Abstract
The amoeba Dictyostelium discoideum has been used as a model organism to study host-pathogen interaction in many intracellular bacteria. Francisella tularensis is a Gram-negative, highly infectious bacterium that causes the zoonotic disease tularemia. The bacterium is able to replicate in different phagocytic and nonphagocytic cells including mammalian, amoebae, and arthropod cells. The aim of this study was to determine the optimal temperature and infection dose in the interaction of Francisella novicida with D. discoideum in order to establish a model of Francisella infection in the social amoeba. The amoeba cells were infected with a different multiplicity of infection (5, 10, and 100) and incubated at different temperatures (22, 25, 27, 30, and 37°C). The number of intracellular bacteria within D. discoideum, as well as cytotoxicity, was determined at 2, 4, 24, 48, and 72 hours after infection. Our results showed that the optimal temperature for Francisella intracellular replication within amoeba is 30°C with the MOI of 10. We can conclude that this MOI and temperature induced the optimal growth of bacteria in Dictyostelium with low cytotoxicity.
1. Introduction
Francisella tularensis is a small Gram-negative bacterium that causes the zoonotic disease tularemia. Type A is designated as a category A select agent due to its low infectious dose [1, 2]. There are three subspecies of F. tularensis that cause human illness: tularensis (Type A), holartica (Type B), and mediasiatica [3]. F. novicida, F. philomiragia, and F. hispaniensis can cause tularemia in immunocompromised people [4]. F. noatunensis has been recognized as a fish pathogen, while recently new environmentally adapted species have been discovered including F. piscidida, F. guangzhouensis, F. opportunistica, F. Salina, F. uliginis, F. frigiditurris, and F. adeliensis [4–7].
Francisella is known as a fastidious organism with many growth requirements in vitro. The bacterium is difficult to culture and grows slowly at 37°C, requiring enriched growth medium [8]. Each species of the genus Francisella require different growth and medium conditions. Francisella requires medium supplemented with cysteine for cultivation. The bacterium grows on Buffered Charcoal-Yeast Extract Agar (BCYE), Chocolate Agar, Thayer–Martin Agar, and on the media containing hemoglobin, such as Cysteine Heart Agar Base (CHAB). Bacterial growth on blood agar is very slow with a narrow zone of alpha-hemolysis. Francisella can also be cultivated in a liquid medium; however it shows a lower replication rate. For example, the most used is Mueller–Hinton medium (MHM) in which bacteria efficiently replicates after incubation of 7 to 10 days. Francisella species can be incubated with 5% CO2, but it only enhances the growth of F. tularensis subsp. holarctica LVS (Live Vaccine Strain). F. novicida, F. noatunensis, and F. philomiragia require less nutritive supplements in the medium for in vitro growth, possibly due to their adaptation to environmental conditions [8, 9].
Further, the optimal temperature for the growth of F. tularensis is 37°C. F. tularensis grows slowly at room temperature, in contrast to F. novicida and F. philomiragia, which survive at 25°C. Because of the complex growth requirement of Francisella, most of the human tularemia cases are misdiagnosed by serology or clinical features [10, 11]. Importantly, previous studies demonstrated the impact of temperature and growth medium on the virulence of bacteria and vaccine design [12].
The exact reservoir of Francisella spp. in the environment has not been definitely determined. However, bacteria have been strongly associated with water environments [13]. It is assumed that the bacterial existence in the aquatic environment is connected with the ability of Francisella to survive and replicate within amoeba cells [14]. Our and other previous in vitro studies showed the survival and replication of F. novicida in H. vermiformis and A. castellanii, showing the possible importance of protozoa in Francisella ecology [15–17].
However, due to limited tools and antigens to study intracellular lifestyle in free-living amoeba, such as A. castellanii and H. vermiformis, D. discoideum has been used as a model organism to study phagocytosis, cell motility, and virulence factors for many bacterial pathogens, such as Pseudomonas, Legionella, Mycobacterium, Salmonella, and Klebsiella [18–23]. Dictyostelium has been established as a model organism for studying the life cycle of the fish pathogen, F. noatunensis, but it has not been established for studying the strains of Francisella that cause the disease in humans [24–26]. It has been shown that Salmonella requires O-antigen and the type VI secretion system (T6SS) for the survival within amoebae [22]. Francisella uses the T6SS to avoid lysosomal fusion within the macrophages [27]. After establishing this model of F. novicida infection in Dictyostelium it would be interesting to investigate the role of T6SS in this social amoeba for prolonged survival of Francisella in nature.
