Clostridioides difficile is an anaerobic bacterium that causes gastrointestinal disease. C. difficile forms dormant spores which can survive harsh environmental conditions, allowing their spread to new hosts. In this study, we determine how intestinally relevant pH conditions impact C. difficile physiology in the two divergent strains, 630Δerm and R20291. Our data demonstrate that low pH conditions reduce C. difficile growth, sporulation, and motility. However, toxin production and spore morphology were differentially impacted in the two strains at low pH. In addition, we observed that alkaline environments reduce C. difficile growth, but increase cell motility. When pH was adjusted rapidly during growth, we observed similar impacts on both strains. This study provides new insights into the phenotypic diversity of C. difficile grown under diverse pH conditions present in the intestinal tract, and demonstrates similarities and differences in the pH responses of different C. difficile isolates.
KEYWORDS: Clostridioides difficile, growth, motility, pH, sporulation, toxins
ABSTRACT
Clostridioides difficile is a pathogenic bacterium that infects the human colon to cause diarrheal disease. Growth of the bacterium is known to be dependent on certain bile acids, oxygen levels, and nutrient availability in the intestine, but how the environmental pH can influence C. difficile is mostly unknown. Previous studies indicated that C. difficile modulates the intestinal pH, and prospective cohort studies have found a strong association between a more alkaline fecal pH and C. difficile infection. Based on these data, we hypothesized that C. difficile physiology can be affected by various pH conditions. In this study, we investigated the impact of a range of pH conditions on C. difficile to assess potential effects on growth, sporulation, motility, and toxin production in the strains 630Δerm and R20291. We observed pH-dependent differences in sporulation rate, spore morphology, and viability. Sporulation frequency was lowest under acidic conditions, and differences in cell morphology were apparent at low pH. In alkaline environments, C. difficile sporulation was greater for strain 630Δerm, whereas R20291 produced relatively high levels of spores in a broad range of pH conditions. Rapid changes in pH during exponential growth impacted sporulation similarly among the strains. Furthermore, we observed an increase in C. difficile motility with increases in pH, and strain-dependent differences in toxin production under acidic conditions. The data demonstrate that pH is an important parameter that affects C. difficile physiology and may reveal relevant insights into the growth and dissemination of this pathogen.
IMPORTANCE Clostridioides difficile is an anaerobic bacterium that causes gastrointestinal disease. C. difficile forms dormant spores which can survive harsh environmental conditions, allowing their spread to new hosts. In this study, we determine how intestinally relevant pH conditions impact C. difficile physiology in the two divergent strains, 630Δerm and R20291. Our data demonstrate that low pH conditions reduce C. difficile growth, sporulation, and motility. However, toxin production and spore morphology were differentially impacted in the two strains at low pH. In addition, we observed that alkaline environments reduce C. difficile growth, but increase cell motility. When pH was adjusted rapidly during growth, we observed similar impacts on both strains. This study provides new insights into the phenotypic diversity of C. difficile grown under diverse pH conditions present in the intestinal tract, and demonstrates similarities and differences in the pH responses of different C. difficile isolates.
INTRODUCTION
Clostridioides difficile is an emerging gastrointestinal pathogen which often infects patients who have recently received antibiotics. Upon ingestion, the dormant spores survive the acidic pH of the stomach and enter the small intestine, where primary bile acids induce the germination of spores and enable subsequent growth of the bacterium (1–4). So far, several factors in the gastrointestinal tract are known to impact C. difficile growth during infection, including secondary bile acids, short-chain fatty acids (SCFA) produced by competing microbiota, host diet, host defense factors, the abundance of oxygen levels, and zinc, as well as iron and nutrient limitations (5–13). Another important factor in the gastrointestinal (GI) tract is the environmental pH, the effects of which are not well characterized for C. difficile. In the GI tract, the pH ranges from as low as 5.2 to as high as 7.88, depending on the region, and can be influenced by diet, transit time, health state, established microbiome, and the intake of drugs (14–21). Furthermore, C. difficile can modulate its own environment by targeting the sodium-proton exchanger 3 (NHE3) in epithelial cells, which usually absorbs nutrients in the colon lumen by creating an H+ gradient (22). The loss of function of NHE3 by toxin B of C. difficile caused an altered intestinal environment with an increase in the luminal and fecal pH (23). A further cohort study reported a strong association between a more alkaline fecal pH and C. difficile infection (CDI), suggesting that higher pH in the GI tract may influence disease symptoms (24).
Based on these prior studies that observed C. difficile-directed pH changes in the intestine, we sought to investigate how the environmental pH impacts C. difficile physiology. To this end, we assessed the growth, sporulation efficiency, cell morphology, toxin production, motility, and pH alteration in vitro for the historical isolate 630Δerm and the epidemic strain R20291. The effects of pH on growth and the ability to respond to rapid pH changes suggested a conserved mechanism for pH adaptation. However, these analyses revealed differences in the pH adaption of strains for sporulation, motility, and toxin production, which may explain differences in pathogenesis between isolates.
RESULTS
pH impacts C. difficile growth and spore formation differentially in 630Δerm and R20291.
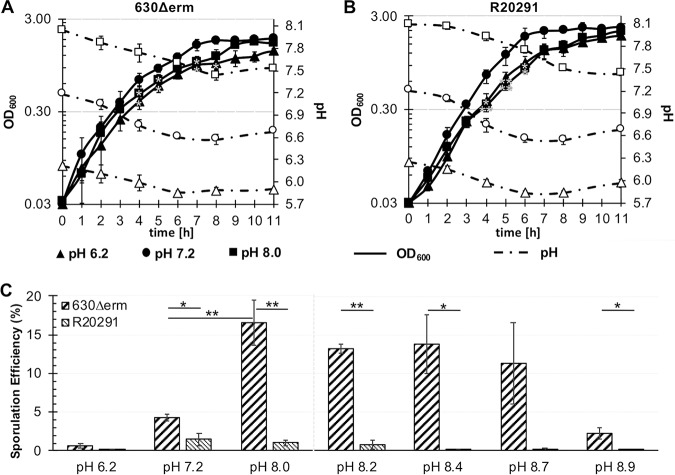
To assess the impact of the environmental pH on C. difficile, we examined the genetically distinct strains 630Δerm and R20291 in 70:30 sporulation broth in a range of pH that is physiologically relevant to the large intestine. Cultures were monitored for effects on growth, change in pH over time, and sporulation in medium at pH 6.2, 7.2, and 8.0, respectively (Fig. 1). For both strains, significant decreases in growth were observed during mid-logarithmic growth at the acidic pH 6.2 and at the alkaline pH 8.0, relative to pH 7.2 (Fig. 1A and B). Analyzing the change in pH of the cultures, the largest drop in pH could be seen for pH 8.0 and pH 7.2 cultures, which decreased from pH 8.0 to 7.4, and from pH 7.2 to 6.5, within 8 h for both strains. For pH 6.2 cultures, similar decreases were observed for both strains during growth, with decreases from pH 6.2 to 5.8 within 6 h, respectively (Fig. 1A and B). In addition, in the pH 7.2 and 6.2 cultures, the pH increased after ∼6 h, around the transition to stationary-phase growth.
FIG 1.
Growth and sporulation of C. difficile is impacted by pH in liquid sporulation medium. Strains 630Δerm (A) and R20291 (B) were cultivated in 70:30 broth at a pH of 6.2, pH 7.2, or pH 8.0 (OD600, solid lines with filled symbols; pH, dashed lines with open symbols). (C) Analysis of the sporulation efficiency of strains grown in pH 6.2, 7.2, and pH 8.0, or in high alkaline pH cultures of 8.2, 8.4, 8.7, and pH 8.9. Data were analyzed by Student’s two-tailed t test comparing strains 630Δerm and R20291, and by one-way ANOVA with Dunnett’s test for multiple comparisons to pH 7.2. *, P value of ≤ 0.05; **, adjusted P value of ≤ 0.01; n = 4.
