Abstract
Study Objective:
To evaluate the association between use of methadone, other central nervous system (CNS) depressants, and QTc interval–prolonging medications and risk of mortality among human immunodeficiency virus (HIV)-infected and at-risk HIV-uninfected women.
Design:
Multicenter, prospective, observational cohort study (Women’s Interagency HIV Study [WIHS]).
Participants:
A total of 4150 women enrolled in the WIHS study between 1994 and 2014 who were infected (3119 women) or not infected (1031 women) with HIV.
Measurements and Main Results:
Data on medication utilization were collected from all study participants via interviewer-administered surveys at 6-month intervals (1994–2014). Mortality was confirmed by National Death Index data. With age defining the time scale for the analysis, Cox proportional hazards models were used to estimate hazard ratios (HRs) for all-cause mortality in HIV-infected and -uninfected women and non–acquired immunodeficiency syndrome (AIDS) deaths in HIV-infected women. A total of 1046 deaths were identified, of which 429 were considered non-AIDS deaths. Use of benzodiazepines, CNS depressants (excluding methadone), and number of medications with conditional QTc interval–prolonging effects were each associated with all-cause mortality in multivariate models of HIV-infected women: hazard ratio (HR) 1.28, 95% confidence interval (CI) 1.01–1.60, p=0.037; HR 1.61, 95% CI 1.35–1.92, p<0.0001; and HR 1.15 per drug, 95% CI 1.00–1.33, p=0.047, respectively. Other explanatory variables for all-cause mortality in this model included HIV viral load, CD4+ cell count, renal function, hemoglobin and albumin levels, HIV treatment era, employment status, existence of depressive symptoms, ever use of injection drugs, and tobacco smoking. Of interest, use of CNS depressants (excluding methadone) was also associated with non-AIDS deaths (HR 1.49, 95% CI 1.49–2.2, p<0.0001). Although use of benzodiazepines and conditional QT interval–prolonging medications were associated with increased risk of non-AIDS mortality (HR 1.32 and 1.25, respectively), the effect was not statistically significant (p>0.05).
Conclusion:
In this cohort of HIV-infected and at-risk HIV-uninfected women, use of benzodiazepines, CNS depressants, and conditional QTc interval–prolonging medications were associated with a higher risk of mortality independent of methadone and other well-recognized mortality risk factors. Care must be taken to assess risk when prescribing these medications in this underserved and at-risk patient population.
Keywords: Methadone, Central nervous system depressants, Benzodiazepines, HIV infections, Mortality, Arrhythmias cardiac
Individuals with chronic health conditions frequently take multiple medications including common use of opioid pain medications, sedatives, and muscle relaxants.(1) The Women’s Interagency HIV Study (WIHS) cohort, which is the largest longitudinal observational study of human immunodeficiency virus (HIV) in women that includes a risk set–matched HIV-seronegative comparison group, has observed mortality rates of 25.2% (1180 women) over a period of 20 years (1994–2014), with 31.3% for HIV-infected and 6.8% for HIV-uninfected women. Reviewing the data over this period, we made a notable observation that the death rate for women who had ever used methadone in the WIHS was 45.0% (468/1039), whereas this rate was 20.0% (712/3567) for non–methadone users. This observation necessitated further investigation.
Methadone, a long-acting, synthetic opioid receptor agonist, is a widely used pharmacotherapy for the treatment of opiate addiction as well as chronic pain. Although an effective and useful drug, methadone use is associated with mortality.(2–5) Whereas deaths related to opioids are primarily a result of overdose leading to respiratory suppression, methadone has been independently associated with prolongation of the QTc interval in electrocardiograms, which increases the risk of developing life-threatening arrhythmias and sudden cardiac death.(6–9) Furthermore, HIV-infected patients receiving combination antiretroviral therapy (cART) are at higher risk of arrhythmia while receiving methadone.(7, 10) This increased risk has the potential to predispose recipients of cART to arrhythmias and sudden mortality. Methadone use may be of a particular concern in HIV-infected women due to the association of female sex with longer baseline QTc intervals (11).
Sedatives and respiratory depressants may also be a particular concern in the HIV-infected population. Respiratory function abnormalities were associated with an increased risk of death in HIV-infected WIHS participants, and impaired pulmonary diffusion capacity was highly prevalent in WIHS participants.(12, 13) In this setting, drugs that diminish respiratory drive may result in marked abnormalities in ventilation.
Given the univariate finding of high incidence of mortality in women in the WIHS who used methadone, we sought to determine in a multivariate analysis whether methadone use may influence mortality among both HIV-infected and HIV-uninfected women in the WIHS. Since methadone has pharmacologic action on the QTc interval, respiratory system, and central nervous system (CNS) system, which each could contribute to mortality, and given the complex patients in our samples, care was taken to adjust the analysis for known nonmedication explanatory variables for mortality as well as other medications that shared the above described undesirable pharmacologic actions of methadone. These medications included benzodiazepines, QTc interval–prolonging medications, and other CNS depressants, as well as drug-drug interactions that could lead to CNS depression, which could influence mortality among women in the WIHS living with and without HIV. In this study, we report the impact of all of these explanatory variables on mortality.
Methods
Study Population
The WIHS is a multicenter, prospective, observational cohort study of women with and at risk for HIV infection; follow-up visits occur every 6 months. HIV-negative women reporting one or more of the following behaviors were considered at risk for HIV: injection drug use; having a sexually transmitted disease; having unprotected sex with three or more men or protected sex with more than five men; or having exchanged sex for drugs, money, or shelter.(14) At each visit, scripted interviews to collect self-reported data, clinical examinations, and various laboratory testing are conducted. The cohort is highly representative of women in the United States who are living with HIV infection.(14) Cohort methods have been described previously.(14, 15) For this analysis, use of methadone, other CNS depressants, QTC interval-prolonging medications and interacting drugs were determined for 4150 WIHS participants over a 20-year period (1994–2014). WIHS participants consented to each visit according to human subject’s protection protocols in place at each of the collaborating institutions.
