Abstract
Background
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease that affects synovial joints, leading to inflammation, joint destruction, loss of function, and disability. Although recent pharmaceutical advances have improved treatment of RA, patients with RA often inquire about dietary interventions to improve RA symptoms, as they perceive rapid changes in their symptoms after consumption of certain foods. There is evidence that some ingredients have pro- or anti-inflammatory effects. In addition, recent literature has shown a link between diet and microbiome changes. Both diet and the gut microbiome are linked to circulating metabolites that may modulate inflammation. However, evidence of the effects of an anti-inflammatory and probiotic-rich diet in patients with RA is scarce. There is also a need for biological data to support its anti-inflammatory effects.
Methods
The main goal of this study is to delineate the design process for a diet tailored to our RA population. To achieve this goal, we collected information on diet, supplements, cooking methods, and intake of different ingredients for each patient. Different groups were interviewed, and their feedback was assessed to design a diet that incorporates suggested anti-inflammatory ingredients in a manner that was easy for patients to adopt based on their lifestyles and backgrounds.
Results
We designed a diet that includes a high intake of potential anti-inflammatory ingredients. Feedback from highly motivated patients was critical in constructing an anti-inflammatory diet (ITIS diet) with elevated adherence.
Conclusion
In order to tailor our diet, we surveyed our patients on several different parameters. We obtained important feedback on how feasible our ITIS diet is for RA patients. Using this feedback, we made minor improvements and finalized the design of the ITIS diet. This diet is being used in an on-going pilot study to determine their anti-inflammatory effect in pain and joint swelling in RA patients.
Trial registration
Not applicable.
Keywords: Rheumatoid arthritis, Diet design, Anti-inflammatory diet, ITIS diet, Microbiome
1. Background
Rheumatoid arthritis (RA) is a systemic, debilitating, chronic inflammatory autoimmune disorder affecting approximately 1% of the world population [1]. RA is a form of arthritis that causes pain, swelling, stiffness, and loss of function in joints. This disease severely impacts quality of life with increased morbidity and mortality. Although recent pharmaceutical advances have improved the treatment of RA, most RA patients need lifelong pharmacological therapy. RA patients often seek additional sources of relief and/or treatments with less side effects, and often inquire about dietary interventions to improve RA symptoms, as they perceive rapid changes in pain and/or swelling after consumption of certain foods [2]. However, rheumatologists lack information to advise RA patients on nutrition.
Diet might modulate RA symptoms by influencing the patient's metabolic profile and increasing antioxidant levels, but also by altering the microflora of the intestine. The gut microbiome is incredibly dynamic and can change rapidly to dietary perturbations [3]. In addition, the gut microbiome is also involved in the metabolism of some dietary components and has the potential to modify circulating pro- or anti-inflammatory mediators [4]. For example, trimethylamine-N-oxide, a pro-inflammatory metabolite that derives from choline and carnitine present in red meat, eggs, and dairy products, is produced by Prevotella copri among other bacteria [5,6]. An increased abundance of Prevotella copri was found in new-onset untreated RA patients suggesting P. copri may be pathogenic [7]. In general, bacteria that have an almost exclusive saccharolytic metabolism, such as lactobacilli and bifidobacterial, are considered potentially beneficial [8]. Both gut microbial species produce a variety of tryptophan catabolites which are critical for intestinal homeostasis by decreasing intestinal permeability [9]. In addition, some of these catabolites enter the bloodstream and have anti-inflammatory and anti-oxidative effects [9]. Various dietary components including miso and yogurt are a source of this beneficial flora. Daily yogurt was shown to reduce biomarkers of chronic inflammation in premenopausal women [10]. Dietary fiber, other complex carbohydrates, and sugar alcohols present in fruits are prebiotics and might also be beneficial by supporting healthy microbiome [11,12]. For instance, microbial degradation of whole-grain complex carbohydrates increases short chain fatty acids (SCFA), which were shown to be beneficial to intestinal immune response [13].
In addition, several foods have been identified as pro-inflammatory, including highly refined flours, gluten [14], trans- and saturated-fatty acids (FA) [[15], [16], [17]], dairy products (milk and cheeses) [18], and red meat [19,20]. Some vegetables such as tomatoes, eggplants, and potatoes contain solanine, a glycoalkaloid, which was suggested to increase intestinal permeability and be detrimental for arthritogenic pathologies [[21], [22], [23], [24], [25], [26]]. In contrast, other nutrients have been suggested to offer numerous health benefits [27], including long chain omega-3 polyunsaturated FA (PUFA) (chia seeds, flaxseeds, fatty fish) [[28], [29], [30], [31], [32]], monounsaturated FA (MUFA) (avocado, sesame) [33,34], antioxidants [35], phytochemicals [36], flavonoids [37,38], vitamin D [39], fruits with enzymatic proteins such as papain and bromelain (papaya, mango, pineapple) [[40], [41], [42]], ginger [43], turmeric [44,45], black pepper [46,47], green tea [[48], [49], [50]], and legumes [46,51]. However, only a few randomized double-blind placebo-controlled clinical trials have attempted to determine whether supplementation with these ingredients [31,34,35,37,38,43] or probiotics [52,53] are beneficial in RA patients. So far, most of the studies are pilot studies with small number of patients, studies on animal models, or in vitro studies.