Although the previous studies demonstrated the survival and replication of Francisella within macrophages and various cell types, little is known about the adaptation of Francisella to protozoa cells [28, 29]. Our study was focused on establishing the optimal external factors required for survival and replication of F. novicida within D. discoideum. We examined the role of various incubation temperatures and the dose of infection on F. novicida capability to survive and replicate within D. discoideum.
2. Materials and Methods
2.1. The Cell Strains and Growth Conditions
The wild type strain of F. novicida was kindly obtained from prof. Anders Sjöstedt (Umeå University, Umeå, Sweden). F. novicida was cultured on buffered charcoal-yeast extract (BCYE) agar (Sigma, USA) at 37°C with 5% CO2 atmosphere for 24 h. D. discoideum strain (AX2) was obtained from prof. Michael Steinert (Technische Universitat Braunschweig, Germany). D. discoideum cells were grown in HL5 medium at 25°C. HL5 medium contains peptone (Biolife, Italy), yeast extract (Oxoid, UK), glucose (Merck, Germany), monopotassium phosphate (Kemika, Croatia), and disodium phosphate (Kemika, Croatia).
2.2. Cultivation of the Bacteria at Different Temperatures
To determine the survival of F. novicida in vitro at different growing temperatures, the bacterial suspensions (103 CFU·mL−1) were inoculated in tubes with 50 mL of the buffered yeast extract (BYE) broth (Sigma, USA). The suspensions were incubated at 22, 25, 27, 30, 37°C, and 42°C for 5 days. Every 24 hours, the number of F. novicida was determined by plating the serial dilutions on BCYE agar at 37°C.
2.3. Infection of Cells
The number of D. discoideum (105 amoebae·mL−1) was counted using a Neubauer chamber (Thermo Fisher Scientific, USA). The cells were seeded in 96-well plates, incubated overnight and infected with F. novicida at a multiplicity of infection (MOI) 5, 10, or 100. For infection, the HL5 medium was diluted with a phosphate buffer (1 : 1). In order to achieve synchronized infection, the cells were centrifuged immediately after infection at 240 g for 3 minutes at room temperature. The cells were then incubated at 27°C for 1 hour and washed 3 times with PBS to remove extracellular bacteria. This was considered as a time point zero. At each time point after infection (2, 4, 24, 48, and 72 h), the cells were treated with 0.9% Triton X-100 (Sigma, USA) for 10 minutes to lyse the cells. The number of intracellular bacteria was determined by plating the serial dilutions on BCYE agar.
2.4. Growth of Bacteria within Amoeba at Different Temperatures
To determine the influence of different incubation temperatures on the growth of bacteria within amoeba cells, the cells were infected with MOI 10 as described previously. The infected cells were incubated at 22, 25, 27, 30, or 37 °C and the number of intracellular bacteria was determined by plating the serial dilutions on BCYE agar.
2.5. Released Lactate Dehydrogenase (LDH) Assay
D. discoideum cells were infected with MOI 5, 10, or 100, washed 3 times and incubated for 1 hour (time point zero). At 2, 4, 24, 48, and 72 h after infection, 50 μL of culture supernatant was transferred to a 96-well plate for monitoring cytotoxicity. The CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega Corporation, USA) was used to quantitatively measure lactate dehydrogenase (LDH) according to the manufacturer's instructions. The absorbance signal was measured at 490 nm using a microplate reader (Tecan Systems, USA).
2.6. Statistics
Statistical significances were determined using two-tailed Student's t-test. Statistical analyses were performed using Statistica (Statsoft) software version 12 or with Graph Pad Prism version 6.0 software. p value < 0.05 were accepted as significantly different and were denoted by ∗. Exact p values are listed in the results.
3. Results
3.1. Prolonged Survival of Bacteria In Vitro Is Temperature Dependent
The effect of different incubation temperatures on the survival of F. novicida in vitro was examined. The bacterial suspensions were incubated at temperatures of 22°C, 25°C, 27°C, 30°C, 37°C, or 42°C for 5 days. By every 24 hours, the number of F. novicida was determined by plating the serial dilutions on BCYE agar at 37°C.