Mortality Outcome
Cause of death was ascertained from various sources including death certificates (National Death Index), medical records, providers, and family/friends. This information was used to construct a final variable of causes of death based on the following four categories: acquired immunodeficiency syndrome (AIDS), non-AIDS, indeterminate, or unknown.(16) Importantly, medications such as methadone or other CNS depressants could contribute to all of these reported causes of death. Therefore, this analysis focused not only on non–AIDS-related deaths but also on all-cause mortality for all HIV-infected WIHS participants, with methadone use being a key predictor. All deaths in HIV-uninfected women were considered to be non-AIDS deaths. Because of a lag in the availability of comprehensive data from the National Death Index, mortality ascertainment for this study was complete for dates of death up to December 31, 2013; no deaths or observation time after that date were included in the analyses. All time following study disenrollment occurrence was also excluded.
Methadone Use
Various survey items were used to ascertain methadone treatment. Six forms of self-reported use (recent, recent prescribed, recent unprescribed, ever, ever prescribed, and ever unprescribed) were created from the available data. Prescribed methadone use was not specifically queried as part of the cohort data collection protocol from visits 17 (2002) to 38 (2013), so variables depending on that information were set to missing after 2 years of carry forward from visit 16.
Other Medication Explanatory Variables
Variables capturing use of QTc interval–prolonging medications, medications that could alter methadone plasma exposure, and use of other combinations of medications that could contribute to CNS depression were defined. Self-reported data from drug-specific multiple-choice and text fields were used to populate these variables. The medications contributing to QTc interval prolongation were divided into three categories: known, possible, or conditional (Supplementary Table S1).(17) By CredibleMeds’(18) (www.Crediblemeds.org); the conditional risk category includes medications that are associated with QTc interval prolongation only under certain conditions of their use that result in QTc interval prolongation. Tables of cytochrome P450 (CYP) 3A4, 2B6, and 2C19 inhibitors, and P-glycoprotein inhibitors were assembled (Supplementary Table S2).(19) Drug-drug interactions that could lead to CNS depression with (Supplementary Table S3) or without (Supplementary Table S4) methadone were identified and categorized as major, moderate, or mild using the Micromedex online drug reference database (20) (Figure 1). These included alcohol, opioids (methadone excluded), H1-antihistamines, first-generation typical antipsychotics, barbiturates, hypnotics, benzodiazepines, and skeletal muscle relaxants.
Figure 1.
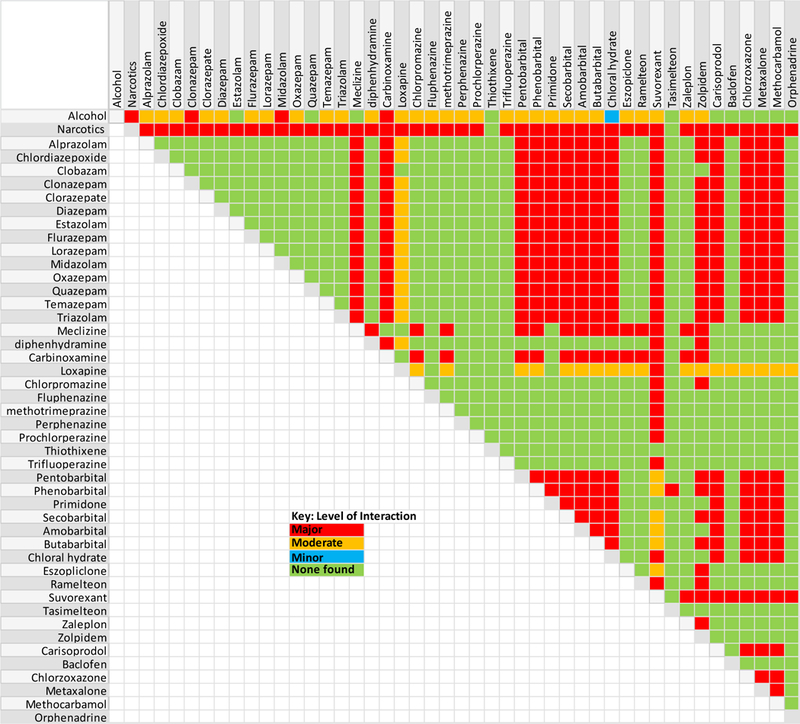
Medication combinations that can increase the risk of central nervous system depression and levels of each drug interaction. The levels of interaction are defined as follows: major (red) = the interaction may be life-threatening and/or require medical intervention to minimize or prevent serious adverse effects); moderate (orange) = the interaction may result in exacerbation of the patient’s condition and/or require an alteration in therapy; minor (blue) = the interaction would have limited clinical effects, and manifestations may include an increase in the frequency or severity of the side effects but generally would not require a major alteration in therapy; and no interaction found (green) according to the Micromedex online drug reference database [20].
Other Explanatory Variables
Care was taken to adjust for factors that might plausibly influence mortality risk, including those previously reported in the WIHS.(16, 21–28) In all, there were 53 candidate explanatory variables (Table 1 and Supplementary Table S5). To account for calendar time as a potential predictor of mortality reflecting changes in HIV care over this project period (1994–2014), a variable dividing this period into three separate treatment eras was created: prior to 1998, 1998–2004, and after 2004.
Table 1.
