Abstract
Sensory neurons of the vagus nerve receive many different peripheral signals that can change rapidly and frequently throughout the day. The ability of these neurons to convey the vast array of nuanced information to the brain requires neuronal adaptability. In this review we discuss evidence for neural plasticity in vagal afferent neurons as a mechanism for conveying nuanced information to the brain important for the control of feeding behavior. We provide evidence that synaptic plasticity, changes in membrane conductance, and neuropeptide specification are mechanisms that allow flexibility in response to metabolic cues that can be disrupted by chronic intake of energy dense diets.
Keywords: Plasticity, Vagus nerve, Obesity, Feeding, Neuropeptide, Conductance
1. Introduction
Vagal afferent neurons peripherally innervate cardiovascular, respiratory, and gastrointestinal organs (de Lartigue, 2014). Their pseudounipolar cell bodies reside in the nodose ganglia with central axons that terminate in the nucleus of the solitary tract (NTS) and convey information on a range of diverse stimuli, including heart rate, blood pressure, lung stretch and irritation, as well as gastrointestinal stretch and nutrient detection (de Lartigue, 2014). Thus, neurons of the nodose ganglia respond to a vast number of stimuli. While individual neurons will only be exposed to stimuli in their milieu, the local environment changes rapidly and frequently throughout the day. For example, vagal afferent neurons innervating the gut are exposed to gastrointestinal stretch, transmitters from enteric neurons, gastrointestinal hormones, the products of digestion and absorption, and bacterial products that all change throughout the day depending on nutrient availability. The response to these signals can be altered depending on the metabolic state, as well as local and systemic immune and inflammatory state. Therefore, vagal afferents display a remarkable degree of adaptability in response to a variety of signals and must be able to convey this information centrally.
Neural plasticity refers to the ability of neurons to change in form and function in response to alterations in their environment. This is essential for the normal development of circuits necessary to learn and to respond appropriately to our internal and external environments. Importantly, neuronal plasticity continues throughout life in order to improve performance and learn to adapt in response to experience both centrally (Ho et al., 2011) and in primary mature sensory neurons (Hubel, 1962). At the cellular level there are three forms of plasticity that have been identified to impact neuronal signaling in the central nervous system, including structural changes in dendritic and axonal anatomy, changes in membrane excitability, and neuropeptide respecification (Fig. 1). There is evidence for these different types of plasticity all occurring in peripheral vagal afferent neurons. In this review we will discuss this evidence focusing on the impact of vagal gut-brain plasticity on feeding.
Fig. 1.
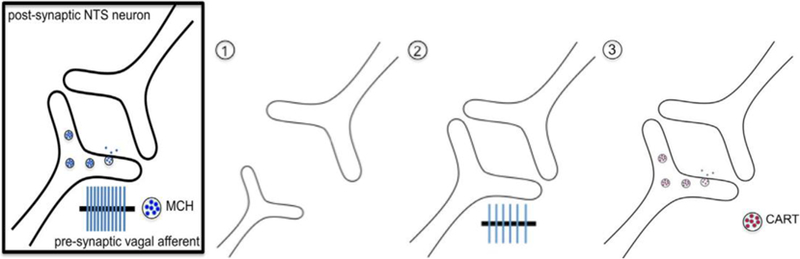
Pre-synaptic mechanisms of plasticity in the vagus nerve. (1) Synaptic plasticity. Prolonged intake of high-fat or high-sugar diets leads to withdrawal of the central vagal afferent fibers. (2) Membrane conductance. Obese animals experience decreased excitability of vagal afferent neurons, leading to reduced firing in response to metabolic signals from the gut. (3) Neuropeptide switching. In a fasted state, MCH (melanin concentrating hormone) is released and motivates food intake. Upon food entering the gut, CCK signals nutrient availability and promotes neurotransmitter switching from MCH to CART (cocaine and amphetamine regulated transcript), which to signals satiety to the hindbrain.
