Abstract
Background
The diminished glucose lowering effect of insulin in obesity, called “insulin resistance,” is associated with glucose intolerance, type 2 diabetes, and other serious maladies. Many publications on this topic have suggested numerous hypotheses on the molecular and cellular disruptions that contribute to the syndrome. However, significant uncertainty remains on the mechanisms of its initiation and long-term maintenance.
Scope of review
To simplify insulin resistance analysis, this review focuses on the unifying concept that adipose tissue is a central regulator of systemic glucose homeostasis by controlling liver and skeletal muscle metabolism. Key aspects of adipose function related to insulin resistance reviewed are: 1) the modes by which specific adipose tissues control hepatic glucose output and systemic glucose disposal, 2) recently acquired understanding of the underlying mechanisms of these modes of regulation, and 3) the steps in these pathways adversely affected by obesity that cause insulin resistance.
Major conclusions
Adipocyte heterogeneity is required to mediate the multiple pathways that control systemic glucose tolerance. White adipocytes specialize in sequestering triglycerides away from the liver, muscle, and other tissues to limit toxicity. In contrast, brown/beige adipocytes are very active in directly taking up glucose in response to β adrenergic signaling and insulin and enhancing energy expenditure. Nonetheless, white, beige, and brown adipocytes all share the common feature of secreting factors and possibly exosomes that act on distant tissues to control glucose homeostasis. Obesity exerts deleterious effects on each of these adipocyte functions to cause insulin resistance.
Keywords: Adipokines, Adipose tissues, Adrenergic receptors, Bioactive lipids, Glucose tolerance, Lipogenesis, Signaling, Thermogenesis, Uncoupling protein
1. Introduction
Insulin is a vital hormone, as both its absence and excess are lethal in humans. Insulin has even been exploited as a murder weapon in notorious crime cases [1]. Its introduction into clinical usage in the early 1920s was designed to replace the insulin lacking in type 1 diabetes, but its use soon revealed confounding paradoxes that engage the field to this day. For example, higher carbohydrate consumption by those with diabetes often unexpectedly decreases the required doses of insulin rather than increasing them. Glucose tolerance tests in humans also showed marked sensitivity to feeding vs fasting, and normal human subjects fed high-fat diets became extraordinarily glucose intolerant [2]. Himsworth et al. subsequently found that insulin was surprisingly ineffective in disposing a glucose load in obese subjects with diabetes, and by 1940 they had categorized diabetes as two types: “insulin sensitive” vs “insulin insensitive” [3]. This insight explained a related paradox, discovered in the 1960s via radioimmunoassay, that circulating insulin was sometimes highly elevated rather than decreased in obese subjects with mild diabetes [4]. Hyperinsulinemia associated with decreased responsiveness to insulin may itself be one of the major drivers of this insulin insensitive state [5,6]. Altogether, these and other findings solidified a strong overall paradigm that peripheral tissue resistance to the actions of insulin is caused by overnutrition and obesity [7].
This impairment in systemic responses to insulin in human and rodent obesity, as defined by its blood glucose lowering effects, reflects decreased insulin sensitivity in one or a combination of the major metabolic organs: liver, muscle, and adipose tissues. In the liver, insulin loses its full ability to inhibit glucose output through glycogenolysis and gluconeogenesis, while in the muscle and adipose tissues, insulin-stimulated glucose uptake and utilization are impaired [5,[8], [9], [10]]. However, the effects of insulin on these tissues are not always cell autonomous (Figure 1), as exemplified by insulin modulation of hepatic metabolism secondarily through its regulation of adipocyte fatty acid release [11,12]. Since the insulin resistant state correlates so closely with serious maladies such as type 2 diabetes and cardiovascular disease in humans [13,14], it has been extensively studied for decades. Scientists new to the field are now confronted by vast literature on the topic, as a PubMed search quickly reveals (Table 1). Searching for the term “insulin resistance” yields 119,349 citations, an overwhelming number of publications. In 2018 alone, more than 8,000 publications referred to insulin resistance. Moreover, there is a multitude of cellular pathways that have been hypothesized to mediate insulin resistance in obesity, each with extensive support from hundreds or even thousands of publications (Table 1). While there have been highly significant improvements in therapies for type 2 diabetes, it could be argued that there has been no breakthrough with the equivalent impact on the discovery of insulin itself in 1921. Meanwhile, the prevalence of diabetes in the US has since increased from approximately 1% to nearly 10% of the population. Thus, it is not difficult to understand why insulin resistance and type 2 diabetes can be daunting for investigators new to the field.
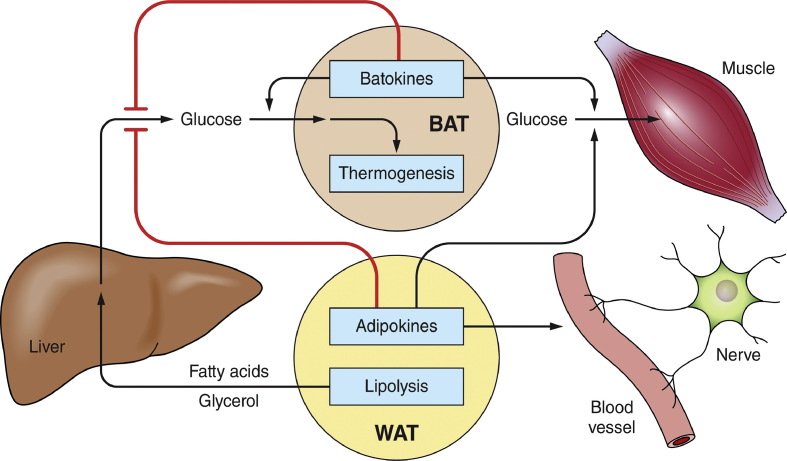
Figure 1.
Pathways by which WAT and BAT depots serve as major nodes of systemic metabolic regulation. Adipokines and batokines regulate hepatic lipogenesis and glucose output as well as glucose uptake and disposal by muscle. Secreted factors from adipocytes can also act in a paracrine fashion to regulate other cell types within adipose depots such as vascular cells and nerve fibers. BAT thermogenesis may contribute to systemic glucose disposal and oxidize lipids to lower systemic toxicity. WAT lipolysis in obesity can contribute to fatty acid and glycerol overload in the liver to enhance gluconeogenesis and glucose output. WAT-derived fatty acids also contribute to skeletal muscle insulin resistance (not shown). The combination of the actions of peptides, lipids, small RNA, and other factors from adipocytes plus the released lipolytic products (fatty acids and glycerol) have major influences on local cell types within adipose tissue as well as on distant tissues.
Table 1.
Number of total and 2018 PubMed citations related to insulin resistance and some of the various major cellular pathways that have been implicated in the induction of insulin resistance in obesity. The terms “insulin resistance” or “insulin resistance and” and each of the terms listed in the table under “Cellular pathway” was searched on August 21, 2019. The number of total citations are provided for each of these searches.
| Cellular Pathway | Total PubMed Citations | 2018 PubMed Citations |
|---|---|---|
| Insulin Resistance | 119,837 | 8376 |
| Insulin Resistance and … | ||
| Protein kinase | 11,449 | 744 |
| Fatty Acids | 10,970 | 668 |
| Cytokines | 9331 | 613 |
| Reactive Oxygen Species | 2207 | 199 |
| Microbiota | 1117 | 228 |
| Hypoxia | 852 | 83 |
| Branched chain amino acids | 773 | 81 |
| miRNA | 752 | 149 |
| Ceramide | 713 | 66 |
| Diacylglycerol | 525 | 32 |
| cAMP | 522 | 21 |
| ER Stress Response | 302 | 29 |
2. Adipose tissue as a central node in systemic metabolism
Can a central theme consolidate the vast amount of research on insulin resistance into a useful framework to guide future strategies? One such major consilience that has emerged over the last two decades is the concept that adipose tissues are major regulators of metabolism and cell signaling in the liver and skeletal muscle [[15], [16], [17]]. Accordingly, adipose tissue dysfunctions under high-fat diet (HFD) or obese conditions are thought to mediate disruptive signals to other metabolic tissues through the circulation [11,12], central nervous system [18], or potentially release of exosomes [17]. This is perhaps unsurprising as adipose tissues are dramatically affected by obesity and significantly expand in mass over the lean condition. Three general mechanisms have been hypothesized to mediate such adipose tissue control over other tissues and systemic glucose homeostasis (Figure 1): 1) lipid sequestration within white adipocyte depots by enhancing synthesis or limiting lipolysis that prevents toxic lipid accumulation in the liver and skeletal muscle [[19], [20], [21]] and is compromised in lipodystrophy [19], 2) high rates of glucose and fatty acid oxidation within specialized beige and brown adipocytes that increase energy expenditure and reduce lipid load [[22], [23], [24]], and 3) secretion of bioactive factors that can target the brain, liver, skeletal muscle, pancreatic islets, or other tissues [[15], [16], [17],[25], [26], [27]]. Each of these complex adipose biology pathways have been shown to modulate whole body glucose tolerance and insulin sensitivity.
The aim of this review article is to provide a broad summary of the field and focus on key questions related to insulin resistance in one affected organ: adipose tissue. Based on the vast literature and the broad perspective covered herein, recent reviews are cited that cover specific areas of investigation in a deeper fashion in addition to the selected original papers. This overview will hopefully promote further research to more fully answer such questions as: What specific steps in the many pathways that control adipocyte functions are actually perturbed in insulin resistant obesity, and how? What contribution do each of these pathways make in causing insulin resistance in adipocytes? How do adipocyte disruptions in obesity cause whole body glucose intolerance? Hopefully, some central features and analytics of insulin resistance in adipocytes will also be applicable to other tissues for a better understanding of systemic metabolic regulation.
3. Insulin-responsive adipose tissue subtypes
Based on the capabilities of adipose tissues to control systemic metabolism, it is unsurprising that there are many subtypes of adipocytes. Indeed, the heterogeneity of adipose tissue depots and the constituent adipocytes within the depots are logically required to execute the various paradigms of systemic metabolic control. The cellular constituents of the many adipose tissue depots in various locations throughout the body are well known to be associated with distinct characteristics and functional differences [[28], [29], [30]]. Most notable in rodents are the contrasting energy storing capacities of white adipose tissue (WAT) depots compared to the energy expenditure capacities of brown adipose tissue depots (BAT). Significant differences between subcutaneous WAT depots and visceral WAT depots have also been extensively documented, including distinct gene expression profiles of the resident adipocytes, immune cell infiltration patterns, and metabolic flux [[31], [32], [33], [34], [35], [36]].
Three major classes of adipocytes in these various depots have been extensively studied. These include white adipocytes specialized for triglyceride storage in WAT, as opposed to beige adipocytes within WAT and brown adipocytes in BAT that display high respiration rates associated with uncoupling protein UCP1 within mitochondria [[37], [38], [39], [40]]. Beige adipocytes appear within certain white adipose depots during cold exposure or even intermittent fasting in mice [25,[39], [40], [41], [42], [43], [44]] and in certain adipose depots in humans, especially in regions that are highly vascularized in the neck and thoracic areas [41,[45], [46], [47], [48], [49], [50]]. Beige adipocytes appear to differ from brown adipocytes not only in their respective location in WAT vs BAT depots, but also in their developmental program, and their responsiveness to adrenergic signaling with respect to mitochondrial regulation and UCP1 expression. Beige cells appear to display a more rapid response and reversion to cold exposure and withdrawal, respectively, whereas brown adipocytes express constitutively high levels of UCP1 and lose this expression over a longer time period during exposure to thermoneutrality [41]. Both beige and brown adipocytes appear to contribute to weight gain and enhanced glucose tolerance in rodents [22,[42], [43], [44],51] and humans [52], either through their ability to enhance energy expenditure or secrete factors beneficial to the liver, skeletal muscle, and other tissues, or via both mechanisms.
Recent work has highlighted further degrees of adipocyte heterogeneity within WAT and BAT, and discoveries along these lines are currently rapidly increasing with the advent of single cell and single nuclei RNA sequencing technology [[53], [54], [55], [56]] as well as single progenitor cell cloning [57]. These findings include substantial heterogeneity of precursor adipocyte progenitor cells [54,55,[57], [58], [59], [60]] and heterogeneity of fully differentiated adipocytes comparing distinct adipose depots [28,29,61] or even within the same depot [[62], [63], [64], [65]]. These studies have revealed a multitude of other cell types that are also present within adipose depots, including endothelial cells and many types of immune cells. Single cell sequencing has also demonstrated a mesenchymal progenitor cell hierarchy in adipose tissue in both mice and humans [66]. Importantly, studies show that adipocyte progenitor cells can proliferate in response to HFD conditions that elicit insulin resistance, and that progenitor proliferation is dependent on adipose depot, dietary lipids, microenvironment, and hormones [[67], [68], [69], [70], [71]]. A stromal cell population has been identified that inhibits adipocyte progenitor proliferation [72]. Signature gene expression profiles in clonal human adipocyte progenitor lines also reveal beige vs white adipocyte fates [57,73]. This area of research is expanding rapidly and will be required to define the detailed dynamics of white and beige adipose tissue depot remodeling under various physiological conditions.
