Abstract
Clinical and preclinical studies have demonstrated that depression, one of the most common psychiatric illnesses, is associated with reduced levels of neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF), contributing to neuronal atrophy in the prefrontal cortex (PFC) and hippocampus, and reduced hippocampal adult neurogenesis. Conventional monoaminergic antidepressants can block/reverse, at least partially, these deficits in part via induction of BDNF and/or VEGF, although these drugs have significant limitations, notably a time lag for therapeutic response and low response rates. Recent studies reveal that ketamine, an N-methyl-D-aspartate receptor antagonist produces rapid (within hours) and sustained (up to a week) antidepressant actions in both patients with treatment-resistant depression and rodent models of depression. Rodent studies also demonstrate that ketamine rapidly increases BDNF and VEGF release and/or expression in the medial PFC (mPFC) and hippocampus, leading to increase in the number and function of spine synapses in the mPFC and enhancement of hippocampal neurogenesis. These neurotrophic effects of ketamine are associated with the antidepressant effects of this drug. Together, these findings provide evidence for a neurotrophic mechanism underlying the rapid and sustained antidepressant actions of ketamine and pave the way for the development of rapid and more effective antidepressants with fewer side effects than ketamine.
Keywords: BDNF, Depression, Ketamine, Synaptogenesis, Rapid antidepressants, VEGF-A
1. Introduction
Major depressive disorder (MDD) is one of the most widespread, debilitating mental illnesses, affecting more than 300 million people worldwide (World Health Organization, 2018), leading to tremendous individual and socioeconomic burden (Greenberg et al., 2015). Depression is closely linked to suicide that is one of the top three leading causes of death for people ages 15-44 years (Aleman and Denys, 2014) with approximately 800,000 people commit suicide every year (World Health Organization, 2018). However, conventional antidepressants based on the monoamine hypothesis of depression, notably selective serotonin reuptake inhibitors (SSRIs), take weeks to months to produce a therapeutic response, while these drugs rapidly block monoamine reuptake and increase extracellular monoamine levels. This delayed onset is associated with an increased risk of suicidal behavior in the first month of antidepressant treatment, especially during the first nine days (Jick et al., 2004). These monoaminergic antidepressants also have limited efficacy: approximately one-third of depressed patients respond to an initial antidepressant agent, and another one-third achieve remission but only after multiple antidepressant trials that takes months to years; the remaining other one-third fail to respond to multiple antidepressant treatments and are considered treatment-resistant depression (Trivedi et al., 2006). These findings emphasize an urgent unmet need for more effective and rapid-acting antidepressants with mechanisms different from conventional antidepressants.
Our understanding of the neurobiology of depression has recently benefited greatly from the discovery of ketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist, as a rapid-acting antidepressant agent in 2000s. A single intravenous infusion of subanesthetic dose of ketamine produces rapid (within hours) and sustained (up to a week) antidepressant effects even in patients with treatment-resistant depression (Berman et al., 2000; Zarate et al., 2006), providing evidence for a promissing new class of rapid and efficacious antidepressants. Indeed, (S)-ketamine, an enantiomer of ketamine, in the form of a nasal spray application has been approved for treatment-resistant depression by the United States Food and Drug Administration in March 2019 (Kaufman, 2019).
The molecular and cellular mechanisms underlying the actions of ketamine have been intensely studied to gain insight for the development of novel, ketamine-like antidepressants but with fewer side effects. One area of interest is neurotrophic factors. In this review, we provide a brief overview of the role and consequence of altered neurotrophic factor signaling, notably brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor-A (VEGF-A, hereafter referred to as VEGF) signaling, in the etiology and treatment of depression. Then, we discuss the roles of BDNF and VEGF signaling in the actions of ketamine.
2. Neurotrophic hypothesis of depression
2.1. Neuronal atrophy and decreased levels of neurotrophic factors in depressed subjects
There is mounting evidence supporting a neurotrophic hypothesis of depression based on evidence that reduced neurotrophic factor support in the depressed subjects and rodent models contributes to neuronal atrophy in brain regions implicated in depression, notably the prefrontal cortex (PFC) and hippocampus (Duman et al., 2016; Duman et al., 1997; Duman and Monteggia, 2006). A wealth of neuroimaging studies have reported decreased volume of the PFC and hippocampus of depressed patients (Botteron et al., 2002; Bremner et al., 2000; Drevets et al., 1997; Frodl et al., 2007; Huang et al., 2013; McKinnon et al., 2009; Sheline et al., 1996; Wise et al., 2017). Rajkowska et al. (1999) report the reduction in neuronal size and cortical thickness in the postmortem PFC of depressed subjects. The number of spine synapses is also decreased in the PFC of subjects with MDD (Kang et al., 2012). Most recently, a study using positron emission tomography with the synaptic vesicle glycoprotein 2A radioligand has demonstrated that lower synaptic density in the dorsolateral PFC, anterior cingulate cortex and hippocampus is associated with depression severity and network alterations in unmedicated patients with MDD, as well as post-traumatic stress disorder (PTSD) and comorbid MDD/PTSD (Holmes et al., 2019). Moreover, it is reported that there are fewer granule cells (Boldrini et al., 2019; Boldrini et al., 2013) in the postmortem hippocampal dentate gyrus (DG) of unmedicated MDD subjects compared with medicated MDD subjects and controls without psychopathology, suggesting less neurogenesis and/or more neuronal loss in the DG of depressed patients. These findings indicate that atrophy and loss of neurons in the PFC and hippocampus are key cellular deficits associated with depression.
BDNF is one of the most widely studied neurotrophic factors in the field of depression and other psychiatric disorders. Postmortem studies demonstrate decreased levels of BDNF and its tyrosine kinase receptor, tropomyosin-related kinase B (TrkB) in the PFC and hippocampus in suicide victims (Dwivedi et al., 2003; Karege et al., 2005) and depressed subjects (Dunham et al., 2009; Qi et al., 2015; Tripp et al., 2012). The BDNF Val66Met polymorphism (valine at codon 66 replaced by methionine) blocks the processing of proBDNF to mature BDNF and activity-dependent release of BDNF (Chen et al., 2004; Chiaruttini et al., 2009; Egan et al., 2003), and is linked with an increased risk of suicide and depression in patients exposed to early life stress (Gatt et al., 2009; Sarchiapone et al., 2008; Youssef et al., 2018). BDNF Met allele carriers are also reported to have smaller PFC (Nemoto et al., 2006; Pezawas et al., 2004) and hippocampal volumes (Frodl et al., 2007; Pezawas et al., 2004). These studies indicate that decreased BDNF signaling is tightly linked with atrophy of the PFC and hippocampus in depressed patients.
VEGF is a pleiotrophic growth factor expressed by neurons and astrocytes, as well as vascular endothelial cells in human and rodent brains (Feast et al., 2012; Greene et al., 2009; Licht and Keshet, 2013; Nagashima et al., 1999). Preclinical studies demonstrate that VEGF exerts potent neurotrophic effects (Deyama et al., 2019a; Deyama et al., 2019b; Rosenstein et al., 2003). The majority of the clinical studies have reported higher serum/plasm VEGF levels in MDD patients than those in controls (although inconsistent results have also been reported (see a review by Sharma et al., 2016)). However, blood VEGF may not directly reflect brain levels of VEGF, since a preclinical study has reported that VEGF levels in the PFC and hippocampus, but not serum, are reduced in Flinder sensitive line rats, a genetic model of depression (Elfving et al., 2010). While preclinical studies indicate the importance of VEGF in the PFC and hippocampus in the neurobiology and treatment of depression (see below), brain VEGF has not been tested as much as BDNF in clinical and postmortem studies of depression. A recent cross-species transcriptional analysis demonstrates dysregulations in hippocampal VEGF, as well as other growth factors (fibroblast growth factor and insulin-like growth factor-1), in both MDD subjects and rat depression models (Carboni et al., 2018). There are also a few studies reporting that VEGF levels are decreased in the cerebrospinal fluid of patients who have attempted suicide (Isung et al., 2012) or in patients with a severe, treatment-resistant depressive episode (Kranaster et al., 2019). It has also been reported that anti-VEGF treatment for age-related macular degeneration increases the risk for anxiety and depression in the patients (Senra et al., 2017). Moreover, a VEGF single nucleotide polymorphism (SNP) is associated with increased risk for depression (Xie et al., 2017) and a recent study has suggested an association between another VEGF SNP and subiculum atrophy in first-episode drug-naïve MDD patients (Nguyen et al., 2018). Together, these findings suggest that a reduction in brain VEGF is associated with depression and suicide, although further clinical and postmortem studies are required.