Despite a large number of publications on the effect of different ingredients and gut microbiome on inflammation, and reports showing an association between poor dietary quality and inflammation in RA patients [54,55], only a few interventional studies have investigated whether specific diet improves RA symptoms (for review [56,57]). The effects of complete diet intervention (including vegetarian and Mediterranean diets) have shown positive effects in ameliorating RA symptoms. One of the studies showed that fasting first and then eating a vegetarian diet for one year was beneficial in RA, particularly in terms of number of swollen joints, stiffness, and C reactive protein (CRP) [58]. A diet trial with Mediterranean diet (MD) also showed a decrease in disease activity (DAS28) of 0.56 (p < 0.001) and in quality of life compared to control diet [59]. Yet, we could not find a trial using a diet that incorporates and combines different potential anti-inflammatory ingredients.
Here, we will describe the steps taken to design our anti-inflammatory diet (ITIS diet) for a complementary therapy in RA patients. In Table 1, we summarized the strategies and ingredients included in the ITIS diet. This diet is an omnivorous diet, based on a Mediterranean diet but with several modifications. Our diet reinforces fast digestions by dissociating grains from proteins, suggests certain cooking methods [60], is a diet low on gluten, and also includes enzymatic fruits, whole grains, a very high consumption of omega 3 PUFA and MUFA, and anti-inflammatory dressings and spices. It introduces green juices in the morning to increase green leafy vegetables and fruit consumption, and daily yogurt and miso as probiotic. It also avoids consumption of processed and red meat, caffeine, dairy products (except yogurt), and vegetables from the solanaceae family. Of note, not all the ingredients and strategies proposed in our ITIS diet are based on interventional diet studies in RA patients. We wanted to introduce ingredients suggested to be beneficial in animal models or other inflammatory diseases as well. Therefore, the design of this diet is intended to give an alternative to non-vegetarian population with higher content of potential anti-inflammatory ingredients than the typical Mediterranean diet.
Table 1.
Strategies and recommendations in the ITIS diet.
| Main recommendations (WHAT/WHAT FOR) | Based on (WHY) | Diet strategies (HOW) |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2. Methods
Patients: Thirty-four adult patients with RA fulfilling the 2010 ACR/EULAR classification criteria for RA, were recruited from the Arthritis Clinics at the University of California at San Diego (UCSD). The study was approved by the UCSD Institutional Review Board. We recruited patients from the two most prevalent populations in our Arthritis Clinics: Hispanic and Caucasian RA patients. For one year, we worked with a total of 20 Hispanic and 14 Caucasian RA patients in collaboration with a nutritionist to put together a 14-day ITIS diet that was feasible and that included our proposed anti-inflammatory/probiotic components (Table 1).
Initial design of the diet: In order to design this first draft, we examined the bibliography relating ingredients and inflammation, and diet intervention in RA patient. Once we decided the strategies and ingredients we wanted to introduce, we designed a Mediterranean-based 7-day draft diet, which also included the main strategies described in Table 1, and was structured as followed: daily green juices in the morning, 3 main meals and 2 snacks.
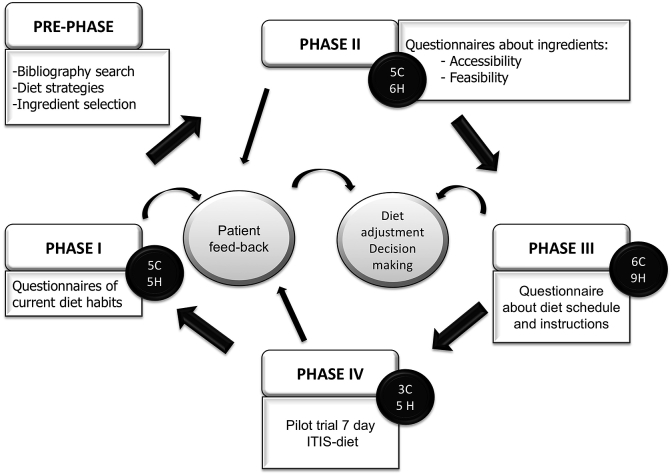
Diet modifications with RA patients was conducted in four phases: Since our main goal was to design a feasible diet, we worked with several small groups of patients to tailor the diet for higher adherence. The information gathered in the four phases of the study contributed to modify the draft until we built the final diet and instructions. Different patients were recruited for each phase. In Phase I, we evaluated the dietary habits of each patient in the last year in order to gather information about their current diet habits. In Phase II, we gathered information about patient knowledge, accessibility, affordability, and taste preferences of our proposed ingredients. In phase III, we designed a first draft of the diet with patient instructions and asked for patient feedback to assess how accurately patients were able to understand and follow the diet. In phase IV, we conducted a 7-day trial with patient feedback for final adjustments on the diet (Fig. 1). Macro and micro-nutrient composition as well as more details of our ITIS diet is available for interested parties.
Fig. 1.
Flow chart showing the different phases of the design of the ITIS diet. From a draft that included potential anti-inflammatory ingredients described in the scientific literature, in phase I, we got information of current dietary habits, in phase II, we collected information about the ingredients that we wanted to include, in phase III, we requested feedback about the instructions and strategies of our ITIS diet, and in phase IV, a few patients followed the ITIS diet for 7 days. The continuous feedback from our patients helped us make adjustments to the diet and build our final version of the ITIS diet.
3. Results
3.1. Phase I
A representative sample of 5 Hispanic and 5 Caucasian patients were included in this phase. We constructed and distributed a diet habit questionnaire (Additional file 1) in order to elucidate dietary habits of the patients in the last year. The questionnaire contained questions about how frequently (how many times per week) our patients had consumed meat, eggs, fish, bread, grains, vegetables, mushrooms, legumes, nuts, fruits, sauces, sweets, snacks, soups, and dairy products in the last year. In addition, patients were asked about their drinking habits and food preparation practices, including time invested in cooking. The questionnaire took approximately 1 h to complete, and RA patients filled the questionnaires individually in a designated room at the clinical facilities.
