Abstract
Purpose
According to the 2015 American Geriatrics Society (AGS) Beers criteria, most antipsychotics are inappropriate in Parkinson’s disease (PD) patients due to the risk of worsening Parkinsonian symptoms. This study examined the incidence and predictors of inappropriate antipsychotic use among long-term care residents with PD and comorbid depression.
Patients and Methods
This retrospective cohort study utilized 2007–2009 Minimum Data Set (MDS) linked to Chronic Condition Warehouse (CCW) Medicare data files involving patients with PD and comorbid depression. Using a 12-month baseline and a 24-month follow-up, the study examined incidence of inappropriate atypical antipsychotics, namely asenapine, brexpiprazole, iloperidone, lurasidone, olanzapine, paliperidone, risperidone, or ziprasidone as specified in the 2015 AGS Beers criteria. Appropriate atypical antipsychotic included aripiprazole, clozapine, or quetiapine. Multivariable logistic regression was used to examine various sociodemographic and clinical factors associated with inappropriate antipsychotic use in PD based on the Andersen Behavioral Model.
Results
The incidence of atypical antipsychotic use was 17.50% (13,352/76,294) among PD patients over a 2-year follow-up. The percentage of inappropriate use among atypical antipsychotic users was 36.32%. The likelihood of inappropriate antipsychotic use was higher for patients who had dementia (OR=1.22, 95% CI: 1.12–1.33) or Chronic Obstructive Pulmonary Disease ((OR=1.13, 95% CI: 1.03–1.24). However, patients who were taking levodopa (OR=0.62, 95% CI: 0.57–0.67), dopamine agonists (OR=0.90, 95% CI: 0.82–0.98), Catechol-O-methyltransferase (COMT) inhibitors (OR=0.77, 95% CI: 0.68–0.86), Monoamine Oxidase (MAO) inhibitors type B (OR=0.72, 95% CI: 0.60–0.86), or amantadine (OR=0.84, 95% CI: 0.71–0.98) were less likely to receive inappropriate antipsychotics.
Conclusion
More than one-third of PD patients used inappropriate antipsychotics among those who were treated with atypical antipsychotic medications. Various socio-demographics and clinical factors were associated with inappropriate antipsychotic use in older patients with PD. Concerted efforts are needed to reduce inappropriate atypical antipsychotic use among PD patients.
Keywords: Parkinson disease, psychotic disorders, antipsychotic agents
Introduction
Parkinson’s disease (PD) is characterized by loss of dopaminergic neurons and associated with various motor and non-motor symptoms.1 The prevalence of PD is estimated to be 0.3% and 1% in general population and older adults aged over 65 years, respectively.2,3 PD primarily affects voluntary movements; however, lack of dopamine leads to neurobehavioral symptoms and other non-motor features.1 Both motor and non-motor symptoms contribute to impaired quality of life of patients with PD.4 Dopaminergic medications act as dopamine replacement (i.e. levodopa) or agonist for dopamine receptors.5 These medications can alleviate movement-related symptoms of PD; however, the use of antiparkinson agents is associated with adverse events such as dopaminergic psychosis.6 For patients with PD, the probability of psychosis is between 25% and 60% in their lifetime course, depending on the diagnostic criteria used.6 The most common symptom of PD psychosis is visual hallucination while delusions and systemized hallucinations are associated with sever psychosis.6 In addition, psychotic features in PD patients might relate to underlying comorbidities such as dementia.7
Although antipsychotics are recommended for the treatment of psychotic symptoms, these medications are implicated for increased risk of adverse events in the elderly population and specifically in patients with PD.8 According to the 2015 American Geriatrics Society (AGS) Beers criteria, most antipsychotics are inappropriate in PD patients due to the risk of worsening Parkinsonian symptoms.9 Three atypical antipsychotics (i.e. aripiprazole, clozapine, and quetiapine) have been excluded from the list of high-risk medications in PD, based on expert review of evidence.9 This is different from the American Academy of Neurology (AAN) guideline which does not recommend aripiprazole for patients with PD psychosis.10 Pimavanserin, a novel treatment for PD psychosis was approved by FDA after these guidelines were published.11
The affinity for dopamine D2 receptors varies across atypical antipsychotics which can explain biological plausibility of interaction between inappropriate antipsychotics and Parkinsonian symptoms.12,13 The efficacy of antipsychotics in patients with dopaminergic psychosis has been evaluated in small clinical trials.2,14 These clinical trials provided convincing evidence that clozapine is effective in the treatment of PD psychosis, while there are mixed findings regarding the efficacy of other atypical antipsychotics.2,14 Additionally, cholinesterase inhibitors can improve psychotic symptoms in PD patients, according to a clinical study of cholinesterase inhibitors.11
Clozapine is not commonly used in clinical practice due to the risk of adverse events such as agranulocytosis and sedation.6,15 Previous studies have shown that quetiapine is the most frequently used antipsychotic drug in PD and a relatively high number of patients with PD receive inappropriate antipsychotics such as risperidone for the treatment of psychotic symptoms.15 A cohort study of Veterans Affairs data found that antipsychotic agents were prescribed for 50% of patients with PD psychosis within one year of follow-up and quetiapine was prescribed for 66% of patients who received antipsychotic agents.15 Risperidone, an inappropriate medication in PD was prescribed for 17.3% of patients who received antipsychotic treatment.15 Fewer studies have evaluated the use of inappropriate atypical antipsychotics, specifically in elderly patients with PD. This study aimed to evaluate the extent of inappropriate antipsychotics use and thus examined the incidence and factors associated with the use of these medications in PD patients residing in nursing homes. The study can help increase the awareness of inappropriate antipsychotic use in patients with PD, and thus help providers to optimize use of these medications.