Analysis of the spore formation under the different pH conditions uncovered strain-dependent differences in sporulation efficiency (Fig. 1C). The 630Δerm strain produced more spores in sporulation broth than the R20291 strain under every pH condition tested. Additionally, 630Δerm demonstrated dramatic increases in sporulation frequency as the pH increased (pH 8.0, ∼17% versus ∼4% at pH 7.2 and less than 1% at pH 6.2) (Fig. 1C). In comparison, R20291 demonstrated relatively low sporulation under all pH conditions (Fig. 1C). Both strains exhibited very low spore formation at pH 6.2, suggesting that acidic conditions do not support sporulation in C. difficile (∼0.7% for 630Δerm and ∼0.1% for R20291).
Based on the observed correlation between spore formation and higher pH, we further assessed growth and sporulation under more alkaline pHs (pH 8.0 to 8.9). Growth was similarly impacted for both strains in high alkaline pH (Fig. S1A and B in the supplemental material). R20291 sporulated at less than 1% efficiency under alkaline conditions, with very few spores formed above pH 8.2 (Fig. 1C). In comparison, 630Δerm maintained robust spore formation up to pH 8.7 and only showed reduced sporulation, down to ∼2%, at a pH of 8.9 (Fig. 1C).
To investigate whether impacts on sporulation were due to the initial pH of the medium versus bacterial-dependent pH changes, we limited the change in pH by buffering the culture medium. Buffers appropriate for the respective pH conditions were utilized and sporulation assessed as described in the Materials and Methods (0.1 M morpholineethanesulfonic acid [MES] at a pH of 6.2, or 0.1 M HEPES at pH 7.2 and pH 8.0). As expected, buffering the medium limited the pH shift of cultures over time; however, it did not alter the growth or sporulation of either strain (Fig. S2). These data suggest that although growth of the strains appears similar in different pH conditions, the effects of pH on sporulation are considerably greater for the 630Δerm strain than for R20291.
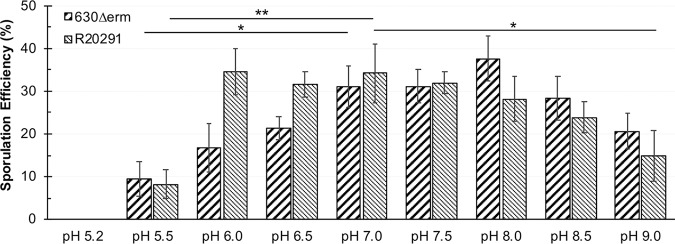
Because of the observed pH effects on C. difficile growth and sporulation in liquid cultures, we asked how pH impacts sporulation on solid medium, which is known to support more robust spore formation (25–28). To test this, sporulation agar plates were prepared with a range of pH (pH 5.2 to 9.0), and the spore formation for 630Δerm and R20291 were determined. Both strains produced considerably more spores on solid medium than were observed in liquid (Fig. 1 and Fig. 2). Consistent with results from broth cultures, the strains showed differences in spore production under different pH conditions. At a low pH of 5.2, both strains were inhibited for growth (Fig. 2). Growth of both strains was observed at a pH of 5.5; however, this low pH resulted in a relatively low sporulation efficiency of 8 to 9% for both strains, compared to the 31 to 34% sporulation observed under neutral pH 7.0 (Fig. 2). The strain 630Δerm exhibited increased sporulation with increases in pH, reaching a maximal sporulation efficiency at pH 8.0 of ∼38% (Fig. 2). In comparison, R20291 demonstrated maximal sporulation efficiency at a pH of 6.0 and exhibited a broader pH range for robust spore formation (pH 6.0 to pH 8.5) (Fig. 2). Beyond pH 8.0, both strains exhibited a stepwise reduction in spore formation. Overall, the data suggest that in liquid or solid medium, 630Δerm sporulated best in more alkaline pH, whereas R20291 sporulated robustly across a broad range of pH conditions.
FIG 2.
Sporulation of C. difficile on solid medium is influenced by pH. Strains 630Δerm and R20291 were analyzed for sporulation on 70:30 plates at the indicated pH. No growth or sporulation was detected at pH 5.2. Data were analyzed by one-way ANOVA with Dunnett’s test for multiple comparisons to pH 7.0, as indicated. *, P value of ≤ 0.05; **, P value of ≤ 0.01; n = 4.
To assess the effects of pH on vegetative cell and spore morphology, phase-contrast microscopy was performed for strains grown in different pH conditions (Fig. 3). Both strains exhibited slightly elongated vegetative cells and the lowest phase-bright spore formation under low pH conditions. Additionally, R20291 formed small, round, phase-dark spores and exhibited more pronounced lysis of cells at pH 6.2 in liquid, compared to the other pH conditions. The spores of R20291 produced at pH 6.2 remained small, round, and phase-dark at 24 to 120 h, and were difficult to purify from the vegetative cells and cell debris in the culture (not shown). To determine the germination and resistance of these spores, 24-h cultures of R20291 and 630Δerm at pH 6.2 and pH 7.2 (control) were removed from the anaerobic cabinet and exposed to oxygen for >10 days to kill the remaining vegetative cells (Fig. S3). Afterward, samples were tested for germination and ethanol resistance (29) (Fig. S3). Most of the small, round spores of R20291 generated at pH 6.2 germinated and were ethanol-resistant (Fig. S3). However, R20291 did not produce these small spores on solid media at low pH, suggesting that growth in liquid or on solid agar medium plays a role in spore development. The 630Δerm strain produced the most abundant phase-bright spores at pH 8.0 for both liquid and solid media, consistent with the results of ethanol-resistance spore assays (Fig. 1 and Fig. 2).
FIG 3.
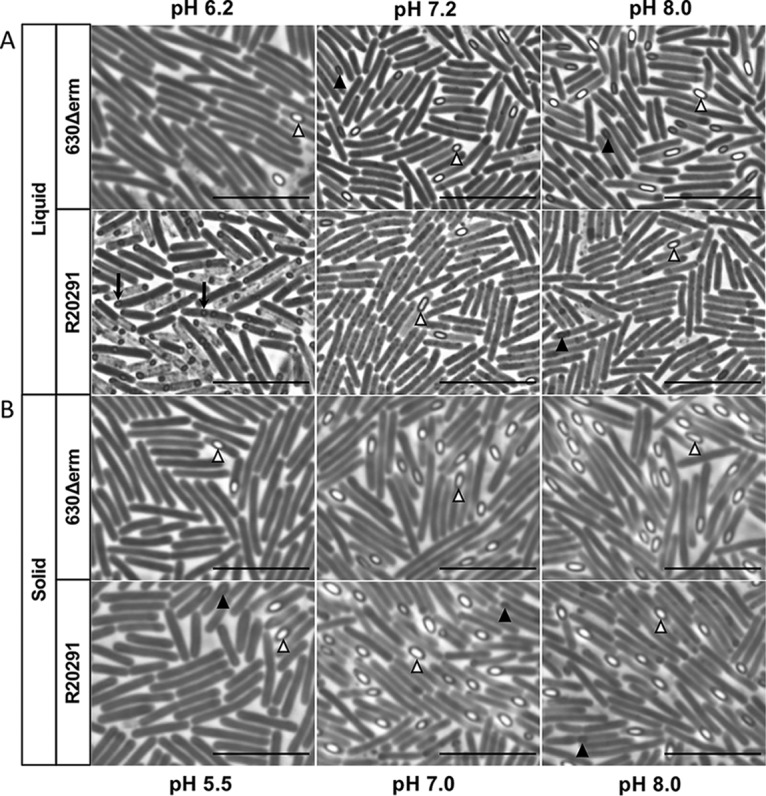
Phase-contrast microscopy of C. difficile strains grown under diverse pH conditions reveals differences in spore morphology and abundance. In liquid culture (A) and on solid medium (B) after 24 h of growth, 630Δerm and R20291 were analyzed for cell morphology. Filled arrowheads (▲), phase-dark prespores; open arrowheads (Δ), phase-bright mature spores; arrow (↓), small, round spores. Bars = 10 μm.
