Abstract
Chromosome segregation in mitosis requires the removal of catenation between sister chromatids. Timely decatenation of sister DNAs at mitotic centromeres by topoisomerase IIα (TOP2A) is crucial to maintain genomic stability. The chromatin factors that recruit TOP2A to centromeres during mitosis remain unknown. Here, we show that histone H2A Thr‐120 phosphorylation (H2ApT120), a modification generated by the mitotic kinase Bub1, is necessary and sufficient for the centromeric localization of TOP2A. Phosphorylation at residue‐120 enhances histone H2A binding to TOP2A in vitro. The C‐gate and the extreme C‐terminal region are important for H2ApT120‐dependent localization of TOP2A at centromeres. Preventing H2ApT120‐mediated accumulation of TOP2A at mitotic centromeres interferes with sister chromatid disjunction, as evidenced by increased frequency of anaphase ultra‐fine bridges (UFBs) that contain catenated DNA. Tethering TOP2A to centromeres bypasses the requirement for H2ApT120 in suppressing anaphase UFBs. These results demonstrate that H2ApT120 acts as a landmark that recruits TOP2A to mitotic centromeres to decatenate sister DNAs. Our study reveals a fundamental role for histone phosphorylation in resolving centromere DNA entanglements and safeguarding genomic stability during mitosis.
Keywords: Bub1, DNA decatenation, histone H2A phosphorylation, Sgo1, TOP2A
Subject Categories: Cell Cycle; Chromatin, Epigenetics, Genomics & Functional Genomics; DNA Replication, Repair & Recombination
Bub1‐mediated mitotic phosphorylation of H2A threonine‐120 is necessary and sufficient for topoisomerase IIα (TOP2A) centromeric localization and decatenation of sister chromatids.

Introduction
Chromosomal DNA is replicated in S phase and segregated during mitotic cell division. These processes are tightly regulated so that both daughter cells receive a full set of genetic information, errors in which cause genome instability that can promote tumorigenesis. Following DNA replication, the sister DNAs are held together until the onset of chromosome segregation by cohesin‐mediated sister chromatid cohesion and DNA catenation (Guacci et al, 1997; Michaelis et al, 1997; Losada et al, 1998; Nasmyth & Haering, 2009). To ensure high‐fidelity chromosome segregation during mitosis, the removal of cohesin from chromosomes must be coordinated with the decatenation of intertwined sister DNAs (Onn et al, 2008; Haarhuis et al, 2014; Bizard & Hickson, 2018).
In vertebrate cells, cohesin is removed from chromosomes in two steps during mitosis (Waizenegger et al, 2000). The first step occurs during prophase, which requires the cohesin release protein Wapl as well as the phosphorylation of cohesin subunits and regulators by mitotic kinases (Losada et al, 2002; Sumara et al, 2002; Gimenez‐Abian et al, 2004; Hauf et al, 2005; Gandhi et al, 2006; Kueng et al, 2006; Nishiyama et al, 2013; Tedeschi et al, 2013). This process, referred to as the prophase pathway, removes the bulk of cohesin from chromosome arms in a proteolysis‐independent manner, resulting in sister chromatid resolution and the classical X‐shaped mitotic chromosomes. Centromeric cohesin is resistant to the prophase pathway due to the actions of the shugoshin protein Sgo1 in complex with protein phosphatase 2A (PP2A) (Kitajima et al, 2004, 2006; Salic et al, 2004; McGuinness et al, 2005; Riedel et al, 2006; Tang et al, 2006), Sororin (Rankin et al, 2005; Nishiyama et al, 2010, 2013; Liu et al, 2013b; Ladurner et al, 2016), and the mitotic kinase Haspin (Dai et al, 2006; Goto et al, 2017; Zhou et al, 2017; Liang et al, 2018a; Yi et al, 2018). At anaphase onset, cohesin at centromeres is removed through proteolytic cleavage by the protease separase, leading to the segregation of sister chromatids toward opposite poles (Uhlmann et al, 2000; Hauf et al, 2001).
Disjunction of sister chromatids in mitosis also requires timely resolution of entanglements between sister DNAs (Shintomi & Hirano, 2010). DNA topoisomerase II (TOP2) plays a key role in this process by catalyzing the DNA strand‐passing reaction that allows transit of one double helix of DNA through another (Luo et al, 2009; Chen et al, 2013; Broderick et al, 2015; Nielsen et al, 2015; Guturi et al, 2016). While low eukaryotes have only one isoform of TOP2, vertebrates have two genetically distinct isoforms (α and β) with the topoisomerase IIα (TOP2A) being the major isoform in proliferating cells (Linka et al, 2007; Nitiss, 2009a). In mammalian cells, TOP2A is found on chromosome arms throughout mitosis, but is most enriched at the centromere region from prophase to metaphase (Earnshaw & Heck, 1985; Earnshaw et al, 1985; Taagepera et al, 1993; Rattner et al, 1996; Christensen et al, 2002; Tavormina et al, 2002; Lane et al, 2013). TOP2A at centromeres has attracted particular attention because of its roles in untangling sister chromatids at mitotic centromeres (Spence et al, 2002, 2005; Wang, 2002; Porter & Farr, 2004; Dawlaty et al, 2008; Dykhuizen et al, 2013). However, the chromatin factors that recruit TOP2A to centromeres during mitosis remain unknown.
As a hallmark of mitosis, histone phosphorylation can act as landmarks and countermarks to recruit and displace regulatory proteins, respectively (Fischle et al, 2005; Hirota et al, 2005; Kawashima et al, 2010; Kelly et al, 2010; Varier et al, 2010; Wang et al, 2010a; Yamagishi et al, 2010; Wang & Higgins, 2013; Liu et al, 2015). During mitosis, histone H2A Thr‐120 phosphorylation (H2ApT120, equivalent to Ser‐121 phosphorylation in fission yeast), which is mediated by the kinetochore‐localized mitotic checkpoint (or spindle assembly checkpoint [SAC] kinase Bub1) (Kawashima et al, 2010), occurs at the kinetochore‐proximal centromere region. This modification is essential for the recruitment of Sgo1, as well as the other shugoshin protein Sgo2, to the centromere region (Kawashima et al, 2010; Ricke et al, 2012; Liu et al, 2013a; Lee et al, 2018; Liang et al, 2018b). Sgo1 is initially enriched at the kinetochore‐proximal centromere region by binding via its C‐terminal basic region to Bub1‐mediated H2ApT120. Upon entry into mitosis, centromeric transcription and Cdk1‐dependent phosphorylation of Sgo1 trigger the re‐localization of Sgo1‐PP2A to inner centromeric cohesin. Then, when sister kinetochores achieve proper amphitelic attachment toward metaphase, Sgo1‐PP2A re‐localizes back from cohesin to H2ApT120 by unknown mechanisms (Lee et al, 2008; Liu et al, 2013a,b, 2015; Hara et al, 2014). While Wapl‐dependent removal of cohesin from the arm of sister chromatids facilitates their decatenation (Haarhuis et al, 2013; Tedeschi et al, 2013; Yu, 2013), the presence of cohesin at mitotic centromeres may impede efficient DNA decatenation (Losada & Hirano, 2001; Wang et al, 2010b; Farcas et al, 2011). It remains unclear how sister DNA decatenation, which is hindered by cohesin, is facilitated at mitotic centromeres.
Here, we show that Bub1‐mediated H2ApT120 localizes TOP2A at mitotic centromeres to promote the untangling of sister chromatids as cells transit into anaphase. Our data uncover a key role for mitotic histone phosphorylation in preventing genomic instability.
Results
Kinetochore‐localized Bub1 is required for the centromeric accumulation of TOP2A in mitosis
In screening for the chromatin factors that recruit TOP2A to mitotic centromeres, we identified a critical role for Bub1. Immunofluorescence microscopy of U2OS cells, a human osteosarcoma cell line which was used throughout the study unless otherwise stated, revealed that knockdown of Bub1 by siRNA prevented TOP2A from localizing to mitotic centromeres, whereas the chromosomal localization was barely affected (Figs 1A–E, and EV1A–E). Immunoblotting showed that Bub1 depletion had little effect on total protein level of TOP2A.
Figure 1. Kinetochore‐localized Bub1 is required for the centromeric accumulation of TOP2A in mitosis.

-
A–EU2OS cells were transfected with siRNA, and subjected to immunofluorescence staining with DAPI, anti‐human centromere autoantibody (ACA), and antibodies for Bub1 and TOP2A. Example images are shown (A). The immunofluorescence intensity ratios of centromeric TOP2A/ACA (B), centromeric TOP2A/arm TOP2A (C), and arm TOP2A/DNA (D) were determined on around 600 (B and C) or 400 (D) chromosomes in 20 cells. Lysates of nocodazole‐arrested mitotic cells were immunoblotted with the indicated antibodies (E). ns, no statistical significance.
-
F, GU2OS cells were transfected with siRNA, and subjected to immunofluorescence staining. Example images are shown (F). The immunofluorescence intensity ratio of centromeric TOP2A/arm TOP2A was determined on around 500 chromosomes in 15 cells (G).
-
H–KU2OS cells treated with STLC and MG132 for 0.5 h were subjected to further treatment with reversine, AZ3146, or dimethyl sulfoxide (DMSO, vehicle control) for 1 h in the continued presence of STLC and MG132. Example images of the immunofluorescence staining are shown (H). The immunofluorescence intensity ratios of centromeric Bub1/ACA (I), centromeric TOP2A/ACA (J), and centromeric TOP2A/arm TOP2A (K) were determined on around 300 chromosomes in 15 cells.
-
LU2OS cells stably expressing CB‐GFP or CB‐Bub1‐K‐GFP were treated as in (H). Example images of the immunofluorescence staining are shown.
Figure EV1. Kinetochore‐localized Bub1 is required for the centromeric accumulation of TOP2A in mitosis. Related to Fig 1 .

-
A–CU2OS cells were transfected with siRNA and subjected to immunofluorescence staining with DAPI, ACA, and antibodies for Bub1 and TOP2A. Example images are shown (A). The immunofluorescence intensity ratio of centromeric TOP2A/arm TOP2A was determined on around 500 chromosomes in 15 cells (B). Lysates of nocodazole‐arrested mitotic cells were immunoblotted with the indicated antibodies (C).
-
D, EU2OS cells were transfected and immunostained as in (A). Example images are shown (D). The immunofluorescence intensity ratio of centromeric TOP2A/arm TOP2A was determined on around 300 chromosomes in 10 cells (E).
-
FSchematic description of the domain and motif organization of human Bub1.
-
G, HU2OS cells were transfected with siRNA and subjected to immunofluorescence staining with DAPI, ACA, and antibodies for Bub1 and H2ApT120 (G) or TOP2A (H). Example images of prophase cells are shown.
Bub1 consists of a C‐terminal kinase domain and a N‐terminal region with which Bub1 binds its partner protein Bub3 and localizes to kinetochores (Fig EV1F) (Taylor et al, 1998; Kawashima et al, 2010; London & Biggins, 2014; Vleugel et al, 2015a; Zhang et al, 2015, 2017). The SAC kinase Mps1 phosphorylates multiple Met‐Glu‐Leu‐Thr (MELT) motifs on KNL1, a scaffold component of the KNL1/Mis12 complex/Ndc80 complex network (Cheeseman et al, 2006), creating the direct docking site for Bub3 (Kiyomitsu et al, 2007; London et al, 2012; Shepperd et al, 2012; Yamagishi et al, 2012; Primorac et al, 2013; Vleugel et al, 2015b). We next investigated whether kinetochore localization of Bub1 is important for the recruitment of TOP2A to centromeres. In line with the role of KNL1 and Bub3 in recruiting Bub1, siRNA‐mediated knockdown of KNL1 or Bub3 clearly reduced Bub1 and TOP2A at mitotic centromeres (Figs 1F and G, and EV1G and H). We further examined the localization of TOP2A in cells treated with reversine and AZ3146, two small‐molecule inhibitors of Mps1 kinase (Hewitt et al, 2010; Santaguida et al, 2010). Cells were first arrested in mitosis with monopolar spindles by the mitotic kinesin Eg5 inhibitor S‐trityl‐L‐cysteine (STLC), together with the proteasome inhibitor MG132 to prevent mitotic exit. When these cells were subsequently treated with reversine and AZ3146, there was a strong reduction in the localization of Bub1 and TOP2A at the kinetochore/centromere regions (Fig 1H–K).
We speculated that Mps1 kinase activity promotes centromeric accumulation of TOP2A through localizing Bub1 at kinetochores. To test this possibility, we established cell lines stably expressing the C‐terminus of Bub1 (residues 630–1,085) containing the kinase domain as a fusion protein with the centromere DNA‐binding domain of CENP‐B and GFP (CB‐Bub1‐K‐GFP). We found that inhibiting Mps1 kinase activity by reversine reduced the centromeric accumulation of TOP2A in cells expressing CENP‐B‐fused GFP (CB‐GFP) but not CB‐Bub1‐K‐GFP (Fig 1L). Thus, centromere‐tethered Bub1 kinase domain bypasses the requirement for Mps1 kinase activity in recruiting TOP2A.
Collectively, these results indicate that kinetochore‐localized Bub1 is required for TOP2A accumulation at mitotic centromeres.
