95% CI=[1.01,1.60], p=0.04) (Fig.2). When considering only non-demented participants (N=332), gross infarcts (OR=1.95, 95% CI=[1.40,2.72], p=10−4) and arteriolosclerosis (OR=1.89, 95% CI=[1.42,2.52], p<10−4) were associated with greater WMH burden, while the association of microscopic infarcts with WMH burden approached significance (OR=1.36, 95% CI=[1.00,1.86], p=0.05) (Fig.2). In NCI participants, gross infarcts (OR=2.28, 95% CI=[1.41,3.69], p=8×10−4) and arteriolosclerosis (OR=1.63, 95% CI=[1.10,2.40], p=0.014) were associated with greater WMH burden (Fig.2). Variance inflation factors for all independent variables in the multiple ordinal logistic regression were lower than 1.5, suggesting that collinearity among independent variables was not a concern.
Figure 2. Neuropathologic correlates of WMH burden.
Odds of higher ex-vivo WMH burden for different neuropathologies, based on a single model including all neuropathologies. The bars denote 95% confidence intervals (solid bars correspond to p<0.05, and dashed bars correspond to p≥0.05).
Association of WMH burden with cognitive decline
When considering the whole group of 603 participants, a linear mixed effects model controlling for neuropathologies and demographics showed that WMH burden was associated with faster decline in perceptual speed (model estimate=−0.014, standard error (SE)=0.007, p=0.038). WMH burden was not associated with decline in cognitive domains other than perceptual speed, or with decline in global cognition, when controlling for neuropathologies and demographics. Multiple comparisons correction was not applied for the six mixed effects models (five cognitive domains plus global cognition). When considering only non-demented participants, WMH burden was also associated with faster decline in perceptual speed (model estimate=−0.015, SE=0.005, p=0.006) above and beyond the effects of neuropathologies and demographics.
WMH burden in-vivo and ex-vivo
Comparison of WMH burden in-vivo and ex-vivo in 79 participants in which WMH burden was assessed both in-vivo and ex-vivo showed the same rating in 59 and higher rating ex-vivo in 20 participants (Table 5). No participants had lower WMH burden ex-vivo compared to in-vivo. A chi-squared test showed no difference in the distribution of participants with unchanged or higher ex-vivo WMH burden to the two magnetic field strengths used for in-vivo MRI, however the sample size may have been too small (only 27% of the 79 participants were imaged at 3T in-vivo) to draw a clear conclusion.
Table 5.
Frequency of WMH burden assessed in-vivo by WMH burden assessed ex-vivo in 79 participants.
| Ex-vivo WMH burden | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| In-vivo WMH burden | 1 | 5 | 7 | 0 |
| 2 | 0 | 17 | 13 | |
| 3 | 0 | 0 | 37 | |
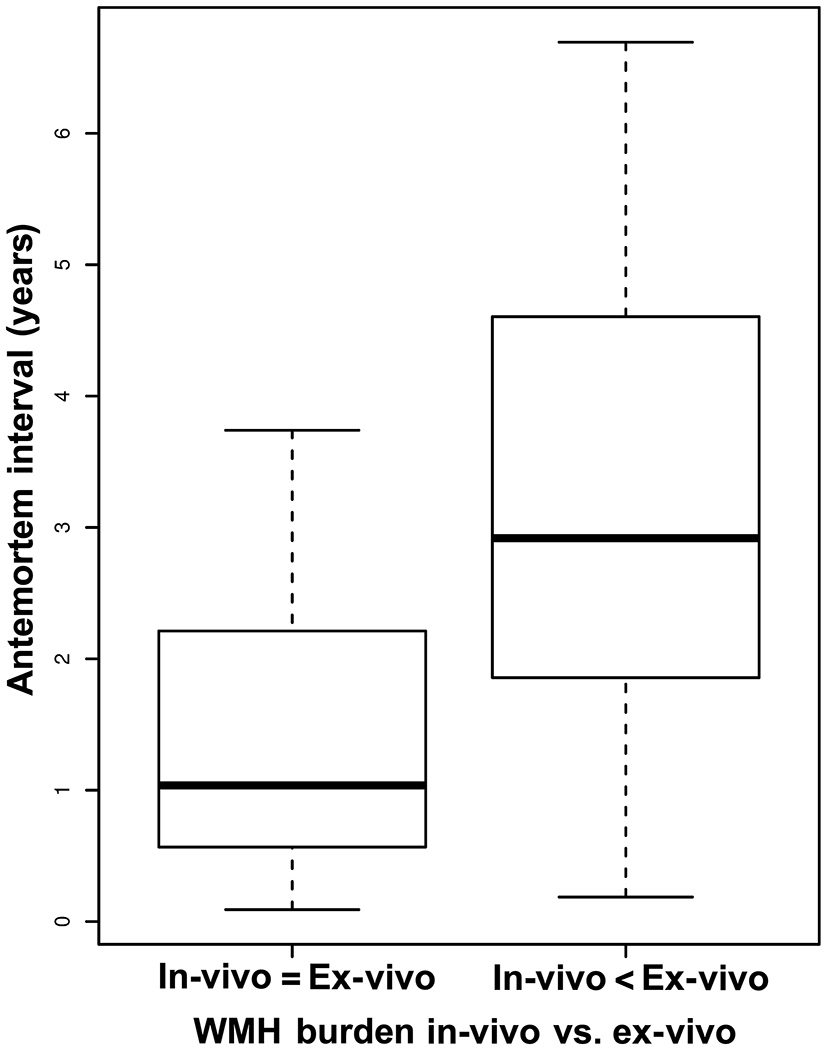