Demographic and Disease Characteristics of the 4150 Participants using the Observation from the Most Recent Completed Visit for Each Participant
| Characteristic | HIV (−) women (n=1031) |
HIV (+) women (n=3119) |
|---|---|---|
| Viral load undetectable | 1031 (100%) | 1206 (40.9%) |
| CD4+ cell counts (cells/µl) (mean ± SD) | NA | 421 ± 302 |
| All-cause death | 70 (6.8%) | 976 (31.3%) |
| Non-AIDS death | 70 (6.8%) | 359/2996 (12%) |
| Methadone | ||
| Recent£ (n=1185)* | 24/204 (11.8%) | 166/981 (16.9%) |
| Prescribed (n=1185)* | 19/204 (9.3%) | 155/981 (15.8%) |
| Not prescribed (n=3798)* | 11/905 (1.2%) | 25/2893 (0.9%) |
| Ever (n=1203)* | 44/211 (20.9%) | 311/992 (31.4%) |
| Prescribed (n=1203)* | 36/211 (17.1%) | 262/992 (26.4%) |
| Not prescribed (n=4140)* | 142/1029 (13.8%) | 426/3111 (13.7%) |
| CES-D score > 16 (n=3869)* | 287/926 (31%) | 1214/2943 (41.3%) |
| Hepatitis C infection | 664 (64.4%) | 1918 (61.5%) |
| Hepatitis B infection | 5 (0.5%) | 81 (2.6%) |
| Injectable illicit drug use | ||
| Recent£ (n=3937)* | 55/945 (5.8%) | 199/2992 (6.3%) |
| Ever | 261 (25.3%) | 982 (31.5%) |
| Smoking status | ||
| Never | 238 (23.1%) | 896 (28.7%) |
| Current | 509 (49.4%) | 1347 (43.2%) |
| Past | 284 (27.5%) | 876 (28.1%) |
| Alcohol use | ||
| Abstain | 423/944 (44.8%) | 1792/2983 (60.1%) |
| 1–7 drinks/week | 367/944 (38.9%) | 912/2983 (30.6%) |
| 8–12 drinks/week | 50/944 (5.3%) | 94/2983 (3.2%) |
| >12 drinks/week | 104/944 (11%) | 185/2983 (6.2%) |
| Race-ethnicity categories | ||
| White | 140 (13.6%) | 450 (14.4%) |
| African-American | 584 (56.6%) | 1812 (58.1%) |
| Hispanic | 266 (25.8%) | 754 (24.2%) |
| Other | 41 (4.0%) | 103 (3.3%) |
| Hemoglobin level (g/dL) | ||
| Critical low: <7 | 0/872 (0%) | 10/2958 (0.3%) |
| Low: ≥7–11.9 | 227/872 (26%) | 1332/2958 (45%) |
| Normal: >11.9–15.6 | 634/872 (72.7%) | 1589/2958 (53.7%) |
| High: >15.6 | 11/872 (1.3%) | 27/2958 (0.9%) |
| Albumin level (g/dL) | ||
| Very low: ≥1.2– 2.1 | 1/889 (0.1%) | 29/2894 (1.0%) |
| Low: >2.1–3.4 | 32/889 (3.6%) | 398/2894 (13.8%) |
| Low normal: >3.4–3.9 | 127/889 (14.3%) | 727/2894 (25.1%) |
| Normal: >3.9–4.2 | 283/889 (31.8%) | 768/2894 (26.5%) |
| High Normal: >4.2–4.8 | 420/889 (47.2%) | 905/2894 (31.3%) |
| High: >4.8 | 26/889 (2.9%) | 67/2894 (2.3%) |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | ||
| Normal: ≥90 | 659/1028 (64.1%) | 1758/3109 (56.5%) |
| Mild kidney disease: ≥60–89 | 306/1028 (29.8%) | 945/3109 (30.4%) |
| Moderate kidney disease: ≥30–59 | 45/1028 (4.4%) | 284/3109 (9.1%) |
| Severe kidney disease: ≥15–29 | 9/1028 (0.9%) | 44/3109 (1.4%) |
| Kidney failure: <15 | 9/1028 (0.9%) | 78/3109 (2.5%) |
| Unemployed | 565/945 (59.8%) | 2205/2998 (73.5%) |
| Hypertension | 629 (61.0%) | 2001 (64.2%) |
| HIV treatment era | ||
| <1998 | 78 (7.6%) | 425 (13.6%) |
| 1998–2004 | 126 (12.2%) | 552 (17.7%) |
| >2004 | 827 (80.2%) | 2142 (68.7%) |
| Medications with major additive CNS depression risk with concurrent methadone use | 192 (18.6%) | 753 (24.1%) |
| Benzodiazepine | 67 (6.5%) | 264 (8.5%) |
| Opioids ¥ | 115 (11.2%) | 448 (14.4%) |
| Medications that can increase risk for CNS depression¥ | 124 (12.0%) | 474 (15.2%) |
| No. of medications with conditional QT interval–prolonging effects¥ | ||
| 0 | 901/1030 (87.5%) | 2578/3114 (82.8%) |
| 1 | 103/1030 (10%) | 465/3114 (14.9%) |
| 2 | 25/1030 (2.4%) | 62/3114 (2%) |
| 3 | 1/1030 (0.1%) | 8/3114 (0.3%) |
| 4 | 0 | 1/3114 (0.0%) |
Data are no. (%) of women unless otherwise specified.
CES-D = Center for Epidemiological Studies–Depression; NA = not applicable.
Because of missing values for participants, the subgroup sample sizes may not sum up to 1031 and 3119 for HIV (−) and HIV (+) women, respectively
Recent was defined as in the past 6 months.
Methadone was excluded.
