Abstract
The microorganism Escherichia coli is commonly used for recombinant protein production. Despite several advantageous characteristics like fast growth and high protein yields, its inability to easily secrete recombinant proteins into the extracellular medium remains a drawback for industrial production processes. To overcome this limitation, a multitude of approaches to enhance the extracellular yield and the secretion efficiency of recombinant proteins have been developed in recent years. Here, a comprehensive overview of secretion mechanisms for recombinant proteins from E. coli is given and divided into three main sections. First, the structure of the E. coli cell envelope and the known natural secretion systems are described. Second, the use and optimization of different one‐ or two‐step secretion systems for recombinant protein production, as well as further permeabilization methods are discussed. Finally, the often‐overlooked role of cell lysis in secretion studies and its analysis are addressed. So far, effective approaches for increasing the extracellular protein concentration to more than 10 g/L and almost 100% secretion efficiency exist, however, the large range of optimization methods and their combinations suggests that the potential for secretory protein production from E. coli has not yet been fully realized.
Keywords: Cell lysis, Escherichia coli, Leaky mutants, Recombinant protein production, Secretion
Abbreviations
- ABC
ATP‐binding cassette
- ATP
adenosine triphosphate
- BRP
bacteriocin release protein
- Cas
CrispR‐associated
- CFU
colony forming units
- CGTase
cyclodextrine glycosyltransferase
- CRISPR
clustered regularly interspaced short palindromic repeats
- DSB
double‐strand break
- gRNA
guide RNA
- HlyA
haemolysine A
- IM
inner membrane
- LPS
lipopolysaccharides
- MFP
membrane fusion protein
- OM
outer membrane
- OMP
outer membrane protein
- PAM
protospacer adjacent motif
- PMF
proton motive force
- POI
protein of interest
- Sec
secretory
- SRP
signal recognition particle
- Tat
twin‐arginine translocation
- TCP
total cellular protein
- T1‐7SS
type 1–7 secretion system
1. Introduction
Escherichia coli is a commonly used microorganism for the industrial production of recombinant proteins. The gram‐negative bacterium serves as a recombinant protein production platform by exhibiting the favourable characteristic of fast growth to high cell densities on low priced carbon sources. A multitude of E. coli strains exist that are well characterized genetically, easy to manipulate 1, 2, 3 and some are even generally regarded as safe (GRAS) 4. Product yields of about 50% of the total cellular protein (TCP) are possible 1, 2, 3. However some drawbacks to this system exist, such as the inability to produce proteins with complex disulfide bonds, the absence of several posttranslational modifications, the accumulation of proteins as inclusion bodies 1, 2, 5, and a low natural secretion of proteins 1, 6.
The lack of effective secretion is a drawback for this host, and has resulted in significant research interest in the optimization of recombinant protein secretion from E. coli. Proteins can be localized in the cytoplasm, periplasm, membranes, or extracellularly in the culture medium. Recombinant protein expression and localization in the cytoplasm results in a high accumulation of proteins that are at risk of degradation by proteases and are often present in the form of inactive inclusion bodies 3. In contrast, secretion of these proteins into the periplasm or culture medium leads to an increased protein solubility and stability due to a dilution of total protein content and is coupled with a reduced risk of proteolysis. In the periplasmic space, correct protein folding and, consequently, higher biological activity, is promoted by a more oxidative environment than in the cytoplasm 7 as well as by the presence of chaperones for correct folding of disulfide‐bonded proteins and peptidyl‐prolyl isomerases 8. During translocation into the periplasm, N‐terminal signal sequences, and methionine are removed, this leads to an authentic N‐terminus of the recombinant protein, potentially avoiding negative impacts on protein activity and stability. The amount of native extracellular proteins in this host is low, reducing the amount of contaminating host proteins and simplifying downstream processing, thereby, reducing process costs 1, 3, 5, 6. For the expression of toxic proteins 9 or for the use of in vivo activity assays, secretion into the culture medium is essential 5.
For these reasons, the aim of this review is to provide an overview of approaches for an optimization of recombinant protein secretion efficiency from E. coli. After a brief introduction to the structure of the cell envelope and the natural protein secretion processes of E. coli, the focus of this review is the description and optimization of existing one‐ or two‐step secretion mechanisms as well as permeabilization methods. The analysis of cell lysis will also be addressed as its occurance can distort research findings and reports on this topic.
2. The cell envelope and natural protein secretion from E. coli
The cell envelope of Gram‐negative bacteria such as E. coli is composed of three layers, the cytoplasmic inner membrane (IM), a peptidoglycan (murein) layer and the outer membrane (OM) 10, 11. The IM is a selectively permeable phospholipid bilayer 12 that contains α‐helical integral proteins and in the outer leaflet anchored lipoproteins. The proteins are involved in the transport of metabolites 10, in cellular functions such as lipid biosynthesis, protein translocation, and secretion.
The peptidoglycan layer is composed of linear glycan strands, cross‐linked with peptides and exhibits a network structure. It provides physical strength to protect the cell and determines the cell shape 11, 13, 14. The peptidoglycan is localized inside the highly viscous periplasmic space between both membranes. The periplasm is characterized by an oxidative environment and among other things by the availability of enzymes to allow correct folding and disulfide bond formation 11, 15.
The OM functions as protective barrier and is essential for the survival of the cell. It is an asymmetric bilayer whose inner leaflet consists of phospholipids and whose outer leaflet is mainly composed of lipopolysaccharides (LPS) that are also the main component of E. coli endotoxins 11, 12, 15. LPS strongly interact among each other, resulting in the essential gel‐like barrier facing the environment 16. They can cause a severe immune response in humans 17. When recombinant proteins are secreted into the cultivation medium almost no endotoxins, except in cases of cell lysis, are found in the medium. The OM also contains integral proteins, called outer membrane proteins (OMPs), and a large amount of lipoproteins. OMPs usually have a β‐barrel conformation and function as enzymes, pores, or transporters 18. Lipoproteins are anchored in the inner leaflet and connect the OM to the peptidoglycan layer covalently 12. Several outer membrane surface appendages exist in E. coli including: the flagella, that enable bacterial motility 15, various pili or fimbriae required for the communication between bacteria, motility as well as host/surface attachment, and curli that participate in biofilm formation, adhesion, and invasion of host cells 19, 20.
Protein secretion, in general, is defined as the translocation of proteins from inside of the cell to its exterior or into other cells. Several studies were conducted in order to determine the composition of the E. coli secretome, including proteomic analyses of the periplasm 21, 22 and of extracellular proteins 23, 24. During secretion, proteins must pass the cell envelope. Various natural secretion systems, most of them involved in pathogenicity, are known for gram‐negative bacteria. Four of them (type 1 secretion system T1SS, T3SS, T4SS, T6SS) transport proteins directly from the cytoplasm into the medium or target cell. The type I secretion system (T1SS) is naturally used for secretion of toxins (e.g. α‐haemolysin), proteases and adhesins into the extracellular space 19, 25. The T3SS has a syringe‐like structure and through it bacterial effector proteins are transported into a target eukaryotic cell in order to alter cellular functions and simplify bacterial invasion 19. The unique T4SS is able to transport DNA, especially plasmid DNA (e.g. pKM101, R388) for conjugation, and virulent proteins through a pilus into eukaryotic and prokaryotic target cells 19, 25, 26. T6SS is also involved in the transport of effector proteins into eukaryotic targets, resulting in pathogenesis, or into prokaryotic target cells, promoting the competition between them 19, 27.
The other known E. coli secretion systems are based on a two‐step mechanism wherein the proteins are first translocated across the IM and then transported through the OM. Translocation into the periplasm occurs via the Sec‐ (general secretory) or Tat‐ (twin‐arginine translocation) pathway 19. For translocation using the Sec‐pathway, the proteins are posttranslationally (SecA/SecB‐dependent) or cotranslationally (SRP‐pathway) targeted to the Sec translocase, a protein complex in the IM. The translocase transports the unfolded proteins in an adenosine triphosphate (ATP)‐dependent manner into the periplasm with the aid of the proton‐motive force (PMF). In contrast, the Tat‐pathway transports folded proteins (often bound to cofactors) across the IM. The required energy is provided by PMF. Both pathways depend on specific signal sequences 6, 25, 28.
Transport through the OM is mediated by T2SS, T5SS, the curli secretion system (T7SS), or the chaperone‐usher pathway 19. The double‐membrane‐spanning T2SS consists of 12–15 proteins (named Gsp‐proteins in E. coli) that have not been found to be expressed under laboratory conditions 29. For secretion via T2SS, ATP is hydrolysed for the assembly of a pseudopilus and protein transport through the OM. T2SS is used for the secretion of, e.g. exoenzymes, toxins, and adhesins. Among other things, T2SS is needed for the acquisition of nutrients, biofilm formation, and motility 19, 30. The OM‐spanning T5SS facilitates secretion of virulence factors as well as proteins involved in biofilm formation and adhesion. Five subclasses are known, including an autotransporter. In the five subclasses of T5SS, secreted proteins are fused to an N‐terminal passenger domain and a C‐terminal translocation domain to enable their own secretion 19, 25. Finally, both the curli secretion system and the chaperone‐usher pathway are involved in the assembly and secretion of surface appendages: curli and pili, respectively 19. In addition to the above‐mentioned secretion systems, in some cases protein secretion cannot be classified and currently remains unexplained.
3. Production of recombinant proteins via secretion using E. coli
In the following sections, focus will be placed on the different mechanisms of secretory production of recombinant proteins and its optimization in E. coli. A short overview of the mechanism and methods to enhance protein production via secretion is given in Fig. 1.
Figure 1.
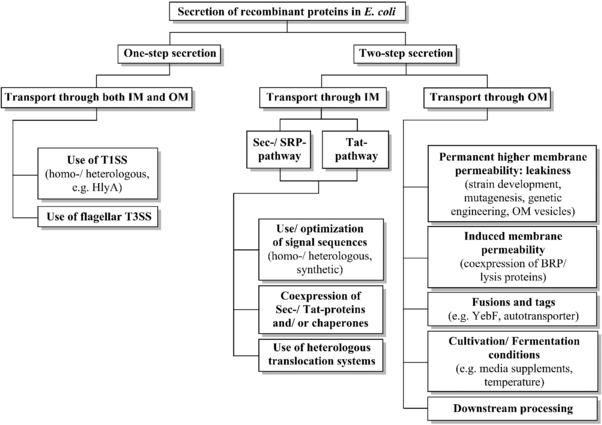
Secretion of recombinant proteins in E. coli: Employed mechanisms and methods to enhance secretion. BRP, bacteriocin release protein; HlyA, haemolysin A; IM, inner membrane; OM, outer membrane; Sec, secretory; SRP, signal recognition particle; Tat, twin‐arginine translocation; T1SS/T3SS, type 1/3 secretion system.
3.1. One‐step secretion
To achieve secretion of recombinant proteins with E. coli, known homologous or heterologous one‐step secretion systems can be leveraged. Natively, the translocation of proteins into the extracellular space can occur via T1SS and flagellar T3SS 31. T1SS was defined by Thomas et al. 32 as a one‐step secretion system consisting of three membrane proteins, namely an ATP‐binding cassette (ABC) protein, a membrane fusion protein (MFP), and an OMP. The secreted protein is characterized by a C‐terminal signal sequence that is not cleaved during translocation. Several reviews describing the function of ATP‐dependent T1SS, often based on the haemolysin (HlyA) secretion system, already exist 19, 32, 33, 34. Interestingly, there is a broad range of different substrates naturally secreted by T1SS including heme‐binding proteins, toxins, proteases, lipases, adhesins, and S‐layer proteins, with molecular weights between 19 kDa up to 900 kDa 32.
A lot of effort has been made in order to establish T1SS for recombinant protein production, especially the use of HlyA T1SS 35, 36, 37, 38, 39, 40. Bypassing the periplasma during protein secretion reduces the physiological impact on the cell and, consequently, the disturbance of protein production in comparison to several two‐step secretion systems based on permeabilization of the OM 31. The HlyA T1SS derived from uropathogenic E. coli strains is well‐characterized, reliable and can be used in nonpathogenic laboratory strains 35. The system consists of the inner membrane ABC translocator protein HlyB, the OMP TolC as well as the MFP HlyD, that serves as a linker between HlyB and TolC 35, 40, 41. While tolC is encoded on the genome, hlyB and hlyD have to be coexpressed for secretory protein expression in nonpathogenic laboratory strains, whereby the stoichiometry between hlyA, hlyB, and hlyD transcripts has a high impact on secretion rate 38, 41. For secretion of recombinant proteins in this way, a fusion to the nontoxic 50–60 AA HlyA C‐terminal domain, which induces the protein translocation, is essential 35, 40. A longer C‐terminal HlyA sequence may facilitate protein secretion 41 due to glycine‐rich repeats upstream of the signal sequence in RTX toxins like HlyA 37, 42.
Several structurally different proteins have already been secreted successfully using the HlyA approach, including, but not limited to, GFPuv 43, β‐galactosidase from E. coli 37, cutinase from Thermobifida fusca 44, single‐chain Fv antibodies (scFvs) 45, and mammalian intestinal fatty acid‐binding protein IFABP 42. Furthermore, it was shown that proteins containing disulfide bonds 49 as well as dimeric proteins can be folded and secreted properly 50. Whether alkaline phosphatase PhoA from E. coli fused to HlyA is secreted efficiently is questionable, since there are controversial publications 41, 46, 47, 48. Potentially, HlyA‐mediated secretion is disturbed by protein interactions with SecB and/or the N‐terminal signal sequence 46. The secretion of maltose‐binding protein MalE fused to HlyA was only possible for slow‐folding mutants, indicating that the rate of protein folding within the cell has an impact on secretion efficiency. It has been suggested that proteins are secreted in unfolded states 39, 51. In general, it is clear that efficiency and yield of secreted protein depends heavily on the particular protein of interest (POI) and its characteristics (length, folding rate, sec‐dependency) 52. Additional disadvantages of HlyA T1SS are; (i) the metabolic stress upon coexpression of hlyB and hlyD and (ii) that the HlyA signal sequence is not removed during secretion, resulting in the requirement of additional downstream processing steps as well as costs 31, 41. Further (iii) the secretion capacity is limited to a few mg per liter and (iv) there is a strong dependency on growth phase as well as oxygen availability 52. Some approaches previously used for optimization are: the assembly of a linker including a protease cleavage site between POI and HlyA C‐terminus (e.g. for OM protease OmpT 48), or random mutagenesis of hlyB and hlyD in order to improve the secretion efficiency 53.
In addition to HlyA T1SS, several ABC transporters from different Gram‐negative bacteria can be used for secretion of their native substrate as well as other heterologous proteins from E. coli 35. These include the ABC transporter TliDEF from Pseudomonas fluorescencs 54, 55, 56, PrtDEF from Erwinia chrysanthemi 54, 56, 57, AprDEF from Pseudomonas aeruginosa 56, 58, LipBCD from Serratia marcenscens 56, HasDEF from S. marcenscens or P. fluorescens 59 and also hybrid exporters thereof 60. The S‐layer protein RsaA secretion system of Caulobacter crescentus may also have great potential for the use in E. coli, due to its similarity to the HlyA system. More than 50 mg/L secreted recombinant protein have been demonstrated with C. crescentus, however, the system has not yet been functionally adopted to E. coli 52, 61.
