Abstract
Cancer immunotherapy by immune checkpoint blockade has proven its great potential by saving the lives of a proportion of late stage patients with immunogenic tumor types. However, even in these sensitive tumor types, the majority of patients do not sufficiently respond to the therapy. Furthermore, other tumor types, including glioblastoma, remain largely refractory. The glioblastoma immune microenvironment is recognized as highly immunosuppressive, posing a major hurdle for inducing immune-mediated destruction of cancer cells. Scattered information is available about the presence and activity of immunosuppressive or immunostimulatory cell types in glioblastoma tumors, including tumor-associated macrophages, tumor-infiltrating dendritic cells and regulatory T cells. These cell types are heterogeneous at the level of ontogeny, spatial distribution and functionality within the tumor immune compartment, providing insight in the complex cellular and molecular interplay that determines the immune refractory state in glioblastoma. This knowledge may also yield next generation molecular targets for therapeutic intervention.
Introduction
During the past decade, immunotherapy of cancer has reached the status of being one of the most effective cancer therapies for defined tumor types. The main progress came from immune checkpoint blockers (ICB), monoclonal antibodies that inhibit the function of molecules involved in downregulating T-cell activation such as CTLA-4 or PD-1. ICB has shown the spectacular potential of curing late stage metastatic patients with highly immunogenic tumors such as melanoma, Merkel cell carcinoma or microsatellite instability (MSI)-high cancers, largely explaining its success. However, the majority of patients, even in responsive tumor types such as melanoma, do not benefit from ICB. Even more troublesome, some tumor types have shown nearly complete refractoriness to ICB, for as yet not fully defined reasons.
Glioblastoma (GBM), the highest-grade, most prevalent and most aggressive glial tumor, is one of the cancers in which ICB has met little success so far. Several underlying mechanisms could be responsible for this failure, including the inherently heterogenous nature of this tumor type within individuals and the establishment of an immunosuppressive tumor microenvironment.
Growth of GBM tumors, but also resistance to radiotherapy and chemotherapies, is mediated by stem-like cells, whose tumor-propagating nature is fully regulated by a core set of neurodevelopmental transcription factors such as POU3F2, SOX2, SALL2, and OLIG2 (Suvà et al., 2014) (Figure 1). Various markers have been suggested for glioblastoma stem cells (Lathia et al., 2015), but it is unclear at present whether different subpopulations of GBM stem cells exist and whether these give rise to tumors with a different cellular composition. In any case, expression profiling of GBM tumors identified at least three GBM subtypes: proneural (TCGA-PN), classical (TCGA-CL) and mesenchymal (TCGA-MES) (Verhaak et al., 2010; Wang et al., 2017), which tend to differentially associate with abnormalities in PDGFRA, IDH1, EGFR and NF1 (Verhaak et al., 2010). This level of heterogeneity is dramatically increased by the notion that different GBM subtypes can be found within the same tumor and are dynamic in function of time or in response to therapy (Sottoriva et al., 2013; Patel et al., 2014; Wang et al., 2017). More recent high-resolution single-cell RNA sequencing provided even more granularity to the concept of intra-tumoral heterogeneity by identifying four cellular states for glioblastoma cells: mesenchymal-like (MES-like), astrocyte-like (AC-like), oligodendrocytic precursor cell-like (OPC-like) and neural progenitor cell-like (NPC-like) (Neftel et al., 2019). There is a preponderance of particular states in each TCGA tumor type, with TCGA-CL and TCGA-MES being enriched in AC-like and MES-like states, respectively, and TCGA-PN encompassing both OPC-like and NPC-like states. Notably, some genetic alterations favor specific cellular states, with for example EGFR overexpression driving an AC-like program (Neftel et al., 2019). Finally, non-genetic heterogeneity within GBM tumors is determined by the relative proximity of cancer cells to blood vessels, with mTOR activity being upregulated in the few cell layers closest to the vessels (Kumar et al., 2019). In these cells, mTOR conveys superior invasive and migratory capabilities and resistance to therapy. Together, this highly heterogeneous nature of GBM strongly undermines the efficacy of therapy, considering the likely presence of cancer cell clones which are able to escape.
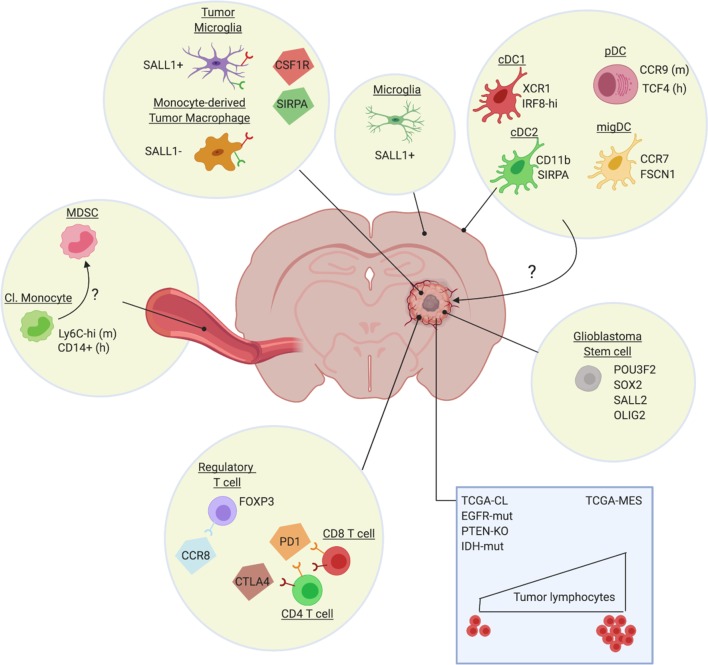
Figure 1. Heterogeneity of the glioblastoma immune microenvironment and potential therapeutic targets.
Within glioblastoma tumors reside ontogenically distinct, immunoregulatory macrophages (Sall1+ tumor microglia, Sall1- monocyte-derived macrophages), immunosuppressive Treg (eg CCR8+) and dysfunctional T-cell populations (CTLA-4/PD-1hi). Not much is known about intratumoral DC subsets, although distinct DC populations are found in other brain regions, such as the dura mater (Van Hove et al., 2019). Glioblastoma also affects the phenotype of classical monocytes (Cl. Monocyte) in the periphery, which acquire an immunosuppressive (MDSC-like?) phenotype. Notably, the genetic make-up of the cancer cells (blue rectangle) and potentially also of the glioblastoma stem cells, affect the immune composition of the tumor, with for example a higher presence of lymphocytes in TCGA-MES tumors. Several potential therapeutic targets (CSF1R, SIRPa, CCR8, PD-1, CTLA-4), either already tested in the clinic or promising for the future, are highlighted.
In addition, defects in anti-tumor T-cell responses are commonly observed in GBM, suggesting the active induction of immunosuppression. In this respect, intracranial tumors of various histological origins (not only GBM) cause an entrapment of T cells in the bone marrow due to a loss of surface spingosine-1-phosphate receptor 1 (S1P1) (Chongsathidkiet et al., 2018). Why only intracranial tumors induce this defect and via which mechanism is currently unknown, but a reversal of this deficiency enables an enhanced migration of T cells into GBM tumors. Along the same line, thymic involution in GBM tumor-bearers results in diminished T-cell production and, hence, reduced availability of T cells for anti-GBM immunity (Andaloussi et al., 2008). Moreover, once T cells do get inside the GBM tumor, they are likely to become dysfunctional via various mechanisms (Woroniecka et al., 2018a). Recent studies provide evidence for the enriched presence of exhausted T cells within GBM tumors, with tumor-infiltrating lymphocytes (TILs) more often co-expressing the immune checkpoint molecules PD-1, LAG3 and TIM-3 than peripheral blood cells of patients or healthy controls (Woroniecka et al., 2018b; Davidson et al., 2019). Interestingly, PD-1+ LAG3+ TIM-3+ T cells showed impaired T-cell functions, while single positive cells for any of these markers did not. A second prominent mechanism of T-cell dysfunctionality is tolerance, mediated by the presence of suppressive cells such as regulatory T cells (Treg) and tumor-associated macrophages (TAM). These aspects will be discussed in detail in this review.