Significant differences in the rates of lipolysis and lipid uptake in white adipocytes derived from visceral vs subcutaneous depots have been well documented and reviewed [29,31]. White adipocyte heterogeneity within the same depot has been observed in both gene expression and insulin sensitivity under normal conditions [62] and in β-adrenergic receptor expression on the cell surface [63]. By isolating preadipocyte clonal lines from human subcutaneous WAT, several subtypes of such precursors and the derived white adipocytes can be defined by distinct gene expression profiles [57]. These included differential expression levels of leptin vs adiponectin in white adipocyte subtypes in addition to the identification of a human thermogenic adipocyte subtype [57]. This approach has led to improved adipose implantation techniques [74]. Single nuclei [56] and single cell RNA [54,55] sequencing methods have led to the identification of multiple white adipocyte subtypes and brown adipocyte subtypes with differing thermogenic potential [75]. A subtype of beige adipocytes with high glycolytic activity independent of adrenergic stimulation has also been identified [76]. Altogether, these new findings and more to come will complicate the understanding of insulin resistance since the contributions of these different adipocyte subtypes will vary considerably.
4. Obesity-mediated disruptions of white adipocyte lipid metabolism
Adipose dysfunctions in genetic obesity and under high-fat diet (HFD) conditions include decreasing the capacity of enlarged white adipocytes to synthesize, esterify, and store triglycerides in a central lipid droplet. These deficiencies are caused in part by the lower expression of enzymes in lipid synthesis, fatty acid esterification, and sequestration, including decreased levels of lipid droplet proteins that normally protect stored triglycerides from lipolysis [reviewed in Refs. [9,[77], [78], [79]]]. Obesity decreases the rates of white adipocyte glycerol 3-phosphate formation from glycolysis due to decreased glucose uptake and a decreased “glyceroneogenesis” pathway (pyruvate conversion to glycerol 3-phosphate) in response to the downregulation of adipocyte phosphoenol phosphate carboxykinase [80]. Obesity also modulates diacylglycerol acyl transferase (DGAT) enzymes, especially DGAT in subcutaneous adipose tissue [81], which catalyze esterification of diacylglycerol with fatty acyl CoA in the final step of triglyceride synthesis [82]. Thus, this fatty acid esterification rate to form and store triglyceride in some depots of white adipocytes is likely reduced in obesity as the overall lipid metabolism enzymes are downregulated in obese, glucose-intolerant subjects (Figure 2, pathway 4) [83]. Importantly, adipose-specific DGAT1 knockout mice show reduced systemic glucose intolerance and apparent lipotoxicity due in part to elicitation of a stress response and inflammation [84,85]. The mechanisms that downregulate overall adipocyte lipid synthesis and lipid storage proteins in obesity are incompletely understood, but likely include attenuation of the activities of transcription factors such as CHREBPα/CHREBPβ [[86], [87], [88]] and PPARg [9,89,90] that regulate the expression of these genes (Figure 2, pathway 5).
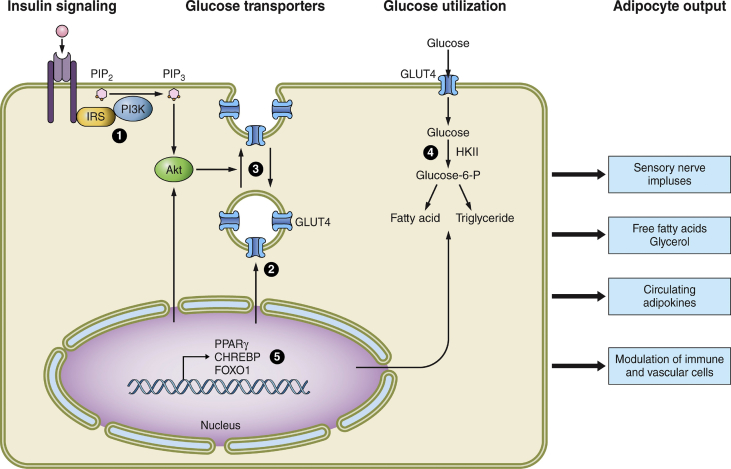
Figure 2.
Sites of disrupted functions in white adipocytes due to genetic and diet-induced obesity. At least 5 major insulin-responsive pathways that affect adipocyte metabolism are dysfunctional in obesity and are numbered in the figure. These include 1) insulin signaling elements from insulin receptor to activation of protein kinase Akt, 2) the expression of glucose transporter GLUT4, 3) the membrane trafficking of GLUT4 that enhances its abundance on the plasma membrane in response to insulin, 4) the entry and metabolism of glucose that is converted to fatty acids through de novo lipogenesis and through esterification of glycerol 3-phosphate with fatty acids, and 5) the regulation of expression of many of the proteins and regulators of these pathways through the actions of transcription factors, some of which are themselves responsive to insulin action (for example, CHREBP and FOXO1). These dysfunctions likely all contribute to adipocyte insulin resistance at some stages of acute and chronic obesity, and the degree to which each contributes continue to be actively debated. A key question for current and future studies that is raised by these considerations is the degree to which obesity-altered metabolite levels within adipocytes are able to signal, directly or indirectly, to the outputs at the right of the figure that regulate other cell types within adipose tissue as well as other distant organs such as the brain, liver, muscle, and pancreatic islets.
Insulin signaling to metabolism in sensitive tissues is largely triggered by insulin receptor tyrosine kinase activation leading to protein kinase Akt stimulation [91]; some of the intermediate signaling elements are listed in Table 2. Downregulation of adipocyte lipid synthesis capacity and lipid droplet proteins in obesity does not necessarily directly affect the activity of insulin signaling itself, but is associated with insulin resistance in other tissues and decreases whole body glucose tolerance [9]. For example, deletion of lipid droplet protein Fsp27 in adipocytes in conditional obese mouse knockout models causes decreased adipocyte fat deposition, lipodystrophy, systemic glucose intolerance, and insulin resistance [92]. However, the latter is due to increased hepatic insulin resistance associated with steatosis and increased liver glucose output, while adipose depots remain insulin responsive with respect to enhanced protein kinase Akt phosphorylation and actually display decreased inflammation [92]. These results support the hypothesis that the decreased ability of adipose depots to sequester fat away from the liver in Fsp27-deficient adipocytes is the cause of hepatic insulin resistance and fat accumulation. Mechanistically, recent results indicate that the link between excess fat entry into the liver and insulin resistance is the buildup of mitochondrial acetyl CoA that can activate a rate limiting enzyme (pyruvate carboxylase) in the gluconeogenesis pathway [11,12]. This apparently increases hepatic glucose production to an extent that cannot be inhibited by direct insulin action on hepatocytes.
Table 2.
Adipocyte-selective gene deletions of components involved in insulin stimulation of glucose utilization and inhibition of lipolysis result in parallel effects on whole body insulin sensitivity or glucose tolerance (GTT). Experimental manipulation of the listed insulin signaling elements or targets specifically in all adipocyte types causes similar effects on systemic glucose tolerance. Thus, gene knockout of elements selectively in adipocytes that drive the pathway of insulin stimulation of glucose uptake and esterification to triglyceride (for example, insulin receptor, Akt) also attenuate whole body insulin sensitivity and enhance obesity-induced insulin resistance. Conversely, adipose-selective deletions of genes that enhance insulin signaling in adipocytes (for example, p85 insufficiency that stimulates PI 3K or PIP3 phosphatase PTEN) cause increased systemic insulin sensitivity and attenuate insulin resistance in obesity. In concert with these results, adipose-selective deletion of genes encoding downstream targets of insulin signaling such as glucose transporter GLUT4 or fatty acid esterification enzyme DGAT1 diminish whole body glucose tolerance and enhance insulin resistance. Adipose-selective gene deletion of ATGL mimics the action of insulin to decrease adipocyte lipolysis, which enhances systemic insulin sensitivity by decreasing hepatic steatosis and insulin resistance. In contrast, when insulin signaling elements are deleted specifically in BAT, mixed results are observed on systemic glucose tolerance.
| Insulin signaling pathway component | Experimental perturbation | Adipocyte insulin action | Systemic insulin sensitivity or GTT | Ref. |
|---|---|---|---|---|
| Insulin Receptor | Adipose-selective KO | Decreased | Decreased | [110] |
| BAT-selective KO | Decreased | Insulin secretion defect | [111] | |
| p85/PI 3 K | Adipose-selective Insufficiency | Increased | Increased | [115] |
| PIP3 | Adipose-selective PTEN KO | Increased | Increased | [116] |
| Akt 1/2 | Adipose-selective KO | Decreased | Decreased | [112] |
| AS160 | Human mutation | Decreased | Decreased | [123] |
| Raptor/mTORC1. | Adipose-selective KO | Decreased | Decreased | [113] |
| Rictor/mTORC2. | BAT/Beige-selective KO. | Decreased | Increased | [195] |
| GLUT4 | Adipose-selective KO | Decreased | Decreased | [114] |
| Adipose-selective Tg | Increased | Increased | [114] | |
| DGAT1 (FA esterification) | Adipose-selective KO | Decreased | Decreased | [84,85] |
| ATGL (lipolysis lipase). | Adipose-selective KO | Mimics Insulin | Increased | [11,117] |
| BAT/Beige-selective KO | Mimics Insulin | Glucose intolerance | [193] |
5. Obesity-mediated disruption of “adipokines” and “batokines”
A second general mode whereby adipocyte disruptions caused by obesity can mediate systemic insulin resistance, even while the adipocytes themselves are not insulin resistant, relates to secretion of bioactive factors [[25], [26], [27]]. Both white adipocytes and brown/beige adipocytes secrete such factors, called “adipokines,” and some are more selectively secreted by BAT, called “batokines” (Figure 1). The canonical white adipocyte adipokine is leptin, which regulates many physiological parameters including appetite and energy expenditure [93,94]. Adipocytes secrete many such factors that do not directly modulate adipocyte insulin sensitivity in a cell autonomous manner but rather act as hormones to effect nearby or distant tissues, and in some cases their production is suppressed in obesity [[15], [16], [17],[25], [26], [27]]. One example is the adipokine adiponectin, which is secreted into the circulation at high concentrations and enhances overall systemic glucose tolerance and insulin sensitivity in obese mice, as evidenced by experiments with adiponectin knockout mice [95]. Another example is neuregulin 4 (Nrg4), which is highly expressed in BAT, less so in WAT, but not in virtually any other examined cell type within peripheral tissues in mice [[96], [97], [98], [99]]. As with adiponectin, adipose Nrg4 production is severely downregulated in obesity in mice and humans. Nrg4 found in the circulation seems largely derived from adipose tissues and functions to decrease hepatic fatty acid synthesis and maintain systemic insulin sensitivity [96]. Nrg4 knockout leads to severe hepatic steatosis, exacerbation of glucose intolerance, and insulin resistance in obese mice [96]. Transgenic Nrg4 overexpression in mice leads to decreased liver steatosis and increased systemic insulin sensitivity and glucose tolerance. Under hyperinsulinemic, euglycemic clamp conditions, increased insulin sensitivity is readily observed in skeletal muscle but not WAT or BAT [96]. These results represent striking examples of adipose dysfunction in obesity driving systemic insulin resistance through downregulation of secreted protein adipokines and batokines, which act on peripheral tissues but not directly on adipocytes themselves.
Recent results have identified several lipid species released from adipocytes that may also regulate systemic glucose tolerance. Experiments that demonstrate systemic effects upon knockout of genes that drive synthesis of such lipids are particularly important in confirming their physiological function. One example relates to the role of 12-hydroxyeicosapentaenoic acid (12-HEPE), which is produced by BAT in response to catecholamine during cold exposure [100]. Importantly, 12-HEPE causes increased glucose uptake into BAT and skeletal muscle and BAT-selective gene ablation of the lipoxygenase (12-LOX) that produces this lipid, causing glucose intolerance in mice [100]. Moreover, 12-LOX KO mice were less able to adapt to the cold. Obesity in humans markedly decreases circulating 12-HEPE levels, and insulin sensitivity varies inversely with levels of this lipokine. The related lipokine, 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME), was found to be increased in the circulation of mice and humans during exercise [101]. It was shown that the main source of this lipokine is also BAT in mice, and that its action on skeletal muscle increased fatty acid uptake and oxidation.
These findings exemplify the overall conclusion from many studies that obesity can downregulate both protein and lipid factors secreted specifically by adipocytes that normally act to maintain normal systemic insulin sensitivity in the liver and skeletal muscle (Figure 1), thereby contributing to systemic glucose intolerance and insulin resistance. Many recent publications have summarized this vast literature on adipokines and batokines and their possible roles in regulating peripheral glucose tolerance [[15], [16], [17],[24], [25], [26], [27],102].