Bub1 kinase activity is necessary for TOP2A localization at mitotic centromeres
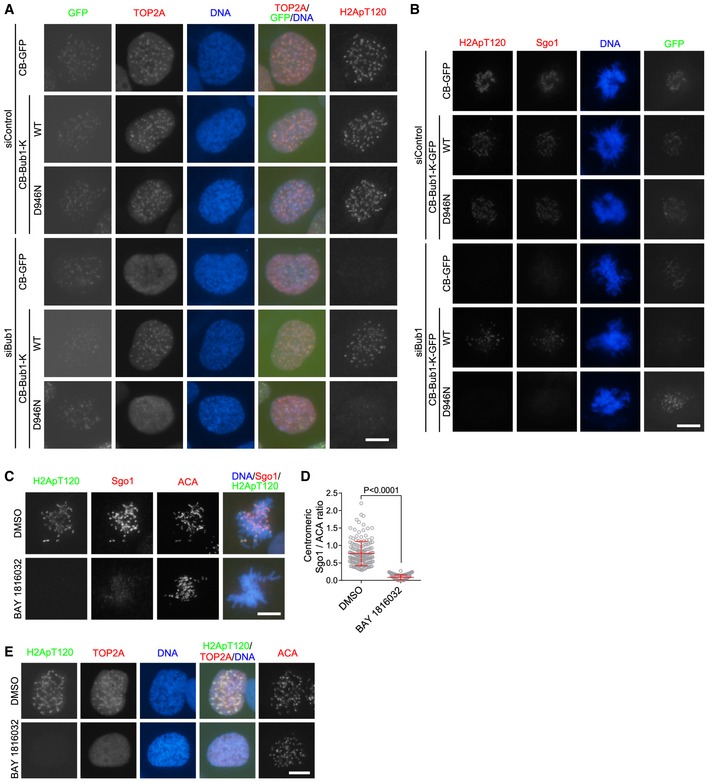
Bub1 executes kinase‐dependent and ‐independent functions during mitosis (London & Biggins, 2014). To test whether the kinase activity of Bub1 is required for TOP2A localization at centromeres, we carried out RNAi rescue assays by depleting endogenous Bub1 in cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP carrying a kinase‐dead Bub1 C‐terminus (Asp‐946 mutated to Asn). As expected, upon depletion of endogenous Bub1 by siRNA targeting the N‐terminal region of Bub1, H2ApT120, and TOP2A were lost at mitotic centromeres in cells expressing CB‐GFP (Figs 2A and B, and EV2A). Importantly, these defects were rescued by expression of CB‐Bub1‐K‐GFP but not CB‐Bub1‐K‐D946N‐GFP. As a positive control, we observed similar results for Sgo1 (Fig EV2B). Thus, an intact kinase domain of Bub1 is important for the centromeric localization of TOP2A.
Figure 2. Bub1 kinase activity is necessary for TOP2A localization at mitotic centromeres.

-
A, BU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were transfected with siRNA, and subjected to immunofluorescence staining with DAPI and the antibodies for H2ApT120 and TOP2A (A). Lysates of nocodazole‐arrested mitotic cells were immunoblotted (B). * represents non‐specific bands.
-
C–FU2OS cells were treated with DMSO or BAY 1816032 at the indicated concentrations for 1 h, and subjected to immunofluorescence staining. Example images are shown (C). The immunofluorescence intensity ratios of centromeric H2ApT120/ACA (D), centromeric TOP2A/ACA (E), and centromeric TOP2A/arm TOP2A (F) were determined on around 450 chromosomes in 15 cells. Means and error bars representing SD are shown (D–F; unpaired t‐test).
Figure EV2. Bub1 kinase activity is necessary for TOP2A localization at mitotic centromeres. Related to Fig 2 .
-
AU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were transfected with siRNA, and subjected to immunofluorescence staining with DAPI and the antibodies for H2ApT120 and TOP2A. Example images of prophase cells are shown.
-
BU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were transfected with siRNA, and subjected to immunofluorescence staining with DAPI and the antibodies for H2ApT120 and Sgo1.
-
C, DU2OS cells were treated with DMSO or 4 μM BAY 1816032 for 1 h, and subjected to immunofluorescence staining. Example images are shown (C). The immunofluorescence intensity ratio of centromeric Sgo1/ACA was determined on around 150 chromosomes in 15 cells (D). Means and error bars representing SD are shown (unpaired t‐test).
-
EU2OS cells were treated with DMSO or 4 μM BAY 1816032 for 1 h, and subjected to immunofluorescence staining. Example images of prophase cells are shown.
We utilized BAY 1816032, a recently developed small‐molecule inhibitor of Bub1 kinase (Siemeister et al, 2018), to corroborate the importance of Bub1 kinase activity for the recruitment of TOP2A to mitotic centromeres. Treatment of cells with BAY 1816032 effectively inhibited Bub1‐mediated H2ApT120 and H2ApT120‐dependent localization of Sgo1 at mitotic centromeres (Fig EV2C and D). Importantly, BAY 1816032 treatment strongly reduced the centromeric accumulation of TOP2A in mitosis (Figs 2C–F, and EV2E).
We conclude from these results that kinetochore‐localized Bub1 acts through its kinase activity to position TOP2A at centromeres during mitosis.
Bub1‐mediated H2ApT120 is sufficient to recruit TOP2A to centromeres and to a non‐centromeric chromatin region
So far, only a few substrates of Bub1 have been identified, including the anaphase‐promoting complex/cyclosome (APC/C) co‐activator Cdc20 (Tang et al, 2004; Jia et al, 2016), histone H2A (Kawashima et al, 2010), and the telomere protein TRF1 (Li et al, 2018). Among them, histone H2A is the best characterized substrate in vivo. We found that the localization of TOP2A at mitotic centromeres was barely affected by siRNA‐mediated knockdown of Cdc20 and TRF1 (Fig EV3A–D), suggesting that they are not involved in the centromeric localization of TOP2A.
Figure EV3. Bub1‐mediated H2ApT120 is sufficient to recruit TOP2A to centromeres and to a non‐centromeric chromatin region. Related to Fig 3 .

-
A–DU2OS cells were transfected with siRNA, and subjected to immunofluorescence staining with DAPI, ACA, and antibodies to TOP2A (A) or Cdc20 (B). Example images of prometaphase cells are shown. Lysates of nocodazole‐arrested mitotic cells were immunoblotted with antibodies for GAPDH and Cdc20 (C) or TRF1 (D).
-
EU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were subjected to immunofluorescence staining. Example images of anaphase cells are shown.
-
F, GU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were subjected to immunofluorescence staining with DAPI, and antibodies for TOP2A and H2ApT120 (F) or INCENP (G). Example images of late‐telophase/early‐G1 cells, as indicated by INCENP staining at the mid‐body, are shown.
-
HA 200‐copy transgene array was integrated into a euchromatic region of chromosome 1p36 in human U2OS cells. The plasmid is composed of 256 copies of the Lac Operator, 96 tetracycline response elements (TRE), a minimal CMV promoter, and the cyan fluorescent protein gene. Expression of EGFP‐LacI fusion proteins allows the DNA to be visualized.
-
IU2OS‐LacO cells transiently expressing EGFP‐LacI or EGFP‐LacI‐Bub1‐K (WT or D946N) were subjected to immunofluorescence staining with DAPI and antibodies for H2ApT120 and Sgo1. Arrows point to the transgene loci.
Immunofluorescence microscopy showed that H2ApT120 co‐localized with TOP2A at the kinetochore‐proximal centromere region of the duplicated chromosomes from prophase till metaphase (Fig 3A). We found that the H2ApT120 signal diminished in anaphase cells, which is a consequence of Bub1 degradation (Qi & Yu, 2007), and that TOP2A was evenly distributed over the whole chromosome. The similarity in the pattern of H2ApT120 and TOP2A accumulation at centromeres prior to anaphase onset is consistent with the requirement of H2ApT120 for TOP2A enrichment at mitotic centromeres, but also raises an intriguing question of whether H2ApT120 is sufficient to recruit TOP2A.
Figure 3. Bub1‐mediated H2ApT120 is sufficient to recruit TOP2A to centromeres and to a non‐centromeric chromatin region.

-
AU2OS cells were subjected to immunofluorescence staining. Example images of mitotic cells at various stages are shown.
-
BU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were subjected to immunofluorescence staining. Example images of anaphase cells are shown.
-
CU2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were subjected to immunofluorescence staining with DAPI, and antibodies for TOP2A and H2ApT120 or H3pS10. Example images of interphase cells are shown. The cells in the top line, which is H3pS10‐positive, are in early prophase/late G2.
-
DU2OS‐LacO cells transiently expressing EGFP‐LacI and EGFP‐LacI‐Bub1‐K (WT or D946N) were subjected to immunofluorescence staining. Arrows point to the transgene loci.
-
E, FMBP‐TOP2A (429–1,531)‐10xHis was subjected to pulldown assay by GST, GST‐H2A, or GST‐H2ApS120, followed by immunoblotting with antibodies for MBP, GST, or H2ApT120 (E). The relative pulldown efficiency was determined (F). Means and error bars representing SD from three independent experiments are shown (unpaired t‐test).
Interestingly, the H2ApT120 signal was largely retained at anaphase centromeres in cells expressing CB‐Bub1‐K‐GFP but not CB‐Bub1‐K‐D946N‐GFP or CB‐GFP (Fig 3B). Importantly, retention of H2ApT120 was accompanied by the enrichment of TOP2A at anaphase centromeres. As the positive control, Sgo1 was also retained at anaphase centromeres in cells expressing CB‐Bub1‐K‐GFP but not CB‐Bub1‐K‐D946N‐GFP or CB‐GFP (Fig EV3E). Similarly, centromere‐tethered Bub1‐K‐GFP but not Bub1‐K‐D946N‐GFP generated H2ApT120 and recruited TOP2A in late‐telophase/early G1 cells (Fig EV3F and G), as well as in non‐late G2 interphase cells (Fig 3C). Thus, the kinase activity of centromere‐tethered Bub1 is sufficient to generate H2ApT120 and recruit TOP2A throughout the cell cycle.
VRK1 (Aihara et al, 2016) and VprBP (Kim et al, 2013) have been implicated in generating H2ApT120 outside mitosis. Surprisingly, when tethered to centromeres as a fusion protein with CENP‐B, neither VRK1 nor VprBP was able to generate H2ApT120 in U2OS and HeLa cells (Appendix Fig S1). Thus, it appears that VRK1 and VprBP are not involved in generating H2ApT120 at least in these two cell lines, which is in line with our observation that H2ApT120 was barely detectable at mitotic centromeres upon Bub1 inhibition.
We next tested whether TOP2A can be recruited by Bub1 kinase to a non‐centromere chromatin region. For this purpose, we used a U2OS‐Lac operator (LacO) cell line in which multiple copies of LacO repeats were stably integrated in a euchromatic region of chromosome 1p36 in U2OS cells (Janicki et al, 2004) (Fig EV3H). As previously showed (Asghar et al, 2015), H2ApT120 was generated at the LacO transgene array targeted by Lac repressor (LacI)‐fused Bub1 kinase domain (EGFP‐LacI‐Bub1‐K), but not by the kinase‐dead mutant EGFP‐LacI‐Bub1‐K‐D946N (Fig 3D). Importantly, endogenous TOP2A was recruited to over 70% of the H2ApT120‐positive transgene array targeted by EGFP‐LacI‐Bub1‐K. By contrast, TOP2A was not recruited to the transgene array targeted by EGFP‐LacI‐Bub1‐K‐D946N. Similar results were observed for Sgo1 (Fig EV3I). Thus, presumably through generating H2ApT120, Bub1 kinase can recruit TOP2A to the LacO transgene array at a non‐centromeric chromatin region.
We further tested whether phosphorylation at Thr‐120 can promote histone H2A binding to TOP2A in vitro. We could not reliably detect the Thr‐120 phosphorylation‐dependent binding of histone H2A peptide (residues 108–127) to MBP‐TOP2A (429–1,531)‐10xHis, a recombinant MBP‐ and 10xHis‐fused human TOP2A protein encompassing residues 429–1,531. We then sought to use phosphorylated and non‐phosphorylated forms of full‐length H2A which were N‐terminally fused to GST (GST‐H2A). Taking advantage of the expanded genetic code of Escherichia coli with phosphoserine (Park et al, 2011; Pirman et al, 2015), as well as the fact that fission yeast H2ApS121 promotes the association of shugoshin proteins with chromatin (Kawashima et al, 2010), we purified a GST‐H2A mutant in which Thr‐120 was replaced by a phosphorylated serine (GST‐H2ApS120). In the GST pulldown assay, we found that GST‐H2ApS120 bound MBP‐TOP2A (429–1,531)‐10xHis more efficiently than GST‐H2A did (Fig 3E and F). Thus, phosphorylation at the residue‐120 can enhance the binding of histone H2A to TOP2A in vitro.
Taken together, these results strongly suggest that Bub1‐mediated H2ApT120 is responsible for the recruitment of TOP2A to mitotic centromeres.
Sgo1 and Sgo2 are not required for the enrichment of TOP2A at mitotic centromeres
Bub1‐mediated H2ApT120 directly binds to Sgo1 and Sgo2 via their C‐terminal basic regions, thereby recruiting them to centromeres during mitosis (Kawashima et al, 2010; Liu et al, 2013a, 2015). To test the possibility that H2ApT120 recruits TOP2A through Sgo1 and/or Sgo2, we examined the localization of TOP2A in cells lacking centromere‐localized Sgo1 and Sgo2.