Immunotherapeutic approaches in glioblastoma
Previous reviews have described the attempts to incorporate ICB in the treatment of GBM (Romani et al., 2018; Simonelli et al., 2018). In summary, preclinical models have provided a proof-of-concept that ICB with anti-CTLA-4 and/or anti-PD-1, either as monotherapy or in conjunction with standard-of-care treatments, improves tumor outcome. This has set the stage for multiple clinical trials in glioblastoma patients, the results of which are up to this date mostly unavailable. Here, we will focus on the most recent progress in this field. One of the most noticeable advances comes from the realization that the timing of ICB administration could majorly impact its effect. Classically, ICB is given in an adjuvant setting, improving recurrence-free survival and overall survival after surgery in cancer types such as melanoma. Neoadjuvant (pre-operative) therapy has proven to be advantageous in breast, bladder and other types of cancer, but this finding remained restricted to chemo- and radiation therapy with very little data available on ICB neoadjuvant therapy until recently (O'Donnell et al., 2019). Liu et al. (2016) demonstrated increased anti-tumor immune responses and survival upon neoadjuvant immunotherapy, in two mouse models of triple-negative breast cancer. This finding has now been reproduced in several other preclinical models (O'Donnell et al., 2019), and importantly, also in the clinical setting of resectable melanoma (Blank et al., 2018; Amaria et al., 2018; Rozeman et al., 2019) and glioblastoma (Cloughesy et al., 2019; Schalper et al., 2019). In resectable glioblastoma, either as primary or recurrent tumor, one neoadjuvant injection of anti-PD-1 monoclonal antibodies, followed by surgical resection of the tumor and repeated adjuvant anti-PD-1 treatment, resulted in a significant increase in overall survival and progression-free survival compared to adjuvant treatment alone (Cloughesy et al., 2019; Schalper et al., 2019). Remarkably, the same conclusions were reached by two independent research groups that used a different monoclonal antibody, either pembrolizumab or nivolumab. Both studies also demonstrated an intratumoral immunomodulatory effect of the neoadjuvant treatment, with an increased interferon response, production of chemokines, and signs of increased TIL activity. However, larger-scale randomized trials should be performed to fully prove the benefit of this neoadjuvant approach.
A second level of innovation comes from the assessment of novel combination therapies in preclinical models, whereby anti-PD-1 efficacy is boosted by a concurrent administration of chemotherapy (Park et al., 2019), radiation therapy (Rajani et al., 2018), other immune checkpoint blocking monoclonal antibodies (Wu et al., 2019), cancer cell-directed immunotoxins (Chandramohan et al., 2019), and nanocarriers that improve the shuttling of ICB across the blood-brain barrier (Galstyan et al., 2019). Most of these studies show a clear benefit of combining different approaches to increase anti-GBM immunity. Indeed, monotherapies usually have little impact, highlighting the strongly immunosuppressive nature of the GBM tumor microenvironment (TME). A novel avenue to increase the immunogenicity of the GBM TME comes from virotherapy, whereby oncolytic viruses not only cause a direct killing of highly proliferative cancer cells but also trigger an (innate) immune response (Iorgulescu et al., 2018). For example, a recent clinical trial applied intratumoral injections of a polio-rhinovirus chimera and could achieve a remarkable 21% of longer term survivors (at least 2 to 3 years), while <4% of such patients is observed in control groups (Desjardins et al., 2018). In a phase I study with DNX-2401, a tumor-selective replication-competent oncolytic adenovirus, 20% of patients survived >3 years from treatment, and three patients had a ≥ 95% reduction of the tumor, which was probably due to direct oncolytic effects of the virus followed by elicitation of an immune-mediated antiglioma response (Lang et al., 2018). A passive transfer of anti-tumor T-effector cells could be an alternative strategy to attack GBM tumors, although such T cells should then be able to withstand the suppressive TME. Novel generations of CAR T-cells are being generated that should show improved functionality in the TME, including the TME from GBM (Petersen and Krenciute, 2019). Finally, the adoptive transfer of antigen-presenting dendritic cells (i.e. DC vaccination) has yielded some efficacy in improving the median overall survival in clinical trials (Srivastava et al., 2019) and further research is warranted to improve the treatment regimens and the choice of the most optimal DC type and the most optimal tumor antigens. Neoantigens are the most tumor-specific and arguably the most promising antigen candidates, that show efficacy as vaccine candidates in glioblastoma patients (Keskin et al., 2019; Hilf et al., 2019).
Heterogeneity of the tumor immune microenvironment in glioblastoma
The immune contexture of human tumors, referring to the exact composition of distinct immune cell types within tumors, changes at each tumor stage and is known to have an impact on clinical outcome (Fridman et al., 2012; Bindea et al., 2013). Hence, understanding the tumor (immune) microenvironment has recently been recognized as one of the future challenges to improve therapy of brain tumors (Aldape et al., 2019). Glioblastoma tumors are known to contain a variety of immune cell types, but with a dominance of immunosuppressive cells. Recent findings further finetune this concept through the identification of distinct subpopulations within the immunoregulatory macrophage compartment of tumors, but also distinct Treg and dendritic cell subsets have been identified and will be discussed in this review.
Correlation between tumor genotype and the glioblastoma immune microenvironment
As mentioned earlier, innovative techniques such as extensive exome and transcriptome sequencing have revealed the existence of molecularly distinct proneural, mesenchymal and classical subtypes of glioblastoma (Verhaak et al., 2010; Wang et al., 2017). In addition, a diverse array of recurrent genomic mutations were found in these tumors, including TP53, IDH1, NF1, PTEN, PDGFRA, EGFR and MAPK pathway mutations, with particular mutations being enriched in particular glioblastoma subtypes.
Of importance in the context of ICB immunotherapy, is the observation that the number of immune cells in the human glioblastoma microenvironment appears to be associated with the predominant genetic alteration (Figure 1). TILs are enriched in TCGA-MES glioblastomas and strongly associated with mutations in NF1 and RB1. Conversely, TILs are depleted in the TCGA-CL class, EGFR-amplified, and homozygous PTEN-deleted tumors and rare in histologies characterized by these alterations (Rutledge et al., 2013). In addition, the IDH mutant status in glioblastoma tumors significantly associates with TIL infiltration. The IDH-wt status is associated with significantly higher TIL infiltration and PD-L1 expression (Berghoff et al., 2017), while IDH mutations result in the opposite phenotype, that is a reduced IFNγ signature and reduced presence of CD8+ and CD4+ cells (Kohanbash et al., 2017; Luoto et al., 2018). Nevertheless, patients bearing IDH-mutated tumors have a more favourable outcome (Ceccarelli et al., 2016), suggesting that other cancer cell-intrinsic or -extrinsic mechanisms are at play beyond the presence of TILs.
One of those mechanisms could be the presence of immunoregulatory macrophages and neutrophils. In a study comparing a small number of treatment-naive IDH-mutated and IDH-wt glioblastoma tumors, IDH-mutants were shown to contain less macrophages which displayed a more pro-inflammatory M1-like activation state, possibly contributing to the longer survival of these patients (Poon et al., 2019). The reduced presence of macrophages in IDH-mutant, but also CDK4-amplified tumors, was also demonstrated via a computational analysis of a large number of TCGA RNAseq samples (Luoto et al., 2018). Hence, although IDH-mutated tumors contain a lower number of T lymphocytes, these cells may be more optimally activated due to the relative paucity of immunosuppressive macrophages and neutrophils. The frequency of macrophages and neutrophils is generally increased in TCGA-MES tumors versus the other TCGA types (Wang et al., 2017). Remarkably, genes for both tumor-promoting M2-like as well as potentially anti-tumoral M1-like macrophages were enriched in TCGA-MES tumors, in particular in the NF1-deficient cases (Wang et al., 2017; Luoto et al., 2018). The absence of NF1 appears to result in chemoattraction of macrophages via an as yet undefined mechanism. In addition, high B-cell and CD4+ T-cell components were commonly observed in TCGA-MES samples, while immune cell components tend to be low in TCGA-PN samples (Luoto et al., 2018). In general terms, computational analysis grouped glioblastoma tumors into three immune response-related subgroups, termed negative (defined by a relative paucity of immune cells; enriched by TCGA-PN and CDK4-MARCH9 amplification), humoral (defined by a high B-cell and CD4+ T-cell compartment; enriched by TCGA-MES) and cellular-like (defined by a higher ‘negative regulation of T-cell activation’ and ‘gamma delta T-cell’ cluster; enriched by TCGA-CL and samples with a high macrophage content) (Luoto et al., 2018).
The question remains how these findings relate to the responsiveness of patients to ICB. Surprisingly, in spite of the immunologically different tumor microenvironment in TCGA subtypes, no association was found with ICB responsiveness (Zhao et al., 2019). Rather, non-responding patients bear significantly more PTEN mutations, which are associated with immunosuppressive gene expression signatures, while responders are enriched in MAPK pathway alterations (PTPN11, BRAF). These findings illustrate that responsiveness to ICB is of a greater complexity than the mere presence of immune cell types within the microenvironment.
Impact of standard-of-care treatments on the Glioblastoma immune microenvironment
Long-standing treatment options for glioblastoma include surgery, radiotherapy and temozolomide chemotherapy, all of which may affect the immune composition of glioblastoma tumors. In addition, brain oedema is most often treated with dexamethasone, a steroid with known immunoregulatory capacities.