Statistical Analysis
All analyses were conducted by using R (R Foundation for Statistical Computing, Vienna, Austria) and SAS (SAS Institute Inc., Cary, NC) software. The mortality analysis did not combine HIV-infected and HIV-uninfected women since we suspect patterns and causes of mortality may differ in these two groups. Separate Cox proportional hazards models were constructed for HIV-infected and HIV-uninfected women. In HIV-infected participants, Cox proportional hazards models estimated hazard ratios (HRs) for all-cause and non-AIDS deaths using data from study enrollment to the end of 2013. Because of the importance of age in the risk of death, we used it to define the time scale for analysis instead of modeling its effect as a covariate. This fully controls for the effect of age while avoiding parametric assumptions about its effects. This same approach was taken to estimate HR in HIV-uninfected participants with the exception that in this group, all-cause and non-AIDS mortality are the same. Analyses were adjusted for various factors including previously described WIHS mortality risks and use of other opioids, CNS depressants, and drugs affecting QTc interval. All variables that were not fixed characteristics were handled as time-varying covariates: at a given time, each woman’s predictor variables were equal to their reported, measured, or derived value at her most recent prior visit, as long as it was within 2 years. After 2 years with no data collection, variables were considered as missing, and those times were not included in the analyses. CD4+ cell counts >1000 were set to =1000. The “ever” variables were defined using data only up to the most recent previous visit, never any later visits. For all-cause mortality, the effect of log10 HIV viral load was nonlinear, so we modeled it as a linear spline, with knots (points where the line can change slope) at 3, 4, and 5. We selected primary multivariate models for presentation using forward stepwise selection with p<0.05 required for entry, with forced inclusion of HIV status, CD4+ cell count, and HIV viral load variables. We then evaluated each remaining unselected candidate predictor as a single addition to the chosen multivariate model. The analysis included all women who had at least one follow-up visit or who were known to have died after their baseline visit. Observation time for these analyses ended at the earliest of the following: time of death, 2 years after the last collection of predictor variables, disenrollment, or December 31, 2013.
Results
Characteristics of the Study Population
Age at study entry ranged from 18–69 years with a median age of 36 years for HIV-infected women, and 18–62 years with median age of 36 years for HIV-uninfected women. The duration of follow-up among those who did not die during the study period ranged from 0.4–20 years, with median of 12 years for HIV-infected and -uninfected groups. Table 1 summarizes the characteristics of the 4150 women who contributed to this study, stratified by their HIV infection status as of their last observation. HIV-infected individuals were more likely (p<0.05) to have died, used prescribed methadone, had symptoms of depression, been infected with hepatitis B virus, ever used injection drugs, smoked, consumed alcohol, been unemployed, and had diminished estimated glomerular filtration rate (eGFR) and abnormal (values above or below normal range) hemoglobin and albumin levels relative to HIV-uninfected participants. There was no statistically significant difference between HIV-infected and -uninfected women at their last visit with respect to hypertension, self-reported race-ethnicity, hepatitis C virus (HCV) infection, recent use of injection drugs, or nonprescribed use of methadone.
The HIV-infected participants were more likely to have been taking medications that inhibit CYP3A4 and CYP2C19 metabolizing enzymes and P-glycoprotein efflux transporters (Supplementary Table S5). Additionally, the use of medications that could have contributed to CNS depression when combined with methadone was common among HIV-infected women. They were also more likely to use medications and opioids other than methadone that are known to increase the risk of CNS depression, benzodiazepines, and QTc interval–prolonging medications (all p<0.05). (Table 1 and Supplementary Table S5).
Explanatory Variables for All-Cause Death
Table 2 summarizes the results of Cox proportional hazards analyses, modeling explanatory variables’ associations with all-cause death in HIV-infected and -uninfected participants. Except for methadone use variables, for brevity, only explanatory variables with p values less than 0.05 are shown in this table. Supplementary Table S6 reports the HRs for all explanatory variables in the models. Factors that contributed independently to increased risk of mortality in the multivariate model, in both HIV-infected and -uninfected participants, can be divided into two groups: nonmedication and medication factors.
Table 2.
Univariate and Multivariate Cox Proportional Hazards Models of All-Cause Death£
| Univariate Model |
Multivariate Model |
|||||||
|---|---|---|---|---|---|---|---|---|
| HIV (−) women | HIV (+) women | HIV (−) women | HIV (+) women | |||||
| Predictor | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) |
