ABSTRACT
Background
Several studies have explored the role of human brown adipose tissue (BAT) in energy expenditure. However, the link between BAT and appetite regulation needs to be more rigorously examined.
Objectives
We aimed to investigate the associations of BAT volume and 18F-fluordeoxyglucose (18F-FDG) uptake after a personalized cold exposure with energy intake and appetite-related sensations in young healthy humans.
Methods
A total of 102 young adults (65 women; age: 22.08 ± 2.17 y; BMI: 25.05 ± 4.93 kg/m 2) took part in this cross-sectional study. BAT volume, BAT 18F-FDG uptake, and skeletal muscle 18F-FDG uptake were assessed by means of static 18F-FDG positron-emission tomography and computed tomography scans after a 2-h personalized exposure to cold. Energy intake was estimated via an objectively measured ad libitum meal and three nonconsecutive 24-h dietary recalls. Appetite-related sensations (i.e., hunger and fullness) were recorded by visual analog scales before and after a standardized breakfast (energy content = 50% of basal metabolic rate) and the ad libitum meal. Body composition was assessed by a whole-body DXA scan.
Results
BAT volume and 18F-FDG uptake were not associated with quantified ad libitum energy intake (all P > 0.088), nor with habitual energy intake estimated from the 24-h dietary recalls (all P > 0.683). Lean mass was positively associated with both the energy intake from the ad libitum meal (β: 17.612, R2 = 0.213; P < 0.001) and the habitual energy intake (β: 16.052, R2 = 0.123; P = 0.001). Neither the interaction BAT volume × time elapsed after meal consumption nor that of BAT 18F-FDG uptake × time elapsed after meal consumption had any significant influence on appetite-related sensations after breakfast or after meal consumption (all P > 0.3).
Conclusions
Neither BAT volume, nor BAT 18F-FDG uptake after cold stimulation, are related to appetite regulation in young adults. These results suggest BAT plays no important role in the regulation of energy intake in humans.
This trial was registered at clinicaltrials.gov as NCT02365129.
Keywords: brown fat, thermogenesis, appetite, energy balance, obesity
Introduction
Brown adipose tissue (BAT) is a highly thermogenic tissue whose main function is to produce heat to maintain mammals’ body temperature (1). BAT plays a central role in the physiological regulation of body temperature in small mammals such as mice (2) and in newborn humans (1), but this tissue was long believed to be absent or metabolically irrelevant in adult humans. However, a decade ago, a number of independent research groups showed that BAT is in fact present and metabolically active in the latter (3–7). Initial observations suggested BAT volume and activity to be negatively associated with age, adiposity, and glycemia (4, 6), and positively associated with whole-body energy expenditure (3). Consequently, BAT has been proposed as a promising therapeutic target for the treatment of obesity and related comorbidities (8–11).
It has been hypothesized that BAT significantly contributes toward human energy expenditure (11–13). Indeed, initial studies showed positive associations between BAT and nonshivering thermogenesis in humans (3, 14–16). However, more recent studies examining human BAT function with more advanced methodologies suggest that the direct contribution of BAT to whole-body energy expenditure in response to both cold (17–20) and overfeeding (17, 21, 22) is negligible. Nonetheless, the technological limitations associated with defining the total amount of BAT in humans (13), and the possible indirect role of BAT in thermogenesis [i.e., via activating muscle thermogenesis through endocrine mechanisms (18, 22)], still preclude any definitive conclusions being drawn with regard to BAT's role in human energy expenditure (13). Nevertheless, energy expenditure is considered to be a major determinant of energy intake (23–27) and, therefore, the link between BAT and appetite regulation needs to be rigorously examined to fully understand the contribution of BAT toward human energy balance (28).
The endocrine mechanisms connecting BAT activity to energy intake regulation have been partially elucidated in murine models (29–31), and some of these mechanisms are conserved in humans (31). In mice, BAT recruitment after cold acclimation is coupled with an increase in energy intake, resulting in no change in these animals’ body composition (27). Importantly, human BAT volume is positively associated with fasting- and cold-induced concentrations of peptides involved in appetite regulation (32). Taken together, these findings suggest a possible role for BAT in human appetite regulation (32). However, energy intake regulation is the result of complex interactions between biological and psychological processes (25), and there is a need to directly study the relation of BAT with energy intake and appetite-related sensations, rather than its physiological predictors.
This study examines the associations of BAT volume and 18F-fluordeoxyglucose (18F-FDG) uptake after a personalized cold exposure, with energy intake and appetite-related sensations in young healthy adults. The associations of skeletal muscle 18F-FDG uptake after this cold exposure with energy intake and appetite-related sensations are also investigated.
Methods
Participants
A total of 102 subjects (65 women) took part in the study (Table 1). All were enrolled in the ACTIBATE study (33), a randomized controlled trial (NCT02365129) aiming to study the effect of a 24-wk exercise training program on BAT volume and 18F-FDG uptake after cold-exposure. The present study includes the baseline measurements of the ACTIBATE study, which were performed in September, October, and November of 2015 and 2016. All participants having valid data for both BAT and ad libitum energy intake (primary variables) were included in the analyses (see the flowchart in Supplemental Figure 1). Table 1 shows the descriptive characteristics of the participants. All participants were young (18–25 y old) and self-reported to be healthy, sedentary (<20 min moderate-to-vigorous-intensity physical activity on <3 d/wk), nonsmokers, not taking any medication, had a stable body weight over the preceding 3 months (<3 kg change), and not be regularly exposed to cold.
TABLE 1.
