Abstract
Yarrowia lipolytica ACA‐YC 5033 was grown on glucose‐based media in which high amounts of olive mill wastewaters (OMWs) had been added. Besides shake‐flask aseptic cultures, trials were also performed in previously pasteurized media while batch bioreactor experiments were also done. Significant decolorization (∼58%) and remarkable removal of phenolic compounds (∼51% w/w) occurred, with the latter being amongst the highest ones reported in the international literature, as far as yeasts were concerned during their growth on phenol‐containing media. In nitrogen‐limited flask fermentations the microorganism produced maximum citric acid quantity ≈19.0 g/L [simultaneous yield of citric acid produced per unit of glucose consumed (YCit/Glc)≈0.74 g/g]. Dry cell weight (DCW) values decreased at high phenol‐containing media, but, on the other hand, the addition of OMWs induced reserve lipid accumulation. Maximum citric acid concentration achieved (≈52.0 g/L; YCit/Glc≈0.64 g/g) occurred in OMW‐based high sugar content media (initial glucose added at ≈80.0 g/L). The bioprocess was successfully simulated by a modified logistic growth equation. A satisfactory fitting on the experimental data occurred while the optimized parameter values were found to be similar to those experimentally measured. Finally, a non‐aseptic (previously pasteurized) trial was performed and its comparison with the equivalent aseptic experiment revealed no significant differences. Yarrowia lipolytica hence can be considered as a satisfactory candidate for simultaneous OMWs bioremediation and the production of added‐value compounds useful for the food industry.
Keywords: Cellular lipids, Citric acid, Modeling, Olive mill wastewaters, Waste bioremediation
Abbreviations
- OMW
olive mill wastewater
1. Introduction
Olive mill wastewaters (OMWs) are one of the most important agro‐industrial residues produced in the European level, with phenolic compounds found into this residue being responsible for its (phyto)‐toxic effect and dark color 1, 2. Besides phenolic compounds, carbohydrates (principally glucose) may be contained in very high quantities 3, also posing significant problems related with their treatment, while recent developments indicate that OMWs should be considered as a fermentation feedstock, rather than a waste to be discharged. Thus, the development of viable treatment technologies is crucial, since OMWs are, in general, discharged directly to the environment, without any prior or other treatment 4.
Due to the significant anti‐cancer and therapeutic properties of the phenolic compounds found in this residue, a technology recently developed, refers to their separation and thereof utilization in several applications 5, 6, 7. Thus, in a proposed theoretical biorefinery scheme, after removal of the phenolic compounds, microbial fermentation related with the removal of the rest of the pollution and the concomitant production of added‐value compounds could follow 8. On the other hand, enriched OMWs (without previous phenol compounds removal) have been used as substrate for the cultivation of yeast and yeast‐like species, molds and prokaryotic microorganisms resulting in the remediation of the waste and also the synthesis of added‐value compounds like microbial mass 9, 10, exo‐polysaccharides 11, enzymes 4, 12, 13, 14, ethanol 9, 15, 16, 17, 18, 19, 20, citric acid 9, 21, 22, and microbial lipids (single cell oils – SCOs) 9, 22, 23. Previous investigations have indicated that several Y. lipolytica strains converted glucose‐enriched OMWs into added‐value compounds (biomass, SCO and citric acid) in carbon‐ and nitrogen‐limited shake‐flask cultures 22. One of the most promising strains was Y. lipolytica ACA‐YC 5033. Goal of the current study was to further investigate the potential of this strain to produce the above‐mentioned metabolites in cultures containing increased OMW (hence initial phenolic compounds) concentrations. Aseptic and non‐aseptic trials were realized, scale‐up in bench top laboratory‐scale bioreactor was performed and kinetic modeling simulating the production of citric acid was carried out.
2. Materials and methods
2.1. Microorganism and media
Yarrowia lipolytica ACA‐YC 5033 22 was used. The strain was isolated from traditional Greek wheat sourdoughs and was identified in the Laboratory of Dairy Research; Department of Food Science and Human Nutrition; Agricultural University of Athens. Conservation of the strain was done on PDA‐containing slants at temperature T = 6 ± 1°C. In order to maintain the viability of the strain, sub‐culture every 3 months was carried out. OMWs that were used in the current investigation were obtained from a three‐phase decanter olive mill of the region Kalentzi (Corinthia, Peloponnissos; Greece). OMWs after their collection were immediately frozen at T = –20 ± 2°C. In order to be used in the performed experiments, OMWs were thawed and the solids were removed by centrifugation (9000 rpm, 15 min, T = 21 ± 1°C) in a Hettich Universal 320R centrifuge. The phenolic content of the wastewaters, expressed as gallic acid equivalent, was c. 10.0 g/L, while its sugar concentration was c. 30.0 g/L, expressed as glucose equivalent. On the other hand, the used wastewaters contained indeed negligible quantities of olive oil (0.4 ± 0.1 g/L; determination of oil conducted after a triple extraction with hexane). Organic acids were also present in small quantities. The principal organic acids detected were acetic acid and gluconic acid (in concentrations of c. 2.0 g/L of each).
OMWs were diluted in several ratios so as various phenolic compound concentrations to be achieved in liquid media. Inexpensive commercial‐type glucose was also added so as to reach the typical initial concentration of reducing sugars found in most OMWs (≈30 g/L) 4. Commercial‐type glucose [the purity of which was c. 95% w/w with impurities being composed of maltose (2% w/w), malto‐dextrines (0.5% w/w), water (1.5% w/w) and salts (0.5% w/w)] was provided by the “Hellenic Industry of Sugar SA” (Thessaloniki, Greece). In all OMW‐diluted media, salts were added as follows (in g/L): KH2PO4 7.0; Na2HPO4 2.5; MgSO4×7H2O 1.5; FeCl3×6H2O 0.15; CaCl2×2H2O 0.15; ZnSO4×7H2O 0.02; MnSO4×H2O 0.06. In nitrogen‐limited fermentations the initial glucose (Glc0) concentration was 35.0 ± 2.0 g/L and the yeast extract and (NH4)2SO4 concentrations were 0.50 g/L (initial molar ratio C/N≈85) of each. In carbon‐limited fermentations Glc0, (NH4)2SO4 and yeast extract concentrations were (in g/L): 28.0 ± 1.0; 4.0; 2.0 respectively (initial C/N≈13).
2.2. Culture conditions
Fermentations were carried out in 250‐mL Erlenmeyer flasks, containing 50 ± 1 mL of previously sterilized or pasteurized growth medium (heat treatment for the pasteurization at T = 80°C for 5 min), inoculated with 1 mL of inoculum (or 3 mL for pasteurized media) of exponential pre‐culture 22. Flasks were incubated in an orbital shaker (New Brunswick Scientific, USA) at an agitation rate of 180 ± 5 rpm and T = 28 ± 1°C. Medium pH was maintained between 5.0 and 6.0 by adding (periodically and aseptically) small quantities (e.g. ranging between 400 and 600 μL) of 5M KOH into the flasks. In fact, the exact volume of KOH solution needed for pH correction was evaluated by measuring the volume of KOH solution required for pH correction in one (at least) flask (collected daily). Then the appropriate volume of KOH solution was aseptically added in the remaining flasks and the value of pH reached was verified to be in the range of 5.0–6.0. Blank (no OMW addition) experimental results were obtained from Sarris et al. 22, and will be presented with the appropriate reference in the section “Results”. Aseptic and previously pasteurized batch fermentations were also conducted in a laboratory scale bioreactor (MBR Bioreactor, AG Switzerland), with total volume 3.5 L and working volume 3.0 L, fitted with four probes and two six‐bladed turbines. The culture vessel was inoculated with 60 mL of exponential pre‐culture (see above). The incubation temperature was adjusted at T = 28 ± 1°C. Agitation rate was adjusted to 300 rpm. The pH was automatically controlled at 5.5 ± 0.1 by adding 5M KOH while media were sparged with air (passing through a 0.2 μm pore size filter) at constant flow rate of 1.0 vvm. All trials were performed in duplicate (each experimental point presented is the mean value of two independent determinations). Finally, no phenolic compounds reduction and very slight increase of color occurred due to agitation employed alone without presence of cells 19.
2.3. Analytical methods
In the shake‐flask experiments, dissolved oxygen tension (DOT) was determined according to Papanikolaou et al. 24. In all trials DOT was >20%, v/v, for all growth steps. Cell mass harvesting, biomass (dry cell weight, DCW) determination and pH measurement was conducted as in Sarris et al. 22. Citric acid and glucose were quantified according to Papanikolaou et al. 21. Total cellular lipid extraction and determination, lipid methanolysis and GC analysis and ammonium concentration into the supernatant fluid was determined as in Papanikolaou et al. 24. Phenolic compounds concentration in the residue was determined according to the Folin‐Ciocalteau method 12. The decolorization assay of the treated residue was performed according to Sayadi and Ellouz 25. Initial phenolic compounds concentration and initial color content were determined after sterilization or pasteurization.
3. Results
3.1. Biomass and lipid production by Yarrowia lipolytica ACA‐YC 5033 growing on OMW‐based media
Fermentations in nitrogen‐limited (Glc0≈35.0 g/L, initial C/N≈85; Table 1) and carbon‐limited (Glc0≈28 g/L, initial C/N≈13; Table 2) conditions were conducted and OMW was added yielding at initial concentration of phenolic compounds at c 2.00 and 2.90 g/L (Tables 1 and 2). Two more trials with higher amounts of OMWs added (initial phenolics at c. 4.50 and 5.50 g/L) were also conducted under nitrogen‐limited conditions (Table 1). Moreover, a pasteurized fermentation (Glc0≈35.0 g/L) and an aseptic trial with (Glc0≈80.0 g/L) with initial phenolics ≈2.9 g/L under nitrogen limited conditions were carried out (Table 1). Finally, aseptic and pasteurized batch bioreactor experiments were carried out under nitrogen‐limited conditions with Glc0≈35.0 g/L and initial phenolic compounds concentration ≈2.90 g/L (Table 2). Results with initial phenolic compounds concentration 0.0 (control experiment; no OMW addition), ≈1.15 g/L and ≈1.55 g/L when/where mentioned are obtained from Sarris et al. 22 for comparison reasons.
Table 1.
