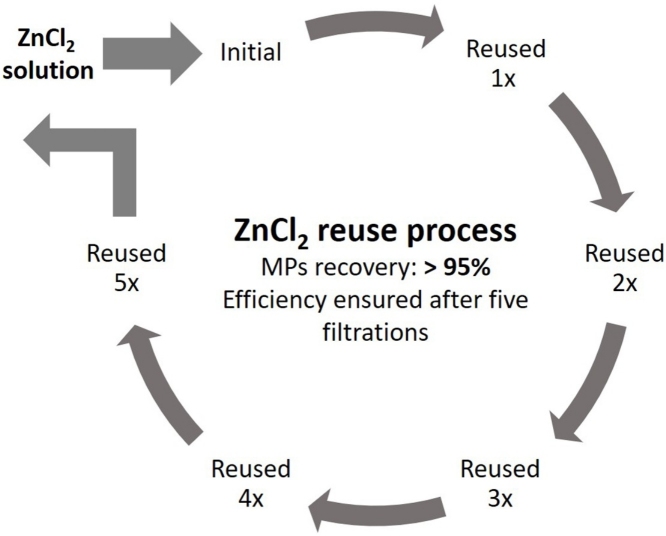
Graphical abstract
Method name: Microplastics isolation using reused zinc chloride solution
Keywords: Microplastics, Separation methodology, Zinc chloride, Aquatic systems, Reuse process
Abstract
The methodology used to extract and quantify microplastics (MPs) in aquatic systems are still not standardized. Salt saturated solutions, such as sodium chloride (NaCl), zinc chloride (ZnCl2) and/or sodium iodide (NaI), are normally added to separate dense plastics from aquatic samples. However, the most effective reagents are also the most expensive (e.g. ZnCl2 and NaI). To decrease this cost, a reuse process of the salt solutions should be applied. The reuse process has been widely investigated for the NaI solution neglecting the ZnCl2. Hence, the aim of this study was to present a simple methodology to reuse the ZnCl2 solution ensuring the efficiency of the product. Results of the present study showed that ZnCl2 solution could be reused at least five times maintaining an efficiency above 95 %.
-
•
The ZnCl2 reuse decreases the cost of the methodology.
-
•
The efficiency of ZnCl2 solution after five filtrations remains above 95 % (all polymers are detected and recovered).
-
•
The use of this salt solution is the most cost-effective methodology to isolate MPs from aquatic samples.
Specification Table
| Subject Area: | Environmental Science |
| More specific subject area: | Aquatic science |
| Method name: | Microplastics isolation using reused zinc chloride solution |
| Name and reference of original method: |
Density separation: J. Masura, J. Baker, G. Foster, C. Arthur, Laboratory methods for the analysis of microplastics in the marine environment: Recommendations for quantifying synthetic particles in waters and sediments., 2015. M. Zobkov, E. Esiukova, Microplastics in Baltic bottom sediments: Quantification procedures and first results, Mar. Pollut. Bull. 114 (2017) 724–732. doi:10.1016/j.marpolbul.2016.10.060. M.O. Rodrigues, A.M.M. Gonçalves, F.J.M. Gonçalves, H. Nogueira, J.C. Marques, N. Abrantes, Effectiveness of a methodology of microplastics isolation for environmental monitoring in freshwater systems, Ecol. Indic. 89 (2018) 488–495. doi:10.1016/j.ecolind.2018.02.038. M. Zobkov, E. Esiukova, Microplastics in Baltic bottom sediments: Quantification procedures and first results, Mar. Pollut. Bull. 114 (2017) 724–732. doi:10.1016/j.marpolbul.2016.10.060. Vacuum filtration: V. Hidalgo-ruz, L. Gutow, R.C. Thompson, M. Thiel, Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification, Environ. Sci. Technol. 46 (2012) 3060–3075. doi:dx.doi.org/10.1021/es2031505. S. Mintenig, Planktonic Microplastic in the North Sea - A new extraction method for the detection by Fourier Transform Infrared Spectroscopy (FTIR), Carl von Ossietzky Universität Oldenburg, Oldenburg, Germany, 2014. W. Urgert, Microplastics in the rivers Meuse and Rhine. Master thesis., Open University of the Netherlands, Heerlen, Netherlands, 2015. M.O. Rodrigues, A.M.M. Gonçalves, F.J.M. Gonçalves, H. Nogueira, J.C. Marques, N. Abrantes, Effectiveness of a methodology of microplastics isolation for environmental monitoring in freshwater systems, Ecol. Indic. 89 (2018) 488–495. doi:10.1016/j.ecolind.2018.02.038. |
