Abstract
Background
The safety and immunogenicity of live respiratory syncytial virus (RSV) candidate vaccine, LID/ΔM2-2/1030s, with deletion of RSV ribonucleic acid synthesis regulatory protein M2-2 and genetically stabilized temperature-sensitivity mutation 1030s in the RSV polymerase protein was evaluated in RSV-seronegative children.
Methods
Respiratory syncytial virus-seronegative children ages 6–24 months received 1 intranasal dose of 105 plaque-forming units (PFU) of LID/ΔM2-2/1030s (n = 21) or placebo (n = 11). The RSV serum antibodies, vaccine shedding, and reactogenicity were assessed. During the following RSV season, medically attended acute respiratory illness (MAARI) and pre- and postsurveillance serum antibody titers were monitored.
Results
Eighty-five percent of vaccinees shed LID/ΔM2-2/1030s vaccine (median peak nasal wash titers: 3.1 log10 PFU/mL by immunoplaque assay; 5.1 log10 copies/mL by reverse-transcription quantitative polymerase chain reaction) and had ≥4-fold rise in serum-neutralizing antibodies. Respiratory symptoms and fever were common (60% vaccinees and 27% placebo recipients). One vaccinee had grade 2 wheezing with rhinovirus but without concurrent LID/ΔM2-2/1030s shedding. Five of 19 vaccinees had ≥4-fold increases in antibody titers postsurveillance without RSV-MAARI, indicating anamnestic responses without significant illness after infection with community-acquired RSV.
Conclusions
LID/ΔM2-2/1030s had excellent infectivity without evidence of genetic instability, induced durable immunity, and primed for anamnestic antibody responses, making it an attractive candidate for further evaluation.
Keywords: live-attenuated viral vaccine, neutralizing antibodies, pediatric RSV vaccine, respiratory syncytial virus (RSV), RNA regulatory protein M2-2
Live respiratory syncytial virus (RSV) vaccine LID/∆M2-2/1030s attenuated by deletion of the RNA regulatory protein M2-2 and temperature-sensitivity mutation 1030s had excellent immunogenicity and genetic stability in RSV-seronegative 6- to 24-month-old children, making it an attractive candidate for further evaluation.
(See the Editorial commentary by Teng et al, on pages 501–3.)
Respiratory syncytial virus (RSV), a major cause of lower respiratory illness (LRI) in children <5 years, results in significant morbidity and mortality worldwide [1, 2]. It is the most frequent cause of mortality among postneonatal infants in low-income countries where it mainly impacts term infants, whereas in high-income countries, mortality risk is associated with prematurity and cardiopulmonary disease [3]. However, 50% to 70% of RSV hospitalizations occur in healthy, full-term children [4]. An RSV vaccine could significantly reduce the burden of RSV [5].
The pipeline of RSV vaccine candidates has expanded [6], and among these are several live-attenuated candidates in development. A live-attenuated RSV vaccine is of particular importance for active immunization of infants and children [7, 8] because this strategy has been shown to avoid the RSV disease enhancement previously observed in RSV-naive recipients of formalin-inactivated RSV vaccines [9–11] and subunit RSV vaccines [12]. In addition, live-attenuated RSV vaccines administered by the intranasal route should stimulate innate, cellular, and antibody responses systemically as well as in the respiratory tract, the latter location being important for restricting a respiratory pathogen [13–16].
A salient challenge for live-attenuated RSV vaccines is achieving a satisfactory balance between safety and immunogenicity. The use of reverse genetics [17] and an improved understanding of RSV gene function [18] have allowed for rational design of attenuated RSV candidate vaccines, whereby well characterized attenuating mutations are combined to achieve desired levels of attenuation, immunogenicity, and genetic stability. Several complementary deoxyribonucleic acid (cDNA)-derived candidate vaccines have been evaluated in children and infants [8, 19–23]. A promising attenuation strategy involves deletion of most of the open reading frame (ORF) encoding the ribonucleic acid (RNA) synthesis regulatory protein M2-2 [21]. The RSV M2-2 protein is a small, nonabundant protein encoded by the second, downstream ORF in the M2 messenger RNA, which slightly overlaps the 5'-proximal, upstream M2-1 ORF [24]. Loss of M2-2 results in increased viral RNA gene transcription and antigen expression, but decreased genome replication [25]. The increased antigen expression results in greater immunogenicity despite lower replication [21]. Attenuating gene-deletion mutations typically are refractory to deattenuation that has been a problem for candidates with point mutations [20, 26]. Reverse genetics also have been used to generate point mutations that are stabilized against deattenuation by the choice of codons, such as the “1030s” temperature-sensitivity attenuating mutation that consists of K(AAA) and S(TCA) at codons 1321 and 1313 in the L polymerase gene [27].
Two candidate vaccines attenuated by M2-2 deletion, MEDI/∆M2-2 and LID/∆M2-2, have been evaluated [21, 22]. These candidates were derived from 2 recombinant parental cDNAs that differ by 21 nucleotide (nt) assignments scattered through the genome. The candidates also differ in the design of the M2-2 deletion [22, 25], and LID/∆M2-2 has silent mutations in the small hydrophobic (SH) gene and the SH ORF introduced to improve the stability of the cDNA during growth in bacteria [28]. Despite these differences, the 2 candidate vaccines had similar phenotypes in vitro and in animals. However, when administered to RSV-seronegative children (ages 6–24 months), MEDI/∆M2-2 exhibited substantial neutralizing antibody responses with very low peak titers of vaccine virus recovered from nasal washes (NWs), whereas LID/∆M2-2 had peak titers of vaccine virus in NWs approximately 100-fold higher than MEDI/∆M2-2 [22]. Thus, modest genetic differences apparently contributed to a different replication phenotype in children.
Although LID/∆M2-2 was well tolerated in the Phase I study, we were concerned that its higher replication might make it poorly tolerated in some recipients when administered to large populations. Therefore, for the present study, we developed LID/∆M2-2/1030s, which contains the mutations present in LID/∆M2-2 plus 1030s, the above-mentioned, genetically stabilized, temperature-sensitivity attenuating mutation in the polymerase protein L [27]. We describe the evaluation of this new intranasal vaccine candidate in RSV-seronegative children aged 6–24 months.