2. Synaptic remodeling
Changes in neuronal connectivity occur constantly in the adult brain in an experience-dependent manner. These occur both pre-synaptically and post-synaptically, including effects on synaptic vesicle release and recycling, transmitter receptor trafficking, cell adhesion, and changes in gene expression (Ho et al., 2011). Under pathologic conditions there can also be changes in synapse number caused by retraction of pre-synaptic processes or neuronal atrophy. For example, in depression, there is evidence for atrophy of pyramidal neurons in the hippocampus and medial prefrontal cortex (Iwata et al., 2013). Further, Alzheimer’s disease is characterized by reorganization of connectivity and loss of synapses (Sw and Da, 2006). Thus, synaptic remodeling is associated with pathologic states and behavioral phenotypes.
Changes in the number of central vagal afferent fibers in mature animals have also been observed under pathologic conditions. Damage to peripheral axons of the vagus nerve is associated with remodeling of central vagal fibers. In response to the vanilloid receptor (TRPV1) channel agonist, capsaicin, the density of lectin IB4, which binds to unmyelinated c-fibers, initially decreases in the NTS, presumably reflecting capsaicin induced neuronal death. Over time, IB4 density in the NTS increases, possibly indicative of neuronal regeneration and sprouting of new fibers (Ballsmide, 2015; Peters et al., 2014). A similar response has been observed in response to chronic consumption of palatable energy-dense diets. After 21 days of either high-fat or high-sugar diet IB4-labeledc-fibers withdraw from the hindbrain, and this is followed by pronounced increase in IB4 density after 8 weeks (Vaughn et al., 2017; Sen et al., 2017). The mechanism for this apparent remodeling and the extent to which it causes changes in feeding behavior and/or leads to obesity require further study.
3. Changes in excitability
The intrinsic excitability of neurons determines their activation in response to an electrical or chemical signal. Regulation of intrinsic excitability can therefore control the dynamic range of stimulus response at the cellular level (Marder et al., 1996). Voltage-gated conductance can change neuronal firing properties such as the threshold (ie. ranging from spontaneously active to inactive until high concentrations of stimulus), frequency (ie. single firing to bursts), or rate of repolarization (ie. how quickly a cell can fire after activation). These properties can change rapidly on the timescale of hours (Aizenman et al., 2003) to days (Desai et al., 1999). Similarly, changing expression of receptors at the terminals would impact the strength of the response. Therefore changing the excitability of a neuron either by altering conductance or receptor expression under physiological conditions can quickly impact behavior.
The threshold of gastric vagal afferents to mechanical stimuli is dynamic and dependent on the combination of GI hormones, feeding state and metabolic state. Gastric vagal afferent fibers can be characterized into two sub-types based on their response to tension or touch, with tension-sensitive fibers terminating predominantly in the muscular layer, and touch-sensitive fibers terminating in the mucosa (de Lartigue et al., 2014a). In lean mice fed ad libitum, leptin increases the threshold of gastric mucosal vagal afferent neurons to tactile stimuli, an effect that is lost in lean fasted mice and HF diet fed obese mice (Kentish et al., 2012). Conversely tension sensitive fibers are depressed by leptin in lean fed mice, an effect that is absent in fasted mice or HF diet fed obese mice. Therefore the threshold is largely determined by the availability and sensitivity to circulating hormones which is shaped by the feeding and metabolic state.
Under fasting conditions, leptin release is reduced (Sinha et al., 1996) while ghrelin levels will increase (Muller et al., 2002). Ghrelin reduces the excitability of mucosal and tension gastric vagal afferents (Kentish et al., 2012) and is also associated with reduced leptin signaling in vagal afferent neurons (de Lartigue et al., 2010). In obesity, ghrelin may not play an important role in reducing gastric vagal afferent sensitivity to leptin since ghrelin levels are reduced (Shiiya et al., 2002; Tschop et al., 2001), instead reduced vagal afferent neurons have been associated with the development of leptin resistance (de Lartigue, 2014; de Lartigue et al., 2012). Importantly loss of leptin receptor the threshold potential of gastric vagal afferents in obesity. In duodenal vagal afferent neurons the resting membrane potential is not significantly different between neurons in lean and obese animals, nor are characteristics of action potentials such as the duration, threshold, maximum rise slope, overshoot amplitude and maximum decay slope; however, the minimum current necessary to elicit a single action potential is nearly twice as high in obese compared to lean mice (Daly et al., 2011). Therefore reduced sensitivity of duodenal vagal afferent neurons in obese animals requires a higher level of stimulus to elicit an action potential. Still lacking is information about the fasted firing properties of gastric vagal afferents in obesity, therefore it remains unclear whether in obesity fibers remain constitutively stuck in a fasted phenotype or whether the basal excitability is reduced.