Experimental data of Yarrowia lipolytica ACA‐YC 5033 in nitrogen‐limited glucose‐based media with OMWs added in various amounts
| Initial phenolic compounds (g/L) | Fermentation time (h) | X (g/L) | L (g/L) | Cit (g/L) | Glccons (g/L) | YX/Glc (g/g) | YL/X (g/g) | YCit/Glc (g/g) | |
|---|---|---|---|---|---|---|---|---|---|
| 0.00c | Shake‐flasks Aseptic | 120 | 5.5 ± 0.4a ) | 0.40 ± 0.05 | 13.8 ± 1.0 | 23.7 ± 1.5 | 0.23 | 0.07 | 0.58 |
| 24 | 4.2 ± 0.3a | 0.50 ± 0.05 | 0.0 | 5.4 ± 0.5 | 0.78 | 0.12 | 0.00 | ||
| 144 | 5.5 ± 0.3b | 0.30 ± 0.05 | 18.9 ± 1.5 | 25.8 ± 1.5 | 0.21 | 0.05 | 0.73 | ||
| 2.00 ± 0.20 | Shake‐flasks Aseptic | 120 | 4.1 ± 0.3a ) | 0.70 ± 0.10 | 13.5 ± 1.0 | 23.3 ± 1.5 | 0.18 | 0.16 | 0.58 |
| 96 | 4.0 ± 0.3a | 0.80 ± 0.05 | 12.2 ± 1.0 | 15.8 ± 1.0 | 0.25 | 0.19 | 0.77 | ||
| 168 | 3.7 ± 0.3b | 0.50 ± 0.05 | 18.2 ± 1.5 | 23.7 ± 1.5 | 0.15 | 0.15 | 0.77 | ||
| 2.90 ± 0.25 | Shake‐flasks Aseptic | 121 | 3.7 ± 0.3a ) | 0.60 ± 0.05 | 17.2 ± 1.0 | 22.5 ± 1.5 | 0.16 | 0.16 | 0.76 |
| 96 | 3.6 ± 0.3a | 1.00 ± 0.10 | 15.1 ± 1.0 | 18.7 ± 1.5 | 0.20 | 0.27 | 0.81 | ||
| 144 | 3.5 ± 0.3b | 0.70 ± 0.05 | 19.0 ± 1.5 | 25.6 ± 2.0 | 0.14 | 0.20 | 0.74 | ||
| Shake‐flasks Pasteurized | 120 | 3.3 ± 0.2a) , a | 0.90 ± 0.10 | 11.6 ± 1.0 | 17.0 ± 1.0 | 0.19 | 0.26 | 0.68 | |
| 178 | 3.2 ± 0.2b | 0.60 ± 0.05 | 15.5 ± 1.0 | 22.8 ± 1.5 | 0.14 | 0.19 | 0.68 | ||
| Shake‐flasks Glc0≈80.0 g/L Aseptic | 288 | 4.6 ± 0.3a ) | 1.20 ± 0.10 | 43.3 ± 3.5 | 70.7 ± 5.0 | 0.07 | 0.26 | 0.61 | |
| 240 | 4.3 ± 0.3a | 1.40 ± 0.10 | 38.3 ± 3.5 | 57.3 ± 4.0 | 0.08 | 0.33 | 0.67 | ||
| 384 | 4.5 ± 0.3b | 0.80 ± 0.10 | 51.9 ± 4.0 | 81.6 ± 5.0 | 0.06 | 0.18 | 0.64 | ||
| Bioreactor Aseptic | 188 | 4.7 ± 0.3a) , b | 0.90 ± 0.10 | 15.2 ± 1.0 | 25.0 ± 2.0 | 0.19 | 0.19 | 0.61 | |
| 138 | 4.3 ± 0.3a | 1.10 ± 0.10 | 13.4 ± 1.0 | 21.7 ± 1.5 | 0.20 | 0.26 | 0.62 | ||
| Bioreactor Pasteurized | 192 | 4.8 ± 0.3a) , b | 0.80 ± 0.05 | 13.9 ± 1.0 | 23.9 ± 1.5 | 0.20 | 0.17 | 0.58 | |
| 144 | 4.4 ± 0.3a | 1.00 ± 0.10 | 12.1 ± 1.0 | 19.7 ± 1.5 | 0.22 | 0.22 | 0.61 | ||
| 4.50 ± 0.35 | Shake‐flasks Aseptic | 243 | 2.1 ± 0.1a) , b | 0.40 ± 0.05 | 4.0 ± 0.5 | 7.4 ± 0.5 | 0.29 | 0.18 | 0.55 |
| 48 | 1.7 ± 0.1a | 0.80 ± 0.05 | 1.0 ± 0.5 | 4.3 ± 0.5 | 0.40 | 0.45 | 0.24 | ||
| 5.50 ± 0.40 | 216 | 3.0 ± 0.3a) , b | 0.90 ± 0.10 | 2.2 ± 0.5 | 6.1 ± 0.5 | 0.50 | 0.30 | 0.36 | |
| 48 | 2.6 ± 0.1a | 1.20 ± 0.10 | 0.4 ± 0.5 | 3.6 ± 0.5 | 0.72 | 0.48 | 0.11 | ||
Representations of total biomass (X, g/L), total cellular lipid (L, g/L), total citric acid (Cit, g/L) and consumed substrate (Glccons, g/L) concentrations at different fermentation points of each trial.
when Xmax concentration was achieved;
when Lmax concentration was achieved;
when Citmax concentration was achieved;
Results regarding control experiment (no OMW addition) were obtained from Sarris et al. 22.
Fermentation time, conversion yield of biomass produced per glucose consumed (YX/Glc, g/g), total lipid in dry biomass (YL/X, g/g) and conversion yield of total citric acid produced per glucose consumed (YCit/Glc, g/g) are presented for all points of the trials. Culture conditions: growth on aseptic and pasteurized 250‐mL flasks at 180 ± 5 rpm, Glc0 = 35.0 ± 2.0 g/L, (NH4)2SO4 = 0.5 g/L, yeast extract = 0.5 g/L, initial pH = 6.0 ± 0.1, pH ranging between 5.0 and 6.0, DOT>20% v/v, incubation temperature T = 28°C; growth on aseptic and pasteurized batch bioreactor, 300 rpm, initial phenolic compounds concentration 2.90 ± 0.25 g/L, initial pH = 6.00 ± 0.02, incubation temperature T = 28°C and sparging of air at 1.0 vvm. Each point is the mean value of two independent measurements.
Table 2.
Experimental data of Yarrowia lipolytica ACA‐YC 5033 in carbon‐limited glucose‐based media with OMWs added in various amounts
| Initial phenolic compounds (g/L) | Fermentation time (h) | X (g/L) | L (g/L) | Glccons (g/L) | YX/Glc (g/g) | YL/X (g/g) |
|---|---|---|---|---|---|---|
| 0.00b | 96 | 10.1 ± 0.7a ) | 0.60 ± 0.05 | 23.4 ± 1.5 | 0.43 | 0.06 |
| 144 | 9.1 ± 0.7a | 1.00 ± 0.05 | 24.5 ± 1.5 | 0.37 | 0.11 | |
| 2.00 ± 0.20 | 70 | 10.9 ± 0.8a ) | 0.70 ± 0.05 | 23.3 ± 1.5 | 0.47 | 0.07 |
| 46 | 9.2 ± 0.7a | 0.90 ± 0.05 | 22.3 ± 1.5 | 0.41 | 0.10 | |
| 2.90 ± 0.25 | 72 | 13.3 ± 1.0a , a | 0.50 ± 0.05 | 25.8 ± 2.0 | 0.52 | 0.04 |
Representations of total biomass (X, g/L), total cellular lipid (L, g/L) and consumed substrate (Glccons, g/L) concentrations at different fermentation points of each trial.
when Xmax concentration was achieved;
when Lmax concentration was achieved;
Results regarding control experiment (no OMW addition) were obtained from Sarris et al. 22.
Fermentation time, conversion yield of biomass produced per glucose consumed (YX/Glc, g/g) and total lipid in dry biomass (YL/X, g/g) are presented for all points of the trials. Culture conditions: growth on 250‐mL flasks at 180 ± 5 rpm, Glc0 = 28.0 ± 1.0 g/L, (NH4)2SO4 = 4.0 g/L, yeast extract = 2.0 g/L initial pH = 6.0 ± 0.1, pH ranging between 5.0 and 6.0, DOT>20% v/v, incubation temperature T = 28°C. Each point is the mean value of two independent measurements.
In the nitrogen‐limited shake‐flask trials, DCW synthesis was affected by the addition of OMW; when compared with the control experiment, Xmax values were depleted in parallel with the phenolic content increase into the medium (Table 1). YX/Glc values seemed to be almost unaffected by the addition of the waste into the medium (Table 1). Total cellular lipids were quantified and in some instances, lipid quantities >25% of lipid in DCW were found (Table 1). Interestingly, the addition of OMWs in the medium seemed to favor the accumulation of storage lipids (Table 1). Lmax quantities up to c. 1.0 g/L, corresponding to lipid in DCW up to c. 27% w/w were obtained when OMW was added into the medium, suggesting stimulation of lipid accumulation process, since in the control experiment clearly lower YL/X and L values were obtained (Table 1). In the trial with initial phenolic compounds concentration ≈2.00 g/L, insignificant quantities of glucose remained unconsumed at the end of the fermentation whereas in the trial with initial phenolic compounds concentration at c. 2.90 g/L the final glucose concentration was ≈10.0 g/L.
For the aseptic flask trials with Glc0≈35.0 g/L and initial phenolics at c. 4.50 and 5.50 g/L, high phenol content clearly inhibited the microbial growth; DCW production reached in both trials the values of 2.1 ± 0.1 g/L and of 3.0 ± 0.3 g/L respectively (Table 1). Moreover, small quantities of glucose were assimilated (7.4 ± 0.5 g/L and 6.1 ± 0.5 g/L respectively). On the other hand, even higher lipid accumulation compared with the previous trials was observed, resulting in an Lmax = 0.80 g/L (YL/X = 0.45 g/g) for the trial with initial phenolics at 4.50 g/L and Lmax = 1.20 g/L (YL/X = 0.48 g/g) for that with initial phenolics at 5.50 g/L [Supporting Information Fig. S1A and B].
In carbon‐limited fermentations, biomass production was stimulated by the OMW addition (Table 2). The kinetics of DCW and glucose evolution for a carbon‐ and a nitrogen‐limited experiment is shown in Fig. 1. Significantly higher DCW values were obtained in the carbon‐limited compared with the nitrogen‐limited trials (Table 2 and Fig. 1). Insignificant quantities of glucose remained unconsumed at the end of all carbon‐limited fermentations. Similarly with previously published information 26, 27, glucose consumption was almost linear for both carbon‐ and nitrogen‐limited experiments, with glucose consumption rate (, in g/L/h) being significantly higher in the carbon‐limited culture than in the nitrogen‐limited one (rGlc≈0.36 against ≈0.17 g/L/h, respectively; see Fig. 1). Finally, total cellular lipids were quantified for all cultures and growth steps, without exceeding 10% w/w in DCW, suggesting, in accordance with literature 28, 29, 30, that no significant lipid accumulation occurred in carbon‐limited conditions (Table 3). Also, trials were performed in previously pasteurized media and were compared with the respective ones conducted when axenic cultures were used (Table 1); between aseptic and pasteurized shake‐flasks cultures, small differences were observed; in the previously pasteurized culture media, the quantity of glucose remained unconsumed was 8.0 ± 0.5 g/L and slightly lower citric acid quantities were reported compared with the previously sterilized media (Table 1).
Figure 1.
