Abstract
Background
The United Nations’ Sustainable Development Goal #3.8 targets ‘access to quality essential healthcare services’. Clinical practice guidelines are an important tool for ensuring quality of clinical care, but many challenges prevent their use in low-resource settings. Monitoring the use of guidelines relies on cumbersome clinical audits of paper records, and electronic systems face financial and other limitations. Here we describe a unique approach to generating digital data from paper using guideline-based templates, rubber stamps and mobile phones.
Intervention
The Guidelines Adherence in Slums Project targeted ten private sector primary healthcare clinics serving informal settlements in Nairobi, Kenya. Each clinic was provided with rubber stamp templates to support documentation and management of commonly encountered outpatient conditions. Participatory design methods were used to customize templates to the workflows and infrastructure of each clinic. Rubber stamps were used to print templates into paper charts, providing clinicians with checklists for use during consultations. Templates used bubble format data entry, which could be digitized from images taken on mobile phones. Besides rubber stamp templates, the intervention included booklets of guideline compilations, one Android phone for digitizing images of templates, and one data feedback/continuing medical education session per clinic each month. In this paper we focus on the effect of the intervention on documentation of three non-communicable diseases in one clinic.
Methods
Seventy charts of patients enrolled in the chronic disease program (hypertension/diabetes, n = 867; chronic respiratory diseases, n = 223) at one of the ten intervention clinics were sampled. Documentation of each individual patient encounter in the pre-intervention (January–March 2016) and post-intervention period (May–July) was scored for information in four dimensions – general data, patient assessment, testing, and management. Control criteria included information with no counterparts in templates (e.g. notes on presenting complaints, vital signs). Documentation scores for each patient were compared between both pre- and post-intervention periods and between encounters documented with and without templates (post-intervention only).
Results
The total number of patient encounters in the pre-intervention (282) and post-intervention periods (264) did not differ. Mean documentation scores increased significantly in the post-intervention period on average by 21%, 24% and 17% for hypertension, diabetes and chronic respiratory diseases, respectively. Differences were greater (47%, 43% and 27%, respectively) when documentation with and without templates was compared. Changes between pre- vs.post-intervention, and with vs.without template, varied between individual dimensions of documentation. Overall, documentation improved more for general data and patient assessment than in testing or management.
Conclusion
The use of templates improves paper-based documentation of patient care, a first step towards improving the quality of care. Rubber stamps provide a simple and low-cost method to print templates on demand. In combination with ubiquitously available mobile phones, information entered on paper can be easily and rapidly digitized. This ‘frugal innovation’ in m-Health can empower small, private sector facilities, where large numbers of urban patients seek healthcare, to generate digital data on routine outpatient care. These data can form the basis for evidence-based quality improvement efforts at large scale, and help deliver on the SDG promise of quality essential healthcare services for all.
Keywords: m-Health, Rubber stamp template, Frugal innovation, Clinical practice guideline, Non-physician clinician
1. Introduction
The majority of primary healthcare clinics (PHC) in Kenya are staffed only by non-physician clinicians (NPCs) such as nurses and clinical officers [1]. NPCs are quickly becoming the mainstay of healthcare service delivery in low and middle-income countries (LMICs). In 2007, the number of NPCs equaled or exceeded that of physicians in nine sub-Saharan countries, and by 2010, NPCs were recognized in 47 of the 54 African countries [2]. There are arguments for clinical officers, a cadre of NPC in Kenya, to play the role of professional ‘primary care clinician’ [3].
Alongside the increasing role for NPCs, another important shift in the landscape of primary healthcare in LMICs is the growth of the private sector, which is likely to play a major role in attempts to achieve universal health coverage (UHC) [4]. For example in Kenya, 60% of urban and 35% of rural primary care facilities are classified as private, for-profit [1]. The private sector in primary healthcare is however diverse and fragmented, ranging from single owner-managed clinics, small general clinic chains managing a few facilities, to large franchise chains (mainly delivering reproductive health services) [5]. Private sector primary healthcare clinics (PS-PHC) in general, and particularly many in challenging settings like urban slums or remote, rural areas, have little support to help improve quality of their care [5,6]. Many are poorly resourced [7], and lack support for engaging in quality improvement (QI) efforts.1
One of the targets under the UN Sustainable Development Goals (SDGs) #3 is to achieve ‘access to quality essential health-care services’ (SDG #3.8). Efforts to achieve this target must therefore focus on the role of NPCs as primary care clinicians, and capacity in the private sector to deliver quality essential healthcare services. Increased utilization of healthcare services does not guarantee improvements in health outcomes, highlighting the need for QI efforts to promote evidence-based care [8].
Clinical practice guidelines (CPG) are an important tool for ensuring quality, evidence-based clinical care. However, there are many challenges to implementing CPGs in low-resource settings, especially in monitoring their use and supporting healthcare professionals to adhere to them [9–11]. Current efforts to measure and improve quality of clinical care are expensive and cumbersome, including manual audits of paper records [12], direct observations of clinical care [13], or standardized patients [14]. As a result, clinical quality in LMICs is commonly measured mostly through service measures (e.g. waiting times, availability of infrastructure, patient satisfaction), and rarely through technical measures (e.g. provider competence, adherence to CPGs) [15,16].
Electronic technologies, and electronic medical record (EMR) systems in particular, are seen as the main solution to improving the generation of data required for QI efforts [17,18]. However, besides functionality and technical infrastructure, few implementations of EMRs in LMIC settings have considered political, ethical or financial criteria that could help improve their use [19]. Use of such systems remains low outside of well-funded, vertical disease programs [20], and many design, human resource, logistical and financial barriers need to be addressed before such technologies can be routinely used to support primary healthcare service delivery in LMICs [21,22]. There are numerous reasons why paper continues to be used as an interface for medical documentation [23,24], and paper-based documentation is almost ubiquitous in LMIC settings. The use of clinical records for QI is therefore tightly linked to paper-based documentation, and the ability to analyze the information contained in paper-based patient charts.
