Abstract
Silicate‐based microporous materials like zeolites are nano enabled particles and used for various applications including pharmaceutical formulations. This study reports on the chemo‐enzymatic functionalization of chitosan‐zeolite particles (CTS‐zeolites) with caffeic acid (CA) and glucose oxidase (GOX) to impart combined antioxidant and antimicrobial properties. CA was grafted on the chitosan moieties by using laccase generating stable particles (zeta potential –36.7 mV) of high antioxidant activity (44% DPPH inhibition). GOX was immobilized both on CTS‐zeolites and on CA modified CTS‐zeolites and creating a hydrogen peroxide generation system continuously and in‐situ producing this oxidative and antimicrobial agent. The system prevented bacterial growth of E. coli and S. aureus over 24 h whereby a steady‐state concentration of around 60 μM hydrogen peroxide in the culture medium was observed. CA and GOX functionalized CTS‐zeolite particles additionally showed combinatorial antioxidant and antimicrobial properties providing a powerful bioactive system for medical applications. These particles proved their suitability for incorporation in bioactive formulations which could be used, inter alia, for topical wound treatments.
Keywords: Caffeic acid, Enzyme immobilization, Glucose oxidase, Hydrogen peroxide, Laccase, Phenolics
Abbreviations
- ATR‐FTIR
Attenuated total reflectance – Fourier transform Infrared spectroscopy
- CA
Caffeic acid
- CTS‐zeolite
Chitosan coated zeolite particles
- DPPH
2,2‐diphenyl‐1‐picrylhydrazyl
- EDX
Energy‐dispersive X‐ray spectroscopy
- GOX
Glucose oxidase
- SEM
Scanning electron microscopy
- ZP
Zeta potential
1. Introduction
Silicate‐based microporous materials gained of great interest for industrial uses, one of the most prevalent being metal‐catalyzed reactions. The most prominent representatives are zeolites, anionic aluminosilicate particles consisting of porous networks of varying sizes and shapes 1. Zeolites are obtained via synthesis in hyperthermal processes but are also available from natural sources 2. The porous nature renders them powerful ion exchangers and scavengers, one of many properties making their use favorable for applications ranging from chemical catalysis to the immobilization of microorganisms 3. Processed zeolites are non‐toxic and slight toxicity was only found for natural zeolites with heterogenous, fibrous shapes 4 which are rarely used in industry. Various biological properties of zeolites are known including long‐term biological stability and immunomodulatory activity. These and other properties rendered zeolites an important constituent in formulations for biomedical applications and for the food and feed industry 5, 6, 7. Pharmaceutical applications of zeolites are of increasing interest exploiting favorable properties like their toxin‐adsorbing and immunomodulatory activities. Antimicrobial properties are of special interest considering zeolites as additives in various bioactive formulations like cosmetics 8 and several reports proof the inherent growth inhibitory effect of zeolites. This property forms the basis for further modifications on zeolites for a target‐oriented design of zeolite materials for biomedical applications.
The physicochemical properties of zeolites are frequently augmented producing composite materials and recently led to the development of chitosan/zeolite co‐polymer systems 9, 10.
Chitosan is one of the most abundant polysaccharides on earth consisting of beta‐1,4‐linked glucosamine units with a minor content of N‐acetyl glucosamine. It is commonly derived via de‐N‐acetylation from chitin (poly N‐acetyl glucosamine) resulting in primary amine groups which cause the cationic charge of chitosan in solution 11, 12. Due to the cationic nature, this polysaccharide is suitable to form stable composites with anionic zeolites. To date chitosan/zeolite composites are reported either in form of chitosan coated zeolites or as composites whereby chitosan is added during hyperthermal zeolite formation 9, 10, 11. These composites have a zwitterionic character and are thus suitable candidates for the incorporation of bioactive compounds ranging from charged small molecule drugs to macromolecules like proteins. Despite physical fixation, chitosan also allows a covalent immobilization of these compounds due to its functional groups, the most prominent anchor for covalent attachment being the amine groups. Various approaches are reported immobilizing macromolecules on the amines of chitosan via different cross linking strategies including chemical crosslinking and enzyme supported strategies 13, 14, 15. Recently, natural phenols were reported as feasible cross linking agents when activated by enzymes like laccases or tyrosinases. These cross linkers do not only enable cross linking of multiple compounds but also show antioxidant properties. The study further proved the biocompatibility of these materials in cytotoxicity test on mice fibroblasts 16.
