Abstract
The NS-Pten knockout (KO) mouse exhibits hyperactivity of the mammalian target of rapamycin (mTOR) and is a model of autism spectrum disorder (ASD). ASD presents with marked deficits in communication which can be elucidated by investigating their counterpart in mice, ultrasonic vocalizations (USVs). While USVs have been found to be altered in NS-Pten KO pups, no study has assessed whether this communication deficit persists into adulthood. In the present study, we investigate female urine-induced USVs, scent marking behavior, and open field activity in NS-Pten KO and wildtype (WT) adult male mice. Results showed that there was no difference in the quantity of vocalizations produced between groups, however, there were extensive alterations in the spectral properties of USVs. KO mice emitted vocalizations of a lower peak frequency, shorter duration, and higher peak amplitude compared to WT mice. KO animals also emitted a significantly different distribution of call-types relative to controls, displaying increased complex and short calls, but fewer upward, chevron, frequency steps, and composite calls. No significant differences between groups were observed for scent marking behavior and there was no difference between groups in the amount of time spent near the female urine. Overall, this study demonstrated that mTOR hyperactivity contributes to communication deficits in adult mice.
Keywords: UV, Communication deficits, mTOR, Call-types, Scent marking
1. Introduction
Autism spectrum disorder (ASD) affects approximately 1 in 42 males and 1 in 182 females, making it one of the most prevalent neurodevelopmental conditions [1, 2]. ASD is characterized by repetitive behaviors and impairments in social interaction and communication [3]. Previous research has found that the communication deficits in individuals with autism are exhibited both early in life, as well as in adulthood [4, 5]. Infants with ASD exhibit a different pattern of crying than neurotypical infants, while displaying an increased pitch of their cries [5, 6]. Autism has also been associated with delayed language development, repetitive speech, echolalia, and poor non-verbal skills, persistently and pervasively hindering communication throughout the lifespan [7–10]. Since humans are social animals, communication is integral to the human experience and vital for healthy functioning and development. Communicative deficits in individuals with autism poses a significant and prevalent threat to their long-term quality of life. However, little is known about the molecular changes underpinning the communication deficits in ASD.
The relationship between ASD and communication deficits can be further elucidated through examining communicative behaviors in murine models. Communication in mice can be studied by assessing a behavior known as ultrasonic vocalizations (USVs) [11]. USVs are whistle-like sounds emitted between 30 and 90 kHz that occur both in early development (postnatal days (PD) 2–14) and adulthood (PD 60–120) [12–15]. Vocalizations are elicited in neonates by separating a pup from its dam, whereas adult vocalizations are emitted during courtship and mating rituals [16–18]. The total quantity of vocalizations emitted and various spectral characteristics such as the amplitude (loudness), peak and fundamental frequency (pitch), and the duration of the USVs can be measured. The vocalizations can also be placed into different categories based on their overall shape to provide qualitative information. Therefore, assessing USVs provides a non-invasive, comprehensive measure of murine communicative behaviors. USVs have been shown to be dysregulated in a variety of autistic-like murine models, including but not limited to BTBR mice and Shank3, Nlgn4, Fmr1, ProSAP1, Tsc1/2, and NS-Pten mutant mice [14, 19–23]. These findings indicate that altered USVs are an important constituent of the autistic-like phenotype and that ASD murine models consistently display deficits in communicative behaviors.
The majority of murine communication research has focused on neonatal vocalizations. Less is known about mouse communicative behaviors in adulthood, with even fewer studies assessing communicative behaviors in adult models expressing an autistic-like phenotype. This omission is significant, as autism persists throughout an individual’s lifespan and negatively impacts their quality of life [24]. Many of the communicative problems present in children with autism are also present in adults with autism, with 85% of adults with autism displaying significant symptomology [4].