2.2. Neuronal atrophy and decreased levels of neurotrophic factors in rodent models of depression
To elucidate the causal relationships between neuronal atrophy, reduced neurotrophic factors and depressive symptoms, rodent chronic stress models, such as chronic restraint stress, chronic unpredictable stress (CUS) and chronic social defeat, are widely used. Rodents exposed to chronic stress exhibit depression-like behaviors, including despair and anhedonia as seen in the forced swim and sucrose preference tests, respectively (Li et al., 2011; Qiao et al., 2014; Yang et al., 2015). Chronic stress-induced behavioral changes are associated with decreases in the number and function of spine synapses and a reduction in dendritic complexity in the medial PFC (mPFC) and hippocampus (Li et al., 2011; Liu and Aghajanian, 2008; Qiao et al., 2014; Yang et al., 2015). Chronic stress also decreases neurogenesis in the DG (Alonso et al., 2004; Czeh et al., 2002; Kiuchi et al., 2012; Pham et al., 2003). These findings indicate that chronic stress causes neuronal atrophy and loss in the mPFC and hippocampus in rodents, consistent with results from neuroimaging and postmortem studies of MDD, as mentioned above. This is supported by evidence that expression of a negative regulator of mechanistic target of rapamycin complex 1 (mTORC1) signaling decreases synaptic number and function in the mPFC and produces depressive behaviors in naïve mice (Ota et al., 2014).
Chronic stress decreases levels of BDNF and phosphorylated/activated TrkB in the PFC and hippocampus, although results from studies regarding the effect of chronic stress on total TrkB levels have been mixed (Barreto et al., 2012; Nibuya et al., 1999; Smith et al., 1995; Yang et al., 2015). BDNF heterozygous knockout mice have reduced hippocampal volume compared with wildtype controls, and decreased length and branching of apical dendrites of CA3 pyramidal neurons, comparable to what is induced by chronic stress (Magariños et al., 2011). BDNF Val66Met knock-in mice are reported to have reduced spine density and dendritic complexity in the mPFC and hippocampus (Chen et al., 2006; Liu et al., 2012) and decreased function of spine synapses on mPFC layer V pyramidal neurons (Liu et al., 2012). Additionally, the role of BDNF signaling in adult hippocampal neurogenesis has been extensively studied using various approaches, although studies in BDNF deletion mutants are conflicting (Bath et al., 2012; Lee et al., 2002; Sairanen et al., 2005). The survival of newborn cells in the DG is reported to be reduced in BDNF Val66Met and TrkB heterozygous deletion mice (Bath et al., 2012; Ieraci et al., 2016). Viral-mediated knockdown of BDNF in the DG blocks the differentiation, but not proliferation, of newborn neurons in the DG (Taliaz et al., 2010). Ablation of TrkB in progenitor cells also blocks hippocampal neurogenesis (Li et al., 2008). Together these findings indicate that reduced BDNF-TrkB signaling in the mPFC and hippocampus contributes to neuronal atrophy and loss associated with chronic stress.
Similar to BDNF, there is also evidence that levels of VEGF and its tyrosine kinase receptor, fetal liver kinase 1 (Flk-1; also known as VEGF receptor 2) are decreased in the PFC and hippocampus of rodent depression models (Elfving et al., 2010; Heine et al., 2005; Howell et al., 2011; Silva et al., 2007), although other studies report that there is no effect of CUS on hippocampal VEGF protein levels (Greene et al., 2009; Kiuchi et al., 2012). Spine density in the apical tuft of mPFC layer V pyramidal neurons is decreased in mice with excitatory neuron-specific deletion of VEGF in the forebrain (α-calcium/calmodulin-dependent protein kinase II (CaMKIIα)-Cre;Vegfaflox/flox mice; hereafter, VegfNEURON−/− mice) (Deyama et al., 2019b), similar to what is observed with chronic stress (Li et al., 2011; Liu and Aghajanian, 2008).
VEGF-Flk-1 signaling is also involved in hippocampal neurogenesis. CUS reduces the survival of newborn neurons in the DG, and this stress effect is improved by regular exercise in a VEGF-Flk-1-dependent manner (Kiuchi et al., 2012). Viral-mediated overexpression of dominant-negative Flk-1 in the rat hippocampus is reported to decrease neurogenesis (Cao et al., 2004). Additionally, viral-mediated knockdown of hippocampal VEGF reduces basal and environmental enrichment-induced neurogenesis (Cao et al., 2004; Choi et al., 2016). However, Licht et al. (2011) reported that hippocampal neurogenesis is not impaired by tetracycline-dependent induction of VEGF-trapping protein (soluble VEGF receptor 1/Fc chimera) in the mouse forebrain in adulthood, although switching off VEGF ablates long-term potentiation in the DG. The discrepancy between these studies could be due to different genetic approaches, as well as species differences (rats vs. mice). Together, although further studies are needed, these findings suggest that decreased VEGF-Flk-1, as well as BDNF-TrkB, signaling is involved in neuronal atrophy and impaired neurogenesis.
However, neither BDNF Val66Met knock-in, BDNF heterozygous deletion, forebrain excitatory neuron-specific TrkB deletion (CaMKIIα-Cre;TrkBflox/flox), VegfNEURON−/− nor forebrain excitatory neuron-specific Flk-1 deletion (CaMKIIα-Cre;Flk-1flox/flox; hereafter, Flk-1NEURON−/−) mice display depression-like behaviors under nonstress baseline conditions (Advani et al., 2009; Deyama et al., 2019b; Duman et al., 2007; Liu et al., 2012; Yu et al., 2012; Zorner et al., 2003). Mice with BDNF heterozygous deletion or heterozygous Val/Met allele exhibit a depressive phenotype only when exposed to mild stress that is insufficient to induce depression-like behaviors in wildtype mice (Advani et al., 2009; Duman et al., 2007; Yu et al., 2012); the stress vulnerability of VegfNEURON−/− and Flk-1NEURON−/− mice has not yet been examined. These findings indicate that loss of one neurotrophic factor and/or its receptor (BDNF-TrkB or VEGF-Flk-1) is not sufficient to induce depression-like behavioral changes, possibly due to the antidepressant-like and neurotrophic actions of the remaining factor. However, some previous studies report that viral-mediated BDNF or VEGF knockdown in the rat DG and female BDNF conditional mutant mice display depression-like behaviors (Choi et al., 2016; Monteggia et al., 2007; Taliaz et al., 2010). The discrepancy among these studies could be due to different knockout/knockdown approaches, as well as behavioral methodology. The depressive effects produced by selective knockdown of BDNF or VEGF in the DG could also result from opposing actions of these factors in other brain regions; BDNF signaling in the mesolimbic dopamine system is reported to promote depression-like behaviors (Berton et al., 2006; Eisch et al., 2003). Further investigation is needed to determine whether reductions of both BDNF and VEGF signaling in neurons and/or glial and endothelial cells in the mPFC or hippocampus are sufficient to elicit depressive symptoms.
