Follicular lymphoma (FL), characterized by a t (14;18)(q32;q21), is one of the most common non-Hodgkin lymphomas (NHL). Although FL is sensitive to chemotherapy, immunotherapy and radiotherapy it currently remains an incurable disease. The variable outcome of FL appears to be determined mainly by the tumor-microenvironment and the non-malignant bystander cells.1,2 The immunomodulatory drug lenalidomide has been shown to modulate the production of various cytokines in the tumor-microenvironment and activates T- and NK-cells.3,4
Impairment of the T-cell immunologic synapse formation in FL seems to be an active immunosuppressive mechanism, which in vitro can be overcome by treatment of FL cells with lenalidomide.5 A synergistic antitumor effect of lenalidomide and rituximab in lymphoma cell killing has been demonstrated in mouse models.2,6,7 Additionally, lenalidomide can have a direct antitumor effect because it inhibits Akt-phosphorylation and increases p21 expression, which are important for tumor cell proliferation and survival. The mechanisms of action of bendamustine include inhibition of the mitotic checkpoints, induction of mitotic catastrophe, activation of DNA damage stress response, intrinsic apoptosis and base-excision DNA repair mechanisms.8
Several studies have demonstrated clinical activity of lenalidomide in indolent lymphoma9 and adding rituximab can improve the response rate with acceptable toxicity.10–12 In mantle cell lymphoma (MCL) and chronic lymphocytic leukaemia (CLL) the combination of lenalidomide, bendamustine and rituximab was found to be effective and feasible in patients in an upfront setting with a high CR rate but also a high rate of infections.13,14 To date there are no data on the combination of rituximab, bendamustine and lenalidomide in patients with FL. With this run-in phase I study we aimed to establish the recommended dose level (RDL) of bendamustine and lenalidomide for the triplet combination with rituximab for relapsed or refractory (R/R) FL patients in a subsequent randomized phase II study.
This is a multicenter, phase I/II study sponsored by HOVON (Hemato-Oncologie voor Volwassenen Nederland) (HOVON110; Netherlands Trial Register: Trial NL2882; EudraCT number: 2011-000097-56). The study was approved by the Ethics Committee of the Amsterdam UMC and was conducted in accordance with the ethical principles of the Declaration of Helsinki.
In the ongoing phase II part, patients are randomized between arm A (lenalidomide and rituximab) and arm B (lenalidomide, bendamustine, rituximab). The aim of the phase I part was to determine the dose limiting toxicity (DLT) and RDL of the combination of lenalidomide, rituximab and bendamustine in a 28-day schedule in R/R FL patients. Eligible patients were ≥18 years, and were required to have biopsy proven, CD20 positive stage II-IV R/R FL grade 1–3a. Rituximab refractory cases (defined as relapse/progression during or within 6 months after a rituximab containing regimen) were not eligible. The maximum number of prior systemic treatment regimens was five. Prior bendamustine was allowed if it was administered >24 months before enrolment and had resulted in at least a partial remission (PR).
A 3+3 dose-escalation design was used. Four carefully designed dose levels were considered: lenalidomide (10 mg, 15 mg or 20 mg) orally, escalating doses of bendamustine (70 mg/m2 or 90 mg/m2) IV, combined with a fixed dose of rituximab (375 mg/m2) IV for a total of 6 induction cycles (Table 1). Patients with complete remission (CR) or partial remission (PR) continued with 2 years of rituximab maintenance treatment (8 infusions, every 3 months).
Table 1.
Dose Levels of Lenalidomide/Rituximab/Bendamustine for Each Cycle, in Total 6 Induction Cycles.

When 3 or 6 patients had been enrolled, inclusion was discontinued until DLT was determined at day 29 of cycle 1. Patients who died of FL within 29 days of starting treatment without a DLT were considered non-evaluable and were replaced by another patient. Dose escalation stopped as soon as at least 2 patients experienced a DLT at the same dose level. Before opening the next dose level, the DLTs at the preceding dose level were reviewed. A DLT was defined as ≥ grade 3 non-hematologic toxicity, grade 4 neutropenia lasting ≥7 days or grade 4 thrombocytopenia (unless due to lymphomatous bone marrow infiltration), febrile neutropenia, or death of any cause except lymphoma. For full details of the protocol see Supplement 1 (Supplemental Digital Content).
Patient and treatment characteristics and safety data were reported per dose level, and were restricted to eligible and evaluable patients. Adverse events were summarized by the maximum CTCAE grade per type of AE over a treatment period. Results of the phase I part will be presented here.