| Resource availability | Material:
|
Method details
Background
Microplastics (particles with a diameter of 1–5000 μm [1]; MPs) are emerging aquatic contaminants since they are persistent, can reach high densities and interact with abiotic and biotic environments. However, there is still not a standardized method for isolating MPs in aquatic systems which could result in inconsistent data not allowing spatial and temporal comparison between studies [2,3]. The choice for a simple, low-cost and accurate methodology is one of the main challenges currently. One of the most adopted methodologies is the density separation with a high concentrated or saturated salt solution (e.g. sodium chloride, zinc chloride and/or sodium iodide) [[3], [4], [5], [6], [7], [8], [9], [10]]. These salts are normally mixed with the samples and shaken [3], notwithstanding they are not equally effective. Zinc chloride (ZnCl2) is the most effective (density of 1.6–1.8 g/cm3) and least expensive method (when compared with sodium iodide; NaI) [5], however the substance is very hazardous, corrosive [9] and requires large amounts of product [7]. Hence, careful handling, disposal and reuse is necessary to guarantee the most efficient and safe use of the product. The reuse process has been widely investigated for the NaI solution [11], while for ZnCl2 there is lack of information. Hence, the aim of this study was to present a methodology for MPs isolation using reused ZnCl2 solution. The methodology proposed focused on the density separation methodology, vacuum filtration and visual inspection, based on [7].
Sample processing
This methodology could be used for MPs isolation (particles with size between 1 and 5000 μm).
Density separation
-
1
Assembly of the density separator.
-
2
Add 50 mL of ZnCl2 solution (concentration = 700 g/L) to the dried sample. Note: Stir the ZnCl2 solution during at least 24 h before adding to the sample.
-
3
Place the sample in the density separator.
-
4
Rinse the glass bottle with ZnCl2 solution to transfer all remaining solids to the density separator.
-
5
Cover loosely with aluminium foil.
-
6
Allow solids to settle 1 h at room temperature.
-
7
Collect floating MPs into a flask with forceps and a glass Pasteur pipette.
-
8
Remove the Mohr’s pinch clamp. Drain settled solids (if existing) and ZnCl2 solution into a flask.
-
9
Rinse the density separator several times with a squirt bottle filled with distilled water to transfer all solids to the flask containing recovered MPs.
-
10
Stir the flask with MPs and the flask with ZnCl2 for 10/15 min.
Vacuum filtration
-
11
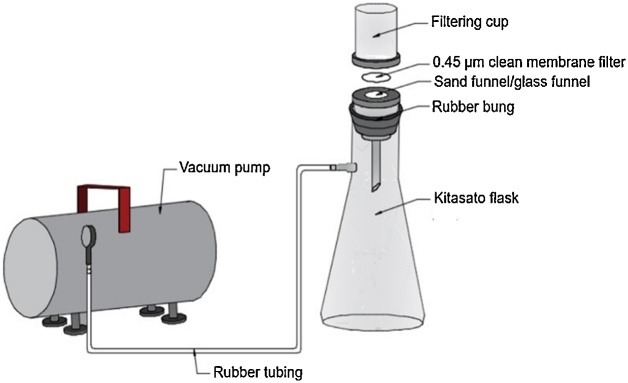
Filter the ZnCl2 solution and settled solids (if existing) through a 0.45 μm clean membrane filter using a sand funnel connected to a vacuum system (see Fig. 1). Note: Do not rinse with distilled water, it can cause precipitation of ZnCl2.