Our results show that F. novicida replicates with prolonged time of incubation at 22, 25, 27, 30, and 37°C. By day 5, the number of bacteria increased up to 8.0 × 105 CFU·mL−1 after incubation at 25°C. At 37°C the number of bacteria increased up to 1.5 × 105 CFU·mL−1, and at 22°C up to 8.0 × 106 CFU·mL−1, while the incubation temperatures of 27 and 30°C resulted in a higher replication of bacteria, 2.0 × 109 CFU·mL−1 and 5.0 × 1012 CFU·mL−1, respectively. In contrast, when the incubation temperature was 42°C, F. novicida was not able to survive after the second day of incubation (Figure 1).
Figure 1.
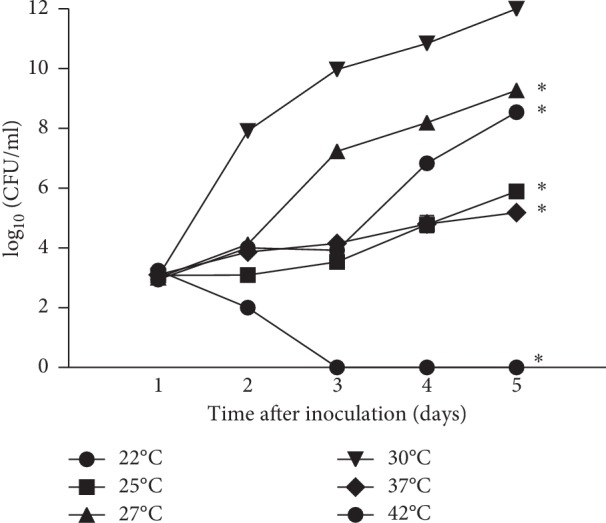
Growth kinetics of F. novicida after exposure to different temperatures. The bacterial suspensions were incubated at 22, 25, 27, 30, 37, or 42°C for 5 days. The number of F. novicida was determined daily by plating the serial dilutions on BCYE agar at 37°C. The experiments were performed in triplicate and the error bars represent standard deviations. ∗p < 0.05.
We can conclude that the optimal temperature for bacterial replication in vitro is 30°C. In comparison with optimal temperatures, the number of bacteria observed after incubation at 22°C (day 2: p=0.003, day 3: p=0.003, day 4: p=0.028, day 5: p < 0.001), 25°C (day 2: p=0.002, day 3: p=0.002, day 4: p=0.012, day 5: p < 0.001), 27°C (day 2: p=0.002, day 3: p=0.002, day 4: p=0.012, day 5: p < 0.001), 37°C (day 2: p=0.003, day 3: p=0.003, day 4: p=0.011, day 5: p < 0.001), and 42°C (day 2, day 3, day 4 and day 5: p < 0.001) was significantly different.
3.2. Bacterial Growth in Amoeba after Different Dose of Infection
The influence of a different dose of infection on the survival and replication of bacteria within D. discoideum was investigated. The amoeba cells were infected with F. novicida with MOI 5, 10, or 100. The infected amoeba cells were incubated at 27°C. At 2, 4, 24, 48, and 72 hours after infection, the number of intracellular bacteria was determined by plating the serial dilutions on BCYE agar.
Our results show that at 2 and 4 hours after infection of the amoeba with MOI 5 and 10, F. novicida did not replicate within Dictyostelium. In contrast, after infection of amoeba cells with MOI 100, bacteria started proliferation after 2 hours of infection and increased up to 106 CFU·mL−1 by 24 hours after infection (Figure 2). Each MOI induced replication of bacteria in Dictyostelium up to 24 hours after infection. The linear growth was followed by the stationary phase, in which the number of bacteria remained constant among 24 and 48 hours after infection. During the stationary bacterial phase, the number of intracellular bacteria was similar to the dose of infection of 5 and 10 (106 CFU·mL−1) (Figure 2). However, the number of intracellular bacteria was higher at the stationary bacterial phase after the infection of the cells with MOI 100 (107 CFU·mL−1) (Figure 2). At 72 hours after infection, the intracellular number of bacteria decreased to 104 CFU·mL−1 with the dose of infection 5, to 105 CFU·mL−1 with the dose of infection 10, and to 106 CFU·mL−1 with the dose of infection 100 (Figure 2).
