Abstract
The electrophysiology of the paralimbic network (“default mode”) for self-awareness has drawn much attention in the past couple of decades. In contrast, knowledge of the molecular organization of conscious experience has only lately come into focus. We here review newer data on dopaminergic control of awareness in humans, particularly in self-awareness. These results implicate mainly dopaminergic neurotransmission and the control of GABAergic function directly in the paralimbic network. The findings are important for understanding addiction, developmental disorders, and dysfunctional consciousness.
Keywords: self-awareness, default mode network, conscious experience, GABA, dopamine, addiction
Introduction
Self-awareness is a conscious experience with the self as an object. It is an essential part of conscious experience of the world, and is a tool for conscious self-monitoring and for controlling behavior. Its default may have grave consequences. The “neural correlates” of self-awareness have been studied by several investigators, including Devue and Brédart (2011) and D’Argembeau (2013). Generally, a cortical, paralimbic network has been proposed as a correlate for self-awareness. It often included prefrontal and medial parietal regions, but it remained unknown whether these regions were indeed instrumental in that function.
The Paralimbic Network: From “Correlations” to Being “Instrumental” in Self-awareness
Based on the effect of temporary dysfunction of brain regions induced by transcranial magnetic stimulation (TMS), we have realized that a paralimbic network is indeed instrumental in self-awareness: the network is not only active during self-awareness (Kjaer et al., 2002; Lou et al., 2004), but is also causal for that function (Lou et al., 2004; Luber et al., 2012). Its core cortical regions, medial prefrontal and medial parietal are interneuron-rich hubs with multiple connections (Freund, 2003). They are usually linked with activity in the angular gyri, insula, and subcortical regions including striatum (Slagter et al., 2017) and thalamus (Rømer Thomsen et al., 2013; Bachman and Hudetz, 2014). The network is often termed the “default mode network” because it defaults when attention is turned away from the self to other tasks (Lou et al., 2017). The medial prefrontal and medial parietal regions are “hubs” of the network. They interact via GABA-induced synchronized gamma oscillations (Rømer Thomsen et al., 2013; Joensson et al., 2015).
Dopamine-GABA Interaction in Conscious Experience
Functional brain imaging has indicated that abnormal conscious experiences in schizophrenia, like hallucinations and delusions, are associated with abnormal dopaminergic neurotransmission (Changeux and Lou, 2011; Palmiter, 2011). In order to test if this association is causal, we used signal detection tasks and subsequent subjective interpretation, i.e., by setting a criterion for when the sensory signal-to-noise ratio provides confidence that a stimulus is present (Lou et al., 2011). We examined the effect of increasing dopamine activation and showed that dopaminergic stimulation with the D1 and D2 receptor agonist pergolide is effective in increasing confidence in seeing words and in improving performance in a forced-choice word recognition task. This demonstrates neurotransmitter regulation of subjective conscious experience of perception and provides the first direct evidence that dopamine is instrumental in conscious experience (Lou et al., 2011; Figure 1). Such activation depends on fast-spiking GABAergic interneurons (Changeux and Lou, 2011), which intermittently inhibit pyramidal cell activity to produce gamma oscillations necessary for effective neurotransmission (Freund, 2003; Lou et al., 2011; Rømer Thomsen et al., 2013; Joensson et al., 2015).
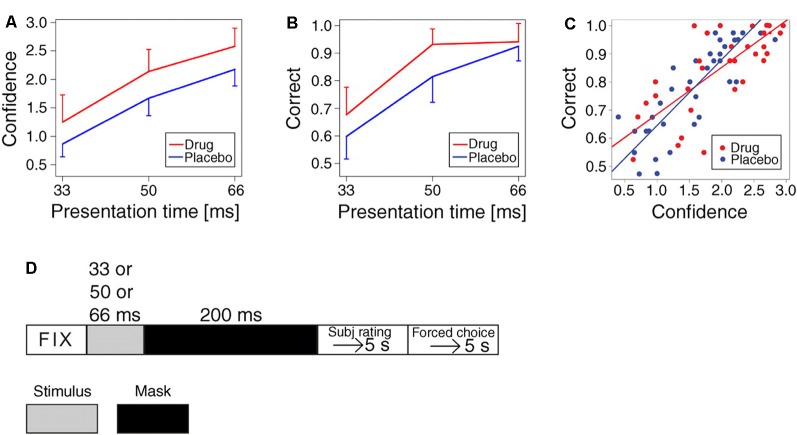
Figure 1.
