Abstract
A wide array of chemokine receptors, including CCR2, are known to control Treg migration. Here, we report that CCR2 regulates Tregs beyond chemotaxis. We found that CCR2 deficiency reduced CD25 expression by FoxP3+ Treg cells. Such a change was also consistently present in irradiation chimeras reconstituted with mixed bone marrow from wild-type (WT) and CCR2−/− strains. Thus, CCR2 deficiency resulted in profound loss of CD25hi FoxP3+ Tregs in secondary lymphoid organs as well as in peripheral tissues. CCR2−/− Treg cells were also functionally inferior to WT cells. Interestingly, these changes to Treg cells did not depend on CCR2+ monocytes/moDCs (the cells where CCR2 receptors are most abundant). Rather, we demonstrated that CCR2 was required for TLR-stimulated, but not TCR- or IL-2-stimulated, CD25 upregulation on Treg cells. Thus, we propose that CCR2 signaling can increase the fitness of FoxP3+ Treg cells and provide negative feedback to counter the proinflammatory effects of CCR2 on myeloid cells.
Subject terms: Regulatory T cells, Chemokines
Introduction
CCR2 is a chemokine receptor known for its role in monocyte chemotaxis. It is most abundant on proinflammatory monocytes and monocyte-derived dendritic cells (moDCs).1–4 Not surprisingly, without CCR2 (or with the loss of CCR2-expressing monocytes), immunity to many bacterial and fungal pathogens is reduced.1–3 Similarly, without CCR2, the severity of experimental autoimmune diseases (including induction of pathogenic Th17 responses) is reduced.4,5 In contrast, CCR2 deficiency has also been found to exacerbate experimental autoimmune arthritis, particularly with regards to its progression.6,7 A plausible explanation for the opposing roles of CCR2 in immune regulation is that CCR2 may also be essential for negative regulatory mechanisms such as those involving T regulatory cells (Tregs), which are key to maintaining immune tolerance.8,9 These opposing roles may explain the disappointing results of the treatment of rheumatoid arthritis and multiple sclerosis with CCR2 antagonists.10
Although less abundant than on monocytes/moDCs, CCR2 is also expressed on Tregs and some activated/memory T cells.11–13 Compared to conventional T cells, Tregs, particularly adipose tissue Tregs, express more CCR2 mRNA.14 Analysis of cell surface expression also indicates that a higher proportion of Tregs, particularly CD62L−FoxP3+ T cells in lymph nodes, express CCR2 than conventional T cells.11 Cell transfer studies have demonstrated that Tregs require CCR2 to migrate to inflamed tissues as well as to migrate from tissues to draining lymph nodes.9 Apart from its well-known roles in chemotaxis, CCR2 is also involved in direct cell signaling.15 When CD4+ T cells are activated by a cognate antigen or anti-CD3, addition of CCL2 increases IL-4 production by T cells. Beyond chemotaxis, no other role has been ascribed to CCR2 in Tregs. Notably, CCR2-deficient Tregs locally transferred to tissue sites are less capable of suppressing alloimmunity than WT Tregs, despite the fact that both are present at graft sites in similar numbers.9 This finding suggests that CCR2 deficiency might impact Tregs independently of cell trafficking.
Treg homeostasis is subject to regulation by myeloid cells, including dendritic cells (DCs). For example, moDCs, but not conventional DCs, have been shown to expand Tregs in vitro and in vivo.16 Indeed, GM-CSF-differentiated DCs, which are akin to moDCs,17 can produce IL-2 to regulate the size of the Treg niche.18 Given that moDCs express high levels of CCR2, it is possible that CCR2 deficiency or antagonism may affect Treg homeostasis and hamper Treg-mediated immune regulation via moDCs.
Although CCR2 clearly participates in Treg homing,9 additional roles for CCR2, either direct or indirect, in the regulation of Treg homeostasis are less clear. Here, we examined how CCR2 might influence Tregs beyond cell homing. In a CCR2-DTR mouse model in which the primate diphtheria toxin receptor is expressed under the control of the CCR2 promoter,2 we found that deletion of CCR2+ monocytes/moDCs did not reduce Treg abundance. Comparisons between CCR2−/− mice and WT mice revealed a selective and profound reduction in CD25hiFoxP3+ Tregs in CCR2−/− mice, while the number of CD25loFoxP3+ Tregs in lymphoid organs was only mildly affected. The reduction in CCR2−/− Tregs was more pronounced in CCR2−/−:WT mixed bone marrow chimeras (which would equalize any impact by CCR2+ myeloid cells). Moreover, the remaining Tregs (largely CD25lo) from CCR2−/− mice were less suppressive in vitro than WT Tregs. In vitro exploration revealed that the effect of CCR2 was mediated through direct reductions in CD25 expression in Tregs in response to TLR stimulation. Thus, we propose that in addition to its chemotactic function, CCR2 plays a signaling role in regulating the fitness of FoxP3+ Tregs.
Materials and methods
Mice
All mice had a C57BL/6 background. Wild-type (WT), CCR2.DTR/CFP mice2 (expressing the primate diphtheria toxin receptor and cyan fluorescent protein under the control of the CCR2 promoter), and B6.FoxP3-GFP mice8 (expressing green fluorescent protein under the control of the FoxP3 promoter) were bred under specific pathogen-free conditions in the animal facility of WEHI. CCR2−/− mice19 were obtained from Jackson Laboratory and maintained in the animal facility of the Peter Doherty Institute together with matched WT mice. Experiments were performed according to the guidelines of the Institute’s Animal Ethics Committee (Ethics numbers 2011.015 and 2013.015).
Flow cytometry and antibodies
Single-cell suspensions were stained with antibodies against CD3 (KT3), CD4 (RM4-5), CD8 (53.67), FoxP3 (FJK-16s) (eBioscience, San Diego, CA), CCR2 (Clone # 475301) (R&D Systems, Minneapolis, MN), Gr-1 (RB6-8CS), CD45.1 (A20) and CD45.2 (104), CD11c (HL3), Siglec H (eBio440c) (eBioscience), CD11b (M1/70), MHC class II I-A/I-E (M5/114.15.2), Ly6C (AL-21), Ly6G (IA8), and CD19 (ID3). All Abs other than those designated otherwise were from BD Biosciences (San Jose, CA). Intracellular FoxP3 protein was detected using a staining kit from eBioscience. Analysis was performed on a BD LSRFortessa and FACSverse, and sorting was performed on BD FACSAria and BD Influx. The data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Mixed bone marrow chimera
Recipient mice were irradiated with two doses of 550 rad. The irradiated mice were then injected with 2×106 cells from a 1:1 mixture of bone marrow cells from WT (Ly5.1) and CCR2−/− (Ly5.2) mice. The irradiated mice were also given i.p. injection of anti-Thy-1 Ab (T24) to deplete irradiation-resistant host T cells. Immune reconstitution was verified 8 weeks later.