Rapid changes in pH impact growth and sporulation in liquid or solid growth medium.
As the pH varies greatly throughout the large intestine, C. difficile is exposed to abrupt shifts in pH during transit through the colon. Considering this, we investigated the impact of rapid changes on growth and spore formation. C. difficile was cultivated at a moderate pH in broth or on agar medium (Fig. 4), and exponentially growing cells were exposed to rapid increases or rapid decreases in pH. For the pH shift experiment in liquid medium, 630Δerm and R20291 cultures were grown to an optical density at 600 nm (OD600) of 0.5 (pH ∼6.8), then the pH of the culture was shifted by the addition of HCl to decrease the pH by 0.5, 1.0, or 1.5 units, or by the addition of NaOH to increase the pH by 0.5, 1.0, or 1.5 units, respectively (Fig. S4). Growth of both strains was minimally impacted by pH shifts of ± 0.5 units (Fig. S4). A moderate reduction in growth was observed for both strains when the pH increased 1.0 or 1.5 units. In comparison, a decrease of 1.0 pH unit drastically impacted growth of both strains, and a 1.5 unit decrease resulted in extinction of the growth (Fig. S4). After a decrease of 1.0 pH unit, growth was inhibited for the next 3 h and the culture densities were significantly lower than the unadjusted culture (Fig. S4A and B). Sporulation was evaluated in pH-adjusted cultures after 24 h of growth and all changes in pH decreased spore formation in both strains (Fig. 4A). As expected, the decreases in sporulation efficiencies correlated directly with the magnitude of the pH shift. These results indicate that C. difficile is able to survive moderate changes in the pH of an aqueous environment, but with significant decreases in spore production.
FIG 4.
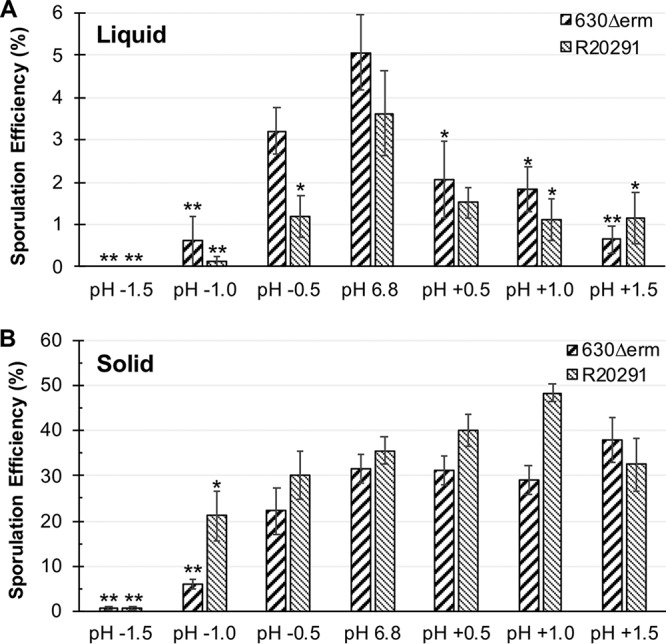
A rapid change in pH influences sporulation of C. difficile strains. (A) Strains 630Δerm and R20291 were cultivated in 70:30 broth medium at an initial pH of 7.2 and upon reaching an OD600 of ∼0.5, the pH (∼6.8) was rapidly shifted (±) 0.5, 1.0, and 1.5 units, as indicated. Sporulation efficiency was assessed after 24 h of growth. No survival of cells or sporulation was detected when the pH was rapidly decreased 1.5 units for either strain in liquid. n = 4. (B) After 6 h of growth on sporulation agar, cells of 630Δerm and R20291 were transferred to sporulation agar at pH 6.8 (control) or to plates with pH shifted (±) 0.5, 1.0, or 1.5 units. Sporulation was determined after 24 h of growth. Data were analyzed by one-way ANOVA and Dunnett’s test for multiple comparisons to the starting pH. *, P value of ≤ 0.05; **, adjusted P value of ≤ 0.01; n = 4.
To test if rapid changes in pH can impact sporulation during growth on solid surfaces, C. difficile strains were similarly cultivated on 70:30 plates at a pH of 6.8 for 6 h, then transferred to a second pH 6.8 plate, as an internal control, or to 70:30 plates with 0.5-, 1.0-, or 1.5-unit increases or decreases in pH, respectively (Fig. 4B). In contrast to growth in liquid medium, sporulation of both strains was marginally impacted when the pH was increased by 0.5, 1.0, or 1.5 units. Conversely, decreases of 1.0 or more pH units impacted sporulation of either strain, with the greatest decrease in spores observed for the 630Δerm strain. The data demonstrate that solid medium enhances C. difficile adaptation to abrupt pH shifts. Overall, C. difficile strains adapted better to increases in pH than to decreases in pH, whether cultured in liquid medium or on solid agar plates, as evidenced by growth and sporulation under all increased pH conditions. In comparison, decreases in pH drastically impaired C. difficile growth and spore formation (Fig. 4A and B). Both strains were able to decrease the pH of the culture when the pH was increased, lowering the pH to a more neutral value (Fig. S4C and D). In contrast, when the pH was rapidly decreased, neither strain could effectively increase the pH over time, resulting in prolonged and deleterious exposure of the cells to acidic conditions.
Toxin production is impacted by pH in a strain-dependent manner.
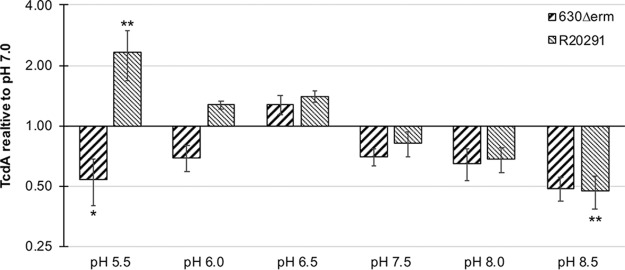
Since sporulation and toxin expression are coregulated in C. difficile (26, 27, 30–33), we considered that the pH during growth may also influence toxin production. Thus, we assessed the production of toxin A from strains grown on 70:30 plates in a range of pH conditions (pH 5.5 to 8.5) by Western blot analysis (Fig. 5). Under acidic conditions, the 630Δerm strain exhibited reduced toxin levels relative to those produced at pH 7.0. In contrast, the R20291 strain demonstrated increased toxin A production under acidic conditions (Fig. 5). Toxin production for 630Δerm was greatest at pH 6.5 and 7.0, while R20291 produced the most toxin at a pH range of 5.5 to 7.0. Both strains exhibited lower toxin production under alkaline conditions, which is in contrast to spore production under the same conditions (Fig. 4B and Fig. 5). These data suggest that sporulation and toxin expression are differentially controlled under alkaline conditions.
FIG 5.
Strain-dependent differences in toxin A production under different pH conditions. Strains 630Δerm and R20291 were analyzed for TcdA production on 70:30 plates at the indicated pH by Western blot analysis after 24 h of growth. Densitometries were normalized to pH 7.0; scale was plotted at log2. Analyzed by one-way ANOVA and Dunnett’s test compared to growth at pH 7.0. *, P value of ≤ 0.05; **, P value of ≤ 0.01; n = 4.