Materials and Methods
Data Source
The study utilized 2007–2009 Minimum Data Set (MDS) cross-linked to Chronic Condition Warehouse (CCW) data files. The original database involved patients with a diagnosis of depression residing in nursing homes. Under a new Data Use Agreement with Center for Medicaid and Medicare Services (CMS), the sub-cohort of PD patients was selected for the purpose of this study. The MDS and CCW datasets contained Research Identifiable Files obtained from Center for Medicaid and Medicare Services (CMS). The MDS was completed by trained nurses via direct face-to-face interviews within 7–14 days of admission/readmission and during subsequent evaluations on a quarterly basis or when any significant change occurred in the resident health status.16,17 The MDS involves individual assessment items covering 17 domains, such as medical diagnosis, behavioral and mood indicators and activities of daily living.16
The data files linked to MDS included the Master Beneficiary Summary Files and Medicare Parts A, B, and D files for each of the study years. It also involved using MedPar and outpatient files for hospitalization and physician services, respectively. Additionally, the master beneficiary summary files were used to obtain information on demographic characteristics and enrollment status in Medicare Parts A and B as well as disease-specific variables. The Part D files capture drug information for each prescription filled by Medicare enrollees during the study period.16 This study was approved by the CMS Privacy Board and was considered under exempt category (exemption four) by the institutional review board for the protection of human subjects at the University of Houston as it involves existing data without direct identifiers.
Study Design and Sample
The study used a retrospective cohort design with a 12-month baseline and a 24-month follow-up. The diagnosis of PD was defined based on both MDS assessment and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 332.0 in the claims database. For all cohort patients, the diagnosis of PD was confirmed in 2007 which was the baseline period. Older adults aged over 65 years with continuous coverage for Medicare parts A, B, and D were included. Patients with a baseline diagnosis of either bipolar disease or schizophrenia were excluded, since those patients were more likely to be chronic users of antipsychotic medications. Patients were excluded from the cohort if they used any atypical antipsychotics in the baseline period or they started taking more than one atypical antipsychotics simultaneously in the follow-up period.
Analytical Approach
The initiation of atypical antipsychotics in PD patients was evaluated over a two-year study period i.e., 2008–2009. The first prescription of atypical antipsychotics in 2008–2009 was considered as new antipsychotics use. The study outcome was the use of inappropriate antipsychotics, based on AGS Beers criteria 2015.9 The use of inappropriate antipsychotics was defined as the prescription of asenapine, brexpiprazole, iloperidone, lurasidone, olanzapine, paliperidone, risperidone, or ziprasidone. As specified in Beers criteria 2015, the use of aripiprazole, clozapine, or quetiapine in PD patients was appropriate in this study. These medications were identified using Generic Name – Short Version (GNN) from the Prescription Drug Event Data. The Prescription Service Date (DOS) was used to determine the first prescription of antipsychotics in the study period.16 Typical antipsychotics users were not excluded from the analysis, rather the baseline use of typical agents was taken into account as a confounding factor.
This study applied Andersen’s Behavioral Model (ABM) to explain the relationship between predisposing, enabling and need factors and antipsychotic medication use. Based on the ABM, predisposing factors included age, sex, race, education, marital status and geographical region.18 The type of insurance coverage was an enabling factor that was applied in the cohort identification process. Need factors encompassed cognitive and functional status, walk assessment, clarity of speech, dyskinesia, dysphagia, abusive behavior, insomnia, depressed mood indicators, along with various comorbidities and co-medications. The MDS assessment contains several measures related to PD severity including cognitive and functional impairment items. These validated measures have been used in previous observational studies involving patients with neurological impairment. The current study utilized MDS-Derived Direct Cognition Scale (MDS-COG) to control for the severity of cognitive impairment in PD. The MDS-COG ranges from 0 to 9, with higher scores indicating greater severity of cognitive impairment.19 The Activities of Daily Living were scored using ADL-Long Form (0–28) with higher scores indicating a worse functional status.20
Based on the ABM, sociodemographic and clinical characteristics were compared between appropriate and inappropriate atypical antipsychotics users. Multivariable logistic regression was used to examine the relationship between predisposing and need factors and the use of inappropriate atypical antipsychotics in elderly patients with PD. All statistical analyses were performed using SAS 9.2 (SAS Institute, Cary, North Carolina) with a statistical significance level of 0.05.