The median antemortem interval (AMI) between in-vivo MRI and death for those participants with unchanged WMH burden ex-vivo compared to in-vivo was 1.04 years (range=0.09-3.74 years), and for those with higher WMH burden ex-vivo compared to in-vivo was 2.92 years (0.19-6.69 years) (Fig.3). Logistic regression showed that longer AMI was associated with a higher WMH burden ex-vivo compared to in-vivo (OR=2.32, 95% CI=[1.43,4.39], p=0.003).
Figure 3. Participants with higher WMH burden ex-vivo compared to in-vivo have longer antemortem intervals.
Boxplot of the antemortem interval from in-vivo MRI to death for participants with unchanged (left) and higher (right) WMH burden ex-vivo compared to in-vivo. In both groups, the bold horizontal lines correspond to the respective medians, the ends of boxes correspond to the 25% and 75% of the observations, and the dashed bars correspond to the minimum and maximum values. This boxplot does not include participants with in-vivo WMH burden rated at the maximum level of 3, because a higher rating ex-vivo was not possible, regardless of AMI.
DISCUSSION
The present study aimed to elucidate the neuropathologic correlates of WMH across cognitive states by combining ex-vivo MRI and detailed pathologic evaluation in a large community-based cohort of older adults. Overall, amyloid-β plaques, arteriolosclerosis, and infarcts (gross and microscopic) were associated with higher WMH burden. However, when considering only non-demented or NCI participants, only vascular pathologies (specifically gross infarcts and arteriolosclerosis) and not Alzheimer’s disease pathology were associated with higher WMH burden. In addition, based on detailed longitudinal cognitive assessments, WMH burden was associated with faster decline in perceptual speed in both the whole group as well as in non-demented participants, above and beyond the effects of neuropathologies and demographics. Finally, in a segment of the cohort with available in-vivo and ex-vivo assessments of WMH burden, a longer interval between in-vivo MRI and death was associated with higher odds of higher burden ex-vivo compared to in-vivo, strengthening the rationale for conducting MRI ex-vivo in this MRI-pathology investigation.
Gross and microscopic infarcts and arteriolosclerosis were related to WMH burden in agreement with extant literature linking WMH to small vessel disease [49]. The fact that the relationship of gross infarcts and arteriolosclerosis with WMH burden persisted when considering only NCI participants demonstrates the strong and early link between WMH and vascular pathologies. This finding, in combination with the absence of any other WMH and pathology associations in NCI, suggests that a cognitively normal person with WMH but no visible infarcts on MRI may have a high probability of having arteriolosclerosis, with the severity of WMH indicating the severity of arteriolosclerosis. Future work will test this hypothesis.