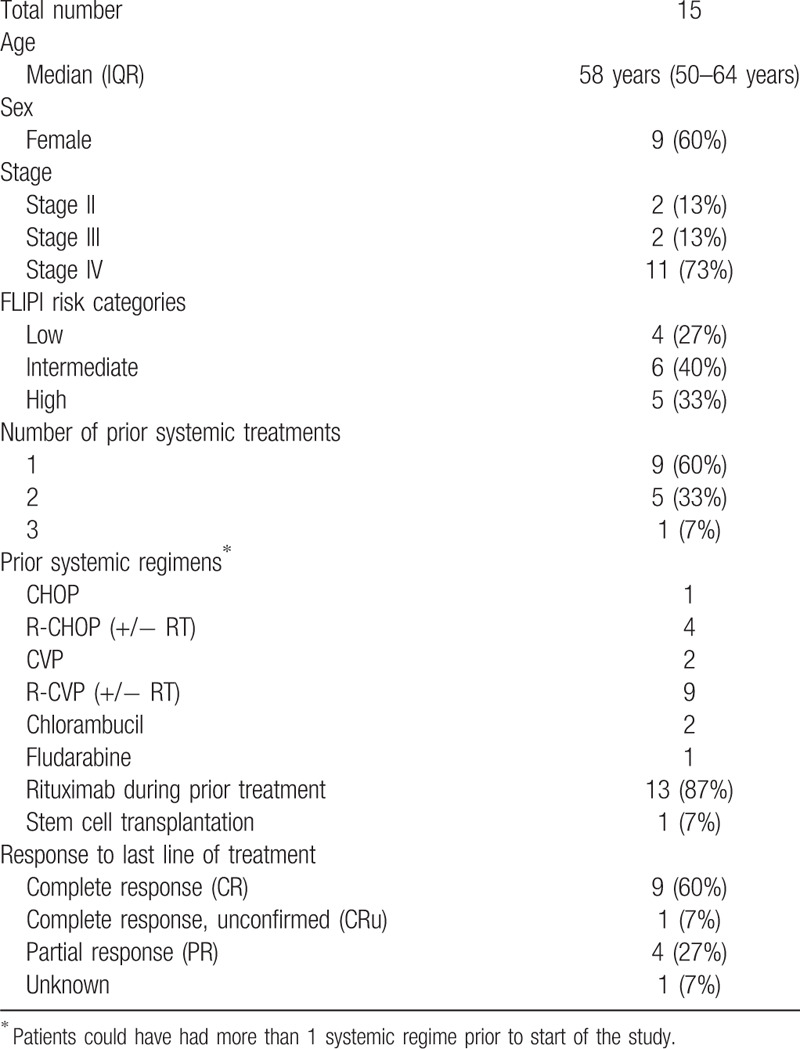
Eighteen patients with R/R FL, from 9 centers in the Netherlands and 1 in Germany, were enrolled between November 2011 and May 2014. Two patients were not evaluable due to a protocol amendment after inclusion, precluding the concurrent use of allopurinol during bendamustine treatment, and one rituximab refractory patient did not fulfill the inclusion criteria, leaving 15 evaluable patients for determination of the DLT and RDL. The median age at baseline was 58 years; 60% were female and the majority of patients had Ann Arbor stage IV disease (73%). The median number of prior treatments was 1 (range 1–3); 2 were rituximab naïve; none of the patients were refractory to their last lymphoma therapy (Table 2).
Table 2.
Demographic and Disease Characteristics of the 15 Patients Enrolled in the Phase I Study.
Among all 15 eligible patients only 1 DLT occurred before day 29 of cycle 1. This event, a grade 3 allergic reaction probably due to bendamustine, occurred at dose level 4 (the highest dose level). One other patient discontinued the study after cycle 4, because of an allergic reaction to rituximab. Despite recommendations against the use of allopurinol, two patients, 1 in dose level 2 and 1 in dose level 3, used allopurinol; none of them experienced any signs of Stevens-Johnsons syndrome.
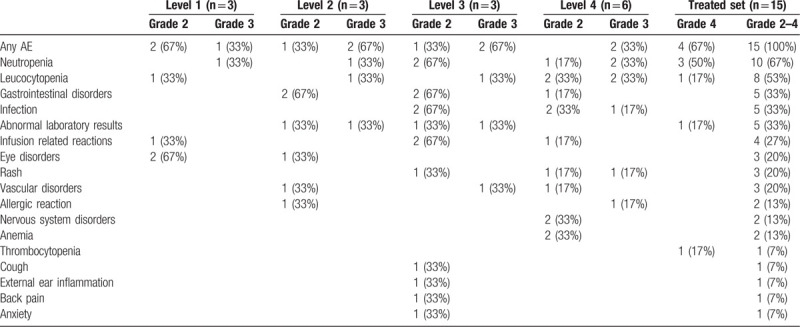
All patients experienced at least 1 AE grade 2–4, in total 57 AEs were reported; 65% (n = 37 grade 2, 24% (n = 14) grade 3 and 10% (n = 6) grade 4. The most common AEs of any grade were: neutropenia, leucocytopenia, gastrointestinal disorders, infections, and abnormal laboratory results (Table 3). Six patients (40%) experienced a total of 13 SAEs; 3 pneumonias, 2 allergic reactions and 5 second primary malignancies; 3 basal cell carcinoma, 1 Merkel cell carcinoma, and 1 lung cancer. The lung cancer was only located in the lung and treated with curative intended radiotherapy. The other SAEs occurred only once; fever, knee pain and thrombocytopenia. Eight were classified as SAEs due to hospitalization, all 13 SAE's resolved completely.