Effect of dopaminergic activation on confidence in seeing words and on accuracy in word recognition task. (A) Increase in the subjective rating of confidence (scale 0–3) for words presented at 33 ms, 50 ms, and 66 ms (means and standard deviations of means, p = 0.0018). (B) Increase in accuracy (percentage of correct responses, in word recognition task by forced choice, one distractor). The expectation from chance: 50%. Note ceiling effect (p = 0.006). (C) All observations of correct answers as a function of confidence, showing a significant effect (p < 0.0001, regression with random effect). The pergolide-treated group and the placebo groups had identical regression lines statistically. (D) Experimental timeline (Lou et al., 2011, p. 3). Adapted with permission from Lou et al. (2011).
Site of Dopamine-GABA Interaction
The site of dopamine-GABA interaction for self-awareness in the human brain was unknown until recently. To clarify this issue, we have used a PET ligand for GABA receptor binding. With this ligand, we were able to detect changes in dopamine-induced GABA binding under different physiological conditions at well-defined brain sites (Lou et al., 2016). It was found that GABA ligand binding was maximal in the medial anterior paralimbic region (cingulate gyrus), indicating empty GABA binding sites in these locations. Orally given dopamine reduced free GABA ligand binding sites throughout the cortex including the paralimbic system (Figure 2). In other words: dopamine increases GABA binding directly in the human paralimbic cortex, concomitantly with increased self-awareness and conscious experience (Lou et al., 2016). The effect is faltering in problem gambling, reflecting faltering self-monitoring and self-awareness (Møller et al., 2019). This development brings the properties of the GABA receptor molecule into focus.
Figure 2.
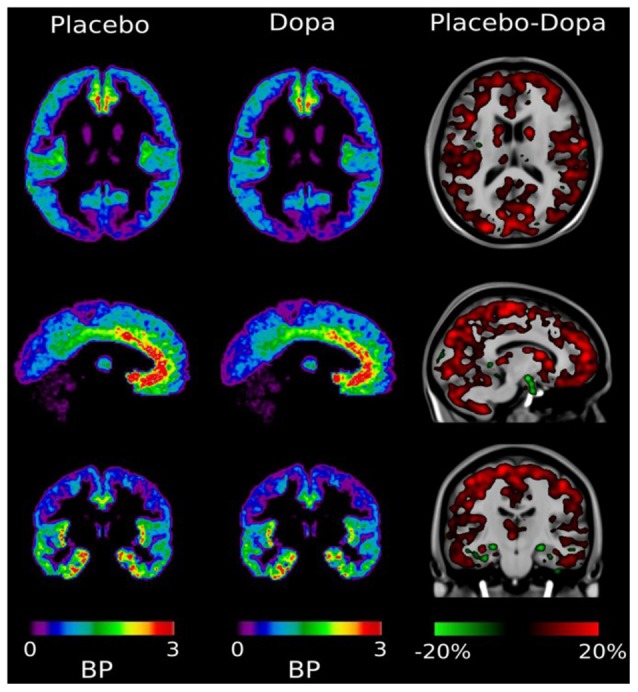
Distribution of [11C] Ro15-4513 GABA receptor ligand. After the placebo, the ligand was mainly bound in the medial-inferior prefrontal cingulate cortex, and right and left insula. After dopamine challenge ligand binding is reduced, with a general reduction of between 5% and 20% throughout the cortex. In hippocampal regions there were small foci of increased binding potential. Adapted with permission from Lou et al. (2016).
Functional Anatomy of GABA Receptors
The GABA receptors are constructed as ligand ion channels. According to Stephens et al. (2017), they are “organized as a pore between five protein complexes. The pore allows passage of negative chloride ions, and, hence, the generation of electrical pulses when stabilized in an open conformation. This stabilization occurs by binding of GABA to the complex. The binding is not specific for GABA. The affinity of other molecules, whether physiologic, including dopamine, and foreign molecules depends on the protein composition of the five pentameric molecules constituting the pore and determine the function of the synapse. The subtype composition of the pentameric pore is abnormal in addiction.” This may explain the abnormal interaction between dopamine and GABA receptors in gambling disorder, a prime example of dysfunctional self-monitoring and self-control (Møller et al., 2019). Related to this, a number of studies suggest that altered GABA neurotransmission plays an important role in substance addiction (Lingford-Hughes et al., 2012, 2016) or gambling (Mick et al., 2017).