2.3. Roles of BDNF and VEGF signaling in the actions of conventional antidepressants
Growing evidence supports the idea that the time lag for the therapeutic action of conventional antidepressants is related to the delayed increase in the expression of BDNF and/or VEGF in the PFC and/or hippocampus. Human postmortem studies report that increased BDNF expression is observed in the hippocampus in depressed subjects with antidepressant medications, compared with unmedicated subjects (Chen et al., 2001; Karege et al., 2005). Preclinical studies also demonstrate that chronic, but not acute, treatment with different classes of antidepressant agents, including tricyclic antidepressants (TCAs), SSRIs, serotonin-noradrenaline reuptake inhibitors (SNRIs), and monoamine oxidase inhibitors, as well as acute/chronic electroconvulsive seizures (ECS), upregulate BDNF in the PFC and hippocampus (Balu et al., 2008; Bath et al., 2012; Calabrese et al., 2007; Duman and Monteggia, 2006; Dwivedi et al., 2006; Nibuya et al., 1995; Nibuya et al., 1996; Song et al., 2019; Zhang et al., 2010). Moreover, the behavioral effects of antidepressant drugs are blocked in BDNF deletion mutant mice (Adachi et al., 2008; Monteggia et al., 2004).
Hippocampal neurogenesis plays a crucial role in the antidepressant effects of conventional antidepressants (Malberg et al., 2000; Santarelli et al., 2003) and deletion of TrkB in progenitor cells blocks both the neurogenic and antidepressant-like effects of exercise or chronic treatment with the SSRI fluoxetine or a TCA imipramine (Li et al., 2008). Similar to BDNF, VEGF expression in the hippocampus is increased by either ECS or chronic treatment with antidepressants, such as fluoxetine and a TCA desipramine (Newton et al., 2003; Warner-Schmidt and Duman, 2007). Notably, ECS and chronic fluoxetine increase VEGF expression in neuronal and endothelial cells, but not astrocytes, in the DG (Greene et al., 2009). Pharmacological blockade of VEGF-Flk-1 signaling also blocks the neurogenic and behavioral effects of ECS, regular exercise or chronic antidepressant treatment (Greene et al., 2009; Kiuchi et al., 2012; Segi-Nishida et al., 2008; Warner-Schmidt and Duman, 2007). Moreover, the antidepressant effects of chronic fluoxetine and repeated desipramine are blocked in VegfNEURON−/− and Flk-1NEURON−/− mice (Deyama and Duman, unpublished results). Taken together, these findings indicate that both BDNF and VEGF signaling play a crucial role in the neurogenic and behavioral actions of conventional antidepressants.
To our knowledge there is no direct evidence that conventional antidepressants reverse the dendritic atrophy and synaptic loss caused by chronic stress via BDNF and/or VEGF signaling, although several studies show the effects of these agents on dendritic arborization and spine density in the mPFC and hippocampus (Ampuero et al., 2010; Bessa et al., 2009; Chen et al., 2016; Davila-Hernandez et al., 2018; Guilloux et al., 2013; Song et al. 2019). Chronic fluoxetine administration is reported to increase spine density in cortical subregions (Ampuero et al., 2010); Song et al. (2019) demonstrate that chronic treatment with fluoxetine increases dendritic arborization and spine density in layer II/III pyramidal neurons in the prelimbic, but not infralimbic, subregion of rat mPFC. Chronic treatment with either fluoxetine or a multimodal antidepressant vortioxetine also increases dendritic length/branching and spine density in DG immature neurons and CA1 pyramidal neurons (Chen et al., 2016; Guilloux et al., 2013). Chronic fluoxetine or imipramine treatment also reverse the spine deficits in hippocampal and cortical regions caused by CUS exposure (Bessa et al., 2009); chronic fluoxetine treatment has been reported to reverse depression-like behavior and the reduction in dendritic length and spine density in CA1 pyramidal neurons caused by chronic social isolation, a rodent depression model (Davila-Hernandez et al., 2018). It would be important in future studies to determine the role of BDNF and VEGF signaling in the synaptic and neurotrophic actions of chronic conventional antidepressants in rodent chronic stress models.
3. Role of neurotrophic factors in the rapid antidepressant actions of ketamine
3.1. Ketamine as a rapid antidepressant agent
Berman et al. (2000) first reported that a single subanesthetic dose (0.5 mg/kg, intravenous infusion over 40 minutes) of ketamine improved depressive symptoms within four hours in double-blind, placebo-controlled trial. A larger double-blind, placebo-controlled study confirmed that ketamine produces rapid (within two hours) and sustained (up to seven days) antidepressant actions in patients with treatment-resistant MDD (Zarate et al., 2006). These beneficial effects are observed after the initial psychotomimetic and dissociative effects of ketamine, which occur during the first hour of treatment. Moreover, ketamine is effective for suicidal ideation (Wilkinson et al., 2018) and bipolar depression (Diazgranados et al., 2010; Zarate et al., 2012). Thus, the discovery of the unique antidepressant effects of ketamine can be considered the biggest breakthrough for the treatment of depression in over 60 years.
Ketamine also produces rapid and sustained antidepressant behavioral effects in rodent models and a number of preclinical studies have attempted to elucidate the underlying cellular mechanisms (Abdallah et al., 2015; Duman, 2018; Duman et al., 2016; Duman et al., 2019; Zanos et al., 2018). The mPFC, hippocampus and the projection from the ventral hippocampus to the mPFC are crucial for the antidepressant actions of ketamine (Autry et al., 2011; Carreno et al., 2016; Li et al., 2010). Our group has found that a single low dose of ketamine rapidly increases the number and function of spine synapses in mPFC layer V pyramidal neurons (Li et al., 2010) and reverses chronic stress-induced synaptic deficits of these neurons (Li et al., 2011), accompanied by increased levels of synaptic proteins, including postsynaptic density protein 95 (PSD95), synapsin-1 and glutamate α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) GluA1 subunit (Li et al., 2010; Li et al., 2011). Notably, activation of Drd1, but not Drd2, dopamine receptor-expressing mPFC pyramidal neurons projecting to the basolateral nucleus of the amygdala plays a crucial role in the antidepressant actions of ketamine (Hare et al., 2019).
A recent study using in vivo two-photon laser-scanning microscopy has revealed that chronic corticosterone (the primary rodent stress hormone) exposure-induced depression-like behavior is associated with targeted, branch-specific elimination of dendritic spines on mPFC pyramidal neurons, and ketamine reverses these effects by selectively rescuing eliminated spines and restoring coordinated activity in multicellular ensembles that predict escape behavior in male mice (Moda-Sava et al., 2019). This study also demonstrates that the effects of ketamine on behavior and ensemble activity in the mPFC precede the effects on spine formation and that optogenetic deletion of ketamine-induced newly formed spines blocks its sustained effects on despair-like behaviors in the tail suspension test, but not on anhedonia in the sucrose preference test (Moda-Sava et al., 2019). These findings indicate that mPFC synaptogenesis is required for some aspects of sustained, but not rapid, antidepressant activity of ketamine (Moda-Sava et al., 2019). However, we have observed increased levels of synaptic proteins, including PSD95, synapsin-1 and GluA1, as early as two hours after ketamine administration, consistent with the onset of the antidepressant actions of ketamine (Li et al., 2010). Together, these findings indicate that activation of mPFC pyramidal neurons and activity-dependent synaptogenesis in mPFC pyramidal neurons plays a crucial role in the antidepressant actions of ketamine.