6. Adipocyte insulin sensitivity influences systemic glucose tolerance
White, beige, and brown adipocytes are all responsive to insulin, and the most widely studied insulin-stimulated anabolic pathways are glucose uptake and esterification, transcription of selected mRNAs, protein synthesis, and de novo lipogenesis. Conversely, lipolytic activity in adipocytes is strongly inhibited by insulin, and the combined effects of these insulin-responsive processes is lipid accumulation in large unilocular (white adipocytes) or multilocular (beige and brown adipocytes) lipid droplets. While systemic insulin resistance is mostly studied in terms of glucose dynamics [103], basal lipolysis and the ability of insulin to lower serum free fatty acids and glycerol through the inhibition of lipolysis in white adipocytes may also be attenuated to some extent in obesity [[104], [105], [106]]. This appears to occur in part through phosphorylation of the β-adrenergic receptor by enhanced ERK MAP kinase in obesity [107]. This effect of insulin on adipocyte lipolysis is thought to be significant in systemic insulin sensitivity and glucose intolerance because circulating fatty acids that are taken up by the liver stimulate hepatic gluconeogenesis [11,12]. Glycerol released from adipocyte lipolysis also enhances hepatic glucose output as a substrate of gluconeogenesis.
Thus, insulin sensitivity of liver glucose output involves direct action on hepatocytes through signaling to the protein kinase Akt and indirect action through insulin action on adipocytes to suppress fatty acid and glycerol release. Mechanistically, the inhibitory effect of insulin on lipolysis is partially mediated through the central nervous system [108] in conjunction with cell autonomous mechanisms [109]. It should be also be noted that insulin stimulation of glucose uptake and glycolysis in adipocytes generates glycerol 3-phosphate for esterification of fatty acids, providing a third mechanism for decreasing the release of fatty acids from adipose tissue. Furthermore, circulating fatty acids are also thought to generate insulin resistance in skeletal muscle through cell autonomous effects on insulin-stimulated glucose uptake and utilization [8,9]. Taken together, it is clear that insulin-sensitive white adipocyte glucose uptake and lipolysis pathways are linked to secondary effects on liver and muscle insulin responsiveness through released fatty acids and other factors in the context of overnutrition.
These considerations suggest that white adipocyte insulin sensitivity is itself important for maintaining whole body glucose homeostasis through indirectly regulating liver or muscle. Accordingly, many laboratories have directly tested the hypothesis that disrupting or amplifying insulin signaling elements or the target systems of such signaling within adipocytes modulates systemic insulin sensitivity. The results of such experiments are summarized in Table 2 and overwhelmingly support this concept. For example, gene deletion of the insulin receptor from all adipocyte types leads to lipodystrophy, liver steatosis, and insulin resistance in the liver and skeletal muscle [110]. Insulin receptor knockout selectively in UCP-1 positive brown and beige adipocytes causes systemic glucose intolerance due to deficits in insulin secretion from beta cells in the pancreas [111]. Similarly, adipose-specific deletion of signaling elements downstream of the insulin receptor such as protein kinase Akt2 cause insulin resistance in the liver and skeletal muscle and systemic glucose intolerance on a high-fat diet [112]. Attenuating adipocyte mTORC1 activity, a target of insulin-sensitive Akt2 signaling, compromises lipid accumulation in the adipocytes and decreases systemic insulin sensitivity in conjunction with lipodystrophy [113]. Adipose-selective knockout of insulin-regulated glucose transporter GLUT4 [114] or downstream enzyme DGAT1 [84,85] that catalyzes esterification of glycerol 3-phosphate and fatty acid into triglycerides also lead to systemic glucose intolerance.
Conversely, increasing adipocyte insulin signaling by partial depletion of the negative regulator p85 subunit of PI 3K [115], PIP3 phosphatase PTEN [116], or overexpressing insulin-sensitive GLUT4 [114] is sufficient to enhance systemic insulin sensitivity under obese conditions in mice. Importantly, mimicking insulin's inhibitory action on lipolysis in adipocytes by knockout of ATGL lipase mimics insulin's suppression of hepatic gluconeogenesis, enhancing systemic glucose tolerance [11,117]. Taken together (Table 2), these results from various laboratories solidify the conclusion that insulin sensitivity of white adipocytes induces profound long-distance regulation of the liver and skeletal muscle to modulate systemic glucose and fatty acid homeostasis under obese conditions.
While experimental perturbations of insulin sensitivity of white adipocytes cause concordant changes in systemic insulin sensitivity in obesity, the relationship to systemic glucose tolerance is more complicated with respect to brown/beige adipocytes. Insulin receptor knockout in brown/beige adipocytes does not cause systemic insulin resistance as does insulin receptor deletion in both white and brown adipocyte types [111]. Furthermore, deletion of the mTORC2 complex subunit RICTOR through UCP1 promoter-driven Cre expression in RICTOR floxed mice, which blocks insulin-stimulated Akt2 activation in BAT and beige adipocytes, actually increases systemic glucose tolerance [118]. This appears to be due to enhanced adipocyte catabolism through unknown mechanisms. Nonetheless, insulin-stimulated fuel utilization may be dominant in BAT during the postprandial period [119,120]. BAT lipolysis does not appear necessary for thermogenesis in vivo, as WAT can supply fatty acids for fuel if BAT lipolysis is disrupted [121]. However, such disruption causes browning of WAT along with increased glucose tolerance, complicating interpretations. Insulin signaling can suppress the process of “browning” of white adipocytes, as experimentally induced hypoinsulinemia causes upregulation of UCP1 [122]. Collectively, these data reveal the complicated mechanisms whereby brown/beige adipocytes are interconnected with systemic glucose homeostasis and the importance of specific environmental conditions in driving various phenotypes related to brown/beige adipocyte perturbations. Nonetheless, these studies demonstrate that cell autonomous perturbations in insulin signaling sensitivity within white or brown/beige adipocytes translate to marked changes in whole body metabolic dynamics.
7. White adipocyte insulin resistance: sites of disruption
7.1. Insulin signaling elements
Based on the convincing results summarized in Table 2 as previously described, a logical therapeutic strategy for alleviating systemic insulin resistance is reversing adipocyte dysfunctions in obesity, including the diminished capacity of white adipocytes to respond to insulin. This strategy would be enhanced by detailed knowledge of the molecular basis of insulin signaling to metabolic targets in white, beige, and brown adipocytes and the steps controlling white adipocyte glucose and lipid metabolism that go awry in obesity. Indeed, several of the aforementioned experimental gene deletions of insulin signaling components have parallels in rare mutations that occur in humans. Examples include the insulin receptor, its downstream target protein kinase Akt, and its substrate TBC1D4 [123]. In the common forms of human obesity associated with insulin resistance that are devoid of such disease mutations, there are often deficiencies in the expression or activation of these same insulin signaling elements (Figure 2) when measured in white adipocytes [8,9,91,124,125]. Thus, insulin-mediated activation of Akt, the required signaling circuit for stimulation of Glut4 translocation and glucose uptake, is often decreased in adipocytes in obese, insulin resistant subjects and obese rodent models of insulin resistance [126]. Conversely, the role of insulin-sensitive Akt in regulating lipolysis has mixed support [[127], [128], [129], [130], [131]], and the ability of obesity to affect the cell autonomous pathway of lipolysis regulation via insulin as opposed to the re-esterification of fatty acids may be relatively small [103,129].
Despite this attenuation of adipocyte insulin signaling to stimulate Akt in chronic obesity, the degree to which deficits in insulin signaling elements, including the insulin receptor, insulin receptor substrate proteins, PI-3K, Akt, and its glucose transport-relevant substrate AS160/TBD1D4 actually contribute to the decreased glucose uptake and utilization in obesity is a subject of continued debate [5,132,133]. Systemic insulin resistance occurs only a few days after the start of high-fat feeding in mice to induce obesity, including strong deficits in insulin stimulation of adipocyte glucose uptake and systemic glucose intolerance [5]. However, both systemic and adipocyte insulin resistance occurs in the absence of apparent decreases in Akt activation in adipose tissue at these early time points [134,135]. Only later in the development of obesity are such deficiencies in Akt activation consistently observed that may contribute to insulin resistance. This lag period prior to the attenuation of Akt regulation may be related to the delayed occurrence of adipose inflammation upon initiating nutritional overload [136], although this is variable [137]. Paracrine signaling from immune cells within adipose tissue in obesity appears to cause such decreases in adipocyte insulin signaling elements as well as in the other pathways shown in Figure 2, which is a major paradigm in the field (Table 1) [for reviews, see [9,124,138,139]. In any case, the mechanisms of adipocyte insulin resistance that are at play early in obesity induction, whatever they are, may continue to be active at later times. Several other considerations indicate that sites downstream of Akt activation and AS160 phosphorylation are significant drivers of insulin resistance of adipocyte glucose uptake, including the very small fraction of Akt signaling needed to maximally stimulate glucose transport and the fact that other signaling pathways to glucose transport are also resistant in obesity [reviewed in detail in Ref. [133].
7.2. GLUT4 translocation and glucose utilization
Adding to the argument that defects downstream of Akt contribute to adipocyte insulin resistance are strong data showing that the machinery driving translocation of GLUT4 to the plasma membrane is impaired [133,140]. Since intricate steps in membrane trafficking that mediate GLUT4 translocation to the plasma membrane remain unknown after almost 40 years of research, neither the exact defective step or steps, nor the signal triggering it in obesity, have yet been revealed. Many exciting hypotheses have been offered, such as the reactive oxygen species generated in insulin resistant states, which has considerable support [133,141], or potentially adipose hypoxia [142]. Even the cellular site and step or steps in membrane trafficking stimulated by insulin signaling is not fully resolved, with competing hypotheses being evaluated [132]. Although insulin action also inhibits the endocytosis of GLUT4 [143,144], adding to its increase in the plasma membrane, the effect of obesity and insulin resistant states on that part of the pathway have not been adequately evaluated. Finally, is it possible that obesity alters the intrinsic catalytic activity of GLUT4 in the plasma membrane? This question also has not been fully evaluated. Additional research tools and approaches will likely be needed to fully resolve these issues regarding GLUT4 translocation and glucose transport and their responsiveness to both insulin and the disruptions of obesity.
Another understudied locus of potential high importance in white adipocyte insulin resistance is the flux of glucose from its cellular uptake through its glycolytic flow and beyond (Figure 2). It is mostly assumed in the field that glucose transport across the adipocyte plasma membrane is strictly rate limiting for glycolysis, which is supported by studies showing that the overexpression of GLUT4 in adipocytes in vivo enhances overall glucose metabolism and even systemic glucose tolerance [114]. Knockout of GLUT4 in adipocytes has the opposite effect [145]. However, a single fully rate limiting step would be an unusual situation in complex metabolic pathways since they are governed by multiple individual rate constants and varying concentrations of intermediate metabolites under various physiological conditions. In the case of adipocyte glucose transport, the concentration of intracellular free glucose within the cells in vivo has not been rigorously measured and defined under various conditions of insulin sensitivity. Intracellular free glucose would have to be near zero for transport to be fully rate limiting in lean and obese conditions. Importantly, increasing glucose phosphorylation capacity by overexpression of a glucose kinase, such as hexokinase II or glucokinase, should not increase glucose utilization if this condition is met. However, this is not the case, as overexpression of glucokinase in adipocytes was able to increase overall glucose uptake into the cells in vivo in a well-controlled study [146]. Therefore, a central assumption in the field is in question, and importantly, these data suggest that alterations in the levels or activities of enzymes in the glycolytic pathway in obesity may be functional in regulating adipocyte glucose utilization.
Adipocyte hexokinase II is subject to inhibition by glucose 6-phosphate, further complicating the potential major role of metabolic enzymes in regulating glucose utilization. Additionally, enzymes in the glycolytic pathway lead to glycerol 3-phosphate production, the substrate for fatty acid esterification, reinforcing the potential importance of changes in expression of glycolytic enzymes in adipose function. Moreover, obesity in humans and rodents significantly decreases the expression of enzymes in the pathway of glucose conversion to fatty acid (de novo lipogenesis), which can lead to changes in the NADPH redox state that affect glucose metabolism in the pentose shunt. Taken together, these results strongly suggest that beyond defective adipocyte GLUT4 translocation in obesity, attenuated glucose metabolism as well as biosynthesis, esterification, and sequestration of fatty acids are key aspects of adipocyte insulin resistance. These considerations also raise the interesting unanswered question of whether such changes in glucose utilization in obesity are mechanistically connected to the expression and secretion of adipokines from adipocytes.
7.3. Adipocyte gene expression in obesity
The previously described effects of obesity to attenuate insulin signaling elements, GLUT4 translocation machinery, and glucose metabolism may be due to allosteric or covalent modifications of the proteins involved in these processes. These concepts have been studied and reviewed in the context of protein kinase-mediated effects in insulin resistant states [8,125,139]. It seems likely, however, that much of the insulin resistance in adipocytes in long-term obesity in humans and rodent model systems is initially driven by the result of transcriptional regulation of many of the gene products illustrated in Figure 2 as well as others not listed [82,83,[147], [148], [149], [150], [151], [152]]. Many genes that are normally responsive to PPARγ, the major regulator of adipocyte differentiation and function [152], are attenuated in the expression of obesity in mice. Recently, important findings have demonstrated that PPARγ-responsive gene promoter regions that are normally occupied by this transcription factor show decreased occupancy under HFD, obese conditions [90]. Other examples of adipocyte transcription factors as targets of obesity are decreases in expression and/or activities of CHREBP [86,114,146] and FOXO1 [147,153,154], which control many genes in the adipocyte pathways as shown in Figure 2. Similarly, transcriptional regulators such as HDAC3 may be targets of obesity [149,155]. Taken together, these and other transcription factors in adipocytes that are targets of disruption in obesity likely play major roles in defining the expression changes in key proteins that regulate insulin signaling, GLUT4 translocation, and glucose metabolism. The mechanisms by which obesity decreases the expressions or activities of such adipocyte transcription factors are poorly understood, and this question is fertile for further investigation.