Cowden syndrome is an autosomal dominant condition that results from a mutation in the phosphatase and tensin homolog on chromosome 10 (PTEN) gene [25]. It is characterized by macrocephaly, benign tumors, developmental delay, intellectual disability, and most notably, is one of the largest single gene contributors to autism, accounting for 8.3% of ASD cases [26–29]. A commonly used murine model of Cowden syndrome is the neuronal subset-specific (NS)-Pten knockout (KO) mouse model, which has a deletion of Pten in a subset of neurons in the cortex, hippocampus, and cerebellum [30, 31]. Deletion of NS-Pten leads to hyperactivation of the mammalian target of rapamycin (mTOR), a commonly implicated molecular mechanism in ASD [32]. Since the NS-Pten deletion is localized to the brain, it allows for a clearer elucidation of the neuronal role mTOR plays in an autistic phenotype than systemic KO models may provide [30, 31]. Our lab has previously demonstrated that neonatal NS-Pten KO mice present with an overall decreased quantity of vocalizations in addition to displaying both spectral and qualitative changes in the calls emitted [19]. When adult NS-Pten KO behavior has been assessed, deficits in social interaction and repetitive behaviors have been found [33, 34]. Altogether, previous studies suggest that the NS-Pten KO model reliably exhibits an autistic phenotype both during development and during adulthood. However, despite the importance of communication deficits in the autistic phenotype, no study has assessed communicative behaviors in NS-Pten adult mice. The purpose of this study is to assess communicative behaviors in NS-Pten adult KO mice in order to further clarify the relationship between mTOR hyperactivity and an autistic-like phenotype.
2. Materials and Methods
2.1. Animals and housing
Heterozygous NS-Pten males and females were used to breed NS-Pten wildtype (WT) and knockout (KO) pups. The NS-Pten mice used were on a FVB-based backcrossed background strain bred for more than 10 generations at Baylor University and have been previously described (RRID:MGI:3714016) [35]. A total of 26 mice were tested in this study, 12 NS-Pten male KO and 14 male WT mice. All animals were tested at 7 weeks of age in the afternoon during the light cycle between 1 and 3 p.m. The mice were kept in a room on a 12-hr light/dark diurnal cycle held at 22°C and given ad libitum access to food and water. All test procedures were carried out in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by Baylor University’s Institutional Animal Care and Use Committee.
2.2. Experimental design
2.2.1. Previous female exposure
One week prior to vocalization recording, male subject mice were introduced to female mice of the same age and background strain. Siblings were not used in the pairing session. Specifically, one male NS-Pten WT or KO mouse was placed with a female NS-Pten WT mouse in a clean polycarbonate cage for a 5-minute duration. The cage was closely monitored for the sessions’ duration and copulation was not allowed. This exposure was done to standardize the history of social experience in the test mice and is a necessary step for eliciting adult vocalizations [14]. Additionally, following standard practice, the estrus cycle of the females were monitored daily after the pairing session [15].
2.2.2. Urine collection
Estrus was induced in females by placing male bedding into a cage of females that had been previously paired with the male test mice. The bedding was placed into the cage 24 hours before testing. The following day, the estrus cycles of the females were checked. If the vaginal area was red, inflamed, and opened, then the females were considered to be in estrus [36]. Fresh urine from the females that were in estrus was generated by removing the female from its cage and gently stroking the abdomen in a superior to inferior direction. The urine was collected in a 1.7 mL Eppendorf tube and 20 uL of the urine was then pipetted onto the center of a piece of Strathmore paper which lined the bottom of the test chamber (Strathmore Drawing Paper Premium, recycled, microperforated, 400 series; Strathmore Artist Papers, Neenah, WI, USA). The female urine used in the test was less than 10 minutes old.
2.2.3. Test procedure
Before testing, the male mice were weighed and allowed to habituate to the testing room for 30 minutes. After the habituation period, the test mice were individually placed into an acrylic, sound-attenuated chamber (40 cm × 40 cm × 30 cm) in an isolated room controlled for temperature, light levels, and background noise per established protocol [15, 23]. Ultrasonic vocalizations, scent marking, and open field activity were simultaneously recorded for a 5-minute duration for each test mouse. The open field activity was recorded with automatic optical animal detection software that was used to assess the total time spent proximal (within 20 cm) to the female urine, as well as the time spent distal (20 cm away) from the female urine (Fusion by Omnitech Electronics, Inc., Columbus, OH). Since both the scent marks and USVs are in response to the urine, knowing the total time near the stimulus for WT and KO mice is an important parameter to ensure a comprehensive assessment of adult communicative behaviors. The urine-induced vocalizations were recorded using a condenser ultrasonic microphone (CM16/CMPA, Avisoft Bioacoustics, Germany, part #40011) connected to an ultrasound-recording interface (UltraSoundGate 116Hb, Avisoft Bioacoustics, part #41161/41162) suspended in the testing chamber. In order to prevent contamination of olfactory cues between subjects, the apparatus was cleaned with 30% isopropyl alcohol between each trial. After the test was complete, the paper was removed then treated with Ninhydrin spray and left to develop for 24 hours (Sigma-Aldrich, St. Louis, MO, USA, ID # 24849098). Ninhydrin turned the urine traces purple and made the scent marks visible, allowing for their measurement. The scent marks were identified, outlined with a pen, and summed. Additionally, the number of scent marks within a 20 cm by 20 cm inner region, proximal to the female urine deposit, were counted, as were the scent marks in the outer region, distal from the female urine [37, 38].