Flagellar T3SS (for reviews see 62, 63, 64) can also be used for extracellular protein production after slight modifications. For this purpose, the E. coli MG1655 derived strain MKS12 was constructed by deletion of fimA‐H as well as fliD and fliC genes, resulting in a loss of the capacity to generate fimbriae and flagella. FliD is the assembly protein for flagella and FliC is the main protein component of flagella with about 20 000 copies per filament. Deletion of both fliD and fliC enables the secretion of proteins into the media and bypass the periplasm. It was found that the 5` untranslated region of FliC has a positive impact on secretion of proteins. Hence, it was suggested that there are further supporting signals within FliC sequence and the flagellar T3SS 65. Several proteins were already expressed and secreted successfully in this manner with a titre of up to 4 mg/L, including α‐enolase from Staphylococcus pneumoniae, GFP (both with about 50% purity defined as percentage of total secreted protein), D1‐D3 repeats from Staphylococcus aureus 65 and PalB lipase B from Pseudozyma antarctica 31. The secretion of Peb1 from Campylobacter jejuni using flagellar T3SS with up to 15 mg/L and more than 50% purity was stated 65 but later disproved by the same group 66. The potential of producing proteins of different origins with high purity and moderate concentration 65 as well as an undisturbed cell growth in comparison to HlyA T1SS 31 may distinguish the flagellar T3SS method. However, the level of expression and secretion strongly depends on each target protein of interest and its similarity to FliC 31, 65.
3.2. Two‐step secretion part one: Transport through the inner membrane
Another approach for extracellular production of recombinant proteins is to direct them into the periplasm first, followed by release outside of the cell. In this chapter, the focus will be on the first secretion step across the inner membrane and its optimization, for which the Sec‐ and Tat‐pathways can be employed.
3.2.1. SecB‐/SRP‐pathway
To date, significant efforts have been invested into uncovering the mechanism of the Sec‐pathway and the function of involved proteins (for reviews see 28, 67, 68, 69). Most secreted proteins are targeted via a signal peptide to the Sec translocase 68, which is known to include, at minimum, the proteins SecYEG and SecA for posttranslational secretion 28. While SecA (for review see 70) constitutes an ATP‐dependent motor, SecYEG complexes form a heterotrimeric protein conduction channel (PCC) for the translocation across the membrane 28. SecB may bind to the preprotein to target it to the Sec translocase, impede the cytoplasmic folding of the protein in order to facilitate the translocation and influence the SecA activity 6, 28, (for review see 71). It also stabilizes the unfolded preprotein 72.
The proteins SecDF, YajC, and YidC may also be involved in translocation. These three proteins form a subcomplex that interacts with SecYEG to facilitate the translocation. SecDF is presumed to be involved in the binding of the motor protein SecA to SecYEG and the regulation of its activity in protein release as well as in converting the energy of the PMF for translocation 73. The accessory protein YajC is not essential for translocation and its role remains unknown 68, whereas YidC is an insertase that is essential for the translocation of membrane proteins. The resulting complex is called a holo‐translocon and has been determined to improve cotranslational translocation to the extracellular space 73.
In contrast to SecA/SecB‐dependent posttranslational secretion of proteins, cotranslational secretion depends on the signal recognition particle (SRP), which consists of a protein named Ffh (fifty‐four homolog), 4.5S RNA, and the membrane‐bound receptor FtsY 70, 74. SRP binds to the ribosome‐bound nascent chain (RNC) and targets it to FtsY, where the RNC is transferred to the Sec translocase 70, 72. The differentiation between SecB‐ and SRP‐dependent pathways occurs competitively and is based on the hydrophobicity of the N‐terminal signal sequence as well as the binding of a trigger factor 6, 72, 75, 76.
Since the translocation capacity of Sec translocase is limited, it is possible that target protein overexpression exceeds its capacity, leading to intracellular protein accumulation. Therefore, inclusion body formation, proteolysis, and decreased protein yields have been reported to occur 77, 78. For this reason, the translocation across the inner membrane may be enhanced by cooverexpression of proteins involved in the Sec‐/SRP‐pathways and of folding chaperones. Examples include the overexpression of SecYE 79, SecYEG 80 and an additional overexpression of SecDF 81. Furthermore, translocation efficiency could be increased by SecB, SecF and, to a lesser extent, SecA overexpression 77. The overexpression of SecB or the chaperone pair DnaK‐DnaJ also resulted in an increased translocation in E. coli, in contrast to the overexpression of chaperone pair GroEL‐GroES. Moreover, it has also been observed that the impact on translocation efficiency can depend on the target protein of interest as well as the signal peptide used 82.
3.2.2. Tat‐pathway
Folded, oligomeric, and/or cofactor‐containing proteins can be transported into the periplasm via the twin‐arginine translocation (Tat‐) pathway (for reviews see e.g. 83, 84, 85). In E. coli, the minimal translocation system consists of three proteins, namely TatABC. The complex TatBC recognizes and binds the specific secretion signal and then recruits multiple TatA monomers, which form a transmembrane channel. The energy for translocation is obtained via PMF 69, 84, 85. It has also been determined that a substrate‐independent binding of TatA to the TatBC complex 85, as well as an early binding of TatA to Tat substrates, can occur and may have a positive effect upon translocation 86. In addition to the minimal E. coli Tat translocase, the protein TatE has been identified. TatE resembles TatA with respect to sequence and function, however, it is expressed at 50–200 x lower levels and is considered less relevant 87. In contrast to the Sec‐system, the Tat‐pathway is slower and less efficient due to a smaller amount of Tat translocons. However, the cytoplasmic folding of proteins prevents aggregation and leads to higher protein stability, even though the signal sequence is sensitive to proteolysis 88. An inherent quality control mechanism ensures that only correctly folded proteins are translocated, which increases the quality of recombinant protein within the periplasm 83, 91.
The amount of Tat translocons is inherently low and one approach to improve recombinant protein yields with these systems is the overexpression of tatABC genes. A positive effect for overexpression and translocation of GFP has been observed by several groups 92, 93. No negative impact on cell growth or target integrity was detected 94, but the translocation is limited by the PMF, which is the main energy source of the translocon. For a review see 89. Placing ptac upstream of the chromosomal tatABCD operon has also been shown to facilitate Tat export. 95 An additional coexpression of phage‐shock protein A (PspA), which maintains the PMF, can further increase the translocation efficiency 96.
Overexpression of nonnative translocation systems in E. coli is another approach to increased recombinant protein secretion yields. Tat‐systems derived from B. subtilis 97, Rhodobacter capsulatus 98, Agrobacterium tumefaciens, and Salmonella typhimurium 99 have already been used successfully for protein translocation from E. coli. For a review see 90. Coexpression of chaperones can also enhance recombinant protein translocation efficiency. For example, improved translocation was detected when a chaperone of TorD was coexpressed in combination with the use of a TorA signal sequence for the GFP protein. It is assumed that proteolysis of the signal sequence is prevented by its binding to TorD 100, 101. Also, the coexpression of chaperones DnaK 102, 103 and SlyD 102 has been shown to exhibit a positive effect in combination with different Tat substrates.
3.2.3. Secretion signals
Many decisions must be considered before attempting to engineer recombinant protein secretion from E. coli. The choice of the host strain and protein of interest are primary decisions, however, the choice of the signal sequences is crucial for efficient recombinant protein expression and translocation 5. Signal sequences for protein targeting to the Sec translocase generally consist of three regions (N‐, H‐, and C‐regions) with an average length of ∼20 amino acids. The short N‐ region contains mainly positively charged amino acids that can enhance the translocation by increased interaction with SecA and by electrostatic interactions with membrane phospholipids. The H‐region represents the core of the signal sequence 28, the hydrophobicity, and length of this sequence have substantial influence on translocation efficiency 104, 105, 106. The short C‐region comprises a conserved recognition motif for the signal peptidase with small and uncharged amino acids in position –1 and –3 relative to the cleavage site. These signals mediate the removal of the signal sequence 28, 107. It has also been shown that it is possible to compensate reduced translocation efficiency of one region of the signal sequence by improving another 108. Signal sequences exhibit a large number of nonoptimal codons 109. They delay the protein folding in order to facilitate the recognition by SRP or SecB. Codon‐optimization of signal sequences affects protein folding and may, therefore, decrease translocation and increase proteolysis 110, 111, 112. However, the general sequence conservation between different signal sequences is low, even if they are targeted to the same secretion apparatus 72, 113.
Several homo‐ and heterologous signal sequences that target proteins to the Sec machinery have already been successfully used for recombinant protein expression in E. coli. Homologous sequences include the signal sequences from LamB 114, MalE 109, 115, OmpA 116, 117, 118, 119, OmpC 120, OmpF 121, OmpT 77, 122, and PhoA 115, 123, 124. For SRP‐dependent secretion, e.g. the ones from DsbA 106, 116, 125, 126, 127, SfmC 127, 128, 129, TolB 116, 127, 128, and TorT 116, 127, 128, 129 can be chosen. Heterologous signal sequences include signal peptides from Bortadetella pertussis 130, G1 (Bacillus sp.) 131, Gene III/pIII (bacteriophage M13) 43, 132, SpA (Staphycococcus) 3, 133, and PelB from Erwinia carotovora 116, 120, 134, 135. The latter has become commonly used in recent years, often in signal peptide comparison studies. Although numerous, the signal sequences mentioned above are only an impression of the wide variety of available options and yet‐unknown sequences from other organisms.
Tat‐dependent signal sequences are also composed of the three (N‐, H‐, C‐) regions and have a variable length of about 25–50 bp on average due to the variable number of base pairs in the N‐region. The main characteristic of these signal sequences is the arginine‐containing motif Z‐R‐R‐x‐Φ‐Φ within the N‐region, where R stands for the conserved arginine, Z for a polar residue, Φ for hydrophobic residues and x for any residue 136. Unusual natural signal sequences also exist that differ from the consensus sequence yet are still functional, even with modifications in the conserved arginines 137. The hydrophobicity of the H‐region is important for the distinction between Sec‐ and Tat‐pathway, hydrophobicity is lower for Tat signal sequences. The specificity of Tat signal sequences is comparatively low, and as long as the proteins are unfolded, they are transported to the Sec translocase 89. The net charge and hydrophobicity downstream of the signal sequence also exerts a high impact on translocation efficiency 138, 139.
About 29 (putative) homologous Tat signal sequences are known for E. coli 140. The highest resemblance toward the consensus motif is found in the signal sequence of the copper oxidase CueO 141. However, the homologous signal sequence of TorA is commonly used for recombinant protein expression 115, 142, 143, 144. Interestingly, the TorA signal sequence was also found to promote inclusion body formation rather than facilitating secretion for several proteins including human epidermal growth factor (hEGF), interleukin‐3 (IL‐3), and even highly soluble proteins such as thioredoxin‐1 as well as maltose‐binding protein (MBP). The addition of multiple TorA signal sequences can enhance the effect 145. Other Tat signal sequences such as CueO 96, Pac 146, 147, and SufI 96, 148, 149 are typically used to investigate the translocation of their natural proteins. Examples for heterologous Tat‐dependent signal sequences that can be used for recombinant protein expression are those of organophosphorus hydrolase (OPH) from Flavobacterium 150, Cel9B signal sequence from Ruminococcus albus major glycoside hydrolase 151, as well as DagA from Streptomyces coelicolor agarase 140.
In addition to the homo‐ or heterologous signal sequences mentioned above, several attempts to increase Sec‐/Tat‐translocation efficiency by modifying the signal sequence have already been made. These include approaches of mutagenesis 131, 146 or the use of synthetic signal peptides 82, 152.
3.3. Two‐step secretion part two: Transport through the outer membrane
This section will focus on the release of proteins from the periplasm to the extracellular culture medium. Several approaches already exist to achieve and optimize the transport of proteins through the outer membrane. For example, strains with a higher permeability of the OM are often used. This higher permeability is either permanent, achieved by both strain development and genetic engineering, or induced by the expression of specific release factors such as BRP (bacteriocin release protein). Several fusion proteins as well as tags enable protein transport through the OM. Also, the choice of cultivation conditions, medium composition, and supplements, may result in an increased release of proteins. The OM can also be opened selectively via physical, chemical, or biological methods. All approaches are described in detail below.
3.3.1. Permanently improved permeability by strain development, mutagenesis, and genomic engineering—leaky mutants
Several strains have been engineered and described that more efficiently release periplasmic proteins. Early publications mainly describe the development and characterization of such strains. Two methods for mutagenesis and selection of strains that leak periplasmic proteins were patented 153, 154. In EP 0338410 B1 153, mutagenesis was performed using N‐Methyl‐N’‐nitro‐N‐nitrosoguanidin (MNNG) combined with D‐Cycloserin. Strain selection was facilitated by the indicator protein cyclodextrin‐glycosyltransferase (CGT), whose activity and secretion could be easily confirmed by the hydrolysis of starch on agar plates. For further selection, indicator plates with amylopectinazur and enzymatic tests were employed. In EP 0497757 B1 154, ultraviolet radiation was used for mutagenesis instead of chemical agents. Selection was performed by addition of T7 bacteriophage and identification of resistant strains. By plating on agar containing RNA from yeast strains that leak RNase I could be identified by surrounding halos. Repeating mutagenesis steps led to improved strains.
More recently, the focus in strain development has shifted toward genomic engineering methods. Several methods for targeted gene knockout are available; phage transduction methods, recombinase‐based systems, and, more recently, clustered regularly interspaced short palindromic repeats (CRISPR)‐Cas9 mediated mutations. By using P1 phage transduction, mutations can be transferred from one E. coli cell with a desired mutation to another strain. This approach is based on the well‐characterized lytic cycle of P1 phages, wherein these phages pack the chromosomal DNA of their host rather than of their own DNA into the capsid with a frequency of about 5%. By infecting the second strain, the bacterial DNA can be transferred into the cell and via RecA‐dependent homologous recombination it can integrate into the chromosomal DNA. Since DNA integration is a rare event, a stringent selection method is important. This method can also be used for multiple insertions 155, 156. It has to be noted that DNA of P1 lysogens can remain as undesired plasmids within the cell. This can be identified by the use of the cross‐streak agar assay, Evans Blue‐Uranine plate assay 156, or directly prevented by the use of the mutant P1vir 155.