P-value | Hazard ratio (95% CI) | P-value |
| Methadone | ||||||||
| Recent | 2.4 (1.07–5.2) | 0.033 | 1.72 (1.42–2.1) | <0.0001 | 1.18 (0.48–2.9) | 0.71 | 0.92 (0.73–1.17) | 0.52 |
| Prescribed | 2.2 (0.98–5.0) | 0.054 | 1.64 (1.34–2.0) | <0.0001 | 1.20 (0.49–2.9) | 0.69 | 0.92 (0.73–1.17) | 0.51 |
| Not prescribed | 3.5 (1.09–11.2) | 0.036 | 2.9 (1.81–4.6) | <0.0001 | 1.50 (0.44–5.1) | 0.52 | 1.08 (0.64–1.82) | 0.78 |
| Ever | 2.4 (1.15–5.0) | 0.020 | 1.38 (1.67–1.64) | 0.0002 | 0.74 (0.29–1.9) | 0.54 | 0.88 (0.70–1.11) | 0.29 |
| Prescribed | 2.0 (0.97–4.2) | 0.062 | 1.50 (1.26–1.79) | <0.0001 | 0.78 (0.32–1.87) | 0.57 | 0.90 (0.71–1.13) | 0.36 |
| Not prescribed | 1.45 (0.84–2.5) | 0.18 | 1.52 (1.30–1.78) | <0.0001 | 0.57 (0.30–1.09) | 0.089 | 0.95 (0.79–1.14) | 0.56 |
| Log10 viral load (copies/ml) | ||||||||
| Effect per 1 log10-increase within ranges | ||||||||
| <3 | NA | 3.7 (3.2–4.3) | <0.0001 | NA | 0.96 (0.66–1.39) | 0.83 | ||
| 3–4 | 6.1 (5.2–7.1) | <0.0001 | 0.69 (0.47–0.99) | 0.049 | ||||
| >4 – 5 | 10.9 (9.4–12.6) | <0.0001 | 1.61 (1.17–2.2) | 0.0034 | ||||
| >5 | 23 (19.4–27) | <0.0001 | 1.94 (1.49–2.5) | <0.0001 | ||||
| Viral load undetectable | NA | 0.25 (0.21–0.30) | <0.0001 | NA | 0.82 (0.57–1.19) | 0.31 | ||
| Log base 2 of CD4+ cell count | NA | 0.48 (0.47–0.50) | <0.0001 | NA | 0.59 (0.56–0.62) | <0.0001 | ||
| Depressive symptoms | 2.9 (1.77–4.8) | <0.0001 | 2.6 (2.2–2.9) | <0.0001 | 2.1 (1.24–3.6) | 0.0062 | 1.39 (1.21–1.60) | <0.0001 |
| Hepatitis B infection | 12.9 (1.69–99) | 0.014 | 1.89 (1.37–2.6) | 0.0001 | 26 (3–229) | 0.0032 | 1.29 (0.92–1.8) | 0.13 |
| Injectable illicit drug use | ||||||||
| Recent | 4.6 (2.4–8.7) | <0.0001 | 2.4 (1.98–3.0) | <0.0001 | 1.91 (0.92–4) | 0.084 | 1.05 (0.83–1.32) | 0.71 |
| Ever | 5.7 (3.4–9.8) | <0.0001 | 2.3 (1.97–2.6) | <0.0001 | 3.6 (2–6.4) | <0.0001 | 1.34 (1.15–1.57) | 0.0001 |
| Smoking status | ||||||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Current | 7.9 (1.91–33) | 0.0043 | 2.2 (1.87–2.7) | <0.0001 | 3.6 (0.82–15.8) | 0.09 | 1.24 (1.01–1.52) | 0.039 |
| Past | 4.7 (1.07–21) | 0.04 | 1.59 (1.30–1.95) | <0.0001 | 2.4 (0.51–11.4) | 0.27 | 1.36 (1.1–1.7) | 0.005 |
| Hemoglobin level (g/dL) | ||||||||
| Normal: >11.9–15.6 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Critical low: <7 | NA | 63 (33–120) | <0.0001 | NA | 3.2 (1.52–6.8) | 0.0022 | ||
| Low: 7–11.9 | 1.52 (0.89–2.6) | 0.12 | 4.5 (3.9–5.2) | <0.0001 | 1.49 (0.83–2.7) | 0.18 | 1.56 (1.33–1.83) | <0.0001 |
| High: >15.6 | 1.10 (0.15–8.1) | 0.92 | 1.62 (0.83–3.1) | 0.15 | 0.56 (0.071–4.4) | 0.58 | 1.61 (0.83–3.2) | 0.16 |
| Albumin level (g/dL) | ||||||||
| Normal:>3.9–4.2 | 1.0 | 1.0 | 1.0 | 1 | ||||
| Very low: ≥1.2– 2.1 | 12.4 (1.59–97) | 0.016 | 19.7 (13.2–30) | <0.0001 | 31 (3.7–254) | 0.0015 | 7.3 (4.7–11.3) | <0.0001 |
| Low: >2.1–3.4 | 7 (3.5–14.1) | <0.0001 | 7.3 (6.1–8.9) | <0.0001 | 4.8 (2.2–10.1) | <0.0001 | 3.1 (2.50–3.8) | <0.0001 |
| Low normal:>3.4–3.9 | 1.34 (0.69–2.6) | 0.38 | 1.92 (1.58–2.3) | <0.0001 | 1.04 (0.53–2) | 0.91 | 1.39 (1.14–1.69) | 0.0013 |
| High Normal:>4.2–4.8 | 0.78 (0.39–1.54) | 0.48 | 0.74 (0.58–0.93) | 0.011 | 0.78 (0.38–1.6) | 0.50 | 0.86 (0.68–1.1) | 0.24 |
| High:>4.8 | 0.99 (0.13–7.5) | 0.99 | 1.04 (0.55–1.97) | 0.91 | 0.83 (0.11–6.4) | 0.85 | 1.37 (0.72–2.6) | 0.34 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | ||||||||
| Normal: ≥90 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| Mild kidney disease: ≤60–89 | 0.59 (0.33–1.07) | 0.085 | 1.21 (1.34–1.41) | 0.016 | 0.7 (0.37–1.3) | 0.25 | 1.02 (0.87–1.2) | 0.81 |
| Moderate kidney disease: ≤30–59 | 2.2 (0.99–4.9) | 0.053 | 3.4 (2.8–4.2) | <0.0001 | 2.3 (0.99–5.3) | 0.05 | 1.86 (1.51–2.3) | <0.0001 |
| 3.6 (0.80–16.6) | 0.093 | 8.8 (6.2–12.6) | <0.0001 | 1.33 (0.25–7.2) | 0.74 | 3.5 (2.4–5.1) | <0.0001 | |
| 9 (3–27) | <0.0001 | 6.1 (4.5–8.1) | <0.0001 | 8 (2.5–26) | 0.0005 | 3.8 (2.8–5.1) | <0.0001 | |
| Severe kidney disease: 15–29 | ||||||||
| Kidney failure: <15 | ||||||||
| Unemployed | 5.5 (2.5–12.1) | <0.0001 | 6.5 (5.0–8.3) | <0.0001 | 2.9 (1.3–6.6) | 0.0094 | 2.4 (1.82–3.1) | <0.0001 |
| HIV treatment era | ||||||||
| <1998 | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 1998–2004 | 0.73 (0.34–1.59) | 0.44 | 0.45 (0.39–0.53) | <0.0001 | 0.74 (0.31–1.74) | 0.49 | 0.68 (0.57–0.8) | <0.0001 |
| >2004 | 0.40 (0.19–0.86) | 0.018 | 0.24 (0.20–0.28) | <0.0001 | 0.46 (0.19–1.09) | 0.077 | 0.55 (0.45–0.68) | <0.0001 |
| Benzodiazepine use | 2.1 (0.99–4.4) | 0.055 | 1.60 (1.30–1.97) | <0.0001 | 0.7 (0.28–1.74) | 0.44 | 1.28 (1.01–1.6) | 0.037 |
| No. of medications with conditional QT interval–prolonging effects¥ | 0.86 (0.47–1.55) | 0.61 | 1.16 (1.02–1.32) | 0.026 | 0.67 (0.36–1.25) | 0.21 | 1.15 (1.00–1.33) | 0.047 |
| No. of medications that can increase risk for CNS depression¥ | 2.9 (1.86–4.6) | <0.0001 | 1.90 (1.66–2.2) | <0.0001 | 2.4 (1.49–3.9) | <0.0003 | 1.09 (0.6–1.97) | 0.77 |
| Medications that can increase risk for CNS depression¥ | 3.3 (1.91–5.6) | <0.0001 | 2.1 (1.79–2.5) | <0.0001 | 1.51 (0.26–8.9) | 0.65 | 1.61 (1.35–1.92) | <0.0001 |
Boldface hazard ratios and p values in the multivariate column are variables present in the final multivariate proportional hazards model. The rest of the values are results for each variable as a single addition to the final multivariate model.