Alzheimer’s pathology, and more specifically amyloid-β plaques, were associated with WMH burden above and beyond contributions from vascular pathologies, when studying the whole group of demented and non-demented older adults. After excluding demented participants, the association between amyloid-β plaques and WMH was lost, while the link between vascular pathologies and WMH remained statistically significant. These findings suggest that a) there is an independent link between Alzheimer’s pathology and WMH, and b) that this link appears later in the progression of Alzheimer’s disease than the link between vascular pathologies and WMH. In support of our finding, previous studies have suggested that there is more than one pathway to WMH [50–52], and that in addition to vascular pathologies, Alzheimer’s pathology may also lead to WMH through axonal degeneration. In particular, a recent study on the composition and aetiology of WMH in the parietal lobes [50], which is a common site of WMH in Alzheimer’s [9], conducted detailed neuropathologic and biochemical evaluation in tissue from patients with Alzheimer’s dementia and non-demented older adults. The authors found that in patients with Alzheimer’s dementia, WMH in the parietal lobes were associated with degenerative axonal loss triggered by Alzheimer’s pathology, while in non-demented older adults WMH were associated with ischemic damage. These results by [50] support our findings of independent links between Alzheimer’s pathology and WMH, and between vascular pathologies and WMH, with the former link present mainly in demented older adults and the latter detectable even in NCI. These differential relationships by cognitive state could have implications in clinical studies and trials, considering that WMH are thought of as a sensitive but not specific biomarker of vascular pathologies.
The link between Alzheimer’s pathology and WMH was shown to involve amyloid-β plaques but not neurofibrillary tangles. Previous work has generated mixed findings, where some studies imply that the link is to plaques [12,52–56], while others suggest that the link is to tangles [32] (some show a negative association to tangles [26]), and yet a third group of studies suggests that the link is to both pathologies [25,29,31]. This discrepancy may be due to differences in the groups of older adults studied, the method for assessing pathology (e.g. in tissue, in CSF, or using positron emission tomography), the method for assessing WMH burden (e.g. visual rating, whole brain WMH volume, regional WMH volumes), as well as the list of pathologies considered in the statistical models (previous studies typically modeled each pathology separately). Although it may be difficult even for the present work to decipher with certainty which of the Alzheimer’s pathologies is responsible for the association with WMH, nevertheless, the current results provide strong evidence on the presence of an independent association of Alzheimer’s pathology with WMH.
WMH burden was associated with faster decline in perceptual speed when considering the whole group or only non-demented participants, above and beyond the effects of neuropathologies and demographics. This extends previous reports on the cognitive profile of WMH [19,22,57,58] and suggests that WMH burden captures contributions to cognitive decline other than those captured by the neuropathology and demographic variables included in the model. These additional contributions are most likely due to the fact that WMH burden represents white matter injury (which is closely related to cognitive decline) that is not captured by WMH-related pathologies.
In participants that had both in-vivo and ex-vivo MRI data, a longer interval between in-vivo MRI and death significantly increased the odds of higher burden ex-vivo compared to in-vivo. This suggests that investigations of WMH combining in-vivo MRI and pathology may image a healthier state of the brain than that assessed at autopsy, weakening the observed relationship between WMH and neuropathologies. This limitation becomes more important as the antemortem interval (AMI) between in-vivo MRI and death increases. The participants that had unchanged WMH burden ex-vivo compared to in-vivo had a median AMI of 1.04 years (range=0.09-3.74 years), and those with higher WMH burden ex-vivo compared to in-vivo had a median AMI of 2.92 years (range=0.19-6.69 years). Based on these findings, AMI<1 year drastically reduces the chances of a change in WMH burden from in-vivo MRI to death, while AMI>3 years increases the chances of an increase in WMH burden after in-vivo MRI data have been collected. The above findings strengthen the rationale for conducting MRI ex-vivo in MRI-pathology investigations of WMH.