The objective of this study was the immobilization of the antimicrobial enzyme glucose oxidase (GOX) on chitosan coated zeolites using the natural phenol caffeic acid as cross linker. Antimicrobial properties should thus be combined with the antioxidant effect of caffeic acid, thereby enhancing the biological activity of the composite material.
2. Materials and methods
All used chemicals were purchased in analytical grade from Sigma Aldrich (Steinheim, Germany) except media components which were purchased from Carl Roth (Karlsruhe, Germany). The chitosan used within this study had a degree of N‐acetylation (DA) of 10% and a number‐average molecular weight (Mn) of 600 kDa. Chitosan coated natural zeolite was prepared by IPUS Mineral‐ und Umwelttechnologie GmbH (Rottenmann, Austria) by impregnation of natural zeolite clinoptilolite with deacetylated chitosan in diluted acetic acid. For this, a 0.4% chitosan solution in 0.5% acetic acid was prepared and mixed with the two‐fold mass of clinoptilolite. After drying at 40°C for 24 h a chitosan‐zeolite composite containing 0.2% (w/w) chitosan was obtained. Ca,K‐Clinoptilolite, a HEU‐type zeolite, was used for the preparation and had a particle size of 1–125 μm with 90% less than 58 μm, the average pore diameter was 20 nm and the total pore volume, calculated from BET measurements, amounted to 0.13 cm3/g. Adsorption of chitosan on zeolite results from electrostatic attraction of anionic zeolite on cationic chitosan. The laccase from Myceliophthora thermophile (MTL) was purchased from Novozymes A/S (Bagsvaerd, Denmark).
All measurements were at least performed in triplicate.
2.1. Laccase mediated grafting of caffeic acid onto chitosan coated zeolites
The chitosan coated zeolite (CTS‐zeolite) (2 g) was suspended in 25 mL of a caffeic acid (CA) solution in 20% ethanol (2,5 mg/mL). Thereafter, 500 μL of a laccase solution (150 U/mL) was added and the solution was gently stirred for 24 h at room temperature. The functionalized material was subsequently washed with EtOH and water until CA could not be detected anymore in the washing solution. The zeolite was freeze dried and stored at 4°C prior to further processing. The laccase activity was determined using an ABTS based assay like previously published 17. The phenol content on the chitosan coated zeolites was determined using the Folin‐Ciocalteu method 18 and the results were expressed in mg phenols per mg zeolite.
2.2. Immobilization of glucose oxidase on chitosan coated zeolites
GOX was immobilized using two different approaches, either directly onto CTS‐zeolite or onto the CA‐functionalized CTS‐zeolite.
GOX immobilization onto CTS‐zeolite: 20 mg of CTS‐zeolite was suspended in sodium phosphate buffer (50 mM, pH 7.4). Subsequently GOX dissolved in buffer was added to yield a total concentration of 0.5 mg/mL before glutaraldehyde as crosslinker (30 μL, 25% solution) was added. The mixture was incubated for 2 h, afterward washed with buffer and water and freeze dried.
GOX immobilization on CA‐CTS‐zeolite and CTS‐zeolite via EDC coupling: To do so, 20 mg of zeolite was suspended in 1.5 mL of MES (2‐(N‐morpholino)ethanesulfonic acid) buffer (50 mM, pH 6.0) containing 20 mM of EDC and NHS. GOX was added to achieve a total concentration of 0.5 mg/mL. The mixture was incubated for 4 h, washed with buffer and water and freeze dried. The course of immobilization was monitored assessing the remaining GOX activity in the reaction supernatant over time and subsequently determining the activity of immobilized GOX on the CTS‐zeolites. GOX activity was determined using the Amplex Red assay of a previously published approach 19.
2.3. Determination of particle size and zeta potential
The zeta potential was analyzed from the CTS‐zeolite particles in water at 25°C using a Malvern zetasizer NS. The particle size distribution was determined via dynamic light scattering on the same device, dispersed in sodium acetate buffer (pH 6.6, 10 mM) 19. Each sample was measured in triplicates.