Characteristics of the study participants1
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| Age, y | 102 | 22.08 ± 2.17 | 37 | 22.20 ± 2.19 | 65 | 22.01 ± 2.17 |
| BMI, kg/m2 | 102 | 25.05 ± 4.98 | 37 | 27.40 ± 5.77 | 65 | 23.72 ± 3.93 |
| Lean mass, kg | 96 | 41.74 ± 9.82 | 35 | 52.17 ± 7.19 | 61 | 35.75 ± 4.82 |
| Fat mass, kg | 96 | 25.28 ± 9.45 | 35 | 26.17 ± 11.68 | 61 | 24.77 ± 7.96 |
| Fat mass, % | 96 | 35.90 ± 7.76 | 35 | 30.95 ± 7.85 | 61 | 38.74 ± 6.15 |
| LMI, kg/m2 | 96 | 14.66 ± 2.49 | 35 | 17.12 ± 2.12 | 61 | 13.25 ± 1.33 |
| FMI, kg/m2 | 96 | 8.95 ± 3.16 | 35 | 8.58 ± 3.80 | 61 | 9.16 ± 2.74 |
| VAT, g | 96 | 343.69 ± 183.44 | 35 | 434.39 ± 187.07 | 61 | 291.65 ± 160.92 |
| BAT volume, mL | 102 | 72.44 ± 60.91 | 37 | 89.26 ± 71.15 | 65 | 62.87 ± 52.44 |
| BAT metabolic activity | 102 | 356.28 ± 350.04 | 37 | 389.75 ± 371.96 | 65 | 337.23 ± 338.42 |
| BAT SUVmean | 102 | 3.85 ± 1.91 | 37 | 3.50 ± 1.41 | 65 | 4.05 ± 2.13 |
| BAT SUVpeak | 102 | 11.62 ± 8.43 | 37 | 11.02 ± 7.77 | 65 | 11.96 ± 8.83 |
| All muscles SUVpeak | 102 | 0.80 ± 0.20 | 37 | 0.80 ± 0.17 | 65 | 0.80 ± 0.21 |
| Deep muscles SUVpeak | 102 | 1.05 ± 0.29 | 37 | 1.05 ± 0.27 | 65 | 1.05 ± 0.31 |
| Cervical muscles SUVpeak | 102 | 1.07 ± 0.31 | 37 | 1.05 ± 0.27 | 65 | 1.07 ± 0.33 |
| Cold-sensitive muscles SUVpeak | 102 | 0.90 ± 0.29 | 37 | 0.87 ± 0.25 | 65 | 0.92 ± 0.31 |
| Descending aorta SUVpeak | 102 | 1.54 ± 0.34 | 37 | 1.65 ± 0.37 | 65 | 1.48 ± 0.31 |
| Ad libitum energy intake, kcal | 102 | 880.71 ± 380.14 | 37 | 1136.10 ± 423.08 | 65 | 735.34 ± 261.13 |
| Ad libitum meal:rate of eating, kcal/min | 100 | 76.91 ± 39.72 | 37 | 105.12 ± 47.12 | 63 | 60.34 ± 21.83 |
| Habitual energy intake, kcal/d | 102 | 1856.41 ± 453.73 | 37 | 2025.99 ± 4732.29 | 65 | 1759.87 ± 415.68 |
Values are n or mean ± SD. All muscles: paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii. Deep muscles: paracervical, scalene, longus colli, parathoracic, and subscapular. Cervical muscles: paracervical, sternocleidomastoid, scalene, and longus colli. Cold-sensitive muscles: sternocleidomastoid, scalene, longus colli, and pectoralis major. BAT, brown adipose tissue; FMI, fat mass index; LMI, lean mass index; SUV, standardized uptake value; VAT, visceral adipose tissue.
All participants gave their written informed consent to be included. The study protocol and design, which were approved by the Human Research Ethics Committee of the University of Granada (n°924) and the Servicio Andaluz de Salud, adhered to the Declaration of Helsinki (last revision 2013).
Procedures
The data reported in this study were collected over 3 subject-visits to our center, all within a period of 3 wk (33). Subjects were required to arrive by bus or by car (i.e., undertaking the minimum physical activity possible), after having slept as usual, and having refrained from stimulant beverages and any moderate physical activity in the previous 24 h, or any vigorous physical activity in the previous 48 h. Moreover, no physical activity was allowed on the testing days, and participants remained still (either lying or sitting) during the assessment.
Figure 1 shows the timeline of the procedures on experimental day 1. Subjects arrived at 08:15 am after an overnight fast (12 h), having consumed a standardized dinner (i.e., boiled rice, tomato sauce, and egg omelet) the evening before. After voiding, they were dressed in standardized clothes (clothing insulation value: 0.20) and entered a warm room (22.78 ± 0.94°C; 43.84 ± 6.74% humidity). Their postabsorptive resting metabolic rate (RMR) was then measured using a CCM Express or Ultima CardiO2 metabolic cart (Medical Graphics Cardiorespiratory Diagnostics) (34, 35) while reclining for 30 min on a bed, as per current methodological recommendations (36). The average of the most stable 5-min period was selected to be representative of the individuals’ RMR (34). Immediately after the 30-min RMR measurement, all subjects were provided with a standardized liquid breakfast which was consumed within a maximum period of 10 min. After finishing, they rested quietly on the bed for another 3 h 30 min (they were allowed to use the toilet, and, in this case, asked to limit their physical activity to light walking). They were then moved into another room in which a whole-body DXA scan was performed. Finally, 4 h 15 min after consuming the breakfast, the subjects were provided with an ad libitum lunch to objectively quantify their energy intake.
FIGURE 1.
Day 1 experimental procedure. Numbers indicate time in minutes. Pen and paper symbols represent visual analogue scales. RMR, resting metabolic rate.
On experimental day 2, the subjects’ shivering threshold was assessed following a previously described method (37). Briefly, in fasted conditions for ≥6 h, the subjects entered a cool room (19.5–20°C) wearing the same standardized clothes as previously and a water-perfused cooling vest (Polar Products Inc.). The water temperature was then progressively reduced until shivering began (self-reported and externally observable). The water temperature at the onset of shivering was recorded as the shivering threshold (5.4 ± 2.2°C and 6.3 ± 2.2°C for men and women, respectively; range: 3.9–12.2°C for both men and women).
Experimental day 3 took place 48–72 h after the shivering threshold test (also after a fasting period of ≥6 h). Subjects underwent a static 18F-FDG positron-emission tomography and computed tomography (PET-CT) scan using a Siemens Biograph 16 PET-CT machine to assess their BAT volume and BAT and skeletal muscle 18F-FDG uptake after a personalized cold exposure based on their shivering threshold (37). Briefly, all subjects entered a cool room (19.5–20°C) with the water in the cooling vest set at 4°C above the individual shivering threshold. After the first hour of cold exposure, they received an injection of ∼185 MBq 18F-FDG and the water temperature was increased by 1°C. After a further hour at this temperature, the PET-CT scan was performed. Self-reported menstrual cycle phase of the female participants was recorded at each visit.
PET-CT analysis
18F-FDG-PET-CT scans were performed and analyzed in agreement with current methodological recommendations (38), following the protocol described elsewhere (37, 39). Images were analyzed using the Beth Israel plug-in for FIJI software (40). The PET-CT images were obtained from cervical vertebra 1 to thoracic vertebra 6 (approximately). For assessing BAT volume and 18F-FDG uptake, voxels with a radiodensity between −190 and −10 Hounsfield units and an 18F-FDG uptake greater than the individualized standardized uptake value (SUV) threshold of 1.2/(lean body mass/body mass) were taken into account (38). BAT volume, BAT metabolic activity (BAT volume × BAT SUVmean), BAT SUVmean, and BAT SUVpeak were all calculated (38). The SUVpeak values of the paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii muscles were also determined from 1-slice regions of interest (ROIs), and the values obtained were averaged for all muscles on either side of the body. These muscles were also grouped as deep (paracervical, scalene, longus colli, parathoracic, subscapular), cervical (paracervical, sternocleidomastoid, scalene, longus colli), and cold-sensitive (sternocleidomastoid, scalene, longus colli, pectoralis major) muscles (20). A ROI (1 slice) was also defined in the descending aorta to be used as reference tissue.