Self-awareness Network During Infancy and Childhood
In premature infants, functional MR imaging together with diffusion tensor imaging-based tractography has been used to study the relationship between performance on the Bayley Scales of Infant Development and early myelination (Cui et al., 2017). The Bayley Scales of Infant Development is a developmental play task that derives a developmental quotient (rather than an intelligence quotient). The authors confirmed such a link by showing that scores on the Bayley Scales of Infant Development were significantly associated with cingulate fractional anisotropy and radial diffusivity (Cui et al., 2017). This suggests that interconnecting axonal pathways within the default mode network are of critical importance already in the early neurocognitive development of infants.
Newborn infants already show a form of “basic consciousness” by establishing rudimentary eye contact with their mother (Lagercrantz and Changeux, 2009). A classical test for the presence of self-awareness in infants (and animals) is the mirror recognition test, where the infant is placed before a mirror with a spot marked on his/her forehead. A positive response is usually present at about 2 years of age. It requires the infant to try to remove the spot on his/her head, and not on the mirror (Anderson, 1984).
A recent review showed that the default mode network follows an inverse U-shape, where it is weaker in children and elderly and stronger in adults. Cognitive function is positively correlated with default mode network functional connectivity (Mak et al., 2017).
Deficient Self-awareness and Pathology
In later childhood and adulthood, disturbance of the paralimbic network is linked to severe pathology. Thus, deficient GABA neurotransmission is prominent in disorders with poor self-awareness and self-monitoring such as addiction (Lingford-Hughes et al., 2012, 2016; Mick et al., 2017; Møller et al., 2019), autism (Hashemi et al., 2017), anosognosia, hallucinations, and delusions (Therriault et al., 2018), progressing to schizophrenia (Rikandi et al., 2018) major depression (Rikandi et al., 2018), and/or dementia (Lin et al., 2015). Even in the vegetative state (also termed unresponsive wakefulness syndrome, Laureys et al., 2010), recovery of the paralimbic network is tightly linked to clinical recovery (Thibaut et al., 2019). Finally, a large clinical study of the possible therapeutic effect of apomorphine, a dopaminergic drug, is now underway (Sanz et al., 2019).
To determine if deficient GABA neurotransmission in pathology could be a primary event or secondary to toxic or pathological effects in more complex disorders, we examined if deficient dopamine-GABA neurotransmission was present in a relatively mono-symptomatic disorder such as gambling disorder (Møller et al., 2019). This was indeed the case. Therefore, normal interaction between these transmitters in the medial paralimbic system seems to be fundamental for brain function.
Dopamine Activity Linked With Other Transmitters
The basal forebrain part of the system is not only regulated by dopamine. It is also a prominent site of cholinergic activity. Interaction between the cholinergic and dopaminergic system via GABA receptors has been well described (Changeux and Lou, 2011; Takács et al., 2018). Thereby it promotes functions related to conscious experiences such as attention, learning and memory, and sleep-wake alternation (Lin et al., 2015).
Vulnerability of Paralimbic Network
The widespread dysfunction of self-awareness in disease is likely to be a consequence of the exceedingly high oxygen demand of the paralimbic network. The high oxygen requirement is considered to be the result of dense concentrations of parvalbumin GABAergic interneurons in the richly connected hubs of the paralimbic network. In particular, the fast gamma oscillations are susceptible to metabolic disruptions because of their high energy-demand (Kann et al., 2016).
Discussion
Until recently, conscious experience and self-awareness were considered off-limits for the natural sciences. Neurobiological research shunned the “hard question” of how conscious experience and self-awareness arise from a physical basis. Hence, it has been fashionable to limit neuroscience to try to identify neural “correlates” of conscious experience and self-awareness. The risk is evident for arriving at two parallel worlds: a mental and a physical, without understanding how they interact. This limitation has impeded our understanding of the biological function of self-awareness, and how it may account for disease. We have here reported data showing that self-awareness and conscious experience can be disturbed by electrophysiological manipulation of the paralimbic network (Lou et al., 2004; Luber et al., 2012). Therefore, we may conclude that the network is instrumental for these functions. Newer data reviewed here also strengthen the conclusion by showing that dopaminergic agents may stimulate conscious experience via GABA receptors in the paralimbic network. This finding has already resulted in a large and promising study of disabled persons with faltering self-awareness and consciousness (Sanz et al., 2019).