-
12
Store the reused solution in a glass bottle at 4 °C.
-
13
Filter the plastic samples through a 0.45 μm clean membrane filter using the same filtration process (see Fig. 1).
-
14
Rinse the filtration setup with a squirt bottle filled with distilled water several times to ensure that no MPs were lost.
-
15
Once filtration was complete, carefully remove the membranes and put them in Petri dishes.
-
16
Place the Petri dishes in 40 °C drying oven for 3–5 days.
Visual inspection
-
17
Stereomicroscope Optika using 1.5× magnification: select the MPs.
-
18
Count and/or weigh the MPs (using an analytical laboratory balance).
Identification
-
19
Fourier-transform infrared spectroscopy (FTIR).
Fig. 1.
Vacuum system (figure copyright: Mariana O. Rodrigues).
Contamination mitigation
To prevent MPs contamination during isolation procedure:
-
•
Wear lab coats (preferably 100 % cotton).
-
•
Change gloves between steps.
-
•
Use glass vials during all steps of sample treatment. However, if plastic ware was used instead, cover it with aluminium foil.
-
•
Clean every material and cover on top immediately (with cap or aluminium foil) after washing and after each step allowing the amount of time that a sample is exposed to air is very limited.
-
•
Clean the workplace before and during each procedure.
-
•
Since all polymers were characterized in the beginning of the experiment, any others, especially fibers, are discarded and not accounted for the final number of recovered MPs.
To reuse the ZnCl2 solution, repeat the methodology described above (density separation, vacuum filtration and visual inspection) using the reused solution (solution obtained in step 12) in the second step of the density separation methodology. It is not necessary to stir again the solution.
Methods validation
MPs and sample preparation
Three different plastic products (i.e. water bottles, pipe and fabric) widely used in everyday life were cut and fragmented using a coffee grinder. These particles were sieved in a 5000 μm mesh and material sized >5000 μm was discarded (also named MPs). Based on previous FTIR analysis, each plastic product was characterized by its polymer type [7]. Table 1 summarizes both chemical and morphological characterization of plastic products (see Table 1).
Table 1.
Morphological and chemical characterization of the 3 types of plastic products used in this experiment.
| Source | Polymer type | Size (μm) | Shape | Colour | Density (g/cm3) |
|---|---|---|---|---|---|
| Water bottles | Polyethylene terephthalate (PET) | Fragment | Blue | 1.37–1.45 | |
| Fabric | <5000 | Fiber | Blue | ||
| Pipe | Polyvinyl chloride (PVC) | Fragment | Grey | 1.16–1.58 |
These three plastic products included some of the most common and dense types of polymers [12] such as polyvinyl chloride (PVC) and polyethylene terephthalate (PET). Five particles of each type of plastic were added per sample. For each sample three replicates were prepared.
Experimental design
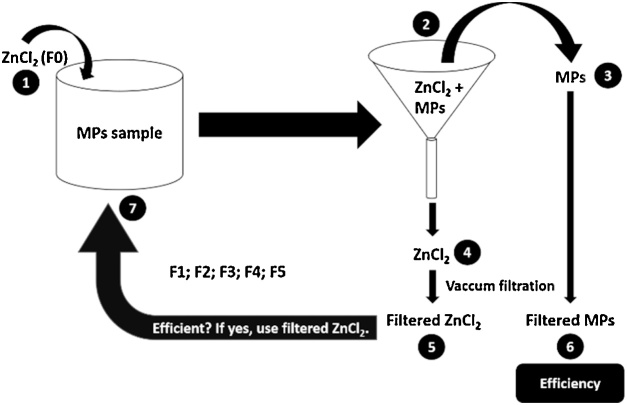
Following the methodology described above, samples were subjected to a density separation methodology with ZnCl2, vacuum filtration and stereomicroscope inspection (see Fig. 2).
Fig. 2.