Mutation of a conserved basic residue Lys‐492 to Ala (K492A) in the C‐terminal basic region renders Sgo1 largely impaired in binding H2ApT120 (Tang et al, 2006; Liu et al, 2013a, 2015). We recently developed a HeLa‐derived cell line in which the K492A mutation was introduced into endogenous Sgo1 by CRISPR/Cas9‐mediated genome editing (Liang et al, 2018b), which prevented Sgo1 from localizing to centromeres (Fig 4A, and Appendix Fig S2A). In accordance with the role of Sgo1 and Sgo2 in promoting the localization of the chromosomal passenger complex (CPC) at mitotic centromeres (Tsukahara et al, 2010), centromeric localization of the INCENP subunit of CPC was reduced in the absence of centromeric Sgo1 (Fig 4A and B, and Appendix Fig S2A and B). By contrast, compared to that in control HeLa cells, centromeric accumulation of TOP2A was not reduced in the Sgo1‐K492A mutant cells (Fig 4C and D). Thus, removal of centromeric Sgo1 does not compromise the localization of TOP2A at mitotic centromeres.
Figure 4. Sgo1 and Sgo2 are not required for the enrichment of TOP2A at mitotic centromeres.

-
A, BHeLa cells and the Sgo1‐K492A mutant cells were immunostained with the indicated antibodies. Example images are shown (A). The immunofluorescence intensity ratio of centromeric INCENP/ACA was determined on around 250 chromosomes in 10 cells (B).
-
C, DHeLa and the Sgo1‐K492A cells were exposed to nocodazole for 4 h. Mitotic cells were cytospun onto coverslips and fixed for immunostaining. Example images are shown (C). The immunofluorescence intensity ratio of centromeric TOP2A/arm TOP2A was determined on around 150 chromosomes in 10 cells (D).
-
E, FHeLa and the Sgo1‐K492A cells were transfected with siRNA, and immunostained with the indicated antibodies. Example images are shown (E). The immunofluorescence intensity ratio of centromeric Aurora B/ACA was determined on around 250 chromosomes in 10 cells (F).
-
G, HHeLa and the Sgo1‐K492A cells were transfected with siRNA, and treated with nocodazole for 5 h. Mitotic cells were cytospun onto coverslips and fixed for immunostaining. Example images are shown (G). The immunofluorescence intensity ratio of centromeric TOP2A/arm TOP2A was determined on around 280 chromosomes in 10 cells (H).
To test whether Sgo2 contributes to the centromeric accumulation of TOP2A when Sgo1 is not localized at centromeres, we further depleted Sgo2 by siRNA in cells expressing the Sgo1‐K492A mutant. As expected, loss of Sgo2 in Sgo1‐K492A cells caused further reduction in the centromeric localization of the Aurora B subunit of CPC (Fig 4E and F, and Appendix Fig S2C and D). However, we did not observe reduction in the centromeric accumulation of TOP2A upon depletion of Sgo2 (Fig 4G and H).
Taken together, these results indicate that Sgo1 and Sgo2 are not required for the centromeric localization of TOP2A, further supporting a role for H2ApT120 in directly recruiting TOP2A to mitotic centromeres.
The C‐gate formed by the coiled‐coil domain is important for TOP2A localization at mitotic centromeres
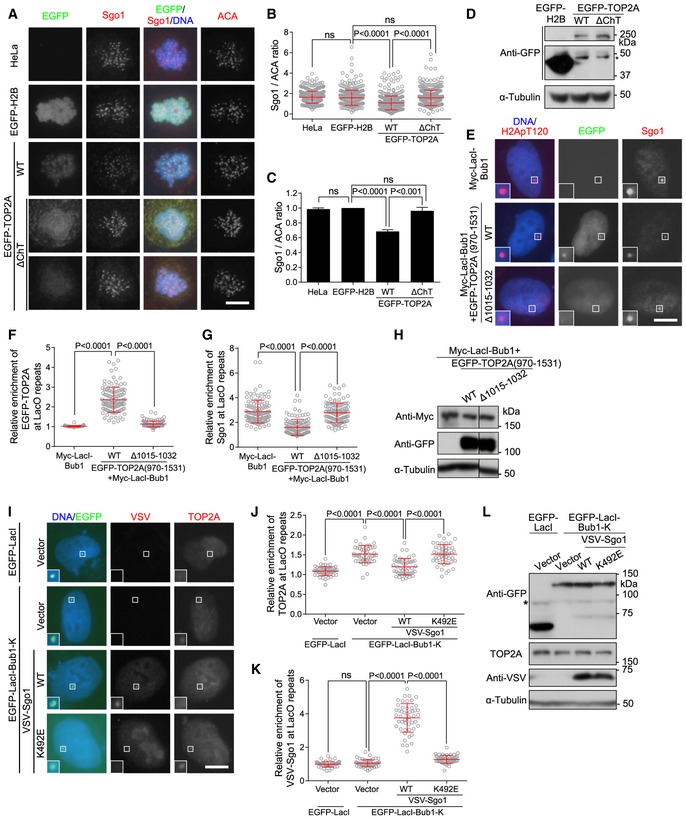
Human TOP2A is formed through dimerization of two monomers consisting of the N‐terminal ATPase gate (N‐gate), the DNA‐binding gate (DNA‐gate), and the C‐gate composed of the coiled‐coil domain (Fig 5A) (Wendorff et al, 2012; Chen et al, 2013). Outside this catalytic core, TOP2A possesses a less conserved and largely disordered C‐terminal domain (CTD) encompassing residues 1,173–1,531. Using a series of truncated mutants, we first examined which domain/region is required for the centromeric localization of EGFP‐fused TOP2A (EGFP‐TOP2A). When expressed in HeLa cells, EGFP‐TOP2A and EGFP‐TOP2A (970–1,531), but not EGFP‐TOP2A (1,200–1,531), localized similarly to mitotic centromeres (Fig EV4A and B). To corroborate this observation, we examined the localization of EGFP‐TOP2A variants in HeLa cells in which endogenous TOP2A was depleted by siRNA. We found that EGFP‐TOP2A (1–1,200) did not localize to mitotic chromosomes (Fig 5B), indicating the necessity of C‐terminal region for the chromosomal localization of TOP2A. Moreover, EGFP‐TOP2A (1,200–1,531) (Fig 5B) and EGFP‐TOP2A‐CTD (1,173–1,531) (Fig EV4C) localized to mitotic chromosomes but not to the centromere. In contrast, EGFP‐TOP2A (970–1,531) localized normally to mitotic centromeres (Fig 5C). These results indicate that residues 970–1,200 are required for the centromeric localization of TOP2A. Indeed, the EGFP‐TOP2A‐Δ (970–1,200) mutant lacking residues 970–1,200 retained some capability of associating with chromosomes, but was incapable of localizing at centromeres. On the contrary, the EGFP‐TOP2A‐Δ (1,212–1,446) mutant lacking residues 1,212–1,446 localized to mitotic centromeres normally (Fig EV4D–F).
Figure 5. The C‐gate formed by the coiled‐coil domain is important for TOP2A localization at mitotic centromeres.

-
ASchematic description of the domain organization of human TOP2A.
-
BHeLa cells were transfected with siRNA and the plasmids encoding the indicated proteins, and then exposed to nocodazole for 5 h. Mitotic cells were cytospun onto coverslips and subjected to immunofluorescence staining with DAPI, ACA, and anti‐GFP antibodies. Example images are shown.
-
C–FHeLa cells were transfected and treated as in (B). Example images are shown (C). The immunofluorescence intensity ratios of centromeric EGFP‐TOP2A/ACA (D), centromeric EGFP‐TOP2A/arm EGFP‐TOP2A (E), and arm EGFP‐TOP2A/DNA (F) were determined on around 160 chromosomes in 8 cells.
-
G, HHeLa cells transiently expressing EGFP‐TOP2A (970–1,531) or EGFP‐TOP2A (970–1,531)‐Δ(1,015–1,032) were treated and immunostained as in (B). Example images are shown (G). The immunofluorescence intensity ratio of centromeric EGFP‐TOP2A/arm EGFP‐TOP2A was determined on around 250 chromosomes in 10 cells (H).
-
I–KU2OS‐LacO cells transiently expressing Myc‐LacI‐Bub1 and EGFP‐TOP2A (970–1,531) were subjected to immunofluorescence staining with DAPI and antibodies for the Myc‐tag and GFP. Example images are shown (I). The relative enrichment of EGFP‐TOP2A at the LacO repeats was quantified in 130 cells (J). Cell lysates were immunoblotted (K).
Figure EV4. The C‐gate formed by the coiled‐coil domain is important for TOP2A localization at mitotic centromeres. Related to Fig 5 .

-
A, BHeLa cells transiently expressing EGFP‐TOP2A were exposed to nocodazole for 5 h. Mitotic cells were cytospun onto coverslips and subjected to immunofluorescence staining with DAPI, ACA, and anti‐GFP antibodies. Example images are shown (A). Lysates of asynchronous cells were immunoblotted (B).
-
CHeLa cells were transfected with siRNA and the plasmids encoding EGFP‐TOP2A (WT or CTD), and then treated and immunostained as in (A). Example images are shown.
-
D–FHeLa cells were transfected with siRNA and the plasmids encoding EGFP‐TOP2A (WT or Δ1212‐1446), and then treated and immunostained as in (A). Example images are shown (D). The immunofluorescence intensity ratios of centromeric EGFP‐TOP2A/ACA (E) and centromeric EGFP‐TOP2A/arm EGFP‐TOP2A (F) were determined on around 160 chromosomes in 8 cells.
-
G–IHeLa cells were transfected with siRNA and plasmids encoding EGFP‐TOP2A (WT or Δ1,015–1,032). Cells were either arrested in mitosis and immunostained as in (A), or were left untreated and fixed for immunostaining. Example images of mitotic (G) and interphase cells (H) are shown. Lysates of asynchronous cells were immunoblotted (I).
-
J, KHeLa cells were transfected with siRNA and plasmids encoding the indicated EGFP‐TOP2A proteins, and then treated and immunostained as in (A). Example images are shown (J). Lysates of asynchronous cells were immunoblotted (K).
We further found that EGFP‐TOP2A (970–1,200) containing the C‐gate did not associate with mitotic chromosomes (Fig 5C), nor did EGFP‐TOP2A (1,447–1,531) composed of residues 1,447–1,531 containing the main nuclear localization signal (NLS). Strikingly, the chimeric protein EGFP‐TOP2A (970–1,200)+(1,447–1,531), in which the 970–1,200 fragment of TOP2A was C‐terminally fused to residues 1,447–1,531, strongly localized to mitotic centromeres, whereas its chromosomal localization was not evidently detectable (Fig 5C–F). This result is consistent with our observation that residues 1,212–1,446 is not required for the centromeric localization of TOP2A.
Amino acids 1,013–1,056 in human TOP2A have previously been shown to form a stable two‐stranded α‐helical coiled‐coil that is important for dimerization (Frere et al, 1995). Two additional regions spanning amino acids 1,053–1,069 and 1,124–1,143 were also reported to be essential for the dimerization of human TOP2A (Bjergbaek et al, 1999). Due to unknown reasons, EGFP‐TOP2A‐Δ(1,015–1,032), a mutant in which residues 1,015–1,032 were internally deleted in full‐length TOP2A, was defective in mitotic chromosome association and in interphase nuclear localization (Fig EV4G–I). Interestingly, deleting this fragment did not affect the chromosomal localization of EGFP‐TOP2A (970–1,531) but prevented it from localizing to mitotic centromeres (Figs 5G and H, and EV4J and K). We further found in U2OS‐LacO cells that targeting Myc‐tagged LacI‐Bub1 (Myc‐LacI‐Bub1) to the LacO transgene array generated H2ApT120 and recruited EGFP‐TOP2A (970–1,531) but not EGFP‐TOP2A (970–1,531)‐Δ(1,015–1,032) lacking residues 1,015–1,032 (Fig 5I–K).
These results together demonstrate that C‐gate formed by the coiled–coil domain is important for the H2ApT120‐dependent localization of TOP2A at mitotic centromeres.
The extreme C‐terminal region contributes to H2ApT120‐dependent localization of TOP2A at centromeres
The data in Fig 5C–F also indicate the contribution of residues 1,447–1,531 to TOP2A localization at mitotic centromeres. A previous study reported that the extreme C‐terminal 31 residues (1,501–1,531) of TOP2A form a so‐called “chromatin tether domain (ChT domain)” that promotes the interaction of TOP2A with chromosomes during mitosis (Lane et al, 2013). We found that, in contrast to EGFP‐TOP2A (970–1,200)+(1,447–1,531), the chimeric protein EGFP‐TOP2A (970–1,200)+(1,447–1,500) lacking the ChT domain did not localize to centromeres (Fig 6A), indicating a critical requirement for the ChT domain. We then assessed the importance of ChT domain for the localization of EGFP‐TOP2A (970–1,200)+(1,447–1,531) at the LacO transgene array in U2OS‐LacO cells. We found that EGFP‐TOP2A (970–1,200)+(1,447–1,531), but not EGFP‐TOP2A (970–1,200)+(1,447–1,500), was efficiently recruited to the LacO repeats by H2ApT120 generated by Myc‐LacI‐Bub1 (Fig 6B–D).
Figure 6. The extreme C‐terminal region contributes to H2ApT120‐dependent localization of TOP2A at centromeres.

-
AHeLa cells were transfected with siRNA and plasmids encoding the indicated proteins, and then exposed to nocodazole for 3 h. Mitotic cells were cytospun onto coverslips and subjected to immunofluorescence staining with DAPI, ACA, and anti‐GFP antibodies. Example images are shown.
-
B–DU2OS‐LacO cells transiently expressing Myc‐LacI‐Bub1 and the indicated EGFP‐TOP2A proteins were subjected to immunofluorescence staining with DAPI and antibodies for the Myc‐tag and H2ApT120. Example images are shown (B). The relative enrichment of EGFP signal at the LacO repeats was quantified in 30 cells (C). Cell lysates were immunoblotted (D). Arrows point to the transgene loci.
