Abstract
The complex nature of inflammatory bowel disease (IBD) often results in treatment failure for many patients. With some patients cycling through multiple therapies before achieving a sustained period of remission, the ability to predict a patient's response to therapeutics could decrease the time from active disease to clinical remission and mucosal healing. The prospect of such individualized treatment of IBD would be aided by accurate biomarkers, both fecal and serological, which have to date shown value as indicators of IBD activity. Here we review the utility of generic biomarkers for inflammation or mucosal healing, such as calprotectin, C-reactive protein (CRP), and fecal hemoglobin (fHb) as predictors of response to treatment of IBD. We further provide a deeper insight into the utility of monitoring the thiopurine treatment by thiopurine metabolites or alternative hematologic parameters. In light of multiple recent publications of biomarkers and biological therapy, our focus in this review is predicting response to thiopurine treatment only, that is, Azathioprine and 6-Mercaptopurine.
Keywords: intestinal inflammation, outcome, predictors, Crohn's disease, ulcerative colitis, thiopurine, azathioprine, 6-mercaptopurine
Introduction
Inflammatory bowel disease (IBD) is a complex disease with multiple risk factors, interactions and treatment options, in the context of the important clinical need to rapidly establish and maintain mucosal healing (MH) (1). The goal of being able to individualize therapy for patients with IBD, so as to maximize effectiveness—including rapid induction of MH—whilst minimizing side effects, has not progressed as quickly in IBD as in other diseases, such as cancer (2). The ability to predict clinical response before the instigation of therapy, or shortly thereafter, would allow for rapid escalation of therapy when required with minimization of side effects. Currently, Crohn's disease (CD) and ulcerative colitis (UC) have multiple different scoring systems using both clinical factors and biomarkers (usually serum-based) to determine disease severity and likely outcome, but less in terms of predicting treatment response. This review article will focus mainly on biomarkers that are currently in clinical use. To date much of the research surrounding response prediction in IBD is centered around treatment with biological agents, such as Infliximab (IFX) (3–10). Given recent reviews published elsewhere in this area (11, 12), our review will only cover studies related to predicting a response to immunomodulator treatment with either azathioprine or 6-mercaptopurine since this was not covered by the before mentioned review articles.
Methodology
The review was performed using PubMed/MEDLINE up to June 2019. The search strategy was using the following search terms alone or in combination: monitoring, biomarker, marker, surrogate, evaluation, prediction, predictor, response, responder, healing, recurrence, relapse, remission, management, efficacy, outcome, flare, immunomodulators, immunosuppressants, thiopurine, azathioprine, 6-mercaptopurine, methotrexate, therapy, treatment, induction, maintenance, serum, fecal, fecal, blood, lactoferrin, S100, C-reactive protein, serological, microbiome, intestinal flora, microflora, gut flora, interleukin, mucosal, mucosa, polymorphisms, inflammatory bowel disease, Crohn's disease, ulcerative colitis. Boolean operators (“not,” “and,” “or”) were also used in succession to narrow or widen the search. The primary outcome was to review the clinical utility of biomarkers for the prediction of response to treatment with immunomodulators in IBD.
Thiopurine Therapy
The thiopurine metabolites, azathioprine (AZA) and 6-mercaptopurine (6-MP), have a long history of use as immunomodulators in patients with IBD (13). Whilst initially having been used based on their known effectiveness in other autoimmune inflammatory diseases, there is now a strong evidence for their use to maintain remission in IBD (13–16). Furthermore, as shown in the SONIC trial, combination therapy of oral AZA with IFX is seen to be superior over monotherapy with either agent for inducing steroid free remission in CD (17).