Results
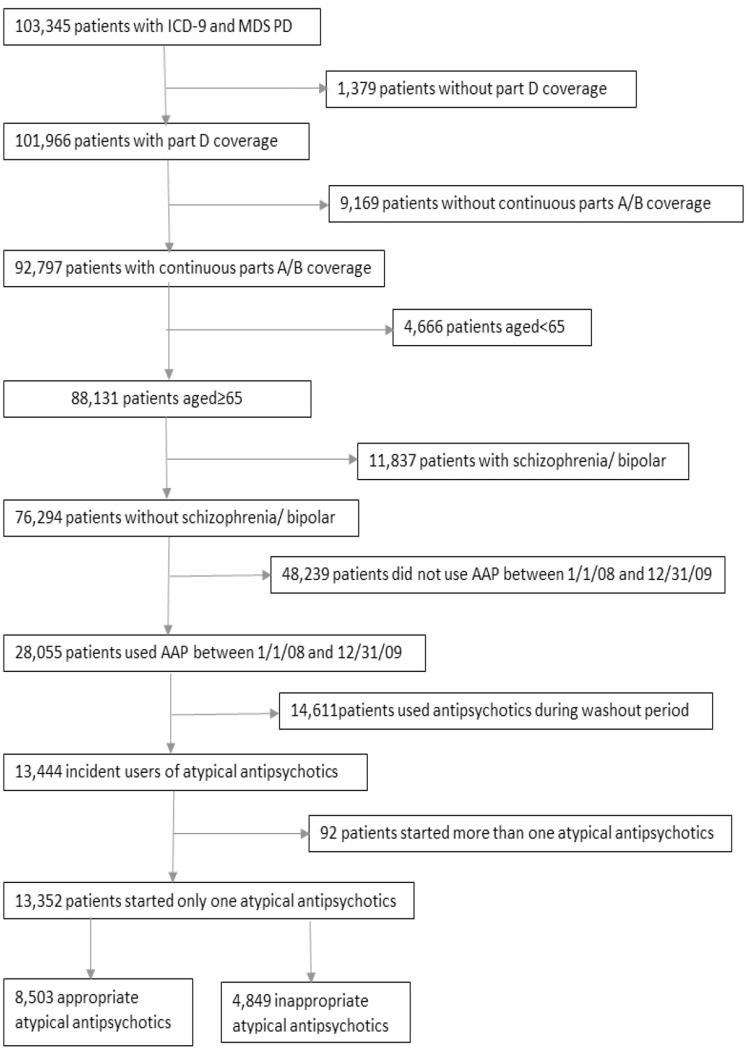
The number of PD patients aged 65 years or older with continuous eligibility for Medicare parts A, B and D coverage was 88,131 from 2007–2009 MDS-linked Medicare data. Excluding those with bipolar disorder or schizophrenia, 76,294 PD patients remained in the cohort and 28,055 patients were found to receive atypical antipsychotics between 1st Jan 2008 and 31st Dec 2009. As shown in the cohort identification flowchart (Figure 1), 14,611 patients were excluded because they used atypical antipsychotics in washout period and 92 patients were excluded because they started more than one antipsychotic drug at the same time in the study period. In the study cohort, 13,352 patients started only one atypical antipsychotic in 2008 and 2009 and the number of inappropriate antipsychotic users was 4,849. The rate of inappropriate use among atypical antipsychotic users was 36.32%.
Figure 1.
Cohort identification flowchart.
Table 1 presents baseline characteristics of the study cohort. The mean age of the study population was over 80 years in both treatment groups. The percentage of persons with dementia was 28.29% in inappropriate antipsychotic users and 22.05% in the appropriate treatment group. Falls and fractures were the most common comorbidities (>85%) in the study population and hypertension was the second most common comorbidity. The rate of baseline typical antipsychotic use was 6.50% and 7.90%, respectively, in appropriate and inappropriate antipsychotic groups. Most study patients received levodopa in the baseline period since the rate of levodopa therapy was 73.17% and 59.79% in the appropriate and inappropriate atypical antipsychotic users, respectively. As shown in Table 2, the percentages use of quetiapine (55.55%), risperidone (22.95%) and olanzapine (11.25%) were the highest among all atypical antipsychotics.
Table 1.