C. difficile motility is affected by pH.
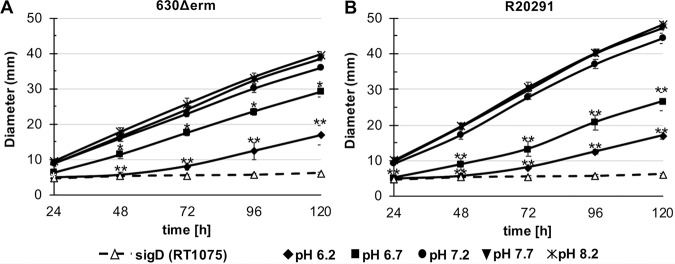
Because toxin expression and motility are both regulated by SigD, the flagellar alternative sigma factor (34–36), we next investigated the impact of pH on C. difficile motility. To test this, we analyzed the motility of C. difficile on soft agar plates at a range of pH conditions between 6.2 and 8.2. Swimming motility was measured every 24 h for 5 days, and the nonmotile sigD mutant strain (RT1075) was used as a negative control (Fig. 6). Both strains demonstrated the lowest motility at low pH (6.2) and the highest motility at pH 7.7 to 8.2 (Fig. 6A and B). However, strain-dependent differences in the distance travelled were observed, with 630Δerm exhibiting greater motility than R20291 at pH 6.2 and 6.7 and with R20291 surpassing 630Δerm at pH 7.2, 7.7, and 8.2. The poor motility for the R20291 strain at low pH contrasts with the higher toxin production observed at low pH for this strain, suggesting that in R20291, factors other than SigD activity restrict motility under acidic conditions. These data demonstrate that pH is an important environmental factor that influences C. difficile motility, and that the bacteria are generally more motile in alkaline conditions and less motile at lower pH.
FIG 6.
C. difficile motility is impacted by pH condition. Strains 630Δerm (A), R20291 (B), and a sigD mutant (RT1075, negative control) were tested for motility on ½ BHI plates (0.3% agar) at pH 6.2, 6.7, 7.2, 7.7, and pH 8.2 by measuring the swimming motility every 24 h for 120 h. Significantly reduced motility was observed at pH 6.2 and pH 6.7, relative to their respective motility at pH 7.2, for both strains. Data were analyzed by one-way ANOVA and Dunnett’s test compared to pH 7.2. *, P value ≤ 0.05; ** adjusted P value of ≤ 0.01; n = 4.
DISCUSSION
C. difficile is exposed to diverse and changing pH conditions during transit through the gastrointestinal tract. Prior studies have shown that the environmental pH impacts spore germination, resulting in inhibition of germination under acidic conditions that is reversed upon exposure to neutral pH (1, 37–39), which highlights a limiting factor for the germination of spores within the small bowel in the presence of bile acids. In this study, we further assessed the impact of diverse pH conditions on C. difficile growth, sporulation, toxin production, and motility.
As strain-dependent phenotypic differences are often described in C. difficile (26, 27, 31, 36, 40–42), we investigated the C. difficile pH response using strains 630Δerm and R20291. The 630Δerm strain is a well-characterized derivative of the historical isolate 630, which is a common laboratory strain, while R20291 is a more recent epidemic-associated strain (43–46). We found that during growth in liquid culture, no strain-dependent differences were observed under different pH conditions (Fig. 1A). However, spore formation differed between the strains grown in broth culture, as evidenced by a dramatic increase in spore production for strain 630Δerm in alkaline conditions (Fig. 1B and C) and only modest sporulation for R20291 at any pH. Thus, despite the greater culture density achieved by R20291 in liquid medium (Fig. 1), sporulation in this strain is hindered in broth culture. Differences in pH-dependent sporulation were also seen on solid sporulation medium, as 630Δerm showed maximal sporulation from pH 7.0 to 8.5, while R20291 sporulated best at a range from pH 6.0 to 8.5.
Under acidic conditions in liquid and on solid medium, both strains produced significantly fewer mature spores. Decreases in spore formation in acidic conditions have also been described for Bacillus spp., including B. weihenstephanensis at pH 5.6, B. licheniformis at pH 6.3, and B. cereus at pH 5.9 to 6.1 (47–51). The clostridia appear more varied in their sporulation efficiency response to pH. In C. perfringens, spores are produced within a narrow pH range of 5.9 to 6.6 (52). In contrast, C. cellulolyticum reached highest sporulation efficiency at the lowest tested pH of 6.4 (53). In accordance with previous studies, no growth of C. difficile was observed below a pH of 5.5 (54–57). Comparative genomic analysis of C. difficile versus C. sordellii revealed the absence of several proteins involved in acid adaptation and broad-range pH survival in C. difficile, such as ureases, glutamate decarboxylase, arginine deaminase, or potassium transport proteins (58), which may explain the inability of C. difficile to grow under more acidic conditions.
Usually C. difficile infects the colon, but cases of C. difficile enteritis involving the small bowel have been described (59–62). From the proximal small bowel to the ascending colon, the pH drops dramatically from 7.88 to 5.26, due to fermentation from colonic bacteria and the production of short-chain fatty acids (63–65). Between the ascending colon and the descending colon, the pH gradually increases as a result of mucosal bicarbonate secretion, colonic ion channel activity, and Na+/H+ exchange (23, 66, 67). To understand how these pH fluctuations impact C. difficile, we investigated the vegetative cell responses to rapid changes in pH. We found that rapid pH changes similarly impacted the growth and spore formation of both strains (Fig. S4 and Fig. 4). In general, both strains adapted better to rapid increases in pH than to pH decreases, which could be explained by deficiencies in acid adaptation mechanisms (58). Emerson et al. (68) analyzed the transcriptional response of C. difficile strain 630 after acid or alkaline shocks of 1.5 pH units. Acid shock induced genes of the general stress response, as well as the heat shock regulon of HrcA, CtsR, GroEL, and GroES (68), which indicates a severe stress for C. difficile. However, the authors did not test whether C. difficile could survive the acid or alkaline shock treatments (68). In this study, we found that C. difficile will adapt and sporulate on agar medium after an alkaline shock of 1.5 units, but cannot survive an acid shock of 1.5 units in broth cultures. Although both strains survived a 1.5-unit acid shock on solid medium, it resulted in drastic reduction in spore formation (Fig. 4).
Differences in phenotypes for cells grown on solid and liquid medium have been observed for B. cereus, with plate-grown cells displaying increased gamma radiation resistance and a more developed S-layer compared to cells grown in liquid (69). Other studies of C. difficile found substantial differences in gene expression profiles between biofilms grown in broth and grown on plates (70), as well as differences in the expression of phase variation genes and the orientation of invertible elements (36, 71). We anticipate that differences in gene expression for liquid and solid medium affected the survival and adaptation of C. difficile after an acid shock; however, this was not explored in this study.
Previous studies that examined the effect of pH on C. difficile toxin production also noted strain-dependent differences in toxin production. For the C. difficile VPI 10463 strain (ribotype 087), toxin production after 24 h and 48 h was highest for cells grown at pH 6.5 to 7.5, and reduced at pH 5.5 and 8.5, respectively (55). In our study, we analyzed the toxin production after 24 h of growth on 70:30 sporulation agar across a range of pH, and found strain-dependent differences in toxin production at different pH. Like the VPI 10463 strain, strain 630Δerm produced the most toxin at a pH of 6.5, and showed a significant reduction of toxin production at either pH 5.5 or pH 8.5. R20291, in comparison, significantly increased toxin production at a pH of 5.5, and demonstrated the greatest toxin production at a pH range of 5.5 to 7.0. The increased toxin production of R20291 (ribotype 027) at a low pH 5.5 is in accordance with the prior observed increase in the expression of toxin genes in an clinical isolate of 027 grown at pH 5 (72).
Our data indicate that low pH conditions reduced motility and high pH increased motility for C. difficile (Fig. 6), similar to observations in B. subtilis (73). As described previously (74), a possible explanation for the reduced motility under acidic conditions could be the necessity to close the flagellar motor-driven proton entrance and the energy costs for flagellum biosynthesis under harmful conditions (75, 76). Another possible explanation for reduced motility might be the incorrect assembly of flagellar proteins or protein instabilities under low pH conditions, as described elsewhere (77, 78).