METHODS
Vaccine
The vaccine, LID/ΔM2-2/1030s, is a cDNA-derived version of RSV subgroup A, strain A2 (the recombinant wild-type [wt] parent is GenBank KT992094), with 241 nts deleted from the M2-2 ORF (nt 8189–8429 relative to GenBank KT992094) and the 3 potential translation initiation codons of the M2-2 ORF silenced (ATG to ACG; the T > C mutations were positions 8161, 8167, and 8179) by site-directed mutagenesis. In addition, 112 nts of the downstream nontranslated region of the SH gene (nt 4499–4610 relative to GenBank KT992094) were deleted, and 5 translationally silent nt changes were present in the 3’ end of the SH ORF (4489C>T, 4492C>T, 4495A>T; 4497A>G; 4498G>A). These changes in the SH gene, described previously, were designed to stabilize RSV full-length cDNA plasmids during propagation in bacteria and appeared phenotypically inconsequential based on replication in mice [28]. Site-directed mutagenesis was used to add the “1030s” mutation in the polymerase protein L, which consists of the temperature-sensitivity attenuating mutation Y1321>K1321(AAA) combined with the stabilizing mutation S1313(AGC)>S1313(TCA) [27], which was genetically stable in preclinical studies as well as in a prior clinical trial [23]. LID/∆M2-2/1030s has a 40°C shut-off temperature (the lowest restrictive temperature at which the reduction in replication in vitro compared with 32°C was 100-fold or greater than that of wt RSV at the 2 temperatures) and a 38°C small plaque temperature (the lowest restrictive temperature at which the small plaque phenotype was observed), compared with values of >40°C for wt RSV.
LID/ΔM2-2/1030s was recovered from cDNA in qualified Vero cells, and clinical trial material (CTM) was manufactured (Charles River Laboratories, Malvern, PA). Sequence analysis confirmed that the seed virus and final drug product were identical. The CTM was diluted onsite before dosing using Leibovitz L15 medium to a dose of 105 plaque-forming units (PFU) in a 0.5-mL volume. This was administered intranasally as a single dose divided between nostrils. Leibovitz L15 medium was used as placebo.
Study Design
This randomized (2:1 vaccine to placebo), double-blind, placebo-controlled study (ClinicalTrials.gov identifiers: NCT02952339 and NCT02794870) was conducted at US clinical trials sites (Johns Hopkins Center for Immunization Research [Baltimore, MD] and 7 domestic International Maternal Pediatric Adolescent AIDS Clinical Trials [IMPAACT] sites), with accrual between July 15, 2016 and October 5, 2016 and surveillance for RSV-medically attended acute respiratory illness (MAARI) during the ensuing RSV season from November 1, 2016 through March 31, 2017. Eligible children were aged ≥6 and <25 months, healthy, and with no history of current or past lung disease. Children born to women living with human immunodeficiency virus (HIV) but proven to be HIV-uninfected were permitted to enroll. Eligible children were RSV seronegative at screening, defined as having a complement-enhanced serum RSV 60% plaque reduction neutralizing titer (PRNT60) <1:40.
Clinical assessments and NWs were performed on study days 0, 3, 5, 7, 10, 12, 14, 17, and 28 (±1), with telephone contact on all intervening days. Additional examinations and NWs were obtained in the event of respiratory illness: namely, upper respiratory illness (URI) defined as rhinorrhea, pharyngitis, or hoarseness; cough; acute otitis media (OM); fever; and LRI, defined as wheezing, rhonchi, or rales, or a diagnosis of pneumonia or laryngotracheobronchitis (croup). All adverse events were collected through day 28; serious adverse events (SAEs) were collected until day 56. During RSV season surveillance, weekly communication identified instances of MAARI, defined as fever, URI, LRI, or OM, and, within 3 days, a NW was obtained. Sera to measure antibodies to RSV were obtained before inoculation, 56 days after inoculation, presurveillance (October 1–31 or at day 56 if that date was after October 1), and postsurveillance (April 1–30).
Written informed consent was obtained from the parents/guardians before enrollment. These studies were approved by each site’s institutional review board, conducted in accordance with the principles of the Declaration of Helsinki and the Standards of Good Clinical Practice (as defined by the International Conference on Harmonization), and monitored by the independent data safety and monitoring board of the National Institute of Allergy and Infectious Diseases, Division of Clinical Research.
Laboratory Assays
Nasal wash specimens collected during illness were tested for adventitious respiratory agents by reverse-transcription quantitative polymerase chain reaction ([RT-qPCR] Respiratory Pathogens 21 multiplex kit; Fast Track Diagnostics, Luxembourg). Vaccine virus in NWs was quantified by immunoplaque assay on Vero cells and by RT-qPCR specific for the RSV matrix (M) protein gene as described [21]. Genetic stability of vaccine isolates was determined as described [22]. Serum RSV PRNT60 were determined by complement-enhanced 60% plaque reduction neutralization assay [29]. Serum immunoglobulin (Ig)G antibody titers to the RSV F glycoprotein (anti-RSV F IgG) were determined by IgG-specific enzyme-linked immunosorbent assay using purified baculovirus-expressed F protein [30, 31], provided by Novavax, Inc. (Gaithersburg, MD), as described [21, 31, 32].
Statistical Analysis
Reciprocal serum PRNT60 and anti-RSV F IgG titers were transformed to log2 values. Even though log transformed, some data deviated from normality; thus, nonparametric methods were used for testing for statistical differences. Medians and interquartile ranges (IQRs) were used to summarize peak NW titers and serum antibody titers to RSV. Mean and standard deviation values were also presented to allow descriptive comparisons with prior studies (Supplemental Tables 1 and 2). The summaries of vaccine virus shed in NW detected by immunoplaque assay and RT-qPCR were restricted to the 18 (of 20 evaluable) vaccine recipients who were infected with vaccine. Infection was defined as the detection of vaccine virus (by plaque assay and/or RT-qPCR) and/or a ≥4-fold rise in either serum RSV PRNT60 or anti-RSV F IgG titer. When comparing the vaccinated and placebo groups, 1-tailed tests were used when there was a clear biological prediction for directionality, and 2-tailed tests were used when testing for differences with no predicted definite directional hypothesis. The Wilcoxon rank-sum test was used to compare peak viral titers and antibody titers between vaccine and placebo recipients. All analyses were performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Accrual and Participant Characteristics
The study accrued 22 vaccine and 11 placebo recipients. Two children in the vaccine group discontinued study after randomization: 1 after inoculation (before assessments) and 1 without inoculation. Of these 2, only the child who was inoculated is included in the demographics table. Baseline characteristics were similar for vaccine and placebo recipients (Table 1).