Standardized breakfast and ad libitum meal
For the standardized liquid breakfast, subjects received a smoothie (Tdiet® energy, Vegenat S.A.) at 4°C. This product has an energy density of 1.6 kcal/mL; carbohydrates made up 47% of the meal's total energy content, fat 35%, proteins 15%, and fiber 3% (http://vegenatnutricion.es/index.php?r=nutricion/producto&id=10). This breakfast was eaten while seated on the same bed in which the RMR was measured. Subjects consumed a quantity equivalent to 50% of their RMR. They were free to drink water during their breakfast, and were allowed to do so over the next 2 h 30 min.
For the ad libitum meal, subjects received a plate of spaghetti with tomato sauce, pork tenderloin, and virgin olive oil, prepared in the research center immediately before its being served. Carbohydrates provided 45.5% of the meal's total energy content, fat 38.5%, and proteins 16%; the energy density was 1.54 kcal/g. The total amount offered was 1500 g for men and 1000 g for women. The subjects were ushered into a quiet, dimly lit room where they found their plate together with a glass of water (450 mL); they were then left alone without external distractions while eating. The participants were instructed to eat until comfortably satisfied (41). Food intake was measured as the difference in the weight of the plate plus food before and after the meal. The energy intake was subsequently calculated.
Appetite sensations
On experimental day 1, subjects’ appetite-related sensations (i.e., hunger and fullness) were recorded using visual analog scales (VASs) at several times. All were asked to provide this information before the RMR assessment, immediately before the standardized breakfast, immediately after it, 65, 135, and 205 min after it, immediately before the ad libitum meal, immediately after it, and 1, 2, 3, and 4 h after it (Figure 1). Every VAS assessment was completed in the laboratory, except for those after the ad libitum meal, which were completed in free-living conditions (subjects were instructed not to eat for the 4 h after consuming their ad libitum meal). The original version of the VAS has been validated as a means of analyzing appetite-related sensations after a meal (42), as has the Spanish version actually employed (43).
Body composition
Height and weight were measured before the RMR assessment, without shoes and with light clothing, with a model 799 Seca scale and stadiometer. Lean mass, fat mass, fat mass percentage, and visceral adipose tissue mass were assessed by a whole-body DXA scan (Discovery Wi, Hologic, Inc.). Body, lean, and fat mass indexes were calculated as kg/m2.
Dietary recalls
Habitual energy intake was estimated using 3 nonconsecutive 24-h dietary recalls (1 of them for a nonworking day). For this, subjects were interviewed by dieticians, recording all the foods and drinks consumed on the previous day. A book with pictures of different food servings and sizes was used to help subjects estimate the amount of food consumed. The nutritional composition of the diet was obtained using EvalFINUT software (FINUT; http://www.finut.org/evalfinut/). The habitual consumption of water and salt was not recorded. To avoid bias, the subjects were not informed when their diets were going to be recorded. Further, they were classified as plausible or nonplausible reporters using the Goldberg cutoff method (44). For this, habitual physical activity was assessed using an ActiGraph GT3X+ wrist-worn accelerometer for 7 consecutive days (24 h/d) (45). Subjects were given detailed information on how to wear the accelerometer. The raw data were processed and analyzed as explained elsewhere (45), and the mean Euclidean Norm Minus One (mG) during the time spent awake was obtained as an overall indicator of physical activity. Only data for those subjects who wore the accelerometer for ≥10 h/d and ≥4 h/night for ≥4 d were included in analyses (46). The most active subject was arbitrarily assigned a physical activity level (PAL) of 1.8, whereas the least active was assigned a PAL of 1.4. For each of the remaining subjects a proportional value between 1.4 and 1.8 was calculated.
Statistical analyses
Descriptive statistics are presented as mean ± SD, unless otherwise stated. Simple linear regression was used to test the associations of BAT volume, BAT and skeletal muscle 18F-FDG uptake, and body composition variables with energy intake. Sex interaction was tested by multiple linear regression models entering the variable of interest, the sex, and the product of variable of interest × sex.
BAT-related variables have been reported to vary across the year (47, 48), with sex (4, 37), and with body composition (4, 49). Therefore, multiple linear regression models were used to test the associations of BAT volume and BAT and skeletal muscle 18F-FDG uptake with energy intake after adjusting for the date when the PET-CT scan was performed (Model 1), as per Model 1 plus sex (Model 2), and as per Model 2 plus fat mass and lean mass (Model 3). One-factor repeated-measures ANCOVA was used to study the relation of BAT volume and BAT and skeletal muscle 18F-FDG uptake with appetite-related sensations over time elapsed since meal consumption (standardized breakfast and ad libitum meal). Subjects were divided into BAT volume and BAT and skeletal muscle 18F-FDG uptake tertiles, and appetite-related sensations over time elapsed since meal consumption for the highest and the lowest tertiles were compared by 2-factor (tertile and time elapsed since meal consumption) ANOVA.
All analyses were conducted using the Statistical Package for the Social Sciences version 21.0 (IBM SPSS Statistics, IBM Corporation). Significance was set at P < 0.05.