CI = confidence interval; CNS = central nervous system; NA = not applicable.
For brevity, only predictors (methadone excluded) with p values <0.05 are reported in this table. Supplemental Table 2 is a comprehensive version of this table.
Methadone was not included.
HIV-Infected Participants
In the final multivariate model (Table 2), nonmedication factors associated with increased mortality (P<0.05) included decreased albumin levels, decreased eGFR, unemployment, depressive symptoms, ever use of injectable illicit drugs, decreased hemoglobin level, and current and past smoking. Medication factors associated with increased risk of included use of medications (other than methadone) that increase risk of CNS depression (HR 1.61, 95% CI 1.35–1.92, p <0.0001), benzodiazepine use (HR 1.28, 95% CI 1.01–1.60, p = 0.037) and number of medications with known conditional QTc interval–prolonging effects (HR 1.15 per drug used, 95% CI 1.00–1.33, p = 0.047). Factors associated with decreased risk of mortality included increasing CD4+ cell count, undetectable HIV viral load, and visit during the more recent HIV treatment era. Although methadone use, prescribed or otherwise, was strongly associated with mortality in the univariate analysis (HR range 1.38–2.9, P≤0.0002), these relationships were greatly attenuated and not statistically significant following adjustment for other explanatory variables in the multivariate analysis (HR range 0.88–1.08, all p ≥ 0.29).
HIV-Uninfected Participants
In the final multivariate model, nonmedication factors associated significantly with increased mortality were depressive symptoms, hepatitis B virus infection, ever use of injectable drugs, decreasing albumin level, decreasing eGFR, and unemployment (Table 2). The only medication factor associated significantly with increased risk of mortality was use of medication combinations associated with increased risk of CNS depression (HR 2.4 per drug used, 95% CI 1.49–3.9, p <0.0003). Methadone association with mortality was not statistically significant when adjusted for other explanatory variables in the final multivariate model (HR range 0.57–1.50, P≥ 0.089).
Explanatory Variables for Non-AIDS Death
Supplementary Table S7 summarizes the results of multivariate Cox proportional hazards models of explanatory variables for non-AIDS death in HIV-infected participants. Note that this table tabulates the same results that are listed in Supplementary Table S6 and Table 2 for HIV-negative participants since for these participants all deaths are non-AIDS deaths. As in the all-cause mortality analysis, all types of methadone use among HIV-infected participants were associated with non-AIDS death by univariate comparison (HR range 1.88–2.6, all p≤0.029). However, this association was attenuated to the null once other factors were considered via multivariate modeling (HR range 0.95–1.09, all p ≥0.65). The explanatory variables that were associated with non-AIDS death can also be considered as nonmedication and medication related. There is considerable overlap in effect size for nonmedication explanatory variables of association with all-cause and non-AIDS mortalities (Table 2 and Supplementary Tables S6 and S7) in HIV-infected participants. For medication-related explanatory variables, use of medications other than methadone that increase risk of CNS depression was the only predictor associated with non-AIDS deaths (HR 1.49, 95% CI 1.49–2.2, p < 0.0001). Although the use of benzodiazepine (HR 1.32, 95% CI 0.83–2.1, p = 0.24) and conditional QT interval–prolonging medications (HR 1.25 per drug, 95% CI 0.97–1.62, p = 0.089) demonstrated some association with increased risk of mortality, the associations failed to meet the a priori significance threshold (p>0.05).
Exploratory Analysis of Methadone Interactions
A secondary analysis of plausible high-risk drug interactions (i.e., use of or number of medications with major additive CNS depression risk with methadone, use of or number of known and possible QTc interval–prolonging medications, or use of CYP3A4 inhibitors) with methadone did not result in statistically significant (p<0.05) associations with all-cause or non-AIDS mortality (results not shown).
Discussion
Although CNS depressants are considered to be high-risk medications, our study is novel in that it identifies important medication and nonmedication risk factors for mortality in a representative population of women living with or at risk for HIV. The knowledge of these risk factors can help clinicians to better manage the pharmacologic needs of their patients who present with combinations of risk factors for mortality identified in this study.
This study was driven by a univariate comparison demonstrating that higher mortality existed among women who had ever used methadone in the WIHS. Although prescribed and unprescribed use of methadone were strongly associated with all-cause and non-AIDS death in univariate analyses, this association was due to confounding with other factors. However, the confidence intervals in the multivariate analysis for methadone explanatory variables were too wide to exclude the possibility of an effect. Use of methadone could also function as a noncausative factor that frequently coexists with other factors that do contribute to mortality; thus, methadone use may be seen as a noncausative indicator of risk.