2.3. Ultrasonic vocalization analyses
The female urine-induced ultrasonic vocalizations were analyzed using Avisoft SASLab Pro software (Avisoft SASLabPro, RRID:SCR_014438). The parameters used consisted of: a fast Fourier transformation (FTT) length of 1024, a time window overlap of 75%, a 100% hamming window, a frequency resolution of 488 Hz, a time resolution of 1 ms, and a sampling frequency of 22050 [14]. Each call was also visually identified and placed into 1 of 10 distinct categories based on their internal pitch changes, length, and shape, as described in Scattoni et al. (2008) [14]. The categories were complex, harmonic, two syllable, upward, downward, flat, chevron, short, composite, and frequency steps [14]. At the time of scoring the experimenter was blinded to the condition of the animal.
2.4. Statistical analysis
GraphPad Prism 7 software (La Jolla, CA) and SPSS 21.0 (IBM, USA) were used to analyze the data. The quantity of the USVs emitted between WT and KO mice was analyzed with an independent t -test. The acoustic and temporal parameters of the ultrasonic vocalizations, as well as the scent marking and open field parameters, were analyzed with independent t-tests and nonparametric Mann-Whitney U tests when homogeneity of variance was violated. Differences in call-types were assessed with a Pearson Chi-Square and accompanying z-tests were used to compare call type proportions between groups. All animals that failed to produce ultrasonic vocalizations were removed from testing and were not included in any analysis. For every analysis, a value of p < 0.05 was considered significant. The data are expressed as the mean ± standard error of the mean (SEM).
3. Results
3.1. Ultrasonic vocalization (USV) recordings
3.1.1. USV quantity and spectral characteristics
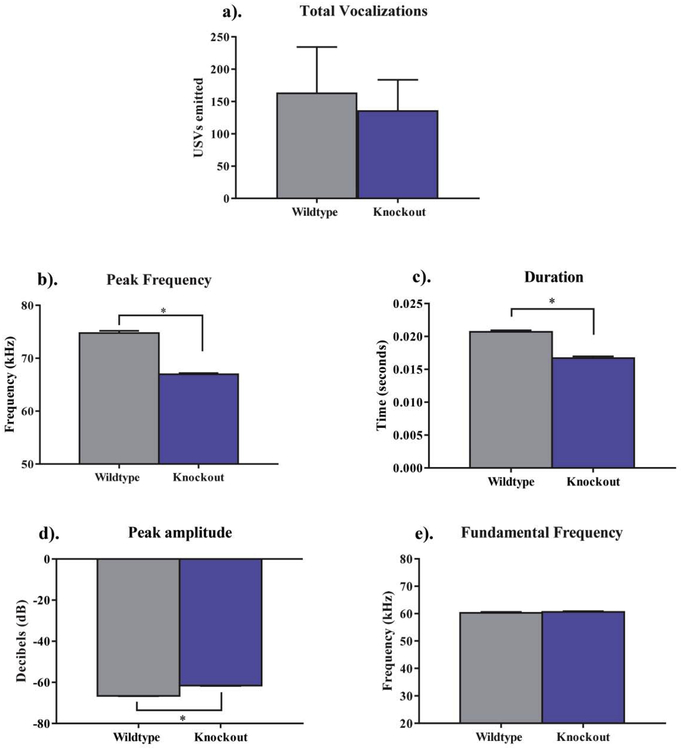
There was no statistically significant difference between the quantity of vocalizations emitted in NS-Pten KO and WT mice (t[24] = .31, p > .05) (Fig. 1a). Analysis of the spectral characteristics of the vocalizations revealed that NS-Pten KO mice emitted USVs of a lower peak frequency (U = 1524074, p < .001), shorter duration (U = 1524074, p < .001), and higher peak amplitude (U = 996497, p < .001) than WT animals (Fig. 1b–d). There was no significant difference between NS-Pten KO and WT mice for the fundamental frequency, (t[4427] = .7, p > .05. (Fig. 1e).