-
E, FHeLa cells transfected with siRNA and plasmids encoding the indicated proteins were treated and immunostained as in (A). Example images are shown (E). The immunofluorescence intensity ratio of centromeric EGFP‐TOP2A/arm EGFP‐TOP2A was determined on around 300 chromosomes in 15 cells (F).
-
G, HHeLa cells transfected with siRNA and plasmids encoding the indicated proteins were treated and immunostained as in (A). Example images are shown (G). The immunofluorescence intensity ratio of centromeric TOP2A‐Myc‐6xHis/arm TOP2A‐Myc‐6xHis was determined on around 200 chromosomes in 10 cells (H).
-
I–KU2OS cells stably expressing the indicated CENP‐B fusion proteins were transfected with the plasmid encoding TOP2A‐Myc‐6xHis or TOP2A‐ΔChT‐Myc‐6xHis, and then subjected to immunofluorescence staining with DAPI and antibodies for the Myc‐tag and H2ApT120. Example images are shown (I). The relative enrichment of anti‐Myc staining signal at the centromere region versus that in the nearby nuclear region was quantified on around 200 chromosomes in 10 cells (J). Cell lysates were immunoblotted (K).
-
LMBP‐TOP2A (429–1,531)‐10xHis and MBP‐TOP2A (429–1,531)‐ΔChT‐10xHis were subjected to pulldown assay by GST, GST‐H2A, or GST‐H2ApS120, followed by immunoblotting with antibodies for MBP or GST.
We also examined the localization of full‐length EGFP‐TOP2A with or without the ChT domain in cells fixed by paraformaldehyde (PFA). While both wild‐type (WT) EGFP‐TOP2A and the EGFP‐TOP2A‐ΔChT mutant lacking residues 1,501–1,531 associated with mitotic chromosomes, the latter was largely impaired in localizing to centromeres in the absence of endogenous TOP2A (Fig 6E and F). Similar results were obtained with the ΔChT mutant of TOP2A tagged at the C‐terminus with the Myc and 6xHis epitopes (TOP2A‐Myc‐6xHis) (Fig 6G and H). Using cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP, we also determined the localization of WT or the ΔChT mutant of TOP2A‐Myc‐6xHis. We found that, compared to TOP2A‐Myc‐6xHis, the TOP2A‐ΔChT‐Myc‐6xHis mutant was much less efficiently recruited to H2ApT120‐positive centromeres in non‐late G2 interphase cells expressing CB‐Bub1‐K‐GFP (Fig 6I–K). These results corroborate the involvement of the ChT domain in TOP2A localization at centromeres.
Comparison of the amino acid sequences of TOP2A and TOP2B indicate that their C‐terminal regions are highly divergent, and that TOP2B does not possess the ChT domain. As previously reported (Linka et al, 2007), TOP2B neither associated with chromosomes nor localized to centromeres during mitosis (Appendix Fig S3A–C). We also found that TOP2B was not recruited by H2ApT120 generated by Bub1 kinase which was tethered to centromeres or the LacO transgene array (Appendix Fig S3D and E). These observations are consistent with the contribution of the ChT domain to H2ApT120‐dependent localization of TOP2A at mitotic centromeres.
We further found in the GST pulldown assay that GST‐H2ApS120 bound MBP‐TOP2A (429–1,531)‐10xHis and MBP‐TOP2A (429–1,531)‐ΔChT‐10xHis with comparable efficiency (Fig 6L). Thus, the extreme C‐terminal 31 residues of TOP2A are not directly involved in the recognition of H2ApS120 in vitro.
Taken together, these results demonstrate the contribution of the extreme C‐terminal region to H2ApT120‐dependent localization of TOP2A at mitotic centromeres.
The H2ApT120‐dependent intracellular localization of TOP2A and Sgo1 is competitive
Since both TOP2A and Sgo1 can bind to H2ApS/T120, we were prompted to investigate whether their recruitment to centromeres are competitive. We first examined the effect of TOP2A overexpression on the localization of Sgo1 at mitotic centromeres. As expected, expression of H2B‐GFP in HeLa cells did not affect centromeric localization of Sgo1 (Fig 7A–D). Interestingly, the level of centromeric Sgo1 in cells expressing EGFP‐TOP2A was 32% lower than that in cells expressing H2B‐GFP, indicating that overexpressed EGFP‐TOP2A can reduce Sgo1 localization at mitotic centromeres. In contrast, overexpression of EGFP‐TOP2A‐ΔChT defective in centromeric localization had little effect on Sgo1 localization at mitotic centromeres. Similarly, centromeric localization of Sgo1 in HeLa cells expressing EGFP‐TOP2A (970–1,531), but not EGFP‐TOP2A (970–1,531)‐Δ(1,015–1,032), was 17% lower than that in cells expressing CB‐GFP (Fig EV5A–D). Thus, the capability of EGFP‐TOP2A in displacing Sgo1 from mitotic centromeres relies on its capacity of localizing at centromeres.
Figure 7. The H2ApT120‐dependent intracellular localization of TOP2A and Sgo1 is competitive.
-
A–DHeLa cells with or without transient expression of H2B‐GFP or EGFP‐TOP2A were exposed to nocodazole for 3 h. Mitotic cells were cytospun onto coverslips and subjected to immunofluorescence staining with DAPI and antibodies for GFP, Sgo1, and ACA. Example images are shown (A). The immunofluorescence intensity ratio of centromeric Sgo1/ACA was determined on 400 chromosomes in 20 cells (B). The means and SDs from three independent experiments with the Sgo1/ACA ratio determined on around total 1,200 chromosomes in 60 cells are shown (C). Lysates of asynchronous cells were immunoblotted (D). * represents non‐specific bands.
-
E–HU2OS‐LacO cells transiently expressing Myc‐LacI‐Bub1, with or without EGFP‐TOP2A (970–1,531), were subjected to immunofluorescence staining with DAPI and antibodies for H2ApT120 and Sgo1. Example images are shown (E). The relative enrichment of EGFP‐TOP2A (F) and Sgo1 (G) at the LacO repeats was quantified in 120 cells. Cell lysates were immunoblotted (H).
-
I–LU2OS‐LacO cells transiently expressing EGFP‐LacI‐Bub1‐K together with VSV‐Sgo1 or a control vector were subjected to immunofluorescence staining with DAPI and antibodies for TOP2A and VSV‐tag. Example images are shown (I). The relative enrichment of TOP2A (J) and VSV‐Sgo1 (K) at the LacO repeats was quantified in 50 cells. Cell lysates were immunoblotted (L). * represents non‐specific bands.
Figure EV5. The H2ApT120‐dependent intracellular localization of TOP2A and Sgo1 is competitive. Related to Fig 7 .

-
A–DHeLa cells transiently expressing CB‐GFP or EGFP‐TOP2A (970–1,531) were exposed to nocodazole for 3 h. Mitotic cells were cytospun onto coverslips and subjected to immunofluorescence staining with DAPI and antibodies for GFP, Sgo1, and CENP‐C. Example images are shown (A). The immunofluorescence intensity ratio of centromeric Sgo1/CENP‐C was determined on around 250 chromosomes in 10 cells (B). The means and SDs on around total 800 chromosomes in 30 cells from three independent experiments with the Sgo1/CENP‐C ratio determined are shown (C). Lysates of asynchronous cells were immunoblotted (D). * represents non‐specific bands.
-
E–HU2OS‐LacO cells transiently expressing EGFP‐LacI or EGFP‐LacI‐Bub1‐K together with VSV‐Sgo1 or a control vector were subjected to immunofluorescence staining with DAPI and antibodies for TOP2A and the VSV‐tag. Example images are shown (E). The relative enrichment of TOP2A (F) and VSV‐Sgo1 (G) at the LacO repeats was quantified in 50 cells. Cell lysates were immunoblotted (H). * represents non‐specific bands.
We further used U2OS‐LacO cells expressing Myc‐LacI‐Bub1 to examine whether the localization of TOP2A and Sgo1 to LacI‐Bub1‐generated H2ApT120 is competitive. We found that overexpression of EGFP‐TOP2A (970–1,531) effectively impeded the recruitment of endogenous Sgo1 to the H2ApT120‐positive LacO transgene array targeted by Myc‐LacI‐Bub1 (Fig 7E–H). By contrast, EGFP‐TOP2A (970–1,531)‐Δ(1,015–1,032) was largely impaired in doing so. Conversely, recruitment of endogenous TOP2A to the LacO transgene array targeted by EGFP‐LacI‐Bub1‐K was obviously compromised by overexpression of VSV‐tagged Sgo1 (VSV‐Sgo1) but not the H2ApT120‐binding‐deficient mutants VSV‐Sgo1‐K492E (mutation of Lys‐492 to Glu; Fig 7I–L) and VSV‐Sgo1‐K492A (Fig EV5E–H). Thus, the recruitment of TOP2A and Sgo1 to the LacO repeats by LacI‐Bub1‐generated H2ApT120 is competitive.
Therefore, the H2ApT120‐dependent intracellular localization of TOP2A and Sgo1 is competitive, which is in line with the fact that they can both bind H2ApS/T120.
Bub1 kinase promotes the timely decatenation of sister chromatids independent of Sgo1 and Sgo2
We then set out to understand the functional significance of centromeric accumulation of TOP2A during mitosis. Timely disjunction of sister chromatids is critical for faithful chromosome segregation. Defects in this process can lead to the persistence of DNA threads known as ultra‐fine bridges (UFBs) between separating sister chromatids during anaphase (Biebricher et al, 2013; Liu et al, 2014; Sarlos et al, 2018). UFBs are devoid of histones and usually cannot be stained with conventional DNA dyes, but can be visualized by detection of associated proteins such as the SNF2 family DNA translocase PICH (Baumann et al, 2007) and the Bloom syndrome helicase BLM (Chan et al, 2007; Ke et al, 2011). Inhibition of TOP2A increases the incidence of anaphases with UFBs, which frequently connect separated centromere pairs (Spence et al, 2007; Wang et al, 2008).
We found that the rate of anaphase cells with UFBs, which were PICH‐positive, increased from 9.3% in control HeLa cells to 19.3% in cells in which Bub1 was depleted by siRNA (Fig 8A–C). Comparably, treatment of asynchronously growing HeLa cells with the Bub1 inhibitor BAY 1816032 caused a 1.9‐fold increase in the percentage of UFBs‐positive anaphase cells (Fig 8D and E, Appendix Fig S4). We observed similar results when HeLa cells were first arrested in a prometaphase‐like state by the spindle microtubule destabilizer nocodazole and then released into anaphase in the presence of BAY 1816032 (Fig 8F and G). Thus, inhibiting Bub1 kinase activity increases the frequency of anaphase UFBs, which is indicative of impaired decatenation of sister chromatids.
Figure 8. Bub1 kinase promotes the timely decatenation of sister chromatids independent of Sgo1 and Sgo2.

-
A–CHeLa cells were transfected with siRNA and subjected to immunofluorescence staining with DAPI, ACA, and the anti‐PICH antibody. Example images of anaphase cells are shown (A). The percentage of PICH‐positive cells was determined in at least 339 anaphase cells in each condition. Means and SDs from three independent experiments are shown (B). Lysates of asynchronously growing cells were immunoblotted (C). Arrows point to the UFBs.
-
D, EHeLa cells were released from single thymidine block, and after 7 h, BAY 1816032 or DMSO was added for 5 h. Cells were then fixed and subjected to immunofluorescence staining as in (A). Example images of anaphase cells are shown (D). The percentage of anaphases with PICH was determined (E). Arrows point to the UFBs.
-
F, GHeLa cells were arrested in a prometaphase‐like state with nocodazole treatment for 5 h, and then BAY 1816032 or DMSO was added for 1 h in the continued presence of nocodazole. Then, nocodazole was removed by washing into fresh medium containing BAY 1816032 or DMSO. After 1 h, cells were fixed and subjected to immunofluorescence staining. Example images are shown (F). The percentage of anaphases with PICH was determined (G). Arrows point to the UFBs.
-
H–KHeLa and the Sgo1‐K492A cells transfected with the indicated siRNA were synchronized and treated with BAY 1816032 or DMSO as in (D), and then immunostained with the indicated antibodies. Example images of anaphase cells are shown (H). The percentage of anaphases with PICH was determined in at least 256 cells in each condition. Means and SDs from three independent experiments are shown (I). Example images of prometaphase cells immunostained with the indicated antibodies are shown (J). Lysates of nocodazole‐arrested mitotic cells were immunoblotted (K). Arrows point to the UFBs.
The established role of Bub1‐mediated H2ApT120 in recruiting Sgo1 and Sgo2 to mitotic centromeres raised the questions of whether loss of centromeric Sgo1 and Sgo2 may increase UFBs. We found comparable frequency of anaphase UFBs in control HeLa cells and in the Sgo1‐K492A mutant cells (Fig 8H–K). Further depletion of Sgo2 by siRNA in Sgo1‐K492A mutant cells did not increase the rate of anaphase UFBs. Moreover, treatment with BAY 1816032 increased anaphase UFBs to a similar extent in HeLa cells, Sgo1‐K492A cells, and Sgo1‐K492A cells depleted of Sgo2.
Collectively, these results indicate that Bub1 kinase limits the occurrence of anaphase UFBs independently of localizing Sgo1 and Sgo2 to mitotic centromeres.