In this study, we identified pH-dependent effects on strain growth, toxin production, motility, and sporulation, all of which can be used to improve growth and phenotypic testing in diverse C. difficile isolates. We discovered differences in the pH adaptation of strains, and impacts of liquid or solid agar growth conditions on C. difficile. This ability of R20291 to adapt to a broad range of pH conditions for sporulation and toxin production (Fig. 2 and Fig. 5), may provide the strain with an advantage for host colonization and pathogenesis. In strain 630Δerm, motility and toxin production were similarly depressed in low pH conditions. This is in contrast to R20291, which demonstrated the greatest toxin production at low pH, but less motility under low pH conditions. Additional genomic and transcriptional studies are necessary to understand how diverse C. difficile strains regulate these individual processes in response to pH changes within the host and how they will impact C. difficile adaptation and survival.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Table 1 lists the bacterial strains used in this study. C. difficile was grown at 37˚C in an anaerobic chamber (Coy Laboratory Products) with an atmosphere of 10% H2, 5% CO2, and 85% N2 as previously described (79, 80). C. difficile strains were routinely cultured in brain heart infusion-supplemented (BHIS) broth or agar plates (81). To induce the germination of C. difficile spores, BHIS medium was supplemented with 0.1% taurocholate (BHIS+TA) (Sigma-Aldrich) (82). d-fructose (0.2%) was added to overnight cultures to prevent sporulation, as needed (83).
TABLE 1.
Bacterial strains used
Sporulation assay for liquid and for solid medium and phase-contrast microscopy.
C. difficile cultures were started in BHIS with 0.1% taurocholate and 0.2% fructose to induce germination of C. difficile spores and prevent sporulation, respectively (79, 83). To determine the sporulation efficiencies of strains in liquid cultures and on agar plates, a slightly modified 70:30 medium (83) was used without the addition of Tris base. The pH of the medium was adjusted before autoclaving using a benchtop Accumet (AB150 pH/MV meter), and the final pH was measured after complete reduction of the medium in the anaerobic chamber (37).
In brief, for sporulation in liquid medium, log-phase BHIS cultures were diluted in 10 ml 70:30 medium adjusted to the indicated pH for ∼45 min and used to inoculate the main culture of 100 ml 70:30 medium (start OD600 = 0.03), which was adjusted to the same pH, respectively. The growth of strains and the pH of the culture was monitored hourly using a spectrophotometer and pH meter, respectively. At time point T2 (2 h after OD600 = 1.00), the total cells (vegetative cells and spores) were serially diluted and plated onto BHIS+TA. After 24 h, samples were prepared for microscopy and the enumeration of spores was performed as previously described (25, 29).
For sporulation on 70:30 agar plates, cultures at log phase were diluted in BHIS broth to an OD600 of 0.5. These cultures (250 μl) were applied to 70:30 plates adjusted to the indicated pH, and spread as a lawn (83). After 24 h, cells were scraped from the plates and suspended in BHIS to an OD600 of 1. Sample preparation, microscopy and enumeration of spores were performed as previously described (25, 29). The results represent four independent experiments and are presented as means with standard errors of the means. A one-way ANOVA and Dunnett’s test were performed for statistical comparison to the standard pH condition.
To determine the sporulation efficiency under buffered medium conditions, 70:30 medium (83) without the addition of Tris base was used. Instead of Tris, the medium was buffered using 0.1 M morpholineethanesulfonic acid (MES) at pH 6.2, or 0.1 M HEPES at pH 7.2 or pH 8.0, respectively. The pH of the medium was adjusted before autoclaving and measured after complete reduction, as described for broth medium. Cultures of C. difficile grown in BHIS broth were diluted into 10 ml 70:30 medium, which was adjusted to the indicated pH, and used to inoculate the main culture of 100 ml 70:30 medium (OD600 = 0.03) at the same pH. At time point T2 as described above for the liquid medium, total cells were diluted, plated onto BHIS+TA, and enumerated.
Preparation of spores from liquid culture and ethanol resistance assay.
C. difficile cultures in liquid 70:30 medium at pH 6.2 and at pH 7.2 were inoculated as described above for the sporulation assay for liquid medium. After 24 h, the cultures were removed from the anaerobic cabinet and exposed to oxygen for 10 days at room temperature to kill all vegetative cells (40). Exponentially growing cells of C. difficile in BHIS were used as a control and exposed to oxygen for the same time, serially diluted in BHIS, and plated onto BHIS agar. No survival of vegetative cells after 10 days exposed to oxygen was observed. Subsequently, 1% bovine serum albumin (BSA) was added to the cultures, to prevent clumping of spores and to facilitate accurate enumeration (84). After vigorous vortexing, 0.5 ml of sample was serially diluted in BHIS with 0.1% taurocholate and plated onto BHIS+TA agar to enumerate germinated spores. In parallel, 0.5 ml of sample was mixed with 0.3 ml of 95% ethanol and 0.2 ml of dH2O to achieve a final concentration of 28.5% ethanol, vortexed, and incubated for 15 min. Ethanol-treated samples were serially diluted in 1× phosphate-buffered saline (PBS) containing 0.1% taurocholate and applied to BHIS+TA agar to determine the number of ethanol-resistant spores (26, 29, 40). Colonies were counted from plates after a minimum of 36 h of incubation. The results represent three independent experiments.
Sporulation assay under rapidly changing pH conditions.
To determine sporulation frequencies under changing pH conditions, 70:30 medium without the addition of Tris base was used. The pH of the medium was adjusted before autoclaving and measured after complete reduction, as described above. For liquid medium, log-phase growing C. difficile in BHIS was diluted into 10 ml of 70:30 medium adjusted to a pH of 7.2, which was used to inoculate the main culture of 100 ml 70:30 medium at pH 7.2 (start OD600 = 0.03). The growth of strains and the pH of the cultures were monitored hourly. At an OD600 of 0.5, the pH (∼pH 6.8) in the cultures was rapidly changed by the addition of 5 N HCl or 5 N NaOH to obtain an increase or decrease of 0.5, 1.0, or 1.5 pH units. At time point T2, as described above for liquid medium, total cells were diluted and plated onto BHIS+TA. Sample preparation, microscopy, and enumeration of spores were performed as described above.
To assess sporulation efficiencies under rapid pH changes on 70:30 plates, log-phase cultures of C. difficile were diluted into BHIS to an OD600 of 0.5- and 250-μl volumes of sample were applied to 70:30 plates at a pH of 6.8 by spreading them as a lawn. After 6 h of growth, the cells were scraped from the plate into BHIS, the OD600 was adjusted to 0.5, and 250-μl volumes were applied to 70:30 plates at a pH of 6.8 as an internal control and to 70:30 plates with 0.5, 1.0, or 1.5 increases in pH units or 0.5, 1.0, or 1.5 decreases in pH units. After 24 h, samples were prepared for microscopy and the enumeration of spores was performed as described above. For the initial and second internal control plate at pH 6.8, no differences in sporulation were observed (not shown). The results represent four independent experiments and are presented as means with standard errors of the means. A one-way ANOVA and Dunnett’s test were performed for statistical comparison to the standard pH condition.
Swimming motility assay.
Strains were grown overnight in BHIS+TA and 0.2% fructose. Active cultures were diluted and grown to an OD600 of 0.5 in BHIS broth, and 5 μl of culture was stabbed into the center of one-half-concentration BHI plates containing 0.3% agar (30). The pH of the BHI plates was adjusted and measured as described above. The cell growth in diameter was measured every 24 h for 5 days. The results represent four independent experiments and are presented as means with standard errors of the means. A one-way ANOVA and Dunnett’s test were performed for statistical comparison to the standard condition of pH 7.2.
Western blot analysis.