Adult hippocampal neurogenesis may also be involved in the antidepressant actions of ketamine, although the results are mixed and somewhat contradictory. Ketamine is reported to rapidly accelerate differentiation of doublecortin (a marker of immature neurons)-positive progenitor cells to newborn neurons and maturation of new neurons in the dentate gyrus, but has no effect on neural progenitor proliferation or transition to doublecortin-positive cells (Ma et al., 2017; Soumier et al., 2016). Ketamine acceleration of late neurogenesis is responsible for the sustained, but not rapid, antidepressant effects of this drug (Ma et al., 2017; Soumier et al., 2016). Consistent with these findings, Yamada and Jinno (2019) have recently reported that ketamine increases the density of neuronal progenitors and newborn granule cells and promotes the maturation of newborn granule cells in the ventral, but not dorsal, hippocampus, although the density of neural stem cells is not affected by ketamine in both regions. In contrast, Choi et al. (2016) reported that ketamine fails to enhance cell differentiation or maturation into neurons, although ketamine accelerates cell proliferation in the DG. Moreover, Michaelsson et al. (2019) reported that ketamine increases the DG proliferation and exerts no effect on synaptic efficacy or induction of long-term potentiation in both dorsal and ventral hippocampus. Together these studies indicate that neurogenesis could be involved in the sustained but not rapid actdions of ketamine, although additional studies needed to further examine this question.
3.2. Role of BDNF in the neurotrophic and antidepressant actions of ketamine and other rapid-acting agents
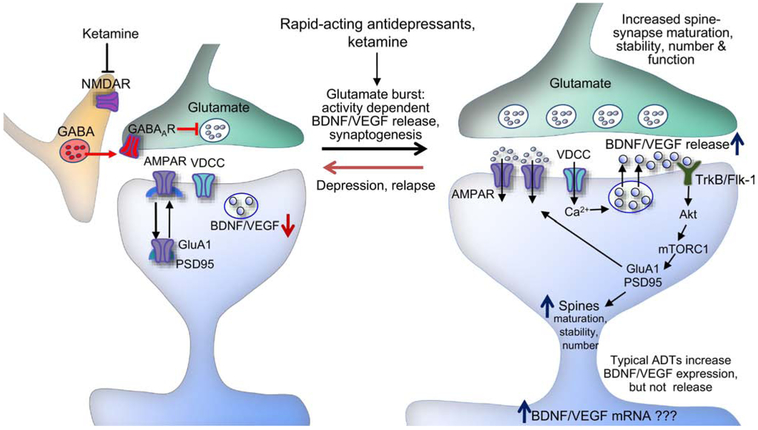
Preclinical studies demonstrate ketamine produces a paradoxial rapid enhancement of glutamate release (glutamate burst) in the mPFC that could stimulate synaptic plasticity (Duman et al., 2016). An in vivo microdialysis study showed that low doses of ketamine rapidly increase extracellular glutamate in the mPFC of rats, whereas higher anesthetic doses have no effects (Moghaddam et al., 1997). This burst of glutamate is thought to occur via blockade of NMDARs on γ-aminobutyric acidergic (GABAergic) interneurons that inhibit glutamatergic neurotransmission; because GABAergic interneurons are tonic firing, which leads to removal of Mg2+ block of NMDAR channels, they are more sensitive to low dose ketamine (Fig. 1). A recent clinical study supported this disinhibition hypothesis and provided direct evidence in human that ketamine increases glutamate release in the PFC by employing carbon-13 (13C) magnetic resonance spectroscopy approach in which ketamine increases the prefrontal rate of conversion of 13C-glutamate to 13C-glutamine, a stoichiometric measure of glutamate release (Abdallah et al., 2018).
Fig. 1.
Model for the cellular mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Ketamine blockade of NMDARs on GABAergic interneurons results in disinhibition and a rapid glutamate burst that activates AMPARs. This leads to activation of L-type voltage-dependent Ca2+ channels (VDCCs) and Ca2+ influx that stimulates BDNF and VEGF release. BDNF and VEGF stimulate TrkB and Flk-1, respectively. Activation of TrkB and Flk-1 stimulates the mTORC1 signaling pathway which controls the translation and synthesis of synaptic proteins, including GluA1 and PSD95, that are required for increases in synaptogenesis and spine maturation. These cellular events are associated with the rapid and sustained antidepressant behavioral actions of ketamine. ADT, antidepressant; GABAAR, GABAA receptor.
The ketamine-induced glutamate burst then stimulates postsynaptic AMPARs, which causes depolarization and activation of L-type voltage-dependent Ca2+ channels, leading to BDNF release (Duman et al., 2016; Lepack et al., 2014; Lepack et al., 2016; Li et al., 2010; Liu et al., 2012) (Fig. 1). The antidepressant effects of ketamine are blocked in conditional BDNF knockout and BDNF Val66Met mice (Autry et al., 2011; Liu et al., 2012). In addition, intra-mPFC infusion of an anti-BDNF neutralizing antibody (nAb) that would bind and sequester BDNF in the extracellular space blocks the antidepressant effects of ketamine (Lepack et al., 2014) and systemic administration of a TrkB inhibitor blocks the antidepressant effects of both the (R)- and (S)-ketamine enantiomers (Yang et al., 2015). Conversely, a single infusion of BDNF into the mPFC produces ketamine-like rapid and sustained antidepressant effects (Deyama et al., 2019a; Kato et al., 2018). Moreover, ketamine-induced synaptogenesis in the apical tuft of mPFC layer V pyramidal neurons is blocked in BDNF Val66Met mice (Liu et al., 2012). Ketamine also rapidly increases hippocampal BDNF expression (Autry et al., 2011; Ma et al., 2017) and BDNF-TrkB-dependent neuronal differentiation in the DG is reported to be important for the sustained antidepressant actions of ketamine (Ma et al., 2017). Additionally, the antidepressant actions of ketamine are attenuated in MDD patients with BDNF Val66Met allele (Laje et al., 2012), although this effect appears to be race-specific (Su et al., 2017). Together, these findings indicate that activity-dependent BDNF release and/or increased expression of BDNF are required for the neurotrophic and antidepressant actions of ketamine.
BDNF activates several intracellular signaling pathways, including mTORC1 signaling pathway. Intracerebroventricular infusion of an mTORC1 inhibitor, rapamycin blocks ketamine-induced increases in the levels of synaptic proteins (PSD95, synapsin-1 and GluA1) in PFC synaptoneurosomes and spine density in the apical tuft of mPFC layer V pyramidal neurons (Li et al., 2010). Infusion of rapamycin into the mPFC also blocks the antidepressant behavioral actions of ketamine (Li et al., 2010). Further evidence for the role of mTORC1 activation is provided by our recent study showing that NV-5138, a blood-brain barrier-permeable synthetic leucine analogue that binds sestrin to directly activate the mTORC1 signaling pathway, produces ketamine-like rapid synaptic and antidepressant behavioral responses (Kato et al., 2019). These findings indicate the key role of BDNF-TrkB-mTORC1 signaling pathway in the actions of ketamine (Fig. 1). Notable sex differences have been reported with females rodents more sensitive to low doses of ketamine, and ketamine-induced synaptic changes in the mPFC more robust in male compared to female rodents (Carrier and Kabbaj, 2013; Sarkar and Kabbaj, 2016; Thelen et al., 2019); however, there are no clinical reports of sex differences in sensitivity to ketamine.