Centromeric localization of TOP2A facilitates the timely decatenation of sister chromatids
To determine whether Bub1 kinase prevents UFBs through localizing TOP2A to centromeres, we tethered TOP2A to centromeres as a CENP‐B and GFP fusion protein (CB‐TOP2A‐GFP) (Fig 9A). As expected, siRNA‐mediated knockdown of Bub1 in HeLa cells expressing CB‐GFP increased the rate of UFBs by 1.8‐fold (Fig 9B–D). In contrast, Bub1 depletion did not increase UFBs in cells expressing CB‐TOP2A‐GFP. Moreover, treatment with BAY 1816032 significantly increased UFBs in control HeLa cells and CB‐GFP‐expressing cells, but not in cells expressing CB‐TOP2A‐GFP (Fig 9E and F). Similar results were obtained when these cells were released from nocodazole into BAY 1816032 (Appendix Fig S5). Thus, centromere‐tethered TOP2A can bypass the requirement for Bub1 kinase in preventing UFBs.
Figure 9. Centromeric localization of TOP2A facilitates the timely decatenation of sister chromatids.

-
AHeLa cells were transfected with plasmids encoding CB‐GFP or CB‐TOP2A‐GFP. Lysates of asynchronously growing cells were immunoblotted with the indicated antibodies.
-
B–DHeLa cells transfected with siRNA and plasmids encoding the indicated proteins were released from single thymidine block. At 12 h post‐release, cells were fixed and subjected to immunofluorescence staining with DAPI and the antibodies for PICH, GFP and CENP‐C. Example images of anaphase cells are shown (B). The percentage of anaphases with PICH was determined in at least 152 cells in each condition. Means and SDs from three independent experiments are shown (C). Lysates of nocodazole‐arrested mitotic cells were immunoblotted (D). * represents non‐specific bands. Arrows point to the UFBs.
-
E, FHeLa cells transiently expressing CB‐GFP or CB‐TOP2A‐GFP were released from single thymidine block, and after 7 h, BAY 1816032 or DMSO was added for 5 h. Cells were then fixed and subjected to immunofluorescence staining as in (B). Example images of anaphase cells are shown (E). The percentage of anaphases with PICH was determined in at least 142 cells in each condition. Means and SDs from three independent experiments are shown (F). Arrows point to the UFBs.
-
G–IHeLa cells transfected with siRNA and plasmids encoding EGFP‐TOP2A (WT or ΔChT) were synchronized as in (B), and then subjected to immunofluorescence staining with DAPI, ACA and the antibodies for PICH and GFP. Example images of anaphase cells are shown (G). The percentage of anaphases with PICH was determined in at least 174 cells in each condition. Means and SDs from three independent experiments are shown (H). Lysates of asynchronous cells were immunoblotted (I). Arrows point to the UFBs.
We further compared the capability of EGFP‐TOP2A and the EGFP‐TOP2A‐ΔChT mutant in preventing UFBs. Consistent with a previous report (Broderick et al, 2015), the rate of anaphase cells with UFBs increased from 11.3% in control HeLa cells to 33.3% in cells in which TOP2A was depleted by siRNA (Fig 9G–I). This defect was largely rescued by exogenous expression of EGFP‐TOP2A but not EGFP‐TOP2A‐ΔChT, which is in line with the role of centromere‐localized TOP2A in suppressing UFBs.
Taken together, these results indicate that, through H2ApT120, Bub1 localizes TOP2A to mitotic centromeres for efficient removal of DNA catenations prior to anaphase.
Discussion
During mitotic cell division, timely disjunction of sister chromatids by TOP2A, particularly at centromeres, is a fundamental requirement for accurate chromosome segregation. However, the nature of the binding site for TOP2A at mitotic centromeres has long remained a mystery. In this study, we reveal a key role for H2ApT120 in the localization of TOP2A in mitosis.
We present several lines of evidence suggesting that TOP2A is a reader protein of H2ApT120. First, centromeric localization of TOP2A in mitosis requires the kinase activity of kinetochore‐localized Bub1. Second, Bub1 kinase activity is sufficient to generate H2ApT120 and recruit TOP2A to centromeres, as well as to a non‐centromeric chromatin region, even during late mitosis and interphase. Third, phosphorylation of histone H2A at the residue‐120 can enhance H2A binding to TOP2A in vitro. Fourth, TOP2A and Sgo1 bind to H2ApT120 in a competitive manner in cells.
We reveal a role for the ChT domain consisting of the extreme C‐terminal 31 residues of human TOP2A in the localization of TOP2A at mitotic centromeres, as well as in the recruitment of TOP2A by H2ApT120 generated by Bub1 tethered to centromeres and a non‐centromeric chromatin region. However, due to unknown reasons, this domain is not directly responsible for the phosphorylation‐dependent binding of histone H2A to TOP2A in vitro. Previous studies have implicated sumoylation in TOP2A localization at centromeres in Xenopus egg extracts and in human cells (Azuma et al, 2005; Diaz‐Martinez et al, 2006). RanBP2, a nucleoporin with SUMO E3 ligase activity, was reported to sumoylate TOP2A in mitosis and promote the localization of TOP2A at centromeres to prevent chromosome segregation errors in mouse embryonic fibroblast cells (Dawlaty et al, 2008). It is currently unclear how sumoylation may facilitate the binding of TOP2A to H2ApT120. Future studies are required to address the molecular basis for the interaction between H2ApT120 and TOP2A.
Though TOP2A preferentially accumulates at mitotic centromeres, it is also found on chromosome arms throughout mitosis where it may play a role in chromosome organization (Earnshaw & Heck, 1985; Earnshaw et al, 1985; Gasser et al, 1986; Meyer et al, 1997; Samejima et al, 2012; Lane et al, 2013; Farr et al, 2014). We show that exogenously expressed TOP2A lacking the ChT domain is capable of associating with mitotic chromosomes in PFA‐fixed cells, but is largely impaired in localizing to mitotic centromeres. Given the reported role of the ChT domain in promoting the interaction between TOP2A and mitotic chromosomes (Lane et al, 2013), the residence time of TOP2A on mitotic chromosomes may influence TOP2A accumulation at the centromere. We further find that the chimeric protein EGFP‐TOP2A (970–1,200)+(1,447–1,531) does not detectably localize to mitotic chromosomes but is highly enriched at the centromere. These results suggest that strong chromosomal association is neither necessary nor sufficient for the centromeric localization of TOP2A during mitosis. There might be two distinct populations of TOP2A on mitotic chromosomes. While one population of TOP2A binds H2ApT120 and localizes to centromeres, the other population distributes over the whole chromosome with an incompletely understood mechanism that may involve the binding to DNA and histone H3 (Gilroy & Austin, 2011; Lane et al, 2013; Clarke & Azuma, 2017).
Then, what is the functional importance of H2ApT120‐dependent localization of TOP2A at mitotic centromeres? As a natural consequence of DNA replication, many catenanes between sister DNAs persist until mitosis. UFBs are normally detectable between sister centromeres in early anaphase, and are thought to be remnants of the catenanes that are maintained by centromeric cohesin until anaphase onset (Wang et al, 2010b; Liu et al, 2014). We find that the frequency of anaphase UFBs is increased by inhibiting Bub1 kinase activity but not by removal of centromeric Sgo1 and Sgo2, two known reader proteins of H2ApT120. Moreover, the requirement for Bub1 kinase in suppressing anaphase UFBs can be bypassed when TOP2A is artificially tethered to centromeres. In addition, the TOP2A‐ΔChT mutant impaired in centromeric localization is incapable of preventing anaphase UFBs. Our data support a model that H2ApT120 recruits TOP2A at mitotic centromeres to limit the amount of catenanes that must be resolved at anaphase onset.
TOP2A is relevant in cancer because it is the target of an important class of anti‐cancer agents (Nitiss, 2009b). Recent studies reported that inhibition of Bub1 kinase activity by small molecules sensitizes tumor cells toward clinically relevant doses of the classic chemotherapy drugs such as paclitaxel, impairing both chromosome segregation and cell proliferation (Baron et al, 2016; Siemeister et al, 2018). Whether the role of H2ApT120‐bound TOP2A in preventing UFBs contributes to the synergistic effect of Bub1 inhibitors with chemotherapeutic agents will be the topic of future studies.
In Xenopus egg extracts and budding yeast (Edgerton et al, 2016; Yoshida et al, 2016), the CTD of TOP2A (Topo II in yeast) has been shown to interact with Haspin, the kinase responsible for histone H3 Thr‐3 phosphorylation (H3pT3) in mitosis (Dai et al, 2005). This modification is directly recognized by the Survivin subunit of the CPC, thereby promoting CPC localization at the inner centromere region of mitotic chromosomes (Kelly et al, 2010; Wang et al, 2010a; Yamagishi et al, 2010). It will be of interest to determine whether the H2ApT120‐bound pool of TOP2A contributes to the centromeric accumulation of the Haspin‐H3pT3‐CPC signaling axis in higher eukaryotes.
In conclusion, our findings lead to a model indicating that kinetochore‐localized Bub1 kinase phosphorylates histone H2A at Thr‐120 during mitosis to recruit TOP2A to centromeres, which promotes timely decatenation of sister DNAs to ensure accurate genome transmission (Fig 10). Our study reveals a fundamental role for a mitotic phospho‐histone mark in safeguarding genomic stability.
Figure 10. Model for the role of Bub1‐mediated H2ApT120 in recruiting TOP2A to mitotic centromeres to decatenate sister DNAs.
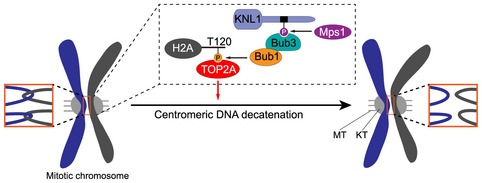
KT represents kinetochore. MT represents microtubule. For simplicity, only one phospho‐MELT motif on KNL1 is shown.
Materials and Methods
Cell culture
U2OS cells, HeLa cells, and the HeLa‐derived Sgo1‐K492A mutant cells were cultured in DMEM supplemented with 1% penicillin/streptomycin and 10% FBS (Gibco), and maintained at 37°C with 5% CO2. U2OS‐LacO cells were kindly provided by Dr. David Spector (Cold Spring Harbor Laboratory, Cold Spring Harbor, USA) (Janicki et al, 2004), and maintained in 100 μg/ml hygromycin B. U2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP were isolated and maintained in 3.0 and 2.0 μg/ml blasticidin (Sigma), respectively.
Plasmids
To construct pBos‐CENP‐B‐GFP encoding CB‐GFP, the H2B fragment in pBos‐H2B‐GFP (BD Biosciences) was replaced with the KpnI/BamHI‐digested PCR fragments encoding the centromere‐targeting domain (residues 1–163) of CENP‐B. To make CB‐Bub1‐K‐GFP (WT or D946N) constructs, the PCR fragments encoding Bub1 residues 630–1,085 were subcloned into the BamHI site of pBos‐CENP‐B‐GFP. The plasmids encoding CB‐TOP2A‐GFP, CB‐VRK1‐GFP, and CB‐VprBP‐GFP were constructed similarly. To make EGFP‐LacI‐Bub1‐K (WT or D946N) constructs, the PCR fragments encoding Bub1 residues 630–1,085 were subcloned into the BsiWI/BamHI sites of pSV2‐EGFP‐LacI (Wang et al, 2006). The plasmid encoding TOP2A‐Myc‐6xHis was constructed by inserting TOP2A cDNA into the BamHI/EcoRV sites of pEF6‐Myc‐6xHis (Invitrogen). The EGFP‐TOP2A plasmid was kindly provided by Dr. Gary Gorbsky (Oklahoma Medical Research Foundation, Oklahoma City, USA) (Tavormina et al, 2002). The Myc‐LacI‐Bub1 (WT or D946N) plasmids were kindly provided by Dr. Sabine Elowe (Université Laval, Québec, Canada) (Asghar et al, 2015). The VSV‐Sgo1 plasmid was kindly provided by Dr. Susanne Lens (Oncode Institute, Utrecht, Netherlands) (Meppelink et al, 2015). All point mutations, deletion mutations, and truncation mutations were introduced with the QuikChange II XL site‐directed mutagenesis kit (Agilent Technologies). All plasmids were sequenced to verify desired mutations and absence of unintended mutations.
siRNAs
The following siRNA duplexes ordered from Integrated DNA Technologies (IDT) or RiboBio were used: siBub1 (5′‐CCCAUUUGCCAGCUCAAGCdTdT‐3′) (Liu et al, 2013a), siBub3 (5′‐ UGACAGAUGCAGAAACAAAdTdT‐3′) (Asghar et al, 2015), siKNL1 (5′‐GCAUGUAUCUCUUAAGGAAdTdT‐3′) (Vleugel et al, 2015b), siSgo2 (5′‐GAACACAUUUCUUCGCCUAdTdT‐3′) (Huang et al, 2007), siTOP2A (5′‐ACACAAUUGGAACAUAUUUdTdT‐3′) (Sakaguchi & Kikuchi, 2004), siTOP2B (5′‐GUUGUCUGUUGAGAGAGUGdTdT‐3′) (Chikamori et al, 2006). siTRF1 (#1, 5′‐AACGUAUUCUGUAAAGCUUdTdT‐3′; #2, 5′‐ACAGUAGUAGUCCUUUGAUdTdT‐3′) (Ohishi et al, 2010, 2014), siCdc20 (5′‐ GGAGCUCAUCUCAGGCCAUdTdT‐3′) (Kidokoro et al, 2008), and control siRNA was previously described (Wang et al, 2010a).