Collectively, the data from thousands of studies on insulin action and resistance in white adipocytes indicate that multiple sites in the pathways of Figure 2 are targets of disruption in obesity in humans and rodent model systems. Decreased expression of GLUT4 itself and its ability to respond to insulin via translocation to the cell surface membrane likely both contribute to adipocyte insulin resistance, at least in long-term obesity, but decreased expression or activities of enzymes in glucose metabolism, especially conversion to lipids, also likely participate in a major way. Probably the most marked example of the latter is the nearly complete downregulation of adipocyte de novo lipogenesis that occurs upon HFD feeding [5,135,156]. Much of this effect is attributed to decreased transcription and expression of ACLY (ATP citrate lyase), ACC1 (acetyl CoA carboxylase), and FASN (fatty acid synthase) enzymes that catalyze the production of palmitate from acetyl CoA. The degree to which this attenuated flux in fatty acid biosynthesis within adipocytes triggers communication to the liver and skeletal muscle through released free fatty acids, circulating adipokines, or perturbations of the nervous system is under investigation [18,135,157]. Deleting FASN selectively in adipocytes causes major changes in systemic metabolism, including increased energy expenditure and glucose tolerance [135,158], while adipocyte-selective ACLY deletion shows only a mild phenotype [159]. These experiments are difficult to interpret in detail at present since the metabolic intermediates in de novo lipogenesis, acetyl CoA, malonyl CoA, and palmitate, also act as signaling molecules that mediate protein acetylation [160], malonylation [161], and palmitoylation [162], respectively. Nonetheless, such experimentation shows that downregulated adipocyte metabolic enzymes through genetic manipulation has a profound influence on liver and skeletal muscle functions.
Altogether, the most reasonable conclusion from the aforementioned considerations is that the data largely nullify the idea that a single step or mechanism alone causes insulin resistance in white adipocytes, as defined by resistance to the action of insulin to increase glucose utilization in these cells. Rather, a multitude of adipocyte-specific transcriptional effects and protein modifications are elicited in obesity that together contribute to the consistent dampening of insulin action on adipocyte glucose metabolism. Much more work needs to be done on adipose tissue metabolism in vivo to be able to extrapolate data from experiments performed in vitro. In particular, there is an urgent need for more detailed metabolic flux measurements in adipocytes in vivo to define the contributions to overall insulin responsiveness of metabolic pathways.
8. Brown/beige adipocyte glucose disposal in obesity
8.1. Relationship of BAT/beige thermogenesis to systemic glucose homeostasis
Initially, thermogenic multilocular brown adipocytes were distinguished from beige adipocytes in mice based on the location of the former in BAT depots vs the latter in WAT depots mixed with white adipocytes [25,[39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50],[163], [164], [165]]. It should be noted that beige adipocytes are also equally referred to as “brite” adipocytes in the literature [166]. Differences were also observed in the gene expression profiles between mouse beige and brown adipocytes [165]. The discovery of thermogenic adipocytes in humans within depots of white adipocytes and subsequent characterizations initially suggested they may be more like beige adipocytes [[167], [168], [169], [170]]. However, more recent findings indicate that mouse and human BAT, as defined by its presence in the interscapular location of mice and the supraclavicular location of humans, are similarly characterized by high levels of UCP1 and thermogenic proteins [171]. In contrast, mouse beige adipocytes are somewhat less thermogenic, even when sympathetic activity within adipose tissue is decreased under “humanized” conditions of thermoneutral temperatures [171]. Taken together, these new findings indicate that human and mouse brown adipocytes may elicit even stronger systemic effects compared to mouse beige adipocytes. Nonetheless, thermogenic beige adipocytes are associated with enhanced glucose tolerance in mice [42] and rats [51] and share newly described mechanisms for this function with BAT [172,173].
Many studies show an association of UCP1-containing thermogenic beige/BAT with lean and glucose tolerance phenotypes in mice and humans. These include BAT transplantation studies in mice that demonstrate significant enhancement of glucose tolerance [174,175]. Obese, glucose-intolerant, or diabetic humans on average display less mass of brown/beige adipocytes than lean individuals as well as less 18F-labeled fluorodeoxyglucose uptake into BAT [170]. Conversely, cold exposure in human subjects causes increased abundance and activity of brown/beige adipocytes and increased glucose tolerance and insulin sensitivity [176]. There is also evidence that a glucose-responsive thermogenic biorhythm in BAT is associated with systemic glycemic control in humans [177]. UCP1 expression regulates insulin-dependent glucose uptake in human adipocytes [178], suggesting along with other data that perhaps the thermogenic activity of BAT may directly influence glucose disposal into BAT in vivo [52,179]. However, mice are not optimal models for human physiology in this regard since HFD in mice can be accompanied by increases in WAT UCP1 rather than decreases, presumably as a compensation mechanism [180]. Nonetheless, chronic administration of β3 adrenergic agonist CL316243 to mice, which activates the lipolytic and thermogenic pathways in brown/beige adipocytes, enhances UCP1 abundance in WAT and BAT while increasing glucose tolerance [180,181].
Initial studies on deletion of UCP1 in mice showed a paradoxical resistance to HFD-induced obesity [182], likely due to compensatory mechanisms of thermogenesis in other tissues. Thus, UCP1 knockout mice do show increased obesity on HFD with age [183] and when housed at thermoneutrality [184]. Subsequent work confirmed the effects of UCP1 on the propensity to become obese even in the obesity resistant 129S mouse strain [185]. Furthermore, UCP1 expression is associated with beneficial effects on glucose tolerance in rats [51], and in female mice it appears independent of weight [186,187]. Other mechanisms of thermogenesis in BAT such as creatine recycling also contribute to both thermogenesis and resistance to HFD-induced obesity [188]. Taken together, these findings indicate that there are favorable effects associated with UCP1-expressing thermogenic adipocytes on glucose tolerance in rodents and humans.
8.2. GLUT1 and GLUT4 translocation in BAT
While both white and brown adipocytes share the feature of secreting factors that act as hormones to affect liver and skeletal muscle metabolism, BAT differs fundamentally from WAT in its ability to directly take up large quantities of glucose [52]. Indeed, BAT transplantation in mice is associated with increased glucose uptake in BAT but not muscle as it improves glucose tolerance [174,175]. Glucose uptake and glycolysis appear to be pathways in driving thermogenesis in mice [189,190], along with fatty acids [[191], [192], [193], [194], [195], [196], [197], [198], [199], [200]]. This may also be the case in human subjects subjected to the cold, as cold exposure also increases BAT glucose uptake based on PET imaging [52,178]. Mechanistically, BAT also differs substantially in the pathways of glucose uptake and metabolism compared to WAT (Figure 3). The canonical insulin-sensitive GLUT4 translocation pathway operates in BAT, but in the context of a more active GLUT1 translocation pathway. Silencing of GLUT4 and GLUT1 in brown adipocytes in culture shows that both contribute significantly to overall glucose uptake [190], as opposed to white adipocytes, which have little GLUT1 in vivo. However, GLUT1-mediated glucose uptake and utilization in BAT is highly responsive to β-adrenergic regulation, and its ability to lower blood glucose appears dependent on catecholamine stimulation [201] and probably less so on insulin stimulation. Nonetheless, insulin stimulates glucose uptake in BAT to a significant extent [[202], [203], [204], [205]]. Interestingly, even though white to beige conversion increases fatty acid metabolism [206], loss of lipolysis [193], or de novo lipogenesis [200] in rodents, it does not impair survival of mice in the cold, suggesting compensation by increases in other fuel sources including glucose. Thus, elucidating the signaling pathways that control GLUT1-mediated glucose uptake and glucose flux through glycolysis, lipid synthesis, and mitochondrial oxidation is important for understanding the insulin resistant state as it impacts BAT in rodents.
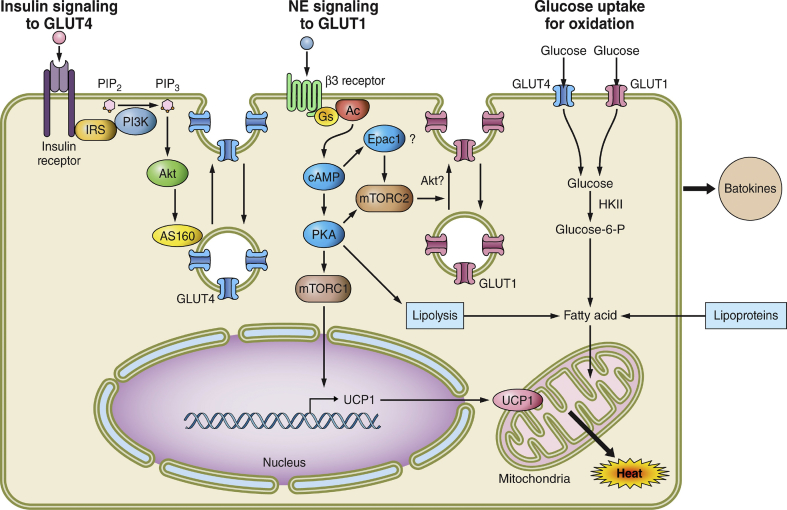
Figure 3.
Mechanisms whereby brown and beige adipocytes take up and utilize glucose to influence whole body insulin sensitivity and glucose tolerance. BAT and beige adipocytes are extremely active in taking up glucose, which may contribute significantly to systemic glucose disposal, at least during cold exposure. Left, insulin signaling to GLUT4: Insulin signaling through Akt to stimulate GLUT4 translocation to the plasma membrane operates as in WAT and BAT, but appears to contribute less to overall glucose uptake in BAT. Obesity does attenuate this pathway in BAT as it does in WAT. Middle, norepinephrine (NE) signaling to GLUT1: Upon stimulation of sympathetic innervation in BAT or WAT, brown and beige adipocytes activate a novel mTORC2-dependent pathway to cause GLUT1 trafficking to the plasma membrane. This significantly stimulates glucose uptake while also activating lipolysis and lipoprotein-based triglyceride hydrolysis to provide fatty acid substrates for uncoupled respiration through UCP1 upregulation. Surprisingly, the mTORC1 complex was found to mediate the expression of UCP1. Right, glucose uptake for oxidation: Glucose uptake in BAT and beige adipocytes is both oxidized and converted to fatty acids by upregulated de novo lipogenesis enzymes during NE stimulation. The newly synthesized fatty acids are thought to be rapidly oxidized in chronic uncoupled respiration. The effects of obesity on GLUT1 translocation and glucose utilization in BAT are less well studied, but the limited data available indicate diminished glucose uptake in BAT in obesity. A key unanswered question is the degree to which altered brown and beige adipocyte metabolites affect the secretion of both beneficial and deleterious batokines that regulate systemic metabolism and the effects of obesity on such regulation.
Early studies showed that glucose uptake was stimulated by GLUT1-mediated glucose uptake in BAT and enhanced by β-adrenergic signaling through increased GLUT1 abundance, even as GLUT4 abundance was decreased [207]. More recently, it was revealed that GLUT1 translocation from intracellular membranes to the plasma membrane is a major mode of increased glucose uptake in BAT [208]. As depicted in Figure 3, translocation of GLUT1 to the plasma membrane in brown adipocytes is mediated by the cAMP pathway, but there is some disagreement about whether PKA [52,208,209] vs Epac1 [210] is the downstream mediator of cAMP on GLUT1 translocation. There is consensus, however, that downstream of cAMP signaling is the requirement that the mTORC2 complex be functional [52,[208], [209], [210], [211], [212], [213]], an unexpected finding since mTORC2 is also known as an intermediate in insulin signaling to Akt (Figure 3). How mTORC2 engages the GLUT1 trafficking machinery is under investigation, as is the question of whether Akt is also involved [210]. Experiments have been reported that show that regulation of Akt2 expression modifies GLUT1-mediated hexose uptake [210], while other research indicated that Akt inhibition does not reduce responsiveness of GLUT1 to catecholamine [208].
Altogether, these studies provide support for the hypothesis that mTORC2 functions in a novel mode to promote GLUT1 translocation in brown adipose cells in response to the cAMP/mTORC2 pathway. Cold exposure markedly stimulates this pathway through activation of sympathetic nerve fibers within BAT, while the administration of β3-adrenergic agonist to mice mimics this effect in part through enhancing the expression of the Dio2 enzyme that produces thermogenic T3 from T4 thyroid hormone [214]. Thus, while insulin and catecholamines are often thought of as antagonistic hormones, catecholamines can paradoxically enhance “apparent” insulin sensitivity. This may in part be through increased glucose uptake into BAT at temperatures below thermoneutrality. Moreover, it has been revealed that adrenergic signaling can cause browning and UCP1 upregulation in WAT through activation of an mTORC1-dependent mechanism [215], which is also an insulin-responsive pathway. This is an exciting area of investigation that will in future studies reveal substantial insight into glucose flux in BAT. The flux through glucose metabolic pathways in BAT may also modulate the secretion of beneficial batokines, providing a dual role for control of systemic glucose tolerance by changes in BAT glucose metabolism. This is an active area of investigation that is likely to yield interesting results.