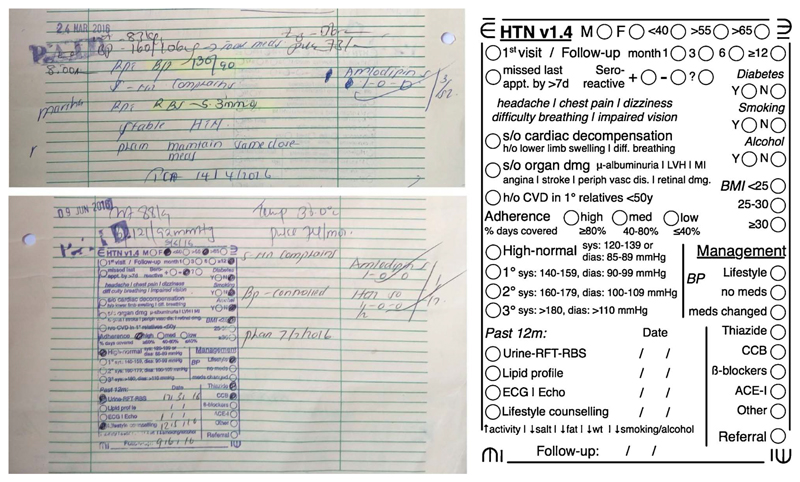
The Guidelines Adherence in Slums Project (GASP) in Nairobi, Kenya, has pioneered an innovative approach of retaining paper as the interface for documentation, while simplifying the digital extraction of medical information from paper [22]. The project uses rubber stamps to print, on demand, specific CPG templates onto paper-based patient charts (Fig. 1). Each rubber stamp CPG templates (RST) is specific to a disease/condition and incorporates important diagnostic and therapeutic recommendations. Extending the use of checklists for medical safety [25], RSTs also include data entry fields which can be digitized using a mobile phone camera and linked software. The approach therefore combines: a) paper-based interfaces for use during clinical encounters, b) condition-specific checklists that aide the clinician in managing a range of commonly encountered illnesses in the outpatient setting, and c) digital data extraction and management.
Fig. 1.
Left: Examples of clinical documentation on paper in cases of hypertension, both before (top) and after the intervention (bottom), which included using a RST to aide documentation. Right: A to-scale illustration of a template for documenting management of hypertension. The template is printed into the paper chart using a rubber stamp, as seen in the image on the bottom left.
In this paper we examine the effect of using RSTs on clinical documentation in paper-based charts. The report focuses on documentation related to the management of three non-communicable diseases (NCD) in one PS-PHC. We discuss the links to improving quality through adherence to CPGs, especially for PS-PHCs, the benefits and limitations of template-based documentation, and the use of paper, rubber stamps and mobile phones as a ‘frugal innovation’ for generating digital health information in LMIC settings. Separate reports will describe the effect of RSTs on improving adherence to CPGs, the acceptance and use of RSTs (and related technology) by NPCs and managers of PS-PHCs, and the technology for extracting digital data from RSTs.
2. Methods
2.1. The intervention
GASP was a mixed methods study targeting ten PS-PHCs serving populations living in the urban informal settlements of Nairobi, Kenya. The intervention included four elements, all of which were implemented at every facility. These included:
-
1)
Rubber stamps of CPG templates: RSTs were developed for some of the commonly encountered clinical conditions in the outpatient setting (Fig. 1). These included, for example, upper respiratory tract infections, urinary tract infections, hypertension, etc. Each facility was free to choose the RSTs to implement, with the possibility to change anytime during the project. RSTs were developed using participatory approaches. Clinicians and/or managers at each facility were provided a draft of the RST. The research team then worked with the facility staff to adapt the RSTs to suit their workflow and available infrastructure. The constraints of rubber stamps (e.g. size limited to ~10 × 7 cm, the size of commonly available ink pads) and template-based documentation (e.g. capturing discrete and not continuous data) were explained to the clinicians, along with their advantages (e.g. printing templates on demand versus managing stacks of pre-printed paper). Each RST included a checklist of selected elements under four broad aspects of clinical documentation: general data (e.g. high-risk age groups, HIV status), assessment (e.g. documentation of complications, if any, of diabetes), testing (e.g. documentation of blood sugar levels), and management (e.g. prescription of drug class, documentation of counselling provided). One set of RSTs and one ink pad were provided for each consultation desk/room at every facility. Clinicians were asked to use a relevant RST for each consultation if possible. More than one RST could be used, for example, to document a case of hypertension and diabetes. Each clinician was trained to ensure a good transfer of ink by applying uniform pressure, and enter data by shading bubbles (and not ticking or crossing).
-
2)
Guideline compilations: CPG compilations were provided in the form of a printed booklet for each target condition. The compilations were created from existing national and international guidelines to provide clinicians with a ready reference at the point of care. The compilations provided additional detail to the limited aspects of CPGs included in the RST. One set of booklets was provided for each consultation desk/room.
-
3)
Budget Android mobile phones: One low-budget (~$70) Android mobile phone was provided to each facility to take images of RSTs. At least one member of staff at each facility was trained to take images of RSTs, ensuring that lighting conditions were adequate and phones were held parallel to the paper. Images were taken typically once each day, coinciding with data entry into registers for reporting to the Ministry of Health. Monthly purchases of internet data bundles were made for each phone. The phones were configured to automatically synchronize the images to a cloud-based database that was accessible only to the research group. A prototype software application has been developed to extract data automatically from the images of RSTs, and while it is possible to automatically extract data filled in the RSTs from the images, the technology and processes involved are being evaluated. All data in this report were extracted manually from the images of RSTs.
-
4)
Data feedback and continuing medical education (CME) sessions: One CME based on RST data was delivered to each facility every month. These were delivered at the facility to all clinical and paraclinical staff. These sessions involved a brief feedback of data from RSTs, and a CME session on a topic related to the data (e.g. signs of organ damage in hypertension). These sessions served not only as continuing education for clinicians, but also as regular reminders of the intervention and the data emerging from RSTs.
2.2. Focus on NCDs
In this paper we focus on the use of RSTs for three illnesses managed in the chronic disease program at one of the intervention sites – Baraka Health Centre in Mathare, Nairobi (BHC). BHC is managed by a German NGO ‘German Doctors’ with head office in Bonn, Germany, and governed under the umbrella of the Christian Health Association of Kenya (CHAK). The outpatient-only facility serves a population of about 180,000.2
BHC was the only intervention clinic to focus their use of RSTs on non-communicable diseases (NCDs), and the only facility with an ongoing chronic disease management program. The chronic disease clinic is managed by one clinical officer. There were 867 cases of hypertension (HTN) and/or diabetes (DM) and 223 cases of chronic respiratory diseases (CRD) enrolled in the chronic disease program at BHC as of January 2016. CRD charts include patients diagnosed with asthma or chronic obstructive pulmonary disease (COPD). Patients enrolled in the chronic disease management program typically visited BHC once every month. All patient charts were stored at the facility, unlike in some of the other intervention clinics where documentation was done on patient-held notebooks.