Table 3.
Maximum Grade of the Adverse Events During the Induction Treatment.
This first clinical study investigating the combination of lenalidomide, bendamustine and rituximab in R/R FL showed an acceptable safety and tolerability profile. Only one DLT was reported and most AEs were grade 2. Hematological toxicity was mostly seen at dose level 4 without severe consequences. The secondary primary malignancies were considered possibly related, but the patients had received prior therapy that could have contributed. We concluded that dose level 4 (lenalidomide 20 mg dag 3–21 day, bendamustine 90 mg/m2 day 1–2, combined with rituximab 375 mg/m2 day 1) is feasible for R/R FL patients. This dose level is currently being studied further in the randomized phase II part of the study for efficacy and toxicity in comparison with 6 cycles of lenalidomide 20 mg dag 1 to 21 day and rituximab 375 mg/m2 day 1.
Supplementary Material
Acknowledgments
The authors acknowledge the HDC trial team (HOVON Data Center) for trial coordination and central data management and Roberto Liu, Amsterdam UMC, location AMC for trial coordination.
Footnotes
Citation: Stevens WBC, Bakunina K, Cuijpers M, Chamuleau M, Beeker A, Fijnheer R, Hebart H, Visser HPJ, Doorduijn JK, Linton K, Dreyling M, de Jong D, Kersten MJ. HOVON110/ReBeL Study: Results of the Phase I part of a Randomized Phase I/II Study of Lenalidomide, Rituximab with or without Bendamustine in Patients with Relapsed/Refractory Follicular Lymphoma. HemaSphere, 2020;00:00. http://dx.doi.org/10.1097/HS9.0000000000000325
This clinical trial is registered with EudraCT number: 2011-000097-56.
The study was financially supported by the Dutch Cancer Society, Celgene and Roche. Study medication was provided by Celgene (lenalidomide), Mundipharma (bendamustine) and Roche (rituximab).
Wendy B.C. Stevens, Katerina Bakunina, Aart Beeker, Marloes Cuijpers, Holger Hebart, Hein P.J. Visser, Rob Fijnheer: nothing to disclose; Marie José Kersten: research support, travel grants, honoraria or advisory boards from Celgene, research support, travel grants, honoraria or advisory boards from Roche; Jeanette K Doorduijn: Financial compensation for clinical study from, and travel costs support from Roche, travel costs support from Celgene; Daphne de Jong: advisory board and research support from Celgene; Martin Dreyling: Research support, Scientific advisory board, speaker's honoraria from Celgene; Research support, Scientific advisory board, speaker's honoraria from Roche; Martine Chamuleau: Research support for study from Celgene; Kim Linton: honoraria and consultation or advisory role from Roche; consultation or advisory role and sponsorship to attend conferences from Celgene.
Supplemental Digital Content is available for this article.
References
- 1.de Jong D. Molecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factors. J Clin Oncol. 2005;23:6358–6363. [DOI] [PubMed] [Google Scholar]
- 2.Herreros B, Sanchez-Aguilera A, Piris MA. Lymphoma microenvironment: culprit or innocent? Leukemia. 2008;22:49–58. [DOI] [PubMed] [Google Scholar]
- 3.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. [DOI] [PubMed] [Google Scholar]
- 4.Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-hodgkin lymphoma. J Clin Oncol. 2015;33:2803–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramsay AG, Clear AJ, Kelly G, et al. Follicular lymphoma cells induce T-cell immunologic synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu L, Adams M, Carter T, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–4657. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Qian Z, Cai Z, et al. Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol. 2009;84:553–559. [DOI] [PubMed] [Google Scholar]
- 8.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. [DOI] [PubMed] [Google Scholar]
- 9.Witzig TE, Wiernik PH, Moore T, et al. Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin's Lymphoma. J Clin Oncol. 2009;27:5404–5409. [DOI] [PubMed] [Google Scholar]
- 10.Tuscano JM, Dutia M, Chee K, et al. Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol. 2014;165:375–381. [DOI] [PubMed] [Google Scholar]
- 11.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zucca E, Rondeau S, Vanazzi A, et al. Short regimen of rituximab plus lenalidomide in follicular lymphoma patients in need of first-line therapy. Blood. 2019;134:353–362. [DOI] [PubMed] [Google Scholar]
- 13.Albertsson-Lindblad A, Kolstad A, Laurell A, et al. Lenalidomide-bendamustine-rituximab in patients older than 65 years with untreated mantle cell lymphoma. Blood. 2016;128:1814–1820. [DOI] [PubMed] [Google Scholar]
- 14.Soumerai JD, Davids MS, Werner L, et al. Phase 1 study of lenalidomide, bendamustine, and rituximab in previously untreated patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2019;60:1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