In support of the idea that vagal afferent excitability in obesity is caused by lack of plasticity, there is a lack of vagal afferent expression that could account for reduced excitability. Cannabinoid 1 receptor (CB1R) and Melanin concentrating hormone 1 receptor (MCH1R) both increase with fasting and after prolonged exposure to energy dense diets (de Lartigue et al., 2012; Dockray and Burdyga, 2011). Activation of either receptor by its ligand inhibits neuronal depolarization in central neurons (Qian et al., 2017). Conversely Y2 receptor, activated by the anorectic gastrointestinal hormone PYY3–36, is downregulated in both fasted and obese conditions (de Lartigue et al., 2012; Burdyga et al., 2008). Thus, switching hormonal sensitivity in vagal afferent neurons in both fasted and obese conditions could reduce satiation by simultaneously promoting orexigenic signaling and reducing general membrane excitability.
4. Neuropeptide switching
Early studies for immunoreactivity and mRNA detection identified the presence of several putative neuropeptides in the nodose ganglia, including substance P (SP) (Lundberg et al., 1978; Gamse et al., 1979), neurokinin A (NKA) (Nagashima et al., 1989; Helke and Niederer, 1990), calcitonin gene-related peptide (CGRP) (Zhuo et al., 1997), somatostatin (SOM) (Czyzyk-Krzeska et al., 1991), vasoactive intestinal peptide (VIP) (Zhuo et al., 1997), cholecystokinin (CCK) (Zhuo et al., 1997), enkephalin (ENK) (Zhuo et al., 1997), neuropeptide Y (NPY) (Zhuo et al., 1997), galanin, (Zhuo et al., 1997; Broberger et al., 1999), cocaine and amphetamine regulated transcript (CART) (de Lartigue et al., 2007), melanin concentrating hormone (MCH) (Burdyga et al., 2006), brain derived neurotrophic factor (BDNF) (Hsieh et al., 2010). A number of amino acid based transmitters have also been identified including glutamate (Dietrich et al., 1982), gamma-aminobutyric acid (GABA) (Dietrich et al., 1982), serotonin (Thor et al., 1988; Gaudin-Chazal et al., 1982), and the machinery to produce dopamine (Katz et al., 1983; Giacobini and Noré, 1971) and acetylcholine (Palouzier et al., 1987) has also been reported. Not only are a wide variety of transmitters present in vagal afferent neurons, but they can also be co-expressed to varying degrees. For example, the tachykinins, SP and NKA colocalize in more than 94% of vagal afferent neurons (Zhuo et al., 1997). Neurons expressing SP also make up approximately 50% of CGRP neurons in the nodose (Zhuo et al., 1997), and the vast majority of vagal afferent neurons are glutaminergic (Wright et al., 2011), suggesting potential for co-expression of glutamate, SP, NKA, and CGRP in the same neurons. The role of individual transmitters, let alone the combination, in shaping behavior in response to peripheral stimuli is poorly understood.