The findings of this study are consistent with previous WIHS reports that used a smaller sample and fewer explanatory variables that identified low CD4+ cell count(21–24, 26, 28), higher quantitative HIV viral RNA load(23, 24, 26, 28), low serum albumin level(23, 24, 26), reduced kidney function(22), and low hemoglobin level(26, 28) as factors associated with increased risk of all-cause mortality in HIV-infected women. Explanatory variables associated with increased risk of non-AIDS mortality in HIV-infected women in our study—decreasing CD4+ cell count, unemployment, depression, smoking, ever use of injectable illicit drugs, and hypertension—also are consistent with the findings of previously published studies of non-AIDS mortality in the WIHS(16, 27). Factors previously identified to be associated with all-cause mortality that were not statistically significantly associated in our study included HCV infection (22) and self-reported black race (22). The lack of a strong association between HCV infection and mortality in our models is similar to some but not all prior WIHS analyses.(16, 22, 27)
Although confirming the findings of previously reported mortality studies of the WIHS, our models also identified novel medication-related risk factors in this population. In the all-cause mortality analysis, benzodiazepine use was associated with a statistically significant increased risk of mortality. The data linking use of these drugs to mortality in various populations are mixed. (29–33) Our study is the first to report the association of benzodiazepines with all-cause mortality among HIV-infected women. Although benzodiazepine use was associated with increased risk of non-AIDS death, the finding did not reach statistical significance. Benzodiazepines as a class have a ceiling effect that limits their lethal toxicities (i.e., CNS and respiratory depression) at higher doses, but lethal effects can occur when they are combined with other drugs such as alcohol, antihistamines, antipsychotics or barbiturates. Thus, the observed association with mortality for benzodiazepines may not be causal but an indicator of other underlying factors, disease and non-disease, that were not studied. Despite inclusion of the aforementioned risk factors of mortality in our analysis, it appears that there exists a complex relationship between benzodiazepine use and mortality. Nevertheless, the association with mortality should prompt careful use of this class of drugs, particularly among patients with other risk factors, including concurrent medications.
Any use of CNS depressant medications was associated with increased risk of all-cause mortality and non-AIDS mortality in HIV-infected women. CNS depressants are widely used in the United States and yet the dangers associated with these medications are often not well appreciated by patients and clinicians. Hypnotics(31), H1-antihistamines(34), opioids(35), and benzodiazepines used in combination with alcohol(36) have been associated with mortality in various populations in the United States.
We also examined combinations of medications that increase the risk of QTc interval prolongation. Use of medications that may prolong the QTc interval in a conditional manner was associated with increased all-cause mortality in HIV-infected women. Although conditional circumstances associated with increased QTc prolongation (e.g., hypokalemia, hypomagnesemia) for these medications has not previously included HIV infection, HIV-infected women are at high risk of having long QTc intervals due to their sex and potentially due to antiretroviral treatments. Many antiretroviral drugs inhibit the CYP system and drug transporters, resulting in decreased metabolism of these QTc interval–prolonging medications. Known, conditional, and possible QTc interval–prolonging medications were all associated with increased risk of non-AIDs death in HIV-infected women, but the confidence intervals were wide and the findings were not statistically significant. Thus, an effect is possible but not well defined. Nevertheless, routine electrocardiographic monitoring should be considered in the care of HIV-infected patients receiving any QTc interval–prolonging medication when combined with cART.
Limitations to the study warrant consideration. First, the data in the WIHS were collected twice a year, so if a patient died months after their last WIHS visit, the available data may not represent the actual values at the time of death. Second, given that methadone response is greatly influenced by the tolerance of the individual to the medication, the absence of antemortem and postmortem methadone blood concentrations verifying a therapeutic or toxic level of methadone, as well as lack of data on dose, limits our understanding of the role of methadone in studying our outcome. Third, there was a considerable amount of missing methadone usage data specifically related to prescribed use of methadone, and this may have significantly limited our ability to capture the methadone effect in our analysis. The period of observation for this study ended in 2014; thus, these results may not reflect changes in the management of patients with opioid use disorders since then. Although patients who have newly identified opioid use disorders may be prescribed suboxone, there still remains a substantial cohort of patients who have longstanding previously diagnosed opioid use disorders and remain on methadone as well as patients who use methadone for chronic pain; therefore, we believe our study findings can still be relevant and useful. Lastly, the source of data for use of medications, including methadone, CNS depressants, and benzodiazepines, are self-reports that are limited to a recall time frame of the participants. Based on this, we anticipate that the point estimates are an underestimation for medication if medication use is underreported.
Conclusion
Assessment of the contribution of methadone, CNS depressants, benzodiazepines, and QTc interval–prolonging medications to mortality is complex. Although our investigation accounted for many factors, this analysis would benefit from a better understanding of the circumstances of death, toxicologic analysis, and more complete data on prescribed use of medications in the WIHS. Nevertheless, our study identified important risk factors—benzodiazepine, CNS depressant, and conditional QTc interval–prolonging medication use—that were associated with mortality in the WIHS. Since the WIHS represents a vulnerable population of women living with HIV in the United States, care must be taken when prescribing these medications in this underserved and at-risk patient population.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to acknowledge the help provided by Likke Chandra for extracting the data for different explanatory variables from the WIHS database and Chengshi Lin for running all statistical models in SAS statistical software.
Disclaimer:
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health.
Funding:
Women’s Interagency HIV Study (WIHS) Principal Investigators: UAB-MS WIHS (Mirjam-Colette Kempf, and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos), U01-AI-035004; Brooklyn WIHS (Howard Minkoff and Deborah Gustafson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye), U01-AI-034994; Miami WIHS (Margaret Fischl and Lisa Metsch), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I - WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection and statistical analysis is also supported by UL1-TR000004 (UCSF CTSA) and data collection by UL1-TR000454 (Atlanta CTSA). Bani Tamraz was also supported by UCSF-Gladstone Center For AIDS Research grant (P30 A1027763).