Activity-dependent BDNF release and downstream mTORC1 signaling are also required for the synaptic and antidepressant actions of other rapid-acting agents, including scopolamine (a nonselective muscarinic acetylcholine receptor antagonist) (Ghosal et al., 2018; Voleti et al., 2013; Wohleb et al., 2016), rapastinel (formerly GLYX-13, an NMDAR positive allosteric modulator) (Kato et al., 2018; Liu et al., 2017), (2R,6R)-hydroxynorketamine (HNK; a ketamine metabolite) (Fukumoto et al., 2019) and NV-5138 (an mTORC1 activator; see above) (Kato et al., 2019). These studies include evidence that intra-mPFC infusion of a BDNF nAb blocks the behavioral actions of ketamine, scopolamine, rapastinel, (2R, 6R)-HNK and NV-5138, as well as evidence that the effects of these agents are blocked in BDNF Met mice. However, unlike ketamine, rapastinel directly enhances postsynaptic NMDA activity and Ca2+ influx in mPFC pyramidal neurons, but does not increase extracellular glutamate levels in the mPFC (Banerjee et al., 2016; Donello et al., 2019; also see a recent review by Kato and Duman (2020)). Further studies are required to determine if NV-5138 increases BDNF release and the related mechanism, although it is notable that AMPAR activity is required, in part for the antidepressant behavioral actions of this agent (Kato et al., 2019). Together these studies indicate that the rapid, as well as sustained actions of ketamine result from activity dependent release of BDNF in the mPFC and possibly the hippocampus. This differentiates rapid acting agents from typical monoaminergic antidepressants that slowly increase the expression of BDNF, but do not cause activity dependent release (Lepack et al., 2016). This appears for be a critical difference as activity dependent release of BDNF is required for synaptic plasiticity (Harward et al., 2016; Hedrick et al., 2016). In addition, ketamine is reported to increase BDNF translation in the hippocampus via deactivation of eukaryotic elongation factor 2 signaling (Autry et al., 2011).
3.3. Role of VEGF in the neurotrophic and antidepressant actions of ketamine
In addition to BDNF, recent studies have demonstrated a key role for VEGF signaling in the antidepressant actions of ketamine. Ketamine is reported to induce VEGF expression in the hippocampus (Choi et al., 2016). In addition, viral-mediated non-celltype-specific knockdown of VEGF in the DG produces depression-like behaviors and reduces neurogenesis (as discussed above), effects that are partially blocked by ketamine (Choi et al., 2016). These findings suggest that ketamine induction of hippocampal VEGF is only partially involved in its neurogenic and antidepressant behavioral actions.
More recently, we have demonstrated that the antidepressant behavioral actions of ketamine are completely blocked by forebrain excitatory neuron-specific deletion of VEGF or Flk-1 (VegfNEURON−/− and Flk-1NEURON−/− mice), intra-mPFC infusion of a VEGF nAb, or viral-mediated knockdown of Flk-1 in mPFC pyramidal neurons (Deyama et al., 2019b). In addition, a single intra-mPFC infusion of recombinant VEGF is sufficient to produce ketamine-like antidepressant effects which are completely blocked by neuron-specific Flk-1 deletion (Deyama et al., 2019b). Moreover, inhibition of neuronal VEGF signaling blocks the neurotrophic and synaptogenic actions of ketamine (Deyama et al., 2019b). These findings indicate that mPFC pyramidal neurons are both the source and the target of VEGF that is released in response to ketamine and that neuronal VEGF-Flk-1 signaling plays a crucial role in the antidepressant and synaptic actions of ketamine (Fig. 1). Taken together, the results indicate that ketamine increases VEGF, as well as BDNF, release and signaling in the mPFC and hippocampus, leading to rapid and sustained antidepressant effects. It would be important in future studies to determine the role of VEGF in the actions of other rapid-acting agents, notably scopolamine, rapastinel, (2R,6R)-HNK and NV-5138.
3.4. Role of BDNF-VEGF interplay in the actions of rapid-acting agents
Both BDNF and VEGF play an essential role in the rapid and sustained antidepressant actions of ketamine as discussed above, and VEGF also activates the mTORC1 signaling pathway (Kim et al., 2008). These findings raise a question of whether BDNF and VEGF act in parallel or sequentially? Since BDNF is reported to stimulate VEGF expression and release via the mTORC1 signaling pathway and induction of hypoxia-inducible factor-1α in neuroblastoma cells (Nakamura et al., 2006), we have recently tested the hypothesis that the antidepressant and neurotrophic actions of BDNF are mediated by VEGF. Intra-mPFC infusion of recombinant BDNF produces ketamine-like rapid and sustained antidepressant actions, and these effects are completely blocked either by co-infusion of VEGF nAb or in VegfNEURON−/− mice, suggesting that VEGF release from pyramidal neurons is required for the antidepressant actions of BDNF (Deyama et al., 2019a). Indeed, BDNF stimulates VEGF release in cultured primary cortical neurons in a TrkB-dependent manner and the neurotrophic effects of BDNF require VEGF (Deyama et al., 2019a). We have also examined the reciprocal interdependence of BDNF in the antidepressant and neurotrophic effects of VEGF and found that the antidepressant effects of intra-mPFC infusion of VEGF are blocked by co-infusion of BDNF nAb, suggesting the essential role of BDNF release in the behavioral actions of VEGF (Deyama et al., 2019a). We have also confirmed that VEGF stimulates BDNF release in an Flk-1-dependent manner, and that BDNF is required for the neurotrophic actions of VEGF in cultured primary cortical neurons (Deyama et al., 2019a). These findings reveal an essential interdependence between BDNF and VEGF signaling in the mPFC and suggest that this interplay plays a key role in the neurotrophic and antidepressant effects of ketamine, and could also be involved in the effects of other rapid-acting agents.
4. Conclusions
The discovery of the rapid antidepressant effects of ketamine opens the door to a new class of rapid-acting and efficacious agents for the treatment of depression, including severe depression considered treatment resistant. Indeed, the (S)-ketamine nasal spray, Spravato has recently been approved in the United States to treat treatment-resistant depression (Kaufman, 2019). Despite the unique antidepressant efficacy, racemic ketamine and (S)-ketamine also have undesirable serious side effects and have the potential for abuse. Recent rodent studies have shown that (R)-ketamine and its metabolite (2R, 6R)-HNK, which have low affinity for the NMDAR, produce rapid antidepressant effects with fewer side effects in rodent models than racemic ketamine or (S)-ketamine (Chang et al., 2019; Masaki et al., 2019; Yang et al., 2015; Yang et al., 2019; Zanos et al., 2016), suggesting that these agents may be safer alternatives although their clinical efficacy and safety have not yet been proven in depressed patients. Clinical trials with other compounds targeting NMDARs with fewer side effects than ketamine, including lanicemine (AZD6765; a low trapping NMDAR antagonist), L-4-chlorokynurenine (AV-101; a prodrug of 7-chlorokynurenic acid, a selective NMDAR glycine site antagonist) and rislenemdaz (MK-0657/CERC-301; a selective GluN2B-NMDAR antagonist), have been negative (Wilkinson and Sanacora, 2019). It will be interesting in future studies to determine the effects of these agents on activity-dependent BDNF and VEGF release. In addition, although rapastinel showed a rapid antidepressant effect in a phase II clinical trial and rodent models with fewer side effects than ketamine, it failed to show a significant beneficial effect in MDD patients in phase III clinical trials (Allergan, 2019; Burgdorf et al., 2013; Kato and Duman, 2020; Preskorn et al., 2015). In September 2019, it was announced that single dose of NV-5138 produces rapid and sustained antidepressant effects with favorable safety and tolerability profile in a phase I clnical trial (Navitor, 2019). Together convergent effects of diverse rapid acting agents on BDNF/VEGF signaling indicates that the adverse side effect profile of racemic and (S)-ketamine is at least partly related to NMDAR blockade and subsequent burst of glutamate (Fig. 1), while agents lacking these side effects act via a different initial target that does not produce the same robust glutamate burst, or that produce more subtle effects on glutamate signaling. Therefore, further unraveling of the cellular and neuronal mechanisms underlying the antidepressant actions of racemic ketamine, ketamine enantiomers and other rapid-acting agents is essential for the identification and characterization of novel targets for the development of safer rapid-acting agents, and could also further elucidate the pathophysiology of depression. As discussed in this review, compounds that can rapidly increase BDNF and VEGF release, as well as expression, and/or activate mTORC1 signaling could be promising candidates for novel rapid antidepressants. At the present time, these would include other NMDAR positive allosteric modulators and metabotropic glutamate receptor 2/3 antagonists, as well as activation of mTORC1 signaling. There could also be other targets that could regulate BDNF/VEGF release and expression, although further studies are needed to identify these targets, which could include sites on both excitatory and inhibitory neurons.