Average frequency of consumption of the ingredients (times per week) of these 10 RA patients are shown in additional file 2. Patient consumption of vegetables, fruits, meat, eggs, fish, grains, legumes and nuts did not differ from our ITIS diet. Of note, red meat was consumed less than once a week among these RA patients. Leeks, onion, peppers, and lettuce were the most consumed vegetables; berries and avocados were the most consumed fruits followed by peaches, nectarines, apricots, oranges, apples, and pears. Enzymatic fruits and ginger were not frequently consumed. Alcohol consumption was low, but they had daily coffee. Patients also reported a high consumption of simple carbohydrates such as cookies and cakes, added sugar, and consumed dairy products almost daily. They did consume yogurt but not miso. They did not frequently add seeds, anti-inflammatory dressings or anti-inflammatory spices in their food. They used olive oil followed by canola oil. Patients mainly steamed or boiled their food, but also fried for longer than 5 min. Though patients mainly prepared their meals at home, pre-cooked food was used frequently.
3.2. Phase II
A total of 6 Hispanic and 5 Caucasian patients were included in this phase. The objective of this phase was to gather information about patient knowledge and assess the affordability and accessibility of the products included in the ITIS diet. We designed a questionnaire that assessed the following categories: familiarity with ingredients, previous consumption of ingredients, knowledge of food and preparation methods, knowledge of where to buy the ingredients, and affordability of the ingredients. The questionnaire took around 30 min to complete and RA patients answered it individually in a designated room at the clinical facilities.
After the questionnaire was administered, we assigned a numeric value based on patient responses: if the response was negative, we assigned 0. If the response was positive, we assigned 100, and if it was doubtful, we assigned 50. Finally, we averaged patient responses for each ingredient and analyzed feasibility of patient use. Responses regarding patient knowledge and accessibility to consumption were averaged for individual ingredients (Additional file 3). We also calculated two final scores: accessibility was calculated as the average of the following questions: ‘do you know where to buy it?’, ‘can you buy it near your home?’, ‘do you know the price?’ and ‘can you afford it?’. Knowledge was calculated as the average of the following questions: ‘do you know what it is?’, ‘do you consume it?’ and ‘do you know how to cook it?’. If final averages fell below 50 for any category, this was interpreted as poor knowledge or accessibility for that ingredient, and we reconsidered introducing the ingredient and/or reevaluated our strategy for introducing the ingredient.
Additional file 3 shows the results of this questionnaire. Most of the ingredients that we intended to include in our ITIS diet were well-known and easily accepted. All of the fruits, most vegetables, chicken, and nuts, were well-known and accessible. Of note, knowledge and accessibility correlated well for each product; this possibly reflects a relationship between knowing how to use an ingredient and knowing where to buy it at the best price. Notably, products that have been promoted recently in the media, such as ginger and chia seeds, were well-known and accessible to patients.
Several ingredients had a value of <50 in knowledge or accessibility. These ingredients were: rye, tahini, kefir, arugula, turmeric, quinoa, miso, tofu, flaxseed oil, omelets, apple cider vinegar, and sweet potato. Among the ingredients that were poorly accepted, we decided to completely remove kefir, and substituted it with yogurt. Other ingredients such as rye (contained in german bread), sweet potato, arugula shoots, quinoa, apple cider vinegar, and tofu were made optional (but recommended). Ingredient that we deemed critical were left as a mandatory ingredient in the ITIS diet: miso, turmeric, flaxseed oil, and tahini.
We also asked patients if they were willing to avoid some products and/or substitute them for others. Of the patients surveyed, one did not respond to this question, and 9 out of 10 patients agreed to substitute: refined cereals for integral cereals, solanaceae vegetables for green vegetables, trans fatty acids and saturated fatty acids for omega 3 PUFA and MUFA, sugar for stevia or honey, soda drinks for home-made juices, and dairy products for vegetable extracts. 8 out of 10 patients were willing to substitute alcoholic drinks and coffee for green tea, and 6 out of 10 patients agreed to reduce gluten consumption.
Finally, patients were asked if they had access to a blender to determine if they were able to prepare smoothies. All Hispanic patients and 3 of the 4 Caucasian patients had one available at home. Average available time to cook per day was 1.5 h (±1.03), with a range from 30 min to 3 h. All but one patient responded that they were willing to learn new recipes, and subjects assigned themselves an average of 8.6/10 in their resolution to follow the diet.
3.3. Phase III
9 Hispanic and 6 Caucasian patients were included in phase III. We asked patients for feedback on a final version of the 14-day diet (which included both the diet and pertinent instructions). We focused on whether the diet could be easily understood and completed by patients. Patients were shown the diet and instructions, and after providing necessary clarifications, we asked patients to answer the diet-related questions shown in additional file 4A. Patients responded individually in a designated room at the clinical facilities.