Figure 2.
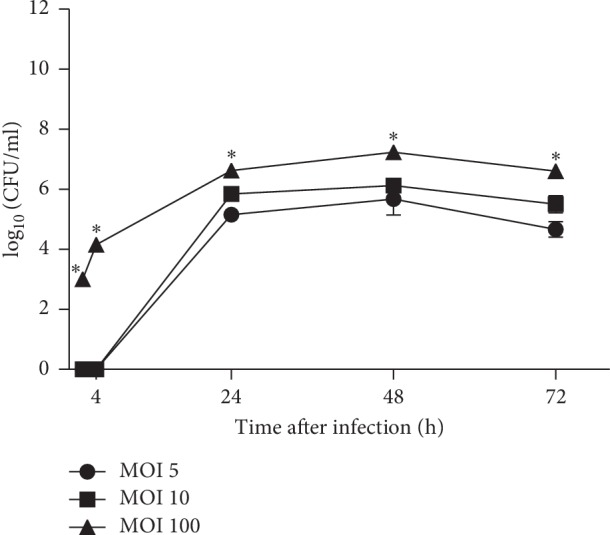
The intracellular growth kinetics of F. novicida within D. discoideum. The D. discoideum cells were infected with F. novicida at MOI 5, 10, and 100. At 2, 4, 24, 48, and 72 h after infection, the cells were lysed and suspension was plated on BCYE agar to determine the number of intracellular bacteria. The experiments were performed in triplicate and the error bars represent standard deviations. ∗p < 0.05.
Our results showed that the number of intracellular bacteria was statistically different after infection of amoeba cells with MOI 10 and MOI 100 (p=0.002) but not with MOI 5 and MOI 10 (p=0.09). The number of intracellular bacteria after infection of Dictyostelium cells with MOI 5 and MOI 100 was statistically different at all observed time points (p < 0.001).
We can conclude that the infection of amoeba cells with MOI 100 induced the highest intracellular growth of F. novicida in Dictyostelium. The number of intracellular bacteria within D. discoideum increased with higher MOI and prolonged incubation times.
3.3. The Induction of Cytopathogenicity in Amoeba Cells after Infection with Different MOI
We examined the ability of the F. novicida to induce a cytopathogenic response in D. discoideum cells. After infection of the amoeba cells with MOI 5, 10, or 100, the cells were washed 3 times and incubated for 1 hour. The supernatants of the infected cells were sampled at 2, 4, 24, 48, and 72 hours after infection and examined for the presence of the LDH.
At 2 and 4 hours after infection, the LDH levels in supernatants of D. discoideum infected with F. novicida did not differ much between different MOI (2 h after infection with MOI 5 = 0.050, MOI 10 = 0.048, MOI 100 = 0.054; 4 h after infection with MOI 5 = 0.059, MOI 10 = 0.0535, MOI 100 = 0.0625) (Figure 3). At 24 and 48 hours after infection, F. novicida induced higher LDH release in comparison to the earlier time points, which is consistent with the results of the intracellular growth of F. novicida within D. discoideum (24 h after infection with MOI 5 = 0.163, MOI 10 = 0.180, MOI 100 = 0.190; 48 h after infection) with MOI 5 = 0.213, MOI 10 = 0.220, MOI 100 = 0.184. F. novicida induced the highest LDH release at 72 h after infection at MOI 100, in comparison to previous time points (74 h after infection with MOI 5 = 0.347, MOI 10 = 0.307, MOI 100 = 0.464) (Figure 3). The LDH release was not statistically different at observed time points with different MOI. Statistical difference was observed only at 72 hours after infection in comparison to cells infected with MOI 5 and MOI 100 (p=0.03), and MOI 10 with MOI 100 (p=0.01).
Figure 3.