Diphtheria toxin (DT) treatment
To deplete CCR2+ cells, CCR2.DTR mice and mixed BM chimeras were given 20 ng/g body weight DT (Sigma).4 The mice were treated four times (CCR2.DTR) over 1 week. The organs were harvested 1 day after the last dose for analysis.
In vitro suppression by Tregs
GITR+CD4+ cells were purified by flow sorting from the spleens of WT and CCR2−/− mice. The cells were labeled with Cell Trace Violet (CTV; Thermo Fisher Scientific). For Treg cell functional assays, 5×104 CTV-labeled CD44-CD4+ T cells, used as responder cells, were cultured with or without 5×104 GITR+CD4+ cells as Tregs in a V-bottom 96-well plate. The cells were stimulated for 96 h with 40,000 anti-CD3/CD28 beads/well (Dynabeads, Thermo Fisher Scientific). Proliferation of effector cells was measured by flow cytometric analysis of CTV dilution. PE-conjugated calibration beads (BD Biosciences) were included to determine the total cell numbers. A division index (DI) was derived using FlowJo software. Suppression by Tregs was calculated as 100 − [(DI with Treg/DI with responders only) × 100].
In vitro stimulation with CpG, IL-2 and anti-CD3/anti-CD28
Spleen cells (2 × 105/well) were cultured in 96-well round-bottom plates with or without graded doses of CpG (ODN 1826; Sigma Genosys, Castle Hill, NSW, Australia), IL-2 (100 U/ml) or 5 μg/ml soluble anti-CD3 Ab (clone 145-2C11) plus 2 μg/ml anti-CD28 Ab (clone 37.58) for 20 or 44 h. A CCR2 inhibitor (RS102895, Sigma, St Louis, MO) was included in some cultures. For cells from FoxP3-reporter mice, the cells were harvested and stained for surface markers. For cells from WT and CCR2−/− mice, the harvested cells were stained for surface markers and then stained intracellularly for FoxP3. In some cell cultures, the cells were preincubated with various concentrations of anti-CCR2 Abs or control rat IgG2b and were then incubated with anti-rat Ig. The cells were cultured and then stimulated with CpG for 20 h before analysis.
Preparation of lymphocytes from tissue
Lungs and livers were collected from matched WT and CCR2−/− mice. The lung tissues were finely cut and suspended in 0.025% collagenase type IV (Thermo Fisher, Waltham, MA). The suspension was incubated for 90 min at 37 °C in a shaker, then diluted tenfold with phosphate-buffered saline containing 2% fetal calf serum and spun at 800 × g for 15 min at 4 °C. The livers were minced and passed through cell strainers. The cell pellets were further purified to obtain lymphocytes through Histopaque (Sigma) density separation.
Statistical analysis
Mean and SEM values were calculated and two-tailed unpaired t tests were performed with GraphPad Prism software. In some circumstances, such as when cells of two genotypes from the same mouse were analyzed, paired t tests were used. The data presented are from a single study of 3–4 repeated experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001; n.s. = not statistically significant.
Results
CD25hiFoxP3+ T cells are selectively reduced in CCR2−/− mice
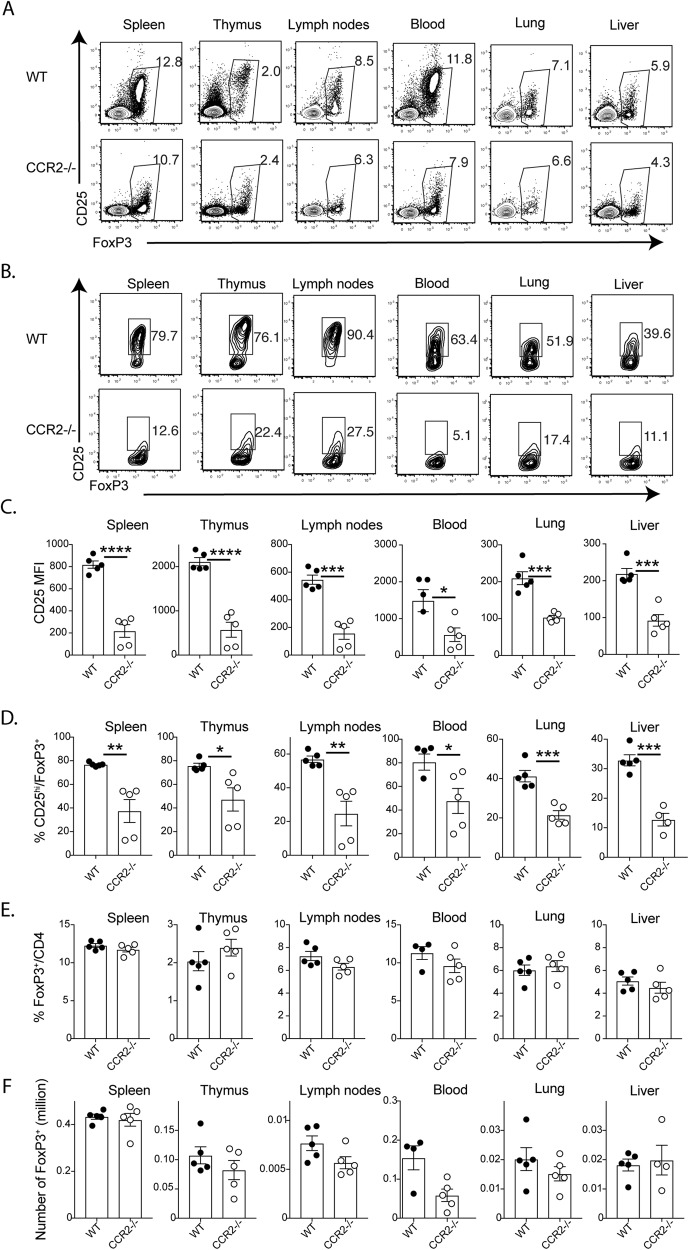
CCR2 has previously been shown to be required for Treg cell migration into inflamed tissues but not into secondary lymphoid organs.9 When we examined the Tregs in CCR2−/− mice, we found that FoxP3+ cells in the spleen, thymus, lymph nodes, blood, lungs and liver expressed significantly lower levels of CD25 than WT cells, resulting in a significant reduction in CD25hiFoxP3+ CD4+ cells (Fig. 1a−d). On the other hand, there were no significant differences in total FoxP3+ CD4+ cells among the different tissues (Fig. 1e, f). Furthermore, the total number of recovered spleen cells was not significantly different between WT and CCR2−/− mice (20.7 ± 1.0 × 106 in WT mice (n = 5) vs. 21.9 ± 1.0 × 106 in CCR2−/− mice (n = 5)). Together, these data suggest that CCR2 plays a role in Treg homeostasis that may extend beyond its previously demonstrated function in cell homing.9
Fig. 1.