The feedback from this phase is shown in additional file 4B. All patients understood the instructions and overall were not worried about satiety and time to prepare the meals. When patients were asked about the diet schedule, all but 3 indicated that they could adhere to it. 12 patients reported that they would be satiated with the diet, while three patients expressed doubt. We also observed that most patients had sufficient time to prepare breakfast correctly. Though most of the questioned patients did not indicate their cooking habits, those who did, indicated that they often steam, bake, or grill. We did encounter a few complaints on certain aspects of the diet plan. Some patients complained about elimination of dairy products, coffee, and alcohol. Yet, we strongly believe that these products would detrimentally affect inflammation and decided to eliminate them from the 14-day diet. We suggested substituting dairy products with nut extracts like almond milk, and coffee with green tea. Finally, patients asked for more recipe examples to facilitate diet completion.
3.4. Phase IV
We conducted a pilot trial of a 7-day ITIS diet on 5 Hispanic and 3 Caucasian patients in order to get their feedback about following the diet. Patients were provided with turmeric, black pepper, powder ginger, flaxseed oil, apple vinegar, tahini, sesame seeds, miso, chai seeds, flaxseeds, rye bread, oatmeal, almond milk, and olive oil to facilitate their consumption. We had 1-h session with groups of two to three patients to explain the diet and instructions in detail. Sessions were conducted in English or Spanish depending on patient preferences. Patients were instructed to keep a diet diary and were given instructions on how to contact us in case of questions.
We obtained feedback from 4 Hispanic patients; one patient dropped out of the trial. All 3 Caucasian patients completed the 7-diet trial. The Hispanic patients surveyed, except for some exceptions attributed to personal tastes, were able to follow the diet and had no critical difficulties. Among Caucasian patients, two patients asked for a shopping list to help with the menu and struggled to accomplish the timings and the cooking expectations for the diet. We plan to give more flexibility in the diet schedule for the interventional study.
Final diet design: Table 2 shows the final version of the ITIS diet. For lunch and dinner, three recommended options were given, and patients were able to distribute those throughout the week. These options and guidelines for the patient are described in additional file 5. Macro and micro-nutrient composition as well as more details of our ITIS diet is available to interested parties.
Table 2.
Final version of the ITIS diet.
| Monday | Tuesday | Wednesday | Thursday | Friday | Saturday | Sunday | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7–8 am | SMOOTHIE | Coconut milk, mango, papaya, pineapple | Pear, lemon, yogurt, vanilla, water | Grapes, celery, spinach, cucumber, lime, water | Almond or oat milk, spinach, strawberries, pear, ginger, chia seeds, cinnamon | Spinach, ginger, turmeric, papaya, flaxseeds, banana, water | Papaya, spinach, almond milk, turmeric, chia seeds, honey | Parsley, pineapple, strawberries, water | ||
| 7–8 am | BREAKFAST | 1-2 spoonful of oats with non-dairy milk (oat milk, almond milk or rice milk). Add berries (optional). Green tea infusion. |
1-2 corn tortillas, spread with avocado, sesame seeds, and flaxseed oil. Green tea infusion. |
1 -2 corn tortillas with tahini (sesame seed extract) with ¼ teaspoon of honey. Green tea infusion |
1-2 spoons of oat with non-dairy milk (oat, almond or rice milk. Add berries (optional). Green tea infusion. |
1-2 corn tortillas, spread with avocado, sesame seeds, and flaxseed oil. Green tea infusion. |
1 -2 corn tortillas with tahini (sesame seed extract) with ¼ teaspoon of honey. Green tea infusion |
1-2 corn tortillas, spread with avocado, sesame seeds, and linseed oil. Green tea infusion. |
||
| 10–11 a.m. | SNACK | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | Plain yogurt (Chobani Brand, no sugar added) | ||
| 12-1 pm | LUNCH | OPTION 1: Salad (generous plate) OPTION 2: Grains with vegetables OPTION 3: Legumes with vegetables |
||||||||
| 4 p.m. | SNACK | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | Mango, papaya, pineapple, apple, pear or banana +4 walnuts | ||
| 6–7 pm | DINNER | OPTION 1: Vegetable soup/cream + protein OPTION 2: Miso soup + baked/steamed/grilled vegetables + protein OPTION 3: Salad + protein |
||||||||
4. Discussion
Though there is some evidence that several foods might be pro-inflammatory while other foods might have anti-inflammatory properties, there are few interventional studies to show their effect in patients affected with diseases whose main symptoms are pain and swelling such as RA. Although Mediterranean and vegetarian diets are beneficial for those suffering from RA, these diets do not include several ingredients shown to be anti-inflammatory in some randomized clinical trials or animal models of arthritis or in vitro experiments. Given the complexity and severity of RA, it seems doubtful that a single change in diet, by adding or eliminating one ingredient, would be sufficient to diminish systemic inflammation. Some metanalyses of individual ingredients or probiotics in RA reflect contradictory results or mild effects of those supplements [44,45,61,62]. Instead, a diet that combines several of the suggested strategies to decrease inflammation may represent a more realistic dietician approach for those suffering from the disease. Yet, the patients might adhere better to a small change or addition of just supplements than a complete change in their diet. Thus, our goal was to design a diet that introduced patient's feedback to increase their adherence and feasibility for a future interventional trial.
Patient feedback in the different phases helped us adjust the diet based on knowledge and accessibility of ingredients, and to improve the quality of the instructions. In phase I, we learned that the strategies our ITIS diet contemplated did not differ drastically from the current diet habits of our patients. Our patients mentioned that they had adjusted their habits since they had been diagnosed, either after getting information from nutrition websites, or eliminating some foods they thought were triggering their symptoms. We hope that this will increase the patient adherence to the ITIS diet. Of note, one study showed an association between specific foods and symptoms in RA patients, and they reported that blueberries, fish, and spinach improved RA symptoms, while soda containing sugar and desserts aggravated their symptoms [2].