Table 1.
Baseline Characteristics of Vaccine and Placebo Recipients
| Characteristics | Number (%) | ||
|---|---|---|---|
| Vaccine | Placebo | Total | |
| N = 21 | N = 11 | N = 32 | |
| Gender | |||
| Female | 7 (33%) | 7 (64%) | 14 (44%) |
| Ethnicity | |||
| Hispanic or Latino | 10 (48%) | 4 (36%) | 14 (44%) |
| Not Hispanic or Latino | 11 (52%) | 7 (64%) | 18 (56%) |
| Race | |||
| African American | 10 (48%) | 5 (45%) | 15 (47%) |
| White | 8 (38%) | 5 (45%) | 13 (41%) |
| American Indian | 1 (5%) | 0 (0%) | 1 (3%) |
| More than one race | 0 (0%) | 1 (9%) | 1 (3%) |
| Unknown | 2 (10%) | 0 (0%) | 2 (6%) |
| Residencea | |||
| California | 6 (29%) | 3 (27%) | 9 (28%) |
| Colorado | 6 (29%) | 3 (27%) | 9 (28%) |
| Illinois | 9 (43%) | 2 (18%) | 11 (34%) |
| Maryland | 0 (0%) | 1 (9%) | 1 (3%) |
| New York | 0 (0%) | 2 (18%) | 2 (6%) |
| HIV exposed, uninfected | 14 (67%) | 6 (55%) | 20 (63%) |
| Age in monthsb | 9 (7–14) | 11 (8–16) | 9 (7–16) |
Abbreviations: HIV, human immunodeficiency virus.
aOf the 8 sites, 2 each were in California, Illinois, and New York.
bAge expressed as median (interquartile range).
Safety and Adverse Events
During the 28 days postinoculation, respiratory and/or febrile illnesses were common in both vaccine and placebo recipients, with 60% (90% CI, 39%–78%) and 27% (90% CI, 8%–56%) having 1 or more illness episodes, respectively (Table 2). Among the 12 vaccinees with illness, Fast Track RT-qPCR of NW detected vaccine virus alone in 3, vaccine virus plus ≥1 other adventitious agent in 4, and no vaccine virus but ≥1 other agent in 5. Among the 3 placebo recipients with illness, rhinovirus was detected in 1 and no agent was detected in 2. Grade 3 fever occurred in 2 participants, 1 in each group. The vaccinee with grade 3 fever (104oF, day 3) and respiratory symptoms (days 5–22) did not have detectable vaccine virus shedding or a serum RSV antibody response (thus no evidence of infection by vaccine virus), but he/she did have 4 other viruses detected in NW collected between days 5 and 22. All other events in both groups were grade 2 or lower in severity. One vaccinee had a grade 2 LRI on day 21 after inoculation, characterized by wheezing, cough, and URI symptoms. This vaccinee had vaccine virus detectable in NW only on day 9. At the time of his LRI, vaccine virus was no longer detected in the NW, but rhinovirus was present. There were no SAEs through day 56.
Table 2.
Vaccine Virus Shedding, Peak Virus Titers, and Clinical Assessment During the First 28 Days After Inoculation
| Group | No. of Children | %SheddingVaccine Virusc | Viral Detectiona | Number (%) With Indicated Symptomb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plaque Assay Log10 PFU/mLd | RT-qPCR Log10 copies/mLe | Fever | URI | LRI | Cough | OM | Respiratory or Febrile Illness | |||
| Vaccine | 20 | 85 | 3.1 (2.1, 3.7) | 5.1 (4.4, 6.0) | 4 (20) | 10 (50) | 1 (5) | 4 (20) | 2 (10) | 12 (60) |
| Placebo | 11 | 0 | 0.5 (0.5, 0.5) | 1.7 (1.7, 1.7) | 1 (9) | 2 (18) | 0 (0) | 1 (9) | 0 (0) | 3 (27) |
Abbreviations and definitions: LRI, lower respiratory illness (defined as wheezing, rhonchi, or rales, or having been diagnosed with pneumonia or laryngotracheobronchitis [croup]); OM, acute otitis media; PFU, plaque-forming units; RT-qPCR, reverse-transcription quantitative polymerase chain reaction; URI, upper respiratory illness (defined as rhinorrhea, pharyngitis, or hoarseness).
aMedian (25th, 75th percentile) peak viral titers detected in nasal washes (NWs). For the vaccine group, these summaries were calculated only for the 18 children who were infected with vaccine virus; infection was defined as the detection of vaccine virus by immunoplaque assay and/or RT-qPCR and/or a ≥4-fold rise in respiratory syncytial virus (RSV) serum-neutralizing antibody titer and/or serum anti-RSV F antibody titer.
bNumber (percentage) of children with indicated respiratory symptoms occurring in the 28 days after inoculation.
cPercentage of children with vaccine virus detected in NW by immunoplaque assay and/or RT-qPCR; 15 children had vaccine virus detected by both immunoplaque assay and PCR, and 2 only by RT-qPCR.
dFor each child, the individual peak (highest) titer, irrespective of day, was selected from among all titers measured in the NW by immunoplaque assay and expressed as log10 PFU/mL. The lower limit of detection was 0.5 log10 PFU/mL.
eFor each participant, the individual peak (highest) titer, irrespective of day, was selected from among all titers measured in NW by RT-qPCR and expressed as log10 copies/mL. The lower limit of detection was 1.7 log10 copies/mL.