Recombinase‐based genomic engineering systems also depend on homologous recombination. Mostly Red recombinases from λ‐phage are used for this approach, but RecET proteins from Rac prophage are an alternative 156. For λ‐Red recombination, cells are transformed with (i) a plasmid containing λ‐Red gam, beta, and exo proteins and (ii) with a PCR‐amplified deletion cassette, containing an antibiotic selection marker flanked by two flippase recognition target (FRT) sites and up to 50‐bp extensions that are homologous to the targeted gene. Via homologous recombination, the deletion cassette can replace the target gene on the chromosome. After selection of positive transformants, the selection marker can be removed through the use of a second plasmid containing a flippase, leaving behind a short scar 157. Using this method, a library containing 3985 E. coli single gene knockout mutants was constructed based on E. coli BW25113, a K‐12 derivative 158. Three years later, the KEIO collection was updated due to changes within gene annotation 159. Of note is that the scar may be problematic for multiple recombination steps, as it constitutes another homologous sequence 157. Further problems of this recombination method may be an undesired integration of DNA, partial gene duplication and the presence of an unstable genomic region. Several methods have been described to identify incorrect mutants, including PCR‐amplification, sequencing, and southern blotting 156. For high‐throughput and automated manipulations of bacterial genomes using this technique, the multiplex automated genome engineering (MAGE) method was invented 160.
Genetic engineering methods based on an introduction of a double‐strand break (DSB) at the locus of interest and its repair via either nonhomologous end joining (NHEJ) or homologous recombination (HR) have been developed. Early targeted DSB strategies involved the use of meganucleases, zinc finger nucleases, or transcription activator‐like effector nucleases (TALENs). Recently, the CRISPR‐Cas9 system has become commonly used 161. The basis for this approach is a guideRNA (gRNA) and a Cas9 nuclease. The gRNA, consisting of a crRNA‐tracrRNA hybrid molecule, directs and binds the Cas9 nuclease to a locus of interest. Targeting is realized with a 20‐bp long sequence (protospacer) at the end of the gRNA. This protospacer is complementary to the locus of interest and 5’ of a short PAM (protospacer adjacent motif) sequence. The PAM sequence is necessary for DNA cleavage performed by the Cas9 protein 161, 162. Employing the transformation of ssDNA or dsDNA and homologous recombination, deletions, insertions, or point mutations can be introduced at the locus of interest. It is possible to introduce several mutations at the same time without the use of selection markers and without leaving scars 163, 164, 165. Nevertheless, off‐target effects can occur and multiple DSBs may be toxic for E. coli cells 164. In E. coli, several fast plasmid‐based systems for CRISPR‐Cas9 with up to 100% editing efficiency have been described 163, 164, 165. Time will show if this approach becomes the method of choice for genetic engineering of E. coli cells.
Numerous publications have reported the use of these or similar methods to develop strains that leak periplasmic proteins. First approaches mainly focused on characteristics like morphology, ability to release different proteins, as well as sensitivity toward antibiotics and detergents 166, 167, 168. Later, identification of the specific mutation responsible for the observed phenotype gained importance. It must be noted that changes of certain gene names have occurred over the years. A selection of relevant changes is presented in Table 1. Based on these results, Dassler and Wich (2008) defined leaky mutants as strains that release periplasmic proteins into the extracellular medium due to mutations within structural elements of the outer membrane. The most prominent are the genes omp, tol, excC, excD, lpp, env, and lky. They listed several leaky strains that possess at least one of these mutations including E. coli JF733 (CGSC #6047), PM61 (CGSC #6628), and 207 (CGSC #6686). Dassler and Wich also named additional strains that do not have an apparent mutation within one of the named gene groups yet are nevertheless capable of increased release of periplasmic proteins, compared to the reference E. coli W3110 (ATCC #27325). These E. coli strains include BLR (Novagen), K802 (CGSC #5610), MM28 (CGSC #5892), RV308 (ATCC #31608), and RR1 (ATCC #31434) 182.
Table 1.
Leaky strains: Mutated genes and their former designations. CGSC = coli genetic stock center (http://cgsc2.biology.yale.edu/)
| Affected gene (cluster) | Mutations | Former designations | Reference |
|---|---|---|---|
| tolA | tolA1 | — | 169, 170, 171, 172 |
| tolA207 | lky207 | ||
| tolA876 | exc876 | ||
| tolB | tolB2 | — | |
| tolB236 | lky236 | ||
| tolB880 | lky880 | ||
| tolB903 | lky903 | ||
| tolQAB cluster | not specified | tolPAB cluster, lky::Mucts | 173 |
| tolQ | tolQ856 | tolP856, excD856 exc856 | 171, 174 |
| tolQ875 | tolP875, excD875, exc875 | ||
| tolQ890 | tolP890, excD890, exc890 | ||
| tolQ925 | tolP925, excD925, exc925 | ||
| pal | pal852 | excC852, exc852 | 171, 174 |
| env | envC | — | 175 |
| envA | — | 176 | |
| lpp | not specified | lpo (lpm+lpp) | 177 |
| mlpA | — | 178 | |
| lpp‐14‐1 | — | 179 | |
| lpp‐1 | lpm | CGSC #6673 | |
| lpp‐254(del) | lpo | CGSC #6672 | |
| ompA | not specified | — | 180, 181 |
| ompF | not specified | — | |
| ompC | not specified | — |
Several of these leaky strains have already been used for efficient recombinant protein production. Most early publications on the subject usually present qualitative findings on whether periplasmic proteins were found outside the cell or not 183, more recent publications present more detailed information about the extracellular protein concentrations achieved and/or secretion efficiency (see Table 2). For secretory strains such as WCM88 184 and WCM100 182, extracellular protein yields of up to 2.6 g/L were observed, however, their genotype and therefore the cause of protein release remain unknown. Strains with lpp mutation or deletion have recently been described and are gainined attention for protein production. Secretion efficiencies of ∼95–96% were stated for penicillin G acylase 186 and for streptavidin 187. Moreover, an extracellular protein yield of up to 3.5 g/L was achieved, but as indicated in EP 1903105 B1, the secretion efficiency depends on the protein of interest 188. Yields could be further increased by additional deletion of relA and spoT coding for guanosine‐tetraphosphate and guanosine‐pentaphosphase synthetases that are necessary for metabolic regulation during deficiency of amino acids or energy sources 189. The expression of human parathyroid hormone 1–84 fused to thioredoxin (Trx‐hPTH) resulted in up to 420 mg/L extracellular protein in lpp deletion strains (71.1% secretion efficiency) and reached a similar protein level for lpp mrcA double knockout mutants with ∼89% secretion efficiency 190. This indicates that secretion efficiency also depends on total protein production, as higher secretion efficiency can be due to lower total protein production or higher extracellular protein concentration. Single or double knockout of pal, mrcA, and mrcB reached only modest extracellular protein concentrations 190. The recently stated extracellular protein concentration for YebF in yaiW and gfcC knockout mutants was only 10 mg/L 191, indicating that lpp mutants are the most robust strains yet described.
Table 2.
Recombinant protein production using leaky strains: Achieved extracellular protein concentrations and secretion efficiencies
| E. coli strain (mutation) | Recombinant protein | Extracellular concentration [g/L] | Secretion efficiency [% of total enzyme activity] | Reference |
|---|---|---|---|---|
| WCM88 | hirudine | 2.6 | — | 184 |
| WCM100 | anti‐αTF | 0.24 | — | 182 |
| BW7261 (ompF627, tonA22 ( = fhuA22)) | cyclodextrine‐glycosyl‐transferase | 0.21 | — | 153 |
| 207T4 (lky207 ( = tolA207)) | alkaline phosphatase | — | 81 | 169 |
| 207c (lky207 ( = tolA207)) | alkaline phosphatase | — | 86.6 | 170 |
| 236c (lky236 ( = tolB236)) | alkaline phosphatase | — | 85.1 | 170 |
| E609Y (Δlpp) | xylanase | — | up to 90 | 185 |
| E609Y (Δlpp) | cellulase | — | up to 70 | 185 |
| JE5505 (Δlpp‐254) | penicillin G acylase | — | 97 | 186 |
| JW1667‐5 (Δlpp‐752::kan) | streptavidin | — | >95 | 187 |
| W3110 (lpp3) | cyclodextrine‐glycosyl‐transferase | 3.5 | — | 188 |
| W3110 (Δlpp) | hirudin | 3.1 | — | 188 |
| W3110 (lpp3) | interferon α2B | 0.57 | — | 188 |
| W3110 (lpp3) | anti‐lysozyme Fab‐fragment | 1.5 | — | 188 |
| W3110 (lpp3) | anti‐αTF antibody | 0.65 | — | 188 |
| JM109 (DE3) (Δlpp) | Trx‐hPTH | 0.42 | 71.1 | 190 |
| JM109 (DE3) (ΔmrcA Δlpp) | Trx‐hPTH | 0.41 | 88.9 | 190 |
| JW0369‐1 (ΔyaiW743::kan) | YebF | 0.001‐0.01 | — | 191 |
| JW0968‐1 (ΔgfcC729::kan) | YebF | 0.001‐0.01 | — | 191 |
Wacker Biotech GmbH also developed proprietary secretion strains with modified outer membranes that are stable in large‐scale fermentations, including E. coli WCM105 192. They offer the patented secretion strains ESETEC® and ESETEC® 2.0, both based on E. coli K‐12. ESETEC® is applicable for prokaryotic and eukaryotic proteins with molecular weights of 5–150 kDa, achieving yields of up to 11 g/L. Several tools to increase the yield of secreted proteins exist, including expression plasmids and further elements for coexpression of chaperones, disulfide‐bridge formation factors, and components of the Sec‐apparatus (https://www.wacker.com/cms/media/publications/downloads/6518_EN.pdf). This strain was further optimized to ESETEC® 2.0, which is more cost‐ and time‐efficient with up to 4x higher yields (https://www.wacker.com/cms/media/publications/downloads/7236_EN.pdf) 193.
An extreme example of cells with a modified outer membrane is L‐form cells, which have an extremely altered, or completely absent, cell wall. Stable and unstable L‐form cells, as well as protoplasts and spheroplasts can be distinguished. Whereas protoplasts completely lack the cell wall and are therefore simply surrounded by the cytoplasma membrane, spheroplasts have some residual cell wall 194. Characteristics of L‐form cells include an altered protein/lipid composition within the membrane, morphological changes, bacteriophage resistance, and resistance toward some cell wall‐active antibiotics 195. The advantages of these cell types are that the cells lack extracellular proteases and protein secretion limitation through the outer membrane is not an issue 196. However, the cells are more vulnerable toward environmental changes as well as substances such as Triton, limiting their potential for large‐scale fermentation 195. With regard to protein production, E. coli L‐form cells were able to express and secrete proteins including penicillin G acylase 196 and a mini‐antibody, however, the yield was comparably low (less than 0.5 mg/L functional mini‐antibody) 197. E. coli L‐form cells were also tested for their use as membrane surface display system, since the lack of the cell wall (components) avoids human inflammatory processes, which is especially advantageous for diagnostic and medical applications. However, the protein yields from this strategy were low, generating only a few mg/L of fusion protein 198. Recently, research focus has moved away from E. coli L‐form cells, presumably due to the low yields and difficult handling. Another very special export strategy of protein is using strains capable of forming outer membrane vesicles, which are more or less filled mainly with the recombinant protein 199.
3.3.2. Induced permeability
In order to secrete the periplasmic proteins into the medium, it is also possible to induce membrane permeability during cultivation by the coexpression of specific proteins. For this purpose, BRP, also named kil or lysis proteins, are most widely used. BRPs are generally required for the release of bacteriocins such as colicins from E. coli, cloacins from E. cloacae, or pesticines from Yersinia pestis 200. Secretion is semispecific, resulting in the additional release of some cytoplasmic and periplasmic proteins 201. BRPs are translocated into the periplasm by the Sec‐pathway, processed by signalpeptidase II and then modified at their N‐terminus. The mature lipoprotein (about 3 kDa) is able to integrate into the outer membrane and disturb its structure, leading to increased membrane fluidity. As a consequence, phospholipase A monomers can dimerize into their active form and counteract the BRP‐derived disturbance. This results in partial membrane degradation, enabling periplasmic proteins to diffuse into the medium 200. Possible side‐effects of BRP‐coexpression are quasi‐lysis and lethality that might result in decreased cell numbers, growth, and decreased productivity, therefore, this strategy requires strict control of BRP‐coexpression 200, 201. For optimal BRP‐coexpression, it is important to find a compromise between the release of proteins and the BRP‐derived influence on cell growth 202.
Several BRPs are known: colicin E1, E2, and A, as well as cloacin DF13. Whereas the majority have stable signal peptides that can block the Sec translocase and are, therefore, unsuitable for Sec‐dependent recombinant protein expression, colicin E1 has an unstable signal peptide 200, 201. Colicin E1 BRP was already used for extracellular production of alkaline phosphatase 200, 202, phytase 203, penicillin amidase 77, and β‐glucanase 204, 205. Another commonly used BRP is cloacin DF13 that was previously modified to contain an unstable lpp signal peptide. Extracellular proteins that have been produced by the coexpression of this BRP are alkaline phosphatase 200, 202, β‐lactamase 200, maltose‐binding protein, hybrids thereof, and also Tat‐dependent GFP 115. The yields of extracellular proteins depend on the promoter strength for BRP and target protein expression, induction time 200, 202, 203, 204, dependency of Sec‐ or Tat‐pathway for translocation into the periplasm 115, and medium composition 205. Via the use of BRP, extracellular protein levels of more than 90% were detected by 115, 123, 200, 202, 204.
Alternatively, phage‐derived lysis proteins can be coexpressed to induce membrane permeability 201. Examples include the coexpression of phage ΦX174 lysis protein E 206, 207, 208, MS2 lysis protein L, T4 phage lysis proteins Gpe and Gpt 209, as well as SRRz lysis operon from phage λ 210. Depending on the lysis protein used, the cells can be either discharged, weakened (for further lysis procedures like osmotic shocks) or completely lysed resulting in lysis efficiencies of up to 99%. This method was already applied for the release of β‐glucuronidase 209 and A. fumigattus amadoriase 210.
In addition to coexpression of lysis proteins, it was determined that the coexpression of Thermobifida fusca cutinase induces membrane permeability without significant cell lysis. It was assumed that proteins are released due to its phospholipid hydrolase activity 211, 212, 213. Employing cutinase coexpression, the extracellular productivity of periplasmic xylanase and α‐amylase could be increased 7.9‐ and 2.0‐fold, respectively. Even cytosolic D‐xylose isomerase and trehalose synthase were found to be extracellular with about 65 and 54%, respectively. However, one adverse effect of cutinase coexpression is the development of foam. This could be avoided using the coexpression of phospholipase C from Bacillus cereus 212. Three recombinant secretory (β‐galactosidase, CGTase (cyclodextrine glycosyltransferase), and xylanase) and cytoplasmic (isoamylase, trehalose synthase and glutamate decarboxylase) enzymes could be produced and secreted. The secretion efficiency of this system depends on the molecular mass of the protein, was up to 4‐fold increased for native secretory enzymes and up to nearly 90% of cytosolic enzymes could be secreted without cell lysis 214.