In phase II, we collected patient feedback about the ingredients that were part of the ITIS diet. To increase the feasibility of our diets, the ingredients that were poorly known or too expensive were either removed, made optional, given more information or provided. Interestingly, some studies have suggested that low socio-economic status has been associated with increased RA morbidity, poorer clinical outcomes, decreased functional ability and reduced quality of life [63]. Although people from lower socio-economic are more likely to smoke, which is a known environmental risk factor for RA, they are also more deficient in certain micronutrients. Of interest, a study from Harvard School of Public Health found that eating a healthy diet (rich in fruits, vegetables, fish, and nuts) cost about $1.50 more per day per person than eating an unhealthy diet (the kind full of processed foods and refined grains) [64]. In phase III, we received feedback about our diet instructions and guideline, and we improved the text for clarity and included more recipes. Finally, in phase IV, a 7-day feasibility trial helped us with the last version of our ITIS diet. Overall, patients were highly motivated and open to trying most of the suggested ingredients. Managing chronic diseases in self-motivated patients with diet and lifestyle changes was shown to be critical to achieve treatment goals in other diseases [65].
The strength of this study is the continuous feedback we got from the community we are planning to treat to build our ITIS diet. Although we expected differences between Hispanic and Caucasian patients given their different socioeconomic status, lifestyle, and accessibility to some stores, our perception was that the diet habits of Hispanic and Caucasian patients were very similar. Additionally, both Caucasians and Hispanics had similar knowledge about ingredients included in the diet. Unfortunately, we were unable to conduct statistical analyses given the small number or patients in each phase. Second, we provided groceries. Participants received around $100 in specific ingredients that help their consumption. Finally, we also provided detailed instructions and dietary counseling for the participants at the phase IV which together with groceries being provided increased adherence and positive feedback in this pilot phase. This diet is being used in an on-going pilot study to determine their anti-inflammatory effect in pain and joint swelling in RA patients.
Ethical approval and consent to participate
Ethical approval was granted by the Institutional Review Board (IRB) at University of California at San Diego.
Consent for publication
Not applicable.
Availability of supporting data
Not applicable.
Funding
Funding sources: Research reported in this publication was supported by Krupp Endowed Foundation to MG and by grant from the Spanish Society of Rheumatology to RC. This work was also supported in part by the Erickson Family.
Authors’ contributors
Study conception and design: MFB, MAP, RN, SG, and MG. Patients recruitment: FC, RC and MG. Analysis and interpretation of data. MFB, MAP, RC, SG and FC. MG supervised the overall project.
Authors’ information
MFB, MAP, FC, RC, RN and MG are of the Department of Medicine University of California San Diego, San Diego, CA, USA; RC and MG are also of the Department of Medicine, Autonomous University of Barcelona, Plaça Cívica, Spain; SG is of the Department of Psychiatry University of California San Diego, San Diego, CA, USA.
Declaration of competing interest
The authors report no conflict of interest.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2020.100524.
List of abbreviations
- RA
Rheumatoid arthritis
- SCFA
Short chain fatty acids
- CRP
C reactive protein
- FA
Fatty acids
- PUFA
polyunsaturated FA
- MUFA
monounsaturated FA
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Firestein G.S. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Tedeschi S.K., Frits M., Cui J., Zhang Z.Z., Mahmoud T., Iannaccone C., Lin T.C., Yoshida K., Weinblatt M.E., Shadick N.A., Solomon D.H. Diet and rheumatoid arthritis symptoms: survey results from a rheumatoid arthritis registry. Arthritis Care Res. 2017;69:1920–1925. doi: 10.1002/acr.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derrien M., Veiga P. Rethinking diet to aid human-microbe symbiosis. Trends Microbiol. 2017;25:100–112. doi: 10.1016/j.tim.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 5.Koeth R.A., Wang Z., Levison B.S., Buffa J.A., Org E., Sheehy B.T., Britt E.B., Fu X., Wu Y., Li L. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2 doi: 10.7554/eLife.01202. e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaucher F., Bonnassie S., Rabah H., Marchand P., Blanc P., Jeantet R., Jan G. Review: adaptation of beneficial propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 2019;10:841. doi: 10.3389/fmicb.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roager H.M., Licht T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018;9:3294. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pei R., DiMarco D.M., Putt K.K., Martin D.A., Gu Q., Chitchumroonchokchai C., White H.M., Scarlett C.O., Bruno R.S., Bolling B.W. Low-fat yogurt consumption reduces biomarkers of chronic inflammation and inhibits markers of endotoxin exposure in healthy premenopausal women: a randomised controlled trial. Br. J. Nutr. 2017;118:1043–1051. doi: 10.1017/S0007114517003038. [DOI] [PubMed] [Google Scholar]