Infectivity and Immunogenicity
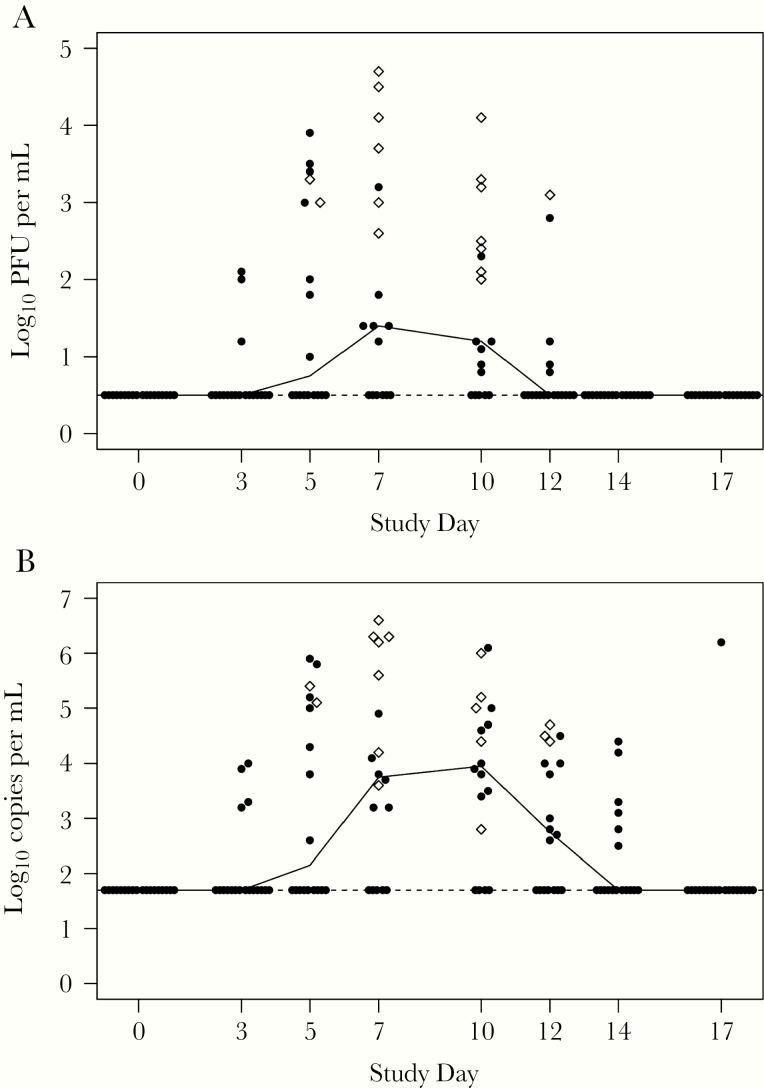
Eighteen of the 20 vaccinees (90%) were infected with vaccine virus (ie, shed vaccine virus and/or had a ≥4-fold rise in serum RSV antibodies). Vaccine virus was detected by immunoplaque assay and/or RT-qPCR for 17 of the 20 vaccinees (85%) (90% CI, 78%–99.7%) and in none of the placebo recipients (Table 2). Vaccine virus was detected for a median duration of 10 days (IQR, 9–12) by immunoplaque assay and 12 days (IQR, 10–14) by RT-qPCR. Daily median viral shedding was highest on study days 7 to 10 (Figure 1). Medians of the peak titers from each vaccinee, irrespective of study day (Figure 1, open diamonds), were 3.1 log10 PFU/mL and 5.1 log10 copies/mL for the 18 infected vaccinees (Table 2). Titers exceeding 4 log10 PFU/mL were detected in 4 (20%) vaccinees (Figure 1). Genetic stability of vaccine isolates was demonstrated by sequence analysis for the ∆M2-2 deletion and the 1030s mutation in the 15 and 14 vaccinees, respectively, comprising all vaccinees in whom the sequences could be determined for the respective regions.
Figure 1.
Vaccine virus shed in nasal washes (NWs) in vaccinees. Titers from individual participants (closed circles and open diamonds) and median titers (solid line) of vaccine virus detected in NWs collected from vaccinees during study visits (indicated study day ±1 day) after inoculation on day 0, determined by immunoplaque assay ([A] log10 plaque-forming units [PFU] per mL) and reverse-transcription quantitative polymerase chain reaction (RT-qPCR) ([B] log10 copies per mL). Peak titers for each participant are indicated by open diamonds; nonpeak titers are indicated by closed circles. Lower limits of detection indicated by dashed lines were 0.5 log10 PFU/mL and 1.7 log10 copies/mL for immunoplaque assay and RT-qPCR, respectively.
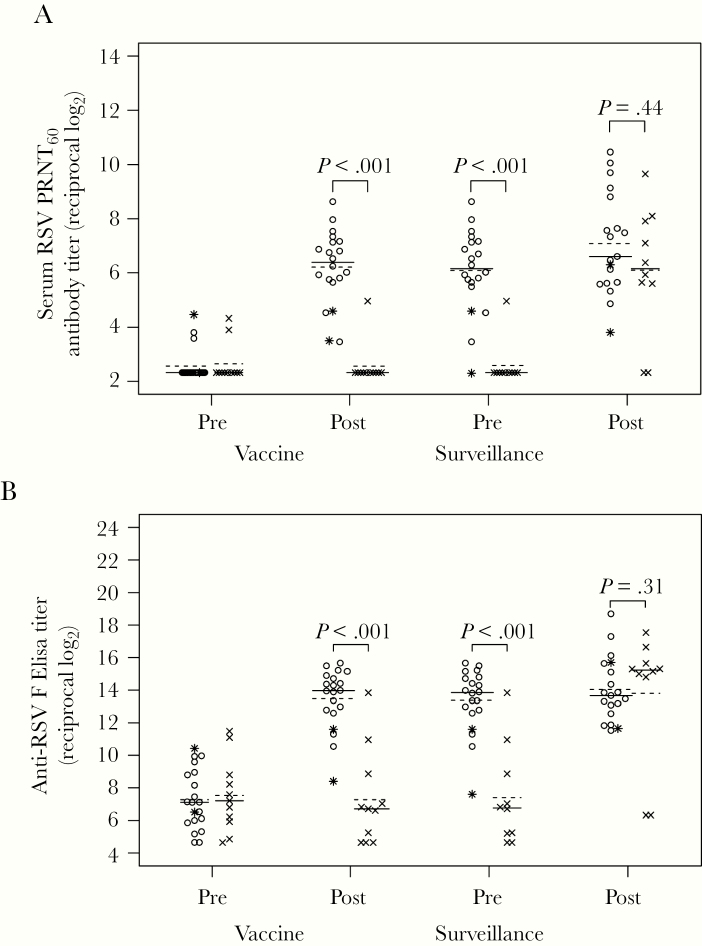
Serum RSV antibody responses were assessed at day 56 postinoculation (Table 3, Figure 2). Eighteen (90%) vaccinees had a ≥4-fold increase in 1 or both serum RSV antibody assays. Four-fold or greater rises in serum RSV PRNT60 were detected in 17 (85%) vaccinees (all the vaccinees with vaccine virus detected in NW) and no placebo recipients (P < .001). Four-fold or greater rises in serum IgG anti-RSV F titers were detected in 17 (85%) vaccinees and 2 (18%) placebo recipients (P < .001). The 2 placebo recipients with increase in anti-RSV IgG, both enrolled in August, were not ill and did not shed RSV in NW during the 28 days postinoculation.