In addition to co-expression there have been several examples of stimuli-mediated changes in neuropeptide expression in vagal afferent neurons. Allergic inflammation in guinea pig airways increases the expression of the neuropeptides substance P and CGRP within neurons of the nodose ganglia (Myers et al., 2002). Axonal damage of the vagus nerve increases expression of the neuropeptides galanin, NPY, VIP and CCK, and decreases CCK-1 receptor expression while increasing expression of CCK-2 and Y2 receptors in vagal afferent neurons (de Lartigue, 2014; Burdyga et al., 2008). Furthermore, there are many examples of metabolic cues from the gut shown that regulate gene expression in vagal afferent neurons. Expression of clock genes in the nodose ganglia oscillate throughout the day, and are entrained by food intake (Kentish et al., 2015). In lean rats, fasting increases expression of MCH receptor 1 (Burdyga et al., 2006), CB1 (Burdyga et al., 2004), and ghrelin receptor (Kentish et al., 2012). Re-feeding reduces expression of these receptors and promotes expression of Y2 receptor. Chronic consumption of high fat diets alters mRNA expression of ghrelin receptor (Kentish et al., 2012; Paulino et al., 2009), CB1 (de Lartigue et al., 2012; Nefti et al., 2009), MCH receptor 1 (de Lartigue et al., 2012), orexin receptor (Nefti et al., 2009), Y2 receptor (de Lartigue et al., 2012), PPAR-gamma receptor (Liu et al., 2014), CCK-1 receptor (Paulino et al., 2009; Nefti et al., 2009), GPR40 (Duca et al., 2013), GPR41 (Duca et al., 2013), and GPR120 (Duca et al., 2013). Therefore vagal afferent neurons express many receptors and transmitters and have a propensity to change their neurochemistry in response to peripheral signals.
It is classically considered that the expression of neurotransmitters is fixed under normal physiological conditions, however, there have been examples of neurotransmitter switching within neurons in the developing and mature central nervous system (Spitzer, 2015). Specifically in the adult rat hypothalamus, neurons of the paraventricular and periventricular nucleus reversibly switch between dopamine and somatostatin expression depending on the length of the photoperiod (Dulcis et al., 2013). The reversible reconfiguration of the neurotransmitter within these neurons changes anxiety in rats (Dulcis et al., 2013). Similarly, there is sensory mediated neuronal adaptation in the expression of the neuropeptides CART and MCH in vagal afferent neurons (de Lartigue et al., 2007). Specifically the gastrointestinal hormone cholecystokinin, released from enteroendocrine cells of the gut in response to fats and proteins, administered to a culture of vagal afferent neurons, will shift the balance in neuropeptide expression within the same neuron from MCH to CART (de Lartigue et al., 2007). In vivo, CART and MCH expression diverges in nodose ganglia depending on the feeding state of the animal (de Lartigue et al., 2014b). The effect of this switch on behavior is not yet clear, but CART and MCH have been reported to have opposing effects on food intake when administered centrally. Intracerebroventricular (ICV) injections of CART inhibit food intake in both rats and mice (Kristensen et al., 1998; Vrang et al., 1999; Asakawa et al., 2001; Stanley et al., 2001; Lambert et al., 1998) while CART antibodies increase food intake during the active dark phase in rats (Kristensen et al., 1998; Lambert et al., 1998). ICV injection of MCH in mice or rats increases food consumption (Qu et al., 1996; Nair et al., 2009), while MCH1 receptor antagonist in rats reduces food intake (Nair et al., 2009). Thus, it is possible that the switch in CART and MCH expression repurposes gut innervating vagal afferent neurons to have opposing effects on feeding behavior depending on peripheral signals.
While a less common form of plasticity than changes in synaptic number or strength (Nelson and Turrigiano, 2008), neurotransmitter switching is particularly suited to sensory neurons innervating distal peripheral sites. By switching neuropeptide expression, individual sensory neurons can provide more nuanced information to the brain about the continually evolving local environment. Neurotransmitter switching in these neurons would enable a more complex array of information than a binomial – all or none – signal. Importantly, neuropeptide switching provides a mechanism that would reduce the burden during development, requiring a single neuron instead of two at each innervation site. This is important given the limited space within the nodose ganglia to accommodate neurons, and the vast distance that vagal afferent axons need to travel to innervate a specific area of the gut and connect with an appropriate second-order neuron involved in feeding control in the nucleus tractus solitarius of the hindbrain.