Highlights.
Reduced levels of BDNF and VEGF are associated with the pathophysiology of depression.
Rapid increases in BDNF and VEGF release in the mPFC are pivotal for the rapid antidepressant actions of ketamine.
Agents that can rapidly increase BDNF/VEGF release and signaling could be promising candidates for novel rapid antidepressants.
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health (grant numbers MH045481, MH093897; RSD), JSPS KAKENHI Grant Number JP19K07120 (SD), the Uehara Memorial Foundation (SD), the Shimabara Science Promotion Foundation (SD), the Pharmacological Research Foundation, Tokyo (SD), Suzuken Memorial Foundation (SD), Takeda Science Foundation (SD) and the State of Connecticut.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, Purohit P, de Graaf RA, Esterlis I, Juchem C, Pittman BP, Krystal JH, Rothman DL, Sanacora G, Mason GF, 2018. The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology 43(10), 2154–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH, 2015. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66, 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM, 2008. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry 63(7), 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advani T, Koek W, Hensler JG, 2009. Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. Int J Neuropsychopharmacol 12(5), 583–588. [DOI] [PubMed] [Google Scholar]
- Aleman A, Denys D, 2014. Mental health: A road map for suicide research and prevention. Nature 509(7501), 421–423. [DOI] [PubMed] [Google Scholar]
- Allergan, 2019. Allergan Announces Phase 3 Results for Rapastinel as an Adjunctive Treatment of Major Depressive Disorder (MDD). https://www.allergan.com/news/news/22homson-reuters/allergan-announces-phase-3-results-for-rapastinel (accessed 30 July 2019).
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P, 2004. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 9(3), 278–286, 224. [DOI] [PubMed] [Google Scholar]
- Ampuero E, Rubio FJ, Falcon R, Sandoval M, Diaz-Veliz G, Gonzalez RE, Earle N, Dagnino-Subiabre A, Aboitiz F, Orrego F, Wyneken U, 2010. Chronic fluoxetine treatment induces structural plasticity and selective changes in glutamate receptor subunits in the rat cerebral cortex. Neuroscience 169(1), 98–108. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM, 2011. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475(7354), 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I, 2008. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res 1211, 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P, Donello J, Yoshitake T, Kehr J, 2016. Rapastinel (Glyx-13), a rapid acting antidepressant, does not increase extracellular levels of dopamine and glutamate in rat medial prefrontal cortex. Neuropsychopharmacology 41, S116–S288. [Google Scholar]
- Barreto RA, Walker FR, Dunkley PR, Day TA, Smith DW, 2012. Fluoxetine prevents development of an early stress-related molecular signature in the rat infralimbic medial prefrontal cortex. Implications for depression? BMC Neurosci 13, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS, Ninan I, 2012. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 37(5), 1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, 2000. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47(4), 351–354. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ, 2006. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311(5762), 864–868. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N, 2009. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14(8), 764–773. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Galfalvy H, Dwork AJ, Rosoklija GB, Trencevska-Ivanovska I, Pavlovski G, Hen R, Arango V, Mann JJ, 2019. Resilience Is Associated With Larger Dentate Gyrus, While Suicide Decedents With Major Depressive Disorder Have Fewer Granule Neurons. Biol Psychiatry 85(10), 850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J, 2013. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology 38(6), 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD, 2002. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 51(4), 342–344. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS, 2000. Hippocampal volume reduction in major depression. Am J Psychiatry 157(1), 115–118. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR, 2013. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38(5), 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Molteni R, Maj PF, Cattaneo A, Gennarelli M, Racagni G, Riva MA, 2007. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology 32(11), 2351–2359. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ, 2004. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 36(8), 827–835. [DOI] [PubMed] [Google Scholar]
- Carboni L, Marchetti L, Lauria M, Gass P, Vollmayr B, Redfern A, Jones L, Razzoli M, Malki K, Begni V, Riva MA, Domenici E, Caberlotto L, Mathe AA, 2018. Cross-species evidence from human and rat brain transcriptome for growth factor signaling pathway dysregulation in major depression. Neuropsychopharmacology 43(10), 2134–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ, 2016. Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21(9), 1298–1308. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M, 2013. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 70, 27–34. [DOI] [PubMed] [Google Scholar]
- Chang L, Zhang K, Pu Y, Qu Y, Wang SM, Xiong Z, Ren Q, Dong C, Fujita Y, Hashimoto K, 2019. Comparison of antidepressant and side effects in mice after intranasal administration of (R,S)-ketamine, (R)-ketamine, and (S)-ketamine. Pharmacol Biochem Behav 181, 53–59. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT, 2001. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 50(4), 260–265. [DOI] [PubMed] [Google Scholar]
- Chen F, du Jardin KG, Waller JA, Sanchez C, Nyengaard JR, Wegener G, 2016. Vortioxetine promotes early changes in dendritic morphology compared to fluoxetine in rat hippocampus. Eur Neuropsychopharmacol 26(2), 234–245. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS, 2006. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 314(5796), 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS, 2004. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24(18), 4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaruttini C, Vicario A, Li Z, Baj G, Braiuca P, Wu Y, Lee FS, Gardossi L, Baraban JM, Tongiorgi E, 2009. Dendritic trafficking of BDNF mRNA is mediated by translin and blocked by the G196A (Val66Met) mutation. Proc Natl Acad Sci U S A 106(38), 16481–16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Lee SH, Chang HL, Son H, 2016. Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim Biophys Acta 1862(7), 1247–1254. [DOI] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME, 2002. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry 52(11), 1057–1065. [DOI] [PubMed] [Google Scholar]
- Davila-Hernandez A, Zamudio SR, Martinez-Mota L, Gonzalez-Gonzalez R, Ramirez-San Juan E, 2018. Antidepressant effects of acupoint stimulation and fluoxetine by increasing dendritic arborization and spine density in CA1 hippocampal neurons of socially isolated rats. Neurosci Lett 675, 48–53. [DOI] [PubMed] [Google Scholar]
- Deyama S, Bang E, Kato T, Li XY, Duman RS, 2019a. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry 86(2), 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyama S, Bang E, Wohleb ES, Li XY, Kato T, Gerhard DM, Dutheil S, Dwyer JM, Taylor SR, Picciotto MR, Duman RS, 2019b. Role of neuronal VEGF signaling in the prefrontal cortex in the rapid antidepressant effects of ketamine. Am J Psychiatry 176(5), 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 67(8), 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donello JE, Banerjee P, Li YX, Guo YX, Yoshitake T, Zhang XL, Miry O, Kehr J, Stanton PK, Gross AL, Burgdorf JS, Kroes RA, Moskal JR, 2019. Positive N-methyl-D-aspartate receptor modulation by rapastinel promotes rapid and sustained antidepressant-like effects. Int J Neuropsychopharmacol 22(3), 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR Jr., Todd RD, Reich T, Vannier M, Raichle ME, 1997. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386(6627), 824–827. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS, 2007. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry 61(5), 661–670. [DOI] [PubMed] [Google Scholar]
- Duman RS, 2018. Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Res 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Aghajanian GK, Sanacora G, Krystal JH, 2016. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22(3), 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ, 1997. A molecular and cellular theory of depression. Arch Gen Psychiatry 54(7), 597–606. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM, 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12), 1116–1127. [DOI] [PubMed] [Google Scholar]
- Duman RS, Shinohara R, Fogaca MV, Hare B, 2019. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham JS, Deakin JF, Miyajima F, Payton A, Toro CT, 2009. Expression of hippocampal brain-derived neurotrophic factor and its receptors in Stanley consortium brains. J Psychiatr Res 43(14), 1175–1184. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN, 2003. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60(8), 804–815. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Pandey GN, 2006. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience 139(3), 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR, 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ, 2003. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry 54(10), 994–1005. [DOI] [PubMed] [Google Scholar]
- Elfving B, Plougmann PH, Wegener G, 2010. Differential brain, but not serum VEGF levels in a genetic rat model of depression. Neurosci Lett 474(1), 13–16. [DOI] [PubMed] [Google Scholar]