Characteristics of PD Patients with Comorbid Depression Residing in Nursing Homes with Incident Use of Appropriate and Inappropriate Atypical Antipsychotics (AAP)
| Characteristic | Appropriate AAP (n= 8503) | Inappropriate AAP (n= 4849) |
P-value |
|---|---|---|---|
| Age (mean ± SD) | 80.85 ± 6.99 | 81.85 ± 7.06 | <0.01* |
| Female, n (%) | 4761 (55.99) | 2891 (59.62) | <0.01* |
| White, n (%) | 7756 (91.21) | 4346 (89.63) | <0.01* |
| College education, n (%) | 1790 (21.05) | 737 (15.20) | <0.01* |
| Married, n (%) | 3343 (39.32) | 1666 (34.36) | <0.01* |
| Region, n (%) | |||
| Midwest | 2299 (27.04) | 1388 (28.62) | <0.01* |
| Northeast | 1811 (21.30) | 870 (17.94) | |
| West | 354 (4.16) | 203 (4.19) | |
| South | 4039 (47.50) | 2388 (49.25) | |
| ADL Score (mean ± SD) | 13.11 ± 7.03 | 12.96 ± 7.22 | 0.25 |
| MDS Cognitive Score (mean ± SD) | 4.69 ± 1.16 | 4.67 ± 1.17 | 0.26 |
| Impaired Walking | 4580 (53.86) | 2519 (51.95) | 0.03* |
| Unclear Speech, n (%) | 1394 (16.39) | 797 (16.44) | 0.95 |
| Dyskinesia, n (%) | 184 (2.16) | 80 (1.65) | 0.04* |
| Dysphagia, n (%) | 3220 (37.87) | 1735 (35.78) | 0.02* |
| Insomnia, n (%) | 1135 (13.35) | 577 (11.90) | 0.02* |
| Abusive Behavior, n (%) | 944 (11.10) | 584 (12.04) | 0.1 |
| Depressed Mood Indicators, n (%) | 2564 (30.15) | 1394 (28.75) | 0.09 |
| Depressive Type Psychosis, n (%) | 71 (0.83) | 43 (0.89) | 0.75 |
| Anxiety, n (%) | 3437 (40.42) | 2100 (43.31) | <0.01* |
| Dementia, n (%) | 1875 (22.05) | 1372 (28.29) | <0.01* |
| Stroke, n (%) | 1805 (21.23) | 1180 (24.33) | <0.01* |
| Falls and Fractures, n (%) | 7310 (85.97) | 4136 (85.30) | 0.28 |
| Coronary Artery Disease, n (%) | 1782 (20.96) | 1116 (23.02) | <0.01* |
| Congestive Heart Failure, n (%) | 1985 (23.34) | 1289 (26.58) | <0.01* |
| Dysrhythmia, n (%) | 1675 (19.70) | 1014 (20.91) | 0.09 |
| Hypertension, n (%) | 6474 (76.14) | 3857 (79.54) | <0.01* |
| Diabetes Mellitus, n (%) | 2539 (29.86) | 1618 (33.37) | <0.01* |
| Osteoarthritis, n (%) | 3565 (41.93) | 2168 (44.71) | <0.01* |
| Cancer, n (%) | 888 (10.44) | 465 (9.59) | <0.11 |
| Pneumonia history, n (%) | 2759 (32.45) | 1665 (34.34) | 0.03* |
| Asthma, n (%) | 402 (4.73) | 253 (5.22) | 0.21 |
| COPD, n (%) | 1673 (19.68) | 1129 (23.28) | <0.01* |
| Charlson Comorbidity Index (mean ± SD) | 5.45 ± 3.42 | 5.68 ± 3.45 | <0.01* |
| Typical antipsychotics, n (%) | 553 (6.50) | 383 (7.90) | <0.01* |
| SSRI/SNRI, n (%) | 5918 (69.60) | 3462 (71.40) | 0.03* |
| Levodopa, n (%) | 6222 (73.17) | 2899 (59.79) | <0.01* |
| Dopamine Agonists, n (%) | 2320 (27.28) | 1050 (21.65) | <0.01* |
| COMT Inhibitors, n (%) | 1441 (16.95) | 474 (9.78) | <0.01* |
| MAO Inhibitors Type B, n (%) | 583 (6.86) | 172 (3.55) | <0.01* |
| Amantadine, n (%) | 649 (7.63) | 244 (5.03) | <0.01* |
| Anticholinergics, n (%) | 396 (4.66) | 226 (4.66) | 0.99 |
Note: *Indicate p value <0.05.
Table 2.