Radiation therapy obviously aims to eliminate the proliferating cancer cells, but one of its side effects is the induction of hypoxia. In turn, hypoxia initiates a lethal cascade of events consisting of the increased production of CXCL12, the subsequent recruitment of CXCR4 and CXCR7-positive bone marrow-derived monocytes and hematopoietic progenitor cells that differentiate into tumor-promoting macrophages and mediate vasculogenesis and tumor recurrence in mice (Tabatabai et al., 2006; Kioi et al., 2010; Tseng et al., 2011). Hence, blockade of CXCL12 (Liu et al., 2014) and CXCR7 (Walters et al., 2014) delays recurrence of tumors upon irradiation in preclinical models. Most importantly, a recent Phase I/II clinical trial with the reversible CXCR4 inhibitor plerixafor – a strategy named Macrophage Exclusion after Radiation Therapy or MERT – in conjunction with chemoirradiation improved local control of tumor recurrence in newly diagnosed glioblastoma patients (Thomas et al., 2019). Another effect of radiation is the upregulation of PD-L1 at the surface of tumor-infiltrating myeloid cells. This effect has been exploited to target anti-PD-L1-functionalized lipid nanoparticles, carrying toxic compounds, to these myeloid cells in conjunction with irradiation, leading to an improved anti-tumor response in mice (Zhang et al., 2019).
Temozolomide is the most widely used chemotherapy for patients with glioblastoma (GBM), whose action not only directly affects cancer cells but also depends on its immunomodulatory properties (Karachi et al., 2018). Temozolomide induces lymphopenia, which, counterintuitively, can be harnessed to improve immunotherapy. Indeed, lymphoablative doses of temozolomide were shown to increase tumor antigen-specific immune responses in GBM patients (Sampson et al., 2011; Batich et al., 2017) and GBM-bearing mice (Sanchez-Perez et al., 2013). Mechanistically, it was suggested that compensatory homeostatic cytokines after temozolomide therapy cause enhanced immune responses by reduction of the T-cell activation threshold and proliferation induction.
In recent years, it became clear that dexamethasone treatment of patients may strongly affect outcome, with a lower overall survival in recurrent glioblastoma patients (Wong et al., 2015). This adverse effect is most likely due to the well-known immunomodulatory function of steroids. Indeed, glioblastoma patients treated with dexamethasone contained significantly less lymphocytes in their blood, but more myeloid cells with potentially suppressive functions (Chitadze et al., 2017).
Heterogeneity of macrophages within the Glioblastoma immune microenvironment
Macrophages are a large component of many tumor types and have been an attractive target for glioblastoma therapy (Pyonteck et al., 2013; Quail et al., 2016). Originally, TAMs were often thought to exclusively originate from tumor-infiltrating monocytes, but both in CNS and peripheral tumor types a fraction can be derived from the tissue-resident macrophage pool (Movahedi and Van Ginderachter, 2016; Laviron and Boissonnas, 2019). Recent work that has relied on single-cell RNA sequencing combined with fate mapping approaches has highlighted the rich diversity of murine tissue-resident brain macrophages. (Van Hove et al., 2019) (Figure 1). In this respect, parenchymal microglia differ from macrophages located in the brain's border regions, such as the meninges and the choroid plexus. Whether border-associated macrophages contribute to glioblastoma or metastatic brain tumors is not known. However, there is now firm evidence that TAMs in GBM tumors are partly derived from embryonic microglia (Figure 1). Tumor-associated microglia and monocyte-derived macrophages can be distinguished via genetic lineage tracing, which was used to sort these populations from transplantable and spontaneous mouse models of glioblastoma followed by RNA-sequencing (Bowman et al., 2016; Chen et al., 2017). These data revealed that the glioblastoma microenvironment instructs the transcriptional landscape of these cells, with prominent differences between microglia and monocyte-derived TAMs at multiple levels, including differential expression of genes involved in IL-1 signaling, chemokine and inflammatory cytokine production and antigen presentation. Whether this transcriptional divergence is reflected at the functional level and whether these subsets differentially affect glioblastoma progression is not known, but seems likely. Indeed, tissue-resident interstitial macrophages and their monocyte-derived counterparts are also found in mouse lung tumors, where resident TAMs are suggested to support cancer cell expansion, while monocyte-derived cells might contribute to cancer cell dissemination (Loyher et al., 2018). Also in mouse mammary carcinoma, a distinction was made between monocyte-derived TAMs and resident mammary tissue macrophages, with only the former contributing to a suppression of anti-tumor cytotoxic T-cell responses (Franklin et al., 2014).
In glioblastomas, monocyte-derived macrophages tend to be more enriched in the tumor core, while microglia-derived TAM are typically found at the tumor periphery (Chen et al., 2017). Live in vivo 2-photon microscopy confirmed the distinction between the two TAM populations in the mouse and demonstrated that monocyte-derived cells are small and motile, while microglia are large sessile cells whose processes are continuously extending and retracting within tumors (Chen et al., 2019a). Importantly, the existence of microglia and monocyte-derived TAM populations has now also been shown in human GBM (Müller et al., 2017; Darmanis et al., 2017). However, it will be interesting to evaluate whether microglia and monocyte-derived TAM further consist of specialized subpopulations, for example instructed by their precise intratumoral localization, as was demonstrated for TAM in other tumor models (Movahedi et al., 2010; Casazza et al., 2013). For this reason, future single-cell RNA-sequencing efforts will provide crucial new insights, and will undoubtedly allow the identification of multiple TAM subsets, as was already shown in mouse and human lung tumors (Zilionis et al., 2019). An interesting possibility is that microglia- and monocyte-derived TAMs may exhibit a differential ability to infiltrate or colonize specific tumor microenvironments or tumor macrophage niches.
Interesting novel findings in preclinical models provide further insights in the molecular mechanisms that govern the attraction of monocyte-derived macrophages to the glioblastoma microenvironment. Cancer cell- and host cell-derived osteopontin mediates macrophage infiltration through the interaction with integrin αvβ5 and instructs a pro-tumoral phenotype in these cells (Wei et al., 2019). Also the Aryl Hydrocarbon Receptor (AHR) is indirectly implicated in recruiting monocytes to the tumor environment by driving CCR2 expression, the receptor for the major monocyte chemoattractants CCL2 and CCL7 (Takenaka et al., 2019). In PTEN-deficient tumor models, enhanced LOX production is responsible for the enhanced attraction of macrophages via the β1 integrin-PYK2 pathway, which subsequently promote tumor growth via SPP1 (Chen et al., 2019b). However, how this finding correlates with the enhanced responsiveness of PTEN-mutant tumors to ICB therapy in patients is not clear and requires further investigation, including the potential differences between the PTEN mutations found in patients and the PTEN-null phenotype in mouse models.
Another point of interest is the systemic influence of brain tumors on peripheral myeloid cells. GBM-secreted IL-6 induces immunosuppressive myeloid cells in the periphery that express high levels of PD-L1 in orthotopic mouse models (Lamano et al., 2019). This finding is in line with CyTOF fingerprinting of patients’ peripheral blood, indicating a significant elevation of myeloid-derived suppressor cells (MDSC), but not Treg in the circulation (Alban et al., 2018) (Figure 1). Hence, the myeloid cells that infiltrate GBM tumors may have already been primed into a tumor-promoting role. The importance of MDSC, both the monocytic and granulocytic subtype (Movahedi et al., 2008), is further confirmed by the finding that their presence in mouse GBM tumors is associated with a reduction in the number of tumor-infiltrating lymphocytes (Raychaudhuri et al., 2015). Moreover, within the murine glioblastoma environment, MDSC were shown to induce regulatory B cells (Lee-Chang et al., 2019). Interestingly, CCL2 produced by TAM mediates the recruitment of suppressive CCR2+ monocytic MDSC and CCR4+ Treg, explaining the clinical observation that elevated intratumoral CCL2 expression levels correlate with reduced overall survival (Chang et al., 2016). Of note, recruited monocytic MDSC may in turn differentiate into TAM. In a preclinical setting, this notion has now been taken further by showing that a small molecule CCR2 antagonist sensitizes otherwise resistant murine gliomas to ICB therapy (Flores-Toro et al., 2020).
Heterogeneity of regulatory T cells (Treg) within the glioblastoma immune microenvironment
The presence of Treg has been amply described in multiple cancer types, but their value as predictors of disease outcome is debatable in glioblastoma. Independent researchers described an increased presence of FOXP3+ Treg in higher tumor grades of various brain tumor types, including glioblastoma (El Andaloussi and Lesniak, 2007; Jacobs et al., 2010). However, the correlation of Treg with decreased survival, which was anticipated based on the T-cell suppressive capacity of these cells, has been very moderate at best (Jacobs et al., 2010; Yue et al., 2014; Thomas et al., 2015). Nevertheless, Treg-directed therapies, such as agonist anti-GITR or anti-LAP (Latency-associated Peptide) mAb treatment, have shown some promise in mouse glioblastoma models (Miska et al., 2016; Patel et al., 2016; Gabriely et al., 2017).