- 11.Lyte M., Chapel A., Lyte J.M., Ai Y., Proctor A., Jane J.L., Phillips G.J. Resistant starch alters the microbiota-gut brain Axis: implications for dietary modulation of behavior. PloS One. 2016;11 doi: 10.1371/journal.pone.0146406. e0146406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5:1417–1435. doi: 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach Knudsen K.E. Microbial degradation of whole-grain complex carbohydrates and impact on short-chain fatty acids and health. Adv. Nutr. 2015;6:206–213. doi: 10.3945/an.114.007450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerner A., Matthias T. Rheumatoid arthritis-celiac disease relationship: joints get that gut feeling. Autoimmun. Rev. 2015;14:1038–1047. doi: 10.1016/j.autrev.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Oteng A.B., Kersten S. Mechanisms of action of trans fatty acids. Adv. Nutr. 2019 Nov 29 doi: 10.1093/advances/nmz125. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B., Leung J.C.K., Chan L.Y.Y., Yiu W.H., Tang S.C.W. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2019;77:101020. doi: 10.1016/j.plipres.2019.101020. [DOI] [PubMed] [Google Scholar]
- 17.Bendsen N.T., Stender S., Szecsi P.B., Pedersen S.B., Basu S., Hellgren L.I., Newman J.W., Larsen T.M., Haugaard S.B., Astrup A. Effect of industrially produced trans fat on markers of systemic inflammation: evidence from a randomized trial in women. J. Lipid Res. 2011;52:1821–1828. doi: 10.1194/jlr.M014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demmer E., Van Loan M.D., Rivera N., Rogers T.S., Gertz E.R., German J.B., Zivkovic A.M., Smilowitz J.T. Consumption of a high-fat meal containing cheese compared with a vegan alternative lowers postprandial C-reactive protein in overweight and obese individuals with metabolic abnormalities: a randomised controlled cross-over study. J. Nutr. Sci. 2016;5 doi: 10.1017/jns.2015.40. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samraj A.N., Pearce O.M., Laubli H., Crittenden A.N., Bergfeld A.K., Banda K., Gregg C.J., Bingman A.E., Secrest P., Diaz S.L. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. U. S. A. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ley S.H., Sun Q., Willett W.C., Eliassen A.H., Wu K., Pan A., Grodstein F., Hu F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014;99:352–360. doi: 10.3945/ajcn.113.075663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman M. Potato glycoalkaloids and metabolites: roles in the plant and in the diet. J. Agric. Food Chem. 2006;54:8655–8681. doi: 10.1021/jf061471t. [DOI] [PubMed] [Google Scholar]
- 22.El-Tawil A.M. Prevalence of inflammatory bowel diseases in the Western Nations: high consumption of potatoes may be contributing. Int. J. Colorectal Dis. 2008;23:1017–1018. doi: 10.1007/s00384-008-0480-6. [DOI] [PubMed] [Google Scholar]
- 23.Mensinga T.T., Sips A.J., Rompelberg C.J., van Twillert K., Meulenbelt J., van den Top H.J., van Egmond H.P. Potato glycoalkaloids and adverse effects in humans: an ascending dose study. Regul. Toxicol. Pharmacol. 2005;41:66–72. doi: 10.1016/j.yrtph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Bemeur C., Desjardins P., Butterworth R.F. Antioxidant and anti-inflammatory effects of mild hypothermia in the attenuation of liver injury due to azoxymethane toxicity in the mouse. Metab. Brain Dis. 2010;25:23–29. doi: 10.1007/s11011-010-9186-x. [DOI] [PubMed] [Google Scholar]
- 25.Patel B., Schutte R., Sporns P., Doyle J., Jewel L., Fedorak R.N. Potato glycoalkaloids adversely affect intestinal permeability and aggravate inflammatory bowel disease. Inflamm. Bowel Dis. 2002;8:340–346. doi: 10.1097/00054725-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Iablokov V., Sydora B.C., Foshaug R., Meddings J., Driedger D., Churchill T., Fedorak R.N. Naturally occurring glycoalkaloids in potatoes aggravate intestinal inflammation in two mouse models of inflammatory bowel disease. Dig. Dis. Sci. 2010;55:3078–3085. doi: 10.1007/s10620-010-1158-9. [DOI] [PubMed] [Google Scholar]
- 27.Sears B. Anti-inflammatory diets. J. Am. Coll. Nutr. 2015;34(Suppl 1):14–21. doi: 10.1080/07315724.2015.1080105. [DOI] [PubMed] [Google Scholar]
- 28.Lorente-Cebrian S., Costa A.G., Navas-Carretero S., Zabala M., Laiglesia L.M., Martinez J.A., Moreno-Aliaga M.J. An update on the role of omega-3 fatty acids on inflammatory and degenerative diseases. J. Physiol. Biochem. 2015;71:341–349. doi: 10.1007/s13105-015-0395-y. [DOI] [PubMed] [Google Scholar]
- 29.Berbert A.A., Kondo C.R., Almendra C.L., Matsuo T., Dichi I. Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. 2005;21:131–136. doi: 10.1016/j.nut.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Gioxari A., Kaliora A.C., Marantidou F., Panagiotakos D.P. Intake of omega-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a systematic review and meta-analysis. Nutrition. 2018;45:114–124. doi: 10.1016/j.nut.2017.06.023. e114. [DOI] [PubMed] [Google Scholar]