3.3.3. Fusions and tags
In certain cases, the transport from the periplasm into the medium seems to depend on fusions with different peptides or specific amino acid tags. One example is the fusion to YebF, a periplasmic protein of 118 aa with no known function 216. It is suspected to be transported through the OM in a porin‐dependent manner. OmpF, OmpC, and OmpX are involved in the export of YebF 217. With this method, human interleukin‐2 (hIL2), a truncated amylase of B. subtilis X23 and a leaderless alkaline phosphatase of E. coli could be secreted 218. YebF has the disadvantage of two cysteine residues in the mature protein which may lead, especially in the periplasm, to disulfide bridges with the target protein. As with most fusion proteins, the target peptide and YebF must be separated by an additional downstream step, if required 219. Another remarkable carrier partner is Spy, also an E. coli protein. It is an ATP‐independent chaperone which suppresses protein aggregation and supports protein refolding in the periplasm 220. Spy is a small periplasmic protein of 138 aa and has no cysteine residues. It is claimed to act as a secretion carrier for a long list of proteins, including Interferon α2b 219. In contrast, OmpF, which is also an E. coli carrier protein, has 362 aa and is considerably larger than YebF or Spy. Because of its large molecular mass, the ratio of this carrier to target is poor, secretion of 5.6 g/L fusion protein only led to 0.33 g/L target protein β‐endorphin 221.
An example of a heterologous carrier protein is hirudin from Hirodo medicinalis. Hirudin is 65 aa and forms three internal disulfide bridges 219. Proinsulin was secreted as the target in fusion with this protein 222. However, hirudin is a thrombin inhibitor and is therefore unsuitable for the production of pharmacological proteins, since it must be completely removed during the purification of the target protein 219.
Tags composed of only a few amino acids are also known to support secretion. Using the pelB leader sequence followed by five molecules aspartic acid, Candida antarctica lipase B (CalB) could be secreted in a gram per liter scale into the medium 223 and E. coli L‐asparaginase isozyme II could be secreted up to 40 U/mL 224.
Some periplasmic proteins in E. coli are transported through the outer membrane by specific autotransporter systems. They consist of a secreted passenger domain and a β‐domain that facilitate transfer of the passenger domain across the OM. Such systems can be used for secretion of recombinant proteins or the surface display of proteins for FACS (fluorescence‐activating cell sorting) applications. The autotransporter Adhesin Involved in Diffuse Adherence (AIDA‐I) was used to transport Affibody molecules through the OM onto the surface of E. coli 225. For secretion into the medium, systems have to be used that do not require exogenous proteases for liberation of the passenger domain from the β‐barrel and that can completely replace the passenger domain with a target protein 226, 227.
3.3.4. Cultivation/Fermentation
Any factor that influences the fluidity and integrity of the outer membrane will effect secretion. Therefore, media composition (including supplements) as well as fermentation conditions can have an influence on the export of proteins.
Well‐known medium additives that support secretion are Triton X‐100 or glycine. The surfactant Triton X‐100 interacts with biological membranes, whereas glycine is suspected to interfere with the peptidoglycan layer. Both were used in various attempts to increase secretion, however, both exhibit negative effects on cell growth and/or increased cell lysis. Elevenfold higher extracellular secretion of α‐CGTase was reached via the addition of 1% glycine into the medium at the middle of the exponential growth phase, with no effect on cell lysis 228. In another case, supplementation of the medium with 0.5% Triton X‐100 resulted in a 48‐fold increased secretion of a pullulanase from Bacillus deramificans, compared to the control without supplementation 229. Both supplements were also combined (2% glycine + 1% Triton X‐100) to increase secretion, leading to a 170‐fold increased excretion of an extracellular fusion protein with a single chain antibody and tumor necrosis factor (sFV/TNF‐alpha) 230. Extracellular production of a recombinant fusion protein between ZZ protein and alkaline phosphatase could be improved by a factor of 18 without apparent lysis by synergistic effects of 5% sucrose, 1% glycine, and 1% Triton X‐100 231.
Excessive foaming was observed for Triton X‐100 supplementation 187, 232 and the shorter length variant Triton X‐45 was found to be an effective alternative 187. Additional supplements that enhance extracellular protein production include SDS, Tween 20 and 80, Lactose 233, EDTA, polyethyleneimine (PEI) 234, and sarkosyl 235.
The cultivation temperature will influence the fluidity of cell membrane 236 and, consequently, the release of periplasmic proteins. A mild heat treatment at up to 42°C led to a 7.5% increased extracellular alkaline phosphatase titer without apparent cell lysis 235, however, temperature oscillations between 35 and 40°C can also reduce the release of alkaline phosphatase 237. A quite interesting approach is the incubation with 350 mM TRIS at constant pH for several hours followed by a mild heat treatment of up to 38°C to trigger protein leakiness of about 30% with only minimal lysis 235. During fed‐batch cultivation, the feeding profile can also influence protein release as shown for the improved production of alkaline phosphatase by 60% via an oscillatory feeding profile 238. Additional attempts to affect the extracellular production of recombinant proteins include changes of medium pH, aeration, and making use of growth‐rate dependent membrane composition 239.
3.3.5. Downstream processing
The release of periplasmic proteins after cell harvest requires methods suitable to open the outer membrane without damaging the inner membrane. Some physical, chemical, and biochemical methods are known, mainly from the isolation of the periplasmic fraction in a small scale. Common methods are freeze‐thaw cycles or osmotic shock treatments. A combination of these methods allowed the extraction of 70% of scFv from E. coli biomass 240. Chemical methods involve the use of different substances and concentrations such as Triton X‐100, Tween 20, sodium deoxycholate, benzalkonium chloride, cetyltrimethylammonium bromide, EDTA, diethanolamine, nitrilotriacetic acid, sodium hexametaphosphate, urea, guanidinium chloride, and solvents including hexane, xylene, toluene, benzene, pyridine, and isoamyl alcohol 241. The opening of the periplasmic space without whole cell lysis on an industrial scale is still a challenge and has several major technical limitations to overcome.
4. Cell lysis and its role in secretion studies
Since the results of secreted recombinant proteins may be distorted due to cell lysis, there is a need for methods for its quantification. Potential reasons for cell lysis can include a high metabolic burden due to overexpression of recombinant proteins and/or stress caused by the formation of toxic by‐products. Further reasons may be improper cultivation conditions such as high shear stresses, insufficient mass, or oxygen transfer. It has also been determined that cells in early stages of fermentations are more robust and less vulnerable to cell lysis than in later stages 215. Since cell lysis results in issues such as higher extracellular protein contamination and loss of productivity, there is a need for methods to monitor it in protein production processes in order to avoid product loss or incorrect results.
Several methods and commercial kits exist to quantify cell lysis or the viability of E. coli cells. One common approach is the growth of the culture on solid media and counting the colony forming units (CFU). Since manual counting is time‐consuming and error‐prone, automated high‐throughput methods were developed 242, 243. As it is possible for viable cells to lose the ability to form colonies, the number of viable cells might be underestimated in the CFU‐counting method 244. A commonly used alternative method for detection of viable cells is flow cytometry for offline quantification. However, this approach is not always available due to high instrument costs 215, 244, 245. Another method of detecting periplasmic leakiness is the measurement of the native periplasmic E. coli alkaline phosphatase in the fermentation supernatant. The phosphatase measurements can be combined with FACS to distinguish between intact, leaky, and lysed cells 246.
Commercial kits based on the membrane integrity of the cells are available for the rapid and easy staining of dead and viable cells. One example is the LIVE/DEAD BacLight Bacterial Viability Kit from ThermoFisher (https://tools.thermofisher.com/content/sfs/manuals/mp07007.pdf) that uses two nucleic acid stains, green‐fluorescent SYTO® 9, and red‐fluorescent propidium iodide. Whereas SYTO® 9 penetrates all cells, propidium iodide is only able to enter and stain cells with damaged membranes. The quantification of viable and dead cells can be performed via fluorescence microplate assay, microscopy, or flow cytometry. Since the staining method is only based on membrane integrity, incorrect results may occur for metabolically inactive cells maintaining their membrane integrity or for viable cells with membrane damages 247. Beside the membrane integrity, also the membrane potential can be used as indicator for the viability of cells 244. Promega developed an assay called CellTiter‐Glo® Luminescent Cell Viability Assay (https://www.promega.de/-/media/files/resources/protocols/technical-bulletins/0/celltiter-glo-luminescent-cell-viability-assay-protocol.pdf) for the quantification of viable cells based on their metabolic activity. The number of viable cells is determined via the quantification of ATP which is detected by the formation of a stable glow‐type luminescence signal of a thermostable luciferase.
A common alternative is the quantification of cytoplasmic proteins like β‐galactosidase in the extracellular medium 185, 202, 214 or their detection using Western blots. This method was performed in reports of nucleoside triphosphate pyrophosphohydrolase MazG 248 and catabolite repressor protein (CRP) production 218. The commercial kit CytoTox96® Non‐Radioactive Cytotoxicity Assay from Promega (https://www.promega.de/-/media/files/resources/protocols/technical-bulletins/0/cytotox-96-nonradioactive-cytotoxicity-assay-protocol.pdf) quantifies the release of cytoplasmic enzyme lactate dehydrogenase (LDH) in supernatants. In a coupled assay, a tetrazolium salt is converted into a red formazan product that can be quantified. The amount of quantified formazan and the number of lysed cells are proportional. Moreover, protein leakage can also be detected using offline high pressure liquid chromatography (HPLC) techniques, but this is also time‐consuming 215, 245.
In addition to proteins, DNA or RNA can be detected in the supernatant as indicators of cell lysis. For DNA quantification, Quanti‐iTTM PicogreenTM dsDNA Assay from ThermoFisher or NanoDropTM spectrophotometer can be used 215. DNA can be detected by polymerase chain reaction (PCR) or by the use of hybridisation‐based methods 244. However, DNA detection methods are prone to error due to loss or fragmentation of DNA while preparing the samples or due to its long persistence in a PCR‐detectable form 215. As an alternative, mRNA detection was used mainly by reverse transcriptase PCR (RT‐PCR) or nucleic acid sequence‐based amplification techniques since it has a shorter half‐life. Whereas the latter method was found to be more sensitive, the RT‐PCR correlation was better. rRNA‐based methods are also available, but due to a longer half‐life, the results are less accurate than in mRNA‐based methods 244. Recently, two new approaches for direct quantification of cell lysis were published. In Rajamanickam et al. 245, nucleic acid impurities in supernatants were used as indicators for lysis. They were detected via UV chromatograms as fingerprints followed by multivariate data analysis techniques that allowed fast and reliable identification of E. coli lysis. In Newton et al. 215 the viscosity of the fermentation broth was successfully used as lysis indicator since released cellular components, including chromosomal DNA and proteins, increase the viscosity. The difficulty in selecting a suitable cell lysis quantification method is that they are not universally appropriate and that all have their advantages and drawbacks.
5. Concluding remarks
The optimization of secretory recombinant protein production in E. coli is of great interest for fundamental research as well as industrial settings. Each target protein may have unique behaviours with each proposed strategy for secretion. Indeed methodology determination and parameter optimization can be a time‐consuming process. A multitude of optimization approaches are available, including genetic engineering approaches in preproduction stages, optimization of the production process itself, as well as of the downstream processing after the cell harvest. Simultaneously, suitable and reliable analytic tools for the detection of cell lysis in secretion studies are necessary to prevent misleading results and ensure target protein quality. The increasing amount of diverse analytical methods and available kits in recent years reflects the strong interest in this topic.
To date, the use of one‐step secretion systems for optimizing recombinant protein production in E. coli has led to extracellular protein titres up to the milligram per liter scale. Even higher extracellular protein concentrations were reached using two‐step secretion systems. Here, researchers have several possibilities to increase the secretion efficiency including the optimization of the Sec‐/Tat‐pathway for the transport through the inner membrane, as well as the release of proteins into the medium for example, by increasing the permeability of the outer membrane. In recent years, the use of mutant strains with modified outer membranes has led to promising extracellular protein concentrations of more than 10 g/L and secretion efficiencies of almost 100% of the total cellular protein. Our experience is that the expression and production of recombinant proteins and peptides is still a case of trial and error. This is even more so for secretion attempts. If an investigator chooses a one‐step secretion strategy it would be advisable to first attempt previously described methods. For two‐step secretion strategies, the investigator must ensure that the proteins are transported through the inner membrane into the periplasm of E. coli, which is frequently the bottleneck of the system. Since the range of optimization methods and their combinations is large, it can be assumed that the potential for secretory protein production in E. coli has not been fully realized yet. Therefore, E. coli remains a promising host for secretory recombinant protein production.
Practical application
Escherichia coli is a frequently used host for recombinant protein production. It provides several advantages, but its inability to secrete proteins into the medium still limits production processes. This review gives a comprehensive overview of approaches to overcome this drawback and to optimize protein secretion in E. coli. In addition, multiple methods for the analysis of cell lysis will be addressed in order to prevent misleading results in secretion studies. The large range of methods and publications indicates that (i) there is a strong interest of researchers, (ii) that the potential for secretory protein production in E. coli has not been fully realized yet and (iii) that E. coli remains a promising host for recombinant protein production. Therefore, this review will facilitate and improve further research on protein secretion in E. coli in both academia and industry.
The authors have declared no conflicts of interest.
Acknowledgments
We would like to thank Dr. Kyle J. Lauersen and Dr. Jan‐Philipp Schwarzhans for their constructive criticism on the manuscript.