A potential reason for the unclear association of Treg with disease outcome could be the existence of distinct Treg subsets which may differ in their suppressive capacity and whose presence in tumors may vary between individual patients. Early preclinical work suggested that the majority of Tregs infiltrating glioblastoma tumors are thymus-derived (Wainwright et al., 2011), although it seems likely that the local de novo induction of Treg in the tumor microenvironment is a possibility as well. As a matter of fact, indirect evidence based on correlative studies proposed glioblastoma-induced PD-L1 expression as a mechanism of Treg induction and maintenance in patients (DiDomenico et al., 2018). The attraction of these Treg to the tumor microenvironment may be mediated by various mechanisms, including IDO activity (Wainwright et al., 2012) and CCL2 production, which interacts with CCR4 at the surface of Treg (Chang et al., 2016). Interestingly, another chemokine receptor, CCR8, has recently been identified as a marker that is specifically expressed in at least a subset of tumor-infiltrating Treg, but not Treg in the periphery, as part of a tumor-infiltrating Treg transcriptional signature that is conserved across species and tumor types (Plitas et al., 2016; De Simone et al., 2016; Zheng et al., 2017; Magnuson et al., 2018) (Figure 1). CCR8+ Treg were reported as very potent suppressors, suggesting that this Treg subset is likely important in the creation of an immunosuppressive tumor microenvironment (Barsheshet et al., 2017). However, to what extent the tumor-infiltrating Treg signature and CCR8+ Treg are present within glioblastoma tumors is currently unknown.
Another marker that is functionally important on Treg within tumors is Neuropilin-1 (Nrp1). The interaction of Nrp1 with its ligand Semaphorin 4a was shown to stabilize the Treg phenotype in the mouse, which turns out to be especially important for the suppression of anti-tumor immune responses (Delgoffe et al., 2013). The absence of Nrp1 in Treg leads to their loss of suppressive potential but also to their production of IFNγ, which subsequently annihilates the suppressive capacity of neighbouring Nrp1+ Treg in murine tumors (Overacre-Delgoffe et al., 2017). This mechanism may be stimulated by enhanced HIF-1α expression in Nrp1-deficient Treg. Interestingly, HIF-1α expression in hypoxic tumor-infiltrating Treg was indeed shown to decrease the intrinsic suppressive activity of these Treg, but was at the same time required for the migration of Treg into mouse glioblastoma tumors (Miska et al., 2019). Consequently, the absence of HIF1α in Treg leads to the reduced growth of glioblastoma tumors.
Altogether, suppressive Treg subsets are induced in the microenvironment of multiple tumor types. As these cells could be prime targets for therapeutic intervention, it will be important to assess their presence and relevance in glioblastoma.
Heterogeneity of dendritic cells within the Glioblastoma immune microenvironment
Recent findings, in other tumor types than glioblastoma, have uncovered the existence of distinct DC populations in solid tumors. Indeed, at least three types of conventional DCs (cDCs), in addition to plasmacytoid DCs (pDCs), are present in the tumor microenvironment of multiple mouse and human tumors (Laoui et al., 2016; Roberts et al., 2016; Zilionis et al., 2019). The functionality of these cells is being explored in mouse tumor models. cDC1 are rare cells within tumors that were repeatedly reported to migrate to the tumor-draining lymph nodes where they cross-present tumor antigens to CD8+ T cells (Broz et al., 2014; Roberts et al., 2016; Laoui et al., 2016). Interestingly, NK cells attract cDC1 to the tumor environment via the secretion of various growth factors and chemokines, such as Flt3L, CCL5 and XCL1 (Böttcher et al., 2018; Barry et al., 2018). cDC2, which are typically more abundant within tumors than cDC1, also have the capacity to drive T-cell responses, but are more directed towards the activation of CD4+ T cells. As such, these cells were shown to initiate a Th17 response (Laoui et al., 2016), but their full T-cell activating potential may only be unleashed in the absence of Treg (Binnewies et al., 2019). Both mouse and human lung tumors also harbor an additional DC subset that exhibits a migratory gene signature (Zilionis et al., 2019), although this cannot be taken as proof for actual migration.
Translating these findings to glioblastoma is not trivial, as a systematic analysis of DC subpopulations in these tumors is currently lacking. Recent work has shown that various dendritic cell subsets are present in the murine homeostatic brain (Mrdjen et al., 2018; Van Hove et al., 2019), where they are thought be critical for initiating neuroinflammation (Mundt et al., 2019; Jordão et al., 2019). In the context of glioblastoma, one study reported that CCR2+ Lin- hematopoietic stem and progenitor cells (HSC) can differentiate into cross-presenting DC within mouse glioblastoma tumors (Flores et al., 2018). These HSC-derived DC can be isolated from glioblastoma tumors to successfully vaccinate mice and induce anti-tumor immunity in the vaccine recipients. In addition, their presence potentiates the effect of immune checkpoint blockade immunotherapy. Along the same line, increasing the number of intratumoral CD103+ cDC1 through the administration of Flt3L (Miao et al., 2018), or activating them via TLR3 agonists (Garzon-Muvdi et al., 2018), magnifies the response of mouse glioblastomas to immune checkpoint blockade, suggesting the importance of this DC type to augment immunity against glioblastoma, similar to other tumor types. However, Fibrinogen-like Protein 2 (FGL2), that is predominantly secreted by the cancer cells, may interfere with the induction of these immunostimulatory DCs (Yan et al., 2019). Indeed, FGL2 interferes with GM-CSF signalling, blunting the differentiation of CD103+ cDC1 and consequently lowering the CD8+ T-cell response. Overall, it can be concluded that interventions which increase the number of CD103+ cDC1 in glioblastoma tumors or augment their activation state may be beneficial. Whether cDC2 can also contribute to anti-glioblastoma immunity remains to be determined.
Perspective on novel immunotherapies directed against cells within the tumor immune microenvironment
Finally, how can the recently acquired knowledge on the heterogeneity of the glioblastoma immune microenvironment be turned into novel therapeutic avenues? An important caveat in this respect is the capacity of drugs to cross the blood-brain barrier (BBB) and to reach sufficiently high concentrations in the brain. A number of very recent studies have highlighted the potential of nanoparticles to deliver therapeutic cargo to the mouse brain. Solid lipid nanoparticles can deliver small interfering RNAs to the mouse glioblastoma microenvironment, following a low-dose irradiation to prime brain uptake (Erel-Akbaba et al., 2019). A novel tumor penetrating peptide that targets cell surface p32, LinTT1 (AKRGARSTA), has also been reported as a GBM targeting ligand for systemically-administered nanoparticles (Säälik et al., 2019). Moreover, nanoscale immunoconjugates (NICs) on a natural biopolymer scaffold, poly(β-L-malic acid), were produced with covalently attached anti-CTLA-4 or anti-PD-1 for systemic delivery across the BBB and activation of local brain anti-tumor immune responses (Galstyan et al., 2019). Notably, nanoparticles often end up being phagocytosed by tumor-associated macrophages and are, hence, interesting tools to modulate the activity of these cells. The fact that modulating the macrophage compartment is indeed a promising approach was shown a couple of years ago by the administration of a brain penetrating CSF1R inhibitor in mice, which repolarized TAM into a more anti-tumoral phenotype resulting in a reduced GBM progression (Pyonteck et al., 2013). Likewise, a blocking anti-CD47 antibody mobilizes the phagocytic capacity of both monocyte-derived and microglial TAMs in mouse glioblastoma models (Hutter et al., 2019). As a matter of fact, this type of approaches could now be combined with strategies to increase the presence of antigen-presenting cells, by the targeted delivery of growth factors or chemokines for cDCs.
Targeting tumor-infiltrating Treg, without affecting their peripheral counterparts to avoid auto-immune complications, could be another interesting approach. The recent identification of molecules, such as CCR8, that are specifically upregulated on these cells within tumors may provide novel therapeutic anchor points in multiple tumor types. It needs to be evaluated whether these tiTreg-specific molecules are implicated in Treg functionality as this will determine the type of compound we need for therapy (agonists, antagonists,...).
Overall, increasing knowledge about subpopulations of immune cells that either promote or inhibit glioblastoma tumor progression will allow more specific therapeutic approaches against this aggressive type of brain tumor.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Kiavash Movahedi, Email: Kiavash.Movahedi@vub.be.
Jo A Van Ginderachter, Email: jo.van.ginderachter@vub.be.
Caetano Reis e Sousa, The Francis Crick Institute, United Kingdom.
Jeffrey Settleman, Pfizer, United States.
Funding Information
This paper was supported by the following grants:
Kom op tegen Kanker to Bart Neyns, Kiavash Movahedi, Jo A Van Ginderachter.
Stichting Tegen Kanker to Jo A Van Ginderachter.
Fonds Wetenschappelijk Onderzoek to Isabelle Scheyltjens, Jo A Van Ginderachter.
Innoviris to Kiavash Movahedi.
Additional information
Competing interests
No competing interests declared.