REFERENCES
- 1.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001–2010. Pharmacoepidemiol Drug Saf. 2015;24(8):885–92. [DOI] [PubMed] [Google Scholar]
- 2.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug poisoning deaths in the United States, 1980–2008. NCHS data brief. 2011(81):1–8. [PubMed] [Google Scholar]
- 3.Paulozzi LJ, Logan JE, Hall AJ, McKinstry E, Kaplan JA, Crosby AE. A comparison of drug overdose deaths involving methadone and other opioid analgesics in West Virginia. Addiction. 2009;104(9):1541–8. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease C, Prevention. Vital signs: risk for overdose from methadone used for pain relief - United States, 1999–2010. Morb Mortal Wkly Rep. 2012;61(26):493–7. [PubMed] [Google Scholar]
- 5.Hall AJ, Logan JE, Toblin RL, Kaplan JA, Kraner JC, Bixler D, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613–20. [DOI] [PubMed] [Google Scholar]
- 6.Petrosillo N, Lisena FP, Chinello P. QTc prolongation in human immunodeficiency virus-infected persons. Arch Internal Medicine. 2006;166(20):2288–9. [DOI] [PubMed] [Google Scholar]
- 7.Kao D, Bucher Bartelson B, Khatri V, Dart R, Mehler PS, Katz D, et al. Trends in reporting methadone-associated cardiac arrhythmia, 1997–2011: an analysis of registry data. Ann Intern Med. 2013;158(10):735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krantz MJ, Martin J, Stimmel B, Mehta D, Haigney MC. QTc interval screening in methadone treatment. Ann Internal Med. 2009;150(6):387–95. [DOI] [PubMed] [Google Scholar]
- 9.Krantz MJ, Kutinsky IB, Robertson AD, Mehler PS. Dose-related effects of methadone on QT prolongation in a series of patients with torsade de pointes. Pharmacotherapy. 2003;23(6):802–5. [DOI] [PubMed] [Google Scholar]
- 10.Vallecillo G, Mojal S, Roquer A, Martinez D, Rossi P, Fonseca F, et al. Risk of QTc prolongation in a cohort of opioid-dependent HIV-infected patients on methadone maintenance therapy. Clin Infect Dis. 2013;57(8):1189–94. [DOI] [PubMed] [Google Scholar]
- 11.Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardio. 1992;8(7):690–5. [PubMed] [Google Scholar]
- 12.Gingo MR, Balasubramani GK, Kingsley L, Rinaldo CR, Alden CB Jr., Detels R, et al. The impact of HAART on the respiratory complications of HIV infection: longitudinal trends in the MACS and WIHS cohorts. PloS one. 2013;8(3):e58812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simonetti JA, Gingo MR, Kingsley L, Kessinger C, Lucht L, Balasubramani G, et al. Pulmonary Function in HIV-Infected Recreational Drug Users in the Era of Anti-Retroviral Therapy. J AIDS Clin Res. 2014;5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–25. [PubMed] [Google Scholar]
- 16.Cohen MH, French AL, Benning L, Kovacs A, Anastos K, Young M, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113(2):91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woosley R, Romero K. www.Crediblemeds.org, QTdrugs List, Accession Date: October 2015, AZCERT, Inc. 1822 Innovation; Park Dr., Oro Valley, AZ: 85755. [Google Scholar]
- 18.Woosley RL, Black K, Heise CW, Romero K. CredibleMeds.org: What does it offer? Trends Cardiovasc Med. 2018;28(2):94–9. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Drug development and drug interactions: Table of substrates, inhibitors and inducers. Accession date: October 2015 Available from: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm093664.htm.
- 20.Drug interactions database. Ann Arbor (MI): Truven Health Analytics; publication year [October 2015]. Available from: www.micromedexsolutions.com. Subscription required to view. [Internet].
- 21.Anastos K, Barron Y, Cohen MH, Greenblatt RM, Minkoff H, Levine A, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140(4):256–64. [DOI] [PubMed] [Google Scholar]
- 22.Estrella MM, Parekh RS, Abraham A, Astor BC, Szczech LA, Anastos K, et al. The impact of kidney function at highly active antiretroviral therapy initiation on mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2010;55(2):217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, et al. Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV study. AIDS. 2000;14(7):863–70. [DOI] [PubMed] [Google Scholar]
- 24.Feldman JG, Gange SJ, Bacchetti P, Cohen M, Young M, Squires KE, et al. Serum albumin is a powerful predictor of survival among HIV-1-infected women. J Acquir Immune Defic Syndr. 2003;33(1):66–73. [DOI] [PubMed] [Google Scholar]
- 25.Gange SJ, Barron Y, Greenblatt RM, Anastos K, Minkoff H, Young M, et al. Effectiveness of highly active antiretroviral therapy among HIV-1 infected women. J Epidemiol Community Health. 2002;56(2):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szczech LA, Hoover DR, Feldman JG, Cohen MH, Gange SJ, Gooze L, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39(8):1199–206. [DOI] [PubMed] [Google Scholar]
- 27.Wada N, Jacobson LP, Cohen M, French A, Phair J, Munoz A. Cause-specific mortality among HIV-infected individuals, by CD4(+) cell count at HAART initiation, compared with HIV-uninfected individuals. AIDS. 2014;28(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt CM, Hoover DR, Shi Q, Seaberg E, Wei C, Tien PC, et al. Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr. 2010;55(1):73–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ. 2014;348:g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality? Sleep Med. 2009;10(3):279–86. [DOI] [PubMed] [Google Scholar]
- 31.Kripke DF, Langer RD, Kline LE. Hypnotics’ association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2(1):e000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vinkers DJ, Gussekloo J, van der Mast RC, Zitman FG, Westendorp RG. Benzodiazepine use and risk of mortality in individuals aged 85 years or older. JAMA. 2003;290(22):2942–3. [DOI] [PubMed] [Google Scholar]
- 33.Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS. Mortality associated with benzodiazepines and benzodiazepine-related drugs among community-dwelling older people in Finland: a population-based retrospective cohort study. Can J Psychiatry. 2011;56(6):377–81. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez CA, Mortensen EM, Makris UE, Berlowitz DR, Copeland LA, Good CB, et al. Association of skeletal muscle relaxers and antihistamines on mortality, hospitalizations, and emergency department visits in elderly patients: a nationwide retrospective cohort study. BMC Geriatr. 2015;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths--United States, 2000–2014. Morb Mortal Wkly Rep. 2016;64(50–51):1378–82. [DOI] [PubMed] [Google Scholar]
- 36.Jones Christopher M., Paulozzi Leonard J., Mack KA. Alcohol Involvement in Opioid Pain Reliever and Benzodiazepine Drug Abuse–Related Emergency Department Visits and Drug-Related Deaths — United States, 2010. Morb Mortal Wkly Rep. 2014;63(40):881–5. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