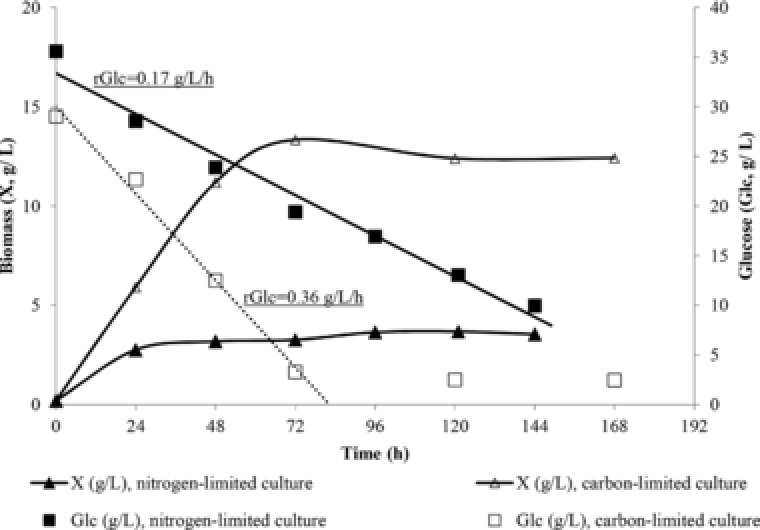
Biomass (X, g/L) and glucose (Glc, g/L) evolution during growth of Yarrowia lipolytica ACA‐YC 5033 on OMW‐based media enriched with commercial glucose, with initial phenolic compounds concentration 2.90 ± 0.25 g/L in nitrogen‐limited [in g/L: Glc0 = 35.0 ± 2.0, (NH4)2SO4 = 0.50 ± 0.05, yeast extract = 0.50 ± 0.05] and carbon‐limited [in g/L: Glc0 = 28.0 ± 1.0, (NH4)2SO4 = 4.00 ± 0.10, yeast extract = 2.00 ± 0.10] conditions. Culture conditions: growth on 250‐mL flasks at 180 ± 5 rpm, initial pH = 6.0 ± 0.1, pH ranging between 5.0 and 6.0, DOT>20% v/v, incubation temperature T = 28°C. Each point is the mean value of two independent measurements.
Table 3.
Fatty acid composition in the total cellular lipid (% w/w) of Yarrowia lipolytica ACA‐YC 5033, cultivated on media containing commercial glucose and various initial OMW concentrations in nitrogen limited fermentations
| Initial phenolic compounds (g/L) | Time (h) | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | U.I |
|---|---|---|---|---|---|---|---|
| 0.0a | 24 | 17.1 | 16.9 | 6.2 | 50.5 | 12.1 | 0.916 |
| 72 | 16.0 | 11.7 | 6.0 | 54.1 | 11.0 | 0.878 | |
| 144 | 13.4 | 10.1 | 4.4 | 57.3 | 9.7 | 0.868 | |
| 2.00 ± 0.20 | 96 | 18.2 | 4.0 | 3.0 | 53.7 | 21.1 | 0.998 |
| 168 | 12.2 | 12.3 | 3.8 | 58.2 | 13.4 | 0.973 | |
| 2.90 ± 0.50 | 24 | 14.7 | 0.0 | 0.0 | 66.7 | 18.6 | 1.039 |
| 96 | 11.5 | 5.1 | 3.7 | 62.7 | 17.0 | 1.018 | |
| 144 | 11.3 | 5.8 | 3.6 | 62.6 | 16.8 | 1.020 | |
| 2.90 ± 0.50 (Pasteurized) | 24 | 0.0 | 0.0 | 0.0 | 78.6 | 21.4 | 1.213 |
| 48 | 13.0 | 0.0 | 0.0 | 66.1 | 20.9 | 1.079 | |
| 96 | 12.6 | 0.0 | 3.8 | 64.6 | 19.0 | 1.026 | |
| 178 | 10.9 | 3.4 | 3.5 | 63.3 | 18.9 | 1.046 | |
| 2.90 ± 0.50 (Bioreactor) | 24 | 13.2 | 0.0 | 0.0 | 69.8 | 22.5 | 1.148 |
| 144 | 11.6 | 4.9 | 3.2 | 63.9 | 15.9 | 1.006 | |
| 4.50 ± 0.35 | 24 | 16.3 | 0.0 | 0.0 | 71.0 | 12.6 | 0.963 |
| 144 | 15.4 | 0.0 | 0.0 | 71.7 | 12.8 | 0.974 | |
| 5.50 ± 0.40 | 24 | 14.0 | 0.0 | 3.1 | 69.0 | 13.9 | 0.969 |
| 144 | 15.8 | 0.0 | 3.2 | 69.0 | 12.0 | 0.930 |
To perform a process scale‐up, an important aspect that should be studied is the comparison between experiments performed in shake‐flask and batch bioreactor cultures, given that agitation and aeration conditions may be different in these fermentation configurations 3, 4, 12. Comparison was performed between aseptic shake‐flask and aseptic bioreactor fermentations of OMWs‐based media that presented almost equal initial glucose (≈35.0 g/L) and phenolic compounds (≈2.90 g/L) concentrations; some differences concerning both maximum DCW and citric acid production were presented (see Table 1). A comparison of the kinetics between the equivalent shake‐flask and bioreactor experiments are seen in Supporting Information Fig. S2, and in both trials, Glc≈10.0 g/L remained unconsumed 160 ± 20 h after inoculation, while the rGlc was almost linear for both types of cultures, being slightly higher in the shake‐flask than in the bioreactor trial (rGlc≈0.17 g/L/h against 0.13 g/L/h, respectively; Supporting Information Fig. S2). Finally, pasteurized batch bioreactor trials (Glc0≈35.0 g/L; initial phenolics ≈2.90 g/L) were performed and between aseptic and pasteurized cultures no significant differences were observed for biomass and total cellular lipids production (Table 1).
Finally, a trial Glc0≈80.0 g/L was performed in shake‐flask aseptic cultures (OMWs added in order to create a medium with phenolics concentration c. 2.90 g/L) (Table 1). Such high Glc0 concentrations can usually be found in OMWs derived from press extraction systems 4, 11, 21. Xmax = 4.6 ± 0.3 g/L and Lmax quantities up to 1.40 ± 0.10 g/L were obtained (Table 1; Supporting Information Fig. S3). On the other hand, lipids in both absolute (g/L) and relative (g per g of DCW) values decreased although significant quantities of sugar remained non‐assimilated into the medium.
The principal cellular fatty acids (FAs) were the C16 and C18 ones. FA composition clearly changed and the UI was increased with the addition of OMWs into the medium but decreased after the initial fermentation hours (Table 3). Specifically, the addition of OMW increased cellular Δ9C18:1 and Δ9,12C18:2, decreasing C18:0. In most of the cases, the addition of OMWs resulted in concentration of Δ9C18:1>65% w/w and in one case (growth of the strain in shake‐flasks under pasteurized conditions) the concentration of this FA was ≈80% w/w. Moreover, Δ9C16:1 was significantly decreased with the addition of OMWs into the medium (Table 3).
3.2. Citric acid production by Yarrowia lipolytica ACA‐YC 5033 growing on OMW‐based media
In nitrogen‐limited experiments, Y. lipolytica ACA‐YC 5033 produced citric acid as the main metabolite synthesized; the highest citric acid concentration achieved was c. 19.0 g/L (when Glc0≈35.0 g/L and initial phenolics at c. 2.90 g/L) with YCit/Glc = 0.74 g/g, while citric acid production remained almost unaffected (by means of Citmax values) by the addition of moderate quantities of phenolic compounds (up to 2.90 g/L) into the culture medium compared with the blank experiments. Comparison between the aseptic and the non‐aseptic trial revealed some reduction of citric acid production in the later case by both means of Citmax and YCit/Glc values (see Table 1). On the other hand, in the aseptic shake‐flask trials with the significantly increased initial phenolic compounds concentrations imposed (viz. 4.50 g/L and 5.50 g/L) the high phenol content seriously inhibited microbial growth. The production of citric acid reached in both trials the values of c. 4.0 and 2.2 g/L respectively, while under these culture conditions the microbial metabolism was mainly shifted toward the synthesis of microbial lipids.
Comparison of the equivalent shake‐flask and batch bioreactor fermentations (Glc0≈35.0 g/L, initial phenolic compounds ≈2.90 g/L), showed that citric acid production was somehow decreased in the bioreactor experiment. Also, comparison of the aseptic and the previously pasteurized batch bioreactor fermentations, showed some citric acid production decrease in the later trial (see Table 1). Moreover, regarding the aseptic shake‐flask trial with initial phenolic compounds adjusted at c. 2.90 g/L and Glc0≈80.0 g/L, Citmax quantities up to c. 52.0 g/L (overall the highest value obtained in the current investigation), corresponding to YCit/Glc values up to 0.64 g/g were obtained (see Table 1). Attempting to compare the above‐mentioned trial with the respective one that presented a lower Glc0 concentration (viz. the trial with initial phenolics at 2.90 g/L and Glc0≈35.0 g/L, thus a trial with a lower initial C/N ratio imposed), one could note that while the production of citrate in absolute values (g/L) clearly increased in the former case, surprisingly the addition of glucose into the medium led to lower values of the conversion yield YCit/Glc. This is somehow surprising as a result, since with a constant initial nitrogen availability, the more the Glc0 concentration is increased, theoretically the more the yield YCit/Glc should be elevated 26, 27, 28, 30, 31, 32. Potentially at Glc0≈80.0 g/L, the carbon flow was directed more efficiently towards lipid and biomass formation. Finally, in the carbon‐limited fermentations insignificant citric acid quantities were produced (Cit<1.5 g/L) since onset of citric acid production occurs only after complete NH4 + exhaustion from the medium (see also: Anastassiadis et al. 33; Papanikolaou and Aggelis 30), and this fact was not reported in the carbon‐limited trials (at the end of growth, a significant quantity of NH4 + ions remained into the medium; data not presented).
3.3. Modeling citric acid production
In the next step it was desirable to apply suitable numerical models capable to predict the kinetic behavior of Y. lipolytica cultivated on glucose‐enriched OMW‐based media. Specifically, given that lipid accumulation was a relatively marginal physiological feature it has been decided to describe by adequate mathematical equations DCW production, glucose and nitrogen consumption, as well as citric acid formation without taking into consideration the process of lipid synthesis. Kinetic modeling was applied only for the runs in which significant secretion of citric acid into the culture medium has been performed. Equations describing the above‐mentioned processes were proposed in the literature. More specifically, cell growth was described through Eq. (1) (Table 4) for X representing the total DCW concentration (X in g/L or gX/L), t the fermentation time (h) and μ the biomass specific growth rate (h−1). The biomass growth rate was related to the biomass concentration through a modified logistic growth equation 34, as depicted on Table 4, Eq. (2). Xmax is the maximum attainable biomass concentration (g/L or gX/L) and n the inhibition exponent, a dimensionless constant.
Table 4.