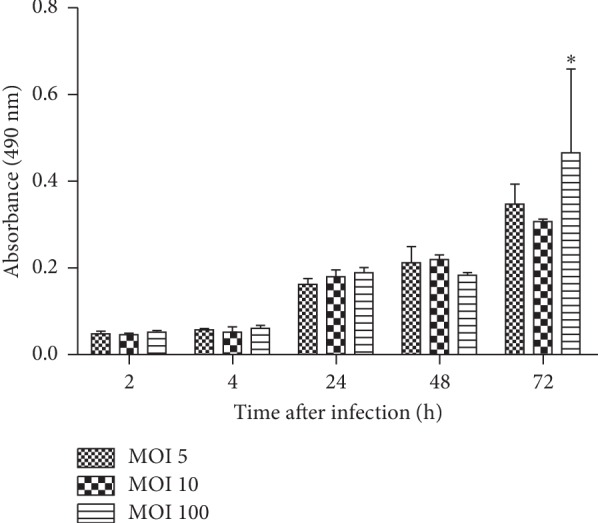
The LDH release from infected D. discoideum. Culture supernatants of infected D. discoideum cells were sampled and tested for LDH activity at 2, 4, 24, 48, and 72 h after infection. The LDH level was measured as absorbance values. The error bars represent standard deviations. ∗p < 0.05.
We can conclude that the increase of the LDH activity in the culture supernatant of the infected amoeba cells is proportional to the intracellular growth of bacteria within D. discoideum. Our results show that the MOI 10 induces the lowest level of cytotoxicity in Dictyostelium, so it will be used in our further experiments.
3.4. The Influence of Incubation Temperature on the Bacterial Growth within Amoeba
The influence of different incubation temperatures on the F. novicida growth within D. discoideum was also examined. Based on our previous results, the optimal dose of infection for the linear growth of bacteria within amoeba with low cytotoxicity is MOI of 10. Therefore, the amoeba cells were infected with MOI 10 and incubated at different temperatures (22°C, 25°C, 27°C, 30°C, and 37°C).
At all observed temperatures, F. novicida showed similar intracellular growth at 2 and 4 hours after infection and the bacterial number increased up to 104 CFU·mL−1. The bacterial cells continued to replicate within amoeba to 24 hours after infection. At 24 h after infection, the highest number of bacteria was determined at a temperature of 30°C (108 CFU·mL−1) (Figure 4). In contrast, the lowest number of intracellular bacteria was observed 24 hours after infection at the incubation temperature of 22°C (105 CFU·mL−1). At 72 hours after infection, the highest number of bacteria was determined after incubation on the temperature of 27 and 30°C (Figure 4).
Figure 4.
The growth kinetics of F novicida in D. discoideum after exposure to different temperatures. The D. discoideum cells were infected with F. novicida at MOI 10. The infected cells were incubated at 22°C, 25°C, 27°C, 30°C, and 37°C. At 2, 4, 24, 48, and 72 h after infection, the cells were lysed and plated on BCYE agar to determine the number of intracellular bacteria. The experiments were performed in triplicate. The error bars represent standard deviations. ∗p < 0.05.
Our results clearly show that the temperature of 30°C is an optimal temperature for Francisella intracellular growth within amoeba. In comparison with optimal temperature, the number of intracellular bacteria was statistically different at 24 hours after infection at 22°C (p=0.001), 25°C (p=0.001), 27°C (p=0.002), and 37°C (0.001). Also, the statistically different number of bacteria was found at 48 hours after infection at 22°C (p=0.001), 25°C (p=0.001), 27°C (p=0.002), and 37°C (0.002). At 72 hours after infection, only the number of intercellular bacteria observed after incubation of infected cells at 37°C was statistically different (p=0.001).
4. Discussion
Numerous studies have shown that the interaction between free-living amoeba and human pathogens, such as F. tularensis, Legionella pneumophila, and Mycobacterium spp., has significant implication in their environmental persistence. It was also shown that after growing within the amoeba, nonpathogenic bacteria gained their pathogenic features and that bacteria become more resistant to antibiotics [30–33]. In addition, many studies described amoeba like “Trojan horse” due to its protective role for bacteria in unfavorable environmental conditions and a role in the transmission of intracellular pathogens to humans [34, 35]. Many factors may play a role in the interaction of free-living amoeba and bacteria, such as incubation temperature and bacterial concentration. Francisella tularensis is a unique pathogen that can survive different environmental conditions and animal reservoirs. To be able to understand this wide range of hosts and the diversity of virulence strategies, there is a need for establishing more models for studying mechanisms required for infecting diverse hosts. This was successfully accomplished using D. discoideum as a host model to study the pathogenesis of Klebsiella, Legionella, Pseudomonas, and Salmonella infections [21–23, 36].