2.4. Scanning electron microscopy (SEM), Energy‐dispersive X‐ray spectroscopy (EDX) and Attenuated total reflectance: Fourier transform Infrared spectroscopy (ATR‐FTIR)
The structure and surface morphology of the functionalized zeolites was visualized using a tabletop SEM TM3030 (Hitachi, Japan) coupled with an EDX spectrometer. Freeze dried zeolites were measured fixing them onto the SEM stub and the pictures were acquired under vacuum at an acceleration voltage of 15 kV without prior sample preparation.
The coupling of CA to chitosan was further confirmed by ATR‐FTIR, recorded on a Perkin Elmer Spectrum 100 spectrophotometer (Perkin Elmer Inc, Germany) equipped with an attenuated total reflection (ATR) sam‐ pling accessory. The absorbance measurements were performed from 650 to 4000 cm−1, 10 scans and a resolution of 4 cm−1.
2.5. Antioxidant activity
The antioxidant activity of the CA‐functionalized CTS‐zeolite was determined using the 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH) assay with slight modifications 20. DPPH, a stable, free radical is reduced in the presence of an antioxidant molecule and the product can be detected at an absorbance of 517 nm. The freeze dried zeolite material (30 mg) was mixed with 1 mL of a 100 μM DPPH stock in methanol. The sample mixture was left to stand in the dark for 30 min. After incubation the sample was centrifuged for 2 min at 11 000 rpm and 200 μL of the sample was taken to perform the absorbance measurements at 517 nm with a spectrophotometer (Tecan Infinite 200Pro, Switzerland). Blanks were performed where only the CTS‐zeolite without immobilized CA was used. The results of the radical scavenging activity were expressed as a percentage of DPPH inhibition. All experiments were carried out in triplicates.
2.6. Antimicrobial activity
The antimicrobial activity of the different CTS‐zeolites was assessed against S. aureus and E. coli. 10 mg of the respective zeolite material was dispersed into 5 mL of Mueller Hinton (MH) broth and a glucose solution was added to add a final concentration of 30 mM. Microbial growth was started by adding a defined amount of overnight culture of the respective bacterium with starting OD600 of 0.1 (equal to 105 CFU/mL). Bacterial growth was monitored for 24 h by regularly removing 250 μL of the culture supernatant. The positive control depicts the common bacterial growth curve without addition of zeolite material to the inoculum. The OD 600 was determined and the amount of viable cells was quantified using the plate drop method as previously published 19. All experiments were performed in triplicates.
3. Results and discussion
3.1. Laccase mediated grafting of caffeic acid onto chitosan coated zeolites (CTS‐zeolites)
The antioxidant activities of various phenols are well‐studied and were recently also confirmed for natural, lignin‐based phenols like caffeic acid (CA) 21, 22, which was thus used as natural antioxidant model compound in this study. CA is efficiently grafted on the primary amines of chitosan via laccase mediated oxidation of CA resulting in a covalent cross‐linkage to chitosan via Michael addition or radical coupling 23. The CA grafting to CTS‐zeolite was performed for 24 h and the decrease of the phenol content in the supernatant was analyzed as well as the total phenol content on the zeolites (Fig. 1). A steady progression of CA immobilization was observed whereby almost all available phenol was consumed after 5 h of incubation and resulted in a total CA concentration of 7 μg immobilized CA per mg CTS‐zeolite. A strong decrease of the CA concentration measured as phenol content in the supernatant indicated an unrealistically high binding to the CTS‐zeolite. However, the CA concentration was most likely concomitantly reduced in the supernatant due laccase catalyzed polymerization. Thus, the exact quantification of the CA‐immobilization yield on CTS‐zeolites was only derived from direct measurements of modified zeolites. The modified material was thoroughly washed with water and ethanol ensuring only covalently bound CA being present on the particles. The coupling success was confirmed via EDX showing an increased carbon content after the reaction (from 11 to 23%). ATR‐FTIR measurements of CA functionalized CTS‐zeolites using different CA concentrations further confirmed the coupling whereby increased absorption was observed in the of 3200–3500 cm−1 (phenolic ring OH stretching) and 1550–1700 cm−1 (stretching vibration of the aromatic C=C bond) (Fig. 2) 24.