Governing equations and corresponding kinetics parameters related to biomass growth, glucose (Glc, g/L) and nitrogen consumption (N, g/L), as well as citric acid formation (Cit, g/L) during citric acid production by Yarrowia lipolytica ACA‐YC 5033
| Equation | Equation Number | Glucose (g/L) | Flasks | Bioreactor | ||
|---|---|---|---|---|---|---|
| ∼35 | ∼80 | ∼35 | ||||
| Number of data points | 7 | 16 | 8 | |||
|
|
(1) | μ max (h−1) | 0.437 | 0.165 | 0.234 | |
| Xmax (gX/L) | 4.06 | 4.23 | 4.09 | |||
|
|
(2) | n | 3.09 | 1.97 | 1.61 | |
| SSE | 0.059 | 1.386 | 0.763 | |||
| R 2 | 0.993 | 0.975 | 0.960 | |||
|
|
(3) | YX|N (gX/gN) | 29.86 | 33.19 | 30.62 | |
| SSE | 4.26×10−4 | 7.89×10−3 | 2.88×10−4 | |||
| R2 | 0.982 | 0.661 | 0.979 | |||
|
|
(4) | q Citmax (gCit/(gX h)) | 0.110 | 9.746 | 0.143 | |
| Citmax (gCit/L) | 20.13 | 48.90 | 14.73 | |||
|
|
(5) | k (gN/L) | 19.89 | 4.50×10−5 | 9.39×10−3 | |
| SSE | 1.419 | 40.101 | 1.273 | |||
| R 2 | 0.996 | 0.994 | 0.995 | |||
|
|
(6) | YX|Glc (gX/gGlc) | 0.85 | 0.92 | 0.83 | |
| YCit/Glc (gCit/gGlc) | 0.96 | 0.67 | 0.81 | |||
| SSE | 13.099 | 337.619 | 12.825 | |||
| R 2 | 0.975 | 0.982 | 0.982 | |||
Culture conditions: growth on aseptic 250‐mL flasks at 180 ± 5 rpm, Glc0 = 35.0 ± 2.0 g/L and Glc0 = 80.0 ± 2.0 g/L, (NH4)2SO4 = 0.50 g/L, yeast extract = 0.5 g/L, initial pH = 6.0 ± 0.1, pH ranging between 5.0 and 6.0, DOT>20% v/v, incubation temperature T = 28°C, initial phenolic compounds concentration 2.90 ± 0.25 g/L; growth on aseptic batch bioreactor (Glc0 = 35.0 ± 2.0 g/L), 300 rpm, initial phenolic compounds concentration 2.90 ± 0.25 g/L, initial pH = 6.0 ± 0.1, incubation temperature T = 28°C and sparging of air at 1.0 vvm.
Nitrogen consumption (for biomass synthesis) was assumed to be related to biomass growth through Eq. (3) (Table 4) for N being the nitrogen concentration (g/L or gN/L). YX/N is defined as the yield coefficient for biomass production with respect to nitrogen (g of dry biomass produced per g of nitrogen consumed, gX/gN). In addition, the rate of citric acid formation was assumed to be analogous to biomass concentration as presented in Eq. (4) of Table 4, for Cit being the citric acid concentration (g/L or gCit/L) and q Cit the specific citric acid formation rate [g of citric acid per g of biomass per hour, gCit/(gX h)]. As suggested by Bellou et al. 23, a reducing factor (viz.: 1‐Cit/Citmax) was incorporated to the q Cit term in order to express the gradual diminishing to zero of the qCit value, as citric acid concentration approached its maximum value, Citmax. Furthermore, a Monod type factor, k/(k+N) 35 was introduced to q Cit indicating low citric acid formation at high nitrogen concentrations. Thus, q Cit was expressed through Eq. (5) (Table 4), for q Citmax being the maximum specific citric acid formation rate [gCit/(gX h)], Citmax the maximum attainable citric acid concentration (gCit/L), and k a constant (gN/L). Glucose was consumed either for biomass or citric acid production at a rate given by Eq. (6) of Table 4. Variable Glc represents the glucose concentration (g/L or gGlc/L), YX/Glc the yield coefficient for total biomass production with respect to glucose (gX/gGlc, g of biomass per g of glucose) and YCit/Glc the yield for citric acid production with respect to glucose (gCit/gGlc, g of citric acid per g of glucose).
The kinetic parameters involved were determined by minimizing the sum of squared errors (SSE) between predicted and experimental values. The governing differential equations presented on Table 4 were discretized through an explicit finite difference scheme and calculations were performed in Microsoft® Office EXCEL. Thus, for example, in its discrete form and in view of Eq. (2), Eq. (1) was transformed into:
| (1) |
where Xi represents the biomass concentration at a certain time, ti, and Xi +1 the total biomass concentration at time ti + Δt. A time step, Δt, equal to 0.1 h was used for the calculations.
Regarding modeling citric acid production by the stain used, the estimated kinetic parameters for the three cases studied, together with the corresponding SSE and R2 values for each equation used, are reported on Table 4. The values of the kinetic parameters presented on Table 4 should be used and interpreted in relation to the associated differential equations. Thus, for example, when referring to q Citmax (the specific citric acid formation rate) its value cannot be directly compared to q Citmax values obtained from a model from which the inhibition term, k/(k+N), used in the present analysis, is omitted. In particular, concerning the estimated q Citmax values reported on Table 4, one should note that the parameter appears in Eq. (5) as the product q Citmax × k and the value of q Citmax can be affected from the value of parameter k. Comparisons between fitted and experimental data are depicted on Fig. 2A and B indicating a rather good agreement between experimental data and theoretical curves.
Figure 2.
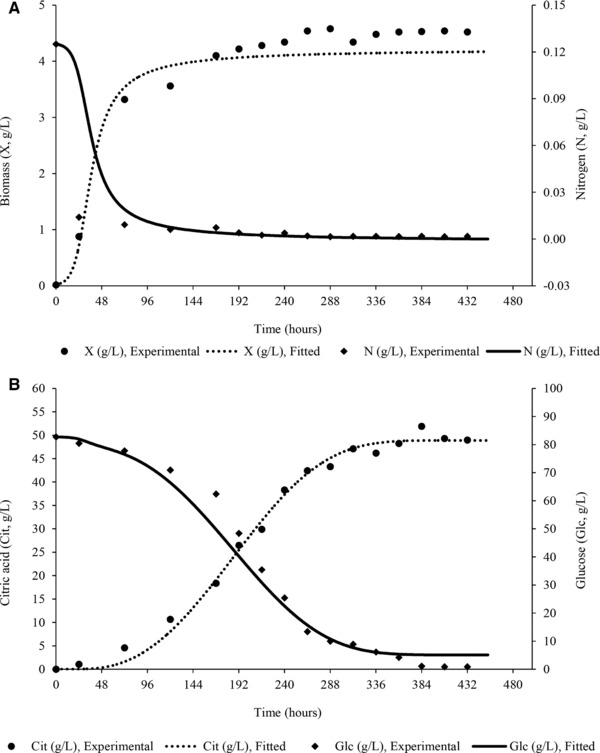
Experimental values and theoretical curves concerning evolution of biomass (X, g/L), ammonium ions (N, g/L) (A), glucose (Glc, g/L), and citric acid (Cit, g/L) (B) concentration during modeling of citric acid bioprocess production by Yarrowia lipolytica ACA‐YC 5033 on OMW‐based media (shake‐flask cultures; Glc0 = 80.0 ± 2.0 g/L, (NH4)2SO4 = 0.50 ± 0.05 g/L, yeast extract = 0.50 ± 0.05 g/L; initial phenolic compounds concentration 2.90 ± 0.25 g/L) enriched with commercial glucose in nitrogen‐limited conditions. Culture conditions as described in Fig. 1.
One must further note that glucose consumption for biomass production is considerably lower than the glucose consumed for citric acid production. For example, for the case of citric acid production in flasks with Glc0≈80.0 g/L, c. 75.0 g/L of glucose are consumed for citric acid production and only 5.0 g/L for biomass production [see: Figs. 5A and B]. Thus, and in view of the interconnection between the YX/Glc and YCit/Glc values and the possible errors in experimental measured concentrations, accurate determination of both YX/Glc and YCit/Glc parameters from the whole range of experimental glucose vs time data might be questionable. For an experimental error in calculating the total glucose consumed of the order of 5%, the error in determining YCit/Glc is not important, while inaccuracies associated with the YX/Glc value can be significant since, for our example, the amount of glucose used for biomass production can be anywhere between almost zero and 10.0 g/L. Thus, at a first step the initial part of the experimental data (glucose versus time) were fitted into Eq. (6) for the determination of both YX/Glc and YCit/Glc parameters. In particular, we used the data up to 72, 120, and 48 h for the case of flasks with Glc0≈35.0 g/L, flasks with Glc0≈80.0 g/L and the bioreactor with Glc0≈35.0 g/L, respectively. These times correspond approximately to the end of the exponential growth of Y. lipolytica ACA‐YC 5033 for the conditions studied. At a second step, the procedure was repeated using the entire data set and the YX/Glc value determined in the first step to estimate the reported values of the YCit/Glc parameter.
3.4. Decolorization and removal of phenolic compounds
The overall maximum decolorization achieved was within the range of 36–58%. The decolorization process seemed to insignificantly decrease by the addition of waste except the aseptic batch bioreactor trial and the experiments with initial phenolic compounds at 4.50 and 5.50 g/L where it was clearly decreased (Table 5). On the other hand, the overall maximum reduction of phenolic compounds ranged between 5 and 51% w/w. In nitrogen‐limited experiments, the maximum decolorization achieved was 55.9 ± 4.0% (at initial phenolics c. 2.00 g/L), while the maximum reduction in phenol compounds obtained was 50.9 ± 2.5% w/w and 29.1 ± 1.5% w/w (at initial phenolics at 5.50 and 4.50 g/L, respectively). Thus, the removal of phenolic compounds increased by the addition of the waste into the medium (Table 5). In carbon‐limited fermentations, the maximum decolorization achieved was 58.1 ± 4.5% (at initial phenolic of 2.00 g/L) whereas the maximum reduction in phenolic compounds was 13.8 ± 1.0% w/w (at initial phenolics of 2.90 g/L), lower, hence, compared to nitrogen‐limited trials (Table 5). The kinetics of color and phenolic compounds removal from the culture medium in the case with initial phenolic compounds at 5.50 g/L is shown in Supporting Information Fig. S4.
Table 5.