- Feast A, Martinian L, Liu J, Catarino CB, Thom M, Sisodiya SM, 2012. Investigation of hypoxia-inducible factor-1alpha in hippocampal sclerosis: a postmortem study. Epilepsia 53(8), 1349–1359. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Moller HJ, Meisenzahl EM, 2007. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry 64(4), 410–416. [DOI] [PubMed] [Google Scholar]
- Fukumoto K, Fogaca MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS, 2019. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A 116(1), 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM, 2009. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 14(7), 681–695. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, Duman RS, 2018. Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine. Biol Psychiatry 83(1), 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC, 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 76(2), 155–162. [DOI] [PubMed] [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS, 2009. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 34(11), 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Mendez-David I, Pehrson A, Guiard BP, Reperant C, Orvoen S, Gardier AM, Hen R, Ebert B, Miller S, Sanchez C, David DJ, 2013. Antidepressant and anxiolytic potential of the multimodal antidepressant vortioxetine (Lu AA21004) assessed by behavioural and neurogenesis outcomes in mice. Neuropharmacology 73, 147–159. [DOI] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, Duman RS, 2019. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nat Commun 10(1), 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harward SC, Hedrick NG, Hall CE, Parra-Bueno P, Milner TA, Pan E, Laviv T, Hempstead BL, Yasuda R, McNamara JO, 2016. Autocrine BDNF-TrkB signalling within a single dendritic spine. Nature 538(7623), 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick NG, Harward SC, Hall CE, Murakoshi H, McNamara JO, Yasuda R, 2016. Rho GTPase complementation underlies BDNF-dependent homo- and heterosynaptic plasticity. Nature 538(7623), 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ, 2005. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 21(5), 1304–1314. [DOI] [PubMed] [Google Scholar]
- Holmes SE, Scheinost D, Finnema SJ, Naganawa M, Davis MT, DellaGioia N, Nabulsi N, Matuskey D, Angarita GA, Pietrzak RH, Duman RS, Sanacora G, Krystal JH, Carson RE, Esterlis I, 2019. Lower synaptic density is associated with depression severity and network alterations. Nat Commun 10(1), 1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KR, Kutiyanawalla A, Pillai A, 2011. Long-term continuous corticosterone treatment decreases VEGF receptor-2 expression in frontal cortex. PLoS One 6(5), e20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Coupland NJ, Lebel RM, Carter R, Seres P, Wilman AH, Malykhin NV, 2013. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry 74(1), 62–68. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Madaio AI, Mallei A, Lee FS, Popoli M, 2016. Brain-derived neurotrophic factor Val66Met human polymorphism impairs the beneficial exercise-induced neurobiological changes in mice. Neuropsychopharmacology 41(13), 3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isung J, Aeinehband S, Mobarrez F, Martensson B, Nordstrom P, Asberg M, Piehl F, Jokinen J, 2012. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2, e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H, Kaye JA, Jick SS, 2004. Antidepressants and the risk of suicidal behaviors. JAMA 292(3), 338–343. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS, Jeong LS, Banasr M, Son H, Duman RS, 2012. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18(9), 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R, 2005. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136(1-2), 29–37. [DOI] [PubMed] [Google Scholar]
- Kato T, Duman RS 2020. Rapastinel, a novel glutamatergic agent with ketamine-like antidepressant actions: convergent mechanisms. Pharmacol Biochem Behav 188, 172827. [DOI] [PubMed] [Google Scholar]
- Kato T, Fogaca MV, Deyama S, Li XY, Fukumoto K, Duman RS, 2018. BDNF release and signaling are required for the antidepressant actions of GLYX-13. Mol Psychiatry 23(10), 2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Pothula S, Liu RJ, Duman CH, Terwilliger R, Vlasuk GP, Saiah E, Hahm S, Duman RS, 2019. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Invest 130, 2542–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MB, 2019. Pharmaceutical Approval Update. P T 44(5), 251–254. [PMC free article] [PubMed] [Google Scholar]
- Kim BW, Choi M, Kim YS, Park H, Lee HR, Yun CO, Kim EJ, Choi JS, Kim S, Rhim H, Kaang BK, Son H, 2008. Vascular endothelial growth factor (VEGF) signaling regulates hippocampal neurons by elevation of intracellular calcium and activation of calcium/calmodulin protein kinase II and mammalian target of rapamycin. Cell Signal 20(4), 714–725. [DOI] [PubMed] [Google Scholar]
- Kiuchi T, Lee H, Mikami T, 2012. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience 207, 208–217. [DOI] [PubMed] [Google Scholar]
- Kranaster L, Blennow K, Zetterberg H, Sartorius A, 2019. Reduced vascular endothelial growth factor levels in the cerebrospinal fluid in patients with treatment resistant major depression and the effects of electroconvulsive therapy-A pilot study. J Affect Disord 253, 449–453. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C Jr., 2012. Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry 72(11), e27–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP, 2002. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem 82(6), 1367–1375. [DOI] [PubMed] [Google Scholar]
- Lepack AE, Bang E, Lee B, Dwyer JM, Duman RS, 2016. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 111, 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS, 2014. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 18(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS, 2010. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329(5994), 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS, 2011. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69(8), 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF, 2008. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59(3), 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Goshen I, Avital A, Kreisel T, Zubedat S, Eavri R, Segal M, Yirmiya R, Keshet E, 2011. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A 108(12), 5081–5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T, Keshet E, 2013. Delineating multiple functions of VEGF-A in the adult brain. Cell Mol Life Sci 70(10), 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK, 2008. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A 105(1), 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E, Burgdorf J, Moskal J, Taylor J, Aghajanian G, Duman RS, 2017. GLYX-13 produces rapid antidepressant responses with key synaptic and behavioral effects distinct from ketamine. Neuropsychopharmacology 42(6), 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK, 2012. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry 71(11), 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zang T, Birnbaum SG, Wang Z, Johnson JE, Zhang CL, Parada LF, 2017. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun 8(1), 1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS, 2011. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 21(3), 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS, 2000. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20(24), 9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki Y, Kashiwagi Y, Watabe H, Abe K, 2019. (R)- and (S)-ketamine induce differential fMRI responses in conscious rats. Synapse 73(12), e22126. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM, 2009. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34(1), 41–54. [PMC free article] [PubMed] [Google Scholar]
- Michaelsson H, Andersson M, Svensson J, Karlsson L, Ehn J, Culley G, Engstrom A, Bergstrom N, Savvidi P, Kuhn HG, Hanse E, Seth H, 2019. The novel antidepressant ketamine enhances dentate gyrus proliferation with no effects on synaptic plasticity or hippocampal function in depressive-like rats. Acta Physiol (Oxf) 225(4), e13211. [DOI] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, Witztum J, Shaver DC, Rosenthal DL, Alway EJ, Lopez K, Meng Y, Nellissen L, Grosenick L, Milner TA, Deisseroth K, Bito H, Kasai H, Liston C, 2019. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 364(6436), eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D, 1997. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17(8), 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ, 2004. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 101(29), 10827–10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ, 2007. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 61(2), 187–197. [DOI] [PubMed] [Google Scholar]
- Nagashima G, Suzuki R, Hokaku H, Takahashi M, Miyo T, Asai J, Nakagawa N, Fujimoto T, 1999. Graphic analysis of microscopic tumor cell infiltration, proliferative potential, and vascular endothelial growth factor expression in an autopsy brain with glioblastoma. Surg Neurol 51(3), 292–299. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ, 2006. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res 66(8), 4249–4255. [DOI] [PubMed] [Google Scholar]
- Navitor, 2019. Navitor’s three phase 1 studies for NV-5138 show antidepressant effects and biomarker impact, supporting further development of direct activator of mTORC1 in depression. http://www.navitorpharma.com/news/press-releases/navitors-three-phase-1-studies-for-nv-5138-show-antidepressant-effects-and-biomarker-impact-supporting-further-development-of-direct-activator-of-mtorc1-in-depression/ (accessed 28 November 2019).