Fig. 1.
The quantity and spectral characteristics of ultrasonic vocalizations (USVs) emitted. (a). There was no significant difference in the total quantity of USVs emitted between wildtype (WT) and knockout (KO) mice (p > .05). (b-d.) Knockout animals emitted USVs of a lower peak frequency, shorter duration, and a higher peak amplitude than wildtype mice. (e). There was no statistically significant difference in the fundamental frequency between groups. Data are presented as the mean ± standard error of the mean (SEM). * = p < 0.05.
3.1.2. USV call type composition
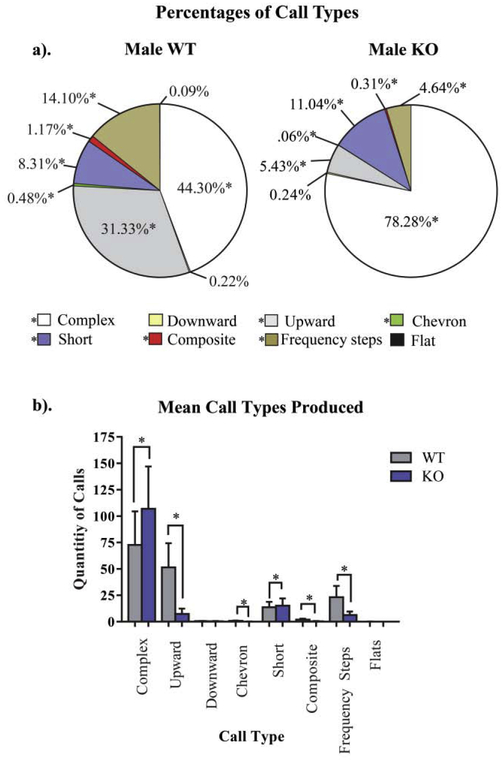
We examined the call-types emitted in each group to assess any qualitative differences in communicative behaviors. A Pearson Chi-Square analysis revealed significant population differences in the composition of calls produced from NS-Pten WT and KO mice (X2[7, N = 3937] = 609.04, p < .001). Accompanying post hoc z-tests were used to detect the quantity and percent of ultrasonic vocalizations emitted in each category of calls. We found that NS-Pten KO animals emitted a greater amount of complex and short calls but a smaller amount of upward, chevron, frequency steps, and composite calls when compared to WT mice (p < .05). The percent of the call-types emitted relative to the whole are depicted in Figure 2a, whereas the quantity of each call type produced is depicted in Figure 2b. No other differences in call type composition were found between groups (p > .05).
Fig. 2.
Call type utilization in adult NS-Pten knockout (KO) and wildtype (WT) mice. (a-b) On average, KO mice produced significantly more complex and short calls relative to WT mice. (a b) KO animals also produced fewer upward, composite, and frequency step call-types when compared to WT mice. No harmonic or two-syllable calls were identified in either group. Data are presented as the mean ± standard error of the mean (SEM). * = p < 0.05.
3.2. Scent marking in response to female urine and time spent near the urine deposit
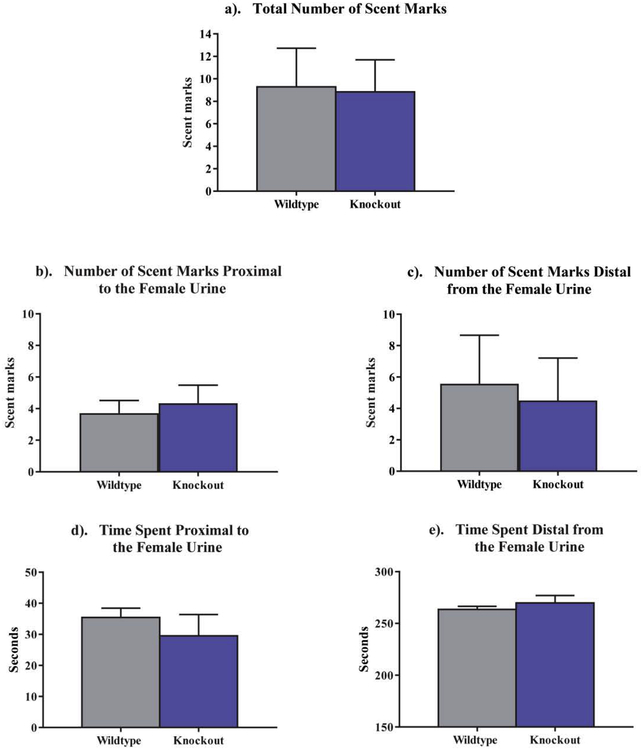
When investigating the male’s response to female urine, we found no difference in the total amount of scent marking between NS-Pten KO mice and WT mice (t[24] = .099, p > .05) (Fig. 3a). We also did not find any significant differences in the amount of scent marking within the center of the testing area (t[24] = .45, p > .05), or in the amount of scent marking surrounding the testing area (t[24] = .26, p > .05) (Fig. 3b,c). There was also no significant difference in the duration of time spent near (within 20 cm) the urine deposit between groups (t[24] = .83, p > .05), or in the amount of time spent distal (20 cm away) from the urine deposit (t[24] = .85, p > .05) (Fig. 3d,e).