Currently there are a number of unanswered questions about the mechanism of action of neuropeptide signaling. Neuropeptides are thought to modulate fast neurotransmitter signaling (van den Pol and Anthony, 2012) although whether by changing presynaptic release or modulating activity of post-synaptic neurons is unclear. Given that they can activate extrasynaptic receptors (Nadler, 2011) and travel further than fast neurotransmitters (van den Pol and Anthony, 2012) they may modulate astrocyte mediated reuptake of fast neurotransmitter and/or coordinate excitability of neighboring neurons. Thus, neuropeptide respecification by vagal afferent neurons could impact more than only a single synapse and therefore have far-reaching effects on behavior. Likewise changes in sensing or processing of metabolic signals by vagal afferent neurons that would prevent neurotransmitter switching could therefore drive behavioral changes leading to pathophysiology.
The challenge with neurochemical plasticity is that it requires careful determination of the conditions necessary to drive expression. Absence of a transmitter only confirms that the current stimuli/stage of development/physiological state are inappropriate for expression. Given the vast number of transmitters expressed by vagal afferent neurons it is plausible that subtle distinctions in metabolic conditions can lead to large expression changes. This could explain much of the discrepancy in the literature in transmitter expression.
5. Conclusion
Vagal afferent neurons are inherently plastic. They are capable of changing synaptic number, neuronal excitability and neuropeptide expression in response to peripheral stimuli. The extent that each type of plasticity is required for normal physiology requires further work. However it is likely important in translating information about the types of macronutrient and their location in the gut into signals that the brain can integrate for meal termination, conditioned reinforcement, and controlling digestion and absorption in the context of the metabolic status. A range of mechanisms for vagal afferent plasticity may reinforce the exquisite ability of vagal afferents to respond appropriately to internal and external environmental cues. The importance of these types of plasticity are highlighted under conditions of chronic dietary change that disrupt vagal synapse number, neuronal excitability and neuropeptide expression which are each associated with overconsumption and obesity.
The plasticity of vagal afferent signaling also has important ramifications for many behavioral paradigms that rely on training animals using food- or water-deprivation to enhance motivation to perform tasks. The altered physiology of the animal must be considered in these cases, as one of the most important roles of neural plasticity is the integration of internal physiological stimuli with external stimuli. For these reasons, experiments testing behavior reliant on internal physiology necessitate dependable controls. Namely, feeding studies must take into account metabolic state of the animal, diet (palatability, caloric content, and food preference) and light-dark phases when the animal is most naturally motivated to consume. Ultimately, more sophisticated naturalistic behavioral paradigms are needed to permit neuropeptide modulation of microcircuits and behavioral choices to be experimentally addressed.
Acknowledgments
Funding
This work was supported by National Institutes of Health Grant R00DK094871.
References
- Aizenman CD, Akerman CJ, Jensen KR, Cline HT, 2003. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron [DOI] [PubMed] [Google Scholar]
- Asakawa A et al. , 2001. Cocaine-amphetamine-regulated transcript influences energy metabolism, anxiety and gastric emptying in mice. Horm. Metab. Res [DOI] [PubMed] [Google Scholar]
- Ballsmider LA et al. , Sleeve gastrectomy and roux-en-Y gastric bypass alter the gut-brain communication. doi: D – NLM: PMC4333325 EDAT- 2015/02/28 06:00 MHDA- 2015/11/05 06:00 CRDT- 2015/02/28 06:00 PHST- 2014/08/11 [received] PHST- 2014/10/15 [revised] PHST- 2014/10/17 [accepted] AID - 10.1155/2015/601985 [doi] PST - ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberger C, Holmberg K, Kuhar MJ, Tomas H, 1999. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: A putative mediator of cholecystokinin-induced satiety. PNAS 96, 13506–13511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G et al. , 2004. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J. Neurosci 24, 2708–2715. 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ, 2006. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neurosci. Res 137, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Burdyga G, 2008. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Bayliss DA, Seroogy KB, Millhorn DE, 1991. Gene expression for peptides in neurons of the petrosal and nodose ganglia in rat. Exp. Brain Res 83, 411–418. [DOI] [PubMed] [Google Scholar]