2.3. Data collection and analysis
The intervention at BHC was initiated in April 2016. A three-month period prior (January-March 2016) was defined as the ‘pre-intervention’ period and the following three months (May-July 2016) as the ‘post-intervention’ period. Sampling of patient charts was done with an aim of being able to demonstrate a 20% difference in documentation scores with a confidence of 95% and power of 80%, which provided a target of 96 clinical encounters per condition. Patient charts classified as HTN/DM and CRD were systematically sampled (every fifth chart) to achieve the target. Clinical documentation of all NCD-related patient encounters in the pre- and post-intervention periods were analyzed in the sampled charts.
Charts were scored using criteria described in Table 1. ‘Scoring’ criteria were developed to allow comparison of charts where clinical information was documented using RSTs and charts with traditional narrative documentation (without RSTs). ‘Control’ criteria included information not possible to document using RSTs (e.g. notes on presenting symptoms, or the exact value of vital signs). One point was awarded for information available in the chart for each criterion (e.g. HIV status documented, notes on any symptoms of hypoglycemia or any other complication of diabetes, etc.). Scoring was initially done in Microsoft Excel, and data exported to R (version 3.3.1) for analysis.
Table 1.
Scoring criteria used to compare documentation in paper-based charts used in the management of three NCDs in BHC. The first set of scoring criteria (A) were used to compare clinical documentation before and after the introduction of RSTs. The second set of criteria (B) were used as control, to score chart documentation irrespective of template use.
| A. Scoring dimensions (Criteria for scoring information in charts, whether templates used or not) | |||
| Dimension | Hypertension | Diabetes | Chronic Respiratory Diseases |
| General data | Sex | Sex | Sex |
| High risk age group | High risk age group | High risk age group | |
| Visit type (First/Follow-up) | Visit type (First/Follow-up) | Visit type (First/Follow-up) | |
| HIV status | HIV status | HIV status | |
| Time since diagnosis | TB status | ||
| Assessment | Comorbidity (Diabetes) | Comorbidity (Hypertension) | Diagnosis group (Asthma/COPD/Recurrent obstructive bronchitis) |
| Smoking | Smoking | Smoking | |
| Alcohol use | Alcohol use | Exposure (domestic/occupational) | |
| Signs of organ damage | Complications of diabetes | Heart disease (COPD) OR Asthma triggers | |
| Severity/control of symptoms | |||
| Testing | Blood pressure | Blood pressure | Chest X-ray |
| RBS/RFT/Urine dipstick | RBS/FBS | Peak Flow | |
| Urine dipstick | ECG/Echo | ||
| Sputum/GeneXpert (COPD only)a | |||
| Management | Prescription/Management instructions | Prescription/Management instructions | Prescription/Management instructions |
| Counselling | Counselling | Counselling | |
| Follow-up date | Follow-up date | Follow-up date | |
| Adherence to medication | Inhaler technique | ||
| # Criteria | 14 | 15 | 17/18 |
| B. Control dimensions (Criteria for scoring information documented outside templates) | |||
| Presentation | Notes on presenting complaints | Notes on presenting complaints | Notes on presenting complaints |
| Vital signs/test results (exact values) | Blood pressure | Blood pressure | Blood pressure |
| Heart rate | Heart rate | Heart rate | |
| Temperature | Temperature | Temperature | |
| Weight | Weight | Weight | |
| RBS/FBS | |||
| Follow-up | Follow-up instructions | Follow-up instructions | Follow-up instructions |
| # Criteria | 6 | 7 | 6 |
Only scored for COPD cases.
A ‘pre versus post’ analysis compared documentation scores for each patient before and after the intervention. Encounters in the pre-intervention period did not involve the use of templates. Post-intervention encounters either used a RST for documentation or did not. All encounters in the post-intervention period were scored. When a RST was used, information in the template was scored. When no RST was used, information in the chart was scored. Mean documentation scores across pre-intervention encounters were compared with mean scores across post-intervention encounters using a paired t-test. Differences in control criteria were also similarly compared. Both mean difference in scores and upper and lower limits are reported.
The effect of RSTs alone on clinical documentation was calculated by focusing the analysis on post-intervention encounters. This analysis included only patient charts with at least one post-intervention encounter documented with a RST and one documented without RST. Scores were analyzed as described above by comparing mean documentation scores with RSTs and mean scores without RSTs using a paired t-test.
Ethical approval for the study was received from the Strathmore University Institutional Review Board (SU-IRB 0015/15). No patient identifiers were collected as part of the chart or template review and scoring process.
3. Results
A total of 70 patient charts were sampled and scored for the analysis. There were, on average, 2.9 encounters per patient in the pre-intervention period and 2.8 encounters in the post-intervention period. We recorded 291 clinical encounters for HTN across 49 patients (149 pre-intervention and 142 post-intervention), 131 encounters for DM across 22 patients (72 pre-intervention and 59 post-intervention), and 124 encounters for CRD across 24 patients (61 pre-intervention and 63 post-intervention). There was no statistically significant difference between the number of pre-intervention and post-intervention encounters. Of the 70 patients, 27 were managed for HTN alone, four for DM alone, and 16 for CRD alone. Fourteen were managed for both HTN and DM, and five for HTN and CRD. Three were managed for all three conditions.
RSTs were used to document 141 of the 264 clinical encounters in the post-intervention period. RST use was highest in CRD cases (67%) and lower for HTN and DM cases (49% and 51%, respectively). In the sample of charts analyzed, use of RSTs was lower in the third month post-intervention (Table 2). However, the total number of RSTs used in the three months (not just in sampled charts) did not reflect this. In the three post-intervention months, the number of templates used in May, June and July respectively were 109, 98 and 101 for HTN, 58, 47 and 47 for DM and 22, 29 and 15 for CRD.
Table 2.