Thiopurine Metabolism and Modes of Action
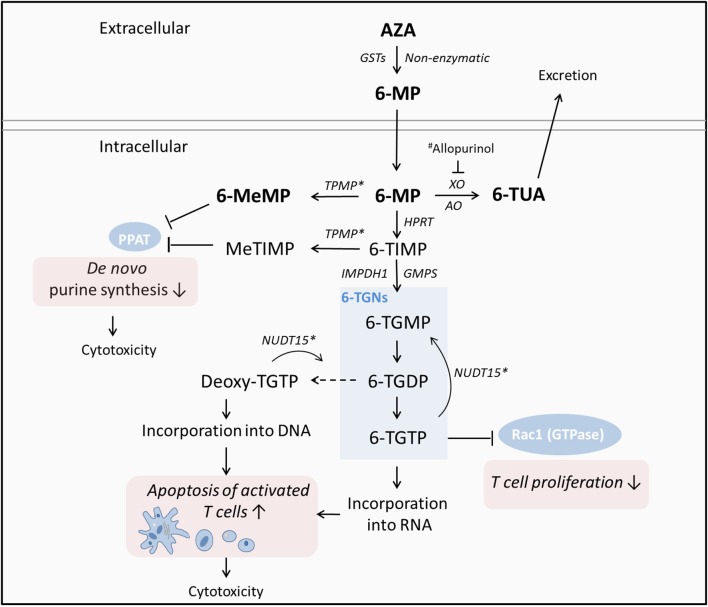
As a prodrug, AZA is converted to 6-MP by glutathione transferases (GSTs) or non-enzymatically upon reaching the systemic blood circulation. Following the intestinal uptake by transporter molecules, 6-MP is metabolized by three competing pathways either in the liver or gut (18, 19) resulting in immunosuppressive effects (Figure 1). Importantly, 6-thioguanine nucleotides (6-TGNs) serve as the active metabolites of thiopurine therapy, incorporating into lymphocyte DNA and thereby inducing apoptosis of activated T-lymphocytes (22) as well as exerting direct cytotoxic effects at higher oncologic doses (23) (Figure 1). In addition, 6-TGN thioguanosine triphosphate (TGTP) inhibits the activity of GTPase Rac1 resulting in suppression of T cell-dependent immune response (22, 24). The conversion of 6-MP into 6-methylmercaptopurine (6-MeMP) or methylthioinosine monophosphate (meTIMP) by thiopurine methyltransferase (TPMT), inhibits the enzyme phosphoribosyl pyrophosphate (PPAT) which catalyzes the first step of de novo purine synthesis (25). As a consequence, there is inhibition of DNA synthesis and cell proliferation along with cytotoxic effects (26).
Figure 1.
Simplified metabolism of thiopurines and modes of action according to (19–21). As a prodrug, AZA is converted to 6-MP upon reaching the systemic circulation. Following the uptake by transporter molecules, 6-MP is metabolized by three competing pathways either in the liver or gut resulting in immunosuppressive effects. Importantly, 6-TGNs serve as the active metabolites of thiopurine therapy, incorporating into lymphocyte DNA and thereby inducing apoptosis of activated T-lymphocytes as well as exerting direct cytotoxic effects at higher doses. In addition, 6-TGTP inhibits the activity of the GTPase Rac1 resulting in suppression of T cell-dependent immune response. The thiopurine metabolites 6-MeMP and MeTIMP inhibit the enzyme PPAT which catalyzes the first step of de novo purine synthesis; resulting in inhibition of DNA synthesis and cell proliferation along with cytotoxic effects. AZA, Azathioprine; 6-MP, 6-mercaptopurine; TPMT, thiopurine S-methyltransferase; TUA, thiouric acid; HPRT, hypoxanthine phosphoribosyltransferase; MeMP, methylmercaptopurine; TIMP, thioinosine monophosphate; TGNs, thioguanine nucleotides; XO, xanthine oxidase; AO, aldehyde oxidase; TGMP, guanosine monophosphate; TGDP, guanosine diphosphate; TGTP, guanosine triphosphate, NUDT15, nudix hydrolase 15; PPAT, phosphoribosyl pyrophosphate aminotransferase; Rac1, Rac family small GTPase 1. *Associated with variability in tolerance to thiopurines. #XO inhibitor allopurinol, applied to induce a switch toward 6-TGN production in patients who do not adequately respond to thiopurine treatment.
Genetic polymorphisms affecting the activity of specific enzymes in the thiopurine pathway are associated with adverse drug reactions due to a shift of metabolite distribution (19). Patients with a complete or partial deficiency of TPMT, where one or two gene copies are defective, have a high risk of developing severe myelosuppression during treatment with standard doses with AZA or 6-MP (27). In Caucasians, ~10% of the population are heterozygous for a TPMT allele causing TPMT deficiency while only 0.3–0.5% are homozygous (20). Due to the preferred metabolism to 6-TGNs, these patients are more responsive to thiopurines where lower dosages are necessary. Thus, patients with complete TPMT deficiency should avoid thiopurine treatment or, if necessary, start with <10% of standard dosage; whereas individuals with a heterozygous genotype should be treated with 50% of standard initiation dose (28). In contrast to TPMT deficiency, the overexpression of TPMTs is associated with an increased accumulation of MeMP, with concurrent lower levels of 6-TGNs. This is referred to as thiopurine hypermethylation and is associated with drug toxicity and non-response to thiopurine treatment (29). In this context it has been shown that the co-therapy with allopurinol, an inhibitor of the enzyme xanthine oxidase, can improve the production of 6-TGNs by modifying 6-MP metabolism (30) promoting clinical remission and mucosal healing (31–34). Regarding the proven relevance of TMPT activity for the outcome of thiopurine treatment, measuring TMPT levels prior to starting of therapy is recommended in Caucasians to prevent potentially life-threatening myelotoxicity (35, 36). In this context, phenotyping using an enzyme assay, is preferred to genotyping, given completely deficient patients can be detected in any case irrespective of the TPMT variant (37). In contrast, the prevalence of non-wild type alleles in the Asian population is much lower, with <5% of population being heterozygous and almost none being completely deficient.