CCR2 deficiency leads to reduced CD25 expression and loss of CD25+ FoxP3+ Tregs. Tissues were harvested from WT and CCR2−/− mice (five each; 9-week-old male mice) for analysis. a Representative plots showing the expression of FoxP3 and CD25 on gated CD4+ T cells from different tissues. b Representative plots showing the expression of FoxP3 and CD25 on gated FoxP3+CD4+ T cells from different tissues. c Bar graphs showing the levels of CD25 expression (as the mean fluorescence index (MFI)) by gated FoxP3+ T cells. d Bar graphs showing the percentages of cells with high CD25 expression among FoxP3+ T cells. e, f. Bar graphs showing the percentages and numbers of FoxP3+ cells. The data shown are the mean ± SEM of four individual animals. All data were analyzed using two-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P<0.0001. Five repeated experiments showed similar results
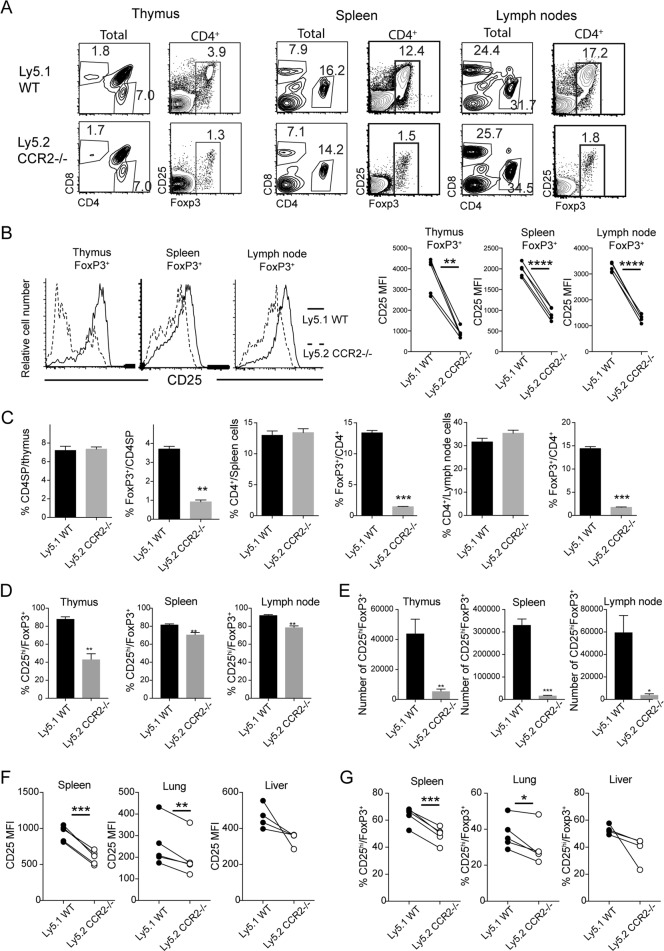
As expected, CCR2 deficiency caused a profound reduction in Ly6C+ monocyte numbers in the blood and spleen but not in the bone marrow, while CCR2 deficiency had little effect on granulocyte numbers (Supplemental F1). As Treg numbers have previously been shown to be affected by DCs,16,18 we wondered whether the reduction in Tregs was secondary to a reduction in monocytes/moDCs. One way to investigate this possibility and to determine whether the effect of CCR2 on Tregs was cell intrinsic was to generate mixed irradiation chimeras using both WT (Ly5.1) and CCR2−/− (Ly5.2) bone marrow. Despite the presence of WT Tregs and monocytic cells, defects in CD25hiCD4+ T cells as well as Ly6C+CD11b+ monocytic cells among CCR2−/−-derived cells were observed (Supplemental F2A and B). Unlike in intact CCR2−/− mice, the reduction in FoxP3+ was not limited to the CD25hi cohort in the mixed chimeras, as we observed a five- to tenfold reduction in the total number of FoxP3+CD4+ cells in secondary lymphoid organs such as the spleen and lymph nodes (Fig. 2a, c). Compared to that in cells from intact CCR2−/− mice, the reduction in CD25 expression by the CCR2−/− FoxP3+ cells from mixed chimeras was less marked but was nevertheless consistent and significant (Fig. 2b). Consequently, the proportion and number of CCR2−/− FoxP3+ cells with high expression of CD25 were significantly lower than those of WT FoxP3+ cells (Fig. 2d). Reduced expression of CD25 by FoxP3+ cells was also observed in peripheral tissue from the lung and liver (Fig. 2f). Accordingly, the proportion of FoxP3+ cells with high expression of CD25 was reduced among CCR2−/− T cells (Fig. 2g). Together, these data suggest that CCR2 has a cell-intrinsic role in regulating Tregs, particularly in regulating the expression of CD25.
Fig. 2.