Patient records and encounters analyzed.
| Condition | #charts | Encounter type | # | Encounters documented with RSTs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | May | June | July | ||||||||
| Hypertension | 46 | Pre-intervention | 149 | NA | |||||||
| Post-intervention | 142 | 69 | (49%) | 31 | (57%) | 23 | (53%) | 15 | (33%) | ||
| Diabetes | 19 | Pre-intervention | 72 | NA | |||||||
| Post-intervention | 59 | 30 | (51%) | 13 | (54%) | 10 | (56%) | 7 | (41%) | ||
| Chronic respiratory diseases | 22 | Pre-intervention | 61 | NA | |||||||
| Post-intervention | 63 | 42 | (67%) | 14 | (70%) | 16 | (73%) | 12 | (57%) | ||
A total of 70 patient charts were sampled for the analysis. Of these, 49 were diagnosed with hypertension, 22 with diabetes and 24 with chronic respiratory diseases. Each patient had, on average, 3.0, 3.3 and 2.5 encounters in the pre-intervention period, and 2.9, 2.7 and 2.6 encounters in the post-intervention period for HTN, DM and CRD, respectively. RST use in the post-intervention period decreased over the three months in the charts sampled. However the total number of templates used did not reflect this (see text).
3.1. Effect of the intervention on clinical documentation
The intervention resulted in improved clinical documentation in the post-intervention period for all three conditions (Table 3). Differences in documentation scores were calculated for each patient between post-intervention and pre-intervention encounters. There was, on average, a 21% improvement in documentation scores for HTN, 24% for DM, and 17% for CRD. These differences were all statistically significant (paired t-test p-values < 0.001). Charts were also scored for information that are not entered in the RST, for example, notes on presenting complaints, documentation of vital signs, etc. Documentation of these ‘control’ criteria were not affected by the intervention in any of the three conditions studied.
Table 3.
Effect of the GASP intervention on clinical documentation.
| Documentation scores, post (with or without RST) vs. pre (without RST) intervention | |||||||
|---|---|---|---|---|---|---|---|
| Condition | n | Comparison | # Criteria | Difference | Lower | Upper | p-value |
| Hypertension | 46 | Scoring criteria | 14 | 21% | 16% | 27% | < 0.001 |
| Control criteria | 6 | −4% | −8% | 1% | 0.1 | ||
| Diabetes | 19 | Scoring criteria | 15 | 24% | 17% | 30% | < 0.001 |
| Control criteria | 7 | −1% | −5% | 2% | 0.4 | ||
| Chronic Respiratory Diseases | 22 | Scoring criteria | 17/18 | 17% | 12% | 22% | < 0.001 |
| Control criteria | 6 | −2% | −8% | 5% | 0.59 | ||
Charts from the same patients (n) were scored both before and after the introduction of the intervention. Pre-intervention scores reflect chart documentation without RSTs. In the three-month pre-intervention period, there were on average 3.0 encounters per patient for HTN, 3.3 for DM, and 2.5 for CRD, and scores are the average across encounters for each patient. In the three months following the introduction of the intervention, there were on average 2.9 encounters per patient for HTN, 2.7 for DM, and 2.6 for CRD. RSTs were used in 49% of clinical encounters for HTN, 51% for DM, and 67% for CRD. Post-intervention scores reflect documentation with or without the use of RSTs. A paired t-test was conducted by taking the differences in scores for each patient between the pre- and post-intervention periods for both ‘Scoring’ and ‘Control’ criteria. The average difference across patients, and the lower and upper limits of the confidence intervals are reported, along with corresponding p-values.
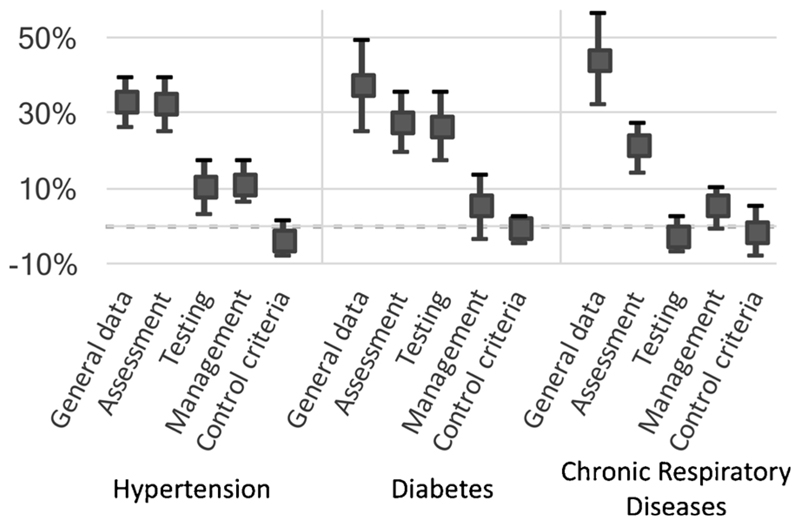
The analysis was also performed on individual dimensions of clinical documentation, as listed in Table 1 (Fig. 2). In general, differences between pre- and post-intervention were highest in the documentation of general information and patient assessment, and lower in the documentation of testing and management. Differences did not reach significance in the documentation of testing (in CRD) and management (both DM and CRD).
Fig. 2. Effect of the intervention on individual scoring criteria.
The filled squares represent the mean difference in documentation scores between pre- and post-intervention periods. Error bars represent 95% confidence intervals. Dimensions of ‘Scoring’ criteria are shown individually, highlighting the variable effect of the intervention on different aspects of clinical documentation.
3.2. Effect of RSTs on clinical documentation
Differences in documentation scores with and without RSTs were significant for all three conditions (Table 4). This analysis was performed on a subset of patients where post-intervention encounters included at least one where a RST was used and one where it was not. These included 30 patients managed for HTN, 14 for DM and 11 for CRD.
Table 4.
Effect of the template use on clinical documentation.
| Documentation scores, with vs. without RST use (post-intervention only) | |||||||
|---|---|---|---|---|---|---|---|
| Condition | n | Comparison | # Criteria | Difference | Lower | Upper | p Value |
| Hypertension | 30 | Scoring criteria | 14 | 47% | 42% | 53% | < 0.001 |
| Control criteria | 6 | 2% | −5% | 8% | 0.62 | ||
| Diabetes | 14 | Scoring criteria | 15 | 43% | 33% | 53% | < 0.001 |
| Control criteria | 7 | −1% | −5% | 3% | 0.59 | ||
| Chronic Respiratory Diseases | 11 | Scoring criteria | 17/18 | 27% | 21% | 34% | < 0.001 |
| Control criteria | 6 | −7% | −14% | 1% | 0.07 | ||
Differences in clinical documentation between encounters where RSTs were used and where they were not. Differences were calculated only in patient charts (n) where post-intervention encounters included at least one encounter where a RST was used and one where it was not. A paired t-test was conducted by taking the differences in scores for each patient between the pre- and post-intervention periods for both ‘Scoring’ and ‘Control’ criteria. The average difference across patients, and the lower and upper limits of the confidence intervals are reported, along with corresponding p-values.