Results
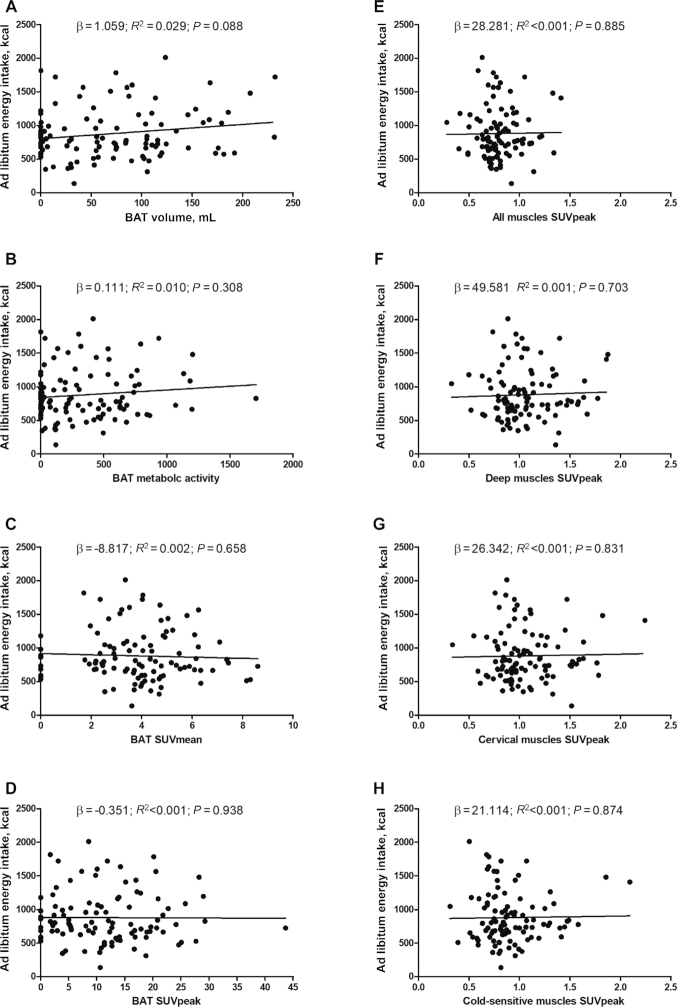
No significant association was seen linking BAT volume and BAT and skeletal muscle 18F-FDG uptake with the energy intake from the objectively measured ad libitum meal (all P > 0.088; Figure 2); this result persisted after adjusting for the date when the PET-CT was performed, and for sex, lean mass, and fat mass (Table 2). Nor was any significant association found linking BAT volume and BAT and skeletal muscle 18F-FDG uptake with habitual energy intake as estimated from the subjects’ dietary recalls (all P < 0.683; Supplemental Figure 2). This finding persisted after adjustment for the date when the PET-CT was performed, for sex, lean mass, and fat mass, and when excluding nonplausible reporters (17 under-reporters, data not shown). The results also persisted when excluding participants with no PET-detectable BAT (n = 20, 19.6%). Moreover, we did not detect any significant sex interaction and the results persisted after adjusting for menstrual cycle phase (data not shown).
FIGURE 2.
Associations of BAT volume and BAT and skeletal muscle 18F-fluorodeoxyglucose uptake with ad libitum energy intake. (A) BAT volume, (B) BAT metabolic activity, (C) BAT SUVmean, (D) BAT SUVpeak, (E) all muscles SUVpeak, (F) deep muscles SUVpeak, (G) cervical muscles SUVpeak, (H) cold-sensitive muscles SUVpeak. Unstandardized β, R2, and P values are from simple linear regression analyses (n = 102). All muscles: paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii. Deep muscles: paracervical, scalene, longus colli, parathoracic, and subscapular. Cervical muscles: paracervical, sternocleidomastoid, scalene, and longus colli. Cold-sensitive muscles: sternocleidomastoid, scalene, longus colli, and pectoralis major. BAT, brown adipose tissue; SUV, standardized uptake value.
TABLE 2.
Associations of BAT volume and BAT and skeletal muscle 18F-fluorodeoxyglucose uptake with ad libitum energy intake1
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | R 2 | P | β | R 2 | P | β | R 2 | P | |
| BAT volume | 1.02 | 0.029 | 0.13 | 0.40 | 0.264 | 0.50 | 0.49 | 0.283 | 0.43 |
| BAT metabolic activity | 0.09 | 0.012 | 0.42 | 0.07 | 0.264 | 0.50 | 0.09 | 0.284 | 0.41 |
| BAT SUVmean | −15.29 | 0.011 | 0.47 | 3.76 | 0.261 | 0.84 | 9.67 | 0.281 | 0.61 |
| BAT SUVpeak | −1.76 | 0.007 | 0.71 | 0.50 | 0.260 | 0.91 | 1.73 | 0.280 | 0.69 |
| All muscles SUVpeak | −6.56 | 0.006 | 0.97 | 19.39 | 0.260 | 0.91 | −41.47 | 0.279 | 0.82 |
| Deep muscles SUVpeak | 26.27 | 0.006 | 0.85 | 36.08 | 0.261 | 0.76 | 12.80 | 0.279 | 0.91 |
| Cervical muscles SUVpeak | 0.71 | 0.006 | 1.00 | 46.26 | 0.262 | 0.68 | 25.90 | 0.279 | 0.82 |
| Cold-sensitive muscles SUVpeak | 2.02 | 0.006 | 0.99 | 72.13 | 0.263 | 0.54 | 53.48 | 0.280 | 0.66 |
Unstandardized β, R2, and P values are from multiple linear regression analyses (n = 102). Model 1: adjusted for the date when the PET/CT scan was performed. Model 2: adjusted as for Model 1 plus sex. Model 3: adjusted as for Model 2 plus fat mass and lean mass. All muscles: paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii. Deep muscles: paracervical, scalene, longus colli, parathoracic, and subscapular. Cervical muscles: paracervical, sternocleidomastoid, scalene, and longus colli. Cold-sensitive muscles: sternocleidomastoid, scalene, longus colli, and pectoralis major. BAT, brown adipose tissue; SUV, standardized uptake value.
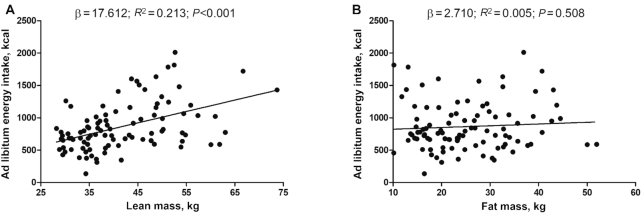
Figure 3 shows the associations of lean and fat mass with the energy intake from the ad libitum meal. Lean mass was positively associated with the energy intake from the ad libitum meal (β: 17.612, R2 = 0.213; P < 0.001) and from the 24-h dietary recalls (β: 16.052, R2 = 0.123; P = 0.001; Supplemental Figure 3). In contrast, fat mass was neither associated with the energy intake from the ad libitum meal (β: 2.710, R2 = 0.005; P = 0.508), nor with the energy intake from the 24-h dietary recalls (β: −0.081, R2 < 0.001; P = 0.987; Supplemental Figure 3).
FIGURE 3.
Associations between body composition and ad libitum energy intake. (A) Lean mass, (B) fat mass. Unstandardized β, R2, and P values are from simple linear regression analyses (n = 96).