- Daly DM, Park Sj Fau - Valinsky WC, Valinsky Wc,M. J. Fau-Beyak & Beyak MJ Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. doi: D - NLM: PMC3112560 EDAT- 2011/04/14 06:00 MHDA- 2011/10/07 06:00 CRDT- 2011/04/14 06:00 AID - jphysiol.2010.204594 [pii] AID - 10.1113/jphysiol.2010.204594 [doi] PST - ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, 2014. Putative roles of neuropeptides in vagal afferent signaling. Physiol. Behav 136, 155–169. 10.1016/j.physbeh.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, Dockray GJ, 2007. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J. Neurosci 27, 2876–2882. 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G et al. , 2010. EGR1 Is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151, 3589–3599. 10.1210/en.2010-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE, 2012. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 7, e32967 10.1371/journal.pone.0032967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, Raybould HE, 2014a. Vagal plasticity the key to obesity. Mol. Metabol 3, 855–856. 10.1016/j.molmet.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, Raybould HE, Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. 2014, Mol. Metab doi: D - NLM: PMC4142400 OTO - NOTNLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG, 1999. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat. Neurosci [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Lowry OH, Loewy AD, 1982. The distribution of glutamate, GABA and aspartate in the nucleus tractus solitarius of the cat. Brain Res 237, 254– 260. 10.1016/0006-8993(82)90576-5. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G, 2011. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol 201, 313–321. [DOI] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Sakar Y, Covasa M, 2013. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int. J. Obes. (Lond.) 37, 375–381. 10.1038/ijo.2012.45. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC, 2013. Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453. 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- Gamse R, Lembeck F, Cuello AC, 1979. Substance P in the vagus nerve. Immunochemical and immunohistochemical evidence for axoplasmic transport. Naunyn Schmiedebergs Arch Pharmacol [DOI] [PubMed] [Google Scholar]
- Gaudin-Chazal G, Portalier P, Barrit MC, Puizillout JJ, 1982. Serotonin-like immunoreactivity in paraffin-sections of the nodose ganglia of the cat. Neurosci. Lett 33, 169–172. 10.1016/0304-3940(82)90246-4. [DOI] [PubMed] [Google Scholar]
- Giacobini E, Noré B, 1971. Dopa-decarboxylase in autonomic and sensory ganglia of the cat. Acta Physiol. Scand 82, 209–217. 10.1111/j.1748-1716.1971.tb04960.x. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Niederer AJ, 1990. Studies on the Coexistence of Substance P with Other Putative Transmitters in the Nodose and Petrosal Ganglia. Synapse 5, 144–151. [DOI] [PubMed] [Google Scholar]
- Ho VM, Lee J-A, Martin KC, 2011. The cell biology of synaptic plasticity. Science 334, 623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H, Robertson C, Vermehren-Schmaedick A, Balkowiec A, 2010. Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J. Neurosci. Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, 1962. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Ota Kristie T ., Duman Ronald S ., The inflammasome: pathways linking psychological stress, depression, and systemic illnesses, 2013, Brain Behav. Immun [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz DM, Markey KA, Goldstein M, Black IB, 1983. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. PNAS 80, 3526–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S et al. , 2012. Diet-induced adaptation of vagal afferent function. J. Physiol 590, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish S et al. in Obesity Week (Los Angeles, CA, 2015) [Google Scholar]
- Kristensen P et al. , 1998. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393, 72–76. [DOI] [PubMed] [Google Scholar]
- Lambert PD, et al. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse, 1998 [DOI] [PubMed] [Google Scholar]