References
- Alban TJ, Alvarado AG, Sorensen MD, Bayik D, Volovetz J, Serbinowski E, Mulkearns-Hubert EE, Sinyuk M, Hale JS, Onzi GR, McGraw M, Huang P, Grabowski MM, Wathen CA, Ahluwalia MS, Radivoyevitch T, Kornblum HI, Kristensen BW, Vogelbaum MA, Lathia JD. Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI Insight. 2018;3:122264. doi: 10.1172/jci.insight.122264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, Gottardo N, Gutmann DH, Hargrave D, Holland EC, Jones DTW, Joyce JA, Kearns P, Kieran MW, Mellinghoff IK, Merchant M, Pfister SM, Pollard SM, Ramaswamy V, Rich JN, Robinson GW, Rowitch DH, Sampson JH, Taylor MD, Workman P, Gilbertson RJ. Challenges to curing primary brain tumours. Nature Reviews Clinical Oncology. 2019;16:509–520. doi: 10.1038/s41571-019-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaria RN, Reddy SM, Tawbi HA, Davies MA, Ross MI, Glitza IC, Cormier JN, Lewis C, Hwu WJ, Hanna E, Diab A, Wong MK, Royal R, Gross N, Weber R, Lai SY, Ehlers R, Blando J, Milton DR, Woodman S, Kageyama R, Wells DK, Hwu P, Patel SP, Lucci A, Hessel A, Lee JE, Gershenwald J, Simpson L, Burton EM, Posada L, Haydu L, Wang L, Zhang S, Lazar AJ, Hudgens CW, Gopalakrishnan V, Reuben A, Andrews MC, Spencer CN, Prieto V, Sharma P, Allison J, Tetzlaff MT, Wargo JA. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nature Medicine. 2018;24:1649–1654. doi: 10.1038/s41591-018-0197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andaloussi AE, Han Y, Lesniak MS. Progression of intracranial glioma disrupts thymic homeostasis and induces T-cell apoptosis in vivo. Cancer Immunology, Immunotherapy. 2008;57:1807–1816. doi: 10.1007/s00262-008-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, Alvarado MD, Wolf DM, Bogunovic D, Bhardwaj N, Daud AI, Ha PK, Ryan WR, Pollack JL, Samad B, Asthana S, Chan V, Krummel MF. A natural killer-dendritic cell Axis defines checkpoint therapy-responsive tumor microenvironments. Nature Medicine. 2018;24:1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J, Lira SA, Karin N. CCR8+FOXp3+ Treg cells as master drivers of immune regulation. PNAS. 2017;114:6086–6091. doi: 10.1073/pnas.1621280114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, Norberg P, Xie W, Herndon JE, Healy P, McLendon RE, Friedman AH, Friedman HS, Bigner D, Vlahovic G, Mitchell DA, Sampson JH. Long-term survival in glioblastoma with Cytomegalovirus pp65-Targeted vaccination. Clinical Cancer Research. 2017;23:1898–1909. doi: 10.1158/1078-0432.CCR-16-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff AS, Kiesel B, Widhalm G, Wilhelm D, Rajky O, Kurscheid S, Kresl P, Wöhrer A, Marosi C, Hegi ME, Preusser M. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro-Oncology. 2017;19:1460–1468. doi: 10.1093/neuonc/nox054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human Cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Binnewies M, Mujal AM, Pollack JL, Combes AJ, Hardison EA, Barry KC, Tsui J, Ruhland MK, Kersten K, Abushawish MA, Spasic M, Giurintano JP, Chan V, Daud AI, Ha P, Ye CJ, Roberts EW, Krummel MF. Unleashing Type-2 dendritic cells to drive protective antitumor CD4+ T Cell Immunity. Cell. 2019;177:556–571. doi: 10.1016/j.cell.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van de Wiel B, Kvistborg P, Krijgsman O, van den Braber M, Philips D, Broeks A, van Thienen JV, Mallo HA, Adriaansz S, Ter Meulen S, Pronk LM, Grijpink-Ongering LG, Bruining A, Gittelman RM, Warren S, van Tinteren H, Peeper DS, Haanen J, van Akkooi ACJ, Schumacher TN. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nature Medicine. 2018;24:1655–1661. doi: 10.1038/s41591-018-0198-0. [DOI] [PubMed] [Google Scholar]
- Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting Cancer immune control. Cell. 2018;172:1022–1037. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, Dhara S, Simpson K, Gardner EE, Iacobuzio-Donahue CA, Brennan CW, Tabar V, Gutin PH, Joyce JA. Macrophage ontogeny underlies differences in Tumor-Specific education in brain malignancies. Cell Reports. 2016;17:2445–2459. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van't Veer LJ, Sperling AI, Wolf DM, Krummel MF. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–652. doi: 10.1016/j.ccell.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor Areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti CG, Tirapelli DP, Rao A, Mikkelsen T, Lau CC, Yung WK, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, Noushmehr H, Iavarone A, Verhaak RG, TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandramohan V, Bao X, Yu X, Parker S, McDowall C, Yu YR, Healy P, Desjardins A, Gunn MD, Gromeier M, Nair SK, Pastan IH, Bigner DD. Improved efficacy against malignant brain tumors with EGFRwt/EGFRvIII targeting immunotoxin and checkpoint inhibitor combinations. Journal for ImmunoTherapy of Cancer. 2019;7:142. doi: 10.1186/s40425-019-0614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, Han Y, Wu M, Zhang L, Horbinski CM, Ahmed AU, Lesniak MS. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and Myeloid-Derived suppressor cells. Cancer Research. 2016;76:5671–5682. doi: 10.1158/0008-5472.CAN-16-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, Rasmussen R, Dwivedi B, Seby S, Wolf SA, Gutmann DH, Hambardzumyan D. Cellular and molecular identity of Tumor-Associated macrophages in glioblastoma. Cancer Research. 2017;77:2266–2278. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ross JL, Hambardzumyan D. Intravital 2-photon imaging reveals distinct morphology and infiltrative properties of glioblastoma-associated macrophages. PNAS. 2019a;116:14254–14259. doi: 10.1073/pnas.1902366116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Zhao D, Li J, Liang X, Li J, Chang A, Henry VK, Lan Z, Spring DJ, Rao G, Wang YA, DePinho RA. Symbiotic Macrophage-Glioma cell interactions reveal synthetic lethality in PTEN-Null glioma. Cancer Cell. 2019b;35:868–884. doi: 10.1016/j.ccell.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitadze G, Flüh C, Quabius ES, Freitag-Wolf S, Peters C, Lettau M, Bhat J, Wesch D, Oberg HH, Luecke S, Janssen O, Synowitz M, Held-Feindt J, Kabelitz D. In-depth immunophenotyping of patients with glioblastoma multiforme: impact of steroid treatment. OncoImmunology. 2017;6:e1358839. doi: 10.1080/2162402X.2017.1358839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, Farber SH, Woroniecka K, Elsamadicy AA, Dechant CA, Kemeny HR, Sanchez-Perez L, Cheema TA, Souders NC, Herndon JE, Coumans JV, Everitt JI, Nahed BV, Sampson JH, Gunn MD, Martuza RL, Dranoff G, Curry WT, Fecci PE. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nature Medicine. 2018;24:1459–1468. doi: 10.1038/s41591-018-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, Wang AC, Ellingson BM, Rytlewski JA, Sanders CM, Kawaguchi ES, Du L, Li G, Yong WH, Gaffey SC, Cohen AL, Mellinghoff IK, Lee EQ, Reardon DA, O'Brien BJ, Butowski NA, Nghiemphu PL, Clarke JL, Arrillaga-Romany IC, Colman H, Kaley TJ, de Groot JF, Liau LM, Wen PY, Prins RM. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature Medicine. 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmanis S, Sloan SA, Croote D, Mignardi M, Chernikova S, Samghababi P, Zhang Y, Neff N, Kowarsky M, Caneda C, Li G, Chang SD, Connolly ID, Li Y, Barres BA, Gephart MH, Quake SR. Single-Cell RNA-Seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Reports. 2017;21:1399–1410. doi: 10.1016/j.celrep.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TB, Lee A, Hsu M, Sedighim S, Orpilla J, Treger J, Mastall M, Roesch S, Rapp C, Galvez M, Mochizuki A, Antonios J, Garcia A, Kotecha N, Bayless N, Nathanson D, Wang A, Everson R, Yong WH, Cloughesy TF, Liau LM, Herold-Mende C, Prins RM. Expression of PD-1 by T cells in malignant glioma patients reflects exhaustion and activation. Clinical Cancer Research. 2019;25:1913–1922. doi: 10.1158/1078-0432.CCR-18-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of Tumor-Infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a Axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, Friedman HS, McSherry F, Muscat AM, Nair S, Peters KB, Randazzo D, Sampson JH, Vlahovic G, Harrison WT, McLendon RE, Ashley D, Bigner DD. Recurrent glioblastoma treated with recombinant poliovirus. New England Journal of Medicine. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, Ampie L, Choy W, Lamano JB, Bloch O. The immune checkpoint protein PD-L1 induces and maintains regulatory T cells in glioblastoma. OncoImmunology. 2018;7:e1448329. doi: 10.1080/2162402X.2018.1448329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Andaloussi A, Lesniak MS. CD4+ CD25+ FoxP3+ T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. Journal of Neuro-Oncology. 2007;83:145–152. doi: 10.1007/s11060-006-9314-y. [DOI] [PubMed] [Google Scholar]