Transfection and drug treatments
Plasmid and siRNA transfections were done with Fugene 6 (Promega) and Oligofectamine or Lipofectamine RNAiMAX (Invitrogen), respectively. For TOP2A RNAi rescue experiments, cells were transfected twice with control or TOP2A siRNAs in a 24‐h interval. At 4 h after the second siRNA transfection, cells were transfected with the EGFP‐TOP2A plasmids harboring silencing mutations that render resistance to the TOP2A siRNA. Cells were arrested in S phase by single thymidine (2 mM; Calbiochem) treatment, or in a prometaphase‐like state with 0.33 μM nocodazole (Selleckchem). Other drugs used in this study were: BAY 1816032 (2–4 μM; Chemietech), STLC (5 μM; Tocris Bioscience), MG132 (20 μM; Sigma), reversine (0.5 μM; MedChem Express), and AZ3146 (3 μM; MedChem Express). Mitotic cells were collected by selective detachment with “shake‐off”.
Antibodies
Rabbit polyclonal antibodies used were to GFP (A11122, Invitrogen), GAPDH (14C10, Cell Signaling Technology, CST), H2ApT120 (Active Motif), INCENP (#2807, CST), VSV (V‐4888, Sigma), Sgo2 (A301‐262, Bethyl Laboratories), MBP (E8032, New England BioLabs), GST (G7781, Sigma), and TOP2B (HPA024120, Sigma). Rabbit anti‐Bub1 and anti‐H3pS10 polyclonal antibodies were produced by immunization with the synthetic peptides NYGLPQPKNKPTGAR and TARK(pS)TGGK (where pS is phosphoserine), respectively. Mouse monoclonal antibodies used were Aurora B (AIM‐1, BD Biosciences), α‐tubulin (T‐6047, Sigma), Sgo1 (3C11, Abnova), TOP2A (M042‐3, MBL), Myc (4A6, Millipore), PICH (H00054821‐B01P, Abnova), TRF‐1 (ab10579, Abcam), and Cdc20 (sc‐13162, Santa Cruz). Guinea pig polyclonal antibodies against CENP‐C were from MBL (PD030). Human centromere autoantibodies were from Immunovision. Sheep anti‐Bub1 antibody was kindly provided by Dr. Stephen Taylor (University of Manchester, UK). Secondary antibodies for immunoblotting were goat anti‐rabbit or horse anti‐mouse IgG‐HRP (CST). Secondary antibodies for immunostaining were donkey anti‐rabbit IgG‐Alexa Fluor 488 or Cy3 (Jackson ImmunoResearch); anti‐mouse IgG‐Alexa Fluor 488 or 546 (Invitrogen); anti‐sheep IgG‐Cy3; anti‐human IgG‐Alexa Fluor 647 (Jackson ImmunoResearch); and goat anti‐guinea pig IgG‐Alexa Fluor 647 (Invitrogen).
Fluorescence microscopy and quantifications
Unless otherwise stated, cells cultured or spun on coverslips were fixed with 2% PFA in PBS for 10 min followed by extraction with 0.5% Triton X‐100 in PBS for 5 min. For images shown in Figs 7A and EV5A, cells were extracted for 5 min with 1% Triton X‐100 in PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, and 2 mM MgCl2, pH 6.9), and then fixed with 4% PFA in PHEM buffer for 20 min. For immunostaining of PICH in anaphase, cells were extracted for 30 seconds with 0.2% Triton X‐100 in PEM buffer (20 mM Pipes, 10 mM EGTA, and 1 mM MgCl2, pH 6.8), and then fixed by 7% PFA with 0.1% Triton X‐100 in PEM buffer for 15 min. Fixed cells were stained with primary antibodies for 1–2 h and secondary antibodies for 1 h, all with 3% BSA in PBS with or without 0.5% Triton X‐100 and at room temperature. DNA was stained for 10 min with DAPI (4′, 6‐diamidino‐2‐phenylindole; 2 μg/ml). Fluorescence microscopy was carried out at room temperature using a Nikon ECLIPSE Ni microscope with a Plan Apo Fluor 60× Oil (NA 1.4) objective lens and a Clara CCD (Andor Technology). For deconvolution microscopy, images were acquired as z stacks at 0.2‐μm intervals on a deconvolution system (DeltaVision Elite Applied Precision/GE Healthcare) with an ×100/1.40 NA UPlanSApo objective (Olympus) using SoftWorx software. After deconvolution, maximum intensity projections of deconvolved stacks were made using SoftWorx.
Quantification of fluorescent intensity was carried out with ImageJ (NIH) using images obtained at identical illumination settings. Briefly, for chromosomes in cells cytospun on the coverslips (Figs 4C, D, G and H, and 5C–H and 6E–H and 7A–C and EV4D–F and EV5A–C, and Appendix Fig S2A and C), the average pixel intensity of TOP2A, Sgo1, INCENP, Aurora B, ACA, or CENP‐C staining at centromeres, defined as circular regions including paired centromeres or on chromosome arms, was determined using ImageJ. After background correction, the ratio of centromeric TOP2A/ACA, centromeric Sgo1/ACA, centromeric Sgo1/CENP‐C, or centromeric TOP2A/arm TOP2A was calculated for each centromere. To quantify the enrichment of proteins at centromeres in cells cultured on coverslips, the average pixel intensity of TOP2A, Bub1, H2ApT120, Sgo1, Aurora B, INCENP, CENP‐C, or ACA staining at centromeres or kinetochore, defined as circular regions including single centromeres or kinetochores, or on chromosome arms, was determined using ImageJ. Sgo1 staining at inner centromeres was defined as circular regions at paired centromeres. After background correction, the ratio of kinetochore or centromeric staining intensity of TOP2A, Bub1, H2ApT120, Sgo1, Aurora B, and INCENP versus that of CENP‐C or ACA, or the ratio of centromeric staining intensity of TOP2A versus arm staining intensity of TOP2A, was calculated for each centromere. To quantify the relative enrichment of proteins at the LacO transgene array in U2OS‐LacO cells (Figs 5I and J, and 6B and C, and 7E–G and I–K, and EV5E–G), the average pixel intensity of various forms of EGFP‐TOP2A and VSV‐Sgo1, as well as endogenous TOP2A and Sgo1, within circles encompassing the Myc‐LacI‐Bub1 or EGFP‐LacI‐Bub1‐K fluorescent signal at LacO transgene array, and in the nearby nucleus, was determined. After background correction, the ratio of average fluorescent protein intensity or immunostaining intensity at LacO repeats versus that in the nuclei was calculated. To quantify the relative enrichment of TOP2A‐Myc‐6xHis at the centromere in U2OS cells stably expressing CB‐GFP, CB‐Bub1‐K‐GFP, or CB‐Bub1‐K‐D946N‐GFP (Fig 6I and J), the average pixel intensity of anti‐Myc staining signal within circles encompassing the centromere region, and in the nearby nucleus, was determined. After background correction, the ratio of average immunostaining intensity at the centromere versus that in the nuclei was calculated. The acquired images were processed using Adobe Photoshop and Adobe Illustrator.
Statistics
Statistical analyses were performed with a two‐tailed unpaired Student's t‐test in GraphPad Prism 6. A P value of < 0.05 was considered significant.
Protein purification
The plasmid expressing GST‐H2A‐10xHis was constructed by subcloning the PCR fragments encoding histone H2A and the 10xHis‐tag into the BamHI/EcoRI sites of pGEX‐4T1 (GE Healthcare). The plasmid expressing GST‐H2ApS120‐10xHis was constructed by point mutation at T120 site, whose codon was changed to “TAG”. The plasmid pEVOL‐SEP bears SEP‐tRNA and SEP synthetase (Park et al, 2011), in which SEP tRNA and SEP synthetase were driven by E. coli proK promoter and the arabinose‐inducible promoter, respectively. The C321▵SerB cells were generated from C321.▵A.exp (Addgene #49018) using Lambda Red‐mediated recombination (Pirman et al, 2015), in which the serB gene was replaced by the kanamycin resistance gene.
The GST‐H2A‐10xHis plasmid was transformed into BL21 (DE3) competent cells (Stratagene). The GST‐H2ApS120‐10xHis and pEVOL‐SEP plasmids were co‐transformed into C321▵SerB. Cells were grown in LB broth under antibiotic selection at 37°C until OD600 at 0.6–0.7. Expression of the GST‐H2A protein was induced by 0.4 mM IPTG for 16 h at 16°C. Expression of the GST‐H2ApS120 protein was induced by 0.4 mM IPTG for 16 h at 16°C in the presence of 0.08% glucose and 0.2% arabinose. Cells were lysed by sonication in 50 mM Tris–HCl, pH 8.0, 300 mM NaCl, 20 mM imidazole. GST‐H2A and GST‐H2ApS120 with the 10xHis‐tag at the C‐terminus were purified by Ni‐NTA column (Smart) according to the manufacturer's instructions, and were eluted by 300 mM imidazole. The eluted protein was incubated with Glutathione‐Sepharose 4B beads (GE Healthcare) at 4°C for 4 h, and then used for the subsequent GST pulldown assays. The expression of GST protein by pGEX‐4T1 was induced similarly as GST‐H2A. Cells were lysed by sonication in 20 mM Tris–HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 1% Triton X‐100. The lysate was clarified by centrifugation and incubated with Glutathione‐Sepharose 4B beads in lysis buffer.
To make the plasmids expressing MBP‐TOP2A (429–1,531)‐10xHis and MBP‐TOP2A (429–1,531)‐ΔChT‐10xHis, the PCR fragments encoding TOP2A residues 429–1,531 and 429–1,500, respectively, were subcloned into the Not1/SfaA1 sites of pGEX‐MBP‐10xHis (Kindly provided by Dr. Zongping Xia). The expression of MBP‐TOP2A (429–1,531)‐10xHis and MBP‐TOP2A (429–1,531)‐ΔChT‐10xHis was induced by 0.4 mM IPTG for 16 h at 16°C. Cells were lysed by sonication in lysis buffer (50 mM Tris–HCl, pH 8.0, 500 mM NaCl). After centrifugation, supernatant was incubated with Ni‐NTA Resin. The resins were washed with lysis buffer and eluted with 300 mM imidazole in lysis buffer.
GST pulldown assay and immunoblotting
About 10 μg samples of GST, GST‐H2A, and GST‐H2ApS120 immobilized to Glutathione‐Sepharose 4B beads were incubated with around 2 μg of eluted MBP‐TOP2A (429–1,531)‐10xHis in 500 μl of buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Triton X‐100, 10 mM MgCl2, 5 mM EDTA, 1 mM DTT). After incubation at 4°C for 1 h, the beads were washed three times (5 min each) in the same buffer. The beads were then boiled in standard SDS sample buffer and subjected to immunoblotting. SDS–PAGE and immunoblotting were carried out using standard procedures. Cell lysates were prepared in standard SDS sample buffer. The intensity of immunoblotting bands of MBP or GST fusion protein was determined by ImageJ. The relative pulldown efficiency was determined by normalizing the ratio of intensity of MBP to GST in the GST‐H2ApS120 pulldown against that in the GST‐H2A pulldown assay.
Author contributions
MZ designed and performed the majority of the experiments and analysis. CL designed and carried out the GST pulldown assays (Figs 3E and F, and 6L), the localization of TOP2A in HeLa and Sgo1‐K492A cells (Fig 4C and D), the competitive localization of EGFP‐TOP2A and Sgo1 (Fig 7A–D), as well as the deconvolution microscopy (Appendix Fig S4). HZ and SL helped express the GST‐H2ApS120 protein. XY performed the experiments shown in Appendix Fig S1. QC, HY, JX, and JL provided technical assistance. WL provided reagents and constructive suggestions. FW conceived and supervised the project, designed the experiments, analyzed the data, and wrote the manuscript with the input from HY.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Expanded View Figures PDF
Review Process File
Acknowledgements
We are grateful to Drs Jonathan Higgins and Xiangwei He for critically reading and commenting on the manuscript; to Drs Dong Fang, Gary Gorbsky, Guohong Li, Wei Tian, and Sheng Ye for discussions; and to Drs Sabine Elowe, Gary Gorbsky, Susanne Lens, David Spector, and Stephen Taylor for kindly providing reagents. We also thank the Core Facility of Life Sciences Institute, Zhejiang University, for technical assistance. This work was supported by grants to F.W. from the National Natural Science Foundation of China (NSFC) (31571393), the National Key Research and Development Program of China (2017YFA0503600), the NSFC (31771499, 31322032, 31371359, 31561130155), the Natural Science Foundation of Zhejiang Province (LZ19C070001 and LR13C070001), a Royal Society Newton Advanced Fellowship (NA140075), the Fundamental Research Funds for the Central Universities in China (2014XZZX003‐35 to F.W. and 2018QN81011 to S.L.), and the NSFC (81702552 to J.X.).