- Liu C et al. , 2014. PPARgamma in vagal neurons regulates high-fat diet induced thermogenesis. Cell Metab 19, 722–730. 10.1016/j.cmet.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM et al. , 1978. Peptide neurons in the vagus, splanchnic and sciatic nerves⁄. Acta Physiol. Scand 104, 499–501. 10.1111/j.1748-1716.1978.tb06307.x. [DOI] [PubMed] [Google Scholar]
- Marder E, Abbott Lf Fau - Turrigiano GG, Turrigiano Gg Fau - Liu, Liu Z Fau – Golowasch ZJ, & Golowasch J Memory from the dynamics of intrinsic membrane currents doi: D - NLM: PMC33634 EDAT- 1996/11/26 MHDA- 1996/11/26 00:01 CRDT- 1996/11/26 00:00 PST - ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller AF et al. , 2002. Ghrelin drives GH secretion during fasting in man. Eur. J. Endocrinol 146, 203–207. [DOI] [PubMed] [Google Scholar]
- Myers AC, Kajekar R, Undem BJ, 2002. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am. J. Physiol. Lung Cell. Mol. Physiol 282, L775–781. 10.1152/ajplung.00353.2001. [DOI] [PubMed] [Google Scholar]
- Nadler JV Aspartate release and signalling in the hippocampus, 2011, Neurochem Res [DOI] [PubMed] [Google Scholar]
- Nagashima A et al. , 1989. Cardiovascular roles of tachykinin peptides in the nucleus tractus solitarii of rats. Brain Res 487, 392–396. 10.1016/0006-8993(89)90848-2. [DOI] [PubMed] [Google Scholar]
- Nair S, Adams-Deutsch T, Pickens C, Smith D, Sahaham Y, 2009. Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology 205, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N, 2009. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol 296, R1681–1686. 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG, 2008. Strength through diversity. Neuron 60, 477–482. 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palouzier B, Barrit-Chamoin MC, Portalier P, Ternaux JP, 1987. Cholinergic neurons in the rat nodose ganglia. Neurosci. Lett 80, 147–152. 10.1016/0304-3940(87)90644-6. [DOI] [PubMed] [Google Scholar]
- Paulino G et al. , 2009. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab 296, E898–E903. 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters James H., Gallaher Zachary R., Ryu Vitaly, and Czaja Krzysztof, 2014, Withdrawal and restoration of central vagal afferents within the dorsal vagal complex following subdiaphragmatic vagotomy, J. Comp. Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian W-J et al. , 2017. Cannabinoid CB1 and CB2 receptors differentially modulate L- and T-type Ca2+ channels in rat retinal ganglion cells. Neuropharmacology 124, 143–156. 10.1016/j.neuropharm.2017.04.027. [DOI] [PubMed] [Google Scholar]
- Qu D et al. , 1996. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature [DOI] [PubMed] [Google Scholar]
- Sen T et al. , 2017. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiiya T et al. , 2002. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab 87, 240–244. 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- Sinha MK et al. , 1996. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Investig 97, 1344–1347. 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC, 2015. Neurotransmitter switching? No surprise. Neuron 86, 1131– 1144. 10.1016/j.neuron.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley SA et al. , 2001. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res [DOI] [PubMed] [Google Scholar]
- Sw S, Da P, 2006. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J. Alzheimers Dis 9, 101–115. [DOI] [PubMed] [Google Scholar]
- Thor KB, Hill KM, Harrod C, Helke CJ, 1988. Immunohistochemical and biochemical analysis of serotonin and substance P colocalization in the nucleus tractus solitarii and associated afferent ganglia of the rat. Synapse 2, 225–231. 10.1002/syn.890020309. [DOI] [PubMed] [Google Scholar]
- Tschop M et al. , 2001. Circulating ghrelin levels are decreased in human obesity. Diabetes 50, 707–709. [DOI] [PubMed] [Google Scholar]
- Van den Pol AN, 2012. Neuropeptide Transmission in Brain Circuits. Neuron 76, 98–115. 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AC et al. , 2017. Energy-dense diet triggers changes in gut microbiota, reorganization of gutbrain vagal communication and increases body fat accumulation. Acta Neurobiol. Exp. (Wars) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P, 1999. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res [DOI] [PubMed] [Google Scholar]
- Wright J. et al. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. doi: 10.1152/ajpregu.00026.2011. doi: D - NLM: PMC3154714 EDAT- 2011/05/13 06:00 MHDA- 2011/10/19 06:00 CRDT- 2011/05/13 06:00 AID - ajpregu.00026.2011 [pii] AID - 10.1152/ajpregu.00026.2011 [doi] PST - ppublish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo H, Ichikawa H, Helke CJ, 1997. Neurochemistry of the nodose ganglion. Prog. Neurobiol 52, 79–107. [DOI] [PubMed] [Google Scholar]