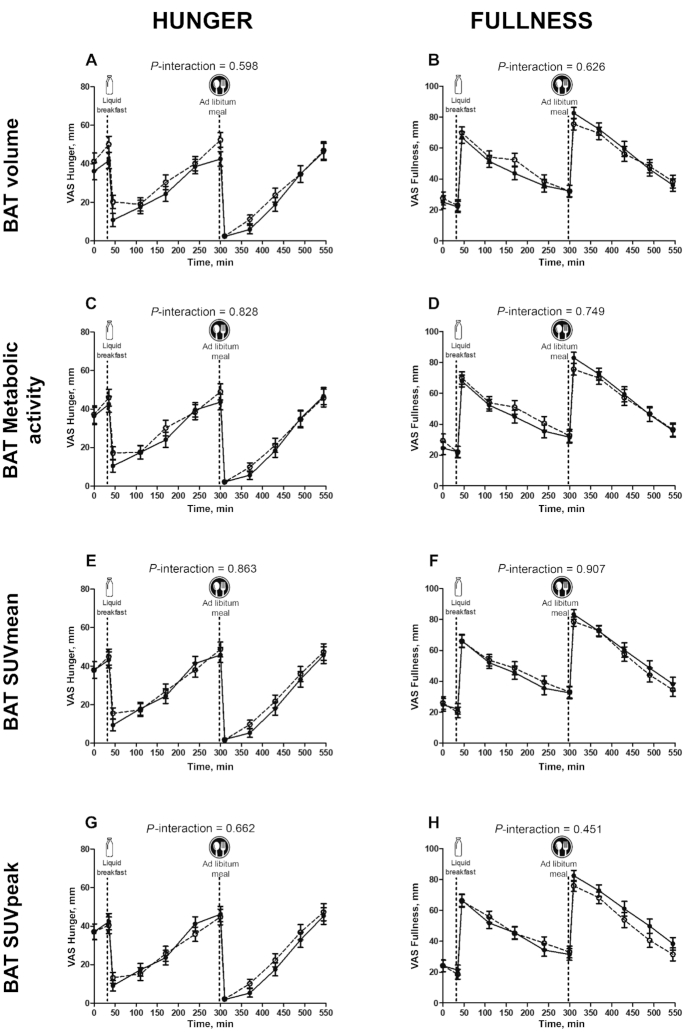
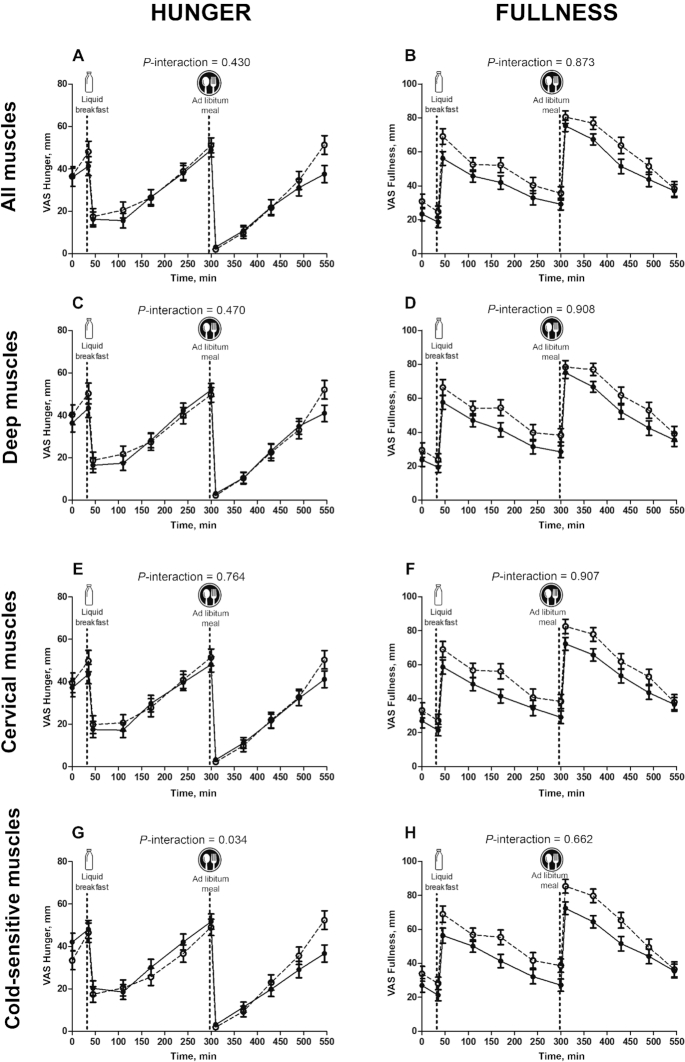
The interactions BAT volume × time elapsed after meal consumption and BAT 18F-FDG uptake × time elapsed after meal consumption had no significant influence on appetite-related sensations after breakfast or after the ad libitum meal (P > 0.3 in both cases; Figure 4, Supplemental Table 1). Neither did the interactions all skeletal muscle 18F-FDG uptake × time elapsed after meal consumption, deep skeletal muscle 18F-FDG uptake × time elapsed after meal consumption, and cervical skeletal muscle 18F-FDG uptake × time elapsed after meal consumption have any significant influence on appetite-related sensations after breakfast or after the ad libitum meal (all P > 0.28; Figure 5, Supplemental Table 1). However, the interaction cold-sensitive skeletal muscle 18F-FDG uptake × time elapsed after meal consumption did have a marginally significant influence on these appetite-related sensations (P = 0.06; Supplemental Table 1). The latter relation was corroborated when comparing, by 2-factor ANOVA, the highest and the lowest cold-sensitive skeletal muscle 18F-FDG uptake tertiles over time after meal consumption (P = 0.034; Figure 5). Moreover, the AUC for fullness was positively correlated with all (r = 0.281, P = 0.011), deep (r = 0.261, P = 0.018), cervical (r = 0.281, P = 0.011), and cold-sensitive (r = 0.257, P = 0.020) muscle 18F-FDG uptakes (data not shown).
FIGURE 4.
Changes in hunger (A–D) and fullness (E–H) sensations over time elapsed since meal consumption, with respect to BAT variables. (A, B) BAT volume, (C, D) BAT metabolic activity, (E, F) BAT SUVmean, (G, H) BAT SUVpeak. Dashed lines represent the high-BAT group (n = 28) and solid lines represent the low-BAT group (n = 26). The “high” and “low” BAT groupings refer to the highest and lowest tertiles for the BAT variables. The P values are for 2-factor (tertile and time elapsed since meal consumption) mixed ANOVAs. BAT, brown adipose tissue; SUV, standardized uptake value; VAS, visual analog scale.
FIGURE 5.