The defect in CCR2−/− Tregs is cell-intrinsic. Lymphoid organs were harvested from five irradiation chimeric mice (Ly5.1/Ly5.2) reconstituted with mixed bone marrow from Ly5.1 WT and Ly5.2 CCR2−/− mice. Single-cell suspensions were analyzed for cell composition. a Representative plots showing CD4+ T cells and CD8+ T cells. The gated CD4+ T cells show expression of FoxP3 and CD25. b Representative histograms showing CD25 expression by gated FoxP3+ T cells (left). The levels of CD25 expression by FoxP3+ T cells are shown (right). **P < 0.01 and ****P < 0.0001 by paired Student’s t tests. c−e The bars show the percentages and numbers of the indicated T-cell populations. *P < 0.05, **P < 0.01, and ***P < 0.0001 by paired Student’s t tests. f, g CD25 expression by tissue Tregs was examined in five chimeric mice. The scatter plots in (f) show the levels of CD25 expression (as the mean fluorescence index (MFI)) by gated FoxP3+ T cells. The scatter plots in (g) show the percentage of cells with high CD25 expression among FoxP3+ T cells. *P < 0.05, **P < 0.01, and ***P < 0.001 by paired Student’s t tests. Five independent experiments were performed in mixed chimeric mice with similar results
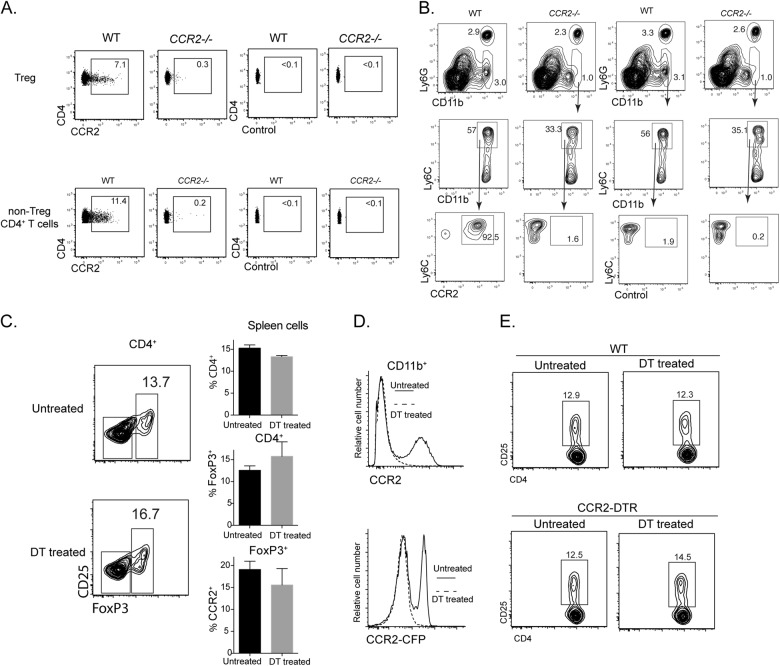
Deletion of CCR2+ Mo/moDCs has minimal impact on Treg abundance
The cell-intrinsic role of CCR2 in regulating Treg abundance implies that Tregs express CCR2. When spleen cells from WT and CCR2−/− mice were evaluated for the expression of CCR2, we found that both conventional CD4 T cells and Tregs from WT mice contained a fraction with appreciable expression of CCR2 (Fig. 3a). Nevertheless, T cells expressed much lower levels of CCR2 than spleen Ly6C+CD11b+ monocytes from WT mice (Fig. 3b). To further clarify the influence of CCR2-expressing monocytic cells (monocytes and moDCs), we employed the CCR2-DTR mouse model, in which the primate diphtheria toxin receptor is transgenically expressed under the CCR2 promoter.2 Previously, we found that Ly6C+ monocytes and moDCs could be readily depleted with DT treatment, while T cells, even of the activated phenotype, were minimally affected, likely due to their lower levels of DTR expression.4 When CCR2-DTR mice were given repeated doses of DT over 7 days, there were no overt changes in CD4+ T cells, including FoxP3+ T cells, in treated mice (Fig. 3c), while the treatment efficiently deleted CCR2+CD11b+ spleen cells (Fig. 3d). Furthermore, in mixed bone marrow chimeras containing WT and CCR2-DTR cells, CD25+CD4+ T cells were also not affected by DT treatment (Fig. 3e). As deficiency in CCR2+ myeloid cells did not impact Treg frequency and CD25 expression, we contend that CCR2 primarily affects Treg abundance independently of CCR2-expressing monocytic cells.
Fig. 3.
Deletion of CCR2 monocytes/moDCs has little impact on Tregs. a, b CCR2 expression by T cells and Ly6C+CD11b+ monocytes. Spleen cells from WT mice and CCR2−/− mice were analyzed for CCR2 expression with an anti-CCR2 Ab and an isotype control. a Plots showing CCR2 expression by Tregs and non-Treg CD4+ T cells from WT mice and CCR2−/− mice. b Plots showing CCR2 expression by gated Ly6C+CD11b+ monocytes. c−e CCR2-DTR mice were either untreated or treated with 200 ng DT/dose every second day over 7 days. Spleens were harvested 1 day after the last dose. Spleen cells were analyzed for T cells and CD11b+ cells. c The contour plots show the distribution of FoxP3 in gated spleen CD4+ T cells with or without DT treatment. The bar graphs show the percentages of the indicated T-cell populations; >4 similar experiments were performed with similar results. d Histograms showing the expression of surface CCR2 (by Ab) and CCR2 reporter (cyan fluorescent protein, CFP) by CD11b+ spleen cells from DT-treated and untreated mice. DT-treated mice (dotted lines) lost CCR2+CD11b+ cells. e Mixed bone marrow chimeras of WT and CCR2-DTR donors were either untreated or treated with 200 ng DT/dose every second day over 7 days. Spleen cells were analyzed for CD25 expression