6 References
- 1. Yoon, S. H. , Kim, S. K. , Kim, J. F , Secretory production of recombinant proteins in Escherichia coli . Recent. Pat. Biotechnol. 2010, 4, 23–29. [DOI] [PubMed] [Google Scholar]
- 2. Baneyx, F. , Mujacic, M. , Recombinant protein folding and misfolding in Escherichia coli . Nat. Biotechnol. 2004, 22, 1399–1408. [DOI] [PubMed] [Google Scholar]
- 3. Mergulhão, F. J. , Monteiro, G. A. , Periplasmic targeting of recombinant proteins in Escherichia coli . Methods Mol. Biol. 2007, 390, 47–61. [DOI] [PubMed] [Google Scholar]
- 4. Fijan, S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int. J. Environ. Res. Publ. Health 2014, 11, 4745–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi, J. H. , Lee, S. Y. , Secretory and extracellular production of recombinant proteins using Escherichia coli . Appl. Microbiol. Biotechnol. 2004, 64, 625–635. [DOI] [PubMed] [Google Scholar]
- 6. Mergulhão, F. J. M. , Summers, D. K. , Moneiro, G. A. , Recombinant protein secretion in Escherichia coli . Biotechnol. Adv. 2005, 23, 177–202. [DOI] [PubMed] [Google Scholar]
- 7. Billen, B. , Vincke, C. , Hansen, R. , Devoogdt, N. et al. Cytoplasmic versus periplasmic expression of site‐specifically and bioorthogonally functionalized nanobodies using expressed protein ligation. Prot. Expr. Purif. 2017, 133, 25–34. [DOI] [PubMed] [Google Scholar]
- 8. Goemans, C. , Denoncin, K. , Collet, J.‐F. , Folding mechanisms of periplasmic proteins. Biochim. Biophys. Acta. 2014, 1843, 1517–1528. [DOI] [PubMed] [Google Scholar]
- 9. Ni, Y. , Chen, R. , Extracellular recombinant protein production from Escherichia coli . Biotechnol. Lett. 2009, 31, 1661–1670. [DOI] [PubMed] [Google Scholar]
- 10. Molloy, M. P. , Herbert, B. R. , Slade, M. B. , Rabilloud, T. et al., Proteomic analysis of the Escherichia coli outer membrane. Eur. J. Biochem. 2000, 267, 2871–2881. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz, N. , Kahne, D. , Silhavy, T. , Advances in understanding bacterial outer‐membrane biogenesis. Nat. Rev. Microbiol. 2006, 4, 57–66. [DOI] [PubMed] [Google Scholar]
- 12. Weiner, J.H. , Li, L. , Proteome of the Escherichia coli envelope and technological challenges in membrane proteome analysis. Biochim. Biophys. Acta. 2008, 1778, 1698–1713. [DOI] [PubMed] [Google Scholar]
- 13. Vollmer, W. , Bertsche, U. , Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli . Biochim. Biophys. Acta. 2008, 1778, 1714–1734. [DOI] [PubMed] [Google Scholar]
- 14. de Pedro, M. , Cava, F. , Structural constraints and dynamics of bacterial cell wall architecture. Front. Microbiol. 2015, 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silhavy, T. , Kahne, D. , Walker, S. , The bacterial cell envelope. Cold. Spring. Harb. Perspect. Biol. 2010, 2, a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nikaido, H. , Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003, 67, 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller, S.I. , Ernst, R.K. , Bader, M.W. , LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 2005, 3, 36–46. [DOI] [PubMed] [Google Scholar]
- 18. Schulz, G.E. , The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta. 2002, 1565, 308–317. [DOI] [PubMed] [Google Scholar]
- 19. Costa, T. R. D. , Felisberto‐Rodrigues, C. , Meir, A. , Prevost, M. S. et al., Secretion systems in Gram‐negative bacteria: structural and mechanistic insights. Nat. Rev. Microbiol. 2015, 13, 343–359. [DOI] [PubMed] [Google Scholar]
- 20. Fronzes, R. , Remaut, H. , Waksman, G. , Architectures and biogenesis of non‐flagellar protein appendages in Gram‐negative bacteria. EMBO J. 2008, 27, 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han, M.‐J. , Kim, J. Y. , Kim, J. A. , Comparison of the large‐scale periplasmic proteomes of the Escherichia coli K‐12 and B strains. J. Biosci. Bioeng. 2014, 117, 437–442. [DOI] [PubMed] [Google Scholar]
- 22. Stancik, L. M. , Stancik, D. M. , Schmidt, B. , Barnhart, D. M. et al., pH‐dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli . J. Bacteriol. 2002, 184, 4246–4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nandakumar, M. P. , Cheung, A. , Marten, M. R . Proteomic analysis of extracellular proteins from Escherichia coli . J. Proteome Res. 2005, 5, 1155–1161. [DOI] [PubMed] [Google Scholar]
- 24. Xia, X.‐X. , Han, M.‐J. , Lee, S. Y. , Yoo, J.‐S. , Comparison of the extracellular proteomes of Escherichia coli B and K‐12 strains during high cell density cultivation. Proteomics 2008, 8, 2089–2103. [DOI] [PubMed] [Google Scholar]
- 25. Dalbey, R. E. , Kuhn, A. , Protein traffic in Gram‐negative bacteria‐how exported and secreted proteins find their way. FEMS Microbiol. Rev. 2012, 36, 1023–1045. [DOI] [PubMed] [Google Scholar]
- 26. Chandran, V. , Type IV secretion machinery: molecular architecture and function. Biochem. Soc. Trans. 2013, 41, 17–28. [DOI] [PubMed] [Google Scholar]
- 27. Cascales, E. , Cambillau, C. , Structural biology of type VI secretion systems. Phil. Trans. R. Soc. B. 2012, 367, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Natale, P. , Brüser, T. , Driessen, A. , Sec‐ and Tat‐mediated protein secretion across the bacterial cytoplasmic membrane—Distinct translocases and mechanisms. Biochim. Biophys. Acta. 2008, 1778, 1735–1756. [DOI] [PubMed] [Google Scholar]
- 29. Francetic, O. , Pugsley, A.P. , The cryptic general secretory pathway (gsp) operon of Escherichia coli K‐12 encodes functional proteins. J. Bacteriol. 1996, 178, 3544–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nivaskumar, M. , Francetic, O. , Type II secretion system: A magic beanstalk or a protein escalator. Biochim. Biophys. Acta. 2014, 1843, 1568–1577. [DOI] [PubMed] [Google Scholar]
- 31. Narayanan, N. , Khan, M. , Chou, C. P. , Enhancing functional expression of heterologous lipase B in Escherichia coli by extracellular secretion. J. Ind. Microbiol. Biotechnol. 2010, 37, 349–361. [DOI] [PubMed] [Google Scholar]
- 32. Thomas, S. , Holland, B. , Schmitt, L. , The type 1 secretion pathway—the hemolysin system and beyond. Biochim. Biophys. Acta. 2014, 1843, 1629–1641. [DOI] [PubMed] [Google Scholar]
- 33. Delepelaire, P. , Type I secretion in gram‐negative bacteria. Biochim. Biophys. Acta. 2004, 1694, 149–161. [DOI] [PubMed] [Google Scholar]
- 34. Holland, I. B. , Schmitt, L. , Young, J. , Type 1 protein secretion in bacteria, the ABC‐transporter dependent pathway. Mol. Membr. Biol. 2005, 22, 29–39. [DOI] [PubMed] [Google Scholar]
- 35. Blight, M. A. , Holland, I. B. , Heterologous protein secretion and the versatile Escherichia coli haemolysin translocator. Trends Biotechnol. 1994, 12, 450–455. [DOI] [PubMed] [Google Scholar]
- 36. Mackman, N. , Baker, K. , Gray, L. , Haigh, R. et al., Release of a chimeric protein into the medium from Escherichia coli using the C‐terminal secretion signal of haemolysin. EMBO J. 1987, 6, 2835–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kenny, B. , Haigh, R. , Holland, I. B. , Analysis of the haemolysin transport process through the secretion from Escherichia coli of PCM, CAT or β;‐galactosidase fused to the Hly C‐terminal signal domain. Mol. Microbiol. 1991, 5, 2557–2568. [DOI] [PubMed] [Google Scholar]
- 38. Kern, I. , Ceglowski, P. , Secretion of streptokinase fusion proteins from Escherichia coli cells through the hemolysin transporter. Gene 1995, 163, 53–57. [DOI] [PubMed] [Google Scholar]
- 39. Bakkes, P. J. , Jenewein, S. , Smits, S. H. , Holland, I. B. et al., The rate of folding dictates substrate secretion by the Escherichia coli hemolysin type 1 secretion system. J. Biol. Chem. 2010, 285, 40573–40580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lenders, M. H. H. , Weidtkamp‐Peters, S. , Kleinschrodt, D. , Jaeger, K. E. et al., Directionality of substrate translocation of the hemolysin A Type I secretion system. Sci. Rep. 2015, 5, 12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Buttkewitz, A. , Entwicklung einer neuen funktionellen Proteintechnologie in E. coli. Dissertation. University Konstanz, 2005. [Google Scholar]
- 42. Schwarz, C. K. , Landsberg, C. D. , Lenders, M. H. H. , Smits, S. H . et al., Using an E. coli Type 1 secretion system to secrete the mammalian, intracellular protein IFABP in its active form. J. Biotechnol. 2012, 159, 155–161. [DOI] [PubMed] [Google Scholar]
- 43. Linton, E. , Walsh, M. K. , Sims, R. C. , Miller, C. D. , Translocation of green fluorescent protein by comparative analysis with multiple signal peptides. Biotechnol. J. 2012, 7, 667–676. [DOI] [PubMed] [Google Scholar]
- 44. Su, L. , Chen, S. , Yi, L. , Woodard, R. W. et al., Extracellular overexpression of recombinant Thermobifida fusca cutinase by alpha‐hemolysin secretion system in E. coli BL21(DE3). Microb. Cell. Fact. 2012, 11, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandez, L. A. , Sola, I. , Enjuanes, L. , de Lorenzo, V. , Specific secretion of active single‐chain Fv antibodies into the supernatants of Escherichia coli cultures by use of the hemolysin system. Appl. Environ. Microbiol. 2000, 66, 5024–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gentschev, I. , Hess, J. , Goebel, W. , Change in the cellular localization of alkaline phosphatase by alteration of its carboxy‐terminal sequence. Mol. Gen. Genet. 1990, 222, 211–216. [DOI] [PubMed] [Google Scholar]
- 47. Hess, J. , Gentschev, I. , Goebel, W. , Jarchau, T. , Analysis of the haemolysin secretion system by PhoA‐HlyA fusion proteins. Mol. Gen. Genet. 1990, 224, 201–208. [DOI] [PubMed] [Google Scholar]
- 48. Hanke, C , Hess, J. , Schumacher, G. , Goebel, W. , Processing by OmpT of fusion proteins carrying the HlyA transport signal during secretion by the Escherichia coli hemolysin transport system. Mol. Gen. Genet. 1992, 233, 42–48. [DOI] [PubMed] [Google Scholar]
- 49. Fernández, L. A. , de Lorenzo, V. , Formation of disulphide bonds during secretion of proteins through the periplasmic‐independent type I pathway. Mol. Microbiol. 2001, 40, 332–346. [DOI] [PubMed] [Google Scholar]
- 50. Fraile, S. , Muñoz, A. , de Lorenzo, V. , Fernández, L. A. , Secretion of proteins with dimerization capacity by the haemolysin type I transport system of Escherichia coli . Mol. Microbiol. 2004, 53, 1109–1121. [DOI] [PubMed] [Google Scholar]
- 51. Schwarz, C. K. , Lenders, M. H. , Smits, S. H. , Schmitt, L. , Secretion of slow‐folding proteins by a type 1 secretion system. Bioengineered 2012, 3, 289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hahn, H. P. , von Specht, B. U. , Secretory delivery of recombinant proteins in attenuated Salmonella strains: Potential and limitations of type I protein transporters. FEMS Immunol. Med. Microbiol. 2003, 37, 87–98. [DOI] [PubMed] [Google Scholar]
- 53. Sugamata, Y. , Shiba, T. , Improved secretory production of recombinant proteins by random mutagenesis of hlyB, an alpha‐hemolysin transporter from E. coli . Appl. Environ. Microbiol. 2005, 71, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chung, C.W. , You, J. , Kim, K. , Moon, Y. et al., Export of recombinant proteins in Escherichia coli using ABC transporter with an attached lipase ABC transporter recognition domain (LARD). Microb. Cell. Fact. 2009, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Park, Y. , Moon, Y. , Ryoo, J. , Kim, N. et al., Identification of the minimal region in lipase ABC transporter recognition domain of Pseudomonas fluorescens for secretion and fluorescence of green fluorescent protein. Microb. Cell. Fact. 2012, 11, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eom, G. T. , Lee, S. H. , Oh, Y. H. , Choi, J. E. et al., Efficient extracellular production of type I secretion pathway‐dependent Pseudomonas fluorescens lipase in recombinant Escherichia coli by heterologous ABC protein exporters. Biotechnol. Lett. 2014, 36, 2037–2042. [DOI] [PubMed] [Google Scholar]
- 57. Palacios, J. L. , Zaror, I. , Martínez, P. , Uribe, F. et al., Subset of hybrid eukaryotic proteins is exported by the type I secretion system of Erwinia chrysanthemi . J. Bacteriol. 2001, 183, 1346–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kawai, E. , Idei, A. , Kumura, H. , Shimazaki, K. et al., The ABC‐exporter genes involved in the lipase secretion are clustered with the genes for lipase, alkaline protease, and serine protease homologues in Pseudomonas fluorescens no. 33. Biochim. Biophys. Acta. 1999, 1446, 377–382. [DOI] [PubMed] [Google Scholar]
- 59. Idei, A. , Kawai, E. , Akatsuka, H. , Omori, K. , Cloning and characterization of the Pseudomonas fluorescens ATP‐binding cassette exporter, HasDEF, for the heme acquisition protein HasA. J. Bacteriol. 1999, 181, 7545–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Akatsura, H. , Binet, R. , Kawai, E. , Wandersman, C. , Lipase secretion by bacterial hybrid ATP‐binding cassette exporters: molecular recognition of the LipBCD, PrtDEF, and HasDEF exporters. J. Bacteriol. 1997, 179, 4754–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bingle, W. H. , Nomellini, J. F. , Smit, J. , Secretion of the Caulobacter crescentus S‐Layer protein: Further localization of the C‐terminal secretion signal and its use for secretion of recombinant proteins. J. Bacteriol. 2000, 182, 3298–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Macnab, R.M. , Type III flagellar protein export and flagellar assembly. Biochim. Biophys. Acta. 2004, 1694, 207–217. [DOI] [PubMed] [Google Scholar]
- 63. Minamino, T. , Imada, K. , Namba, K. , Mechanisms of type III protein export for bacterial flagellar assembly. Mol. Biosyst. 2008, 4, 1105–1115. [DOI] [PubMed] [Google Scholar]
- 64. Minamino, T. , Protein export through the bacterial flagellar type III export pathway. Biochim. Biophys. Acta. 2013, 1843, 1642–1648. [DOI] [PubMed] [Google Scholar]
- 65. Majander, K. , Anton, L. , Antikainen, J. , Lång, H. et al., Extracellular secretion of polypeptides using a modified Escherichia coli flagellar secretion apparatus. Nat. Biotechnol. 2005, 23, 475–481. [DOI] [PubMed] [Google Scholar]