Changes in hunger (A–D) and fullness (E–H) sensations compared with skeletal muscle 18F-FDG uptake. (A, B) All muscles, (C, D) deep muscles, (E, F) cervical muscles, (G, H) cold-sensitive muscles. Dashed lines represent high–muscle 18F-FDG group (n = 24), and solid lines represent low–muscle 18F-FDG group (n = 29). The high and low skeletal muscle 18F-FDG uptake groups are the highest and lowest 18F-FDG uptake tertiles. The P values are for 2-factor (tertile and time elapsed since meal consumption) mixed ANOVAs. All muscles: paracervical, sternocleidomastoid, scalene, longus colli, trapezius, parathoracic, supraspinatus, subscapular, deltoid, pectoralis major, and triceps brachii. Deep muscles: paracervical, scalene, longus colli, parathoracic, and subscapular. Cervical muscles: paracervical, sternocleidomastoid, scalene, and longus colli. Cold-sensitive muscles: sternocleidomastoid, scalene, longus colli, and pectoralis major. VAS, visual analog scale; 18F-FDG, 18F-fluordeoxyglucose.
Finally, a significant association was observed between the energy intake from the ad libitum meal and the habitual energy intake (β: 0.229, R2 = 0.083; P = 0.001; Supplemental Figure 4), a result that persisted after excluding the nonplausible reporters (i.e., 17 participants). In addition, we tested whether fasted and pre–ad libitum meal sensations of hungry and fullness were associated with the ad libitum energy intake, finding no association (all P > 0.06).
Discussion
The present results show that BAT volume and 18F-FDG uptake are not associated with energy intake assessed by an ad libitum meal and by 24-h dietary recalls. It is noteworthy that both methods for assessing energy intake were sensitive enough to detect an already well-established positive association between lean mass and energy intake (50). No role for BAT was detected with respect to the regulation of appetite-related sensations either before or after the 2 meals the subjects ate. This suggests that BAT has little to do with human appetite regulation. Similarly, skeletal muscle 18F-FDG uptake was not associated with energy intake, yet the results suggest that some skeletal muscle groups are associated with the regulation of appetite-related sensations after a meal. More work is needed to confirm this.
The present results reflect the now-recognized robust positive association between fat-free mass and energy intake (23), and the lack of such an association between fat mass and energy intake (50). In this context, BAT could be considered a third component of this body composition model. Certainly, it shares histological characteristics with white adipose tissue (51), as well as an ontogenic origin and thermogenic role with skeletal muscle (52). However, no association was observed between BAT volume or 18F-FDG uptake and energy intake, assessed via either the ad libitum meal or the habitual dietary intake. Fat-free mass is a strong predictor of not only energy intake, but also energy expenditure (53). Therefore, the lack of association between BAT volume or 18F-FDG uptake and energy intake is consistent with previous studies showing that the BAT energy expenditure elicited by mild cold or meal ingestion only accounts for 10–15 kcal/d even when continuously and maximally activated (17, 18, 54). Paradoxically, in these earlier studies, BAT showed a higher energy expenditure, a higher blood flow increase, and a higher fractional substrate uptake per unit volume than any other tissue measured (16, 18, 54). However, the relatively small volume of BAT thought to be present in human adults (39) explains why it accounts for <1% of whole-body energy expenditure (18, 54). Likewise, a scant volume in adults would probably explain why BAT appeared not to be associated with energy intake or appetite-related sensations after the standardized breakfast or ad libitum meal. Nonetheless, new technologies for in vivo BAT quantification are needed to confirm the small volume of BAT observed in previous human studies (13). Moreover, it cannot be ruled out that human BAT has endocrine connections with the appetite regulation system (31, 32), as occurs in mice (29–31). For instance, a recent study has shown that, in these animals, the gut hormone secretin activates BAT thermogenesis, which in turn regulates postprandial thermogenesis and satiety (31). Importantly, serum secretin is also increased in humans under postprandial conditions, and secretin infusions increase BAT 18F-FDG uptake (31). Further studies are needed if we are to fully understand the role of human BAT in appetite regulation.
In the present work, energy intake and appetite-related sensations were measured in a warm environment. It cannot be ruled out, therefore, that BAT is associated with energy intake or appetite sensations in cold environments. Indeed, an earlier study in humans suggested that a cold-induced increase in energy expenditure might not be compensated for by energy intake or appetite-related sensations in the following hours (55). In animal models, however, cold acclimation induces similar increases in energy expenditure and energy intake (27). These findings suggest that cold exposure increases energy intake in the long term, thereby compensating for the increase in energy expenditure, although maybe not in the very short term. Whether this applies to humans remains to be seen.
Muscle thermogenesis seems to be the main contributor to cold-induced thermogenesis, even in mild cold exposures where shivering is minimized (20, 54). An anatomical dimorphism has been reported in several studies, showing that some muscle groups are especially active in response to cold (16, 20, 54). Whether this augmented metabolism is mediated by shivering (20) or nonshivering (56) mechanisms is still unknown. In addition, it has been suggested that this muscle metabolism may be partly mediated by endocrine signals released from the BAT (54, 57). Interestingly, the interaction cold-sensitive skeletal muscle 18F-FDG uptake × time elapsed after meal consumption showed a trend toward having a significant effect on appetite-related sensations after the standardized breakfast and ad libitum meal. Moreover, an association between the AUC for fullness sensations and skeletal muscle 18F-FDG uptake was detected. This raises the possibility that skeletal muscle, rather than BAT, could be the tissue responsible for both energy expenditure and energy intake stimulation in response to cold. Similarly, it seems reasonable that cold exposure is a useful physiological state for studying the mechanistic connections that might explain the link between fat-free mass and energy intake (50).
The present results should be understood with caution because this study is not free from limitations. The study population was made up of young healthy adults, hence it remains unknown whether these findings hold true for older or unhealthy individuals. Although we checked that results remained consistent after adjusting for menstrual cycle phase, we have no record of oral contraceptive use, and therefore could not check its influence on the results. On the other hand, energy intake estimations by dietary recalls can suffer from important bias and imprecision (58), and a single ad libitum meal is less accurate for estimating daily energy intake than a 24-h ad libitum procedure. However, both the ad libitum meal and the dietary recall records were sensitive enough to detect the already known relation between lean mass and energy intake, and they were associated with one another (Supplemental Figure 4). Moreover, both the ad libitum meal and VASs are reliable, valid methods for assessing appetite regulation (41, 42). It should be noted that although 18F-FDG is currently the best available method for assessing human BAT volume, it suffers serious limitations as a method for assessing BAT metabolic activity (13). Finally, it would have been desirable to have known the concentrations of several appetite-related peptides and hormones under fasting and postprandial conditions.