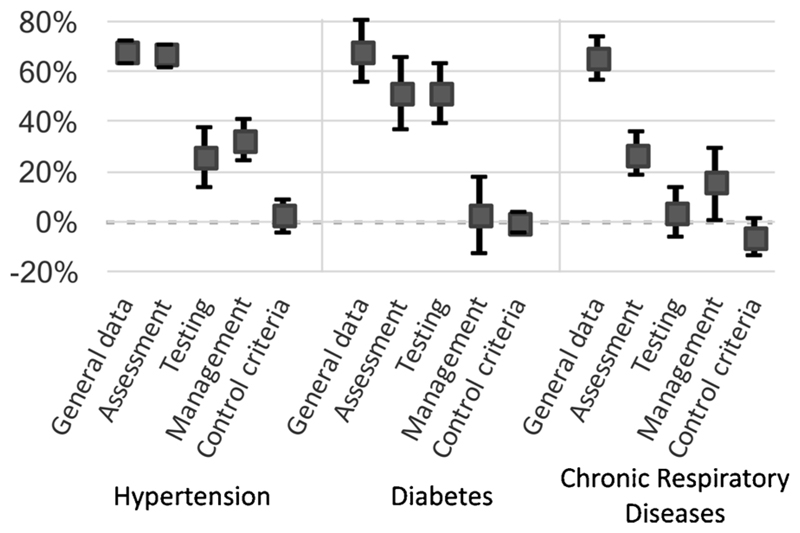
On average the difference was 47% in HTN, 43% in DM and 27% in CRD (paired t-test p-values < 0.001). There were no significant differences in ‘control’ criteria. Here too, we performed an analysis to examine the effect of RST use on individual dimensions of clinical documentation (Fig. 3). Differences in scores were highest for general data and patient assessment and lower in testing and management dimensions. Differences did not reach significance in the documentation of testing (in CRD) and management (both DM and CRD).
Fig. 3. Effect of template use on individual scoring criteria.
The filled squares represent the mean difference in documentation scores between clinical encounters where RSTs were used and those where they were not. Error bars represent 95% confidence intervals. Dimensions of ‘Scoring’ criteria are shown individually, highlighting the variable effect of RST use on different aspects of clinical documentation.
4. Discussion
RSTs were used to manage the majority of NCD cases in the post-intervention period despite a heavy workload at BHC. The chronic disease program in BHC was managed by one clinical officer, who typically saw about 20 patients each day. Various factors affected the use of RSTs for documentation, and these will be described in a separate report based on qualitative interviews with clinicians and managers of intervention PS-PHCs.
The GASP intervention, which included the use of RSTs, guideline compilations for reference, and monthly data feedback/CME sessions, resulted in more clinical information recorded in paper charts. While it is likely that the guideline compilations and monthly data feedback/CME trainings influenced clinical documentation, the analysis of post-intervention data shows that the use of RSTs for documentation resulted in large increases in information available in the chart. The relevance of the guideline compilations and data feedback/CME sessions are being studied using qualitative methods.
As highlighted in Figs. 2 and 3, increases in information were greatest in general data and patient assessment dimensions, with smaller increases in information on testing and patient management. It is likely that documentation, especially in settings where patient volumes are high and clinical audits are rare, is focused mainly on the investigations conducted, the overall impression/diagnosis and the prescription. CPG-based templates could ensure that other aspects of each condition, such as risk factors, signs of any complications, adherence to medicines or counselling needed and provided, are also regularly documented.
4.1. Pros and cons of template-based documentation
The use of templates in documenting healthcare have been mainly studied in the context of electronic systems [26–28]. As the use of templates has increased however, there have been concerns that template-only EMRs, while allowing rapid documentation, do not adequately document a face-to-face visit and medical examination [29]. In both analyses conducted in this study, there were no differences in documentation outside templates (control criteria). The use of templates in paper therefore increased the overall information contained in the charts without affecting the quality of narrative documentation.
Besides the increase in information contained in the chart as shown in this study, there are several arguments to support the use of templates in paper-based clinical documentation as listed in Table 5. Templates can support documentation and case management that are aligned to CPGs, such as documentation of symptoms/tests necessary for a diagnosis, or treatment upon meeting specific criteria. They also provide a standardized and repeatable format for documentation that allows for simpler and faster documentation and review of case history when compared with narrative notes. Templates can be designed to be intuitive, minimizing the need to train clinicians in their use.
Table 5.
Pros and cons of template-based documentation in paper.
| Templates in general | Rubber stamp templates specifically |
|---|---|
| Pros | |
| Rapid documentation | Can print relevant templates on demand |
| Allows incorporation of checklists | Easily adapted to suit each context/facility |
| Standardized care documentation | Cheap, local production (no printing needed) |
| Potential improvements in quality of care | Minimal wastage compared to printed sheets |
| Does not compromise narrative documentation | Minimal training needed to use stamps |
| Minimal training needed to use templates | Designed for automated data digitization |
| Can be modified to suit different needs, and support iterative QI | Logistically simple to manage (Multiple stamps can be placed in a desk/drawer) |
| Cons | |
| Only support discrete data | Limited size (typical ink pads are ~10 × 7 cm |
| Manual data extraction is cumbersome | Ink transfer can vary in quality |
| Multiple templates needed for outpatient care | Mobile phone images can vary in quality |
However, templates also face limitations. From an information perspective template-based data entry rarely supports recording of continuous data. For example, as seen in Fig. 1, instead of the exact values for blood pressure, the clinician is required to record the corresponding hypertension level. Besides posing a challenge in the design of templates, upstream health information systems may also need to be designed with such limitations in mind.
Data in paper-based templates are likely to be easier to audit than narrative notes, but still require cumbersome manual data extraction, with rare examples in the literature of optimization of this process. [30] There are also logistical challenges with using templates for clinical documentation in paper. Multiple templates are required to cover the diversity of cases encountered in typical outpatient settings. If printed on paper, there are likely to be supply chain challenges in maintaining a stock of such sheets, and time spent in locating the relevant template for use.