That GLUT1-regulated glucose flux in BAT in response to sympathetic nerve activation may contribute directly to systemic glucose disposal raises an important question: Is it a target of disruption in obesity? While it appears that overall BAT glucose uptake is diminished in obesity in mice and humans [52,170,216], the detailed analysis of sites in the pathways of GLUT1 function that are affected has not been defined. It should be noted that obese mice also display significantly decreased BAT Akt phosphorylation in response to injected insulin compared to lean mice [217], indicating that insulin signaling defects are present as well. Since it is possible that BAT glucose uptake directly contributes to systemic glucose disposal through GLUT1 regulation, at least in mice under cold stress conditions, the evidence suggests that obesity disrupts this mode of BAT contribution to systemic glucose tolerance. However, neither these nor more recent studies have differentiated GLUT1-mediated vs GLUT4-mediated glucose uptake in BAT in obesity using gene knockout mice. Nor have there been definitive studies defining the relationship in obesity between defects in insulin signaling elements and decreased glucose uptake in BAT. It will be important for future studies to define which sites in the pathways of BAT glucose uptake and utilization are the most important in contributing to systemic insulin sensitivity.
9. Conclusions and therapeutic strategies
The last few years have brought major breakthroughs in both the application of new technologies to adipose biology and novel insights into mechanisms of adipocyte function. The former advances include single cell [54,55] and single nuclei [56] gene expression analysis as well as CRISPR-mediated gene editing [218], while the latter include unexpected molecular mechanisms of brown/beige adipocyte glucose uptake through cAMP/mTORC2-mediated GLUT1 as opposed to GLUT4 translocation. It will be important to uncover the unknown elements in brown/beige cells that act in the translocation of GLUT1 to the cell surface and compare them to those known to be involved in insulin-sensitive GLUT4 membrane trafficking. It will also be important to more thoroughly evaluate the actual contribution of BAT glucose disposal in humans to determine whether some of the rather low estimates obtained [24,219] are underestimates [170]. The discovery of multiple subtypes of both adipocytes and their progenitors within individual adipose depots begs questions about the relative contributions of GLUT1 vs GLUT4 to glucose uptake and responsiveness to catecholamines and insulin in the various adipocyte subtypes. Moreover, the specific contributions made by the various classes of adipocytes to adipokine, batokine, and metabolite [[15], [16], [17],[24], [25], [26], [27],[96], [97], [98], [99], [100], [101], [102],220,221] secretions and lipid storage and sequestration will be important to elucidate. Overall, this progress opens new avenues for deeper investigations into the underlying mechanisms of the regulation of adipose functions that lead to systemic glucose tolerance and insulin sensitivity.
The general concept that enhancing white adipocyte insulin sensitivity translates to improvements in hepatic and skeletal muscle insulin sensitivity as well as systemic glucose tolerance is now firmly established by many experimental approaches and laboratories (Table 2). Equally well established is the concept that insulin sensitivity of adipocytes can be improved by perturbations at many different sites in the pathways of insulin signaling, glucose utilization, lipid metabolism, adipokine secretion, and transcription control. These concepts suggest at least two potentially fruitful therapeutic approaches to exploit this knowledge. First, these considerations reinforce the urgency to develop methods that can selectively direct therapeutic agents to adipocytes, analogous to the achievement of liver-selective delivery of small molecules and biologics. Accomplishing this difficult goal would yield a considerable payoff in terms of therapeutic development, as research in the field has already identified beneficial adipocyte targets. Second, brown/beige adipocytes have tremendous potential as cell therapeutics since they are particularly active in secreting multiple factors beneficial to distant metabolic tissues. Methods for expanding human adipocyte progenitors [57,222] and for genetic manipulation of adipocytes to enhance “browning” prior to implantation [218] are in development. Future gains in insights into these “adipocentric” technologies will reinforce adipocytes as central players in the larger field of regenerative medicine.
Acknowledgments
The author thanks our laboratory group of talented investigators for stimulating discussions on the topics covered in this review and Kerri Miller for excellent technical help in preparing the manuscript. The work referred to from our laboratory was generously funded by NIDDK grants DK30898 and DK116056 from the National Institutes of Health.
Conflict of interest
None declared.
References
- 1.Marks V., Richmond C. 1st ed. Royal Society of Medicine; London: 2007. Insulin murders. [Google Scholar]
- 2.Sweeney J.S. Dietary factors that influence the dextrose tolerance test. A Preliminary Study. Archives of Internal Medicine. 1927;40(6):818–830. [Google Scholar]
- 3.Himsworth H.P. Insulin deficiency and insulin inefficiency. British Medical Journal. 1940;1(4139):719–722. doi: 10.1136/bmj.1.4139.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yalow R.S., Berson S.A. Immunoassay of endogenous plasma insulin in man. Journal of Clinical Investigation. 1960;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czech M.P. Insulin action and resistance in obesity and type 2 diabetes. Nature Medicine. 2017;23(7):804–814. doi: 10.1038/nm.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shanik M.H., Xu Y., Skrha J., Dankner R., Zick Y., Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(Suppl 2):S262–S268. doi: 10.2337/dc08-s264. [DOI] [PubMed] [Google Scholar]
- 7.Kim S.H., Reaven G.M. Insulin resistance and hyperinsulinemia: you can't have one without the other. Diabetes Care. 2008;31(7):1433–1438. doi: 10.2337/dc08-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen M.C., Shulman G.I. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. National Review. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kusminski C.M., Bickel P.E., Scherer P.E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nature Reviews Drug Discovery. 2016;15(9):639–660. doi: 10.1038/nrd.2016.75. [DOI] [PubMed] [Google Scholar]
- 11.Perry R.J., Camporez J.G., Kursawe R., Titchenell P.M., Zhang D., Perry C.J. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160(4):745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Titchenell P.M., Quinn W.J., Lu M., Chu Q., Lu W., Li C. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metabolism. 2016;23(6):1154–1166. doi: 10.1016/j.cmet.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(8):1754–1759. doi: 10.1161/ATVBAHA.111.241885. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgibbons T.P., Czech M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. Journal American Heart Association. 2014;3(2) doi: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Straub L.G., Scherer P.E. Metabolic messengers: adiponectin. Natural Metabolism. 2019;1:334–339. doi: 10.1038/s42255-019-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarroya J., Cereijo R., Gavalda-Navarro A., Peyrou M., Giralt M., Villarroya F. New insights into the secretory functions of brown adipose tissue. Journal of Endocrinology. 2019 doi: 10.1530/JOE-19-0295. pii: JOE-19-0295.R1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Funcke J.B., Scherer P.E. Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. The Journal of Lipid Research. 2019;60(10):1648–1697. doi: 10.1194/jlr.R094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guilherme A., Henriques F., Bedard A.H., Czech M.P. Molecular pathways linking adipose innervation to insulin action in obesity and diabetes mellitus. Nature Reviews Endocrinology. 2019;15(4):207–225. doi: 10.1038/s41574-019-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann J.P., Savage D.B. What lipodystrophies teach us about the metabolic syndrome. Journal of Clinical Investigation. 2019;130:4009–4021. doi: 10.1172/JCI129190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unger R.H., Scherer P.E. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends in Endocrinology and Metabolism. 2010;21(6):345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montgomery M.K., De Nardo W., Watt M.J. Impact of lipotoxicity on tissue "cross talk" and metabolic regulation. Physiology. 2019;34(2):134–149. doi: 10.1152/physiol.00037.2018. [DOI] [PubMed] [Google Scholar]
- 22.Chouchani E.T., Kazak L., Spiegelman B.M. New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metabolism. 2019;29(1):27–37. doi: 10.1016/j.cmet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Nedergaard J., Wang Y., Cannon B. Cell proliferation and apoptosis inhibition: essential processes for recruitment of the full thermogenic capacity of brown adipose tissue. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids. 2019;1864(1):51–58. doi: 10.1016/j.bbalip.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Carpentier A.C., Blondin D.P., Virtanen K.A., Richard D., Haman F., Turcotte E.E. Brown adipose tissue energy metabolism in humans. Frontiers in Endocrinology. 2018;9:447. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajimura S., Spiegelman B.M., Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metabolism. 2015;22(4):546–559. doi: 10.1016/j.cmet.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villarroya F., Cereijo R., Villarroya J., Giralt M. Brown adipose tissue as a secretory organ. Nature Reviews Endocrinology. 2017;13(1):26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 27.Klepac K., Georgiadi A., Tshop M., Herzig S. The role of brown and beige adipose tissue in glycaemic control. Molecular Aspects of Medicine. 2019;68:90–100. doi: 10.1016/j.mam.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Luong Q., Huang J., Lee K.Y. Deciphering white adipose tissue heterogeneity. Biology. 2019;8(2):23. doi: 10.3390/biology8020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee M.J., Wu Y., Fried S.K. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Molecular Aspects of Medicine. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry D.C., Jiang Y., Graff J.M. Emerging roles of adipose progenitor cells in tissue development, homeostasis, expansion and thermogenesis. Trends in Endocrinology and Metabolism. 2016;27(8):574–585. doi: 10.1016/j.tem.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoettl T., Fischer I.P., Ussar S. Heterogeneity of adipose tissue in development and metabolic function. Journal of Experimental Biology. 2018;221(Pt Suppl 1) doi: 10.1242/jeb.162958. pii: jeb162958. [DOI] [PubMed] [Google Scholar]
- 32.Atzmon G., Yang X.M., Muzumdar R., Ma X.H., Gabriely I., Barzilai N. Differential gene expression between visceral and subcutaneous fat depots. Hormone and Metabolic Research. 2002;34(11–12):622–628. doi: 10.1055/s-2002-38250. [DOI] [PubMed] [Google Scholar]
- 33.Passaro A., Miselli M.A., Sanz J.M., Dalla N.E., Morieri M.L., Colonna R. Gene expression regional differences in human subcutaneous adipose tissue. BMC Genomics. 2017;18(1):202. doi: 10.1186/s12864-017-3564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linder K., Amer P., Flores-Morales A., Tollet-Egnell P., Norstedt G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. The Journal of Lipid Research. 2004;45(1):148–154. doi: 10.1194/jlr.M300256-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Hardy O.T., Perugini R.A., Nicoloro S.M., Gallagher-Dorval K., Puri V., Straubhaar J. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surgery for Obesity and Related Diseases. 2011;7(1):60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gealekman O., Guseva N., Hartigan C., Apotheker S., Gorgoglione M., Gurav K. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123(2):186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poher A.L., Altirriba J., Veyrat-Durebex C., Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Frontiers in Physiology. 2015;6(4):1–9. doi: 10.3389/fphys.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology. 2011;214(Pt2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 39.Montanari T., Poscic N., Colitti M. Factors involved in white-to-brown adipose tissue conversion and in thermogenesis: a review. Obesity Reviews. 2017;18(5):495–513. doi: 10.1111/obr.12520. [DOI] [PubMed] [Google Scholar]
- 40.Li G., Xie C., Lu S., Nichols R.G., Tian Y., Li L. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metabolism. 2017;26(4):672–685. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda K., Maretich P., Kajimura S. The common and distinct features of Brown and beige adipocytes. Trends in Endocrinology and Metabolism. 2018;29(3):191–200. doi: 10.1016/j.tem.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen P., Levy J.D., Zhang Y., Frontini A., Kolodin D.P., Svensson K.J. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156(1–2):304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacks H.S., Fain J.N., Bahouth S.W., Ojha S., Frontini A., Budge H. Adult epicardial fat exhibits beige features. Journal of Clinical Endocrinology & Metabolism. 2013;98(9):E1448–E1455. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 44.Carobbio S., Guenantin A.C., Samuelson I., Bahri M., Vidal-Puig A. Brown and beige fat: from molecules to physiology and pathophysiology. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids. 2019;1864(1):37–50. doi: 10.1016/j.bbalip.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Rui L. Brown and beige adipose tissues in health and disease. Comparative Physiology. 2017;7(4):1281–1306. doi: 10.1002/cphy.c170001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jash S., Banerjee S., Lee M.J., Farmer S.R., Puri V. CIDEA transcriptionally regulates UCP1 for britening and thermogenesis in human fat cells. iScience. 2019;20:73–89. doi: 10.1016/j.isci.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scheja L., Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. Journal of Hepatology. 2016;64(5):1176–1186. doi: 10.1016/j.jhep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 48.Scheja L., Heeren J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nature Reviews Endocrinology. 2019;15(9):507–524. doi: 10.1038/s41574-019-0230-6. [DOI] [PubMed] [Google Scholar]