This work has major strengths and also a few weaknesses. The design of the study combined solutions to a number of problems that have traditionally plagued MRI-pathology investigations of WMH. First, studying a large community-based cohort of older adults increased statistical power and generalizability of findings. Second, conducting detailed pathologic evaluation and considering a comprehensive array of age-related neuropathologies in the same model of WMH burden allowed a better control for the effects of comorbid pathologies. Third, performing MRI ex-vivo allowed imaging independent of frailty level thereby increasing generalizability of findings. Ex-vivo MRI also ensured that imaging captures brain characteristics at the same brain condition as neuropathologic examination. Fourth, detailed longitudinal cognitive assessments allowed investigation of the independent association of WMH burden with cognitive decline after accounting for neuropathologies and demographics. One weakness of the present study is the use of a rating scale for assessing WMH burden instead of measuring the total volume of WMH [59,60]. The rationale for choosing this approach was that publicly available software was not able to successfully segment WMH in single cerebral hemispheres imaged ex-vivo while immersed in fixative solution. Efforts are currently underway to develop in-house software that will allow automated segmentation and measurement of WMH volume in such ex-vivo images. Another minor weakness is that laterality was not considered, since only one cerebral hemisphere was imaged per participant.
In conclusion, the findings of this MRI-pathology investigation in a large community-based cohort of older adults suggest that WMH burden has independent associations with small vessel pathologies as well as Alzheimer’s disease pathology. The link between WMH and vascular pathology is pervasive across all cognitive states, appearing early, even in cognitively normal older adults. By contrast, the link between WMH and Alzheimer’s pathology appears later in the progression of Alzheimer’s disease when there is cognitive impairment and specifically dementia. Furthermore, WMH burden is associated with faster decline in perceptual speed above and beyond the effects of neuropathologies and demographics, suggesting important additional tissue injury. These data demonstrate the role ex-vivo MRI can have in both enhancing pathology studies and advancing our understanding of cognitive aging. Finally, the finding that longer intervals between in-vivo MRI and death increase the odds of additional WMH developing after in-vivo imaging, weakening observed WMH-pathology associations, strengthens the rationale for the use of ex-vivo MRI in MRI-pathology investigations of WMH.
ACKNOWLEDGMENTS
The authors would like to thank the participants and staff of the Rush University Memory and Aging Project, Religious Orders Study, and Minority Aging Research Study. This study was supported by National Institutes of Health grants P30AG010161, UH2NS100599, UH3NS100599, R01AG064233, RF1AG022018, R01AG056405, R01AG042210, R01AG17917, R01AG34374.
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
- [1].de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MM (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 70, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA (2005) Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol. Aging 26, 491–510. [DOI] [PubMed] [Google Scholar]
- [3].Habes M, Sotiras A, Erus G, Toledo JB, Janowitz D, Wolk DA, Shou H, Bryan NR, Doshi J, Völzke H, Schminke U, Hoffmann W, Resnick SM, Grabe HJ, Davatzikos C (2018) White matter lesions: Spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology 91, e964–e975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scott JA, Braskie MN, Tosun D, Thompson PM, Weiner M, DeCarli C, Carmichael OT, Alzheimer’s Disease Neuroimaging Initiative (2015) Cerebral Amyloid and Hypertension are Independently Associated with White Matter Lesions in Elderly. Front. Aging Neurosci 7, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dufouil C, Chalmers J, Coskun O, Besançon V, Bousser M-G, Guillon P, MacMahon S, Mazoyer B, Neal B, Woodward M, Tzourio-Mazoyer N, Tzourio C, PROGRESS MRI Substudy Investigators (2005) Effects of Blood Pressure Lowering on Cerebral White Matter Hyperintensities in Patients With Stroke. Circulation 112, 1644–1650. [DOI] [PubMed] [Google Scholar]