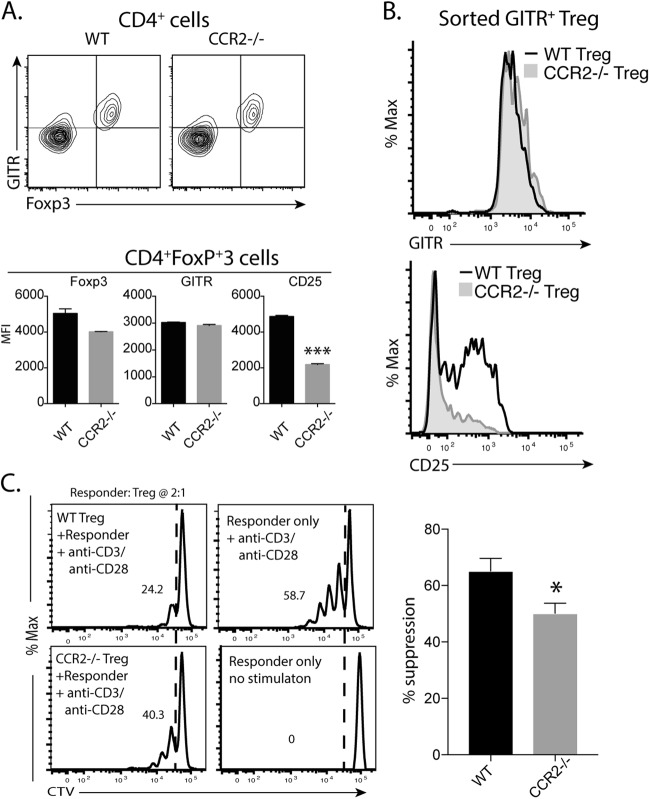
CCR2−/− Tregs have a reduced capacity to inhibit T-cell proliferation
CCR2−/− mice retained a cohort of Tregs with lower CD25 expression. We previously reported that CD25loFoxP3+ T cells had reduced regulatory capacity.20 Thus, we attempted to compare the function of Tregs from WT and CCR2−/– mice. For functional analysis, we used the expression of GITR as a surrogate marker for sorting Tregs. The expression of GITR was closely related to the expression of FoxP3 in both WT and CCR2−/− mice (Fig. 4a). GITR levels on FoxP3+ cells were comparable in WT and CCR2−/− mice, while expression of CD25 was lower in CCR2−/− Tregs than in WT Tregs (Fig. 4a). To determine the ability of the Tregs to inhibit T-cell proliferation, CD4+GITR+ T cells were purified from the spleens of WT and CCR2−/− mice. The cells had similar levels of GITR expression but differed in their CD25 levels (Fig. 4b). When Tregs from both sources were cocultured with naïve CD4+ T cells that were labeled with CTV and stimulated with anti-CD3/CD28 beads, CCR2−/− Tregs were less inhibitory than WT Tregs (Fig. 4c), although both types of Tregs caused the proliferation of responder cells to be lower than that in cultures with responder cells alone.
Fig. 4.
CCR2−/− Tregs are less suppressive in vitro. a The FACS plots shows the coexpression of GITR and FoxP3 by Tregs from WT and CCR2−/− mice. The bars show the mean fluorescence index (MFI) of the indicated molecules on Tregs from WT and CCR2−/− mice. ***P < 0.001 by t test. B&C. GITR+CD4+ Tregs were isolated from WT and CCR2−/− mice. The histograms show the levels of GITR and CD25 expression by Tregs from WT and CCR2−/− mice (b). For the functional assay (c), graded numbers of Tregs were cultured with 5×104 CTV-labeled naïve CD4+ T cells (responders) for 5 days under stimulation with beads coated with anti-CD3 and anti-CD28 Abs. The histograms show the proliferation profiles of responders with or without Tregs (ratio of responders to Tregs: 2:1). The bar graph shows the % of suppression (mean ± SEM) from three pooled experiments. The data were analyzed using two-tailed Student’s t tests. *P<0.05
CCR2 inhibition reduces CpG-induced CD25 expression on FoxP3+ cells
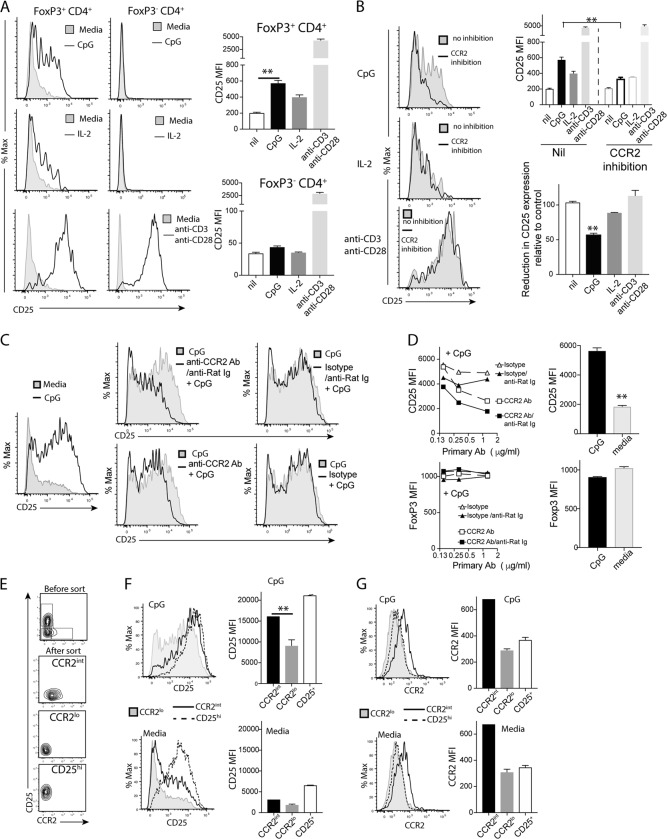
Our findings above suggest that CCR2 may regulate Tregs independently of its chemotactic role, particularly under inflammatory conditions. To mimic inflammation, we stimulated spleen cells from B6.FoxP3-GFP mice8 with CpG. As a control, we also stimulated spleen cells with IL-2 or anti-CD3/anti-CD28 Abs. Using spleen cells from FoxP3-GFP reporter mice, we found that CD25 expression on FoxP3+ T cells was upregulated with TLR-9 agonism (CpG stimulation), but the expression of FoxP3 was not changed (Fig. 5a). As predicted, stimulation with IL-2 or anti-CD3/anti-CD28 Abs also upregulated CD25 expression on FoxP3+ T cells (Fig. 5a). While stimulation with anti-CD3/anti-CD28 upregulated CD25 expression on conventional FoxP3− CD4+ T cells, stimulation with CpG or IL-2 did not (Fig. 5a). To assign a role for CCR2 in CpG-induced CD25 expression, we tested whether a small molecular CCR2 antagonist (RS102895) could inhibit this CD25 upregulation on FoxP3+ T cells. We found that CCR2 antagonism inhibited CD25 upregulation on FoxP3+ cells after CpG stimulation but had a minimal effect on CD25 upregulation that was induced by IL-2 or TCR stimulation (Fig. 5b).