Fig. 3.
Scent marking behavior and duration spent proximal (within 20 cm) to the female urine, and distal (20 cm away) from the female urine deposit. (a-c) There was no difference in the total number of scent marks made, nor any differences in the scent marks proximal to, nor distal from, the female urine. (d,e) There were also no differences in the quantity of time spent proximal to, and distal from, the female urine. Data are presented as the mean ± standard error of the mean (SEM).
4. Discussion
In the present study, we compared the quantity, call-types, and spectral characteristics of female urine-induced ultrasonic vocalizations in WT and NS-Pten KO male mice. We also examined scent marking behavior and the duration of time spent around the urine deposit. We found that NS-Pten KO mice display alterations in the spectral characteristics of the calls while exhibiting a significantly different distribution of call-types relative to WT mice. No difference in scent marking behavior was observed between WT and NS-Pten KO mice, nor was there a difference in the amount of time both groups spent proximal or distal to the urine deposit.
A property of importance in both human and animal studies examining communication in autism is the frequency of the vocalizations emitted, a parameter that is synonymous with pitch. Esposito et al. (2010) previously reported that human infants with autism cry at a higher pitch than neurotypical infants and that these cries are perceived as more aversive to caregivers [6]. Alterations in pitch have also been observed in our study, as well as in other mouse models of ASD. We found that NS-Pten KO adult mice emit vocalizations of a lower peak frequency relative to controls, similar to findings reported in BTBR mice [14] (Table 1). Conversely, NS-Pten KO pups and Fmr1 KO adult mice exhibit an increase in frequency [19, 23] (Table 1). While the specific increase or decrease in peak frequency may change depending on the model or time-point assessed, there are still deviations in peak frequency across a variety of ASD models. The presence of alterations in this parameter in humans and mice indicates that significant changes in peak frequency in an autistic phenotype may be conserved across species.
Table 1.
Quantitative USV Results in ASD Models
| Total | Peak Frequency | Duration | Peak Amplitude | |
|---|---|---|---|---|
| NS-Pten KO (adult) | ---- | ↓ | ↓ | ↑ |
| NS-Pten KO (pup)19 | ↓ | ↑ | ↓ | ↓ |
| Fmr1 KO (adult)23 | ---- | ↑ | ↓ | ↓ |
| Fmr1 KO (pup)39 | ↓ | N/A | ↓ | N/A |
| BTBR (adult)15 | ↓ | N/A | N/A | N/A |
| BTBR (pup)14 | ↑ | ↓ | ↑ | ↑ |
N/A = data not reported
We also observed a decreased duration and an increased peak amplitude of the vocalizations emitted from NS-Pten KO adult mice. A decreased duration has been previously shown in NS-Pten KO pups, and in Fmr1 KO pup and adult mice, whereas an increased duration was reported in BTBR pups [14, 19, 23, 39] (Table 1). A decreased amplitude of vocalizations has been reported in BTBR pups (age: PD 2), Fmr1 KO adults, and NS-Pten KO pups, whereas an increased amplitude was found in our study as well as in BTBR pups (age: PD 4–12) [14, 19, 23] (Table 1). It may be that the specific change in spectral parameter is less relevant than that the parameters are consistently aberrant in autistic models when compared to controls. Furthermore, studies that have assessed crying behavior in human infants with autism have similarly reported a shorter duration of the expiration phase (crying bout) and cries of a lower peak amplitude relative to neurotypical infants [40]. These findings provide further evidence that several spectral parameters that are atypical in murine ASD models may also be atypical in individuals with autism.