The EMBO Journal (2020) 39: e101863
References
- Aihara H, Nakagawa T, Mizusaki H, Yoneda M, Kato M, Doiguchi M, Imamura Y, Higashi M, Ikura T, Hayashi T et al (2016) Histone H2A T120 phosphorylation promotes oncogenic transformation via upregulation of cyclin D1. Mol Cell 64: 176–188 [DOI] [PubMed] [Google Scholar]
- Asghar A, Lajeunesse A, Dulla K, Combes G, Thebault P, Nigg EA, Elowe S (2015) Bub1 autophosphorylation feeds back to regulate kinetochore docking and promote localized substrate phosphorylation. Nat Commun 6: 8364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Arnaoutov A, Anan T, Dasso M (2005) PIASy mediates SUMO‐2 conjugation of topoisomerase‐II on mitotic chromosomes. EMBO J 24: 2172–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AP, von Schubert C, Cubizolles F, Siemeister G, Hitchcock M, Mengel A, Schroder J, Fernandez‐Montalvan A, von Nussbaum F, Mumberg D et al (2016) Probing the catalytic functions of Bub1 kinase using the small molecule inhibitors BAY‐320 and BAY‐524. Elife 5: e12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann C, Korner R, Hofmann K, Nigg EA (2007) PICH, a centromere‐associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell 128: 101–114 [DOI] [PubMed] [Google Scholar]
- Biebricher A, Hirano S, Enzlin JH, Wiechens N, Streicher WW, Huttner D, Wang LH, Nigg EA, Owen‐Hughes T, Liu Y et al (2013) PICH: a DNA translocase specially adapted for processing anaphase bridge DNA. Mol Cell 51: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizard AH, Hickson ID (2018) Anaphase: a fortune‐teller of genomic instability. Curr Opin Cell Biol 52: 112–119 [DOI] [PubMed] [Google Scholar]
- Bjergbaek L, Jensen S, Westergaard O, Andersen AH (1999) Using a biochemical approach to identify the primary dimerization regions in human DNA topoisomerase IIalpha. J Biol Chem 274: 26529–26536 [DOI] [PubMed] [Google Scholar]
- Broderick R, Nieminuszczy J, Blackford AN, Winczura A, Niedzwiedz W (2015) TOPBP1 recruits TOP2A to ultra‐fine anaphase bridges to aid in their resolution. Nat Commun 6: 6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, North PS, Hickson ID (2007) BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J 26: 3397–3409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson‐Kubalek EM, Desai A (2006) The conserved KMN network constitutes the core microtubule‐binding site of the kinetochore. Cell 127: 983–997 [DOI] [PubMed] [Google Scholar]
- Chen SH, Chan NL, Hsieh TS (2013) New mechanistic and functional insights into DNA topoisomerases. Annu Rev Biochem 82: 139–170 [DOI] [PubMed] [Google Scholar]
- Chikamori K, Hill JE, Grabowski DR, Zarkhin E, Grozav AG, Vaziri SA, Wang J, Gudkov AV, Rybicki LR, Bukowski RM et al (2006) Downregulation of topoisomerase IIbeta in myeloid leukemia cell lines leads to activation of apoptosis following all‐trans retinoic acid‐induced differentiation/growth arrest. Leukemia 20: 1809–1818 [DOI] [PubMed] [Google Scholar]
- Christensen MO, Larsen MK, Barthelmes HU, Hock R, Andersen CL, Kjeldsen E, Knudsen BR, Westergaard O, Boege F, Mielke C (2002) Dynamics of human DNA topoisomerases IIalpha and IIbeta in living cells. J Cell Biol 157: 31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke DJ, Azuma Y (2017) Non‐catalytic roles of the topoisomerase IIalpha C‐terminal domain. Int J Mol Sci 18: E2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sultan S, Taylor SS, Higgins JM (2005) The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev 19: 472–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sullivan BA, Higgins JM (2006) Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell 11: 741–750 [DOI] [PubMed] [Google Scholar]
- Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM (2008) Resolution of sister centromeres requires RanBP2‐mediated SUMOylation of topoisomerase IIalpha. Cell 133: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz‐Martinez LA, Gimenez‐Abian JF, Azuma Y, Guacci V, Gimenez‐Martin G, Lanier LM, Clarke DJ (2006) PIASgamma is required for faithful chromosome segregation in human cells. PLoS ONE 1: e53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen EC, Hargreaves DC, Miller EL, Cui K, Korshunov A, Kool M, Pfister S, Cho YJ, Zhao K, Crabtree GR (2013) BAF complexes facilitate decatenation of DNA by topoisomerase IIalpha. Nature 497: 624–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Heck MM (1985) Localization of topoisomerase II in mitotic chromosomes. J Cell Biol 100: 1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Halligan B, Cooke CA, Heck MM, Liu LF (1985) Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol 100: 1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton H, Johansson M, Keifenheim D, Mukherjee S, Chacon JM, Bachant J, Gardner MK, Clarke DJ (2016) A noncatalytic function of the topoisomerase II CTD in Aurora B recruitment to inner centromeres during mitosis. J Cell Biol 213: 651–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farcas AM, Uluocak P, Helmhart W, Nasmyth K (2011) Cohesin's concatenation of sister DNAs maintains their intertwining. Mol Cell 44: 97–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr CJ, Antoniou‐Kourounioti M, Mimmack ML, Volkov A, Porter AC (2014) The alpha isoform of topoisomerase II is required for hypercompaction of mitotic chromosomes in human cells. Nucleic Acids Res 42: 4414–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, Hunt DF, Funabiki H, Allis CD (2005) Regulation of HP1‐chromatin binding by histone H3 methylation and phosphorylation. Nature 438: 1116–1122 [DOI] [PubMed] [Google Scholar]
- Frere V, Sourgen F, Monnot M, Troalen F, Fermandjian S (1995) A peptide fragment of human DNA topoisomerase II alpha forms a stable coiled‐coil structure in solution. J Biol Chem 270: 17502–17507 [DOI] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T (2006) Human Wapl is a cohesin‐binding protein that promotes sister‐chromatid resolution in mitotic prophase. Curr Biol 16: 2406–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser SM, Laroche T, Falquet J, Boy de la Tour E, Laemmli UK (1986) Metaphase chromosome structure. Involvement of topoisomerase II. J Mol Biol 188: 613–629 [DOI] [PubMed] [Google Scholar]
- Gilroy KL, Austin CA (2011) The impact of the C‐terminal domain on the interaction of human DNA topoisomerase II alpha and beta with DNA. PLoS ONE 6: e14693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez‐Abian JF, Sumara I, Hirota T, Hauf S, Gerlich D, de la Torre C, Ellenberg J, Peters JM (2004) Regulation of sister chromatid cohesion between chromosome arms. Curr Biol 14: 1187–1193 [DOI] [PubMed] [Google Scholar]
- Goto Y, Yamagishi Y, Shintomi‐Kawamura M, Abe M, Tanno Y, Watanabe Y (2017) Pds5 regulates sister‐chromatid cohesion and chromosome bi‐orientation through a conserved protein interaction module. Curr Biol 27: 1005–1012 [DOI] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov A (1997) A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae . Cell 91: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guturi KK, Bohgaki M, Bohgaki T, Srikumar T, Ng D, Kumareswaran R, El Ghamrasni S, Jeon J, Patel P, Eldin MS et al (2016) RNF168 and USP10 regulate topoisomerase IIalpha function via opposing effects on its ubiquitylation. Nat Commun 7: 12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarhuis JH, Elbatsh AM, van den Broek B, Camps D, Erkan H, Jalink K, Medema RH, Rowland BD (2013) WAPL‐mediated removal of cohesin protects against segregation errors and aneuploidy. Curr Biol 23: 2071–2077 [DOI] [PubMed] [Google Scholar]
- Haarhuis JH, Elbatsh AM, Rowland BD (2014) Cohesin and its regulation: on the logic of X‐shaped chromosomes. Dev Cell 31: 7–18 [DOI] [PubMed] [Google Scholar]
- Hara K, Zheng G, Qu Q, Liu H, Ouyang Z, Chen Z, Tomchick DR, Yu H (2014) Structure of cohesin subcomplex pinpoints direct shugoshin‐Wapl antagonism in centromeric cohesion. Nat Struct Mol Biol 21: 864–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Waizenegger IC, Peters JM (2001) Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293: 1320–1323 [DOI] [PubMed] [Google Scholar]
- Hauf S, Roitinger E, Koch B, Dittrich CM, Mechtler K, Peters JM (2005) Dissociation of cohesin from chromosome arms and loss of arm cohesion during early mitosis depends on phosphorylation of SA2. PLoS Biol 3: e69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt L, Tighe A, Santaguida S, White AM, Jones CD, Musacchio A, Green S, Taylor SS (2010) Sustained Mps1 activity is required in mitosis to recruit O‐Mad2 to the Mad1‐C‐Mad2 core complex. J Cell Biol 190: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota T, Lipp JJ, Toh BH, Peters JM (2005) Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 438: 1176–1180 [DOI] [PubMed] [Google Scholar]
- Huang H, Feng J, Famulski J, Rattner JB, Liu ST, Kao GD, Muschel R, Chan GK, Yen TJ (2007) Tripin/hSgo2 recruits MCAK to the inner centromere to correct defective kinetochore attachments. J Cell Biol 177: 413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicki SM, Tsukamoto T, Salghetti SE, Tansey WP, Sachidanandam R, Prasanth KV, Ried T, Shav‐Tal Y, Bertrand E, Singer RH et al (2004) From silencing to gene expression: real‐time analysis in single cells. Cell 116: 683–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Li B, Yu H (2016) The Bub1‐Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat Commun 7: 10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y (2010) Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 327: 172–177 [DOI] [PubMed] [Google Scholar]
- Ke Y, Huh JW, Warrington R, Li B, Wu N, Leng M, Zhang J, Ball HL, Li B, Yu H (2011) PICH and BLM limit histone association with anaphase centromeric DNA threads and promote their resolution. EMBO J 30: 3309–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H (2010) Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330: 235–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro T, Tanikawa C, Furukawa Y, Katagiri T, Nakamura Y, Matsuda K (2008) CDC20, a potential cancer therapeutic target, is negatively regulated by p53. Oncogene 27: 1562–1571 [DOI] [PubMed] [Google Scholar]
- Kim K, Kim JM, Kim JS, Choi J, Lee YS, Neamati N, Song JS, Heo K, An W (2013) VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell 52: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima TS, Kawashima SA, Watanabe Y (2004) The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427: 510–517 [DOI] [PubMed] [Google Scholar]
- Kitajima TS, Sakuno T, Ishiguro K, Iemura S, Natsume T, Kawashima SA, Watanabe Y (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441: 46–52 [DOI] [PubMed] [Google Scholar]
- Kiyomitsu T, Obuse C, Yanagida M (2007) Human Blinkin/AF15q14 is required for chromosome alignment and the mitotic checkpoint through direct interaction with Bub1 and BubR1. Dev Cell 13: 663–676 [DOI] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM (2006) Wapl controls the dynamic association of cohesin with chromatin. Cell 127: 955–967 [DOI] [PubMed] [Google Scholar]
- Ladurner R, Kreidl E, Ivanov MP, Ekker H, Idarraga‐Amado MH, Busslinger GA, Wutz G, Cisneros DA, Peters JM (2016) Sororin actively maintains sister chromatid cohesion. EMBO J 35: 635–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AB, Gimenez‐Abian JF, Clarke DJ (2013) A novel chromatin tether domain controls topoisomerase IIalpha dynamics and mitotic chromosome formation. J Cell Biol 203: 471–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kitajima TS, Tanno Y, Yoshida K, Morita T, Miyano T, Miyake M, Watanabe Y (2008) Unified mode of centromeric protection by shugoshin in mammalian oocytes and somatic cells. Nat Cell Biol 10: 42–52 [DOI] [PubMed] [Google Scholar]
- Lee HS, Lin Z, Chae S, Yoo YS, Kim BG, Lee Y, Johnson JL, Kim YS, Cantley LC, Lee CW et al (2018) The chromatin remodeler RSF1 controls centromeric histone modifications to coordinate chromosome segregation. Nat Commun 9: 3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Kim H, Ji Z, Zhang T, Chen B, Ge Y, Hu Y, Feng X, Han X, Xu H et al (2018) The BUB3‐BUB1 complex promotes telomere DNA replication. Mol Cell 70: 395–407 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Chen Q, Yi Q, Zhang M, Yan H, Zhang B, Zhou L, Zhang Z, Qi F, Ye S et al (2018a) A kinase‐dependent role for Haspin in antagonizing Wapl and protecting mitotic centromere cohesion. EMBO Rep 19: 43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Zhang Z, Chen Q, Yan H, Zhang M, Xiang X, Yi Q, Pan X, Cheng H, Wang F (2018b) A positive feedback mechanism ensures proper assembly of the functional inner centromere during mitosis in human cells. J Biol Chem 294: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linka RM, Porter AC, Volkov A, Mielke C, Boege F, Christensen MO (2007) C‐terminal regions of topoisomerase IIalpha and IIbeta determine isoform‐specific functioning of the enzymes in vivo . Nucleic Acids Res 35: 3810–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Jia L, Yu H (2013a) Phospho‐H2A and cohesin specify distinct tension‐regulated Sgo1 pools at kinetochores and inner centromeres. Curr Biol 23: 1927–1933 [DOI] [PubMed] [Google Scholar]
- Liu H, Rankin S, Yu H (2013b) Phosphorylation‐enabled binding of SGO1‐PP2A to cohesin protects sororin and centromeric cohesion during mitosis. Nat Cell Biol 15: 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nielsen CF, Yao Q, Hickson ID (2014) The origins and processing of ultra fine anaphase DNA bridges. Curr Opin Genet Dev 26: 1–5 [DOI] [PubMed] [Google Scholar]
- Liu H, Qu Q, Warrington R, Rice A, Cheng N, Yu H (2015) Mitotic transcription installs Sgo1 at centromeres to coordinate chromosome segregation. Mol Cell 59: 426–436 [DOI] [PubMed] [Google Scholar]