In summary, BAT volume and 18F-FDG uptake after a personalized cold exposure appear not to be associated with energy intake or meal-induced appetite-related sensations in young, healthy adults. These results suggest that BAT plays no important role in the regulation of energy intake in humans.
Supplementary Material
ACKNOWLEDGEMENTS
This study was performed as part of a Ph.D. thesis conducted within the Biomedicine Doctoral Studies Program of the University of Granada, Spain.
The authors’ responsibilities were as follows—GS-D, GF, CG, IL, AG, JEB, and JRR: designed the research; GS-D, FMA, BM-T, JML-E, and JRR: conducted the experiments; GS-D, FMA, and BM-T: analyzed the data; GS-D, JEB, and JRR: wrote the manuscript; GS-D and JRR: were primarily responsible for the final content; and all authors: discussed the results and read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the Spanish Ministry of Economy and Competitiveness via Fondo de Investigación Sanitaria del Instituto de Salud Carlos III grant PI13/01393 (to JRR), Retos de la Sociedad grant DEP2016-79512-R (to JRR), and European Regional Development Funds (ERDF); Spanish Ministry of Education grant FPU13/04365 (to GS-D); the Fundación Iberoamericana de Nutrición (to JRR); Redes Temáticas de Investigación Cooperativa grant Red SAMID RD16/0022 (to AG); the AstraZeneca HealthCare Foundation; the University of Granada Plan Propio de Investigación 2016—Excellence actions: Unit of Excellence on Exercise and Health (to JRR)—and Plan Propio de Investigación 2018—Programa Contratos-Puente (to GS-D); and Junta de Andalucía, Consejería de Conocimiento, Investigación y Universidades grant SOMM17/6107/UGR (to JRR) via the ERDF.
Supplemental Table 1 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
Abbreviations used: BAT, brown adipose tissue; PAL, physical activity level; PET-CT, positron-emission tomography and computed tomography; RMR, resting metabolic rate; ROI, region of interest; SUV, standardized uptake value; VAS, visual analog scale; 18F-FDG, 18F-fluorodeoxyglucose.
References
- 1. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. [DOI] [PubMed] [Google Scholar]
- 2. Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–9. [DOI] [PubMed] [Google Scholar]
- 3. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. [DOI] [PubMed] [Google Scholar]
- 4. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng Y-H, Doria A et al.. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto N, Enerbäck S, et al.. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. [DOI] [PubMed] [Google Scholar]
- 6. Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K et al.. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, Nedergaard J, Cinti S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–20. [DOI] [PubMed] [Google Scholar]
- 8. Lee P, Swarbrick MM, Ho KKY. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013;34:413–38. [DOI] [PubMed] [Google Scholar]
- 9. Thyagarajan B, Foster MT. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig. 2017;31:0016. [DOI] [PubMed] [Google Scholar]
- 10. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ruiz JR, Martinez-Tellez B, Sanchez-Delgado G, Osuna-Prieto FJ, Rensen PCN, Boon MR. Role of human brown fat in obesity, metabolism and cardiovascular disease: strategies to turn up the heat. Prog Cardiovasc Dis. 2018;61(2):232–45. [DOI] [PubMed] [Google Scholar]
- 12. Brychta RJ, Chen KY. Cold-induced thermogenesis in humans. Eur J Clin Nutr. 2017;71:345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte ÉE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne). 2018;9:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, Herscovitch P, Millo CM, Remaley A, Lee P, et al.. Brown fat activation mediates cold-induced thermogenesis in adult humans in response to a mild decrease in ambient temperature. J Clin Endocrinol Metab. 2013;98:E1218–E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoneshiro T, Matsushita M, Nakae S, Kameya T, Sugie H, Tanaka S, Saito M. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. Am J Physiol Regul Integr Comp Physiol. 2016;310(10):R999–R1009. [DOI] [PubMed] [Google Scholar]
- 16. Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. U Din M, Saari T, Raiko J, Kudomi N, Maurer SF, Lahesmaa M, Fromme T, Amri E-Z, Klingenspor M, Solin O, et al.. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 2018;28(2):207–216.e3. [DOI] [PubMed] [Google Scholar]
- 18. Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013;54:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jensen MD. Brown adipose tissue – not as hot as we thought. J Physiol. 2015;593:489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blondin DP, Labbé SM, Phoenix S, Guérin B, Turcotte ÉE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson CM, Orooji M, Johnson DN, Naraghi-Pour M, Ravussin E. Brown adipose tissue does not seem to mediate metabolic adaptation to overfeeding in men. Obesity (Silver Spring). 2017;25:502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlögl M, Piaggi P, Thiyyagura P, Reiman EM, Chen K, Lutrin C, Krakoff J, Thearle MS. Overfeeding over 24 hours does not activate brown adipose tissue in humans. J Clin Endocrinol Metab. 2013;98:1956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lam YY, Ravussin E. Variations in energy intake: it is more complicated than we think. Am J Clin Nutr. 2017;106:1169–70. [DOI] [PubMed] [Google Scholar]
- 24. Hopkins M, Beaulieu K, Myers A, Gibbons C, Blundell JE. Mechanisms responsible for homeostatic appetite control: theoretical advances and practical implications. Expert Rev Endocrinol Metab. 2017;12:401–15. [DOI] [PubMed] [Google Scholar]
- 25. Hopkins M, Finlayson G, Duarte C, Gibbons C, Johnstone AM, Whybrow S, Horgan GW, Blundell JE, Stubbs RJ. Biological and psychological mediators of the relationships between fat mass, fat-free mass and energy intake. Int J Obes. 2019;43(2):233–42. [DOI] [PubMed] [Google Scholar]
- 26. MacLean PS, Blundell JE, Mennella JA, Batterham RL. Biological control of appetite: a daunting complexity. Obesity. 2017;25:S8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravussin Y, Xiao C, Gavrilova O, Reitman ML. Effect of intermittent cold exposure on brown fat activation, obesity, and energy homeostasis in mice. PLoS One. 2014;9(1):e85876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marlatt K, Chen K, Ravussin E. Is activation of human brown adipose tissue a viable target for weight management?. Am J Physiol Integr Comp Physiol. 2018;315(3):R479–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shido O, Yoneda Y, Nagasaka T. Changes in brown adipose tissue metabolism following intraventricular vasoactive intestinal peptide and other gastrointestinal peptides in rats. Jpn J Physiol. 1989;39:359–69. [DOI] [PubMed] [Google Scholar]