- [6].Carmichael O, Schwarz C, Drucker D, Fletcher E, Harvey D, Beckett L, Jack CR, Weiner M, DeCarli C, Alzheimer’s Disease Neuroimaging Initiative (2010) Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch. Neurol 67, 1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].de Bruijn RFAG, Akoudad S, Cremers LGM, Hofman A, Niessen WJ, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA (2014) Determinants, MRI Correlates, and Prognosis of Mild Cognitive Impairment: The Rotterdam Study. J. Alzheimer’s Dis 42, S239–S249. [DOI] [PubMed] [Google Scholar]
- [8].Longstreth WT, Arnold AM, Beauchamp NJ, Manolio TA, Lefkowitz D, Jungreis C, Hirsch CH, O’Leary DH, Furberg CD (2005) Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 36, 56–61. [DOI] [PubMed] [Google Scholar]
- [9].Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS (2006) Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 67, 2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lazarus R, Prettyman R, Cherryman G (2005) White matter lesions on magnetic resonance imaging and their relationship with vascular risk factors in memory clinic attenders. Int. J. Geriatr. Psychiatry 20, 274–279. [DOI] [PubMed] [Google Scholar]
- [11].van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB (2008) Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke 39, 2712–2719. [DOI] [PubMed] [Google Scholar]
- [12].Stenset V, Johnsen L, Kocot D, Negaard A, Skinningsrud A, Gulbrandsen P, Wallin A, Fladby T (2006) Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurology 67, 830–833. [DOI] [PubMed] [Google Scholar]
- [13].Biesbroek JM, Weaver NA, Biessels GJ (2017) Lesion location and cognitive impact of cerebral small vessel disease. Clin. Sci 131, 715–728. [DOI] [PubMed] [Google Scholar]
- [14].Debette S, Markus HS (2010) The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341, c3666–c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van der Flier WM, van Straaten ECW, Barkhof F, Verdelho A, Madureira S, Pantoni L, Inzitari D, Erkinjuntti T, Crisby M, Waldemar G, Schmidt R, Fazekas F, Scheltens P (2005) Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke 36, 2116–2120. [DOI] [PubMed] [Google Scholar]
- [16].Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, Erkinjuntti T, Fazekas F, Ferro JM, Langhorne P, O’Brien J, Scheltens P, Visser MC, Wahlund LO, Waldemar G, Wallin A, Hennerici MG, LADIS Study Group (2008) Association of gait and balance disorders with age-related white matter changes: The LADIS Study. Neurology 70, 935–942. [DOI] [PubMed] [Google Scholar]
- [17].The LADIS Study Group, Poggesi A, Pantoni L, Inzitari D, Fazekas F, Ferro J, O’Brien J, Hennerici M, Scheltens P, Erkinjuntti T, Visser M, Langhorne P, Chabriat H, Waldemar G, Wallin A, Wahlund A (2011) 2001–2011: A Decade of the LADIS (Leukoaraiosis And DISability) Study: What Have We Learned about White Matter Changes and Small-Vessel Disease? Cerebrovasc. Dis 32, 577–588. [DOI] [PubMed] [Google Scholar]
- [18].Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA (2008) Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 71, 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arvanitakis Z, Fleischman DA, Arfanakis K, Leurgans SE, Barnes LL, Bennett DA (2016) Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct. Funct 221, 2135–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brickman AM (2013) Contemplating Alzheimer’s disease and the contribution of white matter hyperintensities. Curr. Neurol. Neurosci. Rep 13, 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brickman AM, Provenzano FA, Muraskin J, Manly JJ, Blum S, Apa Z, Stern Y, Brown TR, Luchsinger JA, Mayeux R (2012) Regional White Matter Hyperintensity Volume, Not Hippocampal Atrophy, Predicts Incident Alzheimer Disease in the Community. Arch. Neurol 69, 1621–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Birdsill AC, Koscik RL, Jonaitis EM, Johnson SC, Okonkwo OC, Hermann BP, LaRue A, Sager MA, Bendlin