Fig. 5.
CCR2 antagonism diminishes CD25 upregulation on Tregs. Spleen cells were harvested from FoxP3 reporter mice. Spleen cells (2×105/well) were cultured in 96-well round-bottom plates and stimulated with CpG, IL-2 or anti-CD3/anti-CD28 Abs for 24 h. a The representative histograms show CD25 expression by FoxP3+CD4+ (left) and Foxp3-CD4+ T cells (right). The shaded areas indicate unstimulated cultures. The bar graphs show the CD25 mean fluorescence index (MFI) of expression by FoxP3+CD4+ (top) and FoxP3-CD4+ (bottom) T cells in 3 replicate wells with or without stimulation. b The above cell cultures with three replicate wells were incubated with a CCR2 inhibitor (RS102895, 0.5 µM). The histograms show the expression of CD25 by FoxP3+ cells with or without the inhibitor. The shaded areas show expression by unstimulated FoxP3+ cells. The bar graph (top) shows the CD25 MFI of FoxP3+ cells with or without the CCR2 inhibitor. The other bar graph (bottom) shows the % CD25 MFI of FoxP3+ cells with the CCR2 inhibitor relative to that of cultures without the inhibitor. **P < 0.01 compared to cultures without inhibitor. The data are from one of three similar experiments. c, d Spleen cells from Foxp3 reporter mice were incubated with various concentrations of anti-CCR2 Ab (rat IgG2b) or isotype control and then with anti-rat IgG as a crosslinking Ab. The cells were then stimulated with CpG for 20 h and analyzed for CD25 expression. The histogram shows the expression of CD25 by CpG-stimulated FoxP3+ cells (c). Line graph and bar graphs showing the MFI of CD25 (top) and FoxP3 (bottom) under different conditions. The data are from one of three similar experiments. E&G. Spleen FoxP3+ CD4+ cells from FoxP3 reporter mice were sorted into three subpopulations: CD25loCCR2int, CD25loCCR2lo, and CD25hiCCR2lo (e). The three types of cells (50,000 cells per well in a U-bottom 96-well plate) were cultured in the presence of CpG and 2×105 spleen cells for 20 h. The CD25 expression (f) and CCR2 expression (g) by the three populations with or without CpG are shown. **P < 0.01 by t tests between the indicated groups
Notably, we also found that crosslinking CCR2 with an anti-CCR2 antibody inhibited CpG-induced CD25 expression on Tregs (Fig. 5c, d). Without crosslinking, inhibition by the anti-CCR2 antibody was less clear (Fig. 5c, d). We noted that purified FoxP3+ T cells showed little upregulation of CD25 when they were stimulated with CpG, suggesting that soluble factors or cell surface molecules of non-FoxP3+ T cells provide the necessary signals to FoxP3+ T cells to upregulate CD25. However, inclusion of the CCR2 prototype ligand MCP-1 did not induce overt CD25 upregulation (data not shown).
As shown in Fig. 3, only a portion of FoxP3+ T cells expressed intermediate levels of CCR2 relative to the high expression of CCR2 by Ly6C+CD11b+ cells. Furthermore, CpG did not uniformly induce CD25 upregulation on all Foxp3+ T cells (Fig. 5a). We wondered whether CCR2 expression correlates with CD25 upregulation. To this end, we sorted spleen Foxp3+ T cells into three subpopulations: CD25loCCR2int (approximately 10%), CD25loCCR2lo (40%), and CD25hiCCR2lo (50%) (Fig. 5e). When the three subpopulations were stimulated with CpG in the presence of spleen cells, all three populations showed increased CD25 expression. Nevertheless, CD25 expression by CD25loCCR2int cells was higher than that by CD25loCCR2lo cells after CpG stimulation (Fig. 5f). Without CpG, the increase in CD25 expression was less conspicuous (Fig. 5f). Interestingly, there was no clear increase in CCR2 expression with or without CpG stimulation (Fig. 5g).
Optimal CpG-induced CD25 expression requires CCR2 signaling on FoxP3+ cells
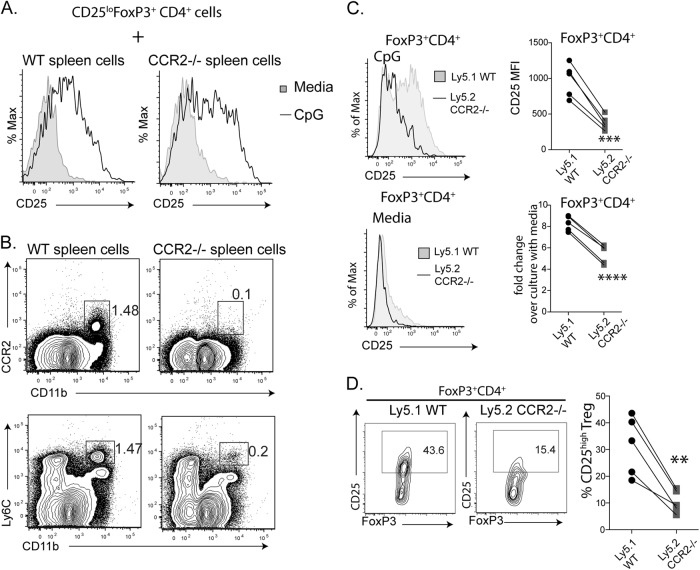
To support the notion that CCR2 signaling on FoxP3+ Tregs is required for CD25 upregulation, we first cultured CD25lo FoxP3+ cells from reporter mice with spleen cells from either WT or CCR2−/− mice under stimulation with CpG. We found that CpG-stimulated CD25 upregulation on CD25loFoxP3+ cells occurred similarly between WT and CCR2−/− spleen cells (Fig. 6a), suggesting that CCR2 deficiency in non-Treg cells is not sufficient to prevent CD25 upregulation on CD25loFoxp3+ cells; i.e., the effect of CCR2 on Tregs is cell intrinsic. As predicted, the spleen cells of CCR2−/− mice contained fewer CCR2+Ly6C+CD11b+ cells than did WT spleen cells (Fig. 6b). We then cocultured WT and CCR2−/− spleen cells from mixed bone marrow chimeric mice so that both WT and CCR2−/− Tregs were exposed to the same environment. Tregs of CCR2−/− origin expressed lower levels of CD25 than WT Tregs, while FoxP3 expression was comparable in both cell types (Fig. 2). Upon stimulation with CpG, the increase in CD25 expression on WT Tregs was significantly higher than that on CCR2−/− Tregs (P < 0.001, paired t test, Fig. 6c). Accordingly, the percentage of Tregs that were CD25hi under CpG stimulation was higher in WT cells than in CCR2−/− cells (P < 0.001, paired t test, Fig. 6d). Thus, CCR2 on Treg cells is required for the upregulation of CD25 expression.