In addition to TMPT, variants of the gene NUDT15 affects the metabolism of thiopurines (38). The enzyme NUDT15 dephosphorylates the active thiopurine metabolites TGTP and deoxyTGTP, thus preventing their incorporation into DNA (39). It is assumed that decreased NUDT15 enzymatic activity or a lower expression level is associated with a higher level of active 6-TGNs resulting in thiopurine-induced myelotoxicity and a reduced thiopurine maintenance dose (38, 40). In addition to toxic effects on bone marrow function, AZA and 6-MP are associated with a high risk of hepatotoxicity (41) with an incidence of 1 in 133 in a population based study from Iceland (42). Although the prognosis is generally favorable, patients with pre-existing cirrhosis should be considered carefully before starting a thiopurine therapy (43).
Monitoring of Thiopurine Treatment
To evaluate the efficacy and potential toxicity of thiopurine treatment, the concentration of thiopurine metabolites can be assessed in red blood cells (RBC). However, there are some analytical pitfalls aggravating the translation of metabolite levels to clinical outcome (44). Although it has been shown, that lower TGN concentrations were associated with the development of active Crohn's disease, there was substantial variation between repeated measurements of 6-TGNs on the same patient (45) revealing that serial 6-TGN analyses are required to evaluate active metabolite levels. Furthermore, due to high costs or limited availability of the analytical tests, the therapeutic monitoring of TGNs is not practical for every clinic (46). Therefore, surrogate markers, such as the white blood cell count (WBC) or the mean corpuscular volume (MCV) are discussed as alternatives in monitoring thiopurine efficacy.
Thiopurine Metabolites
With regard to the metabolism of thiopurines, it is assumed that very high or very low levels of metabolites are associated with adverse reactions or non-response to thiopurine treatment. As a consequence, 6-TGNs and 6-MMP are often evaluated to gauge the success of thiopurine therapy. In spite of some studies identifying a strong association between metabolite levels and clinical outcome, the current viewpoint is not yet clear enough for routine clinical use of metabolite levels as predictors of treatment response (Table 1).
Table 1.
Studies assessing the value of biomarkers in predicting response to thiopurine treatment in IBD.
| Study | Patient population | Subjects (n) | Therapy | Metabolite/Biomarker | Cut off level |
Hazard Ratio Odds Ratio or P-value |
Sensitivity/Specificity (%) | PPV/NPV (%) | Definition of relapse | Definition of response |
|---|---|---|---|---|---|---|---|---|---|---|
| Park et al. (47) | CD Pediatric/Adult |
82 | AZA/6-MP | CRP | ≥5 mg/L | p = 0.009 | – | – | CDAI > 150 | CDAI < 150 (remission) |
| Lémann et al. (48) | CD Adult |
83 | AZA | CRP Hb |
≥20 mg/L <12 g/dL |
p = 0.0002 p = 0.043 |
– | – | CDAI > 250 OR CDAI between 150 and 250 on 3 consecutive weeks with an increase of at least 75 points above baseline OR the need for surgery for CD (with the exception of limited perianal surgery) |
– |
| Treton et al. (49) | CD Adult |
66 | AZA | CRP Hb NC |
≥20 mg/L <12 g/dl >4 × 109/l |
HR 58.6, p = 0.002 HR 4.8, p = 0.04 HR 3.2, p = 0.003 |
– | – | HBI ≥ 4 with the need for treatment | HBI ≤ 3 without any steroid treatment or immunosuppressive agent in the past 3 months and without surgery. |
| Osterman et al. (50) | CD/UC Adult |
971 | AZA/6-MP | 6-TGN | ≥230 pmol/8 × 108 RBCs | OR = 3.27 | 62/67 | – | Not provided | Not provided |
| Nguyen et al. (51) | CD/UC Pediatric |
86 | AZA | 6-TGN | ≥250 pmol/8 × 108 RBCs | OR = 4.4 p = 0.007 |
– | – | Inability to achieve steroid free remission | PCDAI ≤ 10 without corticosteroids PUCAI ≤ 10 without corticosteroids |
| Kwan et al. (52) | CD/UC Adult |
39 | AZA/6-MP | 6-TGN | ≥230 pmol/8 × 108 RBCs | – | 63/67 | 63/67 | Disease flare requiring cyclosporine, infliximab, intravenous corticosteroid | Any reduction in oral steroid use, HBI, SCCAI scores, and clinical assessment |
| Gonzalez-Lama et al. (53)a | CD/UC Adult |
113 | AZA/6-MP | 6-TGN | ≥230 pmol/8 × 108 RBCs | – | 41/56 at 2 weeks 58/50 at 1 month 57/50 at 2 months 68/62 at 4 months |
61/36 at 2 weeks 64/44 at 1 month 67/40 at 2 months 84/40 at 4 months |
Unable to achieve steroid free CDAI <150 | CDAI <150 or mTWAI <11 without corticosteroids for at least 6 months |