Figure 1.
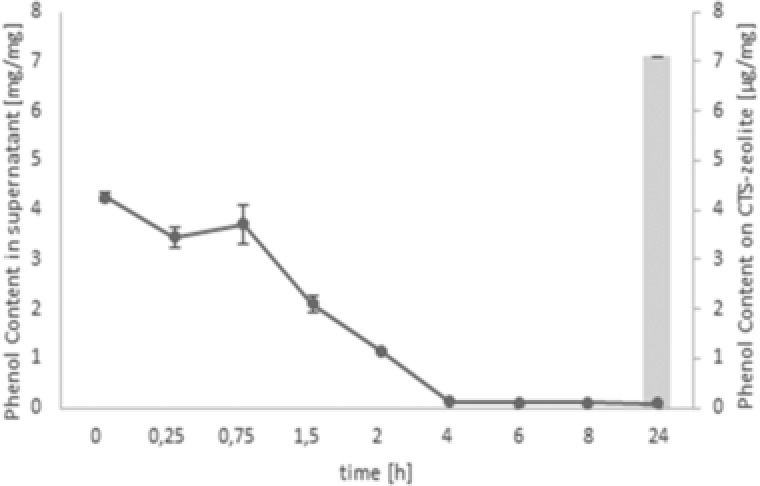
Enzymatic binding of caffeic acid (CA) onto chitosan functionalized zeolites (CTS‐zeolites) Decrease of phenol content in the reaction supernatant (grey circles) and total phenol content grafted on CTS‐zeolites (gray bar) after 24 h of incubation.
Figure 2.
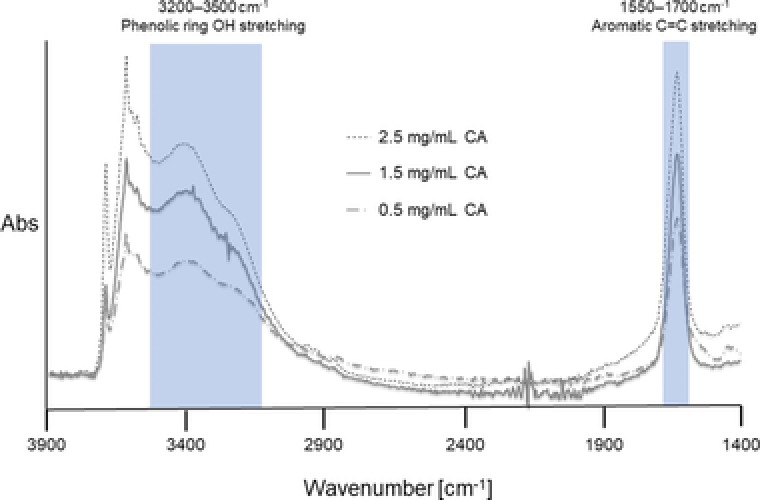
ATR‐FTIR measurement of CA‐CTS‐zeolites. CA solution of different concentration (given in mg/mL) were applied in the laccase mediated functionalization of CTS‐zeolites.
The particle size and zeta potential (ZP) of the modified zeolites was investigated since a significant alteration of these values was supposed after CA grafting. CA grafting did not show an effect on the particles size which remained between 1 and 2 μm with a poly dispersity index (PDI) of 0.5. The zeta potential of the CTS‐zeolite was found to be –28.4 ± 1.3 mV indicating that the zeolite was not heavily covered by chitosan, otherwise a positive ZP would be expected 25, 26. CA grafting (CA‐CTS‐zeolite) dropped the ZP down to –36.7.4 ± 0.5 mV which was expected because of the exhibition of anionic carboxy groups (pKa 4.62). This decrease of the ZP may lead to improved stability of CA‐CTS‐zeolite containing formulations which was a favorable attribute of the CA functionalization of CTS‐zeolites.