In addition to the spectral differences in NS-Pten WT and KO mice, there were also qualitative changes between the groups. Although KO mice emitted different quantities of chevron, short, composite, frequency steps, and upward call-types from WT mice, the most robust effect was in the quantity of complex calls emitted. Complex calls accounted for 44.3% of the total vocalizations produced by WT mice but accounted for 78.2% of the call-types utilized by KO mice. Complex calls have been found to be decreased in NS-Pten KO pups and increased in Fmr1 KO adult mice [14, 19, 23] (Table 2). These studies highlight the importance of examining specific differences in call-types and suggest that qualitative changes may be a more sensitive indicator of communication deficits, though further research is required to parse out this relationship.
Table 2.
Qualitative USV Results in ASD Models
| Complex | Short | Two-syallable | Downward | Upward | Chevron | Composite | Frequency Step | Flat | Harmonic | |
|---|---|---|---|---|---|---|---|---|---|---|
| NS-Pten KO (adult) | ↑ | ↑ | ---- | ---- | ↓ | ↓ | ↓ | ↓ | ---- | ---- |
| NS-Pten KO (pup)19 | ↓ | ---- | ↓ | ---- | ↑ | ↓ | ↓ | ↑ | ---- | ---- |
| Fmr1 KO (adult)23 | ↑ | ---- | ---- | ---- | ---- | ↑ | ↓ | ↓ | ↑ | ---- |
| Fmr1 KO (pup)39 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| BTBR (adult)15 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| BTBR (pup)14 | ---- | ---- | ↑ | ---- | ---- | ---- | ↑ | ---- | ---- | ↑ |
N/A = no call type data reported
When assessing communicative behaviors overall, no differences were found in the total USVs emitted or in scent marking behaviors between WT and NS-Pten KO animals. The lack of a difference in total USVs was surprising, as we have previously shown that NS-Pten KO pups emit significantly fewer vocalizations than WT pups. The discrepancy in results may be due to the differences in the function of the vocalizations. Pup USVs are emitted in an aversive context and are used to elicit a retrieval response from the dam. However, adult USVs are emitted in the context of an appetitive behavior and are used in mating. In addition to our USV results, no significant difference was found in scent marking behavior. Males deposit scent marks in response to female urine, to prevent mating competition, to mark territory, and to attract females [41]. Our lab has previously assessed the olfactory capabilities of NS-Pten WT and KO adult male mice using the olfactory discrimination test. No difference was found between the groups, indicating that the NS-Pten KO mice can detect social and non-social odors [33]. Additionally, our open field analysis revealed no difference in the quantity of time spent proximal and distal to the female urine. Therefore, our results are not due to an inability to detect the female urine scent nor a marked avoidance of the urine, eliminating potential confounds and further indicating that NS-Pten KO mice did not exhibit an overall difference in the total quantity of communicative behaviors produced.
4.1. Conclusions
ASD results in marked deficits in communication in infants and persists throughout the lifespan, affecting 85% of adults with autism [4]. Despite the prevalence and the severity of ASD, little is known about communicative deficits in adulthood and the underlying neural mechanisms that contribute to these deficits. The current study investigated communicative behaviors in adult NS-Pten KO mice that exhibit hyperactivity of mTOR exclusively in the brain. We assessed USVs as well as scent marking behavior and found numerous quantitative and qualitative changes in vocalizations. Our study further implicates hyperactivity of mTOR as playing a crucial role in the communication deficits that accompany an autistic phenotype and is among the first to demonstrate that mTOR hyperactivity is sufficient to impair adult communication. A better understanding of the relationship between mTOR and communicative deficits in adult ASD models can lead to the development of effective treatments capable of mitigating communicative deficits in individuals with autism of all ages.
Highlights:
NS-Pten KO mice display both quantitative and qualitative changes in USVs
NS-Pten KO mice emit USVs of a lower peak frequency than WT mice
NS-Pten KO’s emit USVs of a higher amplitude and shorter duration than WT mice
Spectral analysis reveal NS-Pten KO mice produce different types of calls
Acknowledgements
This work was supported by the National Institutes of Health grant R15S088776. We would like to thank Suzanne Nolan and Paige Womble for their critical review of the paper. The authors do not have any conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].NIH, Autism Spectrum Disorder: Communication Problems in Children, https://www.nidcd.nih.gov/health/autism-spectrum-disorder-communication-problems-children (2018).