| Gonzalez-Lama et al. (53)b | CD/UC Adult |
113 | AZA/6-MP | 6-TGN | 260 pmol/8 × 108 RBCs | – | 35/65 at 2 weeks 49/53 at 1 month 53/57 at 2 months 57/75 at 4 months |
63/37 2 weeks 62/40 1 month 68/41 2 months 87/37 4 months |
Unable to achieve steroid free CDAI <150 | CDAI <150 or mTWAI <11 without corticosteroids for at least 6 months |
| Waljee et al. (54) | CD/UC Adult |
346 | AZA/6-MP | 6-TGN | – | AuROC = 0.594 | – | – | mHBI ≥ 4 on or off steroids or; mHBI <4 requiring steroids | CD—mHBI <4, steroid free, no fistulae for 3 weeks UC—mUCDAI <4 off steroids |
| Nakarai et al. (55) | UC Adult |
158 | AZA/6-MP Biologics |
fHb | <100 ng/mL | – | 92/71 | 37/97 | – | MS = 0 |
| Mooiweer et al. (56) | CD/UC Adult |
164 | AZA/6-MP Biologics |
fHb | 1.5 μg/g | – | 74/84 | 72/84 | – | Assessed ability to predict MS |
| Takashima et al. (57) | UC Pediatric/Adult |
98 | AZA/6MP Biologics |
FIT fCP |
<100 ng/mL <250 μg/g |
– | 95/62 82/62 |
– | – | Assessed ability to predict MES |
AuROC, Area under Receiver Operator Characteristic Curve; AZA, Azathioprine; CD, Crohn's Disease; CDAI, Crohn's Disease Activity Index; CRP, C-Reactive Protein; fCP, fecal calprotectin; fHb, fecal Hemoglobin; FIT, fecal immunochemical tests; Hb, hemoglobin; HBI, Harvey-Bradshaw Index; mHBI, modified Harvey-Bradshaw Index; MES, Mayo endoscopic subscore; MS, Mayo Score; mTWAI, modified Truelove-Witts Activity Index; mUCDAI, modified Ulcerative Colitis Disease Activity Index; NC, neutrophil count; PUCAI, Pediatric Ulcerative Colitis Activity Index; PPV/NPV; negative/positive predictive value; RBCs, Red Blood Cells; SCCAI, Simple Clinical Colitis Activity Index; UC, Ulcerative Colitis; 6-MP, 6-Mercaptopurine; 6-TGN, 6-Thioguanine nucleotides. Gonzalez-Lama et al. (53) tested two different cut-off levels in the same study indicated by the superscript letters a and b.
In 2006, Osterman et al. (50) discussed the results of their meta-analysis of 55 retrospective and cross sectional studies focused on 6-TGN levels and remission. In spite of wide variance in each cohort demographic, results were consistent, providing a cut-off of 230–260 pmol/8 × 108 erythrocytes as a therapeutic target in patients with active disease. Above this level, the authors found patients to be 3.3 times more likely to be in clinical remission. Response prediction had a 62% sensitivity and 72% specificity in this study. However, no optimal time point was suggested for testing 6-TGN levels.
Two years following this review, Kwan et al. (52) published the results of their retrospective analysis of a small cohort (n = 39) of adults receiving either 6-MP or AZA for treatment of IBD. Although mean 6-TGN values were higher in responders than in non-responders a statistical significance was not achieved (p = 0.37). Nevertheless, there was a trend for a better clinical response in patients with 6-TGN levels >230 pmol/8 × 108 RBC, however, the results were statistically insignificant and had a relatively poor accuracy (63% sensitivity, 67% specificity). These results may in part be influenced by the fact that some patients received concomitant corticosteroid and the cohort included the thiopurine treatments 6-MP, AZA, and combined 6-MP/AZA, however, results were collectively analyzed as a whole. Interestingly, in this study, the combination of thiopurine methyltransferase (TPMT) activity (<30.5 U) with 6-TGN metabolite levels (>230 pmol/8 × 108 RBC) was the best predictor of response to thiopurine treatment (p = 0.01). Gonzalez-Lama et al. (53) provide important data in their prospective study of 113 adult IBD patients starting immunosuppression with either 6-MP or AZA given a history of steroid refractory luminal disease. Importantly, 6-TGN levels were taken at 2 weeks and then again at 1, 2, and 4 months after commencing thiopurine therapy. No clinically useful cut off could be identified at any time that would allow 6-TGN levels to predict their primary outcome of 6 months steroid free remission. Sensitivity and specificity values were overall very poor (41% sensitivity and 56% specificity at 2 weeks), but did increase as treatment progressed (68% sensitivity and 62% specificity at 4 months). In being the first study that aims to assess the role of 6-TGN levels early on in therapy as markers of long-term treatment response, further larger studies are required to provide more useful data for translation into clinical practice.