3.2. Immobilization of glucose oxidase on modified zeolites
The covalent immobilization of enzymes is by many means the most efficient strategy for industrial applications considering stability, reutilization and many other aspects 27. Another issue applying enzyme‐based systems in biomedical applications is uncontrolled leaching of biological material from bioactive formulations which is not intended to diffuse into tissue compartments, thereby causing adverse host reactions. Covalent fixation of antimicrobial acting enzymes like GOX is thus the only method efficiently preventing diffusion processes into host tissue. Glutaraldehyde and EDC mediated coupling of oxidases on chitosan‐based scaffolds turned out to be an efficient strategy for this purpose 19, 28.
These two approaches were hence investigated in this study and the activity recovery of GOX on the CTS‐zeolites compared. Glutaraldehyde coupling was performed using the CTS‐zeolites, linking primary amines of chitosan with amine carrying amino acids of GOX. EDC coupling is based on the activation of a carboxy groups to form an amide bond with primary amines. Within a mixture of GOX with modified CTS‐zeolites the carboxy groups of both, GOX and CA grafted on chitosan could be activated for subsequent crosslinking. Figure 3 compares the GOX activities found on CTS‐zeolites and CA‐CTS‐zeolites, respectively, after immobilization of GOX using the different strategies. Glutaraldehyde coupling of GOX onto CTS‐zeolites resulted in the highest GOX activity found on the particles (12.3 μM/min H2O2 per mg zeolites). The EDC approach on the same zeolite material resulted in 50% lower GOX activity which may be a result of the restricted cross‐linking sites available on the surface of GOX (carboxy groups). The suspected low amount of chitosan (and thus primary amines) coating the zeolites is also supposed to impede crosslinking since the small spacer arm length of the acylisourea intermediate results in a decreased degree of freedom during bond formation.
Figure 3.
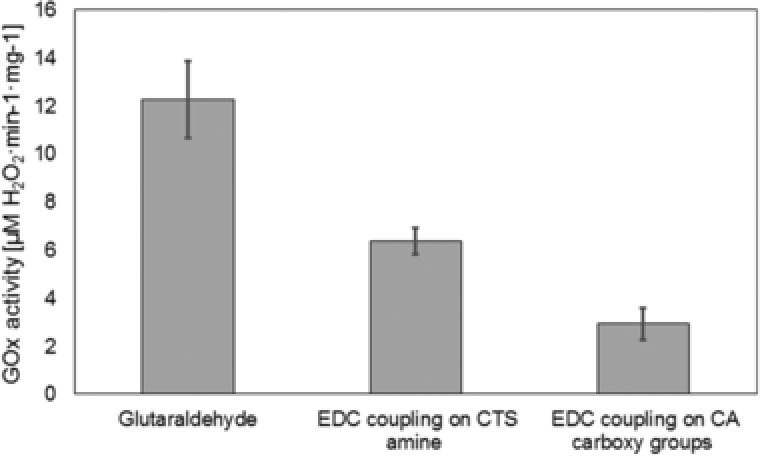
Immobilization of GOX onto CTS‐zeolites and CA grafted CTS‐zeolites using different coupling methods. GOX activity is given as μM H2O2 generated from 1 mg CTS‐zeolite per minute.
EDC coupling was subsequently performed using the CA‐CTS‐zeolite, thereby exploiting additional carboxy groups from CA. Applying the same reactions conditions, 2.9 μM/min H2O2 per mg zeolite were measured which was again a 50% lower in GOX activity when compared to EDC coupling on CTS‐zeolites (6.3 μM/min H2O2 per mg zeolites). Since the residual GOX activity in the reaction supernatant was the same for both EDC immobilizations, multiple enzyme coupling to the zeolites was supposed which often decreases the enzyme´s flexibility (degree of freedom) und thus impedes its activity. However, all GOX functionalized particles generated considerable amounts of H2O2 to ensure an accumulation of the oxidative reagent in solution and thus prevent microbial growth.
The impact of GOX immobilization on the ZP of the CA‐CTS‐zeolites was investigated while no significant effect was found (−37.2.4 ± 0.5 mV). Thus GOX immobilization does not alter the stability of the zeolite suspensions. The maintenance of the ZP may be an indicator that GOX immobilization mainly occurred at the amines of chitosan leaving residual carboxy groups of CA unaltered.