Author Contributions
HL conceptualized the review and wrote the initial draft. KR and J-PC participated in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Henriette Vuust, CFIN, Aarhus University, for help with figures.
Footnotes
Funding. The authors received no financial support for the research and authorship of this article.
References
- Anderson J. R. (1984). The development of self-recognition: a review. Dev. Psychobiol. 17, 35–49. 10.1016/s0273-2297(84)80006-4 [DOI] [PubMed] [Google Scholar]
- Bachman T., Hudetz A. G. (2014). It is time to combine the two main traditions in the research on the neural correlates of consciousness: C = L × D. Front. Psychol. 5:940. 10.3389/fpsyg.2014.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P., Lou H. C. (2011). Emergent pharmacology of conscious experience. FASEB J. 25, 2098–2108. 10.1096/fj.11-0702ufm [DOI] [PubMed] [Google Scholar]
- Cui J., Tymoflyeva O., Desikan R., Flynn T., Kim H., Gano D., et al. (2017). Microstructure of the default mode network in the preterm infant. AJNR Am. J. Neuroradiol. 38, 343–348. 10.3174/ajnr.A4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A. (2013). On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Front. Hum. Neurosci. 7:372. 10.3389/fnhum.2013.00372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devue C., Brédart S. (2011). Neural correlates of visual self-recognition. Conscious. Cogn. 20, 40–51. 10.1016/j.concog.2010.09.007 [DOI] [PubMed] [Google Scholar]
- Freund T. F. (2003). Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 26, 489–495. 10.1016/s0166-2236(03)00227-3 [DOI] [PubMed] [Google Scholar]
- Hashemi E., Ariza J., Rogers N., Noctor S. C., Martínez-Cardeno V. (2017). The number of paralbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cereb. Cortex 27, 1931–1943. 10.1093/cercor/bhw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensson M., Rømer Thomsen K., Andersen L. M., Gross J., Mouridsen K., Sandberg K., et al. (2015). Making sense: dopamine activates conscious self-monitoring through medial prefrontal cortex. Hum. Brain Mapp. 36, 1866–1877. 10.1002/hbm.22742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kann O., Hollnagel J. O., Elzoheiry S., Schneider J. (2016). Energy and potassium homeostasis during γ oscillations. Front. Mol. Neurosci. 9:47. 10.3389/fnmol.2016.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T. W., Nowak M., Lou H. C. (2002). Reflective self-awareness and representation of the mental self. NeuroImage 17, 1080–1086. 10.1006/nimg.2002.1230 [DOI] [PubMed] [Google Scholar]
- Lagercrantz H., Changeux J. P. (2009). The emergence of human consciousness: from fetal to neonatal Life. Pediatr. Res. 65, 255–260. 10.1203/pdr.0b013e3181973b0d [DOI] [PubMed] [Google Scholar]
- Laureys S., Celesia G. G., Cohandon F., Lavrijsen J., León-Carrión J., Sannita W. G., et al. (2010). Unresponsive wakefulness syndrome: a new name for the vegetative state or apallic syndrome. BMC Med. 8:68. 10.1186/1741-7015-8-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. C., Brown R. E., Hussain Shuler M. G., Petersen C. C., Kepec A. (2015). Optogenetic dissection of the basal forebrain. J. Neurosci. 35, 13896–13901. 10.1523/JNEUROSCI.2590-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A., Myers J., Watson B., Reid A. G., Kalk N., Feeney A., et al. (2016). Using [11C]Ro15 4513 PET to characterize GABA-benzodiazepine receptors in opiate addiction: similarities and differences with alcoholism. Neuroimage 132, 1–7. 10.1016/j.neuroimage.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A., Reid A. G., Myers J., Feeney A., Hammers A., Taylor L. G., et al. (2012). A [11C]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced α5 benzodiazepine receptors in limbic regions. J. Psychopharmacol. 26, 273–281. 10.1177/0269881110379509 [DOI] [PubMed] [Google Scholar]
- Lou H. C., Changeux J. P., Rosenstand A. (2017). Towards a cognitive neuroscience of self-awareness. Neurosci. Biobehav. Rev. 83, 765–773. 10.1016/j.neubiorev.2016.04.004 [DOI] [PubMed] [Google Scholar]