Table 3.
Respiratory Syncytial Virus (RSV)-Specific Serum Antibody Responses Before and After Inoculation and RSV Season Surveillance
| Group | No. of Children | Serum RSV-Neutralizing Antibodiesa | Serum IgG ELISA RSV F Antibodiesa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inoculation | Surveillance | Inoculation | Surveillance | ||||||||||
| Preb | Postc | ≥4-Fold Rise N (%)e | Pred | Postd | ≥4-Fold Rise N (%)f | Preb | Postc | ≥4-Fold Rise N (%)e | Pred | Postd | ≥4-Fold Rise N (%)f | ||
| Vaccine | 20 | 2.3 (2.3–2.3) | 6.4 (5.7–7.1) | 17 (85) | 6.2 (5.6–7.1) | 6.6g (5.6–8.8) | 6g (32) | 7.1(5.9–8.9) | 14.0 (12.7–14.8) | 17 (85) | 13.8 (12.7–14.8) | 13.7g (12.6–15.6) | 5g (26) |
| Placebo | 11 | 2.9 (2.3–2.3) | 2.3 (2.3–2.3) | 0 (0) | 2.3g (2.3–2.3) | 6.2g (5.6–7.9) | 8g (80) | 7.2 (5.9–8.8) | 6.7 (4.6–8.9) | 2 (18) | 6.8g (5.2–8.9) | 15.2g (14.8–15.6) | 8g (80) |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; Ig, immunoglobulin.
aSerum RSV 60% plaque reduction neutralizing titer (PRNT60) was determined by complement-enhanced 60% plaque reduction neutralization assay; serum IgG titers to RSV F were determined by ELISA to baculovirus-expressed RSV F protein. Titer results are expressed as median reciprocal log2, with the 25th and 75th percentiles indicated in parenthesis, determined for all participants in each group. Specimens with titers below the limit of detection were assigned reciprocal titers of 2.3 log2 (PRNT60) and 4.6 log2 (ELISA).
bBefore inoculation.
cPostinoculation at study day 56.
dPresurveillance, collected October 1–31 or on day 56 if on or after October 1; postsurveillance, collected April 1 to 30 at approximately 6 months postinoculation (1 vaccine recipient had the last visit in July).
eNumber and percentage of vaccine and placebo recipients with ≥4-fold increase in antibody titers between preinoculation and postinoculation.
f Number and percentage of vaccine and placebo recipients with ≥4-fold increase in antibody titers between presurveillance and postsurveillance.
gOne participant had missing data at this time point.
Figure 2.
Serum respiratory syncytial virus (RSV) antibody titers in vaccine and placebo recipients. Serum RSV 60% plaque reduction neutralizing antibody titers (PRNT60) (A) and anti-RSV F immunoglobulin (Ig)G enzyme-linked immunosorbent assay titers (ELISA) (B) were determined by complement-enhanced 60% plaque reduction neutralization assay and IgG-specific ELISA against purified RSV F protein, respectively, for vaccine (open circles) and placebo (x) recipients in sera collected at preinoculation (screening), postinoculation (study day 56), presurveillance (October 1–31), and postsurveillance (April 1–30, after the RSV season). Two vaccinees who did not shed vaccine virus and did not have a ≥4-fold increase in either antibody response are indicated by asterisks instead of open circles. The lines indicate median (solid line) and mean (dashed line) values. Serum antibody titers are expressed as the reciprocal log2. P values were determined by Wilcoxon rank-sum test. Postsurveillance data are missing for 1 vaccine recipient, and pre- and postsurveillance data are missing for 1 placebo recipient.
Respiratory Syncytial Virus Surveillance
During the surveillance period (the RSV season after vaccination), rates of any MAARI were similar between vaccine and placebo groups: 9 of 20 (45%; 90% CI, 26%–65%) vs 7 of 11 (64%; 90% CI, 35%–86%). The MAARI associated with RSV (all RSV-B) occurred in 1 vaccinee (an LRI) and 2 placebo recipients (1 LRI, 1 URI). The vaccinee with RSV-associated MAARI was 1 of the 2 vaccinees without evidence of infection with vaccine virus.