- 49.Herz C.T., Kiefer F.W. Adipose tissue browning in mice and humans. Journal of Endocrinology. 2019;241(3):R97–R109. [PubMed] [Google Scholar]
- 50.Carobbio S., Pellegrinelli V., Vidal-Puig A. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Advances in Experimental Medicine & Biology. 2017;960:161–196. doi: 10.1007/978-3-319-48382-5_7. [DOI] [PubMed] [Google Scholar]
- 51.Poher A.L., Veyrat-Durebex C., Altirriba J., Montet X., Colin D.J., Caillon A. Ectopic UCP1 overexpression in white adipose tissue improves insulin sensitivity in lou/C rats, a model of obesity resistance. Diabetes. 2015;64(11):3700–3712. doi: 10.2337/db15-0210. [DOI] [PubMed] [Google Scholar]
- 52.Hankir M.K., Cowley M.A., Fenske W.K. A BAT-centric approach to the treatment of diabetes: turn on the brain. Cell Metabolism. 2016;24(1):31–40. doi: 10.1016/j.cmet.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Spallanzani R.G., Zemmour D., Xiao T., Jayewickreme T., Li C., Bryce P.J. Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors. Science Immunology. 2019;4(35) doi: 10.1126/sciimmunol.aaw3658. pii: eaaw3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burl R.B., Ramseyer V.D., Rondini E.A., Pique-Regi R., Lee Y.H., Granneman J.G. Deconstructing adipogenesis induced by b3-adrenergic receptor activation with single cell expression profiling. Cell Metabolism. 2018;28(2):300–309. doi: 10.1016/j.cmet.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X., Xiang Q., Xu F., Huang J., Yu N., Zhang Q. Data Descriptor: single cell RNAseq of cultured human adiposederived mesenchymal stem cells. Sci Data. 2019;6:190031. doi: 10.1038/sdata.2019.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajbhandari P., Arneson D., Hart S.K., Ahn I.S., Diamante G., Santos L.C. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. Elife. 2019;8 doi: 10.7554/eLife.49501. pii: e49501. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min S.Y., Desai A., Sharma A., DeSouza T., Genga R.M.J., Kucukural A. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(36):17970–17979. doi: 10.1073/pnas.1906512116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jespersen N.Z., Feizi A., Andersen E.S., Heywood S., Hattel H.B., Daugaard S. Heterogeneity in the perirenal region of humans suggests presence of dormant brown adipose tissue that contains brown fat precursor cells. Mol Metabolism. 2019;24:30–43. doi: 10.1016/j.molmet.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raajendiran A., Ooi G., Bayliss J., Rodeheffer M.S., Burton P.R., Watt M.J. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Reports. 2019;27(5):1528–1540. doi: 10.1016/j.celrep.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Hepler C., Shan B., Zhang Q., Henry G.H., Shao M., Vishvanath L. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. Elife. 2018;7 doi: 10.7554/eLife.39636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchoukalova Y.D., Votruba S.B., Tchkonia T., Giorgadze N., Kirkland J.L., Jensen M.D. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee K.L., Luong Q., Sharma R., Dreyfuss J.M., Ussar S., Kahn C.R. Developmental and functional heterogeneity of white adipocytes within a single fat depot. The EMBO Journal. 2019;38(3) doi: 10.15252/embj.201899291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hagberg C.E., Li Q., Kutschke M., Boucher J., Thorell A., Spalding K.L. Flow cytometry of mouse and human adipocytes for the analysis of browning and cellular heterogeneity. Cell Reports. 2018;24(10):2746–2756. doi: 10.1016/j.celrep.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dichamp J., Barreau C., Guissard C., Carriere A., Martinez Y., Descombes X. 3D analysis of the whole subcutaneous adipose tissue reveals a complex spatial network of interconnected lobules with heterogeneous browning ability. Scientific Reports. 2019;9(1):6684. doi: 10.1038/s41598-019-43130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C., Hu X., Weston T.A., Jung R.S., Heizer P., Tu Yiping. NanoSIMS imaging reveals unexpected heterogeneity in nutrient uptake by brown adipocytes. Biochemical and Biophysical Research Communications. 2018;504(4):899–902. doi: 10.1016/j.bbrc.2018.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Merrick D., Sakers A., Irgebay Z., Okada C., Calvert C., Morley M.P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364(6438) doi: 10.1126/science.aav2501. pii: eaav2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sebo Z.L., Rodeheffer M.S. Assembling the adipose organ: adipocyte lineage segregation and adipogenesis in vivo. Development. 2019;146(7) doi: 10.1242/dev.172098. pii: dev172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kulenkampff E., Wolfrum C. Proliferation of nutrition sensing preadipocytes upon short term HFD feeding. Adipocyte. 2019;8(1):16–25. doi: 10.1080/21623945.2018.1521229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meln I., Wolff G., Gajek T., Koddebusch J., Lerch S., Harbrecht L. Dietary calories and lipids synergistically shape adipose tissue cellularity during postnatal growth. Molecular Metabolism. 2019;24:139–148. doi: 10.1016/j.molmet.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raajendiran A., Ooi G., Bayliss J., O'Brien P.E., Schittenhelm R.B., Clark A.K. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Reports. 2019;27(5):1528–1540. doi: 10.1016/j.celrep.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Gawronska-Kozak B., Staszkiewicz J., Gimble J.M., Kirk-Ballard H. Recruitment of fat cell precursors during long-term high fat diet in C57BL/6J mice is fat depot specific. Obesity. 2014;22(4):1091–1102. doi: 10.1002/oby.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwalie P.C., Dong H., Zachara M., Russeil J., Alpern D., Akchiche N. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature. 2018;559(7712):103–108. doi: 10.1038/s41586-018-0226-8. [DOI] [PubMed] [Google Scholar]
- 73.Xue R., Lynes M.D., Dreyfuss J.M., Shamsi F., Schulz T.J., Zhang H. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nature Medicine. 2015;21(7):760–768. doi: 10.1038/nm.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rojas-Rodriguez R., Lujan-Hernandez J., Min S.Y., DeSouza T., Teebagy P., Desai A. Generation of functional human adipose tissue in mice from primed progenitor cells. Tissue Engineering Part A. 2019;25(11–12):842–854. doi: 10.1089/ten.tea.2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song A., Dai W., Jang M.J., Medrano L., Li Z., Zhao H. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. Journal of Clinical Investigation. 2019 doi: 10.1172/JCI129167. pii: 129167. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen Y., Ikeda K., Yoneshiro T., Scaramozza A., Tajima K., Wang Q. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature. 2019;565(7738):180–185. doi: 10.1038/s41586-018-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang-Doran I., Sleigh A., Rochford J.J., O'Rahilly S., Savage D.B. Lipodystrophy: metabolic insights from a rare disorder. Journal of Endocrinology. 2010;207(3):245–255. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 78.Czech M.P., Tencerova M., Pederson D.J., Aouadi M. Insulin signaling mechanisms for triacylglycerol storage. Diabetologia. 2013;56(5):949–964. doi: 10.1007/s00125-013-2869-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardy O.T., Czech M.P., Corvera S. What causes the insulin resistance underlying obesity? Current Opinion in Endocrinology Diabetes and Obesity. 2012;19(2):81–87. doi: 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Del Corno M., Baldassarre A., Calura E., Conti L., Martini P., Romualdi C. Transcriptome profiles of human visceral adipocytes in obesity and colorectal cancer unravel the effects of body mass index and polyunsaturated fatty acids on genes and biological processes related to tumorigenesis. Frontiers in Immunology. 2019;10:265. doi: 10.3389/fimmu.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hou X.G., Moser S., Sarr M.G., Thompson G.B., Que F.G., Jensen M.D. Visceral and subcutaneous adipose tissue diacylglycerol acyltransferase activity in humans. Obesity. 2009;17(6):1129–1134. doi: 10.1038/oby.2008.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yen C.L.E., Stone S.J., Koliwad S., Harris C., Farese R.V. DGAT enzymes and triacylglycerol biosynthesis. The Journal of Lipid Research. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ranganathan G., Unal R., Pokrovskaya I., Yao-Borengasser A., Phanavanh B., Lecka-Czernik B. The lipogenic enzymes DGAT1, FAS, and LPL in adipose tissue: effects of obesity, insulin resistance, and TZD treatment. The Journal of Lipid Research. 2006;47(11):2444–2450. doi: 10.1194/jlr.M600248-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chitraju C., Walther T.C., Farese R.V. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. The Journal of Lipid Research. 2019;60(6):1112–1120. doi: 10.1194/jlr.M093112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chitraju C., Mejhert N., Haas J.T., Grisell Diaz-Ramirez L., Grueter C.A., Imbriglio J.E. Triglyceride synthesis by DGAT1 protects adipocytes from lipid-induced ER stress during lipolysis. Cell Metabolism. 2017;26(2):407–418. doi: 10.1016/j.cmet.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ortega-Prieto P., Postic C. Carbohydrate sensing through the transcription factor ChREBP. Frontiers in Genetics. 2019;10:472. doi: 10.3389/fgene.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vijayakumar A., Aryal P., Wen J., Syed I., Vazirani R.P., MoraesVieira P.M. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Reports. 2017;21(4):1021–1035. doi: 10.1016/j.celrep.2017.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang Y., Wallace M., Sanchez-Gurmaches J., Hsiao W.-Y., Li H., Lee P.L. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nature Communications. 2016;7:11365. doi: 10.1038/ncomms11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang F., Mullican S.E., DiSpirito J.R., Peed L.C., Lazar M.A. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18656–18661. doi: 10.1073/pnas.1314863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soccio R.E., Li Z., Chen E.R., Foong Y.H., Benson K.K., Dispirito J.R. Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. Journal of Clinical Investigation. 2017;127(4):1451–1462. doi: 10.1172/JCI91211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boucher J., Kleinridders A., Kahn C.R. Insulin receptor signaling in normal and insulin resistant states. Cold Spring Harb Perspect Biol. 2014;6(1) doi: 10.1101/cshperspect.a009191. pii: a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou L., Park S.Y., Xu L., Xia X., Ye J., Su L. Insulin resistance and white adipose tissue inflammation are uncoupled in energetically challenged Fsp27-deficient mice. Nature Communications. 2015;6:5949. doi: 10.1038/ncomms6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedman J. 20 years of leptin: leptin at 20: an overview. Journal of Endocrinology. 2014;223(1):T1–T8. doi: 10.1530/JOE-14-0405. [DOI] [PubMed] [Google Scholar]
- 94.Caron A., Lee S., Elmquist J.K., Gautron L. Leptin and brain-adipose crosstalks. Nature Reviews Neuroscience. 2018;19(3):153–165. doi: 10.1038/nrn.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nawrocki A.R., Rajala M.W., Tomas E., Pajvani U.B., Saha A.K., Trumbauer M.E. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. Journal of Biological Chemistry. 2006;281(5):2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 96.Wang G.X., Zhao X.Y., Meng Z.X., Kern M., Dietrich A., Chen Z. The brown fat–enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nature Medicine. 2014;20(12):1436–1445. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Z., Wang G.X., Ma S.L., Jung D.Y., Ha H., Altamimi T. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Molecular Metabolism. 2017;6(8):863–872. doi: 10.1016/j.molmet.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nugroho D.B., Ikeda K., Barinda A.J., Wardhana D.A., Yagi K., Miyata K. Neuregulin 4 is an angiogenic factor that is critically involved in the maintenance of adipose tissue vasculature. Biochemical and Biophysical Research Communications. 2018;503(1):378–384. doi: 10.1016/j.bbrc.2018.06.043. [DOI] [PubMed] [Google Scholar]
- 99.Bluher M. Neuregulin 4: a “hotline” between Brown fat and liver. Obesity. 2019;27(10):1555–1557. doi: 10.1002/oby.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Leiria L.O., Wang C.H., Lynes M.D., Yang K., Shamsi F., Sato M. 12-Lipoxygenase regulates cold adaptation and glucose metabolism by producing the omega-3 lipid 12-HEPE from Brown fat. Cell Metabolism. 2019;30(4):768–783. doi: 10.1016/j.cmet.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stanford K.I., Lynes M.D., Takahashi H., Baer L.A., Arts P.J., May F.J. 12,13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metabolism. 2018;27(5):1111–1120. doi: 10.1016/j.cmet.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pellegrinelli V., Peirce V.J., Howard L., Virtue S., Turei D., Senzacqua M. Adipocyte-secreted BMP8b mediates adrenergic-induced remodeling of the neuro-vascular network in adipose tissue. Nature Communications. 2018;9(1):4974. doi: 10.1038/s41467-018-07453-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Howard B.V., Klimes I., Vasquez B., Brady D., Nagulesparan M., Unger R.H. The antilipolytic action of insulin in obese subjects with resistance to it glucoregulatory action. Journal of Clinical Endocrinology & Metabolism. 1984;58(3):544–548. doi: 10.1210/jcem-58-3-544. [DOI] [PubMed] [Google Scholar]