BB (2014) Regional white matter hyperintensities: aging, Alzheimer’s disease risk, and cognitive function. Neurobiol. Aging 35, 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Erten-Lyons D, Woltjer R, Kaye J, Mattek N, Dodge HH, Green S, Tran H, Howieson DB, Wild K, Silbert LC (2013) Neuropathologic basis of white matter hyperintensity accumulation with advanced age. Neurology 81, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV., Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, Kramer JH, Schuff N, DeCarli C, Chui HC (2008) Neuropathological basis of magnetic resonance images in aging and dementia. Ann. Neurol 63, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Polvikoski TM, van Straaten ECW, Barkhof F, Sulkava R, Aronen HJ, Niinistö L, Oinas M, Scheltens P, Erkinjuntti T, Kalaria RN (2010) Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 75, 2071–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shim YS, Yang D- W, Roe CM, Coats MA, Benzinger TL, Xiong C, Galvin JE, Cairns NJ, Morris JC (2015) Pathological Correlates of White Matter Hyperintensities on Magnetic Resonance Imaging. Dement. Geriatr. Cogn. Disord 39, 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259. [DOI] [PubMed] [Google Scholar]
- [28].Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–486. [DOI] [PubMed] [Google Scholar]
- [29].Moghekar A, Kraut M, Elkins W, Troncoso J, Zonderman AB, Resnick SM, O’Brien RJ (2012) Cerebral white matter disease is associated with Alzheimer pathology in a prospective cohort. Alzheimers. Dement 8, S71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Young VG, Halliday GM, Kril JJ (2008) Neuropathologic correlates of white matter hyperintensities. Neurology 71, 804–811. [DOI] [PubMed] [Google Scholar]
- [31].Alosco ML, Sugarman MA, Besser LM, Tripodis Y, Martin B, Palmisano JN, Kowall NW, Au R, Mez J, DeCarli C, Stein TD, McKee AC, Killiany RJ, Stern RA (2018) A Clinicopathological Investigation of White Matter Hyperintensities and Alzheimer’s Disease Neuropathology. J. Alzheimers. Dis 63, 1347–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Erten-Lyons D, Dodge HH, Woltjer R, Silbert LC, Howieson DB, Kramer P, Kaye JA (2013) Neuropathologic Basis of Age-Associated Brain Atrophy. JAMA Neurol. 70, 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 134, 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA (2018) Religious Orders Study and Rush Memory and Aging Project. J. Alzheimer’s Dis 64, S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA (2012) The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr. Alzheimer Res 9, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wilson RS, Boyle PA, Yu L, Segawa E, Sytsma J, Bennett DA (2015) Conscientiousness, dementia related pathology, and trajectories of cognitive aging. Psychol. Aging 30, 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [38].Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J (2002) Natural history of mild cognitive impairment in older persons. Neurology 59, 198–205. [DOI] [PubMed] [Google Scholar]
- [39].Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA (2006) Mild cognitive impairment: risk of Alzheimer disease and rate of cognitive decline. Neurology 67, 441–445. [DOI] [PubMed] [Google Scholar]
- [40].Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA, Golak T, Arfanakis K (2015) Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol. Aging 36, 2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR. Am. J. Roentgenol 149, 351–356. [DOI] [PubMed] [Google Scholar]
- [42].Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA (2007) Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann. Neurol 62, 59–66. [DOI] [PubMed] [Google Scholar]
- [43].Yu L, De Jager PL, Yang J, Trojanowski JQ, Bennett DA, Schneider JA (2015) The TMEM106B locus and TDP-43 pathology in older persons without FTLD. Neurology 84, 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA (2009) The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol 66, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH (1996) Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 47, 1113–1124. [DOI] [PubMed] [Google Scholar]