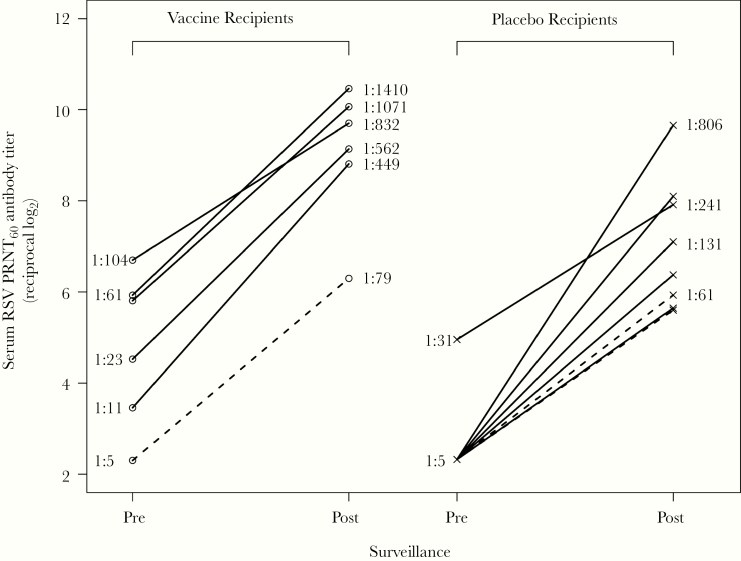
Comparison of serum RSV antibody titers pre- and postsurveillance allowed evaluation of anamnestic responses after presumed infection with wt RSV. A postsurveillance increase of ≥4-fold in PRNT60 occurred in 6 of 19 (32%) vaccinees and 8 of 10 (80%) placebo recipients, including all 3 participants with an RSV-associated MAARI. Two of the 6 vaccinees with wt RSV infection did not have a ≥4-fold increase in RSV PRNT60 at day 56 postvaccine. The median postsurveillance RSV PRNT60 titer among these 6 vaccinees was 6.5-fold higher than the median postsurveillance titer observed in the placebo recipients (median log2 [Q1, Q3] vaccine 9.4 [8.8, 10.1] vs placebo 6.7 [5.8, 8.0]) (Figure 3). The median postsurveillance RSV PRNT60 titer in the placebo recipients (6.7 [5.8, 8.0]), which represents a primary response to wt RSV, was similar to the median day 56 titer in the vaccine recipients (6.4 [5.7, 7.1]), which represents a primary response to the vaccine (Table 3).
Figure 3.
Rises in serum respiratory syncytial virus (RSV)-neutralizing antibody titers, and incidences of medically attended acute RSV illness (RSV-MAARI), during the RSV season surveillance. Serum RSV-neutralizing antibody titer (PRNT60) in sera collected pre- and postsurveillance are shown for vaccine (left) and placebo (right) recipients who had a postsurveillance ≥4-fold increase in either serum RSV PRNT60 or anti-RSV F immunoglobulin G enzyme-linked immunosorbent assay titer. Dashed and solid lines indicate participants with and without RSV-MAARI during surveillance, respectively. Titers are expressed as the reciprocal log2, but for ease of interpretation, titers corresponding to the arithmetic values are indicated for several participants.
Postsurveillance serology also allowed evaluation of the durability of primary vaccine-induced antibody responses in recipients who did not have a 4-fold increase in antibody titer and thus were presumed not to have been infected with RSV during the surveillance. In the 14 vaccinees who did not have boosted responses, the pre- and postsurveillance median RSV PRNT60 was minimally changed (median 6.7 [IQR, 5.8–7.3] log2 vs 6.1 [IQR, 5.6–7.3] log2). Similar results were observed for the pre- and postsurveillance anti-RSV F IgG titer (median 14.1 [IQR, 13.4–15.2] log2 vs 13.2 [IQR, 11.9–13.7] log2).
DISCUSSION
Respiratory syncytial virus vaccine candidate LID/∆M2-2/1030s was well tolerated in seronegative children ages 6–24 months, with no LRIs associated with vaccine shedding, nor with other concerning safety signals. LID/∆M2-2/1030s demonstrated excellent immunogenicity, with serum RSV-neutralizing and/or anti-F IgG responses in 90% of the vaccinees. Titers of RSV antibodies remained robust when evaluated at 6 to 9 months after vaccination. The postvaccination neutralizing antibody response in vaccinees was essentially the same magnitude as in placebo recipients with primary wt RSV infection after RSV season. Rates of induction of neutralizing antibody and anti-RSV F IgG were comparable to those observed in previous studies in similar populations vaccinated with 2 alternative RSV vaccine candidates attenuated by deletions in M2-2 (MEDI/∆M2-2 and LID/∆M2-2) [21, 22], but higher than one third of ∆M2-2-based vaccine (LID/cp/ΔM2-2) that was poorly infectious and highly restricted [33], and higher than vaccine candidates with combinations of cold-passaged, temperature-sensitivity (cpts), and RSV SH protein deletion mutations [20]. Although not measured in the present study, a live vaccine would be expected to induce a variety of immune responses such as local and systemic innate and cellular immunity and mucosal antibody responses [13–16], in addition to the measured serum antibody responses, which would be expected to contribute to preventing and restricting subsequent wt RSV infection. The ∆M2-2 and 1030s mutations were found to be stable, which confirms results from recent preclinical and clinical studies [22, 23, 27] and removes a significant obstacle to developing a live RSV vaccine.
During RSV season, 32% and 80% of the vaccinees and placebo recipients, respectively, demonstrated ≥4-fold increases in their RSV-specific serum antibody titers, indicating that they had been infected with wt RSV. For children with serologic evidence of wt RSV, the postsurveillance PRNT60 was substantially higher for most vaccinees than for the placebo recipients, indicating that the vaccinees experienced anamnestic antibody responses compared with primary responses for the placebo recipients. Evidence of anamnestic responses after RSV vaccination has been observed in prior studies of RSV candidates attenuated by the M2-2 deletion [21, 22]. The 1 vaccinee with an RSV-MAARI was among the minority of vaccinees who had neither a postvaccine serologic response nor detectable vaccine virus shedding.
Based on the frequency of boosted serum RSV antibody responses, infection with wt RSV during the surveillance period was frequent among the placebo recipients (80%) in this study, compared with only 32% among vaccinees. The RSV infections were usually asymptomatic because RSV-MAARI was uncommon in both placebo and vaccine recipients, which may be related to a mild RSV season and/or may reflect that the eligibility criteria for this study selected for healthy children. Given that the 2 groups should have had similar risk of exposure to RSV during the season, the lower rate of wt RSV infection in vaccinees suggests that the vaccine may have provided protection against RSV infection. In previous studies, an increase in RSV-neutralizing antibodies has emerged as a possible correlate of protection against RSV-MAARI/medically attended acute lower respiratory infection [34, 35]. Two of the 6 vaccinees with a 4-fold increase in serum RSV antibody responses during surveillance did not have a vaccine-induced serum RSV-neutralizing antibody response, which would be consistent with this concept.