In general, the influence of CA on the GOX mediated oxidation of glucose must be considered, which could further lower the H2O2 production rate. Whether an eventual influence of antioxidative compounds impedes GOX activity or not, is subject to further studies.
3.3. Antioxidant and antimicrobial properties
The readily synthesized chitosan/zeolite materials were investigated and compared towards their bioactivities. The concept of this study was a combined antioxidant and antimicrobial effect, the former resulting from CA and the latter as function of H2O2 generation by the immobilized GOX. The antioxidant activity was analyzed using the DPPH assay which is based on the reduction of DPPH by an antioxidant compound. The starting material CTS‐zeolite did not show antioxidant activity (data not shown) thus CA grafting was originally chosen to provide the material the desired properties. CA modification resulted in 44% DPPH inhibition as function of the antioxidant effect. Chitosan coated zeolite which was only functionalized with GOX did not show any inhibitory effect on DPPH. A decreased antioxidant effect was observed with both GOX and CA were coupled to CTS‐zeolites (15% inhibition) which may be a reason of GOX immobilization partially on the free carboxy groups of CA. Although the phenol moiety of CA is considered the radical scavenging component, GOX coupling seemed to alter this effect. It is supposed that steric hindrance by the enzyme caused this retarding effect. However, 15% of radical scavenging remains a viable value for a dual bioactive material.
The antimicrobial activity of the zeolite materials is a core parameter and was assessed against E. coli and S. aureus as representatives of Gram‐negative and Gram‐positive bacteria, respectively (Fig. 4). Immobilized GOX oxidizes glucose which results in the generation of H2O2. The culture medium used in this assay contained glucose available for GOX. However, the medium was additionally spiked with glucose preventing substrate limiting effects for the catalytic reaction. Both GOX‐functionalized zeolite materials showed complete growth inhibition of E. coli and S. aureus after 4–6 h incubation. The unmodified chitosan coated zeolites showed a slight inhibitory effect against S. aureus (2 magnitudes) which may be a combinatory effect of the zeolite and chitosan. Existing studies on the inhibitory effects of chitosan against E. coli and S. aureus do not reveal a significant difference in the susceptibility of these two bacteria towards chitosan 29. The H2O2 concentration generated by GOX in the culture media was measured to be between 55 and 68 μM within 8 h incubation whereby a steady state of this concentration range was obtained after 2 h. This concentrations are in agreement with previous studies of immobilized H2O2 producing enzymes 19, 30, 31 and lie above the desired values of 10 μM which are known to be the minimum inhibitory concentration 32. The culture media were incubated with the particles for 24 h and the complete inhibition of bacterial growth could be set upright long this time, proofing a high stability of immobilized GOX in this complex medium.
Figure 4.
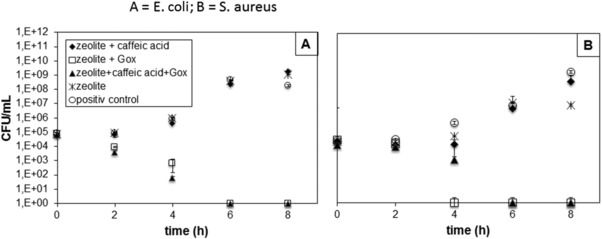
Incubation of 10 mg of the respective CTS‐zeolite particles with E. coli (A) and S. aureus (B) assessing the count of viable cells over time. Complete inhibition of bacterial growth was obtained for the particles functionalized with GOX.
The CA / GOX modified CTS‐zeolite particles proved combined antioxidant and antimicrobial activity which is a concept of great interest for future biomedical applications (Table 1). CA could enzymatically be grafted onto the chitosan moieties of the particles resulting in phenolic spacers with a terminal carboxy group which enabled EDC coupling of GOX on both, the amines of chitosan and on CA. Enzyme immobilization on biomaterials is an important aspect supporting the applicability of various enzymes for different means. The use of cross‐link spacers with bioactivity like CA constitutes a new concept which helps installing further functionality to a bioactive material.
Table 1.