Fig. 6.
CpG-stimulated CD25 upregulation requires CCR2 expression by FoxP3+ cells. a, b CD25loFoxP3+ cells were purified from Foxp3 reporter mice. CD25loFoxP3+ cells (50,000 cells per well) were cocultured with 2×105 spleen cells from WT or CCR2−/− mice in the presence or absence of CpG (1 μM) in a 96-well round-bottom plate for 20 h. The expression of CD25 by FoxP3+ cells was evaluated. The histograms (a) show CD25 expression by FoxP3+ cells with WT spleen cells or CCR2−/− spleen cells. The FACS plots (b) show the differences in myeloid cells between WT and CCR2−/− spleen cells. c, d Spleen cells from five individual chimeric mice were cultured with or without CpG for 20 h. The histograms (c) show the expression of CD25 by FoxP3+ cells cultured with or without CpG. The MFI of CD25 expression is shown for CpG-stimulated WT and CCR2−/− FoxP3+ Tregs (top), and the fold changes in CD25 expression compared to that of unstimulated Tregs are also shown (bottom). The lines indicate data from the same mouse. **P < 0.01, ***P < 0.001, and ****P < 0.0001 by paired t tests. The data are from one of three similar experiments
Discussion
CCR2 is pivotal for chemotaxis of monocytic cells. A chemotactic role of CCR2 in Treg trafficking has also been documented in a transfer study.9 Here, we have revealed a new cell-intrinsic role for CCR2 that is independent of cell trafficking, namely, a role in regulating Treg abundance and function that includes upregulation of CD25.
Given that CCR2 is highly expressed by monocytes and derived dendritic cells, we initially surmised that the Treg defect in CCR2−/− mice was secondary to a defect in monocytes/moDCs, partly because homeostasis of Tregs has been shown to be influenced by various types of DCs.21 It has been documented that loss of DCs leads to a loss of Tregs and a heightened inflammatory response.22 Furthermore, it has been shown that moDCs, but not conventional DCs, expand Tregs.16 However, we found two lines of evidence that did not support the idea that the CCR2−/− Treg defect is largely due to defects in monocytes/moDCs. First, in mixed bone marrow chimeras in which both WT and CCR2−/– monocytes/moDCs coresided, the defect in CCR2−/− Tregs remained. Second, removal of CCR2+ monocytes/moDCs by injection of DT in CCR2-DTR mice over 7–10 days did not cause a reduction in Tregs. Nevertheless, our study does not discount some contribution of the non-Treg compartment to Treg regulation by CCR2. Notably, the expression of CD25 by CCR2−/− Tregs from mixed bone marrow chimeras was generally higher than that by CCR2−/− Tregs from intact CCR2−/− mice. Furthermore, in vitro upregulation of CD25 expression on Tregs induced by CpG requires the presence of non-Treg cells in the culture. Indeed, beyond the influence of CCR2, variations in CD25 expression can also be affected by the activation milieu, e.g., by TCR activation and IL-2 (Fig. 5).
It has been reported that approximately a quarter of CD62L−FoxP3+ T cells in lymph nodes express CCR2.11,23 In a transfer study, CCR2 deficiency was found to hamper Treg trafficking from lymph tissues to graft sites (kidney capsules) as well as from graft sites to draining LNs.9 Notably, intravenous injection of CCR2−/− Tregs did not prevent their traffic to the spleen and lymph nodes.9 On the other hand, we found that CCR2 deficiency resulted in reduced Treg numbers and lower CD25 expression on CD4+FoxP3+ T cells in central lymphoid organs (thymus), peripheral lymphoid organs (spleen and lymph nodes), and peripheral parenchyma (liver and lung), arguing against a defect in cell trafficking underlying this effect.
IL-2 and its receptor CD25 are critical in Treg homeostasis.24 In addition to IL-2 and TCR stimulation, we found that the TLR ligand CpG can also stimulate CD25 expression on Tregs. Upregulation of CD25 by CpG stimulation, but not by IL-2 and TCR stimulation, could be inhibited by a CCR2 antagonist in vitro, suggesting that CCR2 signaling is required. Coculture of WT and CCR2−/− Tregs showed that upregulation of CD25 by CpG was more prominent in WT Tregs than in CCR2−/− Tregs, suggesting that direct signaling of CCR2 may be required for upregulation of CD25. Furthermore, WT Tregs had high CD25 expression and hence responded strongly to IL-2, which in turn upregulated CD25. Thus, in CCR2−/− Tregs, this virtuous circle was defective, and this alone could explain the outcome of reduced Treg abundance. The reduced fitness of CCR2−/− Tregs was also evident by their lower regulatory function. We observed that CCR2−/− Tregs were not optimal in suppressing the proliferative response of naïve T cells. This reduced regulatory function of CCR2−/− Tregs is in accord with what we and others have reported previously: that Tregs with low CD25 expression are functionally inferior20 and that CD25 on Tregs behaves as an IL-2 “sink” to induce cytokine deprivation of effector T cells.25 Notably, although CCR2−/− Tregs locally transferred to graft sites are retained in the tissue, they are less capable of suppressing alloimmunity compared to WT Tregs.9 Interestingly, the proportion of Tregs that bear CCR2 increases during tumor development (21% in draining lymph nodes and 50% in tumors),23 implying that CCR2 deficiency might impact Treg function in vivo.