The median peak titer of vaccine virus shed by vaccinees in this study was slightly lower than observed in a study of LID/∆M2-2 (3.1 vs 3.8 log10 PFU/mL) [22]. Although this comparison is limited by sample size and because the vaccines were not studied concurrently, the studies shared the same study design, research sites, laboratory assays, and participant baseline characteristics [22]. Because these vaccines had the same nt and amino acid sequences except for the 2 codons of the 1030s mutation in LID/∆M2-2/1030s, the 1030s mutation likely accounts for the lower peak titers of vaccine shedding titer, thereby achieving the goal of further attenuation of the parent LID/∆M2-2. Although the optimal peak shedding titer for an RSV vaccine has not been determined, experience with live-attenuated RSV vaccines suggests that median peak vaccine shedding should be <3.0 log10 PFU/mL, with peak titers in almost all recipients of ≤4.0 log10 PFU/mL. In support of these values, a prior study of a different vaccine virus (cpts 248/404), in which titers were 4.0–4.9 log10 PFU/mL, had high rates of nasal congestion in young infants, resulting in difficulty feeding and sleeping [19]. By contrast, a derivative of that vaccine virus with additional attenuating mutations (cpts 248/202/1030∆SH), in which titers were 2.4–3.5 log10 PFU/mL, was well tolerated in infants [20]. In the current study, the observations that the median peak titer was 3.1 log10 PFU/mL and <4.0 log10 PFU/mL in 80% of vaccinees suggest that LID/∆M2-2/1030s may have an appropriate level of attenuation. Because the rate of mild respiratory illness is frequent in both vaccine and placebo recipients, tolerability of this vaccine can only be determined by studies that enroll larger numbers of children.
The present study has several limitations. The small sample size precludes firm estimates of rates of vaccine-associated events, infectivity, immunogenicity, and viral replication. The immune assessment included the most established correlate of protection against RSV disease, namely, the serum RSV-neutralizing antibody titer. We also measured serum anti-RSV F IgG titers. Future studies could include measurements such as NW RSV-neutralizing antibodies, serum, and NW antibodies specific to RSV prefusion protein, and cellular immunity.
CONCLUSIONS
In summary, in seronegative young children, the LID/∆M2-2/1030s vaccine had high rates of vaccine virus shedding and serum RSV antibody responses. The vaccine-induced titers of serum RSV-neutralizing antibodies were essentially equivalent to primary wt RSV infection and were durable across the subsequent RSV season. In some individuals, these titers were strongly boosted during the subsequent RSV season, suggesting that the vaccine primed for potent anamnestic responses upon RSV exposure. The LID/∆M2-2/1030s vaccine was more attenuated than its LID/∆M2-2 parent, indicating that addition of the temperature sensitivity mutation (1030s) had the desired effect of further reducing viral replication. This study demonstrates that incremental modifications by rational design of a candidate RSV vaccine generated a version with increased attenuation. The LID/∆M2-2/1030s vaccine virus is a very attractive candidate for further development as a live-attenuated intranasal pediatric RSV vaccine.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We especially thank the children and families for their commitment to advancing research in the prevention of respiratory syncytial virus (RSV). We acknowledge members of the protocol team for their expert contributions including Data Managers Benjamin Johnston, Linda Marillo, Oswald Dadson, and Andee Fox (Frontier Science and Technology Research); Protocol Pharmacist Dr. Lynette Purdue (Pharmaceutical Affairs Branch, Division of AIDS); Laboratory Technologist Paul Harding (University of Colorado School of Medicine); Network Laboratory Center Specialist Dale Dayton (Children’s Hospital of Los Angeles); Westat Clinical Research Associate Scott Watson (Westat, Inc.); the companion protocol (Johns Hopkins Center for Immunization Research [CIR] 311) Medical Monitor Dr. Shirley Jankelevich and the CIR 311 Clinical Research Oversight Managers Kelly Cahill, Susan Vogel, and John Tierney (Regulatory Compliance and Human Subjects Protection Branch, Division of Clinical Research, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]). We thank the members of the NIAID, Division of Clinical Research, Data and Safety Monitoring Board. Dr. Gregory Glenn (Novavax) graciously provided the gift of baculovirus-expressed RSV F protein used as enzyme-linked immunosorbent assay antigen. Finally, we thank the dedicated site investigators and research professionals at the following institutions, in alphabetical order. Ann & Robert H. Lurie Children’s Hospital: Brize Morales, Ruth Williams, and Lynn Heald. Jacobi Medical Center: Dr. Joanna Dobroszycki, Marlene Burey, and Dr. Heesun Huh. Johns Hopkins University Center for Immunization Research: Kimberli Wanionek and Suzanne Woods. Laboratory of Infectious Diseases, NIAID, NIH: Lijuan Yang and Thomas McCarty. Rush University: Maureen McNichols and Dr. Kenneth Boyer. SUNY Stony Brook: Dr. Sharon Nachman and Erin Infanzon. University of California San Diego: Kimberly Norris and Megan Loughran. University of Colorado School of Medicine: Carrie Chambers, Jennifer Englund, Juliana Darrow, and Kacey Navarro. University of Southern California: Sara Villanueva.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. Overall funding for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institute of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC), and UM1AI106716 (IMPAACT LC) and by NICHD contract number HHSN275201800001I. R. A. K., B. T., and E. S. were funded by NIAID contract HHSN272200900010C. C. L., P. C., and U. B. were funded by the Intramural Program of the National Institute of Allergy and Infectious Disease. This work also received funding from a Cooperative Research and Development Agreement between NIAID, NIH, and Sanofi Pasteur, Inc. Work at University of Colorado Anschutz Medical Campus and Children’s Hospital Colorado was funded in part by a Clinical and Translational Science Award from the National Center for Advancing Translational Sciences/NIH (UL1 (UL1 TR002535). Work at Duke University was funded in part by the Duke University Center for AIDS Research (CFAR), an NIH-funded program (5P30 AI064518).