- 30. Mano-Otagiri A, Ohata H, Iwasaki-Sekino A, Nemoto T, Shibasaki T. Ghrelin suppresses noradrenaline release in the brown adipose tissue of rats. J Endocrinol. 2009;201:341–9. [DOI] [PubMed] [Google Scholar]
- 31. Li Y, Schnabl K, Gabler S-M, Willershäuser M, Reber J, Karlas A, Laurila S, Lahesmaa M, U Din M, Bast-Habersbrunner A et al.. Secretin-activated brown fat mediates prandial thermogenesis to induce satiation. Cell. 2018;175:1561–74..e12. [DOI] [PubMed] [Google Scholar]
- 32. Chondronikola M, Porter C, Malagaris I, Nella AA, Sidossis LS. Brown adipose tissue is associated with systemic concentrations of peptides secreted from the gastrointestinal system and involved in appetite regulation. Eur J Endocrinol. 2017;177:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Labayen I, Ortega FB, Chillon P, Fernandez-Reguera C, Alcantara JMA, Martinez-Avila WD et al.. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: rationale, design and methodology. Contemp Clin Trials. 2015;45:416–25. [DOI] [PubMed] [Google Scholar]
- 34. Sanchez-Delgado G, Alcantara JMA, Ortiz-Alvarez L, Xu H, Martinez-Tellez B, Labayen I, Ruiz JR. Reliability of resting metabolic rate measurements in young adults: impact of methods for data analysis. Clin Nutr. 2018;37:1618–24. [DOI] [PubMed] [Google Scholar]
- 35. Alcantara JMA, Sanchez-Delgado G, Martinez-Tellez B, Merchan-Ramirez E, Labayen I, Ruiz JR. Congruent validity and inter-day reliability of two breath by breath metabolic carts to measure resting metabolic rate in young adults. Nutr Metab Cardiovasc Dis. 2018;28(9):929–36. [DOI] [PubMed] [Google Scholar]
- 36. Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, Lee PSP, Piemonte T, Trabulsi J. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J Acad Nutr Diet. 2015;115:1417–46..e2. [DOI] [PubMed] [Google Scholar]
- 37. Martinez-Tellez B, Sanchez-Delgado G, Garcia-Rivero Y, Alcantara JMA, Martinez-Avila WD, Muñoz-Hernandez MV, Olza J, Boon MR, Rensen PCN, Llamas-Elvira JM et al.. A new personalized cooling protocol to activate brown adipose tissue in young adults. Front Physiol. 2017;8:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, Enerbäck S, Kinahan PE, van Marken Lichtenbelt W, Lin FI et al.. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab. 2016;24:210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Martinez-Tellez B, Nahon KJ, Sanchez-Delgado G, Abreu-Vieira G, Llamas-Elvira JM, van Velden FHP, Pereira Arias-Bouda LM, Rensen PCN, Boon MR, Ruiz JR. The impact of using BARCIST 1.0 criteria on quantification of BAT volume and activity in three independent cohorts of adults. Sci Rep. 2018;8:8567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B et al.. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregersen NT, Flint A, Bitz C, Blundell JE, Raben A, Astrup A. Reproducibility and power of ad libitum energy intake assessed by repeated single meals. Am J Clin Nutr. 2008;87:1277–81. [DOI] [PubMed] [Google Scholar]
- 42. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 43. Gonzalez-Anton C, Lopez-Millan B, Rico MC, Sanchez-Rodriguez E, Ruiz-Lopez MD, Gil A, Mesa MD. An enriched, cereal-based bread affects appetite ratings and glycemic, insulinemic, and gastrointestinal hormone responses in healthy adults in a randomized, controlled trial. J Nutr. 2015;145:231–8. [DOI] [PubMed] [Google Scholar]
- 44. Ambrus, A, Horvath Z, Doroghazi E, Cseh J, Petrova S, Dimitrov P, Duleva V, Rangelova L, Chikova-Iscener E, et al. Pilot study in the view of a Pan-European dietary survey-adolescents, adults and elderly. EFSA Support Publ. Wiley; 2017:10. [Google Scholar]
- 45. Acosta FM, Martinez-Tellez B, Sanchez G, Contreras-Gomez MA, Martinez-Avila WD, Merchan-Ramirez E, Alcantara JMA, Amaro-Gahete FJ, Llamas-Elvira JM, Ruiz JR. Association of objectively measured physical activity with brown adipose tissue volume and activity in young adults. J Clin Endocrinol Metab. 2019;104(2):223–33. [DOI] [PubMed] [Google Scholar]
- 46. Migueles JH, Cadenas-Sanchez C, Ekelund U, Delisle Nyström C, Mora-Gonzalez J, Löf M, Labayen I, Ruiz JR, Ortega FB. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. 2017;47:1821–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Au-Yong ITH, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bahler L, Deelen JW, Hoekstra JB, Holleman F, Verberne HJ. Seasonal influence on stimulated BAT activity in prospective trials: a retrospective analysis of BAT visualized on 18F-FDG PET-CTs and 123I-mIBG SPECT-CTs. J Appl Physiol. 2016;120:1418–23. [DOI] [PubMed] [Google Scholar]
- 49. Wang Q, Zhang M, Xu M, Gu W, Xi Y, Qi L, Li B, Wang W. Brown adipose tissue activation is inversely related to central obesity and metabolic parameters in adult human. PLoS One. 2015;10:e0123795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blundell JE, Caudwell P, Gibbons C, Hopkins M, Näslund E, King NA, Finlayson G. Body composition and appetite: fat-free mass (but not fat mass or BMI) is positively associated with self-determined meal size and daily energy intake in humans. Br J Nutr. 2012;107:445–9. [DOI] [PubMed] [Google Scholar]
- 51. Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510:76–83. [DOI] [PubMed] [Google Scholar]
- 52. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H et al.. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Müller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3:113–22. [DOI] [PubMed] [Google Scholar]
- 54. U Din M, Raiko J, Saari T, Kudomi N, Tolvanen T, Oikonen V, Teuho J, Sipilä HT, Savisto N, Parkkola R et al.. Human brown adipose tissue [15O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016;43:1878–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Langeveld M, Tan CY, Soeters MR, Virtue S, Ambler GK, Watson LPE, Murgatroyd PR, Chatterjee VK, Vidal-Puig A. Mild cold effects on hunger, food intake, satiety and skin temperature in humans. Endocr Connect. 2016;5:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Betz MJ, Enerbäck S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol. 2018;14(2):77–87. [DOI] [PubMed] [Google Scholar]
- 57. Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, Dawes BA, Tsai L, Kahn BB, Spiegelman BM et al.. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 2018;28:631–43.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beaton GH, Milner J, Corey P, McGuire V, Cousins M, Stewart E, de Ramos M, Hewitt D, Grambsch PV, Kassim N, et al.. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32:2546–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.