- Erel-Akbaba G, Carvalho LA, Tian T, Zinter M, Akbaba H, Obeid PJ, Chiocca EA, Weissleder R, Kantarci AG, Tannous BA. Radiation-Induced targeted Nanoparticle-Based gene delivery for brain tumor therapy. ACS Nano. 2019;13:4028–4040. doi: 10.1021/acsnano.8b08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores CT, Wildes TJ, Drake JA, Moore GL, Dean BD, Abraham RS, Mitchell DA. Lin-CCR2+ hematopoietic stem and progenitor cells overcome resistance to PD-1 blockade. Nature Communications. 2018;9:4313. doi: 10.1038/s41467-018-06182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M, Jain RK, Mitchell DA, Harrison JK. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. PNAS. 2020;117:1129–1138. doi: 10.1073/pnas.1910856117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, Pamer EG, Li MO. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–925. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature Reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- Gabriely G, da Cunha AP, Rezende RM, Kenyon B, Madi A, Vandeventer T, Skillin N, Rubino S, Garo L, Mazzola MA, Kolypetri P, Lanser AJ, Moreira T, Faria AMC, Lassmann H, Kuchroo V, Murugaiyan G, Weiner HL. Targeting latency-associated peptide promotes antitumor immunity. Science Immunology. 2017;2:eaaj1738. doi: 10.1126/sciimmunol.aaj1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galstyan A, Markman JL, Shatalova ES, Chiechi A, Korman AJ, Patil R, Klymyshyn D, Tourtellotte WG, Israel LL, Braubach O, Ljubimov VA, Mashouf LA, Ramesh A, Grodzinski ZB, Penichet ML, Black KL, Holler E, Sun T, Ding H, Ljubimov AV, Ljubimova JY. Blood-brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nature Communications. 2019;10:3850. doi: 10.1038/s41467-019-11719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon-Muvdi T, Theodros D, Luksik AS, Maxwell R, Kim E, Jackson CM, Belcaid Z, Ganguly S, Tyler B, Brem H, Pardoll DM, Lim M. Dendritic cell activation enhances anti-PD-1 mediated immunotherapy against glioblastoma. Oncotarget. 2018;9:20681–20697. doi: 10.18632/oncotarget.25061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, Platten M, Tabatabai G, Dutoit V, van der Burg SH, Thor Straten P, Martínez-Ricarte F, Ponsati B, Okada H, Lassen U, Admon A, Ottensmeier CH, Ulges A, Kreiter S, von Deimling A, Skardelly M, Migliorini D, Kroep JR, Idorn M, Rodon J, Piró J, Poulsen HS, Shraibman B, McCann K, Mendrzyk R, Löwer M, Stieglbauer M, Britten CM, Capper D, Welters MJP, Sahuquillo J, Kiesel K, Derhovanessian E, Rusch E, Bunse L, Song C, Heesch S, Wagner C, Kemmer-Brück A, Ludwig J, Castle JC, Schoor O, Tadmor AD, Green E, Fritsche J, Meyer M, Pawlowski N, Dorner S, Hoffgaard F, Rössler B, Maurer D, Weinschenk T, Reinhardt C, Huber C, Rammensee HG, Singh-Jasuja H, Sahin U, Dietrich PY, Wick W. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- Hutter G, Theruvath J, Graef CM, Zhang M, Schoen MK, Manz EM, Bennett ML, Olson A, Azad TD, Sinha R, Chan C, Assad Kahn S, Gholamin S, Wilson C, Grant G, He J, Weissman IL, Mitra SS, Cheshier SH. Microglia are effector cells of CD47-SIRPα antiphagocytic Axis disruption against glioblastoma. PNAS. 2019;116:997–1006. doi: 10.1073/pnas.1721434116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorgulescu JB, Reardon DA, Chiocca EA, Wu CJ. Immunotherapy for glioblastoma: going viral. Nature Medicine. 2018;24:1094–1096. doi: 10.1038/s41591-018-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JF, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJ, Wesseling P, Adema GJ. Prognostic significance and mechanism of treg infiltration in human brain tumors. Journal of Neuroimmunology. 2010;225:195–199. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Jordão MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai YH, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science. 2019;363:eaat7554. doi: 10.1126/science.aat7554. [DOI] [PubMed] [Google Scholar]
- Karachi A, Dastmalchi F, Mitchell DA, Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro-Oncology. 2018;20:1566–1572. doi: 10.1093/neuonc/noy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, Oliveira G, Giobbie-Hurder A, Felt K, Gjini E, Shukla SA, Hu Z, Li L, Le PM, Allesøe RL, Richman AR, Kowalczyk MS, Abdelrahman S, Geduldig JE, Charbonneau S, Pelton K, Iorgulescu JB, Elagina L, Zhang W, Olive O, McCluskey C, Olsen LR, Stevens J, Lane WJ, Salazar AM, Daley H, Wen PY, Chiocca EA, Harden M, Lennon NJ, Gabriel S, Getz G, Lander ES, Regev A, Ritz J, Neuberg D, Rodig SJ, Ligon KL, Suvà ML, Wucherpfennig KW, Hacohen N, Fritsch EF, Livak KJ, Ott PA, Wu CJ, Reardon DA. Neoantigen vaccine generates intratumoral T cell responses in phase ib glioblastoma trial. Nature. 2019;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of Vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. Journal of Clinical Investigation. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, Chheda ZS, Downey KM, Watchmaker PB, Beppler C, Warta R, Amankulor NA, Herold-Mende C, Costello JF, Okada H. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. Journal of Clinical Investigation. 2017;127:1425–1437. doi: 10.1172/JCI90644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sharife H, Kreisel T, Mogilevsky M, Bar-Lev L, Grunewald M, Aizenshtein E, Karni R, Paldor I, Shlomi T, Keshet E. Intra-Tumoral metabolic zonation and resultant phenotypic diversification are dictated by blood vessel proximity. Cell Metabolism. 2019;30:201–211. doi: 10.1016/j.cmet.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Lamano JB, Lamano JB, Li YD, DiDomenico JD, Choy W, Veliceasa D, Oyon DE, Fakurnejad S, Ampie L, Kesavabhotla K, Kaur R, Kaur G, Biyashev D, Unruh DJ, Horbinski CM, James CD, Parsa AT, Bloch O. Glioblastoma-Derived IL6 induces immunosuppressive peripheral myeloid cell PD-L1 and promotes tumor growth. Clinical Cancer Research. 2019;25:3643–3657. doi: 10.1158/1078-0432.CCR-18-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, Prabhu SS, Rao G, Fuller GN, Aldape KD, Gumin J, Vence LM, Wistuba I, Rodriguez-Canales J, Villalobos PA, Dirven CMF, Tejada S, Valle RD, Alonso MM, Ewald B, Peterkin JJ, Tufaro F, Fueyo J. Phase I study of DNX-2401 (Delta-24-RGD) Oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. Journal of Clinical Oncology. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoui D, Keirsse J, Morias Y, Van Overmeire E, Geeraerts X, Elkrim Y, Kiss M, Bolli E, Lahmar Q, Sichien D, Serneels J, Scott CL, Boon L, De Baetselier P, Mazzone M, Guilliams M, Van Ginderachter JA. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nature Communications. 2016;7:13720. doi: 10.1038/ncomms13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes & Development. 2015;29:1203–1217. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviron M, Boissonnas A. Ontogeny of Tumor-Associated macrophages. Frontiers in Immunology. 2019;10:1799. doi: 10.3389/fimmu.2019.01799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chang C, Rashidi A, Miska J, Zhang P, Pituch KC, Hou D, Xiao T, Fischietti M, Kang SJ, Appin CL, Horbinski C, Platanias LC, Lopez-Rosas A, Han Y, Balyasnikova IV, Lesniak MS. Myeloid-Derived suppressive cells promote B cell-Mediated immunosuppression via transfer of PD-L1 in glioblastoma. Cancer Immunology Research. 2019;7:1928–1943. doi: 10.1158/2326-6066.CIR-19-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SC, Alomran R, Chernikova SB, Lartey F, Stafford J, Jang T, Merchant M, Zboralski D, Zöllner S, Kruschinski A, Klussmann S, Recht L, Brown JM. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncology. 2014;16:21–28. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, Young A, O'Donnell JS, Allen S, Smyth MJ, Teng MW. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discovery. 2016;6:1382–1399. doi: 10.1158/2159-8290.CD-16-0577. [DOI] [PubMed] [Google Scholar]