4.2. Rubber stamps for printing templates
The intervention described in this study was an attempt to overcome some of the limitations described above to using templates in paper-based clinical documentation. We used rubber stamps to print templates into paper, on demand. BHC used three RSTs for their chronic disease program, and other general outpatient PS-PHCs in our study used up to eight RSTs to cover a typical outpatient case mix in urban slums (e.g. upper respiratory tract infections, urinary tract infections, sexually transmitted infections, malaria and illnesses presenting as fevers, diarrheal diseases, etc.). Stamps and an ink pad were stored on the desk or in a drawer, and the relevant stamps could be easily identified and retrieved. This eliminated the need to pre-print templates on paper, and allowed templates to be printed onto any sheet of paper, including patient-held notebooks.
Rubber stamps are ubiquitously available and used in LMICs, and almost no instructions are needed to use them. Each template in our study (Supplementary Fig. 4) was manufactured locally for under US$5. Clinicians were trained briefly (~5 min) to ensure a good ink transfer by applying pressure on the edges of the stamp, and shown how to shade the bubbles. There was a high-level of acceptance for the intervention among the clinicians,1 and similar methods could be employed to expand the use of templates widely in LMICs, where paper-based clinical documentation is still widely used.
4.3. Automated data extraction
RSTs were also designed for easy data extraction using optical mark recognition technology [31,32]. While the data presented here were extracted manually from images of RSTs taken on mobile phones, the research team have since developed prototype software for automatic extraction of data from RST images. Testing of the software and processes is ongoing, along with efforts to deploy the software on mobile phones and data transmission using SMS. Once complete, this approach will be able to create a digital repository of individual case management data, in near-real time, in facilities using paper for clinical documentation. The processes and technologies involved are intended for clinics in low-resource settings, without reliable electricity or mobile internet.
Similar paper-to-digital approaches are used in high-income settings where hand-filled forms are part of clinical workflows. Captricity,3 a commercial solution, combines machine learning algorithms with human verification to achieve a high degree of accuracy, including recognition of handwriting. The solution described here does not aim to digitize all data entered on paper, but takes the ‘middle ground’ by placing constraints on the information set that is needed in digital form. The paper interfaces for data entry – RSTs for optical mark recognition – are designed with user participation to adapt them to the constraints and workflows in each facility and increase clinician ‘buy-in’. The combination of robust data entry formats with ‘mobile-first’ and ‘offline-first’ technologies to digitize images accurately could provide a sustainable approach to generating digital health data in demanding LMIC settings.
4.4. A case of frugal innovation in m-Health
The approach described in this study, combining the use of rubber stamps and mobile phones to deliver digital data on clinical case management, can be described under various terms such as inclusive, base-of-the-pyramid, or frugal innovation [33]. While the term ‘frugal innovation’ can trace its history to the ‘appropriate technology’ movement [34], it also combines broader issues like the growth of emerging markets, information and communications, and movements like social entrepreneurship [35]. Our tools for QI are frugal, both in the resources they use, but also in the data and impact they aim to deliver. For example, while EMRs could support a diversity of healthcare management needs including clinical decision support, RSTs and mobile phone application are specifically designed to deliver data that can support a continuous and targeted QI process at low cost.
The case described here fits well into wider scenarios of m-Health adoption [36,37]. Paper-based tools working in combination with mobile phone-based applications can provide a framework for generating ‘big data’ for health, combining facility-based healthcare and population health. The approach could be used to streamline clinical workflows (e.g. by combining data from ‘paper-first’ and ‘computer-first’ workflows), expand patient involvement through user-friendly interfaces, and integrate clinical care data with other m-Health applications that can potentially transform healthcare [38,39].
4.5. Limitations of the study
The GASP intervention was designed to fit the QI needs of small PS-PHCs, with no prescribed set of templates or CME training sessions. Staff in each clinic could choose the templates for use in their facility from a broad set, and work with the research team to modify the templates to suit their needs and workflows. For example, the diabetes template could be modified to include glycated haemoglobin (HbA1c) data for a clinic performing the test routinely. Some PS-PHCs in the study like BHC stored patient charts in the facility, while others relied on patient-held notebooks for documentation. The variation in the intervention in each clinic limits the analyses possible across facilities. BHC and the documentation of management of NCDs was chosen for this report due to the relatively large numbers of patients enrolled in the chronic disease program, and the possibility of comparing the same patient charts before and after the intervention.
The analysis in this study was focused on clinical documentation, as this is the prerequisite for technical QI efforts. We will report the effect of the intervention on clinical quality in a separate manuscript focusing on data on infectious disease management across multiple clinics.
5. Conclusion
Evidence-based healthcare management relies on easy availability of local data for decision making [40]. Generation of this data in healthcare relies predominantly on digital systems. There are widespread, however uncoordinated, attempts to digitize medical records in LMIC settings [20,41]. Easier generation of data (compared to manual aggregation of data from paper) for evidence-based decision making is frequently cited as a reason to adopt electronic systems [41,42]. However, paper continues to be the main interface for documenting case management in primary care settings in LMICs, and the introduction and use of electronic systems face numerous challenges [21,43].
This paper describes a frugal innovation for generating data needed to improve quality of care (Supplementary Fig. 5), while continuing to use paper as the documentation interface. We have demonstrated that this approach can indeed improve routine documentation of care for NCDs. While rubber stamps represent a simple, ancient, but overlooked technology for printing on demand, mobile phones and m-Health approaches are the current technologies that promise to revolutionize healthcare [37–39]. The ubiquitous availability of both in LMIC settings at low cost, and the minimal training needed to use either technology, creates the potential for widespread use of such approaches to healthcare documentation.
Improved clinical documentation, and digital data from this documentation, both achieved at low cost, are likely to be the first steps in delivering on the SDG promise of quality essential healthcare services for all. While much more work is needed to explore the applications and limits to this approach, the idea of using two cheap and ubiquitous technologies for generating digital data on case management is promising and merits further testing.
Supplementary Material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijmedinf.2017.10.014.
Summary points.