Potential conflicts of interest. U. J. B., C. L., and P. C. are listed as inventors on patents related to live-attenuated RSV vaccines, including vaccines made by ablating expression of the M2-2 open reading frame and vaccines containing genetically stabilized attenuating mutations, and received research support and royalties paid by Sanofi Pasteur. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Pediatric Academic Society (PAS) 2018 Conference, May 5–8, 2018, Toronto, Canada; RSV Vaccines for the World (ReSViNET) 2017, November 29–December 1, 2017, Malaga, Spain.
References
- 1. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017; 390:946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karron RA, Black RE. Determining the burden of respiratory syncytial virus disease: the known and the unknown. Lancet 2017; 390:917–8. [DOI] [PubMed] [Google Scholar]
- 3. Geoghegan S, Erviti A, Caballero MT, et al. Mortality due to respiratory syncytial virus. burden and risk factors. Am J Respir Crit Care Med 2017; 195:96–103. [DOI] [PubMed] [Google Scholar]
- 4. Boyce TG, Mellen BG, Mitchel EF Jr, Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000; 137:865–70. [DOI] [PubMed] [Google Scholar]
- 5. Giersing BK, Vekemans J, Nava S, Kaslow DC, Moorthy V. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8-10th June 2016. Vaccine 2019; 37:7315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graham BS. Vaccines against respiratory syncytial virus: the time has finally come. Vaccine 2016; 34:3535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Polack FP, Karron RA. The future of respiratory syncytial virus vaccine development. Pediatr Infect Dis J 2004; 23:S65–73. [DOI] [PubMed] [Google Scholar]
- 8. Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol 2013; 372:259–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–34. [DOI] [PubMed] [Google Scholar]
- 10. Collins PL, Murphy BR. Vaccines against human respiratory syncytial virus. In: Cane P, ed. Perspectives in Medical Virology. Vol. 14 Amsterdam, Netherlands: Elsevier; 2006. pp 233–78. [Google Scholar]
- 11. Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine 2007; 25:7372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schneider-Ohrum K, Cayatte C, Bennett AS, et al. Immunization with low doses of recombinant postfusion or prefusion respiratory syncytial virus f primes for vaccine-enhanced disease in the cotton rat model independently of the presence of a Th1-biasing (GLA-SE) or Th2-biasing (Alum) adjuvant. J Virol 2017; 91:e02180–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frederik WvG, Huan HN, Jerry RM. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerging Infect Dis J 2000; 6:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol 2004; 59:1–15. [DOI] [PubMed] [Google Scholar]
- 15. Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults. Drugs 2011; 71:1591–622. [DOI] [PubMed] [Google Scholar]
- 16. Hoft DF, Babusis E, Worku S, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis 2011; 204:845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5’ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A 1995; 92:11563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: still crazy after all these years. Virus Res 2011; 162:80–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J Infect Dis 2000; 182:1331–42. [DOI] [PubMed] [Google Scholar]
- 20. Karron RA, Wright PF, Belshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis 2005; 191:1093–104. [DOI] [PubMed] [Google Scholar]
- 21. Karron RA, Luongo C, Thumar B, et al. A gene deletion that up-regulates viral gene expression yields an attenuated RSV vaccine with improved antibody responses in children. Science Transl Med 2015; 7:312ra175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McFarland EJ, Karron RA, Muresan P, et al. ; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 2000 Study Team Live-attenuated respiratory syncytial virus vaccine candidate with deletion of RNA synthesis regulatory protein M2-2 is highly immunogenic in children. J Infect Dis 2018; 217:1347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buchholz UJ, Cunningham CK, Muresan P, et al. ; International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) P1114 Study Team Live respiratory syncytial virus (RSV) vaccine candidate containing stabilized temperature-sensitivity mutations is highly attenuated in RSV-seronegative infants and children. J Infect Dis 2018; 217:1338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collins PL, Hill MG, Johnson PR. The two open reading frames of the 22K mRNA of human respiratory syncytial virus: sequence comparison of antigenic subgroups A and B and expression in vitro. J Gen Virol 1990; 71 (Pt 12):3015–20. [DOI] [PubMed] [Google Scholar]
- 25. Bermingham A, Collins PL. The M2-2 protein of human respiratory syncytial virus is a regulatory factor involved in the balance between RNA replication and transcription. Proc Natl Acad Sci U S A 1999; 96:11259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Malkin E, Yogev R, Abughali N, et al. Safety and immunogenicity of a live attenuated RSV vaccine in healthy RSV-seronegative children 5 to 24 months of age. PLoS One 2013; 8:e77104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luongo C, Winter CC, Collins PL, Buchholz UJ. Increased genetic and phenotypic stability of a promising live-attenuated respiratory syncytial virus vaccine candidate by reverse genetics. J Virol 2012; 86:10792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bukreyev A, Belyakov IM, Berzofsky JA, Murphy BR, Collins PL. Granulocyte-macrophage colony-stimulating factor expressed by recombinant respiratory syncytial virus attenuates viral replication and increases the level of pulmonary antigen-presenting cells. J Virol 2001; 75:12128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol 1966; 83:299–313. [DOI] [PubMed] [Google Scholar]
- 30. Karron RA, Wright PF, Crowe JE Jr, et al. Evaluation of two live, cold-passaged, temperature-sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. J Infect Dis 1997; 176:1428–36. [DOI] [PubMed] [Google Scholar]
- 31. Smith G, Raghunandan R, Wu Y, et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS One 2012; 7:e50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Karron RA, Talaat K, Luke C, et al. Evaluation of two live attenuated cold-adapted H5N1 influenza virus vaccines in healthy adults. Vaccine 2009; 27:4953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cunningham CK, Karron R, Muresan P, et al. Live-Attenuated Respiratory Syncytial Virus Vaccine With Deletion of RNA Synthesis Regulatory Protein M2-2 and Cold Passage Mutations Is Overattenuated. Open Forum Infect Dis 2019; 6:ofz212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98:708–15. [DOI] [PubMed] [Google Scholar]
- 35. Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140:543–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.