Data of Yarrowia lipolytica ACA‐YC 5033 concerning removal of phenol compounds and color during kinetics in media (sterile and pasteurized shake‐flask cultures and sterile batch bioreactor cultures) containing commercial glucose and various initial O.M.W. concentrations
| Initial phenolics (g/L) | Final phenolics (g/L) | Phenolic compounds reduction (% w/w) | Color removal (%) | ||
|---|---|---|---|---|---|
| (A) | Flasks | 2.10 ± 0.20 | 1.83 ± 0.20 | 12.9 ± 1.0 | 55.9 ± 4.0 |
| 2.82 ± 0.25 | 2.31 ± 0.20 | 17.9 ± 1.0 | 55.7 ± 4.0 | ||
| 2.83 ± 0.25 Pasteurized | 2.36 ± 0.20 | 16.7 ± 1.0 | 54.9 ± 4.0 | ||
| 3.05 ± 0.25 Glc0∼80.0 g/L | 2.53 ± 0.20 | 17.1 ± 1.0 | 56.7 ± 4.0 | ||
| Bioreactor | 2.96 ± 0.25 | 2.50 ± 0.20 | 15.4 ± 1.0 | 35.6 ± 3.5 | |
| 3.04 ± 0.25 Pasteurized | 2.51 ± 0.20 | 17.4 ± 1.0 | 37.8 ± 3.5 | ||
| Flasks | 4.51 ± 0.35 | 3.20 ± 0.30 | 29.0 ± 1.5 | 46.5 ± 4.0 | |
| 5.47 ± 0.40 | 2.68 ± 0.25 | 50.9 ± 2.5 | 45.3 ± 4.0 | ||
| (B) | Flasks | 2.10 ± 0.20 | 2.00 ± 0.20 | 4.8 ± 0.5 | 58.1 ± 4.5 |
| 2.90 ± 0.25 | 2.50 ± 0.20 | 13.8 ± 1.0 | 55.3 ± 4.0 |
Representation of initial and final phenol compounds concentration in the culture medium, phenol compounds removal (% w/w) and color removal (%) from the medium in nitrogen‐limited (A) and carbon‐limited (B) experiments. Each point is the mean value of two independent measurements.
4. Discussion
The ability of Yarrowia lipolytica ACA‐YC 5033 to reduce the phenolic content and the color of glucose‐enriched OMW‐based media and simultaneously produce added‐value products (cellular lipids, citric acid and biomass) was assessed in the current study. Non‐negligible DCW production in both nitrogen‐ and carbon‐limited shake‐flask cultures occurred when various amounts of OMWs were added into the medium. In nitrogen‐limited trials, satisfactory biomass and citric acid quantities were obtained. Finally, as in some cases, significant phenolic compounds removal (reaching at c. 51% w/w) and a remarkable decolorization (up to 58%) were observed.
In carbon‐limited media, significant DCW formation (Xmax = 10.9–13.3 g/L; YX/Glcmax = 0.52 g/g) was observed, while OMWs addition enhanced biomass production compared with the control experiment (Table 2). Similar increment of DCW production due to OMWs addition compared with the trials with no OMW added have already been reported for Saccharomyces cerevisiae strain MAK 1 19. Apparently, in order such microbial behavior to be observed, strains should be significantly robust and resistant against recalcitrant and toxic substances found into the medium like the phenolics, while OMWs should also contain micro‐elements or nutrients (e.g. vitamins) that enhance the production of biomass in comparison with the control experiments. On the other hand, trials performed in carbon‐limited conditions showed significantly higher sugar uptake than in the nitrogen‐limited trials (see i.e. Fig. 1), in accordance with the theory and the literature 22, 26, 29.
Various microbial strains (including but not limited to Y. lipolytica) gradually turn the metabolism from cell growth to citric acid and/or storage lipid formation when cultured on glucose or similarly metabolized compounds (e.g. glycerol) and when nitrogen limitation had been previously imposed 21, 27, 28, 30, 32, 33, 36, 37, 38, 39. Noticeable citric acid production has been reported by Candida oleophila, C. guillermondii or Y. lipolytica strains during growth on glucose or other compounds (e.g. glycerol, vegetable oils, ethanol, etc.) after nitrogen depletion from the media 21, 26, 28, 32, 33, 40, 41, 42, 43, 44. Culture conditions [viz. the initial C/N molar ratio, the carbon source, the nitrogen source (i.e. corn‐steep liquor significantly enhances the production of citric acid compared with other nitrogen sources), the pH drop into the medium, etc] and the potentiality of the strain itself have serious impact upon citric acid production process 21, 27, 32, 33, 41, 42, 43, 45, 46. In the current investigation, the overall Citmax concentration achieved (∼52 g/L), although satisfactory, it is somehow at the lower limits in comparison with the highest literature values reported so far (i.e. ranging between 50 and 160 g/L) when other Y. lipolytica and C. oleophila strains were cultivated on various carbon sources 32, 33, 40, 41, 42, 43, 44, 45, 47, 48, 49, 50, 51. On the other hand, the yield of citric acid produced per unit of glucose consumed (YCit/Glc) in the current investigation ranged between 0.6 and 0.8 g/g (see Table 1). Values of citric acid produced per substrate consumed reported in the literature by strains growing on sugars or similarly metabolized compounds such as glycerol ranges between 0.40 and 0.80 g/g 21, 33, 39, 44, 45, 47, 48, 52. Only in a very limited number of reports this conversion yield was >0.90 g/g 27, 46, 53. It is evident that substances having a higher carbon content or an increased reductance degree compared with glucose (i.e. fatty materials, hydrocarbons, etc.) can easily present a conversion yield that is equal or higher than 1.0 g/g 42, 54. An overview of citric acid production by yeasts when grown under various fermentation configurations including the current investigation is presented in Table 6.
Table 6.
Experimental results of citric acid‐producing yeasts during growth under various fermentation configurations and comparisons with the present investigation
| Strain | Substrate | Culture mode | Cit (g/L) | YCit/S a (g/g) | Reference |
|---|---|---|---|---|---|
| Yarrowia lipolytica LGAM S (7) | OMW/glycerol blend | Shake flasks | 30.3 | 0.62 | Dourou et al. 9 |
| Yarrowia lipolytica ACA‐DC 50109 | OMW/glucose blend | Shake flasks | 28.9 | 0.53b | Papanikolaou et al. 21 |
| Yarrowia lipolytica W29 | OMW/glucose blend | Shake flasks | 15.8 | 0.46b | Sarris et al. 22 |
| Yarrowia lipolytica ATCC 20346 | Glucose | Fed‐batch culture, bioreactor | 50.0–69.0 | 0.52 | Moresi 26 |
| Yarrowia lipolytica JMY1203d | Crude glycerol | Shake flasks | 57.7 | 0.92 | Papanikolaou et al. 27 |
| Yarrowia lipolytica LGAM S(7)1 | Crude glycerol | Shake flasks | 35.1 | 0.44 | Papanikolaou et al. 31 |
| Candida oleophila ATCC 20177 | Glucose | Batch culture, flasks, bioreactor | 50.1–79.1 | 0.55 | Anastassiadis et al. 33 |
| Yarrowia lipolytica N1 | Ethanol | Fed‐batch culture, bioreactor | 120.0 | 0.85 | Kamzolova et al. 41 |
| Yarrowia lipolytica 187/1 | Rapeseed oil | Fed‐batch culture, bioreactor | 135.1 | 1.55 | Kamzolova et al. 42 |
| Yarrowia lipolytica N15 | Pure glycerol | Fed‐batch bioreactor | 98.0 | 0.70 | Kamzolova et al. 43 |
| Candida oleophila ATCC 20177 | Glucose | Continuous culture bioreactor | 37.6–57.8 | 0.40 | Anastassiadis and Rehm 45 |
| Yarrowia lipolytica SWJ‐1b | Glucose | Batch bioreactor | 52.3 | 0.87 | Liu et al. 46 |
| Candida lipolytica Y1095 | Glucose | Fed‐batch culture bioreactor | 13.6–78.5 | 0.79 | Rane and Sims 47 |
| Yarrowia lipolytica Wratislavia 1.31 | Crude glycerol | Batch bioreactor | 124.5 | 0.62 | Rymowicz et al. 49 |
| Yarrowia lipolytica A‐101‐1.22 | Crude glycerol | Batch bioreactor | 112.0 | 0.60 | Rymowicz et al. 50 |
| Yarrowia lipolytica Wratislavia AWG7 | Crude glycerol | Repeated batch | 154.0 | 0.78 | Rywińska and Rymowicz 51 |
| Yarrowia lipolytica NG40/UV7 | Pure glycerol | Fed‐batch bioreactor | 115.0 | 0.64 | Morgunov et al. 53 |
| Candida lipolytica NRRL Y‐1095 | n‐Paraffins | Fed‐batch culture bioreactor | 30.0–40.0 | 1.00 | Crolla and Kennedy 54 |
| Saccharomycopsis lipolytica D 1805 | Glucose | Batch culture, bioreactor | 95.0 | 0.75 | Briffaud and Engasser 55 |
| Saccharomycopsis lipolytica NRRL Y‐7576 | Glucose | Batch culture, bioreactor | 51.5 | 0.71 | Klasson et al. 56 |
| Candida guillermondii IMK 1 | Galactose | Shake flasks | 13.5 | 0.38 | Gutierrez et al. 57 |
| Candida lipolytica Y 1095 | Glucose | Continuous culture recycling, bioreactor | 40.0–50.0 | 0.72 | Rane and Sims 58 |
| Yarrowia lipolytica ACA‐DC 50109 | Commercial glucose | Shake flasks | 42.9 | 0.56 | Papanikolaou et al. 59 |
| Yarrowia lipolytica AWG‐7 | Glucose syrup | Shake flasks | 36.7 | 0.31 | Rymowicz and Cibis 60 |
| Yarrowia lipolytica NRRL YB‐423 | Pure glycerol | Shake flasks | 21.6 | 0.55 | Levinson et al. 61 |
| Yarrowia lipolytica LFMB 20 | Crude glycerol | Shake flasks | 42.0 | 0.39 | Papanikolaou et al. 62 |
| Commercial glucose | 58.1 | 0.55 | |||
| Yarrowia lipolytica A‐101 | Crude glycerol | Batch bioreactor | 66.8 | 0.43 | Rywińska et al. 63 |
| Yarrowia lipolytica Wratislavia 1.31 | Crude glycerol | Fed‐batch bioreactor | 126.0 | 0.63 | Rywińska et al. 64 |
| 157.5 | 0.58 | ||||
| Yarrowia lipolytica Wratislavia AWG7 | Pure glycerol | Continuous bioreactorc | 86.5 | 0.59 | Rywińska et al.65 |
| Yarrowia lipolytica PR32e | Glucose | Fed‐batch bioreactor | 111.1 | 0.93 | Fu et al.66 |
| Yarrowia lipolytica ACA‐YC 5033 | OMW/glucose blend | Shake flasks | 52.0 | 0.64b | Present study |
YCit/S: g of citric acid produced per gram of carbon substrate consumed (maximum values).
In the case of OMW/glucose blends, the conversion yield was based on the reducing sugars (OMWs contained some quantities of reducing sugars) consumed by the strains.
D = 0.009 h–1.
Strain JMY1203 is genetically engineered strain derivative from W29, with inactivated 2‐methyl‐citrate dehydratase.
Strain PR32 is genetically engineered strain derivative from SWJ‐1b, with over‐expressed pyruvate carboxylase.