- Nemoto K, Ohnishi X, Mori T, Moriguchi Y, Hashimoto R, Asada X, Kunugi H, 2006. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neurosci Lett 397(1-2), 25–29. [DOI] [PubMed] [Google Scholar]
- Newton SS, Collier EF, Hunsberger I, Adams D, Xerwilliger R, Selvanayagam E, Duman RS, 2003. Gene profile of electroconvulsive seizures: induction of neurotrophic and angiogenic factors. J Neurosci 23(34), 10841–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Kakeda S, Katsuki A, Sugimoto K, Otsuka Y, Ueda I, Igata R, Watanabe K, Kishi X, Iwata N, Korogi Y, Yoshimura R, 2018. Relationship between VEGF-related gene polymorphisms and brain morphology in treatment-naive patients with first-episode major depressive disorder. Eur Arch Psychiatry Clin Neurosci. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS, 1995. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15(11), 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS, 1996. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci 16(7), 2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Takahashi M, Russell DS, Duman RS, 1999. Repeated stress increases catalytic TrkB mRNA in rat hippocampus. Neurosci Lett 267(2), 81–84. [DOI] [PubMed] [Google Scholar]
- Ota KT, Liu RJ, Voleti B, Maldonado-Aviles JG, Duric V, Iwata M, Dutheil S, Duman C, Boikess S, Lewis DA, Stockmeier CA, DiLeone RJ, Rex C, Aghajanian GK, Duman RS, 2014. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 20(5), 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR, 2004. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci 24(45), 10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS, 2003. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17(4), 879–886. [DOI] [PubMed] [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, Group, G.-C.S., 2015. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract 21(2), 140–149. [DOI] [PubMed] [Google Scholar]
- Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN, 2015. Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 25(1), 75–83. [DOI] [PubMed] [Google Scholar]
- Qiao H, An SC, Ren W, Ma XM, 2014. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behav Brain Res 275, 191–200. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA, 1999. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45(9), 1085–1098. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM, 2003. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci 23(35), 11036–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E, 2005. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25(5), 1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R, 2003. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301(5634), 805–809. [DOI] [PubMed] [Google Scholar]
- Sarchiapone M, Carli V, Roy A, Iacoviello L, Cuomo C, Latella MC, di Giannantonio M, Janiri L, de Gaetano M, Janal MN, 2008. Association of polymorphism (Val66Met) of brain-derived neurotrophic factor with suicide attempts in depressed patients. Neuropsychobiology 57(3), 139–145. [DOI] [PubMed] [Google Scholar]
- Sarkar A, Kabbaj M, 2016. Sex differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol Psychiatry 80(6), 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS, 2008. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A 105(32), 11352–11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senra H, Balaskas K, Mahmoodi N, Aslam T, 2017. Experience of anti-VEGF treatment and clinical levels of depression and anxiety in patients with wet age-related macular degeneration. Am J Ophthalmol 177, 213–224. [DOI] [PubMed] [Google Scholar]
- Sharma AN, da Costa e Silva BF, Soares JC, Carvalho AF, Quevedo J, 2016. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: A comprehensive review of human studies. J Affect Disord 197, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW, 1996. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A 93(9), 3908–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R, Martins L, Longatto-Filho A, Almeida OF, Sousa N, 2007. Lithium prevents stress-induced reduction of vascular endothelium growth factor levels. Neurosci Lett 429(1), 33–38. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM, 1995. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15(3 Pt 1), 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Wu H, Li R, Xu H, Rao X, Gao L, Zou Y, Lei H, 2019. Repeated fluoxetine treatment induces long-lasting neurotrophic changes in the medial prefrontal cortex of adult rats. Behav Brain Res 365, 114–124. [DOI] [PubMed] [Google Scholar]
- Soumier A, Carter RM, Schoenfeld TJ, Cameron HA, 2016. New hippocampal neurons mature rapidly in response to ketamine but are not required for its acute antidepressant effects on neophagia in rats. eNeuro 3(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Chen MH, Li CT, Lin WC, Hong CJ, Gueorguieva R, Tu PC, Bai YM, Cheng CM, Krystal JH, 2017. Dose-related effects of adjunctive ketamine in Taiwanese patients with treatment-resistant depression. Neuropsychopharmacology 42(13), 2482–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A, 2010. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry 15(1), 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, Pitychoutis PM, 2019. Sex differences in the temporal neuromolecular and synaptogenic effects of the rapid-acting antidepressant drug ketamine in the mouse brain. Neuroscience 398, 182–192. [DOI] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E, 2012. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169(11), 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M, Team, S.D.S., 2006. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163(1), 28–40. [DOI] [PubMed] [Google Scholar]
- Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, Sanacora G, Eid T, Aghajanian G, Duman RS, 2013. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 74(10), 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS, 2007. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A 104(11), 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA Jr., Sanacora G, 2018. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 175(2), 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson ST, Sanacora G, 2019. A new generation of antidepressants: an update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 24(2), 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T, Radua J, Via E, Cardoner N, Abe O, Adams TM, Amico F, Cheng Y, Cole JH, de Azevedo Marques Perico C, Dickstein DP, Farrow TFD, Frodl T, Wagner G, Gotlib IH, Gruber O, Ham BJ, Job DE, Kempton MJ, Kim MJ, Koolschijn P, Malhi GS, Mataix-Cols D, McIntosh AM, Nugent AC, O'Brien JT, Pezzoli S, Phillips ML, Sachdev PS, Salvadore G, Selvaraj S, Stanfield AC, Thomas AJ, van Tol MJ, van der Wee NJA, Veltman DJ, Young AH, Fu CH, Cleare AJ, Arnone D, 2017. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol Psychiatry 22(10), 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, Duman RS, 2016. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest 126(7), 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2018. Depression. https://www.who.int/news-room/fact-sheets/detail/depression (accessed 30 July 2019).
- Xie T, Stathopoulou MG, de Andres F, Siest G, Murray H, Martin M, Cobaleda J, Delgado A, Lamont J, Penas LE, A LL, Visvikis-Siest S, 2017. VEGF-related polymorphisms identified by GWAS and risk for major depression. Transl Psychiatry 7(3), e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Jinno S, 2019. Potential link between antidepressant-like effects of ketamine and promotion of adult neurogenesis in the ventral hippocampus of mice. Neuropharmacology 158, 107710. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K, 2015. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5, e632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Yang J, Luo A, Hashimoto K, 2019. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry 9(1), 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef MM, Underwood MD, Huang YY, Hsiung SC, Liu Y, Simpson NR, Bakalian MJ, Rosoklija GB, Dwork AJ, Arango V, Mann JJ, 2018. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol 21(6), 528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY, 2012. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 32(12), 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr., Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533(7604), 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Thompson SM, Duman RS, Zarate CA Jr., Gould TD, 2018. Convergent mechanisms underlying rapid antidepressant action. CNS Drugs 32(3), 197–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, 2012. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71(11), 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63(8), 856–864. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu F, Chen J, Dong W, 2010. Chronic antidepressant administration alleviates frontal and hippocampal BDNF deficits in CUMS rat. Brain Res 1366, 141–148. [DOI] [PubMed] [Google Scholar]
- Zorner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, Grunwald I, Lipp HP, Klein R, Henn FA, Gass P, 2003. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than "depressive". Biol Psychiatry 54(10), 972–982. [DOI] [PubMed] [Google Scholar]