Under CpG stimulation, the expression of CD25 on FoxP3+ cells became much higher. This upregulation was reduced by CCR2 antagonism and CCR2 deficiency. There are at least two explanations for these findings. The first is that CpG stimulates the production of CCR2 ligands in culture. Such CCR2 ligands then enhance CCR2 signaling on Tregs to increase CD25. In support of this possibility, we found that CpG did not stimulate the overt upregulation of CD25 when FoxP3+ T cells were isolated and cultured alone. A previous study reported that the classic CCR2 ligand, CCL2, costimulates T cells.15 Transgenic CCL2 expression in the central nervous system results in preferential local accumulation of Treg,26 although it is not clear whether CCL2 increases Treg via recruitment or direct signaling. We found that CCL2 alone did not robustly stimulate CD25 upregulation on Tregs. Apart from CCL2, there are many ligands, such as CCL7, CCL8, CCL12, and CCL16, that can bind CCR2. It remains to be tested whether any or the sum of these are required to orchestrate the response. The second explanation is that CpG stimulates CCR2 expression on Tregs, either directly or indirectly. Tregs can express various TLRs, including TLR9, and can be stimulated directly by CpG.27 However, we did not detect a strong induction of CCR2 on highly purified FoxP3+ cells after CpG stimulation.
The impact of CCR2 on Tregs, either via well-known chemotactic functions or the nonchemotactic functions revealed in this study, may offer an immunological explanation for the failure of CCR2 antagonism (mainly aimed at monocytic cells) in interventions for inflammatory diseases.10 Notably, there are reports showing that CCR2 deficiency can exacerbate experimental autoimmune arthritis,6,28 suggesting a protective role for CCR2 in autoimmune diseases. In CCR2−/− mice with arthritis, the levels of Th17 cell cytokines are increased. These studies did not address how a protective effect of CCR2 was achieved. Our findings suggest that the effect of CCR2 may be due to an effect on Tregs. CCR2 is also expressed on some activated/memory T cells,12,13 but we noted that CCR2 deficiency has less impact on conventional T cells in lymphoid organs and adipose tissue than on Tregs. Perhaps the differential impact reflects the difference in CCR2 expression. FoxP3– T cells, even CD62L− ones (activated T cells), contained a smaller cohort expressing CCR2 than Tregs.11
In summary, in addition to its likely role in cell migration, CCR2 may be required for optimal CD25 expression in Tregs and for the fitness of Tregs in general. The finding that Tregs use the same chemokine axis to constrain the proinflammatory effect of such an interaction on monocytic cells during inflammation may represent a novel negative feedback mechanism. The precise signaling events mediated by CCR2 that impact Tregs remain to be defined. Our findings suggest that the development of CCR2 antagonism for the treatment of autoimmune diseases should take into account the protective role of CCR2 in immune regulation. The significance of CCR2-mediated Treg regulation under steady-state and inflammatory conditions, particularly in human diseases, also beckons to be explored.
Electronic supplementary material
Acknowledgements
We thank M. Dayton, Li Sun, and Lisa Reid for technical assistance. This work was supported by the Rebecca L. Cooper Foundation, National Health and Medical Research Council of Australia (NHMRC) grants (1037321, 1080321, 1105209, 1143976), an NHMRC Independent Research Institutes Infrastructure Support Scheme grant (361646) and a Victorian State Government Operational Infrastructure Support grant. We acknowledge the Wurundjeri people of the Kulin nation as the traditional owners and custodians of the land on which most of the work was performed.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0187-8) contains supplementary material.
References
- 1.Fiorina P, et al. Phenotypic and functional differences between wild-type and CCR2-/- dendritic cells: implications for islet transplantation. Transplantation. 2008;85:1030–1038. doi: 10.1097/TP.0b013e31816843a0. [DOI] [PubMed] [Google Scholar]
- 2.Hohl TM, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawai CM, et al. Transcription factor Runx2 controls the development and migration of plasmacytoid dendritic cells. J. Exp. Med. 2013;210:2151–2159. doi: 10.1084/jem.20130443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko HJ, et al. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J. Immunol. 2014;192:2202–2209. doi: 10.4049/jimmunol.1302040. [DOI] [PubMed] [Google Scholar]
- 5.Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J. Exp. Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quinones MP, et al. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 2004;113:856–866. doi: 10.1172/JCI200420126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruhl H, et al. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+T cells. J. Immunol. 2004;172:890–898. doi: 10.4049/jimmunol.172.2.890. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 9.Zhang N, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pease J, Horuk R. Chemokine receptor antagonists. J. Med. Chem. 2012;55:9363–9392. doi: 10.1021/jm300682j. [DOI] [PubMed] [Google Scholar]
- 11.Lee JH, Kang SG, Kim CH. FoxP3+T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J. Immunol. 2007;178:301–311. doi: 10.4049/jimmunol.178.1.301. [DOI] [PubMed] [Google Scholar]
- 12.Bonecchi R, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–574. doi: 10.1016/S0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 14.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpus WJ, et al. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 16.Yamazaki S, et al. Direct expansion of functional CD25+CD4+regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Zhan Y, Lew AM, Naik SH, Kershaw MH. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J. Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 18.Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat. Immunol. 2015;16:635–641. doi: 10.1038/ni.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boring L, et al. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan Y, et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+anergic CD4+T cells. J. Immunol. 2011;187:1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darrasse-Jeze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loyher PL, et al. CCR2 influences T regulatory cell migration to tumors and serves as a biomarker of cyclophosphamide sensitivity. Cancer Res. 2016;76:6483–6494. doi: 10.1158/0008-5472.CAN-16-0984. [DOI] [PubMed] [Google Scholar]
- 24.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 25.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+T cells. Nat. Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 26.Trujillo JA, Fleming EL, Perlman S. Transgenic CCL2 expression in the central nervous system results in a dysregulated immune response and enhanced lethality after coronavirus infection. J. Virol. 2013;87:2376–2389. doi: 10.1128/JVI.03089-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X, et al. Excessive TLR9 signaling contributes to the pathogenesis of spontaneous abortion through impairment of Treg cell survival by activation of Caspase 8/3. Int. Immunopharmacol. 2015;29:285–292. doi: 10.1016/j.intimp.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Rampersad RR, et al. Enhanced Th17-cell responses render CCR2-deficient mice more susceptible for autoimmune arthritis. PLoS ONE. 2011;6:e25833. doi: 10.1371/journal.pone.0025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.