A larger 2010 retrospective study of 346 IBD patients from Waljee et al. (54) compared the predictive accuracy of thiopurine metabolites compared to common laboratory markers. Two hundred and eighteen patients diagnosed with CD were included in the study, where the authors developed an algorithm of laboratory values and patient demographics, comparing results to thiopurine metabolite levels in both responders and non-responders. Such laboratory values included neutrophil count, C-reactive protein (CRP), mean corpuscular volume, alkaline phosphatase and thrombocyte count among others. The areas under the receiver operator characteristics curve (AuROC) of the algorithm using laboratory values and patient age was 0.856 (95%CI 0.793–0.919) and was statistically significantly more predictive of treatment response to thiopurines (p = 0.001) than the measurement of 6-TGN [AuROC = 0.594 (95%CI 0.546–0.642)]. However, as acknowledge by the authors, the patients in the study were perhaps more likely to have thiopurine refractory disease given that recruitment was from a pool of patients at a single tertiary care center where referral of complex patients is common. Further validation of such a model in a prospective analysis is required prior to treatment being adjusted accordingly.
In 2013, Nguyen et al. (51) retrospectively analyzed 86 pediatric IBD patients whose 6-TGN and 6-MeMP levels were taken at 2 monthly intervals during a median of 18 months of AZA therapy. Their analysis of 6-TGN levels was in accordance with the review article provided by Osterman et al. (50), having attained a statistically significant cut off of 250 pmol/8 × 108 erythrocytes at which disease remission was 4.4 times as likely (p = 0.007). Again however, no accurate time point for the cut off was suggested. When evaluating the role of 6-MMP, Nguyen et al. (51) found it was not able to predict response to AZA.
The studies discussed above are an example of both the heterogeneity of populations analyzed and the differing results published when it comes to thiopurine metabolite use as a novel biomarker for predicting response to immunomodulator therapy in IBD.
Yet, in what is perhaps the most comprehensive meta-analysis of 6-TGN levels and remission in IBD, Estevinho et al. (58) systematically reviewed all published evidence of thiopurine use in IBD up to 2017. After screening over 1,000 studies identified by searching four online databases, 25 studies were ultimately deemed eligible for analysis of 6-TGN mean value and cut off levels. Naturally, there was marked heterogeneity in study design and size analyzed, yet mean 6-TGN levels were seen to be higher in those in clinical remission across the board, with a pooled difference of 63 pmol/8 × 108 RBC. A global analysis of cut of levels used found that patients with a 6-TGN level above cut off were 3.95 times more likely to be in remission. Additionally, when the thresholds were analyzed separately, the highest odds ratio (4.71) was found when using a cut off of 250 pmol/8 × 108 RBC.
With these results being similar to the aforementioned 2006 meta-analysis from Osterman et al. (50), the current evidence surrounding the relationship between 6-TGN levels and clinical remission may be used to guide clinical decisions. However, the ability of 6-TGN levels to accurately predict response to therapy with thiopurines is currently clinically insignificant given the modest sensitivity and specificity values published to date.
Blood Cell Counts
As a simple and low-cost alternative to measure concentrations of 6-TGNs in patients during AZA/6-MP treatment, the evaluation of hematologic parameters, such as counts of white or red blood cells are discussed here as surrogate markers.
Studies in 166 patients with inflammatory bowel disease reveal that the mean corpuscular volume (MCV) correlated with the RBC 6-TGN concentration (r = 0.33, p < 0.001) (59). This was supported by a 5-years database study (60) revealing a positive correlation between MCV and 6-TGN. Furthermore, it has been shown that the change of MCV (ΔMCV) correlates with intracellular 6-TGN levels which may be useful for monitoring of intracellular metabolite levels (46). In contrast to MCV, RBC count, WBC and absolute neutrophil count (ANC) correlated negatively with RBC 6-TGN concentrations during AZA or 6-MP treatment (r = −0.16, −0.28, −0.19, respectively). Interestingly, the concentration of the thiopurine metabolite 6-MeMP did not correlate with the specified hematologic parameters. Although in patients, who received a 6-TGN treatment the WBC and the platelet count correlated positively with the 6-TGN concentration, this was, however, not reproducible in different disease subgroups indicating the effect of 6-TGNs alone on bone marrow function is rather slight compared to a combination of different downstream metabolites of AZA or 6-MP.
According to the assumption that 6-TGN levels correlate with the efficacy of thiopurine therapy it was evaluated whether hematologic surrogate markers for thiopurine metabolites are suitable for prediction of remission or relapse. In this context, a meta-analysis in 2002 included 424 patients with IBD revealed that the ANC, WBC as well as the MCV were predictive factors of achieving remission after 6 months of AZA treatment (61). Therefore, the ANC and the WBC were higher in patients which achieved remission while the MCV was lower compared with patients where remission was not achieved (p = 0.0001). Furthermore, Cox regression analysis revealed that in patients who achieved remission a WBC < 5.0 × 109 was associated with a lower risk of relapse (p = 0.03). In the SONIC trial, an ΔMCV > 7 after 26 weeks of oral AZA therapy was associated with a higher proportion of steroid-free remission (62). However, the analysis of 15 studies did not provide sufficient evidence that ΔMCV can predict clinical remission after AZA or 6-MP treatment (46).