Characterization of the produced zeolite derivatives
| CTS‐zeolite modification | Phenol content [μg per mg zeolite] | Antioxidant activity [%] | GOX activity [μM/min per mg zeolite] | Antimicrobial activity |
|---|---|---|---|---|
| CA | 5.0 ± 1 | 44 ± 1 | – | No |
| GOx | – | – | 5.48 ± 1.73 | Yes |
| GOx‐CA | 7.0 ± 0.3 | 15 ± 0.2 | 0.95 ± 0.15 | Yes |
4. Concluding remarks
A new approach for the functionalization of chitosan coated zeolites for biomedical applications was developed within this study. Caffeic acid (CA) was successfully grafted on chitosan by the aid of laccase providing the material antioxidant properties. Immobilization of glucose oxidase rendered these particles a hydrogen peroxide generation machinery in‐situ producing the oxidative agent and thus showing antimicrobial activity. In a combined approach, CA grafted chitosan zeolites were functionalized with GOX resulting in modified zeolites that revealed both antioxidant and antimicrobial properties. The modification led to a change of the zeta potential from –28.3 to –37.2 mV which consequently increases the stability of the particles dispersion, a favorable property for future applications. The combined antioxidant and antimicrobial activity in combination with high stability renders these particles a functional material for incorporation in bioactive formulations.
Practical application
Natural zeolites are aluminosilicate‐based materials nowadays frequently used in cosmetics and dermatology due to the known biological activities like UV protection and wound healing promoting activities.
The use of zeolites is thus divers and enzyme modified zeolites systems, like the presented, offer a great opportunity expanding and refining current application strategies.
Supporting information
Supporting Information Figure S1
Acknowledgments
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/ 2007–2013) under grant agreement no. 604278 and the Austrian Centre of Industrial Biotechnology (ACIB).
The authors have declared no conflict of interest.
5 References
- 1. Ma, Y. , Tong, W. , Zhou, H. , Suib, S. L. , A review of zeolite‐like porous materials. Microporous Mesoporous Mater. 2000, 37, 243–252. [Google Scholar]
- 2. Bogdanov, B. , Georgiev, D. , Angelova, K. , Yaneva, K. , Natural zeolites: clinoptilolite review, in: Economics and Society Development on the Base of Knowledge, Union of Bulgarian Scientists in Stara Zagora, Stara Zagora: 2009. [Google Scholar]
- 3. Ennaert, T. , Van Aelst, J. , Dijkmans, J. , De Clercq, R. et al., Potential and challenges of zeolite chemistry in the catalytic conversion of biomass. Chem. Soc. Rev. 2016, 45, 584–611. [DOI] [PubMed] [Google Scholar]
- 4. Thomas, J. , Ballantyne, B. , Toxicological assessment of Zeolites. Int. J. Toxicol. 1992, 11, 259–273. [Google Scholar]
- 5. Appendini, P. , Hotchkiss, J. H. , Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar]
- 6. Mumpton, F. A. , Fishman, P. H. , The application of natural zeolites in animal science and aquaculture. J. Anim. Sci. 1977, 45, 1188–1203. [Google Scholar]
- 7. Pavelić, K. , Hadžija, M. , Medical applications of zeolites. Handb. Zeolites Sci. Technol. 2003, 24, 1141–1172. [Google Scholar]
- 8. Bobb, S. , Zeolites for Personal Care Prodcts, 1986.