- 31.Dawczynski C., Dittrich M., Neumann T., Goetze K., Welzel A., Oelzner P., Volker S., Schaible A.M., Troisi F., Thomas L. Docosahexaenoic acid in the treatment of rheumatoid arthritis: a double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018;37:494–504. doi: 10.1016/j.clnu.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Abdulrazaq M., Innes J.K., Calder P.C. Effect of omega-3 polyunsaturated fatty acids on arthritic pain: a systematic review. Nutrition. 2017;39–40:57–66. doi: 10.1016/j.nut.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto Y., Sugioka Y., Tada M., Okano T., Mamoto K., Inui K., Habu D., Koike T. Monounsaturated fatty acids might be key factors in the Mediterranean diet that suppress rheumatoid arthritis disease activity: the TOMORROW study. Clin. Nutr. 2018;37:675–680. doi: 10.1016/j.clnu.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Helli B., Shahi M.M., Mowla K., Jalali M.T., Haghighian H.K. A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytother Res. 2019;33:2421–2428. doi: 10.1002/ptr.6433. [DOI] [PubMed] [Google Scholar]
- 35.Batooei M., Tahamoli-Roudsari A., Basiri Z., Yasrebifar F., Shahdoust M., Eshraghi A., Mehrpooya M., Ataei S. Evaluating the effect of oral N-acetylcysteine as an adjuvant treatment on clinical outcomes of patients with rheumatoid arthritis: a randomized, double blind clinical trial. Rev. Recent Clin. Trials. 2018;13:132–138. doi: 10.2174/1574887113666180307151937. [DOI] [PubMed] [Google Scholar]
- 36.Zhu F., Du B., Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit. Rev. Food Sci. Nutr. 2018;58:1260–1270. doi: 10.1080/10408398.2016.1251390. [DOI] [PubMed] [Google Scholar]
- 37.Javadi F., Ahmadzadeh A., Eghtesadi S., Aryaeian N., Zabihiyeganeh M., Rahimi Foroushani A., Jazayeri S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017;36:9–15. doi: 10.1080/07315724.2016.1140093. [DOI] [PubMed] [Google Scholar]
- 38.Ghavipour M., Sotoudeh G., Tavakoli E., Mowla K., Hasanzadeh J., Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017;71:92–96. doi: 10.1038/ejcn.2016.151. [DOI] [PubMed] [Google Scholar]
- 39.Lo Gullo A., Mandraffino G., Bagnato G., Aragona C.O., Imbalzano E., D'Ascola A., Rotondo F., Cinquegrani A., Mormina E., Saitta C. Vitamin D status in rheumatoid arthritis: inflammation, arterial stiffness and circulating progenitor cell number. PloS One. 2015;10 doi: 10.1371/journal.pone.0134602. e0134602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller S., Marz R., Schmolz M., Drewelow B., Eschmann K., Meiser P. Placebo-controlled randomized clinical trial on the immunomodulating activities of low- and high-dose bromelain after oral administration - new evidence on the antiinflammatory mode of action of bromelain. Phytother Res. 2013;27:199–204. doi: 10.1002/ptr.4678. [DOI] [PubMed] [Google Scholar]
- 41.Hale L.P., Chichlowski M., Trinh C.T., Greer P.K. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm. Bowel Dis. 2010;16:2012–2021. doi: 10.1002/ibd.21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey S., Cabot P.J., Shaw P.N., Hewavitharana A.K. Anti-inflammatory and immunomodulatory properties of Carica papaya. J. Immunot. 2016;13:590–602. doi: 10.3109/1547691X.2016.1149528. [DOI] [PubMed] [Google Scholar]
- 43.Aryaeian N., Shahram F., Mahmoudi M., Tavakoli H., Yousefi B., Arablou T. Jafari Karegar S: the effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene. 2019;698:179–185. doi: 10.1016/j.gene.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 44.Daily J.W., Yang M., Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J. Med. Food. 2016;19:717–729. doi: 10.1089/jmf.2016.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandran B., Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 46.Bang J.S., Oh D.H., Choi H.M., Sur B.J., Lim S.J., Kim J.Y., Yang H.I., Yoo M.C., Hahm D.H., Kim K.S. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res. Ther. 2009;11 doi: 10.1186/ar2662. R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panahi Y., Hosseini M.S., Khalili N., Naimi E., Majeed M., Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: a randomized controlled trial and an updated meta-analysis. Clin. Nutr. 2015;34:1101–1108. doi: 10.1016/j.clnu.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Alghadir A.H., Gabr S.A., Al-Eisa E.S. Green tea and exercise interventions as nondrug remedies in geriatric patients with rheumatoid arthritis. J. Phys. Ther. Sci. 2016;28:2820–2829. doi: 10.1589/jpts.28.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohishi T., Goto S., Monira P., Isemura M., Nakamura Y. Anti-inflammatory action of green tea. Anti-Inflammatory Anti-Allergy Agents Med. Chem. 2016;15:74–90. doi: 10.2174/1871523015666160915154443. [DOI] [PubMed] [Google Scholar]
- 50.Sung S., Kwon D., Um E., Kim B. Could polyphenols help in the control of rheumatoid arthritis? Molecules. 2019:24. doi: 10.3390/molecules24081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salehi-Abargouei A., Saraf-Bank S., Bellissimo N., Azadbakht L. Effects of non-soy legume consumption on C-reactive protein: a systematic review and meta-analysis. Nutrition. 2015;31:631–639. doi: 10.1016/j.nut.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Zamani B., Golkar H.R., Farshbaf S., Emadi-Baygi M., Tajabadi-Ebrahimi M., Jafari P., Akhavan R., Taghizadeh M., Memarzadeh M.R., Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. Int. J. Rheum. Dis. 2016;19:869–879. doi: 10.1111/1756-185X.12888. [DOI] [PubMed] [Google Scholar]
- 53.Alipour B., Homayouni-Rad A., Vaghef-Mehrabany E., Sharif S.K., Vaghef-Mehrabany L., Asghari-Jafarabadi M., Nakhjavani M.R., Mohtadi-Nia J. Effects of Lactobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int. J. Rheum. Dis. 2014;17:519–527. doi: 10.1111/1756-185X.12333. [DOI] [PubMed] [Google Scholar]