Proportion of Atypical Antipsychotic Agents Initiated for Elderly Nursing Home Patients with PD and Comorbid Depression (n=13,352)
| Antipsychotics | Frequency | Percent |
|---|---|---|
| Aripiprazole | 1036 | 7.76 |
| Clozapine | 50 | 0.37 |
| Olanzapine | 1502 | 11.25 |
| Paliperidone | 23 | 0.17 |
| Quetiapine | 7417 | 55.55 |
| Risperidone | 3064 | 22.95 |
| Ziprasidone | 260 | 1.95 |
The odds of receiving inappropriate vs appropriate antipsychotics are presented in Table 3. Among predisposing factors, patient’s age was associated with higher likelihood of receiving inappropriate antipsychotics with adjusted Odds Ratio (OR): 1.01 (1.01–1.02). Patients were less likely to use inappropriate antipsychotics, if they were highly educated [OR=0.76, 95% Confidence Interval (CI): 0.69–0.84], married (OR=0.91, 95% CI: 0.84–0.99) or lived in northeast (OR=0.83, 95% CI: 0.75–0.92). Several need factors including clinical characteristics were associated with inappropriate antipsychotic use among elderly patients with PD. The likelihood of inappropriate antipsychotic use was higher for patients who had dementia (OR=1.22, 95% CI: 1.12–1.33), Chronic Obstructive Pulmonary Disease (COPD)(OR=1.13, 95% CI: 1.03–1.24), or those who received Selective Serotonin Reuptake Inhibitor (SSRI) or Serotonin–Norepinephrine Reuptake Inhibitor (SNRI) (OR=1.14, 95% CI: 1.06–1.24). On the contrary, patients who were taking levodopa (OR=0.62, 95% CI: 0.57–0.67), dopamine agonists (OR=0.90, 95% CI: 0.82–0.98), Catechol-O-methyltransferase (COMT) inhibitors (OR=0.77, 95% CI: 0.68–0.86), Monoamine Oxidase (MAO) inhibitors type B (OR=0.72, 95% CI: 0.60–0.86), or amantadine (OR=0.84, 95% CI: 0.71–0.98) were less likely to receive inappropriate antipsychotics.
Table 3.
Factors Associated with Use of Inappropriate vs Appropriate Atypical Antipsychotics in Elderly Nursing Home Patients with PD and Comorbid Depression
| Characteristic | Unadjusted OR (%95 OR) | Adjusted OR (%95 OR) |
|---|---|---|
| Age | 1.02 (1.02–1.03) | 1.01 (1.01–1.02) |
| Female vs Male | 1.16 (1.08–1.25) | 1.04 (0.96–1.13) |
| White vs Others | 0.83 (0.74–0.94) | 0.88 (0.77–1.00) |
| College vs Lower education | 0.67 (0.61–0.74) | 0.76 (0.69–0.84) |
| Married vs Unmarried | 0.81 (0.75–0.87) | 0.91 (0.84–0.99) |
| Region | ||
| Midwest | 1.02 (0.94–1.11) | 1.07 (0.98–1.17) |
| Northeast | 0.81 (0.74–0.89) | 0.83 (0.75–0.92) |
| West | 0.97 (0.81–1.16) | 1.11 (0.93–1.34) |
| South | Ref | Ref |
| ADL Score | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) |
| MDS Cognitive Score | 0.98 (0.96–1.01) | 0.98 (0.95–1.02) |
| Impaired Walking (Yes vs No) | 0.93 (0.86–0.99) | 1.00 (0.93–1.08) |
| Unclear Speech (Yes vs No) | 1.00 (0.91–1.10) | 1.07 (0.96–1.18) |
| Dyskinesia, n (%) | 0.76 (0.58–0.99) | 0.96 (0.73–1.26) |
| Dysphagia, n (%) | 0.91 (0.85–0.98) | 0.93 (0.86–1.00) |
| Abusive Behavior (Yes vs No) | 1.10 (0.98–1.22) | 1.12 (0.99–1.25) |
| Insomnia (Yes vs No) | 0.88 (0.79–0.98) | 0.90 (0.80–1.00) |
| Depressed Mood Indicators (Present vs Absent) | 0.94 (0.87–1.01) | 0.93 (0.86–1.01) |
| Depressive Type Psychosis (Yes vs No) | 1.06 (0.73–1.56) | 1.06 (0.72–1.56) |
| Anxiety (Yes vs No) | 1.13 (1.05–1.21) | 1.08 (1.00–1.17) |
| Dementia (Yes vs No) | 1.40 (1.29–1.51) | 1.22 (1.12–1.33) |
| Stroke (Yes vs No) | 1.19 (1.10–1.30) | 1.09 (1.00–1.19) |
| Falls and Fractures (Yes vs No) | 0.95 (0.86–1.05) | 0.92 (0.83–1.02) |
| Coronary Artery Disease (Yes vs No) | 1.13 (1.04–1.23) | 1.04 (0.95–1.14) |
| Congestive Heart Failure (Yes vs No) | 1.19 (1.10–1.29) | 1.02 (0.93–1.11) |
| Dysrhythmia (Yes vs No) | 1.08 (0.99–1.18) | 1.00 (0.91–1.09) |
| Hypertension (Yes vs No) | 1.22 (1.12–1.33) | 1.05 (0.96–1.15) |
| Diabetes Mellitus (Yes vs No) | 1.18 (1.09–1.27) | 1.06 (0.98–1.15) |
| Osteoarthritis (Yes vs No) | 1.12 (1.04–1.20) | 1.02 (0.94–1.10) |
| Cancer (Yes vs No) | 0.91 (0.81–1.02) | 0.88 (0.78–0.99) |
| Pneumonia history (Yes vs No) | 1.01 (1.01–1.17) | 1.01 (0.93–1.10) |
| Asthma (Yes vs No) | 1.11 (0.94–1.30) | 1.00 (0.84–1.18) |
| COPD (Yes vs No) | 1.24 (1.14–1.35) | 1.13 (1.03–1.24) |
| Charlson Comorbidity Index | 1.02 (1.01–1.03) | 1.01 (1.00–1.02) |
| Typical antipsychotics, n (%) | 1.23 (1.08–1.41) | 1.09 (0.95–1.25) |
| SSRI/SNRI (Used vs Not Used) | 1.09 (1.01–1.18) | 1.14 (1.06–1.24) |