- 9. Yu, L. , Gong, J. , Zeng, C. , Zhang, L. , Synthesis of monodisperse zeolite A/chitosan hybrid microspheres. Ind. Eng. Chem. Res. 2012, 51, 2299−2308. [Google Scholar]
- 10. Wan Ngah, W. S. , Teong, L. C. , Toh, R. H. , Hanafiah, M. A. K. M. , Utilization of chitosan‐zeolite composite in the removal of Cu(II) from aqueous solution: adsorption, desorption and fixed bed column studies. Chem. Eng. J. 2012, 209, 46–53. [Google Scholar]
- 11. Zargar, V. , Asghari, M. , Dashti, A. , A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. Chem. Bio. Eng. Rev. 2015, 2, 204–226. [Google Scholar]
- 12. Pillai, C. K. S. , Paul, W. , Sharma, C. P. , Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar]
- 13. Biró, E. , Németh, A. S. , Sisak, C. , Feczkó, T. et al., Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [DOI] [PubMed] [Google Scholar]
- 14. Chiou, S. H. , Wu, W. T. , Immobilization of Candida rugosa lipase on chitosan with activation of the hydroxyl groups. Biomaterials 2004, 25, 197–204. [DOI] [PubMed] [Google Scholar]
- 15. Hoven, V. , Tangpasuthadol, V. , Angkitpaiboon, Y. , Vallapa, N. et al., Surface‐charged chitosan: preparation and protein adsorption. Carbohydr. Polym. 2007, 68, 44–53. [Google Scholar]
- 16. Huber, D. , Tegl, G. , Baumann, M. , Sommer, E. et al., Chitosan hydrogel formation using laccase activated phenolics as cross‐linkers. Carbohydr. Polym. 2017, 157, 814–822. [DOI] [PubMed] [Google Scholar]
- 17. Nugroho Prasetyo, E. , Kudanga, T. , Steiner, W. , Murkovic, M. et al., Antioxidant activity assay based on laccase‐generated radicals. Anal. Bioanal. Chem. 2009, 393, 679–687. [DOI] [PubMed] [Google Scholar]
- 18. Scalbert, A. , Monties, B. , Janin, G. , Tannins in wood: comparison of different estimation methods. J. Agric. Food Chem. 1989, 37, 1324–1329. [Google Scholar]
- 19. Tegl, G. , Thallinger, B. , Beer, B. , Sygmund, C. et al., Antimicrobial cellobiose dehydrogenase‐chitosan particles. ACS Appl. Mater. Interfaces 2016, 8, 967–973. [DOI] [PubMed] [Google Scholar]
- 20. Yu, L. , Nanguet, A.‐L. , Beta, T. , Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barclay, L. R. C. , Xi, F. , Norris, J. Q. , Antioxidant properties of phenolic lignin model compounds. J. Wood Chem. Technol. 1997, 17, 73–90. [Google Scholar]
- 22. Faustino, H. , Gil, N. , Baptista, C. , Duarte, A. P. , Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kudanga, T. , Nyanhongo, G. S. , Guebitz, G. M. , Burton, S. , Potential applications of laccase‐mediated coupling and grafting reactions: a review. Enzyme Microb. Technol. 2011, 48, 195–208. [DOI] [PubMed] [Google Scholar]
- 24. Boẑiĉ, M. , Gorgieva, S. , Kokol, V. , Laccase‐mediated functionalization of chitosan by caffeic and gallic acids for modulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012, 87, 2388–2398. [DOI] [PubMed] [Google Scholar]
- 25. Chatterjee, T. , Chatterjee, S. , Woo, S. H. , Enhanced coagulation of bentonite particles in water by a modified chitosan biopolymer. Chem. Eng. J. 2009, 148, 414–419. [Google Scholar]
- 26. Ing, L. Y. , Zin, N. M. , Sarwar, A. , Katas, H. , Antifungal activity of Chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cantone, S. , Ferrario, V. , Corici, L. , Ebert, C. et al., Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [DOI] [PubMed] [Google Scholar]
- 28. Krajewska, B. , Application of chitin‐ and chitosan‐based materials for enzyme immobilizations: a review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar]
- 29. Goy, R. C. , Morais, S. T. B. , Assis, O. B. G. , Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. Coli and S. aureus growth. Brazilian J. Pharmacogn. 2016, 26, 122–127. [Google Scholar]
- 30. Öhlknecht, C. , Tegl, G. , Beer, B. , Sygmund, C. et al., Cellobiose dehydrogenase and chitosan‐based lysozyme responsive materials for antimicrobial wound treatment. Biotechnol. Bioeng. 2017, 114, 416–422. [DOI] [PubMed] [Google Scholar]
- 31. Smith, V. J. , Dyrynda, E. A. , Antimicrobial proteins: from old proteins, new tricks. Mol. Immunol. 2015, 68, 383–398. [DOI] [PubMed] [Google Scholar]
- 32. Hyslop, P. A. , Hinshaw, D. B. , Scraufstatter, I. U. , Cochrane, C. G. et al., Hydrogen Peroxide as a potent bacteriostatic antibiotic: implications for the host defense. Free Radic. Biol. Med. 1995, 19, 3–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1