The effect of incubation temperature on the replication of Gram-negative bacteria in vitro was previously discussed in many researches. Although previous studies showed that the optimal temperature for studying the host-pathogen interaction within Dictyostelium cells is 21–23°C [21–23, 36], Francisella shows a little bit different behavior in comparison to other bacterial pathogens. The optimal growth of bacteria and D. discoideum was achieved at temperature 27–30°C. At lower temperatures, the growth of bacteria was significantly lower. Giving the concern that lower temperate may influence the expression of virulence traits, probably F. novicida somehow needs higher incubation temperature to be able to successfully replicate in this host. Our data show that F. novicida does not survive at 42°C, but it grows at 25 and 37°C. However, previous studies showed that subspecies holartica is more tolerant to a higher temperature (42°C) [8]. Each species of the genus Francisella requires different incubation temperatures. Various researches indicated that the findings of incubation temperature might improve the understanding of pathogenicity potential of the pathogens with the fastidious nature, such as L. pneumophila [37].
In addition, incubation temperature is crucial for the interaction between bacteria and amoeba. For the first time, in this study, we investigated the influence of incubation temperature on the replication of F. novicida within D. discoideum. The growth of bacteria within amoeba was temperature-dependent. Higher survival of Francisella within Dictyostelium was noted at a higher temperature (30°C) at the dose of infection 10. Due to F. novicida adaptation to environmental conditions, bacteria also have the ability to survive within amoeba at lower temperatures (22°C). In contrast, studies showed that Legionella pneumophila is unable to replicate intracellularly at room temperature, while it grew significantly within amoeba at 30, 32, and 37°C [38, 39]. Since it was shown that the growth of Gram-negative bacteria within amoeba depends on incubation temperatures, understanding the temperature dependence of F. novicida growth within amoeba will result in a better understanding of F. novicida ecological niches.
5. Conclusion
Our results demonstrate that the higher MOI (100) induce higher replication of F. novicida within amoeba with a high level of cytotoxicity. The optimal dose of infection is 10 at the incubation temperature of 30°C since it induces linear growth of F. novicida with low cytotoxicity in Dictyostelium.
Altogether, the survival and replication of F. novicida within amoeba depend on temperature and bacterial concentration. Since the free-living amoeba may be a reservoir for bacterial pathogens, growth factors in the interaction between bacteria and amoeba need to be clarified in future research for different Francisella subspecies.
Acknowledgments
This work was supported by the University of Rijeka (UNIRI-BIOMED-18-128) and the Croatian Science Foundation's funding (HRZZ-IP-2016-06-9003).
Data Availability
The Graph Pad data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Ina Kelava, Valentina Marecic, Petra Fucak, Elena Iveka, and Dominik Kolaric equally contributed to the work.