| Levodopa (Used vs Not Used) | 0.55 (0.51–0.59) | 0.62 (0.57–0.67) |
| Dopamine Agonists (Used vs Not Used) | 0.74 (0.68–0.80) | 0.90 (0.82–0.98) |
| COMT Inhibitors (Used vs Not Used) | 0.53 (0.48–0.59) | 0.77 (0.68–0.86) |
| MAO Inhibitors Type B (Used vs Not Used) | 0.50 (0.42–0.59) | 0.72 (0.60–0.86) |
| Amantadine (Used vs Not Used) | 0.64 (0.55–0.75) | 0.84 (0.71–0.98) |
| Anticholinergics (Used vs Not Used) | 1.00 (0.85–1.18) | 1.08 (0.91–1.28) |
Discussion
In this study, the prevalence of atypical antipsychotic use in a cohort of older patients with PD and depression was 36.77% and the incidence rate was 17.50% in a 2-year follow-up. These prevalence and incidence rates are not directly comparable to previous estimates of antipsychotic use in PD due to the clinical setting and comorbid depression. The prevalence and incidence rates of antipsychotic use are possibly high as the study population was nursing home residents with comorbid depression diagnosis. The high rate of inappropriate atypical antipsychotic use could partly be due to the fact that current recommendations for antipsychotic use might not have been implemented in previous guidelines when the study data were collected.21
Although clozapine is an effective treatment in PD psychosis, only 0.37% of patients with PD were treated with this atypical antipsychotic which is consistent with previous studies on antipsychotic use in PD.15 This is possibly due to the adverse side effect profile of clozapine. Quetiapine was the most frequent appropriate antipsychotic (55.55%) and risperidone was the most commonly used inappropriate antipsychotic (22.95%) in this study. The use of quetiapine was consistently higher than other antipsychotics in previous studies of elderly patients with PD, possibly because quetiapine was considered to be safer than other antipsychotics for those patients.22
Multivariable logistic regression was used to examine various predisposing and need factors associated with inappropriate antipsychotic use in PD. Older age was associated with higher use of inappropriate antipsychotics, which could be related to higher rates of behavioral symptoms other than PD psychosis in old age.23 The likelihood of receiving inappropriate antipsychotics was lower for college educated and married patients in comparison to others. This indicates that treatment of PD psychosis might vary across different sociodemographic groups. The study found that inappropriate antipsychotics were less prescribed in the Northeast geographical region in comparison to the South, indicating potentially important regional variations in the quality of care.
Amongst clinical characteristics dementia and COPD were associated with higher risk of inappropriate antipsychotics use. Antipsychotics might be administrated for behavioral symptoms of dementia or for treating delirium in COPD when dementia or COPD coexist with PD.24 These clinical conditions are not associated with dopaminergic medication use, thus, antipsychotics such as risperidone might be prescribed to treat behavioral symptoms of dementia or COPD. The use of inappropriate antipsychotics was higher in typical antipsychotics and antidepressant (SSRI/SNRI) users and this might refer to the use of atypical antipsychotics for preexisting mental disorders.25 On the other hand, patients who were taking levodopa or other antiparkinson drugs with dopaminergic properties were more likely to receive appropriate vs inappropriate antipsychotics with respect to Beers criteria.
Generalizability of Findings
The study population consisted of nursing home residents with a diagnosis of PD and depression, since the original data source was a cohort of nursing home residents with depression. Patients with major depressive disorder might use antipsychotics for a number of reasons including augmentation of antidepressant therapy and treatment of comorbid insomnia or anxiety symptoms.26,27 This potentially leads to higher rates of antipsychotic use in patients with depression when compared to those without depression. Potential confounding by indication might limit the generalizability of study findings for PD patients without depression. However, the multivariable model included various covariates such as depressed mood indicators and anxiety to control for the reasons of antipsychotic use in depression.