Experimental setup for the ZnCl2 reuse, based on a density separation methodology: Step 1 – Addition of the ZnCl2 solution to the sample; Step 2 – Density separation; Step 3 – Recovering of MPs; Step 4 – Recovering of ZnCl2 solution; Step 5 – Filtered ZnCl2 (after vacuum filtration); Step 6 – MPs recovered (after vacuum filtration and stereomicroscope inspection); Step 7 – Reuse of the filtered solution in a new sample (after calculation of the solution efficiency). Solutions were reused to a maximum of five times (F1 to F5): F0 = Initial ZnCl2 solution (concentration = 700 g/L); F1 = ZnCl2 solution after being reused once; F2 = ZnCl2 solution after being reused twice; F3 = ZnCl2 solution after being reused three times; F4 = ZnCl2 solution after being reused four times; F5 = ZnCl2 solution after being reused five times (figure copyright: Mariana O. Rodrigues).
After vacuum filtration (step 5), ZnCl2 was available to be reused. The reuse process was repeated five times, membranes (steps 5 and 6) were recovered and inspected for MPs presence. The MPs recovered were counted for further calculation of the efficiency of initial and filtered/reused solutions (%).
.
Since normality test failed, One-way ANOVA on Ranks was used to assess the significant diff ;erences between the efficiency of solutions. The significance level of all statistical analyses was set at 0.05.
Results
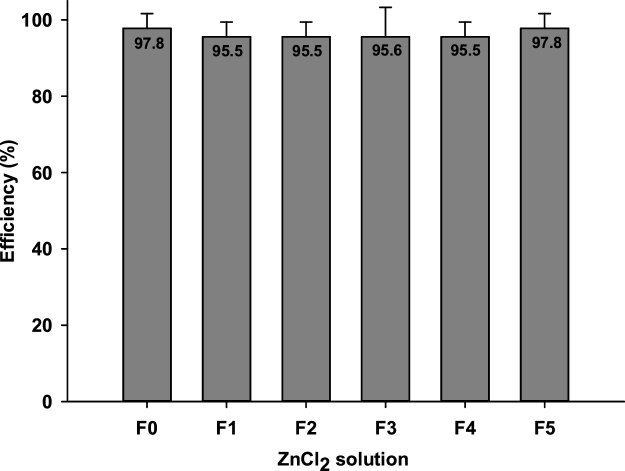
-
•
The efficiency of the density separation was not affected by the reuse process (H= 1.289; DF = 5; p value = 0.936; see Fig. 3).
-
•
All types of polymers and shapes (PVC and PET – fragments and fibers) were recovered from the top of the funnel.
-
•
Fibers (from fabric – PET) tend to deagglomerate making difficult their recovery.
-
•
Pipe (PVC) and water bottle (PET) particles were fully recovered in all treatments.
Fig. 3.
Density separation efficiency (based on the number of particles recovered) of the initial and reused ZnCl2 solutions (F0 = Initial ZnCl2 solution (concentration = 700 g/L); F1 = ZnCl2 solution after being reused once; F2 = ZnCl2 solution after being reused twice; F3 = ZnCl2 solution after being reused three times; F4 = ZnCl2 solution after being reused four times; F5 = ZnCl2 solution after being reused five times). Each bar has an indication, at the top, of the efficiency (%) of the initial and reused ZnCl2 solutions.
Hence, results pointed-out that ZnCl2 solution could be reused at least five times with an efficiency above 95 %. Moreover, it is important to note that although this work was done with pristine MPs, there is no scientific evidence that aging or complexation processes change their density and therefore this process can be used for all MPs particles as long as they have a density lower than 1.6–1.8 g/cm3.
Disadvantage of the reuse process:
-
•
During recovering of MPs, the volume of ZnCl2 solution decreased in approximately 10 %.