References
- 1.Ellis J., Oyston P. C. F., Green M., Titball R. W. Tularemia. Clinical Microbiology Reviews. 2002;15(4):631–646. doi: 10.1128/cmr.15.4.631-646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snowden J., Simonsen K. A. Tularemia. Treasure Island, FL, USA: Statpearls Publishing; 2019. [PubMed] [Google Scholar]
- 3.Rohmer L., Brittnacher M., Svensson K., et al. Potential source of Francisella tularensis live vaccine strain attenuation determined by genome comparison. Infection and Immunity. 2006;74(12):6895–6906. doi: 10.1128/iai.01006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thelaus J., Lundmark E., Lindgren P., Sjodin A., Forsman M. Galleria mellonella reveals niche differences between highly pathogenic and closely related strains of Francisella spp. Frontiers in Cellular and Infection Microbiology. 2018;8:p. 188. doi: 10.3389/fcimb.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brevik Ø. J., Ottem K. F., Kamaishi T., Watanabe K., Nylund A. Francisella halioticida sp. nov., a pathogen of farmed giant abalone (Haliotis gigantea) in Japan. Journal of Applied Microbiology. 2011;111(5):1044–1056. doi: 10.1111/j.1365-2672.2011.05133.x. [DOI] [PubMed] [Google Scholar]
- 6.Challacombe J. F., Petersen J. M., Gallegos-Graves V. L., Hodge D., Pillai S., Kuske C. R. Whole-genome relationships among Francisella bacteria of diverse origins define new species and provide specific regions for detection. Applied and Environmental Microbiology. 2017;83(6) doi: 10.1128/aem.00174-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottem K. F., Nylund A., Karlsbakk E., Friis-Møller A., Krossøy B., Knappskog D. New species in the genus Francisella (Gammaproteobacteria; Francisellaceae); Francisella piscicida sp. nov. isolated from cod (Gadus morhua) Archives of Microbiology. 2007;188(5):547–550. doi: 10.1007/s00203-007-0274-1. [DOI] [PubMed] [Google Scholar]
- 8.Payne M. P., Morton R. J. Effect of culture media and incubation temperature on growth of selected strains of Francisella tularensis. Journal of Veterinary Diagnostic Investigation. 1992;4(3):264–269. doi: 10.1177/104063879200400307. [DOI] [PubMed] [Google Scholar]
- 9.Sunagar R., Kumar S., Rosa S. J., Hazlett K. R. O., Gosselin E. J. Differential in vitro cultivation of Francisella tularensis influences live vaccine protective efficacy by altering the immune response. Frontiers in Immunology. 2018;9:p. 1594. doi: 10.3389/fimmu.2018.01594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labayru C., Palop A., Lopez-Urrutia L., et al. Francisella tularensis: update on microbiological diagnosis after an epidemic outbreak. Enfermedades Infecciosas y Microbiología Clínica. 1999;17(9):458–462. [PubMed] [Google Scholar]
- 11.Yates R. M., Hermetter A., Russell D. G. The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic. 2005;6(5):413–420. doi: 10.1111/j.1600-0854.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 12.Hazlett K. R. O., Cirillo K. A. Environmental adaptation of Francisella tularensis. Microbes and Infection. 2009;11(10-11):828–834. doi: 10.1016/j.micinf.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broman T., Thelaus J., Andersson A.-C., et al. Molecular detection of persistent Francisella tularensis subspecies holarctica in natural waters. International Journal of Microbiology. 2011;2011:10. doi: 10.1155/2011/851946.851946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delafont V., Mougari F., Cambau E., et al. First evidence of amoebae-mycobacteria association in drinking water network. Environmental Science & Technology. 2014;48(20):11872–11882. doi: 10.1021/es5036255. [DOI] [PubMed] [Google Scholar]
- 15.Abd H., Johansson T., Golovliov I., Sandstrom G., Forsman M. Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Applied and Environmental Microbiology. 2003;69(1):600–606. doi: 10.1128/aem.69.1.600-606.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Etr S. H., Margolis J. J., Monack D., et al. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Applied and Environmental Microbiology. 2009;75(23):7488–7500. doi: 10.1128/aem.01829-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santic M., Ozanic M., Semic V., Pavokovic G., Mrvcic V., Kwaik Y. A. Intra-vacuolar proliferation of F. Novicida within H. Vermiformis. Frontiers in Microbiology. 2011;2:p. 78. doi: 10.3389/fmicb.2011.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosson P., Zulianello L., Join-Lambert O., et al. Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system. Journal of Bacteriology. 2002;184(11):3027–3033. doi: 10.1128/jb.184.11.3027-3033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagedorn M., Soldati T. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cellular Microbiology. 2007;9(11):2716–2733. doi: 10.1111/j.1462-5822.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- 20.Solomon J. M., Isberg R. R. Growth of Legionella pneumophila in Dictyostelium discoideum: a novel system for genetic analysis of host-pathogen interactions. Trends in Microbiology. 2000;8(10):478–480. doi: 10.1016/s0966-842x(00)01852-7. [DOI] [PubMed] [Google Scholar]