- Loyher PL, Hamon P, Laviron M, Meghraoui-Kheddar A, Goncalves E, Deng Z, Torstensson S, Bercovici N, Baudesson de Chanville C, Combadière B, Geissmann F, Savina A, Combadière C, Boissonnas A. Macrophages of distinct origins contribute to tumor development in the lung. The Journal of Experimental Medicine. 2018;215:2536–2553. doi: 10.1084/jem.20180534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoto S, Hermelo I, Vuorinen EM, Hannus P, Kesseli J, Nykter M, Granberg KJ. Computational characterization of suppressive immune microenvironments in glioblastoma. Cancer Research. 2018;78:5574–5585. doi: 10.1158/0008-5472.CAN-17-3714. [DOI] [PubMed] [Google Scholar]
- Magnuson AM, Kiner E, Ergun A, Park JS, Asinovski N, Ortiz-Lopez A, Kilcoyne A, Paoluzzi-Tomada E, Weissleder R, Mathis D, Benoist C. Identification and validation of a tumor-infiltrating treg transcriptional signature conserved across species and tumor types. PNAS. 2018;115:E10672–E10681. doi: 10.1073/pnas.1810580115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao X, Chen Y, Hao K, Zheng M, Chen B, Li K, Wang Y, Zhang W, Zhang Y, Mou X, Jiang S, Wang Z. CD103 + Cell Growth Factor Flt3L Enhances the Efficacy of Immune Checkpoint Blockades in Murine Glioblastoma Model. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics. 2018;26:173–182. doi: 10.3727/096504017X14841698396865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska J, Rashidi A, Chang AL, Muroski ME, Han Y, Zhang L, Lesniak MS. Anti-GITR therapy promotes immunity against malignant glioma in a murine model. Cancer Immunology, Immunotherapy. 2016;65:1555–1567. doi: 10.1007/s00262-016-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska J, Lee-Chang C, Rashidi A, Muroski ME, Chang AL, Lopez-Rosas A, Zhang P, Panek WK, Cordero A, Han Y, Ahmed AU, Chandel NS, Lesniak MS. HIF-1α is a metabolic switch between Glycolytic-Driven migration and oxidative Phosphorylation-Driven immunosuppression of tregs in glioblastoma. Cell Reports. 2019;27:226–237. doi: 10.1016/j.celrep.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Laoui D, Gysemans C, Baeten M, Stangé G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Research. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- Movahedi K, Van Ginderachter JA. The ontogeny and microenvironmental regulation of Tumor-Associated macrophages. Antioxidants & Redox Signaling. 2016;25:775–791. doi: 10.1089/ars.2016.6704. [DOI] [PubMed] [Google Scholar]
- Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B. High-Dimensional Single-Cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity. 2018;48:599. doi: 10.1016/j.immuni.2018.02.014. [DOI] [PubMed] [Google Scholar]
- Müller S, Kohanbash G, Liu SJ, Alvarado B, Carrera D, Bhaduri A, Watchmaker PB, Yagnik G, Di Lullo E, Malatesta M, Amankulor NM, Kriegstein AR, Lim DA, Aghi M, Okada H, Diaz A. Single-cell profiling of human gliomas reveals macrophage ontogeny as a basis for regional differences in macrophage activation in the tumor microenvironment. Genome Biology. 2017;18:234. doi: 10.1186/s13059-017-1362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B. Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Science Immunology. 2019;4:eaau8380. doi: 10.1126/sciimmunol.aau8380. [DOI] [PubMed] [Google Scholar]
- Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, Dewitt J, Gritsch S, Perez EM, Gonzalez Castro LN, Lan X, Druck N, Rodman C, Dionne D, Kaplan A, Bertalan MS, Small J, Pelton K, Becker S, Bonal D, Nguyen QD, Servis RL, Fung JM, Mylvaganam R, Mayr L, Gojo J, Haberler C, Geyeregger R, Czech T, Slavc I, Nahed BV, Curry WT, Carter BS, Wakimoto H, Brastianos PK, Batchelor TT, Stemmer-Rachamimov A, Martinez-Lage M, Frosch MP, Stamenkovic I, Riggi N, Rheinbay E, Monje M, Rozenblatt-Rosen O, Cahill DP, Patel AP, Hunter T, Verma IM, Ligon KL, Louis DN, Regev A, Bernstein BE, Tirosh I, Suvà ML. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell. 2019;178:835–849. doi: 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JS, Hoefsmit EP, Smyth MJ, Blank CU, Teng MWL. The promise of neoadjuvant immunotherapy and surgery for Cancer treatment. Clinical Cancer Research. 2019;25:5743–5751. doi: 10.1158/1078-0432.CCR-18-2641. [DOI] [PubMed] [Google Scholar]
- Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-γ drives Tregfragility to promote Anti-tumor immunity. Cell. 2017;169:1130–1141. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim CG, Shim JK, Kim JH, Lee H, Lee JE, Kim MH, Haam K, Jung I, Park SH, Chang JH, Shin EC, Kang SG. Effect of combined anti-PD-1 and temozolomide therapy in glioblastoma. OncoImmunology. 2019;8:e1525243. doi: 10.1080/2162402X.2018.1525243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suvà ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344:1396–1401. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MA, Kim JE, Theodros D, Tam A, Velarde E, Kochel CM, Francica B, Nirschl TR, Ghasemzadeh A, Mathios D, Harris-Bookman S, Jackson CC, Jackson C, Ye X, Tran PT, Tyler B, Coric V, Selby M, Brem H, Drake CG, Pardoll DM, Lim M. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. Journal for ImmunoTherapy of Cancer. 2016;4:28. doi: 10.1186/s40425-016-0132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CT, Krenciute G. Next generation CAR T cells for the immunotherapy of High-Grade glioma. Frontiers in Oncology. 2019;9:69. doi: 10.3389/fonc.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T cells exhibit distinct features in human breast Cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CC, Gordon PMK, Liu K, Yang R, Sarkar S, Mirzaei R, Ahmad ST, Hughes ML, Yong VW, Kelly JJP. Differential microglia and macrophage profiles in human IDH-mutant and -wild type glioblastoma. Oncotarget. 2019;10:3129–3143. doi: 10.18632/oncotarget.26863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine. 2013;19:1264–1272. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, Holland EC, Sutton JC, Joyce JA. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science. 2016;352:aad3018. doi: 10.1126/science.aad3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani KR, Carlstrom LP, Parney IF, Johnson AJ, Warrington AE, Burns TC. Harnessing radiation biology to augment immunotherapy for glioblastoma. Frontiers in Oncology. 2018;8:656. doi: 10.3389/fonc.2018.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri B, Rayman P, Huang P, Grabowski M, Hambardzumyan D, Finke JH, Vogelbaum MA. Myeloid derived suppressor cell infiltration of murine and human gliomas is associated with reduction of tumor infiltrating lymphocytes. Journal of Neuro-Oncology. 2015;122:293–301. doi: 10.1007/s11060-015-1720-6. [DOI] [PubMed] [Google Scholar]
- Roberts EW, Broz ML, Binnewies M, Headley MB, Nelson AE, Wolf DM, Kaisho T, Bogunovic D, Bhardwaj N, Krummel MF. Critical role for CD103(+)/CD141(+) Dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell. 2016;30:324–336. doi: 10.1016/j.ccell.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Pistillo MP, Carosio R, Morabito A, Banelli B. Immune checkpoints and innovative therapies in glioblastoma. Frontiers in Oncology. 2018;8:464. doi: 10.3389/fonc.2018.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van de Wiel BA, Scolyer RA, Krijgsman O, Sikorska K, Eriksson H, Broeks A, van Thienen JV, Guminski AD, Acosta AT, Ter Meulen S, Koenen AM, Bosch LJW, Shannon K, Pronk LM, Gonzalez M, Ch'ng S, Grijpink-Ongering LG, Stretch J, Heijmink S, van Tinteren H, Haanen J, Nieweg OE, Klop WMC, Zuur CL, Saw RPM, van Houdt WJ, Peeper DS, Spillane AJ, Hansson J, Schumacher TN, Long GV, Blank CU. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. The Lancet Oncology. 2019;20:948–960. doi: 10.1016/S1470-2045(19)30151-2. [DOI] [PubMed] [Google Scholar]
- Rutledge WC, Kong J, Gao J, Gutman DA, Cooper LA, Appin C, Park Y, Scarpace L, Mikkelsen T, Cohen ML, Aldape KD, McLendon RE, Lehman NL, Miller CR, Schniederjan MJ, Brennan CW, Saltz JH, Moreno CS, Brat DJ. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clinical Cancer Research. 2013;19:4951–4960. doi: 10.1158/1078-0432.CCR-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Säälik P, Lingasamy P, Toome K, Mastandrea I, Rousso-Noori L, Tobi A, Simón-Gracia L, Hunt H, Paiste P, Kotamraju VR, Bergers G, Asser T, Rätsep T, Ruoslahti E, Bjerkvig R, Friedmann-Morvinski D, Teesalu T. Peptide-guided nanoparticles for glioblastoma targeting. Journal of Controlled Release. 2019;308:109–118. doi: 10.1016/j.jconrel.2019.06.018. [DOI] [PubMed] [Google Scholar]