- 66. Anton, L. , Majander, K. , Savilahti, H. , Laakkonen, L. et al., Two distinct regions in the model protein Peb1 are critical for its heterologous transport out of Escherichia coli . Microb. Cell. Fact. 2010, 9, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beckwith, J. , The Sec‐dependent pathway. Res. Microbiol. 2013, 164, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lycklama a Nijeholt, J. A. , Driessen, A. J. M. , The bacterial Sec‐translocase: Structure and mechanism. Phil. Trans. R. Soc. B. 2012, 367, 1016–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Collinson, I. , Corey, R. A. , Allen, W. J. , Channel crossing: How are proteins shipped across the bacterial plasma membrane? Phil. Trans. R. Soc. B. 2015, 370, 20150025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chatzi, K. E. , Frantzeskos Sardis, M. , Economou, A. , Karamanou, S. , SecA‐mediated targeting and translocation of secretory proteins. Biochim. Biophys. Acta. 2014, 1843, 1466–1474. [DOI] [PubMed] [Google Scholar]
- 71. Bechtluft, P. , Nouwen, N. , Tans, S. J. , Driessen, A. J. M. , SecB—A chaperone dedicated to protein translocation. Mol. Biosyst. 2010, 6, 620–627. [DOI] [PubMed] [Google Scholar]
- 72. Driessen, A. J. M. , Nouwen, N. , Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 2008, 77, 643–667. [DOI] [PubMed] [Google Scholar]
- 73. Schulze, R. J. , Komar, J. , Botte, M. , Allen, W. J. et al., Membrane protein insertion and proton‐motive‐force‐dependent secretion through the bacterial holo‐translocon SecYEG‐SecDF‐YajC‐YidC. Prot. Natl. Acad. Sci. USA 2014, 111, 4844–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Egea, P. F. , Stroud, R. M. , Walter, P. , Targeting proteins to membranes: Structure of the signal recognition particle. Curr. Opin. Struct. Biol. 2005, 15, 213–220. [DOI] [PubMed] [Google Scholar]
- 75. Beck, K. , Wu, L. F. , Brunner, J. , Müller, M. , Discrimination between SRP‐ and SecA/SecB‐dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J. 2000, 19, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee, H. C. , Bernstein, H. D. , The targeting pathway of Escherichia coli presecretory and integral membrane proteins is specified by the hydrophobicity of the targeting signal. Proc. Natl. Acad. Sci. USA 2001, 98, 3471–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ignatova, Z. , Mahsunah, A. , Georgieva, M. , Kasche, V. , Improvement of posttranslational bottlenecks in the production of penicillin amidase in recombinant Escherichia coli strains. Appl. Environ. Microbiol. 2003, 69, 1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang, Z. , Kuipers, G. , Niemiec, L. , Baumgarten, T. et al., High‐level production of membrane proteins in E. coli BL21(DE3) by omitting the inducer IPTG. Microb. Cell. Fact. 2015, 14, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pérez‐Pérez, J. , Márquez, G. , Barbero, J. L. , Gutiérrez, J. , Increasing the efficiency of protein export in Escherichia coli . Biotechnology 1994, 12, 178–180. [DOI] [PubMed] [Google Scholar]
- 80. Douville, K. , Price, A. , Eichler, J. , Economou, A . et al., SecYEG and SecA are the stoichiometric components of preprotein translocase. J. Biol. Chem. 1995, 270, 20106–20111. [DOI] [PubMed] [Google Scholar]
- 81. Duong, F. , Wickner, W. , Distinct catalytic roles of the SecYE, SecG and SecDFyajC subunits of preprotein translocase. EMBO J. 1997, 16, 2756–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berges, H. , Joseph‐Liauzun, E. , Fayet, O. , Combined effects of the signal sequence and the major chaperone proteins on the export of human cytokines in Escherichia coli . Appl. Environ. Microbiol. 1996, 62, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. DeLisa, M.P. , Tullman, D. , Georgiou, G . Folding quality control in the export of proteins by the bacterial twin‐arginine translocation pathway. PNAS 2003. 100 6115–6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Patel, R. , Smith, S. M. , Robinson, C. , Protein transport by the bacterial Tat pathway. Biochim. Biophys. Acta. 2014, 1843, 1620–1628. [DOI] [PubMed] [Google Scholar]
- 85. Berks, B. C. , The twin‐arginine protein translocation pathway. Annu. Rev. Biochem. 2014, 84, 843–864. [DOI] [PubMed] [Google Scholar]
- 86. Taubert, J. , Risselada, H.J. , Mehner, D. , Lünsdorf, H. et al., TatBC‐independent TatA/Tat substrate interactions contribute to transport efficiency. PLoS One. 2015, 10, e0119761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jack, R. L. , Sargent, F. , Berks, B. C. , Sawers, G. et al., Constitutive expression of Escherichia coli tat genes indicates an important role for the twin‐arginine translocase during aerobic and anaerobic growth. J. Bacteriol. 2001, 183, 1801–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sturm, A. , Schierhorn, A. , Lindenstrauss, U. , Lilie, H. et al., YcdB from Escherichia coli reveals a novel class of Tat‐dependently translocated hemoproteins. J. Biol. Chem. 2006, 281, 13972–13978. [DOI] [PubMed] [Google Scholar]
- 89. Natale, P. , Brüser, T. , Driessen, A. J. M . Sec‐ and Tat‐mediated protein secretion across the bacterial cytoplasmic membrane—‐Distinct translocases and mechanisms. Bioch. Biophys. Acta 2008,. 1778, 1735–1756. [DOI] [PubMed] [Google Scholar]
- 90. Brüser, T. , The twin‐arginine translocation system and its capability for protein secretion in biotechnological protein production. Appl. Microbiol. Biotechnol. 2007, 76, 35–45. [DOI] [PubMed] [Google Scholar]
- 91. Albiniak, A. M. , Matos, C. F. , Branston, S. D. , Freedman, R. B. et al., High‐level secretion of a recombinant protein to the culture medium with a Bacillus subtilis twin‐ arginine translocation system in Escherichia coli . FEBS J. 2013, 280, 3810–3821. [DOI] [PubMed] [Google Scholar]
- 92. Barrett, C. M. , Ray, N. , Thomas, J. D. , Robinson, C. , Quantitative export of a reporter protein, GFP, by the twin‐arginine translocation pathway in Escherichia coli . Biochem. Biophys. Res. Commun. 2003, 304, 279–284. [DOI] [PubMed] [Google Scholar]
- 93. Matos, C. F. , Branston, S. D. , Albiniak, A. , Dhanoya, A. et al., High‐yield export of a native heterologous protein to the periplasm by the tat translocation pathway in Escherichia coli . Biotechnol. Bioeng. 2012, 109, 2533–2542. [DOI] [PubMed] [Google Scholar]
- 94. Branston, S. D. , Matos, C. F. , Freedman, R. B. , Robinson, C. et al., Investigation of the impact of Tat export pathway enhancement on E. coli culture, protein production and early stage recovery. Biotechnol. Bioeng. 2012, 109, 983–991. [DOI] [PubMed] [Google Scholar]
- 95. Browning, D. F. , Richards, K. L. , Peswani, A. R. , Roobol, J. , Busby, S. J. W. , Robinson, C. , Escherichia coli “TatExpress” strains super‐secrete human growth hormone into the bacterial periplasm by the Tat pathway. Biotechnol. Bioeng. 2017, 114, 2828–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. DeLisa, M. P. , Lee, P. , Palmer, T. , Georgiou, G. , Phage shock protein PspA of Escherichia coli relieves saturation of protein export via the Tat pathway. J. Bacteriol. 2004, 186, 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pop, O. , Martin, U. , Abel, C. , Müller, J. P. , The twin‐arginine signal peptide of PhoD and the TatAd/Cd proteins of Bacillus subtilis form an autonomous Tat translocation system. J. Biol. Chem. 2002, 277, 3268–3273. [DOI] [PubMed] [Google Scholar]
- 98. Lindenstraus, U. , Brüser, T. , Conservation and variation between Rhodobacter capsulatus and Escherichia coli Tat systems. J. Bacteriol. 2006, 188, 7807–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Oates, J. , Mathers, J. , Mangels, D. , Kühlbrandt, W. et al., Consensus structural features of purified bacterial TatABC complexes. J. Mol. Biol. 2003, 330, 277–286. [DOI] [PubMed] [Google Scholar]
- 100. Jack, R. L. , Buchanan, G. , Dubini, A. , Hatzixanthis, K. et al., Coordinating assembly and export of complex bacterial proteins. EMBO J. 2004, 23, 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li, S. Y. , Chang, B. Y. , Lin, S. C. , Coexpression of TorD enhances the transport of GFP via the TAT pathway. J. Biotechnol. 2005, 122, 412–421. [DOI] [PubMed] [Google Scholar]
- 102. Graubner, W. , Schierhorn, A. , Brüser, T. , DnaK plays a pivotal role in Tat targeting of CueO and functions beside SlyD as a general Tat signal binding chaperone. J. Biol. Chem. 2007, 282, 7116–7124. [DOI] [PubMed] [Google Scholar]
- 103. Pérez‐Rodríguez, R. , Fisher, A. C. , Perlmutter, J. D. , Hicks, M. G. et al., An essential role for the DnaK molecular chaperone in stabilizing over‐expressed substrate proteins of the bacterial twin‐arginine translocation pathway. J. Mol. Biol. 2007, 367, 715–730. [DOI] [PubMed] [Google Scholar]
- 104. Chou, M. M. , Kendall, D. A. , Polymeric sequences reveal a functional interrelationship between hydrophobicity and length of signal peptides. J. Biol. Chem. 1990, 265, 2873–2880. [PubMed] [Google Scholar]
- 105. Michaelis, S. , Hunt, J. F. , Beckwith, J. , Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli . J. Bacteriol. 1986, 167, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhou, Y. , Liu, P. , Gan, Y. , Sandoval, W. et al., Enhancing full‐length antibody production by signal peptide engineering. Microb. Cell. Fact. 2016, 15, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Paetzel, M. , Karla, A. , Strynadka, N. C. J. , Dalbey, R. E. , Signal peptidases. Chem. Rev. 2002, 102, 4549–4579. [DOI] [PubMed] [Google Scholar]
- 108. Hikita, C. , Mizushima, S. , The requirement of a positive charge at the amino terminus can be compensated for by a longer central hydrophobic stretch in the functioning of signal peptides. J. Biol. Chem. 1992, 267, 12375–12379. [PubMed] [Google Scholar]
- 109. Power, P. M. , Jones, R. A. , Beacham, I. R. , Buchholtz, C. et al., Whole genome analysis reveals a high incidence of non‐optimal codons in secretory signal sequences of Escherichia coli . Biochem. Biophys. Res. Commun. 2004, 322, 1038–1044. [DOI] [PubMed] [Google Scholar]
- 110. Zalucki, Y. M. , Jennings, M. P. , Experimental confirmation of a key role for non‐optimal codons in protein export. Biochem. Biophys. Res. Commun. 2007, 335, 143–148. [DOI] [PubMed] [Google Scholar]
- 111. Zalucki, Y. M. , Gittins, K. L. , Jennings, M. P. , Secretory signal sequence non‐optimal codons are required for expression and export of beta‐lactamase. Biochem. Biophys. Res. Commun. 2008, 366, 135–141. [DOI] [PubMed] [Google Scholar]
- 112. Zalucki Y. M., Jones C. E., Ng, P. S. , Schulz, B. L. et al., Signal sequence non‐optimal codons are required for the correct folding of mature maltose binding protein. Biochim. Biophys. Acta. 2010, 1798, 1244–1249. [DOI] [PubMed] [Google Scholar]
- 113. Liu, G. , Topping, T. B. , Randall, L. L. , Physiological role during export for the retardation of folding by the leader peptide of maltose‐binding protein. Proc. Natl. Acad. Sci. USA 1989, 86, 9213–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pratab, J. , Dikshit, K. L. , Effect of signal peptide changes on the extracellular processing of streptokinase from Escherichia coli: requirement for secondary structure at the cleavage junction. Mol. Gen. Genet. 1998, 258, 326–333. [DOI] [PubMed] [Google Scholar]
- 115. Sommer, B. , Friehs, K. , Flaschel, E. , Reck, M. et al., Extracellular production and affinity purification of recombinant proteins with Escherichia coli using the versatility of the maltose binding protein. J. Bacteriol. 2009, 140, 194–202. [DOI] [PubMed] [Google Scholar]
- 116. Thie, H. , Schirrmann, T. , Paschke, M. , Dübel, S. et al., SRP and Sec pathway leader peptides for antibody phage display and antibody fragment production in E. coli . J. Biotechnol. 2008, 25, 49–54. [DOI] [PubMed] [Google Scholar]
- 117. Pechsrichuang, P. , Songsiriritthigul, C. , Haltrich, D. , Roytrakul, S. et al., OmpA signal peptide leads to heterogenous secretion of B. subtilis chitosanase enzyme from E. coli expression system. SpringerPlus. 2016, 5, 1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Dresler, K. , van den Heuvel, J. , Müller, R.J. , Deckwer, W.D. , Production of a recombinant polyester‐cleaving from Thermobifida fusca in Escherichia coli . Bioprocess. Biosyst. Eng. 2006, 29, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Loo, T. , Patchett, M. L. , Norris, G. E. , Lott, J. S. , Using secretion to solve a solubility problem: High‐yield expression in Escherichia coli and purification of the bacterial glycoamidase PNGase F. Protein Expr. Purif. 2002, 24, 90–98. [DOI] [PubMed] [Google Scholar]
- 120. Wang, P. , Ma, J. , Zhang, Y. , Zhang, M. et al., Efficient secretory overexpression of endoinulinase in Escherichia coli and the production of inulooligosaccharides. Appl. Biochem. Biotechnol. 2016, 179, 880–894. [DOI] [PubMed] [Google Scholar]
- 121. Förster, S. , Brandt, M. , Mottok, D. S. , Zschüttig, A. et al., Secretory expression of biologically active human Herpes virus interleukin‐10 analogues in Escherichia coli via a modified Sec‐dependent transporter construct. BMC Biotechnol. 2013, 13, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kurokawa, Y. , Yanagi, H. , Yura, T. , Overproduction of bacterial protein disulphide isomerase (DsbC) and its modulator (DsbD) markedly enhances periplasmic production of human nerve growth factor in Escherichia coli . J. Biol. Chem. 2001, 276, 14393–14399. [DOI] [PubMed] [Google Scholar]
- 123. Miksch, G. , Ryu, S. , Risse, J. M. , Flaschel, E. , Factors that influence the extracellular expression of streptavidin in Escherichia coli using a bacteriocin release protein. Appl. Microbiol. Biotechnol. 2008, 81, 319–326. [DOI] [PubMed] [Google Scholar]
- 124. Xu, R. , Du, P. , Fan, J. J. , Zhang, Q. et al., High‐level expression and secretion of recombinant mouse endostatin by Escherichia coli . Protein Expr. Purif. 2002, 24, 453–459. [DOI] [PubMed] [Google Scholar]
- 125. Schierle, C. F. , Berkmen, M. , Huber, D. , Kumamoto, C. et al., The DsbA signal sequence directs efficient, cotranslational export of passenger proteins to the Escherichia coli periplasm via the signal recognition particle pathway. J. Bacteriol. 2003, 185, 5706–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Durrani, F. G. , Gul, R. , Sadaf, S. , Akhtar, M. W. , Expression and rapid purification of recombinant biologically active ovine growth hormone with DsbA targeting to Escherichia coli inner membrane. Appl. Microbiol. Biotechnol. 2015, 99, 6791–6801. [DOI] [PubMed] [Google Scholar]
- 127. Huber, D. , Boyd, D. , Xia, Y. , Olma, M. H. et al., Use of thioredoxin as a reporter to identify a subset of Escherichia coli signal sequences that promote signal recognition particle‐dependent translocation. J. Bacteriol. 2005, 187, 2983–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Steiner, D. , Forrer, P. , Stumpp, M. T. , Plückthun, A. , Phage display using cotranslational translocation of fusion polypeptides. US Patent 8846577 B2, 2014.