Strengths and Limitations
This study has some strengths such as use of a large nationally representative data, robust methodology and utilization of a large sample size. The MDS provides comprehensive assessment of nursing home residents on their functional status and clinical conditions which shares many common features with Parkinson’s disease assessments.28,29 The study linked MDS to Medicare claims to obtain additional information regarding individual and clinical characteristics. This provided enough flexibility to define a washout period to exclude prevalent users of atypical antipsychotics and to avoid prevalent user bias.30 The MDS and Medicare claims are nationally representative data with good generalizability for the elderly population. Utilizing a large sample size provided adequate power to examine the various factors associated with inappropriate antipsychotics use.
The limitations of the study include those inherent to the nature of the secondary data analysis and data availability. Miscoding and under-coding might occur in the process of administrative data collection.31 The study findings were limited by the data source, definitions, and data analytical approaches. This study utilized data from a cohort of nursing home residents with a diagnosis of depression. This might affect utilization pattern of atypical agents in the study cohort but it would not be a differential bias across the study groups. The methodological limitations also related to the availability of specific variables. One such factor was rating the severity of Parkinson's disease, which is commonly performed using disability scales such as the Unified Parkinson Disease Rating Scale (UPDRS). Since Medicare data did not contain information on PD severity, relevant variables and proxies were used to control for the severity of PD in multivariable models. Also, antipsychotic doses were not involved in the analysis. Finally, this study cannot establish a causal relationship between predictors and the outcome of inappropriate antipsychotic use due to the nature of the study.
Conclusion
The incidence rate of inappropriate atypical antipsychotic use was relatively high, as more than one-third of PD patients received inappropriate agents among those who were treated with atypical antipsychotics in this study. Various socio-demographics and clinical factors were associated with inappropriate antipsychotic use among elderly patients with PD. Users of dopaminergic antiparkinson agents were more likely to be treated with appropriate antipsychotics and those with comorbidities such as dementia or COPD were more likely to receive inappropriate antipsychotics. Further research is warranted to evaluate rates and patterns of antipsychotic use after the implementation of new guidelines for PD psychosis.
Acknowledgment
Portions of this paper were presented as a poster at ISPOR 2018 Baltimore, May 19-23, 2018.
Disclosure
Dr. Rajender R Aparasu reports grants from Astellas, Incyte, and Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- 2.Weerkamp N, Tissingh G, Poels P, Et A. Parkinson disease in long term care facilities: a review of the literature. J Am Med Dir Assoc. 2014;15(2):90–94. doi: 10.1016/j.jamda.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 3.Kowal S, Dall T, Chakrabarti R, Storm M, Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Mov Disord. 2013;28(3):311–318. doi: 10.1002/mds.25292 [DOI] [PubMed] [Google Scholar]
- 4.Skorvanek M, Rosenberger J, Minar M, et al. Relationship between the non-motor items of the MDS–UPDRS and quality of life in patients with Parkinson’s disease. J Neurol Sci. 2015;353(1):87–91. doi: 10.1016/j.jns.2015.04.013 [DOI] [PubMed] [Google Scholar]
- 5.Factor S. Current status of symptomatic medical therapy in Parkinson’s disease. Neurotherapeutics. 2008;5(2):164–180. doi: 10.1016/j.nurt.2007.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman J, Holden S. Treatment of psychosis and dementia in Parkinson’s disease. Curr Treat Options Neurol. 2014;16(3):281. doi: 10.1007/s11940-013-0281-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Der Mussele S, Mariën P, Saerens J, et al. Psychosis associated behavioral and psychological signs and symptoms in mild cognitive impairment and Alzheimer’s dementia. Aging Ment Health. 2015;19(9):818–828. doi: 10.1080/13607863.2014.967170 [DOI] [PubMed] [Google Scholar]
- 8.Ballard C, Isaacson S, Mills R, et al. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16(10):898. E891-898. E897. doi: 10.1016/j.jamda.2015.06.021 [DOI] [PubMed] [Google Scholar]
- 9.Ags. American Geriatrics Society. 2015 Updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–2246. doi: 10.1111/jgs.13702 [DOI] [PubMed] [Google Scholar]
- 10.Miyasaki J, Shannon K, Voon V, et al. Practice parameter: evaluation and treatment of depression, psychosis, and dementia in Parkinson disease (an evidence-based review):[retired]: report of the quality standards subcommittee of the American Academy Of Neurology. Neurology. 2006;66(7):996–1002. doi: 10.1212/01.wnl.0000215428.46057.3d [DOI] [PubMed] [Google Scholar]
- 11.Chen J. Treatment of psychotic symptoms in patients with Parkinson disease. Mental Health Clinician. 2017;7(6):262–270. doi: 10.9740/mhc.2017.11.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chekani F, Holmes H, Johnson M, Chen H, Sherer J, Aparasu R. Risk of pneumonia associated with atypical antipsychotic use in nursing home residents with Parkinson’s disease. J Psychiatr Res. 2019;117:116–121. doi: 10.1016/j.jpsychires.2019.07.005 [DOI] [PubMed] [Google Scholar]
- 13.Kuroki T, Nagao N, Nakahara T. Neuropharmacology of second-generation antipsychotic drugs: a validity of the serotonin–dopamine hypothesis. Prog Brain Res. 2008;172:199–212. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J, Aguilar L. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr Dis Treat. 2008;4(4):743–757. doi: 10.2147/NDT.S2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weintraub D, Chen P, Ignacio R, Mamikonyan E, Kales H. Patterns and trends in antipsychotic prescribing for Parkinson disease psychosis. Arch Neurol. 2011;68(7):899. doi: 10.1001/archneurol.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resdac. Cms Mds and Oasis Assessment Data; 2013.