- 21.Weber S., Wagner M., Hilbi H. Live-cell imaging of phosphoinositide dynamics and membrane architecture during Legionella infection. mBio. 2014;5(1) doi: 10.1128/mbio.00839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riquelme S., Varas M., Valenzuela C., et al. Relevant genes linked to virulence are required for Salmonella typhimurium to survive intracellularly in the social amoeba Dictyostelium discoideum. Frontiers in Microbiology. 2016;7:p. 1305. doi: 10.3389/fmicb.2016.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcoleta A. E., Varas M. A., Ortiz-Severin J., et al. Evaluating different virulence traits of Klebsiella pneumoniae using Dictyostelium discoideum and zebrafish larvae as host models. Frontiers in Cellular and Infection Microbiology. 2018;8:p. 30. doi: 10.3389/fcimb.2018.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenz Y., Ohnezeit D., Winther-Larsen H. C., Hagedorn M. Nramp1 and NrampB contribute to resistance against Francisella in Dictyostelium. Frontiers in Cellular and Infection Microbiology. 2017;7:p. 282. doi: 10.3389/fcimb.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenz Y., Winther-Larsen H. C., Hagedorn M. Expanding Francisella models: pairing up the soil amoeba Dictyostelium with aquatic Francisella. International Journal of Medical Microbiology. 2018;308(1):32–40. doi: 10.1016/j.ijmm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Lampe E. O., Brenz Y., Herrmann L., et al. Dissection of francisella-host cell interactions in Dictyostelium discoideum. Applied and Environmental Microbiology. 2016;82(5):1586–1598. doi: 10.1128/aem.02950-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clemens D. L., Lee B. Y., Horwitz M. A. The Francisella type VI secretion system. Frontiers in Cellular and Infection Microbiology. 2018;8:p. 121. doi: 10.3389/fcimb.2018.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolger C. E., Forestal C. A., Italo J. K., Benach J. L., Furie M. B. The live vaccine strain of Francisella tularensis replicates in human and murine macrophages but induces only the human cells to secrete proinflammatory cytokines. Journal of Leukocyte Biology. 2005;77(6):893–897. doi: 10.1189/jlb.1104637. [DOI] [PubMed] [Google Scholar]
- 29.Bosio C. M., Dow S. W. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. The Journal of Immunology. 2005;175(10):6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 30.Barker J., Scaife H., Brown M. R. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrobial Agents and Chemotherapy. 1995;39(12):2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown M., Barker J. Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends in Microbiology. 1999;7(1):46–50. doi: 10.1016/s0966-842x(98)01425-5. [DOI] [PubMed] [Google Scholar]
- 32.Miltner E. C., Bermudez L. E. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrobial Agents and Chemotherapy. 2000;44(7):1990–1994. doi: 10.1128/aac.44.7.1990-1994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winiecka-Krusnell J., Linder E. Bacterial infections of free-living amoebae. Research in Microbiology. 2001;152(7):613–619. doi: 10.1016/s0923-2508(01)01240-2. [DOI] [PubMed] [Google Scholar]
- 34.Barker J., Brown M. R. W. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology. 1994;140(6):1253–1259. doi: 10.1099/00221287-140-6-1253. [DOI] [PubMed] [Google Scholar]
- 35.Molmeret M., Horn M., Wagner M., Santic M., Abu Kwaik Y. Amoebae as training grounds for intracellular bacterial pathogens. Applied and Environmental Microbiology. 2005;71(1):20–28. doi: 10.1128/aem.71.1.20-28.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bravo-Toncio C., Álvarez J. A., Campos F., et al. Dictyostelium discoideum as a surrogate host-microbe model for antivirulence screening in Pseudomonas aeruginosa PAO1. International Journal of Antimicrobial Agents. 2016;47(5):403–409. doi: 10.1016/j.ijantimicag.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Sharaby Y., Rodriguez-Martinez S., Oks O., et al. Temperature-dependent growth modeling of environmental and clinical Legionella pneumophila multilocus variable-number tandem-repeat analysis (MLVA) genotypes. Applied and Environmental Microbiology. 2017;83(8) doi: 10.1128/aem.03295-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buse H. Y., Ashbolt N. J. Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Letters in Applied Microbiology. 2011;53(2):217–224. doi: 10.1111/j.1472-765x.2011.03094.x. [DOI] [PubMed] [Google Scholar]
- 39.Zeybek Z., Binay A. R. Growth ability of gram negative bacteria in free-living amoebae. Experimental Parasitology. 2014;145:S121–S126. doi: 10.1016/j.exppara.2014.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Graph Pad data used to support the findings of this study are available from the corresponding author upon request.