Use of templates can improve information recorded in paper charts without compromising narrative documentation
Templates provide clinicians with checklists to improve adherence to clinical practice guidelines
Rubber stamps are a low-cost and efficient tool to print templates on demand into paper charts
Rubber stamp templates can be designed for automatic data extraction using mobile phone cameras and image recognition technologies
This paper-to-digital, m-Health approach could generate regular data on case management at low cost, enabling continuous quality improvement of clinical care in LMIC settings
Acknowledgements
PK, AM, MN and PW are funded by Health Systems Research Initiative grants (MR/N005015/1) jointly supported by the Department for International Development (DFID), the Economic and Social Research Council (ESRC), the Medical Research Council (MRC) and the Wellcome Trust (WT). BK and GR were funded by the following grants to the World Friends Onlus: The ‘In Buone Mani’ (In good hands) project (731/2014) funded by the CEI (Italian Episcopal Conference); The F.A.R.E. (Facilities, Advancement and Referral Enhancement) project funded by the Tuscany Region. PM is funded by the KEMRI-Wellcome Trust. We acknowledge guidance from Philip Ayieko on data analysis strategies and Meghan Kumar’s comments on the final manuscript. We thank Amici del Mondo – World Friends Onlus for their support in testing of early-stage concepts. We thank the staff and management of GASP intervention clinics, and Baraka Health Centre specifically, for their participation in the study.
Footnotes
Authors; Manuscript in preparation.
Authorship
BK was involved in the conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the article, and revising it critically for important intellectual content.
AM was involved in the acquisition of data, and analysis.
GR was involved in the acquisition of data, and interpretation of data.
PW was involved in the acquisition of data.
PM was involved in the analysis and interpretation of data.
MM provided final approval of the version to be submitted.
PK was involved in the conception and design of the study, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content, and providing final approval of the version to be submitted
Declaration of interest
PK is the CEO of Health-E-Net Limited, a social enterprise in Kenya focused on healthcare quality improvement. BK and GR were employed by Health-E-Net at periods during this study.
References
- [1].Kenya Master Health Facility List. [Accessed April 7 2017];2017 ( http://kmhfl.health.go.ke/#/home, Published 2017)
- [2].Eyal N, Cancedda C, Kyamanywa P, Hurst SA. Non-physician clinicians in Sub-Saharan africa and the evolving role of physicians. Int J Heal Policy Manage. 2015;5(3):149–153. doi: 10.15171/ijhpm.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mbindyo P, Blaauw D, English M. The role of Clinical Officers in the Kenyan health system: a question of perspective. Hum Resour Health. 2013;11(1):32. doi: 10.1186/1478-4491-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].McPake B, Hanson K. Managing the public–private mix to achieve universal health coverage. Lancet. 2016;388(10044):622–630. doi: 10.1016/S0140-6736(16)00344-5. [DOI] [PubMed] [Google Scholar]
- [5].Mackintosh M, Channon A, Karan A, Selvaraj S, Cavagnero E, Zhao H. What is the private sector? Understanding private provision in the health systems of low-income and middle-income countries. Lancet. 2016;388(10044):596–605. doi: 10.1016/S0140-6736(16)00342-1. [DOI] [PubMed] [Google Scholar]
- [6].Fotso JC, Mukiira C. Perceived quality of and access to care among poor urban women in Kenya and their utilization of delivery care: harnessing the potential of private clinics? Health Policy Plan. 2012;27(6):505–515. doi: 10.1093/heapol/czr074. [DOI] [PubMed] [Google Scholar]
- [7].Government of Kenya. Kenya Service Availability and Readiness Assessment Mapping (SARAM) Ministry of Health; Nairobi, Kenya: 2013. [Google Scholar]
- [8].Koblinsky M, Moyer CA, Calvert C, et al. Quality maternity care for every woman, everywhere: a call to action. Lancet. 2016;388(10057):2307–2320. doi: 10.1016/S0140-6736(16)31333-2. [DOI] [PubMed] [Google Scholar]
- [9].Rowe SY, Kelly JM, Olewe MA, et al. Effect of multiple interventions on community health workers’ adherence to clinical guidelines in Siaya district, Kenya. Trans R Soc Trop Med Hyg. 2007;101(2):188–202. doi: 10.1016/j.trstmh.2006.02.023. [DOI] [PubMed] [Google Scholar]
- [10].Rashidian A, Eccles MP, Russell I. Falling on stony ground? A qualitative study of implementation of clinical guidelines’ prescribing recommendations in primary care. Health Policy (New York) 2008;85(2):148–161. doi: 10.1016/j.healthpol.2007.07.011. [DOI] [PubMed] [Google Scholar]
- [11].Higuchi M, Okumura J, Aoyama A, Suryawati S, Porter J. Application of standard treatment guidelines in rural community health centres, Timor-Leste. Health Policy Plan. 2012;27(5):396–404. doi: 10.1093/heapol/czr051. [DOI] [PubMed] [Google Scholar]
- [12].Pirkle CM, Dumont A, Zunzunegui M-V. Criterion-based clinical audit to assess quality of obstetrical care in low- and middle-income countries: a systematic review. Int J Qual Health Care. 2011;23(4):456–463. doi: 10.1093/intqhc/mzr033. [DOI] [PubMed] [Google Scholar]
- [13].Rannan-Eliya RP, Wijemanne N, Liyanage IK, et al. The quality of outpatient primary care in public and private sectors in Sri Lanka – how well do patient perceptions match reality and what are the implications? Health Policy Plan. 2015;30:i59–i74. doi: 10.1093/heapol/czu115. [DOI] [PubMed] [Google Scholar]
- [14].Das J, Holla A, Das V, Mohanan M, Tabak D, Chan B. In urban and rural India, a standardized patient study showed low levels of provider training and huge quality gaps. Health Aff. 2012;31(12):2774–2784. doi: 10.1377/hlthaff.2011.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Berendes S, Heywood P, Oliver S, Garner P. Jenkins R, editor. Quality of private and public ambulatory health care in low and middle income countries: systematic review of comparative studies. PLoS Med. 2011;8(4):e1000433. doi: 10.1371/journal.pmed.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Morgan R, Ensor T, Waters H. Performance of private sector health care: implications for universal health coverage. Lancet. 2016;388(10044):606–612. doi: 10.1016/S0140-6736(16)00343-3. [DOI] [PubMed] [Google Scholar]