In a number of reports, there have been attempts to perform kinetic modeling of citric acid production in several types of fermentation configurations. In the current investigation, a logistic‐type equation (modification of the Verhust‐type equation; see Messens et al. 34), was employed in order to simulate the production of biomass. On the other hand, as far as the specific rate of citric acid production was concerned, although citric acid was a perfectly non‐growth associated product synthesized after depletion of nitrogen from the medium, it was decided that q Cit would not be constant throughout the fermentation, but a reducing factor (1‐Cit/Citmax) and a Monod type factor, k/(k+N) would be incorporated to the q Cit term in order to better express the gradual diminishing to zero of the q Cit value (in accordance with Bellou et al. 23 and Economou et al. 35; in the above‐mentioned cases it was the lipid accumulation process that had been simulated, while lipid and citric acid production bioprocesses present fundamental similarities at least at their first stages; Papanikolaou and Aggelis 30). A comparison between the optimized parameter values estimated from the kinetic model of the present study and those obtained in the literature during citric acid production process is presented in Table 7, and similarities exist between the optimized parameter values achieved in the current investigation and those reported in the literature. Only for the optimized q Cit value in one of the trials of the current investigation (Glc0≈80.0 g/L) a significantly higher value than the literature was seen [ = 9.746 gCit/(gX h)]. However, as previously stated, in the proposed modeling approach q Citmax value can be affected from the value of parameter k and, in fact, there should be the product q Citmax × k that should be taken into consideration in order to more correctly present citric acid specific production rate.
Table 7.
Comparison between the optimized kinetic parameters estimated from the kinetic model of the present study and those obtained in the literature during production of citric acid from yeasts and molds cultivated in various carbon sources
| Optimized parameter value | Present study | Literature |
|---|---|---|
| μ max (h–1) | 0.17–0.44 | 0.21 (Briffaud and Engasser 55)a |
| 0.24 (Wojtatowicz et al. 67)b | ||
| 0.28 (Klasson et al. 56)a | ||
| 0.18 (Arzumanov et al. 68)c | ||
| 0.18–0.36 (Papanikolaou and Aggelis 69)d | ||
| 0.10–0.15 (Papanikolaou et al. 59)a | ||
| 0.24 (Anastassiadis et al. 33)e | ||
| 0.15–0.19 (Papanikolaou et al. 21)f | ||
| 0.07–0.12 (Karasu‐Yalcin et al. 70)a | ||
| 0.26 (Karasu‐Yalcin et al. 70)g | ||
| YX/Sugar (g/g) (YX/Glycerol g/g) | 0.83–0.92 | 0.37 (Briffaud and Engasser 55)a |
| 0.40 (Klasson et al. 56)a | ||
| 0.41 (Moresi 26)a | ||
| 0.29–0.30 (Papanikolaou and Aggelis 69)d | ||
| 0.27–0.70 (Papanikolaou et al. 59)a | ||
| 0.21–0.29 (Papanikolaou et al. 21)f | ||
| YX/N (g/g) | 29.86–33.19 | 40 (Briffaud and Engasser 55)a |
| 45 (Hossain et al. 71)h , i | ||
| 35 (Rane and Sims 47)a | ||
| 19.67 (Arzumanov et al. 68)c | ||
| 31.97–33.62 (Papanikolaou and Aggelis 69)d | ||
| 46.2–47.3 (Papanikolaou et al. 59)a | ||
| 47.62–67.72 (Papanikolaou et al. 21)f | ||
| Xmax (g/L) | 4.06–4.22 | 8.5–12.5 (Klasson et al. 56)a |
| 12.0–17.5 (Arzumanov et al. 68)c | ||
| 5.89 (Papanikolaou and Aggelis 69)d | ||
| 5.0–6.7 (Papanikolaou et al. 21)f | ||
| 8.78–11.09 (Karasu‐Yalcin et al. 72)a | ||
| YCit/Sugar (g/g) (YCit/Glycerol g/g) | 0.66–0.96 | 0.60–0.75 (Briffaud and Engasser 55)a |
| 0.71 (Klasson et al. 56)a | ||
| 0.48 (Gutierrez et al. 57)j | ||
| 0.50–0.60 (Mayilvahanan et al. 73)k | ||
| 0.62–0.63 (Papanikolaou and Aggelis 69)d | ||
| 0.72–0.82 (Papanikolaou et al. 21)f | ||
| 0.14–0.38 (Karasu‐Yalcin et al. 70)g | ||
| 0.28–0.39 (Karasu‐Yalcin et al. 72)a | ||
| q Cit (g/(g h)) | 0.11–9.74 | 0.01 (Maddox and Kingston 74)a |
| 0.051 (Klasson et al. 56)a | ||
| 0.02 (Roukas and Kotzekidou 75)l | ||
| 3.05 × 10−2 (Papanikolaou and Aggelis 69)d | ||
| 0.120 (Kamzolova et al. 41)c | ||
| 0.127 (Kamzolova et al. 42)m | ||
| 1.29–1.64 × 10−2 (Papanikolaou et al. 59)a | ||
| 1.33–2.12×10−2 (Papanikolaou et al. 21)f | ||
| 0.009–0.030 (Karasu‐Yalcin et al. 72)a |
Values presented in the literature were in most of the cases the optimized ones that derived from the application of various kinetic models.
Yarrowia (Candida – Saccharomycopsis) lipolytica on glucose.
Y. lipolytica on starch hydrolysates.
Y. lipolytica on ethanol.
Y. lipolytica on biodiesel‐derived glycerol.
C. oleophila on glucose.
Y. lipolytica on OMW‐glucose blends.
Y. lipolytica on fructose.
Aspergillus niger on sucrose.
A. niger on lactose.
C. guillermondii on galactose.
A. niger on molasses.
A. niger on brewery sugar residues.
Y. lipolytica on rapeseed oil.
Strain ACA‐YC 5033 produced quantities of intra‐cellular lipids (up to 48% w/w in DCW) in nitrogen‐limited media supplemented with OMWs while in the control experiment small lipid quantities (up to 12% w/w) were accumulated (Table 1). The presence of OMWs into the medium indeed favored storage lipid production process (Fig. 3). This result further fortifies the findings recorded also for the strain Y. lipolytica W29 22, whereas similar physiological feature has been reported for several oleaginous Zygomycetes 23, suggesting that OMWs can act as a “lipogenic” medium. Up‐to‐date, only a limited number of studies indicate that the addition of natural compounds (e.g. Teucrium polium L. aqueous extracts, Origanum extracts, etc) can significantly enhance the process of lipid accumulation in yeast cells 76, 77, and it is evident that this type of studies will have a significant academic and economic interest in the future.
Figure 3.
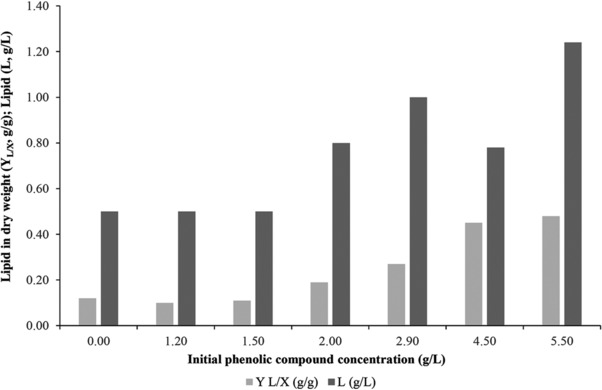
Cellular lipids (L, g/L) and total cellular lipid in dry weight (YL/X, g/g) overall evolution during growth of Y. lipolytica strain ACA‐YC 5033 on OMW‐based media [initial phenolic compounds concentration 0.00 g/L (no OMW addition), 1.20 ± 0.10 g/L, 1.50 ± 0.15 g/L, 2.00 ± 0.20 g/L, 2.90 ± 0.25 g/L, 4.50 ± 0.35 g/L and 5.50 ± 0.40 g/L] enriched with commercial glucose in nitrogen‐limited conditions. Culture conditions as described in Fig. 1. Results regarding trials with initial phenolic compounds concentration 0.00 g/L (control experiment; no OMW addition), 1.20 ± 0.10 g/Land 1.50 ± 0.15 g/L obtained from Sarris et al. 22.
In the present study, the strain used led to decolorization (up to c. 58%) and removal of phenol content (up to c. 51% w/w) on glucose‐enriched OMW‐based media. The reduction of OMWs color and the removal of their phenolic content by yeasts appear to be a strain‐dependent process. The bioremediation of OMW‐based media by various yeast strains and the comparison with the present study is summarized in Supporting Information Table S1. Yeast strains compared to higher fungi, do not possess the mechanisms of producing the appropriate extra‐cellular oxidases to break down phenolic compounds 2, 12, 82, 83, 84. Though, production of laccases has been achieved only in genetically engineered Y. lipolytica strains 85.
Only in a very limited number of reports, significant reduction of phenolic compounds has been observed in cultures performed by yeast species 13, 79, 81, with phenolic compounds removal of 51% w/w (present study), being one of the highest values reported in the international literature. Only in the case of the report by Lopes et al. 81 much higher removal of phenolic compounds compared with the current investigation had been reported for the Y. lipolytica strains W29 and IMUFRJ 50682, but in the above‐mentioned experiment the initial phenolic content of the OMWs used was much lower (c. 0.8 g/L) compared with the current investigation. Since, yeasts lack the existence of phenol‐oxidizing enzymes 12, the OMW decolorization and removal of phenol compounds by yeasts should not be achieved by such mechanisms. Potentially, adsorption of phenolic compounds in the yeast cells may exist 86. It could be also supposed, that partial utilization of phenolic compounds as carbon source by the microorganism might occur.
The main cellular FA of lipid produced by Y. lipolytica during all trials was the Δ9C18:1. Amounts of this FA (together with Δ9,12C18:2) increased with the addition of OMWs (Table 3), in accordance with Papanikolaou et al. 21 and Sarris et al. 22. In several cases, high quantities of Δ9C18:1 (>65% w/w of total lipids) were found into the cellular lipids, specifically in media supplemented with OMWs. This feature seems also to happen with other yeast species (e.g. S. cerevisiae) besides Y. lipolytica, where supplementation of the medium with OMWs increased the cellular lipid content of the FA Δ9C18:1 19.
5. Concluding remarks
Yarrowia lipolytica ACA‐YC 5033 presented efficient growth when cultivated on glucose‐enriched OMWs. Satisfactory citric acid quantities were produced in nitrogen‐ limited media while non‐negligible biomass production was observed in carbon‐limited media. The addition of OMWs in the medium favored the accumulation of storage lipids suggesting that OMWs seemed to be a “lipogenic” substrate. Both nitrogen and carbon‐ limited fermentations resulted in a remarkable decolorization and a non‐negligible reduction of phenolic compounds in the media. The tested Y. lipolytica strain can be considered as satisfactory candidate for simultaneous OMWs bioremediation and the production of added‐value compounds useful for the food industry.
Practical application
Olive mill wastewaters (OMWs) are one of the most important agro‐industrial residues produced in the European level. Despite the efforts that have been carried out up‐to‐date, none of the proposed physico‐chemical or biotechnological methods related with their treatment has been revealed as truly satisfactory. In the current investigation, a new Yarrowia lipolytica strain has been cultivated on commercial glucose‐supplemented media in which OMWs had been added in high concentrations (presenting thus increased phenolic compounds quantities), and it has been revealed capable to grow and present remarkable lipid‐enriched biomass and citric acid production despite the high concentration of phenolic compounds imposed into the medium. Therefore, Y. lipolytica can be considered as a promising microbial cell factory amenable to produce citric acid and lipid‐containing biomass and to simultaneously detoxify OMW‐based media, potentially under non‐aseptic conditions.