Biomarkers of IBD Activity
Induction and maintenance of clinical remission, characterized by the absence of mucosal damage and inflammation is one main focus of IBD treatment (63). In this context, hematologic biomarkers, non-specific to IBD or treatment, such as CRP are commonly used to monitor states of inflammation. Additionally, fecal biomarkers, such as fecal calprotectin (fCP), fecal immunochemical tests (FIT) or fecal hemoglobin (fHb) are discussed as biomarkers for mucosal healing.
Hematological Biomarkers
CRP is an acute phase protein primarily synthesized in the liver and released from hepatocytes following proinflammatory signaling via interleukin (IL-) 1 and IL-6 along with tumor necrosis factor-alpha (TNF-α) (7, 8). In a non-inflammatory state, CRP levels are often <1 mg/L with rapid increases in concentration observed following acute inflammatory events (64). As such, CRP is a long-known serum marker for inflammation within the body and is currently used for diagnostic purposes and as a means of measuring disease activity in IBD. However, the high titres of CRP often observed in CD are not common in UC patients, where it is common to see little to no elevation in CRP concentration (5, 65–67). With CRP concentrations shown to fluctuate with disease activity, results regarding its utility in predicting relapse are to date variable (Table 1). Furthermore, due to genetic polymorphisms in the CRP gene, 20–25% of CD patients do not produce increased CRP levels during acute inflammation (68). In these patients CRP levels would not reflect internal states of inflammation and are therefore not suitable as a surrogate marker of (intestinal) inflammation.
Whilst there is limited literature solely focused on CRP predicting response to thiopurine treatment, a 2012 study from Park et al. (47) retrospectively observed 82 CD patients who received their first course of AZA or 6-MP with initial fortnightly follow up for the first month before commencing three monthly reviews. Aged between 14 and 45 years, patients with a CRP >5 mg/L at the time of remission induced by thiopurine treatment were more likely to experience relapse than those with a CRP <5 mg/L (p = 0.009). The vast majority of patients with an elevated CRP experienced relapse within 24 months of remission induction.
From a different stand point, Lémann et al. published in 2005 their results of observing 83 adult CD patients over the first 18 months after withdrawal of AZA or 6-MP treatment (48). Whilst the primary outcome of their study was to identify any difference in the time to relapse between patients who continued AZA or received a placebo after an initial period of 2 years on AZA, the authors noted that a CRP level >20 mg/L as well as a Hb level <12 g/dL after 2 years of AZA treatment, did in fact predict relapse to occur within the next 18 months of treatment when it came to sub-analysis.
Additionally, Treton et al. (49) followed the Lemann et al. (48) cohort further to assess response to AZA treatment in patients in clinical remission after continuous treatment with AZA for at least 42 months. After AZA interruption the median follow-up period were 47 months. Univariate analysis identified that a CRP level >20 mg/L after AZA interruption predicted relapse (p = 0.002). Independently from CRP, the blood levels of Hb < 12 g/dl and NC > 4 × 109/l were associated with an increased risk of relapse (p = 0.04, p = 0.003, respectively).
Whilst we discuss the role of biomarkers for prediction of relapse elsewhere (63), these results support a high CRP following long term AZA treatment as being predictive of treatment failure.
Fecal Biomarkers
As an indicator of underlying mucosal damage, gastrointestinal blood loss is an important feature of IBD and is beginning to gather interest as a future biomarker of disease activity. In spite of its use as a screening tool in colorectal cancer (69), the utility of fecal immunochemical tests (FITs) as a marker of occult blood loss in IBD has been studied to a far lesser extent. In ulcerative colitis patients, FIT and calprotectin effectively reflect the state of mucosal inflammation and detect active UC better than remission (70). As a result, FITs may be useful in reducing the need for invasive endoscopic examination. The degree of research focused on fHb as a biomarker of intestinal inflammation is itself limited, with only a handful of studies assessing its utility in comparison with FC. McDonald et al. showed low fHb to be an accurate rule out study for IBD activity when comparing to colonoscopy (71). Similar finding were shown elsewhere where negative fHb was concluded as a good indicator of no significant bowel disease (72). Considering the fact that fHb is a general marker for mucosal healing and not specific for thiopurine therapy, we provide a brief account of studies where a large proportion of participants received thiopurine therapy and identify fHb as a biomarker able to predict disease activity with accuracy comparative to that of the well-studied fCP (Table 1). In these studies, all data was analyzed as a collective irrespective of treatment, as such the accuracy in which results predict response to thiopurines is confounded by participants receiving other therapeutic agents.