- Lou H. C., Luber B., Crupain M., Keenan J. P., Nowak M., Kjaer T. W., et al. (2004). Parietal cortex representation of the mental Self. Proc. Natl. Acad. Sci. U S A 101, 6827–6832. 10.1073/pnas.0400049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. C., Rosentand A., Brooks D. J., Bender D., Jakobsen S., Blicher J. U., et al. (2016). Exogenous dopamine reduces GABA receptor availability in the human brain. Brain Behav. 6:e00484. 10.1002/brb3.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou H. C., Skewes J. C., Thomsen K. R., Overgaard M., Law H. C., Mouridsen K., et al. (2011). Dopaminergic stimulation enhances confidence and accuracy in seeing rapidly presented words. J. Vis. 11:15. 10.1167/11.2.15 [DOI] [PubMed] [Google Scholar]
- Luber B., Lou H. C., Keenan J. P., Lisanby S. H. (2012). Self-enhancement in the default network. Exp. Brain Res. 223, 177–187. 10.1007/s00221-012-3249-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak L. E., Minuzzi L., MacQueen G., Hall G., Kennedy S. H., Milev R. (2017). The default mode network in healthy individuals. Brain Connect. 7, 25–33. 10.1089/brain.2016.0438 [DOI] [PubMed] [Google Scholar]
- Mick I., Ramos A. C., Myers J., Stokes P. R., Chandrasekera S., Mendez M. A., et al. (2017). Evidence for GABA-A receptor dysregulation in gambling disorder: correlation with impulsivity. Addicti. Biol. 22, 1601–1609. 10.1111/adb.12457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller A., Rømer Thomsen K., Brooks D. J., Blicher J. U., Hansen K. V., Mouridsen K., et al. (2019). Attenuation of dopamine-induced GABA release in problem gamblers. Brain Behav. 9:e01239. 10.1002/brb3.1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. (2011). Dopamine signaling as a neural correlate of consciousness. Neuroscience 198, 213–220. 10.1016/j.neuroscience.2011.06.089 [DOI] [PubMed] [Google Scholar]
- Rikandi E., Mäntylä T., Lindgren M., Kieseppä T., Suvisaari J., Raij T. T. (2018). Connectivity of the precuneus-posterior cingulate cortex with the anterior cingulate-medial prefrontal cortex differs consistently between control subjects and first-episode psychosis patients during a movie stimulus. Schizophr. Res. 199, 235–242. 10.1016/j.schres.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K., Joensson M., Lou H. C., Møller A., Gross J., Kringelbach M. L., et al. (2013). Altered paralimbic interaction in behavioral addiction. Proc. Natl. Acad. Sci. U S A 110, 4744–4749. 10.1073/pnas.1302374110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L. R. D., Lejeune N., Blandiaux S., Bonin E., Thibaut E., Stenter J., et al. (2019). Treating disorders of consciousness with apomorphine: protocol for a double-blind randomized controlled trial using multimodal assessments. Front. Neurol. 10:248. 10.3389/fneur.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter H. A., Mazaheri A., Reteig L. C., Smolders R., Figee M., Mantione M., et al. (2017). Contributions of the ventral striatum to conscious perception: an intracranial EEG study of the attentional blink. J. Neurosci. 37, 1081–1089. 10.1523/JNEUROSCI.2282-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. N., King S. L., Lambert J. J., Belelli D., Duka T. (2017). GABAA receptor subtype involvement in addictive behavior. Genes Brain Behav. 16, 149–184. 10.1111/gbb.12321 [DOI] [PubMed] [Google Scholar]
- Takács V. T., Cserép C., Schlingloff D., Pósfai B., Szonyi A., Sos K. E., et al. (2018). Co-transmission of acetylcholine and GABA regulates hippocampal states. Nat. Commun. 9:2848. 10.1038/s41467-018-05136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therriault J., Ng K. P., Pascoal T. A., Mathotaarachchi S., Kang M. S., Struys H., et al. (2018). Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology 90, e932–e939. 10.1212/wnl.0000000000005120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut A., Schiff N., Glacino J., Laureys S., Gosseries O. (2019). Therapeutic interventions in patients with prolonged disorders of consciousness. Lancet Neurol. 18, 600–614. 10.1016/s1474-4422(19)30031-6 [DOI] [PubMed] [Google Scholar]