- Sampson JH, Aldape KD, Archer GE, Coan A, Desjardins A, Friedman AH, Friedman HS, Gilbert MR, Herndon JE, McLendon RE, Mitchell DA, Reardon DA, Sawaya R, Schmittling R, Shi W, Vredenburgh JJ, Bigner DD, Heimberger AB. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro-Oncology. 2011;13:324–333. doi: 10.1093/neuonc/noq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Perez LA, Choi BD, Archer GE, Cui X, Flores C, Johnson LA, Schmittling RJ, Snyder D, Herndon JE, Bigner DD, Mitchell DA, Sampson JH. Myeloablative temozolomide enhances CD8⁺ T-cell responses to vaccine and is required for efficacy against brain tumors in mice. PLOS ONE. 2013;8:e59082. doi: 10.1371/journal.pone.0059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, López-Janeiro A, Porciuncula A, Idoate MA, Inogés S, de Andrea C, López-Diaz de Cerio A, Tejada S, Berraondo P, Villarroel-Espindola F, Choi J, Gúrpide A, Giraldez M, Goicoechea I, Gallego Perez-Larraya J, Sanmamed MF, Perez-Gracia JL, Melero I. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nature Medicine. 2019;25:470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- Simonelli M, Persico P, Perrino M, Zucali PA, Navarria P, Pessina F, Scorsetti M, Bello L, Santoro A. Checkpoint inhibitors as treatment for malignant gliomas: "A long way to the top". Cancer Treatment Reviews. 2018;69:121–131. doi: 10.1016/j.ctrv.2018.06.016. [DOI] [PubMed] [Google Scholar]
- Sottoriva A, Spiteri I, Piccirillo SG, Touloumis A, Collins VP, Marioni JC, Curtis C, Watts C, Tavaré S. Intratumor heterogeneity in human glioblastoma reflects Cancer evolutionary dynamics. PNAS. 2013;110:4009–4014. doi: 10.1073/pnas.1219747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Jackson C, Kim T, Choi J, Lim M. A characterization of dendritic cells and their role in immunotherapy in glioblastoma: from preclinical studies to clinical trials. Cancers. 2019;11:537. doi: 10.3390/cancers11040537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvà ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV, Curry WT, Martuza RL, Rivera MN, Rossetti N, Kasif S, Beik S, Kadri S, Tirosh I, Wortman I, Shalek AK, Rozenblatt-Rosen O, Regev A, Louis DN, Bernstein BE. Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell. 2014;157:580–594. doi: 10.1016/j.cell.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai G, Frank B, Möhle R, Weller M, Wick W. Irradiation and hypoxia promote homing of haematopoietic progenitor cells towards gliomas by TGF-beta-dependent HIF-1alpha-mediated induction of CXCL12. Brain. 2006;129:2426–2435. doi: 10.1093/brain/awl173. [DOI] [PubMed] [Google Scholar]
- Takenaka MC, Gabriely G, Rothhammer V, Mascanfroni ID, Wheeler MA, Chao CC, Gutiérrez-Vázquez C, Kenison J, Tjon EC, Barroso A, Vandeventer T, de Lima KA, Rothweiler S, Mayo L, Ghannam S, Zandee S, Healy L, Sherr D, Farez MF, Prat A, Antel J, Reardon DA, Zhang H, Robson SC, Getz G, Weiner HL, Quintana FJ. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nature Neuroscience. 2019;22:729–740. doi: 10.1038/s41593-019-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AA, Fisher JL, Rahme GJ, Hampton TH, Baron U, Olek S, Schwachula T, Rhodes CH, Gui J, Tafe LJ, Tsongalis GJ, Lefferts JA, Wishart H, Kleen J, Miller M, Whipple CA, de Abreu FB, Ernstoff MS, Fadul CE. Regulatory T cells are not a strong predictor of survival for patients with glioblastoma. Neuro-Oncology. 2015;17:801–809. doi: 10.1093/neuonc/nou363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RP, Nagpal S, Iv M, Soltys SG, Bertrand S, Pelpola JS, Ball R, Yang J, Sundaram V, Lavezo J, Born D, Vogel H, Brown JM, Recht LD. Macrophage Exclusion after Radiation Therapy (MERT): A First in Human Phase I/II Trial using a CXCR4 Inhibitor in Glioblastoma. Clinical Cancer Research. 2019;25:6948–6957. doi: 10.1158/1078-0432.CCR-19-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. British Journal of Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, Aerts J, Berx G, Boeckxstaens GE, Vandenbroucke RE, Vereecke L, Moechars D, Guilliams M, Van Ginderachter JA, Saeys Y, Movahedi K. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nature Neuroscience. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN, Cancer Genome Atlas Research Network Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Sengupta S, Han Y, Lesniak MS. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro-Oncology. 2011;13:1308–1323. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon K-S, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clinical Cancer Research. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MJ, Ebsworth K, Berahovich RD, Penfold ME, Liu SC, Al Omran R, Kioi M, Chernikova SB, Tseng D, Mulkearns-Hubert EE, Sinyuk M, Ransohoff RM, Lathia JD, Karamchandani J, Kohrt HE, Zhang P, Powers JP, Jaen JC, Schall TJ, Merchant M, Recht L, Brown JM. Inhibition of CXCR7 extends survival following irradiation of brain tumours in mice and rats. British Journal of Cancer. 2014;110:1179–1188. doi: 10.1038/bjc.2013.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, Barthel F, Cho HJ, Lin YH, Satani N, Martinez-Ledesma E, Zheng S, Chang E, Sauvé CG, Olar A, Lan ZD, Finocchiaro G, Phillips JJ, Berger MS, Gabrusiewicz KR, Wang G, Eskilsson E, Hu J, Mikkelsen T, DePinho RA, Muller F, Heimberger AB, Sulman EP, Nam DH, Verhaak RGW. Tumor evolution of Glioma-Intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Marisetty A, Schrand B, Gabrusiewicz K, Hashimoto Y, Ott M, Grami Z, Kong LY, Ling X, Caruso H, Zhou S, Wang YA, Fuller GN, Huse J, Gilboa E, Kang N, Huang X, Verhaak R, Li S, Heimberger AB. Osteopontin mediates glioblastoma-associated macrophage infiltration and is a potential therapeutic target. Journal of Clinical Investigation. 2019;129:137–149. doi: 10.1172/JCI121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. British Journal of Cancer. 2015;113:1642. doi: 10.1038/bjc.2015.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clinical Cancer Research. 2018a;24:3792–3802. doi: 10.1158/1078-0432.CCR-18-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woroniecka K, Chongsathidkiet P, Rhodin K, Kemeny H, Dechant C, Farber SH, Elsamadicy AA, Cui X, Koyama S, Jackson C, Hansen LJ, Johanns TM, Sanchez-Perez L, Chandramohan V, Yu YA, Bigner DD, Giles A, Healy P, Dranoff G, Weinhold KJ, Dunn GP, Fecci PE. T-Cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clinical Cancer Research. 2018b;24:4175–4186. doi: 10.1158/1078-0432.CCR-17-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Maxwell R, Xia Y, Cardarelli P, Oyasu M, Belcaid Z, Kim E, Hung A, Luksik AS, Garzon-Muvdi T, Jackson CM, Mathios D, Theodros D, Cogswell J, Brem H, Pardoll DM, Lim M. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. Journal of Neuro-Oncology. 2019;143:241–249. doi: 10.1007/s11060-019-03172-5. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhao Q, Gabrusiewicz K, Kong LY, Xia X, Wang J, Ott M, Xu J, Davis RE, Huo L, Rao G, Sun SC, Watowich SS, Heimberger AB, Li S. FGL2 promotes tumor progression in the CNS by suppressing CD103+ dendritic cell differentiation. Nature Communications. 2019;10:448. doi: 10.1038/s41467-018-08271-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Zhang X, Ye HX, Wang Y, Du ZG, Yao Y, Mao Y. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. Journal of Neuro-Oncology. 2014;116:251–259. doi: 10.1007/s11060-013-1314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Miska J, Lee-Chang C, Rashidi A, Panek WK, An S, Zannikou M, Lopez-Rosas A, Han Y, Xiao T, Pituch KC, Kanojia D, Balyasnikova IV, Lesniak MS. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. PNAS. 2019;116:23714–23723. doi: 10.1073/pnas.1906346116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Chen AX, Gartrell RD, Silverman AM, Aparicio L, Chu T, Bordbar D, Shan D, Samanamud J, Mahajan A, Filip I, Orenbuch R, Goetz M, Yamaguchi JT, Cloney M, Horbinski C, Lukas RV, Raizer J, Rae AI, Yuan J, Canoll P, Bruce JN, Saenger YM, Sims P, Iwamoto FM, Sonabend AM, Rabadan R. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nature Medicine. 2019;25:462–469. doi: 10.1038/s41591-019-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, Liu Z, Dong M, Hu X, Ouyang W, Peng J, Zhang Z. Landscape of infiltrating T cells in liver Cancer revealed by Single-Cell sequencing. Cell. 2017;169:1342–1356. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, Krishnan I, Maroni G, Meyerovitz CV, Kerwin CM, Choi S, Richards WG, De Rienzo A, Tenen DG, Bueno R, Levantini E, Pittet MJ, Klein AM. Single-Cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019;50:1317–1334. doi: 10.1016/j.immuni.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]