In 2012, Nakarai et al. (55) published their findings having evaluated the relationship between colonoscopy findings and FIT result in 158 adult patients with UC. With 42% of these patients treated with thiopurines, fecal samples were taken on the day of colonoscopy or within a month of the colonoscopy for FIT. The authors found a fHb level <100 ng/mL predicted mucosal healing as defined by a Mayo score (73) of 0 or 1 at colonoscopy with a sensitivity of 60% and specificity of 87%. Sensitivity increased to 92% when predicting a Mayo score of 0 at the same cut off with a reduction in specificity to 71%. When using a cut off of <60 ng/mL the predictive value was not any more superior for Mayo score 0 or 1 with a modest increase when predicting a Mayo score of 0. Two further studies have been published comparing the predictive value of FIT to fCP in adult CD and UC patient populations. The first of which compared the two biomarkers against the level of intestinal inflammation found during surveillance colonoscopies (56). Forty percent of the 164 patients received thiopurine therapy, 63% of all patients had a Mayo score of 0. The ability to predict inflammation in both UC and CD patients was seen to be comparable between both fCP and FIT. Using a cut-off of 1.51 μg/g, fHb predicted inflammation with 74% sensitivity and 84% specificity relative to the 86% sensitivity and 72% specificity provided by a fCP level above 140 mg/g. Pertinent to our review, the authors did not provide data regarding the long-term treatment response in these patients. Similar results were found by Takashima et al. (57) in 2015, however they found the FIT values to be slightly more sensitive (95%) in predicting a Mayo score of 0 at a level <100 ng/ml compared to multiple fCP cut-offs (77% <200 μg/g and 82% at 250 μg/g). Furthermore, detailed reviews on the broader role of FIT in IBD compared to fCP (74) described a similar school of thought where an increase in sensitivity seen between FIT compared to fCP is often noted when predicting the more strict Mayo score of 0. Given the large body of evidence surrounding fCP and IBD more broadly, the current comparative accuracy of FIT makes it promising low-cost way of measuring disease activity and with more tailored future investigation, a candidate biomarker for predicting treatment response upon the publication of such studies in the future.
Calprotectin is a calcium-binding protein complex of two damage-associated molecular pattern proteins (DAMP), S100A8/S100A9, mainly found in neutrophils (75, 76). It can be measured in feces by enzyme-linked immunosorbent assay (ELISA), and is known to correlate well with intestinal inflammation (75). Its use in distinguishing between irritable bowel syndrome and IBD is well-established, and it is being increasingly used to inform management of those with established IBD (77). Its potential use for the prediction of treatment responses is a new area of investigation for the use of calprotectin (Table 1). The stated cut-off values of fCP for clinical significance reported in many studies is not standardized, and they are often quoted in individual studies at levels that optimize the sensitivity and specificity seen in each individual cohort. In clinical practice, given not only the wide variety of presentation and severity of disease, but also the known variability of intra-individual fCP levels with active UC (78, 79), a percentage decrease from baseline fCP level might be more informative. With regard to the role of fCP in predicting response to thiopurine therapy, there are to our knowledge to date no published studies that interpret its predictive utility. The detailed role of fCP in IBD along with its ability to predict response to other forms of treatment than thiopurines alone are described in a number of up to date reviews (11, 12, 63, 80–84).
Conclusions
Currently, the role of biomarkers in predicting treatment response to thiopurine therapy is not as well-studied compared to the large body of current evidence surrounding the role of biomarkers in biological therapies (11, 12, 63, 85). The current understanding of fCP in particular as not only a marker of disease activity but also as predictor of relapse and treatment response is well-studied in the context of biological therapy but not yet in patients treated with immunomodulator monotherapy. Fecal CP has been clearly shown to correlate with intestinal inflammation. With strong evidence for its use as a surrogate marker for intestinal inflammation (77) we have previously discussed the increasing evidence for its use in predicting relapse (63). Although more evidence is required, it appears that fCP is the most promising of all biomarkers studied to date in the context of individualizing IBD treatment (Figure 2). However, its role in predicting thiopurine response is poorly studied. Unfortunately, whilst many studies assessing the predictive value of fCP include patients on thiopurines, there is no sub-analyses of these cohorts alone and as such there is no real knowledge of how it represents the efficacy of immunomodulation. To see future studies assessing the role of fCP as predictor of thiopurine response would be important not only for guiding thiopurine treatment, but also to provide more robust knowledge of the potential use of fCP in all IBD patients.
Figure 2.