- 104.Opie L.H., Walfish P.G. Plasma free fatty acid concentrations in obesity. New England Journal of Medicine. 1963;268(14):757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- 105.Horowitz J.F., Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. American Journal of Physiology. Endocrinology and Metabolism. 2000;278(6):E1144–E1152. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 106.Jensen M.D., Haymond M.W., Rizza R.A., Cryer P.E., Miles J. Influence of body fat distribution on free fatty acid metabolism in obesity. Journal of Clinical Investigation. 1989;83(4):1168–1173. doi: 10.1172/JCI113997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong S., Song W., Zushin P.J.H., Liu B., Jedrychowski M.P., Mina A.I. Phosphorylation of Beta-3 adrenergic receptor at serine 247 by ERK MAP kinase drives lipolysis in obese adipocytes. Molecular Metabolism. 2018;12:25–38. doi: 10.1016/j.molmet.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shin A.C., Filatova N., Lindtner C., Chi T., Degann S., Oberlin D. Insulin receptor signaling in POMC, but not AgRP, neurons controls adipose tissue insulin action. Diabetes. 2017;66(6):1560–1571. doi: 10.2337/db16-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zechner R., Madeo F., Kratky D. Cytosolic lipolysis and lipophagy: two sides of the same coin. Nature Reviews Molecular Cell Biology. 2017;18(11):671–684. doi: 10.1038/nrm.2017.76. [DOI] [PubMed] [Google Scholar]
- 110.Softic S., Boucher J., Solheim M.H., Fujisaka S., Haering M.F., Homan E.P. Lipodystrophy due to adipose tissue specific insulin receptor knockout results in progressive NAFLD. Diabetes. 2016;65(8):2187–2200. doi: 10.2337/db16-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guerra C., Navarro P., Valverde A.M., Arribas M., Bruning J., Kozak L.P. Brown adipose tissue–specific insulin receptor knockout shows diabetic phenotype without insulin resistance. Journal of Clinical Investigation. 2001;108(8):1205–1213. doi: 10.1172/JCI13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shearin A.L., Monks B.R., Seale P., Birnbaum M.J. Lack of AKT in adipocytes causes severe lipodystrophy. Molecular Metabolism. 2016;5(7):472–479. doi: 10.1016/j.molmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee P.L., Tang Y., Li H., Guertin D.A. Raptor/mTORC1 loss in adipocytes causes progressive lipodystrophy and fatty liver disease. Molecular Metabolism. 2016;5(6):422–432. doi: 10.1016/j.molmet.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Herman M.A., Peroni O.D., Villoria J., Schon M.R., Abumrad N.A., Bluher M. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484(7394):333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mauvais-Jarvis F., Ueki K., Fruman D.A., Hirshman M.F., Sakamoto K., Goodyear L.J. Reduced expression of the murine p85α subunit of phosphoinositide 3-kinase improves insulin signaling and ameliorates diabetes. Journal of Clinical Investigation. 2002;109(1):141–149. doi: 10.1172/JCI13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morley T.S., Xia J.Y., Scherer P.E. Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements. Nature Communications. 2015;6:7906. doi: 10.1038/ncomms8906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schoiswohl G., Stefanovic-Racic M., Menke M.N., Wills R.C., Surlow B.A., Basantani M.K. Impact of reduced ATGL-mediated adipocyte lipolysis on ObesityAssociated insulin resistance and inflammation in male mice. Endocrinology. 2015;156(10):3610–3624. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jung S.M., Hung C.M., Hildebrand S.R., Sanchez-Gurmaches J., Martinez-Pastor B., Gengatharan J.M. Non-canonical mTORC2 signaling regulates Brown adipocyte lipid catabolism through SIRT6-FoxO1. Molecular Cell. 2019;75(4):807–822. doi: 10.1016/j.molcel.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Glick Z., Teague R.J., Bray G.A. Brown adipose tissue: thermic response increased by a single low protein, high carbohydrate meal. Science. 1981;213(4512):1125–1127. doi: 10.1126/science.7268419. [DOI] [PubMed] [Google Scholar]
- 120.Din M.U., Saari T., Raiko J., Kudomi N., Maurer S.F., Lahesmaa M. Postprandial oxidative metabolism of human Brown fat indicates thermogenesis. Cell Metabolism. 2018;28(2):207–216. doi: 10.1016/j.cmet.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 121.Shin H., Ma Y., Chanturiya T., Cao Q., Wang Y., Kadegowda A.K.G. Lipolysis in Brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metabolism. 2017;26(5):764–777. doi: 10.1016/j.cmet.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mehran A.E., Templeman N.M., Brigidi G.S., Lim G.E., Chu K.Y., Chu X. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metabolism. 2012;16(6):723–737. doi: 10.1016/j.cmet.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 123.Melvin A., O'Rahilly S., Savage D.B. Genetic syndromes of severe insulin resistance. Current Opinion in Genetics & Development. 2018;50:60–67. doi: 10.1016/j.gde.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Lackey D.E., Olefsky J.M. Regulation of metabolism by the innate immune system. Nature Reviews Endocrinology. 2016;12(1):15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 125.Kahn C.R., Wang G., Lee K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. Journal of Clinical Investigation. 2019;129(10):3990–4000. doi: 10.1172/JCI129187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang X., Liu G., Guo J., Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. International Journal of Biological Sciences. 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Choi S.M., Tucker D.F., Gross D.N., Easton R.M., DiPilato L.M., Dean A.S. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Molecular and Cellular Biology. 2010;30(21):5009–5020. doi: 10.1128/MCB.00797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Koren S., DiPilato L.M., Emmett M.J., Shearin A.L., Chu Q., Monks B. The role of mouse Akt2 in insulin-dependent suppression of adipocyte lipolysis in vivo. Diabetologia. 2015;58(5):1063–1070. doi: 10.1007/s00125-015-3532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jonsson C., Castor Batista A.P., Kjolhede P., Stralfors P. Insulin and β3-adrenergic receptors mediate lipolytic and anti-lipolytic signalling that is not altered by type 2 diabetes in human adipocytes. Biochemical Journal. 2019;476(19):2883–2908. doi: 10.1042/BCJ20190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stockli J., Zadoorian A., Cooke K.C., Deshpande V., Yau B., Hermann G. ABHD15 regulates adipose tissue lipolysis and hepatic lipid accumulation. Molecular Metabolism. 2019;25:83–94. doi: 10.1016/j.molmet.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia W., Pessentheiner A.R., Hofer D.C., Amor M., Schreiber R., Schoiswohl G. Loss of ABHD15 impairs the anti-lipolytic action of insulin by altering PDE3B stability and contributes to insulin resistance. Cell Reports. 2018;23(7):1948–1961. doi: 10.1016/j.celrep.2018.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klip A., McGraw T.E., James D.E. Thirty sweet years of GLUT4. Journal of Biological Chemistry. 2019;294(30):11369–11381. doi: 10.1074/jbc.REV119.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fazakerley D.J., Krycer J.R., Kearney A.L., Hocking S.L., James D.E. Muscle and adipose tissue insulin resistance: malady without mechanism? The Journal of Lipid Research. 2019;60(10):1720–1732. doi: 10.1194/jlr.R087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Turner N., Kowalski G.M., Leslie S.J., Risis S., Yang C., Lee-Young R.S. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56(7):1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 135.Guilherme A., Pedersen D.J., Henchey E., Henriques F.S., Danai L.V., Shen Y. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Molecular Metabolism. 2017;6(8):781–796. doi: 10.1016/j.molmet.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee Y.S., Li P., Huh J.Y., Hwang I.J., Lu M., Kim J.I. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60(10):2474–2483. doi: 10.2337/db11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiedemann M.S.F., Wueest S., Item F., Schoenle E.J., Konrad D. Adipose tissue inflammation contributes to short-term high-fat diet-induced hepatic insulin resistance. American Journal of Physiology. Endocrinology and Metabolism. 2013;305(3):E388–E395. doi: 10.1152/ajpendo.00179.2013. [DOI] [PubMed] [Google Scholar]
- 138.Ferrante A.W., Jr. Macrophages, fat, and the emergence of immunometabolism. Journal of Clinical Investigation. 2013;123(12):4992–4993. doi: 10.1172/JCI73658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reilly S., Saltiel A.R. Adapting to obesity with adipose tissue inflammation. Nature Reviews Endocrinology. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 140.Tan S.X., Fisher-Wellman K.H., Fazakerley D.J., Ng Y., Pant H., Li J. Selective insulin resistance in adipocytes. Journal of Biological Chemistry. 2015;290(18):11337–11348. doi: 10.1074/jbc.M114.623686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barbato D.L. Redox control of non-shivering thermogenesis. Molecular Metabolism. 2019;25:11–19. doi: 10.1016/j.molmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lempesis I.G., Meijel R.L.J., Manolopoulos K.N., Goossens G.H. Oxygenation of adipose tissue: a human perspective. Acta Physiologica. 2019;11 doi: 10.1111/apha.13298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Czech M.P., Buxton J.M. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. Journal of Biological Chemistry. 1993;268(13):9187–9190. [PubMed] [Google Scholar]
- 144.Huang S., Czech M.P. The GLUT4 glucose transporter. Cell Metabolism. 2007;5(4):237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 145.Abel E.D., Peroni O., Kim J.K., Kim Y.B., Boss O., Hadro E. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409(6821):729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 146.Munoz S., Franckhauser S., Elias I., Ferre T., Hidalgo A., Monteys A.M. Chronically increased glucose uptake by adipose tissue leads to lactate production and improved insulin sensitivity rather than obesity in the mouse. Diabetologia. 2010;53(11):2417–2430. doi: 10.1007/s00125-010-1840-7. [DOI] [PubMed] [Google Scholar]
- 147.Kang S., Tsai L.T., Rosen E.D. Nuclear mechanisms of insulin resistance. Trends in Cell Biology. 2016;26(5):341–351. doi: 10.1016/j.tcb.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosen E.D. Epigenomic and transcriptional control of insulin resistance. Journal of Internal Medicine. 2016;280(5):443–456. doi: 10.1111/joim.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Emmett M., Lazar M.A. Integrative regulation of physiology by histone deacetylase 3. Nature Reviews Molecular Cell Biology. 2019;20(2):102–115. doi: 10.1038/s41580-018-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K. PPARγ mediates high-fat diet–induced adipocyte hypertrophy and insulin resistance. Molecular Cell. 1999;4(4):597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- 151.Ryden M., Hrydziuszko O., Mileti E., Raman A., Bornholdt J., Boyd M. The adipose transcriptional response to insulin is determined by obesity, not insulin sensitivity. Cell Reports. 2016;16(9):2317–2326. doi: 10.1016/j.celrep.2016.07.070. [DOI] [PubMed] [Google Scholar]
- 152.Tontonoz P., Spiegelman B.M. Fat and beyond: the diverse biology of PPARgamma. Annual Review of Biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 153.Rajan M.R., Nyman E., Brannmark C., Olofsson C.S., Stralfors P. Inhibition of FOXO1 transcription factor in primary human adipocytes mimics the insulin resistant state of type 2 diabetes. Biochemical Journal. 2018;475(10):1807–1820. doi: 10.1042/BCJ20180144. [DOI] [PubMed] [Google Scholar]
- 154.Nakae J., Cao Y., Oki M., Sawa H., Kiyonari H., Suga K. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57(3):563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 155.Emmett M.J., Lim H.W., Jager J., Richter H.J., Adlanmerini M., Peed L.C. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature. 2017;546(7659):544–548. doi: 10.1038/nature22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Richardson D.K., Czech M.P. Primary role of decreased fatty acid synthesis in insulin resistance of large rat adipocytes. American Journal of Physiology. 1978;234(2):E182–E189. doi: 10.1152/ajpendo.1978.234.2.E182. [DOI] [PubMed] [Google Scholar]
- 157.Zhou P., Santoro A., Peroni O.D., Nelson A.T., Saghatelian A., Siegel D. PAHSAs enhance hepatic and systemic insulin sensitivity through direct and indirect mechanisms. Journal of Clinical Investigation. 2019;129(10):4138–4150. doi: 10.1172/JCI127092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lodhi I.J., Yin L., Jensen-Urstad A.P.L., Funai K., Coleman T., Baird J.H. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metabolism. 2012;16(2):189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fernandez S., Viola J.M., Torres A., Wallace M., Trefely S., Zhao S. Adipocyte ACLY facilitates dietary carbohydrate handling to maintain metabolic homeostasis in females. Cell Reports. 2019;27(9):2772–2784. doi: 10.1016/j.celrep.2019.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Verdin E., Ott M. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nature Reviews Molecular Cell Biology. 2015;16(4):258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]
- 161.Hirschey M.D., Zhao Y. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Molecular & Cellular Proteomics. 2015;14(9):2308–2315. doi: 10.1074/mcp.R114.046664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ko P.J., Dixon S.J. Protein palmitoylation and cancer. EMBO Reports. 2018;19(10) doi: 10.15252/embr.201846666. pii: e46666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Young P., Arch J.R., Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. FEBS Letters. 1984;167(1):10–14. doi: 10.1016/0014-5793(84)80822-4. [DOI] [PubMed] [Google Scholar]