CRediT authorship contribution statement
M.O. Rodrigues: Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Funding acquisition. A.M.M. Gonçalves: Conceptualization, Methodology, Validation, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. F.J.M. Gonçalves: Methodology, Validation, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. N. Abrantes: Methodology, Validation, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by Fundação para a Ciência e a Tecnologia (FCT/MCTES) through national funds UID/AMB/50017/2019 granted to Centro de Estudos do Ambiente e do Mar (CESAM) and UID/MAR/04292/2019 granted to Marine and Environmental Sciences Centre (MARE), and through the Regional Operational Programme CENTRO2020 within the scope of the project CENTRO-01-0145FEDER-000006. A.M.M. Gonçalves acknowledges FCT for the financial support provided through the post-doctoral grant (SFRH/BPD/ 97210/2013) co-funded by the Human Potential Operational Programme (National Strategic Reference Framework 2007–2013), European Social Fund (EU), and the program POPH/FSE, and University of Coimbra for the contract (IT057-18-7253). N. Abrantes is recipient of an FCT researcher contract (IF/01198/2014) and M.O. Rodrigues is recipient of a research grant from FCT (SFRH/BD/136931/2018) funded by National Funds and Community Funds through FSE.
References
- 1.Hidalgo-ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/es2031505. [DOI] [PubMed] [Google Scholar]
- 2.Duis K., Coors A. Microplastics in the aquatic and terrestrial environment: sources (with a specific focus on personal care products), fate and effects. Environ. Sci. Eur. 2016;28:2. doi: 10.1186/s12302-015-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Löder M.G.J., Gerdts G. Methodology used for the detection and identification of microplastics — a critical appraisal. In: Bergmann M., M G., L K., editors. Mar. Anthropog. Litter. Springer International Publishing; Cham: 2015. pp. 201–227. [Google Scholar]
- 4.Nuelle M.-T., Dekiff J.H., Remy D., Fries E. A new analytical approach for monitoring microplastics in marine sediments. Environ. Pollut. 2014;184:161–169. doi: 10.1016/j.envpol.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Coppock R.L., Cole M., Lindeque P.K., Queirós A.M., Galloway T.S. A small-scale, portable method for extracting microplastics from marine sediments. Environ. Pollut. 2017;230:829–837. doi: 10.1016/j.envpol.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Claessens M., Van Cauwenberghe L., Vandegehuchte M.B., Janssen C.R. New techniques for the detection of microplastics in sediments and field collected organisms. Mar. Pollut. Bull. 2013;70:227–233. doi: 10.1016/j.marpolbul.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues M.O., Gonçalves A.M.M., Gonçalves F.J.M., Nogueira H., Marques J.C., Abrantes N. Effectiveness of a methodology of microplastics isolation for environmental monitoring in freshwater systems. Ecol. Indic. 2018;89:488–495. [Google Scholar]
- 8.Prata J.C., da Costa J.P., Duarte A.C., Rocha-Santos T. Methods for sampling and detection of microplastics in water and sediment: a critical review. TrAC Trends Anal. Chem. 2019;110:150–159. [Google Scholar]
- 9.Frias J., Pagter E., Nash R., O’Connor I., Carretero O., Filgueiras A., Viñas L., Gago J., Antunes J., Bessa F., Sobral P., Goruppi A., Tirelli V., Pedrotti M.L., Suraia G., Aliani S., Lopes C., Raimundo J., Caetano M., Palazzo L., de Lucia G.A., Camedda A., Muniategui S., Grueiro G., Fernandez V., Andrade J., Dris R., Laforsch C., Scholz-Böttcher B.M., Gerdts G. Standardised protocol for monitoring microplastics in sediments. JPI-Oceans BASEMAN Proj. 2018 [Google Scholar]
- 10.Zobkov M., Esiukova E. Microplastics in Baltic bottom sediments: quantification procedures and first results. Mar. Pollut. Bull. 2017;114:724–732. doi: 10.1016/j.marpolbul.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 11.Kedzierski M., Le Tilly V., César G., Sire O., Bruzaud S. Efficient microplastics extraction from sand. A cost effective methodology based on sodium iodide recycling. Mar. Pollut. Bull. 2017;115:120–129. doi: 10.1016/j.marpolbul.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 12.PlasticsEurope . 2018. Plastics – the Facts 2018: An Analysis of European Plastics Production, Demand and Waste Data; p. 38. [Google Scholar]