- 129. Zhou, Y. , Ueda, T. , Müller, M. , Signal recognition particle and SecA cooperate during export of secretory proteins with highly hydrophobic signal sequences. PLoS ONE 2014, 9, e92994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Knapp, S. , Amann, E. , Abel, K. J. , Signalpeptid für die Sekretion von Peptiden in Escherichia coli, Verfahren zu seiner Gewinnung und seine Verwendung. EP 0391022 A2, 1990.
- 131. Low, K. O. , Jonet, M. A. , Ismail, N. F. , Illias, R. M. , Optimization of a Bacillus sp signal peptide for improved recombinant protein secretion and cell viability in Escherichia coli: Is there an optimal signal peptide design? Bioengineered 2012, 3, 334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sroga, G. E. , Dordick, J. S. , A strategy for in vivo screening of subtilisin E reaction specificity in E. coli periplasm. Biotechnol. Bioeng. 2002, 78, 761–769. [DOI] [PubMed] [Google Scholar]
- 133. Mergulhão, F. J. , Monteiro, G. A. , Analysis of factors affecting the periplasmic production of recombinant proteins in Escherichia coli . J. Microbiol. Biotechnol. 2007, 17, 1236–1241. [PubMed] [Google Scholar]
- 134. Ujiie, A. , Nakano, H. , Iwasaki, Y. , Extracellular production of Pseudozyma (Candida) Antarctica lipase B with genuine primary sequence in recombinant Escherichia coli . J. Biosci. Bioeng. 2016, 121, 303–309. [DOI] [PubMed] [Google Scholar]
- 135. Takemori, D. , Yoshino, K. , Eba, C. , Nakano, H. et al., Extracellular production of phospholipase A2 from Streptomyces violaceoruber by recombinant Escherichia coli . Protein Expr. Purif. 2012, 81, 145–150. [DOI] [PubMed] [Google Scholar]
- 136. Berks, B.C. , Palmer T., Sargent, F. , Protein targeting by the bacterial twin‐arginine translocation (Tat) pathway. Curr. Opin. Microbiol. 2005. 8 174–181 [DOI] [PubMed] [Google Scholar]
- 137. Ignatova, Z. , Hörnle, C. , Nurk, A. , Kasche, V. , Unusual signal peptide directs penicillin amidase from Escherichia coli to the Tat translocation machinery. Biochem. Biophys. Res. Commun. 2002, 291, 146–149. [DOI] [PubMed] [Google Scholar]
- 138. Cristóbal, S. , de Gier, J. W. , Nielsen, H. , von Heijne, G. , Competition between Sec‐ and TAT‐dependent protein translocation in Escherichia coli . EMBO J. 1999, 18, 2982–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tullman‐Ercek, D. , DeLisa, M. P. , Kawarasaki, Y. , Iranpour, P. et al., Export pathway selectivity of Escherichia coli twin arginine translocation signal peptides. J. Biol. Chem. 2007, 282, 8309–8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li, Y. D. , Zhou, Z. , Lv, L. X. , Hou, X. P. et al., New approach to achieve high‐level secretory expression of heterologous proteins by using Tat signal peptide. Protein Pept. Lett. 2009, 16, 706–710. [DOI] [PubMed] [Google Scholar]
- 141. Fröbel, J. , Rose, P. , Müller, M. , Twin‐arginine‐dependent translocation of folded proteins. Philos. Trans. R Soc. Lond. B Biol. Sci. 2012, 367, 1029–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yang, C. , Freudl, R. , Qiao, C. , Export of methyl parathion hydrolase to the periplasm by the twin‐arginine translocation pathway in Escherichia coli . J. Agric. Food Chem. 2009, 57, 8901–8905. [DOI] [PubMed] [Google Scholar]
- 143. Kreutzenbeck, P. , Kröger, C. , Lausberg, F. , Blaudeck, N. et al., Escherichia coli twin arginine (Tat) mutant translocases possessing relaxed signal peptide recognition specificities. J. Biol. Chem. 2007, 282, 7903–7911. [DOI] [PubMed] [Google Scholar]
- 144. Kang, D. G. , Lim, G. B. , Cha, H. J. , Functional periplasmic secretion of organophosphorous hydrolase using the twin‐arginine translocation pathway in Escherichia coli . J. Biotechnol. 2005, 118, 379–385. [DOI] [PubMed] [Google Scholar]
- 145. Jong, W. S. P. , Vikström, D. , Houben, D. , van den Berg van Saparoea, H. B. et al., Application of an E. coli signal sequence as a versatile inclusion body tag. Microb. Cell Fact. 2017, 16, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Monroy‐Lagos, O. , Soberon, X. , Gaytan, P. , Osuna, J. , Improvement of an unusual twin‐ arginine transporter leader peptide by a codon‐based randomization approach. Appl. Environ. Microbiol. 2006, 72, 3797–3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ostendorp, R. , Popp, A. , Fischer, M. , Expression of full length IgG and secretion into the culture medium of prokaryotic cells. WO Patent 2009021548 A1, 2009.
- 148. Tarry, M. , Arends, S.J. , Roversi, P. , Piette, E. et al., The Escherichia coli cell division protein and model Tat substrate SufI (FtsP) localizes to the septal ring and has a multicopper oxidase‐like structure. J. Mol. Biol. 2009, 386, 504–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Holzapfel, E. , Moser, M. , Schiltz, E. , Ueda, T. et al., Twin‐arginine‐dependent translocation of SufI in the absence of cytosolic helper proteins. Biochemistry 2009, 48, 5096–5105. [DOI] [PubMed] [Google Scholar]
- 150. Kang, D. G. , Seo, J. H. , Jo, B. H. , Kim, C. S. et al., Versatile signal peptide of Flavobacterium‐originated organophosphorus hydrolase for efficient periplasmic translocation of heterologous proteins in Escherichia coli . Biotechnol. Prog. 2016, 32, 848–854. [DOI] [PubMed] [Google Scholar]
- 151. Esbelin, J. , Martin, C. , Forano, E. , Mosoni, P. , Differential translocation of green fluorescent protein fused to signal sequences of Ruminococcus albus cellulases by the Tat and Sec pathways of Escherichia coli . FEMS Microbiol Lett. 2009, 294, 239–244. [DOI] [PubMed] [Google Scholar]
- 152. Velaithan, V. , Chin, S. C. , Yusoff, K. , Illias, R. M. et al., Novel synthetic signal peptides for the periplasmic secretion of green fluorescent protein in Escherichia coli . Ann. Microbiol. 2014, 64, 543–550. [Google Scholar]
- 153. Böck, A. , Seydel, W. , Schmid, G. , Müller, G. , Sekretormutante von Escherichia coli. EP 0338410 B1, 1994.
- 154. Lunn, C. A. , Narula, S. K. , Reim, R. L. , E. coli secretory strains. EP 0497757 B1, 1994.
- 155. Thomason, L. C. , Costantino, N. , Court, D. L. , E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 2007, 79, 1.17.1–1.17.8. [DOI] [PubMed] [Google Scholar]
- 156. Tiruvadi Krishnan, S. , Moolman, M. C. , van Laar, T. , Meyer, A. S. et al., Essential validation methods for E. coli strains created by chromosome engineering. J. Biol. Eng. 2015, 9, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Datsenko, K.A. , Wanner, B.L. , One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Baba, T. , Ara, T. , Hasegawa, M. , Takai, Y. et al., Construction of Escherichia coli K‐12 in‐ frame, single‐gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yamamoto, N. , Nakahigashi, K. , Nakamichi, T. , Yoshino, M. et al., Update on the Keio collection of Escherichia coli single‐gene deletion mutants. Mol. Syst. Biol. 2009, 5, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gallagher, R. R. , Li, Z. , Lewis, A. O. , Isaacs, F. J. Rapid editing and evolution of bacterial genomes using libraries of synthetic DNA. Nat. Protocols 2014, 9, 2301–2316 [DOI] [PubMed] [Google Scholar]
- 161. Sander, J. D. , Joung, J. K. , CRISPR‐Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Damian, M. , Porteus, M. H. , A crisper look at genome editing: RNA‐guided genome modification. Mol. Ther. 2013, 21, 720–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Pyne, M. E. , Moo‐Young, M. , Chung, D. A. , Chou, C. P. , Coupling the CRISPR/Cas9 system with lambda Red recombineering enables simplified chromosomal gene replacement in Escherichia coli . Appl. Environ. Microbiol. 2015, 81, 5103–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Jiang, Y. , Chen, B. , Duan, C. , Sun, B. et al., Multigene editing in the Escherichia coli genome via the CRISPR‐Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li, Y. , Lin, Z. , Huang, C. , Zhang, Y. et al., Metabolic engineering of Escherichia coli using CRISPR‐Cas9 meditated genome editing. Metab. Eng. 2015, 31, 13–21. [DOI] [PubMed] [Google Scholar]
- 166. Lopes, J. , Gottfried, S. , Rothfield, L. , Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: Isolation of “periplasmic leaky” mutants. J. Bacteriol. 1972, 109, 520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Munekazu, T. , Udaka, S. , Isolation and characterization of protein‐leaky mutants of Escherichia coli . Agric. Biol. Chem. 1978, 42, 1157–1166. [Google Scholar]
- 168. Lazzaroni, J. C. , Portalier, R. C. , Atlan, D. , Isolation and preliminary characterization of periplasmic‐leaky mutants of Escherchia coli K‐12. FEMS Microbiol. Lett. 1979, 5, 411–416. [DOI] [PubMed] [Google Scholar]
- 169. Atlan, D. , Portalier, R. C. , Optimized extracellular production of alkaline phosphatase by lky mutants of Escherichia coli K12. Appl. Microbiol. Biotechnol. 1984, 19, 5–12. [Google Scholar]
- 170. Lazzaroni, J. C. , Portalier, R. C. , Genetic and biochemical characterization of periplasmic‐leaky mutants of Escherichia coli K‐12. J. Bacteriol. 1981, 145, 1351–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lazzaroni, J.C. , Fognini‐Lefebvre, N. , Portalier, R. , Cloning of the excC and excD genes involved in the release of periplasmic proteins by Escherichia coli K12. Mol. Gen. Genet. 1989, 218, 460–464. [DOI] [PubMed] [Google Scholar]
- 172. Fognini‐Lefebvre, N. , Lazzaroni, J. C. , Portalier, R. , tolA, tolB and excC, three cistrons involved in the control of pleiotropic release of periplasmic proteins by Escherichia coli K12. Mol. Gen. Genet. 1987, 209, 391–395. [DOI] [PubMed] [Google Scholar]
- 173. Anderson, J.J. , Wilson, J.M. , Oxender, D.L. , Defective transport and other phenotypes of a periplasmic “leaky” mutant of Escherichia coli K‐12. J. Bacteriol. 1979, 140, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lazzaroni, J.C. , Vianney, A. , Amouroux, C. , Portalier, R. , The excC and excD genes of Escherichia coli K‐12 encode the peptidoglycan‐associated lipoprotein (PAL) and the TolQ protein, respectively. In: James R., Lazdunski C., Pattus F. (Eds.), Bacteriocins, Microcins and Lantibiotics. Springer‐Verlag, Berlin Heidelberg, 1992. pp. 487–491. [Google Scholar]
- 175. Rodolakis, A. , Casse, F. , Starka, J. , Morphological mutants of Escherichia coli K12. Mapping of the envC mutation. Mol. Gen. Genet. 1974, 130, 177–181. [DOI] [PubMed] [Google Scholar]
- 176. Young, K. , Silver, L. L. , Leakage of periplasmic enzymes from envA1 strains of Escherichia coli . J. Bacteriol. 1991, 173, 3609–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hirota, Y. , Suzuki, H. , Nishimura, Y. , Yasuda, S. , On the process of cellular division in Escherichia coli: A mutant of E. coli lacking a murein‐lipoprotein. Proc. Natl. Acad. Sci. USA 1977, 74, 1417–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Yem, D. W. , Wu, H. C. , Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J. Bacteriol. 1978, 133, 1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Giam, C. Z. , Chai, T. , Hayashi, S. , Wu, H. C. , Prolipoprotein modification and processing in Escherichia coli. A unique secondary structure in prolipoprotein signal sequence for the recognition by glyceryl transferase. Eur. J. Biochem. 1984, 141, 331–337. [DOI] [PubMed] [Google Scholar]
- 180. Sonntag, I. , Schwarz, H. , Hirota, Y. , Henning, U. , Cell envelope and shape of Escherichia coli: Multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J. Bacteriol. 1978, 136, 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Van Alphen, W. , Lugtenberg, B. , Berendsen, W. , Heptose‐deficient mutants of Escherichia coli K12 deficient in up to three major outer membrane proteins. Mol. Gen. Genet. 1976, 147, 263–269. [DOI] [PubMed] [Google Scholar]
- 182. Dassler, T. , Wich, G. , Verfahren zur fermentativen Herstellung von Antikörpern. EP 1903115 A1, 2008.
- 183. Bernadac, A. , Gavioli, M. , Lazzaroni, J. C. , Raina, S. , Escherichia coli tol‐pal mutants form outer membrane vesicles. J. Bacteriol. 1998, 180, 4872–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Schmid, G. , Habermann, P. , Sekretion von Hirudinderivaten. EP0448093 B1, 1996.
- 185. Shin, H. D. , Chen, R. R. , Extracellular recombinant protein production from an Escherichia coli lpp deletion mutant. Biotechnol. Bioeng. 2008, 101, 1288–1296. [DOI] [PubMed] [Google Scholar]
- 186. Orr, V. , Scharer, J. , Moo‐Young, M. , Honeyman, C. H. et al., Integrated development of an effective bioprocess for extracellular production of penicillin G acylase in Escherichia coli and its subsequent one‐step purification. J. Biotechnol. 2012, 161, 19–26. [DOI] [PubMed] [Google Scholar]
- 187. Müller, J. M. , Wetzel, D. , Flaschel, E. , Friehs, K. et al., Constitutive production and efficient secretion of soluble full‐length streptavidin by an Escherichia coli ‘leaky mutant’. J. Biotechnol. 2016, 221, 91–100. [DOI] [PubMed] [Google Scholar]
- 188. Dassler, T. , Wich, G. , Schmid, G. , Verfahren zur fermentativen Herstellung von Proteinen. EP 1903105 B1, 2010.
- 189. Dassler, T. , Mitterweger, S. , Wich, G. , Verfahren zur fermentativen Herstellung von heterologen Proteinen mittels Escherichia coli. EP2204441 B1, 2011.