- 54.Barebring L., Winkvist A., Gjertsson I., Lindqvist H.M. Poor dietary quality is associated with increased inflammation in Swedish patients with rheumatoid arthritis. Nutrients. 2018:10. doi: 10.3390/nu10101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tedeschi S.K., Bathon J.M., Giles J.T., Lin T.C., Yoshida K., Solomon D.H. Relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res. 2018;70:327–332. doi: 10.1002/acr.23295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khanna S., Jaiswal K.S., Gupta B. Managing rheumatoid arthritis with dietary interventions. Front. Nutr. 2017;4:52. doi: 10.3389/fnut.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grygielska J., Raciborski F., Klak A., Owoc J. The impact of nutrition and generally available products such as nicotine and alcohol on rheumatoid arthritis - review of the literature. Reumatologia. 2018;56:121–127. doi: 10.5114/reum.2018.75524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kjeldsen-Kragh J., Haugen M., Borchgrevink C.F., Laerum E., Eek M., Mowinkel P., Hovi K., Forre O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet. 1991;338:899–902. doi: 10.1016/0140-6736(91)91770-u. [DOI] [PubMed] [Google Scholar]
- 59.Skoldstam L., Hagfors L., Johansson G. An experimental study of a Mediterranean diet intervention for patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003;62:208–214. doi: 10.1136/ard.62.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner K.H., Elmadfa I. Chemical and biological modulations of food due to the frying process. Int. J. Vitam. Nutr. Res. 2012;82:163–167. doi: 10.1024/0300-9831/a000107. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y., Alookaran J.J., Rhoads J.M. Probiotics in autoimmune and inflammatory disorders. Nutrients. 2018:10. doi: 10.3390/nu10101537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammed A.T., Khattab M., Ahmed A.M., Turk T., Sakr N., Mk A., Abdelhalim M., Sawaf B., Hirayama K., Huy N.T. The therapeutic effect of probiotics on rheumatoid arthritis: a systematic review and meta-analysis of randomized control trials. Clin. Rheumatol. 2017;36:2697–2707. doi: 10.1007/s10067-017-3814-3. [DOI] [PubMed] [Google Scholar]
- 63.Verstappen S.M.M. The impact of socio-economic status in rheumatoid arthritis. Rheumatology. 2017;56:1051–1052. doi: 10.1093/rheumatology/kew428. [DOI] [PubMed] [Google Scholar]
- 64.Rao M., Afshin A., Singh G., Mozaffarian D. Do healthier foods and diet patterns cost more than less healthy options? A systematic review and meta-analysis. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-004277. e004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nadeau D.A. Management of type 2 diabetes mellitus in self-motivated patients: optimized diet, exercise, and medication for weight loss and cardiometabolic fitness. Physician Sportsmed. 2014;42:49–59. doi: 10.3810/psm.2014.11.2091. [DOI] [PubMed] [Google Scholar]
- 66.Boudry G., Hamilton M.K., Chichlowski M., Wickramasinghe S., Barile D., Kalanetra K.M., Mills D.A., Raybould H.E. Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J. Dairy Sci. 2017;100:2471–2481. doi: 10.3168/jds.2016-11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salgado E., Bes-Rastrollo M., de Irala J., Carmona L., Gomez-Reino J.J. High sodium intake is associated with self-reported rheumatoid arthritis: a cross sectional and case control analysis within the SUN cohort. Medicine (Baltim.) 2015;94 doi: 10.1097/MD.0000000000000924. e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharif K., Amital H., Shoenfeld Y. The role of dietary sodium in autoimmune diseases: the salty truth. Autoimmun. Rev. 2018;17:1069–1073. doi: 10.1016/j.autrev.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 69.Vanegas S.M., Meydani M., Barnett J.B., Goldin B., Kane A., Rasmussen H., Brown C., Vangay P., Knights D., Jonnalagadda S. Substituting whole grains for refined grains in a 6-wk randomized trial has a modest effect on gut microbiota and immune and inflammatory markers of healthy adults. Am. J. Clin. Nutr. 2017;105:635–650. doi: 10.3945/ajcn.116.146928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roager H.M., Vogt J.K., Kristensen M., Hansen L.B.S., Ibrugger S., Maerkedahl R.B., Bahl M.I., Lind M.V., Nielsen R.L., Frokiaer H. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: a randomised cross-over trial. Gut. 2019;68:83–93. doi: 10.1136/gutjnl-2017-314786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tilg H., Zmora N., Adolph T.E., Elinav E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020 Jan;20(1):40–54. doi: 10.1038/s41577-019-0198-4. [DOI] [PubMed] [Google Scholar]
- 72.Sharif K., Watad A., Bragazzi N.L., Adawi M., Amital H., Shoenfeld Y. Coffee and autoimmunity: more than a mere hot beverage! Autoimmun. Rev. 2017;16:712–721. doi: 10.1016/j.autrev.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Bae S.C., Lee Y.H. Coffee consumption and the risk of rheumatoid arthritis and systemic lupus erythematosus: a Mendelian randomization study. Clin. Rheumatol. 2018;37:2875–2879. doi: 10.1007/s10067-018-4278-9. [DOI] [PubMed] [Google Scholar]
- 74.Heliovaara M., Aho K., Knekt P., Impivaara O., Reunanen A., Aromaa A. Coffee consumption, rheumatoid factor, and the risk of rheumatoid arthritis. Ann. Rheum. Dis. 2000;59:631–635. doi: 10.1136/ard.59.8.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.