The authors have declared no conflict of interest. The manuscript does not contain experiments using animals or human studies.
Nomenclature
| X | [g/L] | biomass |
| Glc | [g/L] | glucose |
| Cit | [g/L] | citric acid |
| L | [g/L] | total lipid |
| YX/Glc | [g formed per g of glucose consumed] | biomass yield on glucose consumed |
| YCit/Glc | [g formed per g of glucose consumed] | citric acid yield on glucose consumed |
| YL/X | [g formed per g of biomass formed] | total lipid on biomass |
| YX/N | [g formed per g of ammonium ions consumed] | biomass yield on nitrogen consumed |
| μ | [per h] | specific growth rate |
| q Cit | [g formed per g biomass formed per h] | specific rate of citric acid produced |
| UI | unsaturation index | |
| subscripts | 0, cons and max indicate the initial, consumed and maximum quantity of the components in the kinetics performed |
Supporting information
Supplementary Material
Table S1. Decolorization and reduction of phenolic compounds of OMW‐based media by yeasts and processes used; comparison with the present study.
Acknowledgments
Financial support was provided by the Agricultural University of Athens.
6 References
- 1. Aly, A. A. , Hasan, Y. N. , Al‐Farraj, A. S. , Olive mill wastewater treatment using a simple zeolite‐based low‐cost method. J. Εnviron. Μanage. 2014, 145, 341–348. [DOI] [PubMed] [Google Scholar]
- 2. Zerva, A. , Zervakis, G. I. , Christakopoulos, P. , Topakas, E. , Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood‐rot fungi. J. Environ. Manage. 2016, in press, DOI: 10.1016/j.jenvman.2016.02.042. [DOI] [PubMed] [Google Scholar]
- 3. Mantzavinos, D. , Kalogerakis, N. , Treatment of olive mill effluents, Part I organic matter degradation by chemical and biological processes‐an overview. Environ. Int. 2005, 31, 289–295. [DOI] [PubMed] [Google Scholar]
- 4. Crognale, S. , D'Annibale, A. , Federici, F. , Fenice, M. et al., Olive oil mill wastewater valorization by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar]
- 5. Apostolakis, A. , Grigorakis, S. , Makris, D. P. , Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar]
- 6. Cavdarova, M. , Makris, D. P. , Extraction kinetics of phenolics from carob (Ceratonia siliqua L.) kibbles using environmentally benign solvents. Waste Biomass. Valor. 2014, 5, 773–779. [Google Scholar]
- 7. Chouchouli, V. , Kalogeropoulos, N. , Konteles, S. J. , Karvela, E. et al., Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT‐Food Sci. Technol. 2013, 53, 522–529. [Google Scholar]
- 8. Mateo, J. J. , Maicas, S. , Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015, 73, 13–25. [Google Scholar]
- 9. Dourou, M. , Kancelista, A. , Juszczyk, P. , Sarris, D. et al., Bioconversion of olive mill wastewater into high‐added value products. J. Cleaner Prod. 2016, 139, 957–969. [Google Scholar]
- 10. Sassi, A. B. , Ouazzani, N. , Walker, G. M. , Ibnsouda, S. et al., Detoxification of olive mill wastewaters by Moroccan yeast isolates. Biodegrad. 2008, 19, 337–346. [DOI] [PubMed] [Google Scholar]
- 11. Crognale, S. , Federici, F. , Petruccioli, M. , β‐Glucan production by Botryosphaeria rhodina on undiluted olive‐mill wastewaters. Biotechnol. Lett. 2003, 25, 2013–2015. [DOI] [PubMed] [Google Scholar]
- 12. Aggelis, G. , Iconomou, D. , Christou, M. , Bokas, D. et al., Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [DOI] [PubMed] [Google Scholar]
- 13. D'Annibale, A. , Sermanni, G. G. , Federici, F. , Petruccioli, M. , Olive‐mill wastewaters: a promising substrate for microbial lipase production. Bioresour. Technol. 2006, 97, 1828–1833. [DOI] [PubMed] [Google Scholar]
- 14. Tsioulpas, A. , Dimou, D. , Iconomou, D. , Aggelis, G. , Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [DOI] [PubMed] [Google Scholar]
- 15. Bambalov, G. , Israilides, C. , Tanchev, S. , Alcohol fermentation in olive oil extraction effluents. Biolo. Waste 1989, 27, 71–75. [Google Scholar]
- 16. Massadeh, M. I. , Modallal, N. , Ethanol production from olive mill wastewater (OMW) pretreated with Pleurotus sajor‐caju . Energy Fuel. 2008, 22, 150–154. [Google Scholar]
- 17. Sarris, D. , Papanikolaou, S. , Biotechnological production of ethanol: biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar]
- 18. Sarris, D. , Matsakas, L. , Aggelis, G. , Koutinas, A. A. et al., Aerated vs non‐aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non‐aseptic conditions. Ind. Crops. Prod. 2014, 56, 83–93. [Google Scholar]
- 19. Sarris, D. , Giannakis, M. , Philippoussis, A. , Komaitis, M. et al., Conversions of olive mill wastewater‐based media by Saccharomyces cerevisiae through sterile and non‐sterile bioprocesses. J. Chem. Technol. Biotechnol. 2013, 88, 958–969. [Google Scholar]
- 20. Zanichelli, D. , Carloni, F. , Hasanaj, E. , D'Andrea, N. et al., Production of ethanol by an integrated valorization of olive oil byproducts. The role of phenolic inhibition (2 pp). Environ. Sci. Pollut. Res. 2007, 14, 5–6. [DOI] [PubMed] [Google Scholar]
- 21. Papanikolaou, S. , Galiotou‐Panayotou, M. , Fakas, S. , Komaitis, M. et al., Citric acid production by Yarrowia lipolytica cultivated on olive‐mill wastewater‐based media. Bioresour. Technol. 2008, 99, 2419–2428. [DOI] [PubMed] [Google Scholar]
- 22. Sarris, D. , Galiotou‐Panayotou, M. , Koutinas, A. A. , Komaitis, M. et al., Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater‐based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar]
- 23. Bellou, S. , Makri, A. , Sarris, D. , Michos, K. et al., The olive mill wastewater as substrate for single cell oil production by Zygomycetes. J. Biotechnol. 2014, 170, 50–59. [DOI] [PubMed] [Google Scholar]
- 24. Papanikolaou, S. , Sarantou, S. , Komaitis, M. , Aggelis, G. , Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple‐limited media. J. Appl. Microbiol. 2004, 97, 867–875. [DOI] [PubMed] [Google Scholar]
- 25. Sayadi, S. , Ellouz, R. , Decolourization of olive mill waste‐waters by the white‐rot fungus Phanerochaete chrysosporium: involvement of the lignin‐degrading system. Appl. Microbiol. Biotechnol. 1992, 37, 813–817. [Google Scholar]
- 26. Moresi, M. , Effect of glucose concentration on citric acid production by Yarrowia lipolytica . J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar]
- 27. Papanikolaou, S. , Beopoulos, A. , Koletti, A. , Thevenieau, F. et al., Importance of the methyl‐citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica . J. Biotechnol. 2013, 168, 303–314. [PubMed] [Google Scholar]
- 28. Fakas, S. , Lipid biosynthesis in yeasts: a comparison of the lipid biosynthetic pathway between the model nonoleaginous yeast Saccharomyces cerevisiae and the model oleaginous yeast Yarrowia lipolytica . Eng. Life Sci. 2016, in press, DOI: 10.1002/elsc.201600040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Papanikolaou, S. , Aggelis, G. , Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- 30. Papanikolaou, S. , Aggelis, G. , Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica . Lipid Technol. 2009, 21, 83–87. [Google Scholar]
- 31. Papanikolaou, S. , Muniglia, L. , Chevalot, I. , Aggelis, G. et al., Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [DOI] [PubMed] [Google Scholar]
- 32. Papanikolaou, S. , Fakas, S. , Fick, M. , Chevalot, I. et al., Biotechnological valorisation of raw glycerol discharged after bio‐diesel (fatty acid methyl esters) manufacturing process: Production of 1, 3‐propanediol, citric acid and single cell oil. Biomass Bioenerg. 2008, 32, 60–71. [Google Scholar]
- 33. Anastassiadis, S. , Aivasidis, A. , Wandrey, C. , Citric acid production by Candida strains under intracellular nitrogen limitation. Appl. Microbiol. Biotechnol. 2002, 60, 81–87. [DOI] [PubMed] [Google Scholar]
- 34. Messens, W. , Verluyten, J. , Leroy, F. , De Vuyst, L. , Modelling growth and bacteriocin production by Lactobacillus curvatus LTH 1174 in response to temperature and pH values used for European sausage fermentation processes. Int. J. Food Microbiol. 2003, 81, 41–52. [DOI] [PubMed] [Google Scholar]
- 35. Economou, C. N. , Aggelis, G. , Pavlou, S. , Vayenas, D. , Modeling of single‐cell oil production under nitrogen‐limited and substrate inhibition conditions. Biotechnol. Bioeng. 2011, 108, 1049–1055. [DOI] [PubMed] [Google Scholar]
- 36. André, A. , Chatzifragkou, A. , Diamantopoulou, P. , Sarris, D. et al., Biotechnological conversions of bio‐diesel‐derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar]
- 37. André, A. , Diamantopoulou, P. , Philippoussis, A. , Sarris, D. et al., Biotechnological conversions of bio‐diesel derived waste glycerol into added‐value compounds by higher fungi: production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010, 31, 407–416. [Google Scholar]
- 38. Dobrowolski, A. , Mituła, P. , Rymowicz, W. , Mirończuk, A. M. , Efficient conversion of crude glycerol from various industrial wastes into single cell oil by yeast Yarrowia lipolytica. Bioresour. Technol. 2016, 207, 237–243. [DOI] [PubMed] [Google Scholar]
- 39. Makri, A. , Fakas, S. , Aggelis, G. , Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [DOI] [PubMed] [Google Scholar]
- 40. Antonucci, S. , Bravi, M. , Bubbico, R. , Di Michele, A. et al., Selectivity in citric acid production by Yarrowia lipolytica . Enzyme Microb. Technol. 2001, 28, 189–195. [DOI] [PubMed] [Google Scholar]
- 41. Kamzolova, S. V. , Shishkanova, N. V. , Morgunov, I. G. , Finogenova, T. V. , Oxygen requirements for growth and citric acid production of Yarrowia lipolytica . FEMS Yeast Res. 2003, 3, 217–222. [DOI] [PubMed] [Google Scholar]