- [46].Schneider JA, Arvanitakis Z, Yu L, Boyle PA, Leurgans SE, Bennett DA (2012) Cognitive impairment, decline and fluctuations in older community-dwelling subjects with Lewy bodies. Brain 135, 3005–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA (2011) Microinfarct pathology, dementia, and cognitive systems. Stroke 42, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA (2011) Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann. Neurol 69, 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wardlaw JM, Valdés Hernández MC, Muñoz‐Maniega S (2015) What are White Matter Hyperintensities Made of? J. Am. Heart Assoc 4, 001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McAleese KE, Walker L, Graham S, Moya ELJ, Johnson M, Erskine D, Colloby SJ, Dey M, Martin-Ruiz C, Taylor J-P, Thomas AJ, McKeith IG, De Carli C, Attems J (2017) Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 134, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].McAleese KE, Firbank M, Dey M, Colloby SJ, Walker L, Johnson M, Beverley JR, Taylor JP, Thomas AJ, O’Brien JT, Attems J (2015) Cortical tau load is associated with white matter hyperintensities. Acta Neuropathol. Commun 3, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Scott JA, Braskie MN, Tosun D, Maillard P, Thompson PM, Weiner M, DeCarli C, Carmichael OT, ADNI (2016) Cerebral amyloid is associated with greater white-matter hyperintensity accrual in cognitively normal older adults. Neurobiol. Aging 48, 48–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TLS, Marcus DS, Fagan AM, Goate A, Fox NC, Cairns NJ, Holtzman DM, Buckles V, Ghetti B, McDade E, Martins RN, Saykin AJ, Masters CL, Ringman JM, Ryan NS, Förster S, Laske C, Schofield PR, Sperling RA, Salloway S, Correia S, Jack C, Weiner M, Bateman RJ, Morris JC, Mayeux R, Brickman AM, Dominantly Inherited Alzheimer Network (2016) White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann. Neurol 79, 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Brickman AM, Guzman VA, Gonzalez-Castellon M, Razlighi Q, Gu Y, Narkhede A, Janicki S, Ichise M, Stern Y, Manly JJ, Schupf N, Marshall RS (2015) Cerebral autoregulation, beta amyloid, and white matter hyperintensities are interrelated. Neurosci. Lett 592, 54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Graff-Radford J, Arenaza-Urquijo EM, Knopman DS, Schwarz CG, Brown RD, Rabinstein AA, Gunter JL, Senjem ML, Przybelski SA, Lesnick T, Ward C, Mielke MM, Lowe VJ, Petersen RC, Kremers WK, Kantarci K, Jack CR, Vemuri P (2019) White matter hyperintensities: relationship to amyloid and tau burden. Brain 142, 2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Al-Janabi OM, Brown CA, Bahrani AA, Abner EL, Barber JM, Gold BT, Goldstein LB, Murphy RR, Nelson PT, Johnson NF, Shaw LM, Smith CD, Trojanowski JQ, Wilcock DM, Jicha GA (2018) Distinct White Matter Changes Associated with Cerebrospinal Fluid Amyloid-β1–42 and Hypertension. J. Alzheimers. Dis 66, 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Boyle PA, Yu L, Fleischman DA, Leurgans S, Yang J, Wilson RS, Schneider JA, Arvanitakis Z, Arfanakis K, Bennett DA (2016) White matter hyperintensities, incident mild cognitive impairment, and cognitive decline in old age. Ann. Clin. Transl. Neurol 3, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hedden T, Mormino EC, Amariglio RE, Younger AP, Schultz AP, Becker JA, Buckner RL, Johnson KA, Sperling RA, Rentz DM (2012) Cognitive profile of amyloid burden and white matter hyperintensities in cognitively normal older adults. J. Neurosci 32, 16233–16242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].van Straaten ECW, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, Inzitari D, Waldemar G, Erkinjuntti T, Mäntylä R, Wahlund L- O, Barkhof F, LADIS Group (2006) Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke 37, 836–840. [DOI] [PubMed] [Google Scholar]
- [60].Smith CD, Johnson ES, Van Eldik LJ, Jicha GA, Schmitt FA, Nelson PT, Kryscio RJ, Murphy RR, Wellnitz CV. (2016) Peripheral (deep) but not periventricular MRI white matter hyperintensities are increased in clinical vascular dementia compared to Alzheimer’s disease. Brain Behav. 6, e00438. [DOI] [PMC free article] [PubMed] [Google Scholar]