- 164.Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Penicaud L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. Journal of Cell Science. 1992;103(Pt 4):931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 165.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. Journal of Biological Chemistry. 2010;285(10):7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sharp L.Z., Shinoda K., Ohno H., Scheel D.W., Tomoda E., Ruiz L. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cypess A.M., White A.P., Vernochet C., Schulz T.J., Xue R., Sass C.A. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature Medicine. 2013;19(5):635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jespersen N.Z., Larsen T.J., Daugaard S., Homoe P., Loft A., de Jong J. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metabolism. 2013;17(5):798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 170.Leitner B.P., Huang S., Brychta R.J., Duckworth C.J., Baskin A.S., McGehee S. Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences. 2017;114(32):8649–8654. doi: 10.1073/pnas.1705287114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Jong J.M.A., Sun W., Pires N.D., Frontini A., Balaz M., Jespersen N.Z. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Natural Metabolism. 2019;1:830–843. doi: 10.1038/s42255-019-0101-4. [DOI] [PubMed] [Google Scholar]
- 172.Mottillo E.P., Desjardins E.M., Crane J.D., Smith B.K., Green A.E., Ducommun S. Lack of adipocyte AMPK exacerbates insulin resistance and hepatic steatosis through Brown and beige adipose tissue function. Cell Metabolism. 2016;24(1):118–129. doi: 10.1016/j.cmet.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sustarsic E.G., Ma T., Lynes M.D., Larsen M., Karavaeva I., Havelund J.F. Cardiolipin synthesis in Brown and beige fat mitochondria is essential for systemic energy homeostasis. Cell Metabolism. 2018;28(1):159–174. doi: 10.1016/j.cmet.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stanford K.I., Middelbeek J.W., Townsend K.L., An D., Nygaard E.B., Hitchcox K.M. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. Journal of Clinical Investigation. 2013;123(1):215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.White J.D., Dewal R.S., Stanford K.I. The beneficial effects of brown adipose tissue transplantation. Mol Aspects of Med. 2019;68:74–81. doi: 10.1016/j.mam.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hanssen M.J.W., Wierts R., Hoeks J., Gemmink A., Brans B., Mottaghy F.M. Glucose uptake in human brown adipose tissue is impaired upon fasting-induced insulin resistance. Diabetologia. 2015;58(3):586–595. doi: 10.1007/s00125-014-3465-8. [DOI] [PubMed] [Google Scholar]
- 177.Lee P., Bova R., Scholfield L., Bryant W., Dieckmann W., Slattery A. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metabolism. 2016;23(4):602–609. doi: 10.1016/j.cmet.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 178.Tews D., Pula T., Funcke J.B., Jastroch M., Keuper M., Debatin K.M. Elevated UCP1 levels are sufficient to improve glucose uptake in human white adipocytes. Redox Biology. 2019;26:101286. doi: 10.1016/j.redox.2019.101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Giralt M., Villarroya F. Mitochondrial uncoupling and the regulation of glucose homeostasis. Current Diabetes Reviews. 2017;13(4):386–394. doi: 10.2174/1573399812666160217122707. [DOI] [PubMed] [Google Scholar]
- 180.Xiao C., Goldgof M., Gavrilova O., Reitman M.L. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22°C. Obesity. 2015;23(7):1450–1459. doi: 10.1002/oby.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Souza C.J., Hirshman M.F., Horton E.S. CL-316,243, a β3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes. 1997;46(8):1257–1263. doi: 10.2337/diab.46.8.1257. [DOI] [PubMed] [Google Scholar]
- 182.Liu X., Rossmeisl M., McClaine J., Kozak L.P. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice. Journal of Clinical Investigation. 2003;111(3):399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kontani Y., Wang Y., Kimura K., Inokuma K., Saito M., Suzuki-Miura T. UCP1 deficiency increases susceptibility to diet-induced obesity with age. Aging Cell. 2005;4(3):147–155. doi: 10.1111/j.1474-9726.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- 184.Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9(2):203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 185.Luijten I.H.N., Feldmann H.M., von Essen G., Cannon B., Nedergaard J. In the absence of UCP1-mediated diet-induced thermogenesis, obesity is augmented even in the obesity resistant 129S mouse strain. American Journal of Physiology. Endocrinology and Metabolism. 2019;316(5):E729–E740. doi: 10.1152/ajpendo.00020.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Winn N.C., Vieira-Potter V.J., Gastecki M.L., Welly R.J., Scroggins R.J., Zidon T.,M. Loss of UCP1 exacerbates Western diet-induced glycemic dysregulation independent of changes in body weight in female mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2017;312(1):R74–R84. doi: 10.1152/ajpregu.00425.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Winn N.C., Acin-Perez R., Woodford M.L., Hansen S.A., Haney M.M., Ayedun L.A. A thermogenic-like Brown adipose tissue phenotype is dispensable for enhanced glucose tolerance in female mice. Diabetes. 2019;68(9):1717–1729. doi: 10.2337/db18-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kazak L., Rahbani J.F., Samborska B., Lu G.Z., Jedrychowski M.P., Lajoie M. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Natural Metabolism. 2019;1(3):360–370. doi: 10.1038/s42255-019-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jeong J.H., Chang J.S., Jo Y.H. Intracellular glycolysis in brown adipose tissue is essential for optogenetically induced nonshivering thermogenesis in mice. Scientific Reports. 2018;8(1):6672. doi: 10.1038/s41598-018-25265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Winther S., Isidor M.S., Basse A.L., Skjoldborg N., Cheung A., Quistorff B. Restricting glycolysis impairs brown adipocyte glucose and oxygen consumption. American Journal of Physiology. Endocrinology and Metabolism. 2017;314:E214–E223. doi: 10.1152/ajpendo.00218.2017. [DOI] [PubMed] [Google Scholar]
- 191.Mottillo E.P., Balasubramanian P.B., Lee Y.H., Weng C., Kershaw E.E., Granneman J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. The Journal of Lipid Research. 2014;55(11):2276–2286. doi: 10.1194/jlr.M050005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gonzalez-Hurtado E., Lee J., Choi J., Wolfgang M.J. Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing. Molecular Metabolism. 2018;7:45–56. doi: 10.1016/j.molmet.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schreiber R., Diwoky C., Schoiswohl G., Feiler U., Wongsiriroj N., Abdellatif M. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not Brown adipose tissue. Cell Metabolism. 2017;26(5):753–763. doi: 10.1016/j.cmet.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ahmadian M., Abbott M.J., Tang T., Hudak C.S.S., Kim Y., Bruss M. Desnutrin/ATGL is regulated by AMPK and is required for a Brown adipose phenotype. Cell Metabolism. 2011;13(6):739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sanchez-Gurmaches J., Tang Y., Jespersen N.Z., Nielsen S., Scheele C., Guertin D.A. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metabolism. 2018;27(1):195–209. doi: 10.1016/j.cmet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shin H., Shi H., Xue B., Yu L. What activates thermogenesis when lipid droplet lipolysis is absent in brown adipocytes? Adipocyte. 2018;7(2):143–147. doi: 10.1080/21623945.2018.1453769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Singh A.K., Aryal B., Chaube B., Rotllan N., Varela L., Horvath T.L. Brown adipose tissue derived ANGPTL4 controls glucose and lipid metabolism and regulates thermogenesis. Molecular Metabolism. 2018;11:59–69. doi: 10.1016/j.molmet.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Heeren J., Scheia L. Brown adipose tissue and lipid metabolism. Current Opinion in Lipidology. 2018;29(3):180–185. doi: 10.1097/MOL.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 199.Hoeke G., Kooijman S., Boon M.R., Rensen P.C., Berbee J.F. Role of Brown fat in lipoprotein metabolism and atherosclerosis. Circulation Research. 2016;118(1):173–182. doi: 10.1161/CIRCRESAHA.115.306647. [DOI] [PubMed] [Google Scholar]
- 200.Guilherme A., Pedersen D.J., Henriques F., Bedard A.H., Henchey E., Kelly M. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Molecular Metabolism. 2018;16:116–125. doi: 10.1016/j.molmet.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mossenbock K., Vegiopoulos A., Rose A.J., Sijmonsma T.P., Herzig S., Schafmeier T. Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Orava J., Nuutila P., Lidell M.E., Oikonen V., Noponen T., Viljanen T. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism. 2011;14(2):272–279Crossref. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 203.Ferre P., Burnol A.F., Leturque A., Terretaz J., Penicaud L., Jeanrenaud B. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochemical Journal. 1986;233(1):249–252. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Cooney G.J., Caterson I.D., Newsholme E.A. The effect of insulin and noradrenaline on the uptake of 2-[1-14C)deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Letters. 1985;188(2):257–261. doi: 10.1016/0014-5793(85)80383-5. [DOI] [PubMed] [Google Scholar]
- 205.Marette A., Bukowiecki L.J. Stimulation of glucose transport by insulin and norepinephrine in isolated rat brown adipocytes. American Journal of Physiology. 1989;257(4 Pt 1):C714–C721. doi: 10.1152/ajpcell.1989.257.4.C714. [DOI] [PubMed] [Google Scholar]
- 206.Barquissau V., Beuzelin D., Pisani D.F., Beranger G.E., Mairal A., Montagner A. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Molecular Metabolism. 2016;5(5):352–365. doi: 10.1016/j.molmet.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Dallner O.S., Chernogubova E., Brolinson K.A., Bengtsson T. β3-Adrenergic receptors stimulate glucose uptake in Brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology. 2006;147(12):5730–5739. doi: 10.1210/en.2006-0242. [DOI] [PubMed] [Google Scholar]
- 208.Olsen J.M., Sato M., Dallner O.S., Sandstrom A.L., Pisani D.F., Chambard J.C. Glucose uptake in brown fat cells is dependent on mTOR complex 2–promoted GLUT1 translocation. The Journal of Cell Biology. 2014;207(3):365–374. doi: 10.1083/jcb.201403080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chia L.Y., Evans B.A., Mukaida S., Bengtsson T., Hutchinson D.S., Sato M. Adrenoceptor regulation of the mechanistic target of rapamycin in muscle and adipose tissue. British Journal of Phamacology. 2019;176(14):2433–2448. doi: 10.1111/bph.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Albert V., Svensson K., Shimobayashi M., Colombi M., Munoz S., Jimenez V. mTORC2 sustains thermogenesis via Akt-induced glucose uptake and glycolysis in brown adipose tissue. EMBO Molecular Medicine. 2016;8(3):232–246. doi: 10.15252/emmm.201505610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Garcia-Casarrubios E., de Moura C., Arroba A.I., Pescador N., Calderon-Dominguez M., Garcia L. Rapamycin negatively impacts insulin signaling, glucose uptake and uncoupling protein-1 in brown adipocytes. Biochimica et Biophysica Acta. 2016;1861(12 Pt A):1929–1941. doi: 10.1016/j.bbalip.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 212.Mukaida S., Evans B.A., Bengtsson T., Hutchinson D.S., Sato M. Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2. Pharmacological Research. 2017;116:87–92. doi: 10.1016/j.phrs.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 213.Olsen J.M., Csikasz R.I., Dehvari N., Lu L., Sandstrom A., Oberg A.I. β3-Adrenergically induced glucose uptake in brown adipose tissue is independent of UCP1 presence or activity: mediation through the mTOR pathway. Molecular Metabolism. 2017;6(6):611–619. doi: 10.1016/j.molmet.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fonseca T.L., Werneck-De-Castro J.P., Castillo M., Bocco B.M., Fernandes G.W., McAninch E.A. Tissue-specific inactivation of type 2 deiodinase reveals multilevel control of fatty acid oxidation by thyroid hormone in the mouse. Diabetes. 2014;63(5):1594–1604. doi: 10.2337/db13-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu D., Bordicchia M., Zhangm C., Fang H., Wei W., Li J.L. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. Journal of Clinical Investigation. 2016;126(5):1704–1716. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Roberts-Toler C., O'Neill B.T., Cypess A.M. Diet-induced obesity causes insulin resistance in mouse Brown adipose tissue. Obesity. 2015;23(9):1765–1770. doi: 10.1002/oby.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sakamoto T., Nitta T., Maruno K., Yeh Y.S., Kuwata H., Tomita K. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. American Journal of Physiology. Endocrinology and Metabolism. 2016;310(8):E676–E687. doi: 10.1152/ajpendo.00028.2015. [DOI] [PubMed] [Google Scholar]
- 218.Shen Y., Cohen J.L., Nicoloro S.M., Kelly M., Yenilmez B., Henriques F. CRISPR-delivery particles targeting nuclear receptor-interacting protein 1 (Nrip1) in adipose cells to enhance energy expenditure. Journal of Biological Chemistry. 2018;293(44):17291–17305. doi: 10.1074/jbc.RA118.004554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Blondin D.P., Labbe S.M., Phoenix S., Guerin B., Turcotte E.E., Richard D. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. Journal of Physiology. 2015;593(3):701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Carriere A., Jeanson Y., Berger-Muller S., Andre M., Chenouard V., Arnaud E. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Diabetes. 2014;63(10):3253–3265. doi: 10.2337/db13-1885. [DOI] [PubMed] [Google Scholar]
- 221.Mills E.L., Pierce K.A., Jedrychowski M.P., Garrity R., Winther S., Vidoni S. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560(7716):102–106. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Min S.Y., Kady J., Nam M., Rojas-Rodriguez R., Berkenwald A., Kim J.H. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nature Medicine. 2016;22(3):312–318. doi: 10.1038/nm.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]