- 42. Kamzolova, S. V. , Morgunov, I. G. , Aurich, A. , Perevoznikova, O. A. et al., Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- 43. Kamzolova, S. V. , Fatykhova, A. R. , Dedyukhina, E. G. , Anastassiadis, S. G. et al., Citric acid production by yeast grown on glycerol‐containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65. [Google Scholar]
- 44. Moeller, L. , Strehlitz, B. , Aurich, A. , Zehnsdorf, A. et al., Optimization of citric acid production from glucose by Yarrowia lipolytica . Eng. Life. Sci. 2007, 7, 504–511. [Google Scholar]
- 45. Anastassiadis, S. , Rehm, H. J. , Continuous citric acid secretion by a high specific pH dependent active transport system in yeast Candida oleophila ATCC 20177. Electron. J. Biotechnol. 2005, 8, 26–42. [Google Scholar]
- 46. Liu, X. , Wang, X. , Xu, J. , Xia, J. et al., Citric acid production by Yarrowia lipolytica SWJ‐1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind. Crops. Prod. 2015, 78, 154–160. [Google Scholar]
- 47. Rane, K. D. , Sims, K. A. , Production of citric acid by Candida lipolytica Y1095: effect of glucose concentration on yield and productivity. Enzyme Microb. Technol. 1993, 15, 646–651. [Google Scholar]
- 48. Rane, K. D. , Sims, K. A. , Citric acid production by Yarrowia lipolytica: effect of nitrogen and biomass concentration on yield and productivity. Biotechnol. Lett. 1996, 18, 1139–1144. [Google Scholar]
- 49. Rymowicz, W. , Rywińska, A. , Żarowska, B. , Juszczyk, P. , Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica . Chem. Pap. 2006, 60, 391–394. [Google Scholar]
- 50. Rymowicz, W. , Fatykhova, A. R. , Kamzolova, S. V. , Rywińska, A. et al., Citric acid production from glycerol‐containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [DOI] [PubMed] [Google Scholar]
- 51. Rywińska, A. , Rymowicz, W. , High‐yield production of citric acid by Yarrowia lipolytica on glycerol in repeated‐batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [DOI] [PubMed] [Google Scholar]
- 52. Morgunov, I. , Solodovnikova, N. Y. , Sharyshev, A. , Kamzolova, S. et al., Regulation of NAD+‐Dependent isocitrate dehydrogenase in the citrate producing yeast Yarrowia lipolytica . Biochemistry (Mosc.) 2004, 69, 1391–1398. [DOI] [PubMed] [Google Scholar]
- 53. Morgunov, I. G. , Kamzolova, S. V. , Lunina, J. N. , The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [DOI] [PubMed] [Google Scholar]
- 54. Crolla, A. , Kennedy, K. , In‐line mixing for production of citric acid by Candida lipolytica grown on n‐paraffins. J. Chem. Technol. Biotechnol. 2004, 79, 720–728. [DOI] [PubMed] [Google Scholar]
- 55. Briffaud, J. , Engasser, J. , Citric acid production from glucose. I. Growth and excretion kinetics in a stirred fermentor. Biotechnol. Bioeng. 1979, 21, 2083–2092. [Google Scholar]
- 56. Klasson, T. , Clausen, E. , Gaddy, J. , Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 20, 491–509. [Google Scholar]
- 57. Gutierrez, N. A. , McKay, I. A. , French, C. E. , Brooks, J. D. et al., Repression of galactose utilization by glucose in the citrate‐producing yeast Candida guilliermondii . J. Ind. Microbiol. 1993, 11, 143–146. [Google Scholar]
- 58. Rane, K. D. , Sims, K. A. , Citric acid production by Candida lipolytica Y 1095 in cell recycle and fed‐batch fermentors. Biotechnol. Bioeng. 1995, 46, 325–332. [DOI] [PubMed] [Google Scholar]
- 59. Papanikolaou, S. , Galiotou‐Panayotou, M. , Chevalot, I. , Komaitis, M. et al., Influence of glucose and saturated free‐fatty acid mixtures on citric acid and lipid production by Yarrowia lipolytica. Curr. Microbiol. 2006, 52, 134–142. [DOI] [PubMed] [Google Scholar]
- 60. Rymowicz, W. , Cibis, E. , Optimization of citric acid production from glucose syrup by Yarrowia lipolytica using response surface methodology. Electron. J. Pol. Agric. Univ. Series: Biotechnol. 2006, 9. [Google Scholar]
- 61. Levinson, W. E. , Kurtzman, C. P. , Kuo, T. M. , Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb. Technol. 2007, 41, 292–295. [Google Scholar]
- 62. Papanikolaou, S. , Rontou, M. , Belka, A. , Athenaki, M. et al., Conversion of biodiesel‐derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2016, in press, DOI: 10.1002/elsc.201500191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rywińska, A. , Rymowicz, W. , Żarowska, B. , Skrzypiński, A. , Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica . World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [DOI] [PubMed] [Google Scholar]
- 64. Rywińska, A. , Rymowicz, W. , Marcinkiewicz, M. , Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 9–10. [Google Scholar]
- 65. Rywińska, A. , Juszczyk, P. , Wojtatowicz, M. , Rymowicz, W. , Chemostat study of citric acid production from glycerol by Yarrowia lipolytica . J. Biotechnol. 2011, 152, 54–57. [DOI] [PubMed] [Google Scholar]
- 66. Fu, G.‐Y. , Lu, Y. , Chi, Z. , Liu, G.‐L. et al., Cloning and characterization of a pyruvate carboxylase gene from Penicillium rubens and overexpression of the gene in the yeast Yarrowia lipolytica for enhanced citric acid production. Mar. Biotechnol. 2016, 18, 1–14. [DOI] [PubMed] [Google Scholar]
- 67. Wojtatowicz, M. , Rymowicz, W. , Kautola, H. , Comparison of different strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrol. Appl. Biochem. Biotechnol. 1991, 31, 165–174. [DOI] [PubMed] [Google Scholar]
- 68. Arzumanov, T. E. , Sidorov, I. A. , Shishkanova, N. V. , Finogenova, T. V. , Mathematical modeling of citric acid production by repeated batch culture. Enzyme Microb. Technol. 2000, 26, 826–833. [DOI] [PubMed] [Google Scholar]
- 69. Papanikolaou, S. , Aggelis, G. , Modelling aspects of the biotechnological valorization of raw glycerol: production of citric acid by Yarrowia lipolytica and 1,3‐propanediol by Clostridium butyricum . J. Chem. Technol. Biotechnol. 2003, 78, 542–547. [Google Scholar]
- 70. Karasu‐Yalcin, S. , Bozdemir, M. , Ozbas, Z. , A comparative study of citric acid production kinetics of two Yarrowia lipolytica strains in two different media. Indian J. Biotechnol. 2009, 8, 408–417. [Google Scholar]
- 71. Hossain, M. , Brooks, J. , Maddox, I. , The effect of the sugar source on citric acid production by Aspergillus niger . Appl. Microbiol. Biotechnol. 1984, 19, 393–397. [Google Scholar]
- 72. Karasu‐Yalcin, S. , Tijen Bozdemir, M. , Yesim Ozbas, Z. , Effects of different fermentation conditions on growth and citric acid production kinetics of two Yarrowia lipolytica strains. Chem. Biochem. Eng. Q. 2010, 24, 347–360. [Google Scholar]
- 73. Mayilvahanan, D. , Annadurai, G. , Raju, V. , Chellapandian, M. et al., Citric acid production. Bioprocess Eng. 1996, 15, 323–326. [Google Scholar]
- 74. Maddox, I. , Kingston, P. , Use of immobilized cells of the yeast Saccharomycopsis lipolytica for the production of citric acid. Biotechnol. Lett. 1983, 5, 795–798. [Google Scholar]
- 75. Roukas, T. , Kotzekidou, P. , Influence of some trace metals and stimulants on citric acid production from brewery wastes by Aspergillus niger . Enzyme Microb. Technol. 1987, 9, 291–294. [Google Scholar]
- 76. Aggelis, G. , Komaitis, M. , Enhancement of single cell oil production by Yarrowia lipolytica growing in the presence of Teucrium polium L. aqueous extract. Biotechnol. Lett. 1999, 21, 747–749. [Google Scholar]
- 77. Chatzifragkou, A. , Petrou, I. , Gardeli, C. , Komaitis, M. et al., Effect of Origanum vulgare L. essential oil on growth and lipid profile of Yarrowia lipolytica cultivated on glycerol‐based media. J. Am. Oil. Chem. Soc. 2011, 88, 1955–1964. [Google Scholar]
- 78. Ettayebi, K. , Errachidi, F. , Jamai, L. , Tahri‐Jouti, M. A. et al., Biodegradation of polyphenols with immobilized Candida tropicalis under metabolic induction. FEMS Microbiol. Lett. 2003, 223, 215–219. [DOI] [PubMed] [Google Scholar]
- 79. Chtourou, M. , Ammar, E. , Nasri, M. , Medhioub, K. , Isolation of a yeast, Trichosporon cutaneum, able to use low molecular weight phenolic compounds: application to olive mill waste water treatment. J. Chem. Technol. Biotechnol. 2004, 79, 869–878. [Google Scholar]
- 80. Lanciotti, R. , Gianotti, A. , Baldi, D. , Angrisani, R. et al., Use of Yarrowia lipolytica strains for the treatment of olive mill wastewater. Bioresour. Technol. 2005, 96, 317–322. [DOI] [PubMed] [Google Scholar]
- 81. Lopes, M. , Araújo, C. , Aguedo, M. , Gomes, N. et al., The use of olive mill wastewater by wild type Yarrowia lipolytica strains: medium supplementation and surfactant presence effect. J. Chem. Technol. Biotechnol. 2009, 84, 533–537. [Google Scholar]
- 82. D'Annibale, A. , Ricci, M. , Quaratino, D. , Federici, F. et al., Panus tigrinus efficiently removes phenols, color and organic load from olive‐mill wastewater. Res. Microbiol. 2004, 155, 596–603. [DOI] [PubMed] [Google Scholar]
- 83. Fountoulakis, M. , Dokianakis, S. , Kornaros, M. , Aggelis, G. et al., Removal of phenolics in olive mill wastewaters using the white‐rot fungus Pleurotus Ostreatus. Water Res. 2002, 36, 4735–4744. [DOI] [PubMed] [Google Scholar]
- 84. Lakhtar, H. , Ismaili‐Alaoui, M. , Philippoussis, A. , Perraud‐Gaime, I. et al., Screening of strains of Lentinula edodes grown on model olive mill wastewater in solid and liquid state culture for polyphenol biodegradation. Int. Biodeterior. Biodegrad. 2010, 64, 167–172. [Google Scholar]
- 85. Jolivalt, C. , Madzak, C. , Brault, A. , Caminade, E. et al., Expression of laccase IIIb from the white‐rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl. Microbiol. Biotechnol. 2005, 66, 450–456. [DOI] [PubMed] [Google Scholar]
- 86. Rizzo, M. , Ventrice, D. , Varone, M. , Sidari, R. et al., HPLC determination of phenolics adsorbed on yeasts. J. Pharm. Biomed. Anal. 2006, 42, 46–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Table S1. Decolorization and reduction of phenolic compounds of OMW‐based media by yeasts and processes used; comparison with the present study.