- [2].Wingate M, Kirby RS, Pettygrove S, Cunniff C, Schulz E, Ghosh T, Robinson C, Lee LC, Landa R, Constantino J, Fitzgerald R, Zahorodny W, Daniels J, Nicholas J, Charles J, McMahon W, Bilder D, Durkin M, Baio J, Christensen D, Van K, Braun N, Clayton H, Goodman A, Doernberg N, Yeargin-Allsopp M, Monitoring ADD, Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010, Mmwr Surveillance Summaries 63(2) (2014). [PubMed] [Google Scholar]
- [3].DSM-5, Diagnostic and statistical manual of mental disorders (5th ed.), (Arlington, VA: American Psychiatric Publishing; … (American Psychoatric Association, 2013). [Google Scholar]
- [4].Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS, Trajectory of development in adolescents and adults with autism, Ment Retard Dev Disabil Res Rev 10(4) (2004) 234–47. [DOI] [PubMed] [Google Scholar]
- [5].Esposito G, Venuti P, Comparative Analysis of Crying in Children with Autism, Developmental Delays, and Typical Development, Focus on Autism and Other Developmental Disabilities 24(4) (2009) 240–247. [Google Scholar]
- [6].Esposito G, Venuti P, Developmental changes in the fundamental frequency (f0) of infants’ cries: A study of children with Autism Spectrum Disorder, Early Child Development and Care 180(8) (2010) 1093–1102. [Google Scholar]
- [7].Mody M, Belliveau JW, Speech and Language Impairments in Autism: Insights from Behavior and Neuroimaging, North American journal of medicine & science 5(3) (2013) 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].van Santen JPH, Sproat RW, Hill AP, Quantifying Repetitive Speech in Autism Spectrum Disorders and Language Impairment, Autism Research 6(5) (2013) 372–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prizant Barry M, Rydell Patrick J, Analysis of Functions of Delayed Echolalia in Autistic Children, Journal of Speech, Language, and Hearing Research 27(2) (1984) 183–192. [DOI] [PubMed] [Google Scholar]
- [10].Mundy P, Sigman M, Ungerer J, Sherman T, DEFINING THE SOCIAL DEFICITS OF AUTISM: THE CONTRIBUTION OF NON-VERBAL COMMUNICATION MEASURES, Journal of Child Psychology and Psychiatry 27(5) (1986) 657–669. [DOI] [PubMed] [Google Scholar]
- [11].Ehret G, Infant Rodent Ultrasounds – A Gate to the Understanding of Sound Communication, Behavior Genetics 35(1) (2005) 19–29. [DOI] [PubMed] [Google Scholar]
- [12].Branchi I, Santucci D, Alleva E, Ultrasonic vocalisation emitted by infant rodents: a tool for assessment of neurobehavioural development, Behavioural Brain Research 125(1–2) (2001) 49–56. [DOI] [PubMed] [Google Scholar]
- [13].Lai JKY, Sobala-Drozdowski M, Zhou L, Doering LC, Faure PA, Foster JA, Temporal and spectral differences in the ultrasonic vocalizations of fragile X knock out mice during postnatal development, Behavioural Brain Research 259 (2014) 119–130. [DOI] [PubMed] [Google Scholar]
- [14].Scattoni ML, Gandhy SU, Ricceri L, Crawley JN, Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism, PLoS ONE 3(8) (2008) e3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wöhr, Roullet FI, Crawley JN, Reduced scent marking and ultrasonic vocalizations in the BTBR T+tf/J mouse model of autism, Genes, Brain and Behavior 10(1) (2011) 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].D’Amato FR, Scalera E, Sarli C, Moles A, Pups Call, Mothers Rush: Does Maternal Responsiveness Affect the Amount of Ultrasonic Vocalizations in Mouse Pups?, Behavior Genetics 35(1) (2005) 103–112. [DOI] [PubMed] [Google Scholar]
- [17].Nyby J, Ultrasonic vocalizations during sex behavior of male house mice (Mus musculus): a description, Behavioral and neural biology 39(1) (1983) 128–34. [DOI] [PubMed] [Google Scholar]
- [18].Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, Lahvis GP, Affiliative Behavior, Ultrasonic Communication and Social Reward Are Influenced by Genetic Variation in Adolescent Mice, PLOS ONE 2(4) (2007) e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Binder MS, Lugo JN, NS- Pten knockout mice show sex- and age- specific differences in ultrasonic vocalizations, Brain and Behavior (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M, Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice, Nature 488(7413) (2012) 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jamain S, Radyushkin K, Hammerschmidt K, Granon S, Boretius S, Varoqueaux F, Ramanantsoa N, Gallego J, Ronnenberg A, Winter D, Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism, Proceedings of the National Academy of Sciences 105(5) (2008) 1710–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wöhr M, Ultrasonic vocalizations in Shank mouse models for autism spectrum disorders: Detailed spectrographic analyses and developmental profiles, Neuroscience & Biobehavioral Reviews 43 (2014) 199–212. [DOI] [PubMed] [Google Scholar]