Strains were grown in BHIS broth to an OD600 of 0.5 and 250-μl volumes of cultures were applied as a lawn to 70:30 plates at different pHs, as indicated. After 24 h of incubation, cells were scraped from the plates, suspended in SDS-PAGE loading buffer, and mechanically disrupted using a Mini-BeadBeater and 0.1-mm-diameter silica beads (Biospec Products) (27, 30). Following bead beating, the samples were centrifuged for 15 min at 16,100 × g and total protein of the supernatant was quantified using the Pierce Micro BCA protein assay kit (Thermo Fisher Scientific). Eight micrograms of total protein was separated by electrophoresis on a precast 4 to 15% TGX gradient gel (Bio-Rad) and transferred to a 0.45-μm nitrocellulose membrane. Western blot analysis was conducted using a mouse anti-TcdA antibody (Novus Biologicals), followed by a goat anti-mouse Alexa Fluor 488 (Life Technologies) secondary antibody. Imaging and densitometry were performed with a ChemiDoc and Image Lab Software (Bio-Rad). A one-way ANOVA, followed by Dunnett’s multiple-comparison test, was performed to assess statistical differences in TcdA protein levels compared to the standard condition of pH 7.0. Four independent biological replicates were analyzed for each strain.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of McBride lab for helpful suggestions and discussions during the course of this work.
This research was supported by the U.S. National Institutes of Health through research grants AI116933 and AI121684 to S.M.M.
The content of the manuscript is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Wilson KH, Sheagren JN, Freter R. 1985. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J Infect Dis 151:355–361. doi: 10.1093/infdis/151.2.355. [DOI] [PubMed] [Google Scholar]
- 2.Giel JL, Sorg JA, Sonenshein AL, Zhu J. 2010. Metabolism of bile salts in mice influences spore germination in Clostridium difficile. PLoS One 5:e8740. doi: 10.1371/journal.pone.0008740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandhu BK, McBride SM. 2018. Clostridioides difficile. Trends Microbiol 26:1049–1050. doi: 10.1016/j.tim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen A. 2015. A gut odyssey: the impact of the microbiota on Clostridium difficile spore formation and germination. PLoS Pathog 11:e1005157. doi: 10.1371/journal.ppat.1005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buffie CG, Bucci V, Stein RR, McKenney PT, Ling L, Gobourne A, No D, Liu H, Kinnebrew M, Viale A, Littmann E, van den Brink MR, Jenq RR, Taur Y, Sander C, Cross JR, Toussaint NC, Xavier JB, Pamer EG. 2015. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517:205–208. doi: 10.1038/nature13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fachi JL, Felipe JS, Pral LP, da Silva BK, Correa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Camara NOS, de Sales E, Dos Santos Martins F, Guima SES, Thomas AM, Setubal JC, Magalhaes YT, Forti FL, Candreva T, Rodrigues HG, de Jesus MB, Consonni SR, Farias ADS, Varga-Weisz P, Vinolo M. 2019. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep 27:750–761. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 7.Collins J, Danhof H, Britton RA. 2018. The role of trehalose in the global spread of epidemic Clostridium difficile. Gut Microbes doi: 10.1080/19490976.2018.1491266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hryckowian AJ, Van Treuren W, Smits SA, Davis NM, Gardner JO, Bouley DM, Sonnenburg JL. 2018. Microbiota-accessible carbohydrates suppress Clostridium difficile infection in a murine model. Nat Microbiol 3:662–669. doi: 10.1038/s41564-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woods EC, Edwards AN, Childress KO, Jones JB, McBride SM. 2018. The C. difficile clnRAB operon initiates adaptations to the host environment in response to LL-37. PLoS Pathog 14:e1007153. doi: 10.1371/journal.ppat.1007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Verstraete I, Peltier J, Dupuy B. 2016. The regulatory networks that control Clostridium difficile toxin synthesis. Toxins (Basel) 8:E153. doi: 10.3390/toxins8050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berges M, Michel A-M, Lassek C, Nuss AM, Beckstette M, Dersch P, Riedel K, Sievers S, Becher D, Otto A, Maaß S, Rohde M, Eckweiler D, Borrero-de Acuña JM, Jahn M, Neumann-Schaal M, Jahn D. 2018. Iron regulation in Clostridioides difficile. Front Microbiol 9:3183. doi: 10.3389/fmicb.2018.03183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giordano N, Hastie JL, Smith AD, Foss ED, Gutierrez-Munoz DF, Carlson PE Jr.. 2018. Cysteine desulfurase IscS2 plays a role in oxygen resistance in Clostridium difficile. Infect Immun 86:e00326-18. doi: 10.1128/IAI.00326-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zackular JP, Moore JL, Jordan AT, Juttukonda LJ, Noto MJ, Nicholson MR, Crews JD, Semler MW, Zhang Y, Ware LB, Washington MK, Chazin WJ, Caprioli RM, Skaar EP. 2016. Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 22:1330–1334. doi: 10.1038/nm.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dudek-Wicher RK, Junka A, Bartoszewicz M. 2018. The influence of antibiotics and dietary components on gut microbiota. Prz Gastroenterol 13:85–92. doi: 10.5114/pg.2018.76005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EA, Macfarlane GT. 1998. Enumeration of amino acid fermenting bacteria in the human large intestine: effects of pH and starch on peptide metabolism and dissimilation of amino acids. FEMS Microbiol Ecol 25:355–368. doi: 10.1111/j.1574-6941.1998.tb00487.x. [DOI] [Google Scholar]
- 16.Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. 2012. Microencapsulation of probiotics for gastrointestinal delivery. J Control Release 162:56–67. doi: 10.1016/j.jconrel.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Brinkworth GD, Noakes M, Clifton PM, Bird AR. 2009. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr 101:1493–1502. doi: 10.1017/S0007114508094658. [DOI] [PubMed] [Google Scholar]
- 18.Melamed P, Melamed F. 2014. Chronic metabolic acidosis destroys pancreas. JOP 15:552–560. doi: 10.6092/1590-8577/2854. [DOI] [PubMed] [Google Scholar]
- 19.Koziolek M, Grimm M, Becker D, Iordanov V, Zou H, Shimizu J, Wanke C, Garbacz G, Weitschies W. 2015. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J Pharm Sci 104:2855–2863. doi: 10.1002/jps.24274. [DOI] [PubMed] [Google Scholar]
- 20.Yoon MY, Yoon SS. 2018. Disruption of the gut ecosystem by antibiotics. Yonsei Med J 59:4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldman SA, Camilleri M. 2018. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut 67:1543–1552. doi: 10.1136/gutjnl-2018-316029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He P, Yun CC. 2010. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010:238080. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR, Worrell RT. 2015. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol 308:G497–509. doi: 10.1152/ajpgi.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta P, Yakubov S, Tin K, Zea D, Garankina O, Ghitan M, Chapnick EK, Homel P, Lin YS, Koegel MM. 2016. Does alkaline colonic pH predispose to Clostridium difficile infection? South Med J 109:91–96. doi: 10.14423/SMJ.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 25.Edwards AN, Nawrocki KL, McBride SM. 2014. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect Immun 82:4276–4291. doi: 10.1128/IAI.02323-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards AN, Anjuwon-Foster BR, McBride SM, Edwards AN, Anjuwon-Foster BR, McBride SM. 2019. RstA is a major regulator of Clostridioides difficile toxin production and motility. mBio 10:e01991-18. doi: 10.1128/mBio.01991-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nawrocki KL, Edwards AN, Daou N, Bouillaut L, McBride SM. 2016. CodY-dependent regulation of sporulation in Clostridium difficile. J Bacteriol 198:2113–2130. doi: 10.1128/JB.00220-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Childress KO, Edwards AN, Nawrocki KL, Woods EC, Anderson SE, McBride SM. 2016. The phosphotransfer protein CD1492 represses sporulation initiation in Clostridium difficile. Infect Immun 84:3434–3444. doi: 10.1128/IAI.00735-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards AN, McBride SM. 2017. Determination of the in vitro sporulation frequency of Clostridium difficile. Bio Protoc 7:e2125. doi: 10.21769/BioProtoc.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edwards AN, Tamayo R, McBride SM. 2016. A novel regulator controls Clostridium difficile sporulation, motility and toxin production. Mol Microbiol 100:954–971. doi: 10.1111/mmi.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackin KE, Carter GP, Howarth P, Rood JI, Lyras D. 2013. Spo0A differentially regulates toxin production in evolutionarily diverse strains of Clostridium difficile. PLoS One 8:e79666. doi: 10.1371/journal.pone.0079666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girinathan BP, Monot M, Boyle D, McAllister KN, Sorg JA, Dupuy B, Govind R. 2017. Effect of tcdR mutation on sporulation in the epidemic Clostridium difficile strain R20291. mSphere 2:e0083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antunes A, Camiade E, Monot M, Courtois E, Barbut F, Sernova NV, Rodionov DA, Martin-Verstraete I, Dupuy B. 2012. Global transcriptional control by glucose and carbon regulator CcpA in Clostridium difficile. Nucleic Acids Res 40:10701–10718. doi: 10.1093/nar/gks864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Meouche I, Peltier J, Monot M, Soutourina O, Pestel-Caron M, Dupuy B, Pons JL. 2013. Characterization of the SigD regulon of C. difficile and its positive control of toxin production through the regulation of tcdR. PLoS One 8:e83748. doi: 10.1371/journal.pone.0083748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anjuwon-Foster BR, Tamayo R. 2018. Phase variation of Clostridium difficile virulence factors. Gut Microbes 9:76–83. doi: 10.1080/19490976.2017.1362526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjuwon-Foster BR, Maldonado-Vazquez N, Tamayo R. 2018. Characterization of flagellum and toxin phase variation in Clostridioides difficile ribotype 012 isolates. J Bacteriol 200:e00056-18. doi: 10.1128/JB.00056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeldon LJ, Worthington T, Hilton AC, Elliott TS, Lambert PA. 2008. Physical and chemical factors influencing the germination of Clostridium difficile spores. J Appl Microbiol 105:2223–2230. doi: 10.1111/j.1365-2672.2008.03965.x. [DOI] [PubMed] [Google Scholar]
- 38.Paredes-Sabja D, Bond C, Carman RJ, Setlow P, Sarker MR. 2008. Germination of spores of Clostridium difficile strains, including isolates from a hospital outbreak of Clostridium difficile-associated disease (CDAD). Microbiology 154:2241–2250. doi: 10.1099/mic.0.2008/016592-0. [DOI] [PubMed] [Google Scholar]
- 39.Kochan TJ, Shoshiev MS, Hastie JL, Somers MJ, Plotnick YM, Gutierrez-Munoz DF, Foss ED, Schubert AM, Smith AD, Zimmerman SK, Carlson PE Jr, Hanna PC. 2018. Germinant synergy facilitates Clostridium difficile spore germination under physiological conditions. mSphere 3:e00335-18. doi: 10.1128/mSphere.00335-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. 2016. Chemical and stress resistances of Clostridium difficile spores and vegetative cells. Front Microbiol 7:1698. doi: 10.3389/fmicb.2016.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naaber P, Smidt I, Stsepetova J, Brilene T, Annuk H, Mikelsaar M. 2004. Inhibition of Clostridium difficile strains by intestinal Lactobacillus species. J Med Microbiol 53:551–554. doi: 10.1099/jmm.0.45595-0. [DOI] [PubMed] [Google Scholar]
- 42.Lyon SA, Hutton ML, Rood JI, Cheung JK, Lyras D. 2016. CdtR regulates TcdA and TcdB production in Clostridium difficile. PLoS Pathog 12:e1005758. doi: 10.1371/journal.ppat.1005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Eijk E, Anvar SY, Browne HP, Leung WY, Frank J, Schmitz AM, Roberts AP, Smits WK. 2015. Complete genome sequence of the Clostridium difficile laboratory strain 630Δerm reveals differences from strain 630, including translocation of the mobile element CTn5. BMC Genomics 16:31. doi: 10.1186/s12864-015-1252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Collery MM, Kuehne SA, McBride SM, Kelly ML, Monot M, Cockayne A, Dupuy B, Minton NP. 2017. What’s a SNP between friends: the influence of single nucleotide polymorphisms on virulence and phenotypes of Clostridium difficile strain 630 and derivatives. Virulence 8:767–781. doi: 10.1080/21505594.2016.1237333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dannheim H, Riedel T, Neumann-Schaal M, Bunk B, Schober I, Sproer C, Chibani CM, Gronow S, Liesegang H, Overmann J, Schomburg D. 2017. Manual curation and reannotation of the genomes of Clostridium difficile 630Δerm and C. difficile 630. J Med Microbiol 66:286–293. doi: 10.1099/jmm.0.000427. [DOI] [PubMed] [Google Scholar]
- 46.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. 2016. Clostridium difficile infection. Nat Rev Dis Primers 2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yazdany S, Lashkari KB. 1975. Effect of pH on sporulation of Bacillus stearothermophilus. Appl Microbiol 30:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dawes IW, Mandelstam J. 1970. Sporulation of Bacillus subtilis in continuous culture. J Bacteriol 103:529–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazas M, Lopez M, Gonzalez I, Bernardo A, Martin R. 1997. Effects of sporulation pH on the heat resistance and the sporulation of Bacillus cereus. Lett Appl Microbiol 25:331–334. doi: 10.1046/j.1472-765x.1997.00240.x. [DOI] [PubMed] [Google Scholar]
- 50.Monteiro SM, Clemente JJ, Henriques AO, Gomes RJ, Carrondo MJ, Cunha AE. 2005. A procedure for high-yield spore production by Bacillus subtilis. Biotechnol Prog 21:1026–1031. doi: 10.1021/bp050062z. [DOI] [PubMed] [Google Scholar]
- 51.Baril E, Coroller L, Couvert O, El Jabri M, Leguerinel I, Postollec F, Boulais C, Carlin F, Mafart P. 2012. Sporulation boundaries and spore formation kinetics of Bacillus spp. as a function of temperature, pH and aw. Food Microbiol 32:79–86. doi: 10.1016/j.fm.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 52.Philippe VA, Mendez MB, Huang IH, Orsaria LM, Sarker MR, Grau RR. 2006. Inorganic phosphate induces spore morphogenesis and enterotoxin production in the intestinal pathogen Clostridium perfringens. Infect Immun 74:3651–3656. doi: 10.1128/IAI.02090-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desvaux M, Petitdemange H. 2002. Sporulation of Clostridium cellulolyticum while grown in cellulose-batch and cellulose-fed continuous cultures on a mineral-salt based medium. Microb Ecol 43:271–279. doi: 10.1007/s00248-001-0043-7. [DOI] [PubMed] [Google Scholar]
- 54.May T, Mackie RI, Fahey GC Jr, Cremin JC, Garleb KA. 1994. Effect of fiber source on short-chain fatty acid production and on the growth and toxin production by Clostridium difficile. Scand J Gastroenterol 29:916–922. doi: 10.3109/00365529409094863. [DOI] [PubMed] [Google Scholar]
- 55.Woo TD, Oka K, Takahashi M, Hojo F, Osaki T, Hanawa T, Kurata S, Yonezawa H, Kamiya S. 2011. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol 60:1617–1625. doi: 10.1099/jmm.0.033423-0. [DOI] [PubMed] [Google Scholar]