- London N, Ceto S, Ranish JA, Biggins S (2012) Phosphoregulation of Spc105 by Mps1 and PP1 regulates Bub1 localization to kinetochores. Curr Biol 22: 900–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N, Biggins S (2014) Signalling dynamics in the spindle checkpoint response. Nat Rev Mol Cell Biol 15: 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (1998) Identification of Xenopus SMC protein complexes required for sister chromatid cohesion. Genes Dev 12: 1986–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano T (2001) Intermolecular DNA interactions stimulated by the cohesin complex in vitro: implications for sister chromatid cohesion. Curr Biol 11: 268–272 [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T (2002) Cohesin release is required for sister chromatid resolution, but not for condensin‐mediated compaction, at the onset of mitosis. Genes Dev 16: 3004–3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K, Yuan J, Chen J, Lou Z (2009) Topoisomerase IIalpha controls the decatenation checkpoint. Nat Cell Biol 11: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness BE, Hirota T, Kudo NR, Peters JM, Nasmyth K (2005) Shugoshin prevents dissociation of cohesin from centromeres during mitosis in vertebrate cells. PLoS Biol 3: e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meppelink A, Kabeche L, Vromans MJ, Compton DA, Lens SM (2015) Shugoshin‐1 balances Aurora B kinase activity via PP2A to promote chromosome bi‐orientation. Cell Rep 11: 508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KN, Kjeldsen E, Straub T, Knudsen BR, Hickson ID, Kikuchi A, Kreipe H, Boege F (1997) Cell cycle‐coupled relocation of types I and II topoisomerases and modulation of catalytic enzyme activities. J Cell Biol 136: 775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K (1997) Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91: 35–45 [DOI] [PubMed] [Google Scholar]
- Nasmyth K, Haering CH (2009) Cohesin: its roles and mechanisms. Annu Rev Genet 43: 525–558 [DOI] [PubMed] [Google Scholar]
- Nielsen CF, Huttner D, Bizard AH, Hirano S, Li TN, Palmai‐Pallag T, Bjerregaard VA, Liu Y, Nigg EA, Wang LH et al (2015) PICH promotes sister chromatid disjunction and co‐operates with topoisomerase II in mitosis. Nat Commun 6: 8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T, Ladurner R, Schmitz J, Kreidl E, Schleiffer A, Bhaskara V, Bando M, Shirahige K, Hyman AA, Mechtler K et al (2010) Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell 143: 737–749 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Sykora MM, Huis in ‘t Veld PJ, Mechtler K, Peters JM (2013) Aurora B and Cdk1 mediate Wapl activation and release of acetylated cohesin from chromosomes by phosphorylating Sororin. Proc Natl Acad Sci USA 110: 13404–13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL (2009a) DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer 9: 327–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL (2009b) Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 9: 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi T, Hirota T, Tsuruo T, Seimiya H (2010) TRF1 mediates mitotic abnormalities induced by Aurora‐A overexpression. Cancer Res 70: 2041–2052 [DOI] [PubMed] [Google Scholar]
- Ohishi T, Muramatsu Y, Yoshida H, Seimiya H (2014) TRF1 ensures the centromeric function of Aurora‐B and proper chromosome segregation. Mol Cell Biol 34: 2464–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn I, Heidinger‐Pauli JM, Guacci V, Unal E, Koshland DE (2008) Sister chromatid cohesion: a simple concept with a complex reality. Annu Rev Cell Dev Biol 24: 105–129 [DOI] [PubMed] [Google Scholar]
- Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, Soll D (2011) Expanding the genetic code of Escherichia coli with phosphoserine. Science 333: 1151–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirman NL, Barber KW, Aerni HR, Ma NJ, Haimovich AD, Rogulina S, Isaacs FJ, Rinehart J (2015) A flexible codon in genomically recoded Escherichia coli permits programmable protein phosphorylation. Nat Commun 6: 8130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Farr CJ (2004) Topoisomerase II: untangling its contribution at the centromere. Chromosome Res 12: 569–583 [DOI] [PubMed] [Google Scholar]
- Primorac I, Weir JR, Chiroli E, Gross F, Hoffmann I, van Gerwen S, Ciliberto A, Musacchio A (2013) Bub3 reads phosphorylated MELT repeats to promote spindle assembly checkpoint signaling. Elife 2: e01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi W, Yu H (2007) KEN‐box‐dependent degradation of the Bub1 spindle checkpoint kinase by the anaphase‐promoting complex/cyclosome. J Biol Chem 282: 3672–3679 [DOI] [PubMed] [Google Scholar]
- Rankin S, Ayad NG, Kirschner MW (2005) Sororin, a substrate of the anaphase‐promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell 18: 185–200 [DOI] [PubMed] [Google Scholar]
- Rattner JB, Hendzel MJ, Furbee CS, Muller MT, Bazett‐Jones DP (1996) Topoisomerase II alpha is associated with the mammalian centromere in a cell cycle‐ and species‐specific manner and is required for proper centromere/kinetochore structure. J Cell Biol 134: 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke RM, Jeganathan KB, Malureanu L, Harrison AM, van Deursen JM (2012) Bub1 kinase activity drives error correction and mitotic checkpoint control but not tumor suppression. J Cell Biol 199: 931–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel CG, Katis VL, Katou Y, Mori S, Itoh T, Helmhart W, Galova M, Petronczki M, Gregan J, Cetin B et al (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441: 53–61 [DOI] [PubMed] [Google Scholar]
- Sakaguchi A, Kikuchi A (2004) Functional compatibility between isoform alpha and beta of type II DNA topoisomerase. J Cell Sci 117: 1047–1054 [DOI] [PubMed] [Google Scholar]
- Salic A, Waters JC, Mitchison TJ (2004) Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell 118: 567–578 [DOI] [PubMed] [Google Scholar]
- Samejima K, Samejima I, Vagnarelli P, Ogawa H, Vargiu G, Kelly DA, de Lima AF, Kerr A, Green LC, Hudson DF et al (2012) Mitotic chromosomes are compacted laterally by KIF4 and condensin and axially by topoisomerase IIalpha. J Cell Biol 199: 755–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Tighe A, D'Alise AM, Taylor SS, Musacchio A (2010) Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J Cell Biol 190: 73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlos K, Biebricher AS, Bizard AH, Bakx JAM, Ferrete‐Bonastre AG, Modesti M, Paramasivam M, Yao Q, Peterman EJG, Wuite GJL et al (2018) Reconstitution of anaphase DNA bridge recognition and disjunction. Nat Struct Mol Biol 25: 868–876 [DOI] [PubMed] [Google Scholar]
- Shepperd LA, Meadows JC, Sochaj AM, Lancaster TC, Zou J, Buttrick GJ, Rappsilber J, Hardwick KG, Millar JB (2012) Phosphodependent recruitment of Bub1 and Bub3 to Spc7/KNL1 by Mph1 kinase maintains the spindle checkpoint. Curr Biol 22: 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintomi K, Hirano T (2010) Sister chromatid resolution: a cohesin releasing network and beyond. Chromosoma 119: 459–467 [DOI] [PubMed] [Google Scholar]
- Siemeister G, Mengel A, Fernandez‐Montalvan AE, Bone W, Schroder J, Zitzmann‐Kolbe S, Briem H, Prechtl S, Holton SJ, Monning U et al (2018) Inhibition of BUB1 Kinase by BAY 1816032 Sensitizes Tumor Cells towards Taxanes, ATR and PARP Inhibitors in vitro and in vivo . Clin Cancer Res 25: 1404–1414 [DOI] [PubMed] [Google Scholar]
- Spence JM, Critcher R, Ebersole TA, Valdivia MM, Earnshaw WC, Fukagawa T, Farr CJ (2002) Co‐localization of centromere activity, proteins and topoisomerase II within a subdomain of the major human X alpha‐satellite array. EMBO J 21: 5269–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JM, Fournier RE, Oshimura M, Regnier V, Farr CJ (2005) Topoisomerase II cleavage activity within the human D11Z1 and DXZ1 alpha‐satellite arrays. Chromosome Res 13: 637–648 [DOI] [PubMed] [Google Scholar]
- Spence JM, Phua HH, Mills W, Carpenter AJ, Porter AC, Farr CJ (2007) Depletion of topoisomerase IIalpha leads to shortening of the metaphase interkinetochore distance and abnormal persistence of PICH‐coated anaphase threads. J Cell Sci 120: 3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM (2002) The dissociation of cohesin from chromosomes in prophase is regulated by Polo‐like kinase. Mol Cell 9: 515–525 [DOI] [PubMed] [Google Scholar]
- Taagepera S, Rao PN, Drake FH, Gorbsky GJ (1993) DNA topoisomerase II alpha is the major chromosome protein recognized by the mitotic phosphoprotein antibody MPM‐2. Proc Natl Acad Sci USA 90: 8407–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Shu H, Oncel D, Chen S, Yu H (2004) Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell 16: 387–397 [DOI] [PubMed] [Google Scholar]
- Tang Z, Shu H, Qi W, Mahmood NA, Mumby MC, Yu H (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell 10: 575–585 [DOI] [PubMed] [Google Scholar]
- Tavormina PA, Come MG, Hudson JR, Mo YY, Beck WT, Gorbsky GJ (2002) Rapid exchange of mammalian topoisomerase II alpha at kinetochores and chromosome arms in mitosis. J Cell Biol 158: 23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SS, Ha E, McKeon F (1998) The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1‐related protein kinase. J Cell Biol 142: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi A, Wutz G, Huet S, Jaritz M, Wuensche A, Schirghuber E, Davidson IF, Tang W, Cisneros DA, Bhaskara V et al (2013) Wapl is an essential regulator of chromatin structure and chromosome segregation. Nature 501: 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Tanno Y, Watanabe Y (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi‐orientation. Nature 467: 719–723 [DOI] [PubMed] [Google Scholar]
- Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K (2000) Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103: 375–386 [DOI] [PubMed] [Google Scholar]
- Varier RA, Outchkourov NS, de Graaf P, van Schaik FM, Ensing HJ, Wang F, Higgins JM, Kops GJ, Timmers HT (2010) A phospho/methyl switch at histone H3 regulates TFIID association with mitotic chromosomes. EMBO J 29: 3967–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleugel M, Hoek TA, Tromer E, Sliedrecht T, Groenewold V, Omerzu M, Kops GJ (2015a) Dissecting the roles of human BUB1 in the spindle assembly checkpoint. J Cell Sci 128: 2975–2982 [DOI] [PubMed] [Google Scholar]
- Vleugel M, Omerzu M, Groenewold V, Hadders MA, Lens SMA, Kops G (2015b) Sequential multisite phospho‐regulation of KNL1‐BUB3 interfaces at mitotic kinetochores. Mol Cell 57: 824–835 [DOI] [PubMed] [Google Scholar]
- Waizenegger IC, Hauf S, Meinke A, Peters JM (2000) Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in anaphase. Cell 103: 399–410 [DOI] [PubMed] [Google Scholar]
- Wang JC (2002) Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 3: 430–440 [DOI] [PubMed] [Google Scholar]
- Wang F, Koyama N, Nishida H, Haraguchi T, Reith W, Tsukamoto T (2006) The assembly and maintenance of heterochromatin initiated by transgene repeats are independent of the RNA interference pathway in mammalian cells. Mol Cell Biol 26: 4028–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Schwarzbraun T, Speicher MR, Nigg EA (2008) Persistence of DNA threads in human anaphase cells suggests late completion of sister chromatid decatenation. Chromosoma 117: 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, Gorbsky GJ, Higgins JM (2010a) Histone H3 Thr‐3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Mayer B, Stemmann O, Nigg EA (2010b) Centromere DNA decatenation depends on cohesin removal and is required for mammalian cell division. J Cell Sci 123: 806–813 [DOI] [PubMed] [Google Scholar]
- Wang F, Higgins JM (2013) Histone modifications and mitosis: countermarks, landmarks, and bookmarks. Trends Cell Biol 23: 175–184 [DOI] [PubMed] [Google Scholar]
- Wendorff TJ, Schmidt BH, Heslop P, Austin CA, Berger JM (2012) The structure of DNA‐bound human topoisomerase II alpha: conformational mechanisms for coordinating inter‐subunit interactions with DNA cleavage. J Mol Biol 424: 109–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y (2010) Two histone marks establish the inner centromere and chromosome bi‐orientation. Science 330: 239–243 [DOI] [PubMed] [Google Scholar]
- Yamagishi Y, Yang CH, Tanno Y, Watanabe Y (2012) MPS1/Mph1 phosphorylates the kinetochore protein KNL1/Spc7 to recruit SAC components. Nat Cell Biol 14: 746–752 [DOI] [PubMed] [Google Scholar]
- Yi Q, Chen Q, Liang C, Yan H, Zhang Z, Xiang X, Zhang M, Qi F, Zhou L, Wang F (2018) HP1 links centromeric heterochromatin to centromere cohesion in mammals. EMBO Rep 19: e45484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida MM, Ting L, Gygi SP, Azuma Y (2016) SUMOylation of DNA topoisomerase IIalpha regulates histone H3 kinase Haspin and H3 phosphorylation in mitosis. J Cell Biol 213: 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H (2013) Chromosome biology: Wapl spreads its wings. Curr Biol 23: R923–R925 [DOI] [PubMed] [Google Scholar]
- Zhang G, Lischetti T, Hayward DG, Nilsson J (2015) Distinct domains in Bub1 localize RZZ and BubR1 to kinetochores to regulate the checkpoint. Nat Commun 6: 7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Kruse T, Lopez‐Mendez B, Sylvestersen KB, Garvanska DH, Schopper S, Nielsen ML, Nilsson J (2017) Bub1 positions Mad1 close to KNL1 MELT repeats to promote checkpoint signalling. Nat Commun 8: 15822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Liang C, Chen Q, Zhang Z, Zhang B, Yan H, Qi F, Zhang M, Yi Q, Guan Y et al (2017) The N‐terminal non‐kinase‐domain‐mediated binding of haspin to Pds5B protects centromeric cohesion in mitosis. Curr Biol 27: 992–1004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Expanded View Figures PDF
Review Process File