- [23].Hodges SL, Nolan SO, Reynolds CD, Lugo JN, Spectral and temporal properties of calls reveal deficits in ultrasonic vocalizations of adult Fmr1 knockout mice, Behavioural Brain Research 332 (2017) 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Murphy CM, Wilson CE, Robertson DM, Ecker C, Daly EM, Hammond N, Galanopoulos A, Dud I, Murphy DG, McAlonan GM, Autism spectrum disorder in adults: diagnosis, management, and health services development, Neuropsychiatric disease and treatment 12 (2016) 1669–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liaw D, Marsh DJ, Li J, Dahia PLM, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacoke M, Eng C, Parsons R, Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome, Nature Genetics 16(1) (1997) 64–67. [DOI] [PubMed] [Google Scholar]
- [26].Smpokou P, Fox VL, Tan W-H, PTEN hamartoma tumour syndrome: early tumour development in children, Archives of Disease in Childhood 100(1) (2015) 34. [DOI] [PubMed] [Google Scholar]
- [27].Hanssen AM, Fryns JP, Cowden syndrome, Journal of Medical Genetics 32(2) (1995) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, Herman GE, Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly, Autism Research 3(3) (2010) 137–141. [DOI] [PubMed] [Google Scholar]
- [29].Varga EA, Pastore M, Prior T, Herman GE, McBride KL, The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly, Genetics In Medicine 11 (2009) 111. [DOI] [PubMed] [Google Scholar]
- [30].Kwon, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ, Pten regulates neuronal soma size: a mouse model of Lhermitte-Duclos disease, Nature Genetics 29 (2001) 404. [DOI] [PubMed] [Google Scholar]
- [31].Backman, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao M-S, Shannon P, Bolon B, Ivy GO, Mak TW, Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease, Nature Genetics 29 (2001) 396. [DOI] [PubMed] [Google Scholar]
- [32].Sato A, mTOR, a Potential Target to Treat Autism Spectrum Disorder, CNS & neurological disorders drug targets 15(5) (2016) 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lugo, Smith GD, Arbuckle EP, White J, Holley AJ, Floruta CM, Ahmed N, Gomez MC, Okonkwo O, Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins, Frontiers in Molecular Neuroscience 7(27) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhou J, Blundell J, Ogawa S, Kwon C-H, Zhang W, Sinton C, Powell CM, Parada LF, Pharmacological Inhibition of mTORC1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific Pten Knock-Out Mice, The Journal of Neuroscience 29(6) (2009) 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF, Pten regulates neuronal arborization and social interaction in mice, Neuron 50(3) (2006) 377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Byers SL, Wiles MV, Dunn SL, Taft RA, Mouse Estrous Cycle Identification Tool and Images, PLOS ONE 7(4) (2012) e35538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ, A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J mice, Behavioural brain research 190(1) (2008) 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ, Scent marking behavior as an odorant communication in mice, Neuroscience and biobehavioral reviews 32(7) (2008) 1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reynolds, Nolan SO, Jefferson T, Lugo JN, Sex-specific and genotype-specific differences in vocalization development in FMR1 knockout mice, Neuroreport 27(18) (2016) 1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sheinkopf SJ, Iverson JM, Rinaldi ML, Lester BM, Atypical Cry Acoustics in 6- Month- Old Infants at Risk for Autism Spectrum Disorder, Autism Research 5(5) (2012) 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hurst JL, Urine marking in populations of wild house mice Mus domesticus rutty. I. Communication between males, Animal Behaviour 40(2) (1990) 209–22. [Google Scholar]