- 56.Scaria J, Chen JW, Useh N, He H, McDonough SP, Mao C, Sobral B, Chang YF. 2014. Comparative nutritional and chemical phenome of Clostridium difficile isolates determined using phenotype microarrays. Int J Infect Dis 27:20–25. doi: 10.1016/j.ijid.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fredua-Agyeman M, Stapleton P, Basit AW, Beezer AE, Gaisford S. 2017. In vitro inhibition of Clostridium difficile by commercial probiotics: a microcalorimetric study. Int J Pharm 517:96–103. doi: 10.1016/j.ijpharm.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 58.Scaria J, Suzuki H, Ptak CP, Chen JW, Zhu Y, Guo XK, Chang YF. 2015. Comparative genomic and phenomic analysis of Clostridium difficile and Clostridium sordellii, two related pathogens with differing host tissue preference. BMC Genomics 16:448. doi: 10.1186/s12864-015-1663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freiler JF, Durning SJ, Ender PT. 2001. Clostridium difficile small bowel enteritis occurring after total colectomy. Clin Infect Dis 33:1429–1431; discussion 1432. doi: 10.1086/322675. [DOI] [PubMed] [Google Scholar]
- 60.Navaneethan U, Giannella RA. 2009. Thinking beyond the colon-small bowel involvement in Clostridium difficile infection. Gut Pathog 1:7. doi: 10.1186/1757-4749-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dineen SP, Bailey SH, Pham TH, Huerta S. 2013. Clostridium difficile enteritis: a report of two cases and systematic literature review. World J Gastrointest Surg 5:37–42. doi: 10.4240/wjgs.v5.i3.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aujla AK, Averbukh LD, Potashinsky A, Rossi L. 2019. A rare case of Clostridium difficile enteritis: a common bug in an uncommon place. Cureus 11:e4519. doi: 10.7759/cureus.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zarate N, Mohammed SD, O'Shaughnessy E, Newell M, Yazaki E, Williams NS, Lunniss PJ, Semler JR, Scott SM. 2010. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol 299:G1276–G1286. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]
- 64.Farmer AD, Mohammed SD, Dukes GE, Scott SM, Hobson AR. 2014. Caecal pH is a biomarker of excessive colonic fermentation. World J Gastroenterol 20:5000–5007. doi: 10.3748/wjg.v20.i17.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cummings JH, Macfarlane GT. 1997. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr 21:357–365. doi: 10.1177/0148607197021006357. [DOI] [PubMed] [Google Scholar]
- 66.Nugent SG, Kumar D, Rampton DS, Evans DF. 2001. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, de Jonge HR, Estes MK, Donowitz M. 2016. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem 291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Emerson JE, Stabler RA, Wren BW, Fairweather NF. 2008. Microarray analysis of the transcriptional responses of Clostridium difficile to environmental and antibiotic stress. J Med Microbiol 57:757–764. doi: 10.1099/jmm.0.47657-0. [DOI] [PubMed] [Google Scholar]
- 69.Kotiranta AK, Ito H, Haapasalo MP, Lounatmaa K. 1999. Radiation sensitivity of Bacillus cereus with and without a crystalline surface protein layer. FEMS Microbiol Lett 179:275–280. doi: 10.1111/j.1574-6968.1999.tb08738.x. [DOI] [PubMed] [Google Scholar]
- 70.Maldarelli GA, Piepenbrink KH, Scott AJ, Freiberg JA, Song Y, Achermann Y, Ernst RK, Shirtliff ME, Sundberg EJ, Donnenberg MS, von Rosenvinge EC. 2016. Type IV pili promote early biofilm formation by Clostridium difficile. Pathog Dis 74:ftw061. doi: 10.1093/femspd/ftw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrett EM, Sekulovic O, Wetzel D, Jones JB, Edwards AN, Vargas-Cuebas G, McBride SM, Tamayo R. 2019. Phase variation of a signal transduction system controls Clostridioides difficile colony morphology, motility, and virulence. PLoS Biol 17:e3000379. doi: 10.1371/journal.pbio.3000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart DB, Hegarty JP. 2013. Correlation between virulence gene expression and proton pump inhibitors and ambient pH in Clostridium difficile: results of an in vitro study. J Med Microbiol 62:1517–1523. doi: 10.1099/jmm.0.059709-0. [DOI] [PubMed] [Google Scholar]
- 73.Wilks JC, Kitko RD, Cleeton SH, Lee GE, Ugwu CS, Jones BD, BonDurant SS, Slonczewski JL. 2009. Acid and base stress and transcriptomic responses in Bacillus subtilis. Appl Environ Microbiol 75:981–990. doi: 10.1128/AEM.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soutourina OA, Krin E, Laurent-Winter C, Hommais F, Danchin A, Bertin PN. 2002. Regulation of bacterial motility in response to low pH in Escherichia coli: the role of H-NS protein. Microbiology 148:1543–1551. doi: 10.1099/00221287-148-5-1543. [DOI] [PubMed] [Google Scholar]
- 75.Adler J, Templeton B. 1967. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol 46:175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- 76.Li C, Louise CJ, Shi W, Adler J. 1993. Adverse conditions which cause lack of flagella in Escherichia coli. J Bacteriol 175:2229–2235. doi: 10.1128/jb.175.8.2229-2235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang KC, Cheng SJ, Chen YC, Huang HR, Liou JW. 2013. Nanoscopic analysis on pH induced morphological changes of flagella in Escherichia coli. J Microbiol Immunol Infect 46:405–412. doi: 10.1016/j.jmii.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Stocker BA, Campbell JC. 1959. The effect of non-lethal deflagellation on bacterial motility and observations on flagellar regeneration. J Gen Microbiol 20:670–685. doi: 10.1099/00221287-20-3-670. [DOI] [PubMed] [Google Scholar]
- 79.Sorg JA, Dineen SS. 2009. Laboratory maintenance of Clostridium difficile. Curr Protoc Microbiol 12:9A.1.1–9A.1.10. doi: 10.1002/9780471729259.mc09a01s12. [DOI] [PubMed] [Google Scholar]
- 80.Edwards AN, Suarez JM, McBride SM. 2013. Culturing and maintaining Clostridium difficile in an anaerobic environment. J Vis Exp 2013:e50787. doi: 10.3791/50787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith CJ, Markowitz SM, Macrina FL. 1981. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother 19:997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sorg JA, Sonenshein AL. 2008. Bile salts and glycine as cogerminants for Clostridium difficile spores. J Bacteriol 190:2505–2512. doi: 10.1128/JB.01765-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Putnam EE, Nock AM, Lawley TD, Shen A. 2013. SpoIVA and SipL are Clostridium difficile spore morphogenetic proteins. J Bacteriol 195:1214–1225. doi: 10.1128/JB.02181-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edwards AN, McBride SM. 2016. Isolating and purifying Clostridium difficile spores. Methods Mol Biol 1476:117–128. doi: 10.1007/978-1-4939-6361-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wust J, Hardegger U. 1983. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob Agents Chemother 23:784–786. doi: 10.1128/aac.23.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hussain HA, Roberts AP, Mullany P. 2005. Generation of an erythromycin-sensitive derivative of Clostridium difficile strain 630 (630Δerm) and demonstration that the conjugative transposon Tn916ΔE enters the genome of this strain at multiple sites. J Med Microbiol 54:137–141. doi: 10.1099/jmm.0.45790-0. [DOI] [PubMed] [Google Scholar]
- 87.Stabler RA, He M, Dawson L, Martin M, Valiente E, Corton C, Lawley TD, Sebaihia M, Quail MA, Rose G, Gerding DN, Gibert M, Popoff MR, Parkhill J, Dougan G, Wren BW. 2009. Comparative genome and phenotypic analysis of Clostridium difficile 027 strains provides insight into the evolution of a hypervirulent bacterium. Genome Biol 10:R102. doi: 10.1186/gb-2009-10-9-r102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bordeleau E, Purcell EB, Lafontaine DA, Fortier LC, Tamayo R, Burrus V. 2015. Cyclic di-GMP riboswitch-regulated type IV pili contribute to aggregation of Clostridium difficile. J Bacteriol 197:819–832. doi: 10.1128/JB.02340-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.