- 190. Chen, Z. Y. , Cao, J. , Xie, L. , Li, X. F. et al., Construction of leaky strains and extracellular production of exogenous proteins in recombinant Escherichia coli . Microb. Biotechnol. 2014, 7, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Natarajan, A. , Haitjema C. H., Lee, R. , Boock, J. T. et al., An engineered survival‐selection assay for extracellular protein expression uncovers hypersecretory phenotypes in Escherichia coli . ACS Synth. Biol. 2017, 6, 875–883. [DOI] [PubMed] [Google Scholar]
- 192. Leonhardsberger, S. , E. coli expression system efficiently secretes recombinant proteins into culture broth. BioProcess Int. 2006, 4, 64–66. [Google Scholar]
- 193. Richter, H. , Koebsch, I. , Microbial secretion via Esetec technology: System was developed to creaste a cost‐efficient alternative to mammalian cell culture. Genet. Ent. Biotechn. N. 2017, 37, 22–23. [Google Scholar]
- 194. Hoischen, C. , Gumpert, J. , Kujau, J. M. , Fritsche, C. et al., Novel l‐form bacterial strains, method for producing same and the use thereof for producing gene products,WO patent 2001066776 A2, 2001.
- 195. Gumpert, J. , Hoischen, C. , Use of cell wall‐less bacteria (L‐forms) for efficient expression and secretion of heterologous gene products. Curr. Opin. Biotechnol. 1998, 9, 506–509. [DOI] [PubMed] [Google Scholar]
- 196. Gumpert, J. , Cron, H. , Plapp, R. , Niersbach, H. et al., Synthesis and secretion of recombinant penicillin G acylase in bacterial L‐forms. J. Basic Microbiol. 1996, 36, 89–98. [DOI] [PubMed] [Google Scholar]
- 197. Kujau, M. J. , Hoischen, C. , Riesenberg, D. , Gumpert, J. , Expression and secretion of functional miniantibodies McPC603scFvDhlx in cell‐wall‐less L‐form strains of Proteus mirabilis and Escherichia coli: A comparison of the synthesis capacities of L‐form strains with an E. coli producer strain. Appl. Microbiol. Biotechnol. 1998, 49, 51–58. [DOI] [PubMed] [Google Scholar]
- 198. Hoischen, C. , Fritsche, C. , Gumpert, J. , Westermann, M. et al., Novel bacterial membrane surface display system using cell wall‐less L‐forms of Proteus mirabilis and Escherichia coli . Appl. Environ. Microbiol. 2002, 68, 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Ojima, Y. , Yamaguchi, K. , Taya, M . Quantitative evaluation of recombinant protein packaged into outer membrane vesicles of Escherichia coli cells. Biotechnol. Prog. 2018, 34, 51–57. [DOI] [PubMed] [Google Scholar]
- 200. Sommer, B. , Neue Strategien zur extrazellulären Produktion rekombinanter Proteine mit Escherichia coli. Dissertation. Bielefeld University, 2008. [Google Scholar]
- 201. Van der Wal, F.J. , Luirink, J. , Oudega, B. , Bacteriocin release proteins: Mode of action, structure, and biotechnological application. FEMS Microbiol. Rev. 1995, 17, 381–399. [DOI] [PubMed] [Google Scholar]
- 202. Sommer, B. , Friehs, K. , Flaschel, E. , Efficient production of extracellular proteins with Escherichia coli by means of optimized coexpression of bacteriocin release proteins. J. Biotechnol. 2010, 145, 350–358. [DOI] [PubMed] [Google Scholar]
- 203. Miksch, G. , Kleist, S. , Friehs, K. , Flaschel, E. , Overexpression of the phytase from Escherichia coli and its extracellular production in bioreactors. Appl. Microbiol. Biotechnol. 2002, 59, 685–694. [DOI] [PubMed] [Google Scholar]
- 204. Beshay, U. , Miksch, G. , Friehs, K. , Flaschel, E. , Increasing the secretion ability of the kil gene for recombinant proteins in Escherichia coli by using a strong stationary‐phase promoter. Biotechnol. Lett. 2007, 29, 1893–1901. [DOI] [PubMed] [Google Scholar]
- 205. Spexard, M. , Beshay, U. , Risse, J.M. , Miksch, G. et al., Screening for conditions of enhanced production of a recombinant β‐glucanase secreted into the medium by Escherichia coli . Biotechnol. Lett. 2010, 32, 243–248. [DOI] [PubMed] [Google Scholar]
- 206. Bläsi, U. , Kalousek, S. , Lubitz, W. , A bifunctional vector system for controlled expression and subsequent release of the cloned gene product by phi X174 lysis protein‐E. Appl. Microbiol. Biotechnol. 1990, 33, 564–568. [DOI] [PubMed] [Google Scholar]
- 207. Jechtlinger, W. , Szostak, M. P. , Witte, A. , Lubitz, W. , Altered temperature induction sensitivity of the lambda pR/cI857 system for controlled gene E expression in Escherichia coli . FEMS Microbiol. Lett. 1999, 173, 347–352. [DOI] [PubMed] [Google Scholar]
- 208. Ehgartner, D. , Sagmeister, P. , Langemann, T. , Meitz, A. et al., A novel method to recover inclusion body protein from recombinant E. coli fed‐batch processes based on phage phi X174‐derived lysis protein E. Appl. Microbiol. Biotechnol. 2017, 101, 5603–5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Morita, M. , Asami, K. , Tanji, Y. , Unno, H. , Programmed Escherichia coli cell lysis by expression of cloned T4 phage lysis genes. Biotechnol. Prog. 2001, 17, 573–576. [DOI] [PubMed] [Google Scholar]
- 210. Cai, Z. , Xu, W. , Xue, R. , Lin, Z. , Facile, reagentless and in situ release of Escherichia coli intracellular enzymes by heat‐inducible autolytic vector for high‐throughput screening. Protein Eng. Des. Sel. 2008, 21, 681–687. [DOI] [PubMed] [Google Scholar]
- 211. Su, L. , Xu, C. , Woodard, R. W. , Chen, J. et al., A novel strategy for enhancing extracellular secretion of recombinant proteins in Escherichia coli . Appl. Microbiol. Biotechnol. 2013, 97, 6705–6713. [DOI] [PubMed] [Google Scholar]
- 212. Su, L. , Woodard, R. W. , Chen, J. , Wu, J. , Extracellular location of Thermobifida fusca cutinase expressed in Escherichia coli BL21(DE3) without mediation of a signal peptide. Appl. Environ. Microbiol. 2013, 79, 4192–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Su, L. , Yu, L. , Xu, C. , Wu, J. , Extracellular expression of Thermobifida fusca cutinase with pelB signal peptide depends on more than type II secretion pathway in Escherichia coli . J. Biotechnol. 2015, 204, 47–52. [DOI] [PubMed] [Google Scholar]
- 214. Su, L. , Jiang, Q. , Yu, L. , Wu, J. , Enhanced extracellular production of recombinant proteins in Escherichia coli by co‐expression with Bacillus cereus phospholipase C. Microb. Cell. Fact. 2017, 16, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Newton, J. M. , Schofield, D. , Vlahopoulou, J. , Zhou, Y . Detecting cell lysis using viscosity monitoring in E. coli fermentation to prevent product loss. Biotechnol. Prog. 2016, 32, 1069–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Zhang, G. , Brokx, S. , Weiner, J. H. , Extracellular accumulation of recombinant proteins fused to the carrier protein YebF in Escherichia coli . Nat. Biotechnol. 2006, 24, 100–104. [DOI] [PubMed] [Google Scholar]
- 217. Prehna, G. , Zhang, G. , Gong, X. , Duszyk, M. et al., A protein export pathway involving Escherichia coli porins. Structure 2012, 20, 1154–1166. [DOI] [PubMed] [Google Scholar]
- 218. Weiner, J. , Zhang, G. , Protein production method utilizing yebf. WO Patent 2006017929 A1, 2006.
- 219. Schlösser, T. , Dassler, T. , Pfeiffer‐Schwiesow, K. , DNS‐Konstrukt und Verfahren zur fermentativen Herstellung von Fusionsproteinen. EP 1916305 A1, 2010.
- 220. Quan, S. , Koldewey, P. , Tapley, T. , Kirsch, N. et al., Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat. Struct. Mol. Biol. 2011, 18, 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Jeong, K. J. , Lee, S. Y. , Excretion of human β‐endorphin into culture medium by using outer membrane protein F as a fusion partner in recombinant Escherichia coli . Appl. Environ. Microbiol. 2002, 86, 4979–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Ertl, J. , Habermann, P. , Fusion protein comprising hirudin and proinsulin or insulin. EP 1364029 B1, 2005.
- 223. Kim, S. K. , Min, W. K. , Park, Y. C. , Seo, J. H. , Application of repeated aspartate tags to improving extracellular production of Escherichia coli L‐asparaginase isozyme II. Enzyme Microb. Technol. 2015, 70–80, 49–54. [DOI] [PubMed] [Google Scholar]
- 224. Kim, S. K. , Park, Y. C. , Lee, H. H. , Jeon, S. T. et al., Simple amino acid tags improve both expression and secretion of Candida antarctica lipase B in recombinant Escherichia coli . Biotechnol. Bioeng. 2015, 112, 346–355. [DOI] [PubMed] [Google Scholar]
- 225. Fleetwood, F. , Andersson, K. G. , Stahl, S. , Löfblom, J. , An engineered autotransporter‐based surface expression vector enables efficient display of Affibody molecules on OmpT‐negative E. coli as well as protease‐mediated secretion in OmpT‐positive strains. Microb. Cell Fact. 2014, 13, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sevastsyanovic, Y. R. , Leyton, D. L. , Wells, T. J. , Wardius, C. A. et al., A generalised module for the selective extracellular accumulation of recombinant proteins. Microb. Cell Fact. 2012, 11, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Jong, W. S. , Soprova, Z. , de Punder, K. , ten Hagen‐Jongman, C. M. et al., A structurally informed autotransporter platform for efficient heterologous protein secretion and display. Microb. Cell Fact. 2012, 11, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Li, Z. , Gu, Z. , Wang, M. , Du, G. et al., Delayed supplementation of glycine enhances extracellular secretion of the recombinant alpha‐cyclodextrin glycosyltransferase in Escherichia coli . Appl. Microbiol. Biotechnol. 2010, 85, 553–561. [DOI] [PubMed] [Google Scholar]
- 229. Duan, X. , Zou, C. , Wu, J. , Triton X‐100 enhances the solubility and secretion ratio of aggregation‐prone pullulanase produced in Escherichia coli . Bioresour. Technol. 2015, 194, 137–143. [DOI] [PubMed] [Google Scholar]
- 230. Yang, J. , Moyana, T. , MacKenzie, S. , Xia, Q. et al., One hundred seventy‐fold increase in excretion of an FV fragment‐tumor necrosis factor alpha fusion protein (sFV/TNF‐alpha) from Escherichia coli caused by the synergistic effects of glycine and triton X‐100. Appl. Environ. Microbiol. 1998, 64, 2869–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Bao, R. M. , Yang, H. M. , Yu, C. M. , Zhang, W. F. et al., An efficient protocol to enhance the extracellular production of recombinant protein from Escherichia coli by the synergistic effects of sucrose, glycine, and Triton X‐100. Protein Expr. Purif. 2016, 126, 9–15. [DOI] [PubMed] [Google Scholar]
- 232. Fu, X. Y. , Tong, W. Y. , Wei, D. Z. , Extracellular production of human parathyroid hormone as a thioredoxin fusion form in Escherichia coli by chemical permeabilization combined with heat treatment. Biotechnol. Prog. 2005, 21, 1429–1435. [DOI] [PubMed] [Google Scholar]
- 233. Liu, Z. , Tian, L. , Chen, Y. , Mou, H. , Efficient extracellular production of κ‐carrageenase in Escherichia coli: effects of wild‐type signal sequence and process conditions on extracellular secretion. J. Biotechnol. 2014, 185, 8–14. [DOI] [PubMed] [Google Scholar]
- 234. Voulgaris, I. , Finka, G. , Uden, M. , Hoare, M. , Enhancing the selective extracellular location of a recombinant E. coli domain antibody by management of fermentation conditions. Appl. Microbiol. Biotechnol. 2015, 99, 8441–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wurm, D. J. , Slouka, C. , Bosilj, T. , Herwig, C. et al., Comparative analysis of how to trigger periplasmic release in recombinant E. coli cultivations. Eng. Life Sci. 2017, 17, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Brooke, B. , Hopkins, J. , Laflamme, E. , Wark, P. , Effects of temperature‐induced changes in membrane composition on transformation efficiency in E. coli DH5α. JEMI. 2009, 13, 71–74. [Google Scholar]
- 237. Jazini, M. , Herwig, C. , Effects of temperature shifts and oscillations on recombinant protein production expressed in Escherichia coli . Bioprocess Biosyst. Eng. 2013, 36, 1571–1577. [DOI] [PubMed] [Google Scholar]
- 238. Jazini, M. , Herwig, C. , Substrate oscillations boost recombinant protein release from Escherichia coli . Bioprocess Biosyst. Eng. 2013, 37, 881–890. [DOI] [PubMed] [Google Scholar]
- 239. Shokri, A. , Sandén, A.M. , Larsson, G. , Growth rate‐dependent changes in Escherichia coli membrane structure and protein leakage. Appl. Microbiol. Biotechnol. 2002, 58, 386–392. [DOI] [PubMed] [Google Scholar]
- 240. Lindner, R. , Moosmann, A. , Dietrich, A. , Böttinger, H. et al., Process development of periplasmatically produced single chain fragment variable against epidermal growth factor receptor in Escherichia coli . J. Biotechnol. 2014, 192 Pt A, 136–145. [DOI] [PubMed] [Google Scholar]
- 241. Jalalirad, R. , Selective and efficient extraction of recombinant proteins from the periplasm of Escherichia coli using low concentrations of chemicals. J. Ind. Microbiol. Biotechnol. 2013, 40, 1117–1129. [DOI] [PubMed] [Google Scholar]
- 242. Sieuwerts, S. , de Bok, F. A. , Mols, E. , de Vos, W.M. et al., A simple and fast method for determining colony forming units. Lett. Appl. Microbiol. 2008, 47, 275–278. [DOI] [PubMed] [Google Scholar]
- 243. Brugger, S.D. , Baumberger, C. , Jost, M. , Jenni, W. et al., Automated counting of bacterial colony forming units on agar plates. PLoS One. 2012, 7, e33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Keer, J.T. , Birch, L. , Molecular methods for the assessment of bacterial viability. J. Microbiol. Methods. 2003, 53, 175–183. [DOI] [PubMed] [Google Scholar]
- 245. Rajamanickam, V. , Wurm, D. , Slouka, C. , Herwig, C. et al., A novel toolbox for E. coli lysis monitoring. Anal. Bioanal. Chem. 2016, 409, 667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Wurm, D.J. , Marschall, L. , Sagmeister, P. , Herwig, C. , Spadiut, O. , Simple monitoring of cell leakiness and viability in Escherichia coli bioprocesses—A case study. Eng. Life Sci. 2017, 17, 598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Stiefel, P. , Schmidt‐Emrich, S. , Maniura‐Weber, K. , Ren, Q . Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Kwong, K. W. , Wong, W. K. , A revolutionary approach facilitating co‐expression of authentic human epidermal growth factor and basic fibroblast growth factor in both cytoplasm and culture medium of Escherichia coli . Appl. Microbiol. Biotechnol. 2013, 97, 9071–9080. [DOI] [PubMed] [Google Scholar]