- [17].Wildeman MA, Zandbergen J, Vincent A, et al. Can an online clinical data management service help in improving data collection and data quality in a developing country setting? Trials. 2011;12:190. doi: 10.1186/1745-6215-12-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haskew J, Rø G, Turner K, Kimanga D, Sirengo M, Sharif S. Implementation of a cloud-based electronic medical record to reduce gaps in the HIV treatment continuum in rural Kenya. PLoS One. 2015;10(8):e0135361. doi: 10.1371/journal.pone.0135361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fritz F, Tilahun B, Dugas M. Success criteria for electronic medical record implementations in low-resource settings: a systematic review. J Am Med Inf Assoc. 2015;22(2):479–488. doi: 10.1093/jamia/ocu038. [DOI] [PubMed] [Google Scholar]
- [20].Lewis T, Synowiec C, Lagomarsino G, Schweitzer J. E-health in low- and middle-income countries: findings from the Center for Health Market Innovations. Bull World Health Organ. 2012;90(5):332–340. doi: 10.2471/BLT.11.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Landis-Lewis Z, Manjomo R, Gadabu OJ, et al. Barriers to using eHealth data for clinical performance feedback in Malawi: a case study. Int J Med Inform. 2015;84(10):868–875. doi: 10.1016/j.ijmedinf.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kumar P, Paton C, Kirigia D. I’ve got 99 problems but a phone ain’t one: electronic and mobile health in low and middle income countries. Arch Dis Child. 2016;101(10):974–979. doi: 10.1136/archdischild-2015-308556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tange HJ. The paper-based patient record: is it really so bad? Comput Methods Programs Biomed. 1995;48(1–2):127–131. doi: 10.1016/0169-2607(95)01672-G. [DOI] [PubMed] [Google Scholar]
- [24].Ludwick DA, Doucette J. Adopting electronic medical records in primary care: lessons learned from health information systems implementation experience in seven countries. Int J Med Inform. 2009;78(1):22–31. doi: 10.1016/j.ijmedinf.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [25].Haynes A, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360(5):491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- [26].Crist-Grundman D, Douglas K, Kern V, Gregory J, Switzer V. Evaluating the impact of structured text and templates in ambulatory nursing. Proc Annu Symp Comput Appl Med Care. 1995:712–716. [PMC free article] [PubMed] [Google Scholar]
- [27].Henry SB, Douglas K, Galzagorry G, Lahey A, Holzemer WL. A template-based approach to support utilization of clinical practice guidelines within an electronic health record. J Am Med Inform Assoc. 1998;5(3):237–244. doi: 10.1136/jamia.1998.0050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vawdrey DK. Assessing usage patterns of electronic clinical documentation templates. [Accessed 22.04.17];AMIA Annu Symp Proceedings AMIA Symp. 2008 Nov;:758–762. [PMC free article] [PubMed] [Google Scholar]
- [29].Sheber S. CMS revisions increase scrutiny for EHR templates. [Accessed 21.04.17];J AHIMA. 2017 ( http://journal.ahima.org/2012/12/28/cms-revisions-increase-scrutiny-for-ehr-templates/, Published 2012.) [Google Scholar]
- [30].Klein EW, Hunt JS, Leblanc BH. Depression screening interfaced with an electronic health record: a feasibility study in a primary care clinic using optical mark reader technology. [Accessed 21.04.17];Prim Care Companion J Clin Psychiatry. 2006 8(6):324–328. doi: 10.4088/pcc.v08n0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bergeron BP. Optical mark recognition. Postgrad Med. 1998;104(2):23–25. doi: 10.3810/pgm.1998.08.550. [DOI] [PubMed] [Google Scholar]
- [32].Fifolt M, Blackburn J, Rhodes DJ, et al. Man versus machine: comparing double data entry and optical mark recognition for processing CAHPS survey data. Qual Manage Health Care. 2017;26(3):131–135. doi: 10.1097/QMH.0000000000000138. [DOI] [PubMed] [Google Scholar]
- [33].Tran V-T, Ravaud P. Frugal innovation in medicine for low resource settings. BMC Med. 2016;14(1):102. doi: 10.1186/s12916-016-0651-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kaplinsky R. Schumacher meets Schumpeter: appropriate technology below the radar. Res Policy. 2010;40:193–203. doi: 10.1016/j.respol.2010.10.003. [DOI] [Google Scholar]
- [35].Bhatti Y, Ventresca M. The Emerging Market for Frugal Innovation: Fad, Fashion, or Fit? Oxford; 2012. [Accessed 10.08.17]. http://ssrn.com/abstract=2005983) [Google Scholar]
- [36].WHO Global Observatory for eHealth. mHealth: New Horizons for Health Through Mobile Technologies, Second Global Survey on eHealth. Geneva: 2011. [Accessed 14.08.17]. http:/www.who.int/goe/publications/goe_mhealth_web.pdf. [Google Scholar]
- [37].Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J. 2016;37(18):1428–1438. doi: 10.1093/eurheartj/ehv770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Silva BMC, Rodrigues JJPC, de la Torre Díez I, López-Coronado M, Saleem K. Mobile-health: a review of current state in 2015. J Biomed Inf. 2015;56:265–272. doi: 10.1016/j.jbi.2015.06.003. [DOI] [PubMed] [Google Scholar]
- [39].Park Y-T. Emerging new era of mobile health technologies. Healthc Inf Res. 2016;22(4):253–254. doi: 10.4258/hir.2016.22.4.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rycroft-Malone J. The PARIHS framework—a framework for guiding the implementation of. J Nurs Care Qual. 2004;19(4):297–304. doi: 10.1097/00001786-200410000-00002. [DOI] [PubMed] [Google Scholar]
- [41].Tierney WM, Achieng M, Baker E, et al. Experience implementing electronic health records in three East African countries. Stud Health Technol Inform. 2010;160(Part 1):371–375. doi: 10.3233/978-1-60750-588-4-371. [DOI] [PubMed] [Google Scholar]
- [42].Douglas GP, Gadabu OJ, Joukes S, et al. Using Touchscreen electronic medical record systems to support and monitor national scale-up of antiretroviral therapy in Malawi. PLoS Med. 2010;7(8) doi: 10.1371/journal.pmed.1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tilahun B, Fritz F. Comprehensive evaluation of electronic medical record system use and user satisfaction at five low-resource setting hospitals in ethiopia. JMIR Med Inf. 2015;3(2):e22. doi: 10.2196/medinform.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.