- 17.Morris J, Hawes C, Fries B, et al. Designing the national resident assessment instrument for nursing homes. Gerontologist. 1990;30(3):293–307. doi: 10.1093/geront/30.3.293 [DOI] [PubMed] [Google Scholar]
- 18.Andersen R, Newman J. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4):Online‐Only-Online‐Only. doi: 10.1111/milq.2005.83.issue-4 [DOI] [PubMed] [Google Scholar]
- 19.Morris J, Fries B, Mehr D, et al. Mds cognitive performance scale. J Gerontol. 1994;49(4):M174–M182. doi: 10.1093/geronj/49.4.M174 [DOI] [PubMed] [Google Scholar]
- 20.Morris J, Fries B, Morris S. Scaling ADLs within the MDS. J Gerontol a Biol Sci Med Sci. 1999;54(11):M546–M553. doi: 10.1093/gerona/54.11.M546 [DOI] [PubMed] [Google Scholar]
- 21.Fick D, Cooper J, Wade W, Waller J, Maclean JR, Beers M. Updating the beers criteria for potentially inappropriate medication use in older adults: results of a us consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. doi: 10.1001/archinte.163.22.2716 [DOI] [PubMed] [Google Scholar]
- 22.Kim K, Bader G, Kotlyar V, Gropper D. Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol. 2003;16(1):29–31. doi: 10.1177/0891988702250533 [DOI] [PubMed] [Google Scholar]
- 23.Inouye S, Westendorp R, Saczynski J. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomichek J, Stollings J, Pandharipande P, Chandrasekhar R, Ely E, Girard T. Antipsychotic prescribing patterns during and after critical illness: a prospective cohort study. Crit Care. 2016;20(1):378. doi: 10.1186/s13054-016-1557-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright B, Eiland E, Lorenz R. Augmentation with atypical antipsychotics for depression: a review of evidence‐based support from the medical literature. Pharmacotherapy. 2013;33(3):344–359. doi: 10.1002/phar.1204 [DOI] [PubMed] [Google Scholar]
- 26.Fetveit A. Late‐life insomnia: a review. Geriatr Gerontol Int. 2009;9(3):220–234. doi: 10.1111/ggi.2009.9.issue-3 [DOI] [PubMed] [Google Scholar]
- 27.Nemeroff C. Use of atypical antipsychotics in refractory depression and anxiety. J Clin Psychiatry. 2005;66:13–21. [PubMed] [Google Scholar]
- 28.Mor V. A comprehensive clinical assessment tool to inform policy and practice: applications of the minimum data set. Med Care. 2004;42(4):Iii-50-Iii-59. doi: 10.1097/01.mlr.0000120104.01232.5e [DOI] [PubMed] [Google Scholar]
- 29.Goetz C, Tilley B, Shaftman S, et al. Movement disorder society‐sponsored revision of the unified Parkinson’s disease rating scale (Mds‐Updrs): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–2170. doi: 10.1002/mds.v23:15 [DOI] [PubMed] [Google Scholar]
- 30.Ray W. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231 [DOI] [PubMed] [Google Scholar]
- 31.Resdac. Sources and use of medicare enrollment information; 2017. Available from: http://Resdac.Umn.Edu/Sites/Resdac.Umn.Edu/Files/Sources%20and%20use%20of%20medicare%20enrollment%20information%20(Slides).Pdf. Accessed June23, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Resdac. Sources and use of medicare enrollment information; 2017. Available from: http://Resdac.Umn.Edu/Sites/Resdac.Umn.Edu/Files/Sources%20and%20use%20of%20medicare%20enrollment%20information%20(Slides).Pdf. Accessed June23, 2017.