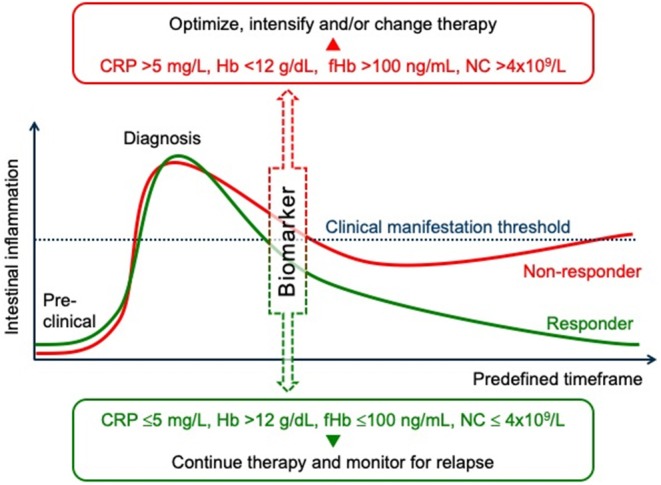
Prediction of treatment responses in inflammatory bowel diseases. An outline of disease activity, from preclinical symptoms through to remission as indicated by a clinical manifestation threshold. At a predefined stage of the induction therapy (e.g., after 1–3 months according to the treat to target strategy), biomarkers, such as C-reactive protein (CRP), hemoglobin (Hb), fecal hemoglobin (fHb), or neutrophile count (NC) correlate with intestinal inflammation and can predict the response to treatment. Early assessment of treatment efficacy using such surrogate markers, and in conjunction with other biochemical tests, clinical signs, and/or imaging studies, can help to adjust treatment in case of persistent inflammatory disease activity. Such an individualized approach/algorithm can help to achieve mucosal healing and thus to avoid long-term bowel damage and subsequent disability.
Similarly, the role of CRP is to date studied in a small number of modest sized cohorts with CD. Its ability to predict response to thiopurine treatment is encouraging based upon on the findings of our review and even more so given the wider evidence base for its role in guiding IBD treatment.
The measurement of thiopurine metabolite levels is currently the most well-studied potential biomarker for predicting response to thiopurine treatment. Yet, the level of evidence is still well below what would be required for a safe translation into clinical practice. A greater number of large prospective trials and randomization is yet required prior to 6-TGN and 6-MeMP levels being used to individualize IBD treatment.
Future Directions
The focus of this article has been predominantly in terms of investigations that are already currently in clinical use. However, development of basic science knowledge, particularly in genomics and immunology, does suggest to us what clinicians will use in the future. The reader is directed to some excellent reviews on the use of genetics and immunology in predicting treatment responses and disease course (1), including the use of gene expression signatures from involved tissue and genetic variability at non-IBD susceptibility loci, for example, apoptosis-related genes for T cells (86, 87). The early detection of treatment failure and prediction of treatment responses are important in maximizing efficiency, and minimizing cost and side effects, and can be considered to be the first step in achieving the goal of individualized IBD management (88). The low population response rate of most treatment regimens for IBD lends itself to the emerging paradigm of CD and UC having many subtypes (89), therefore, increasing the importance of tailoring treatment to the individual. It is likely that the four described “-omes” that together determine the development of IBD and its clinical course (exposome, microbiome, immunome, and genome) (90) may well also affect treatment response (1). Therefore, as we gain further insights into each of these domains our ability to predict treatment outcome will improve. Given the many treatment options for IBD which are approaching on the horizon further research in the prediction and accurate determination of treatment response will be ever more critical.
Author Contributions
JC and JD analyzed and interpreted the data. JC wrote the first draft of the article. JD and EW created the figures. JD and EW contributed to the writing of the article. All authors performed the literature review, read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- 6-MP
6-mercaptopurine
- 6-TGNs
6-thioguanine nucleotides
- 6-MeMP
6-methylmercaptopurine
- AuROC
area under the receiver operator characteristics curve
- ANC
absolute neutrophil count
- AZA
azathioprine
- CRP
C-reactive protein
- CD
Crohn's disease
- CDAI
Crohn's Disease Activity Index
- DNA
deoxyribonucleic acid
- GTP
Guanosin triphosphate
- GTPase Rac1
Rac family small GTPase 1
- fCP
fecal calprotectin
- fHb
fecal hemoglobin
- FIT
fecal immunochemical tests
- Hb
hemoglobin
- HBI
Harvey-Bradshaw Index
- IBD
inflammatory bowel disease
- IFX
infliximab
- IL
interleukin
- meTIMP
methylthioinosine monophosphate
- MCV
mean corpuscular volume
- MH
mucosal healing
- mHBI
Modified Harvey-Bradshaw Index
- MS
Mayo score
- mTWAI
Modified Truelove-Witts Activity Index
- mUCDAI
Modified Ulcerative Colitis Disease Activity Index
- NC
neutrophil count
- NPV
negative predictive value
- NUDT15
nudix hydrolase 15
- PPAT
phosphoribosyl pyrophosphate
- PPV
positive predictive value
- PUCAI
Pediatric Ulcerative Colitis Activity Index
- RBC
red blood cells
- SCCAI
Simple Clinical Colitis Activity Index
- TGTP
thioguanosine triphosphate
- TPMT
thiopurine methyltransferase
- UC
ulcerative colitis
- WBC
white blood cell count.
Footnotes
Funding. The publication of this article was funded by the Open Access Fund of the University of Rostock.
References
- 1.Gerich ME, McGovern DP. Towards personalized care in IBD. Nat Rev Gastroenterol Hepatol. (2014) 11:287. 10.1038/nrgastro.2013.242 [DOI] [PubMed] [Google Scholar]
- 2.Mosli MH, Sandborn WJ, Kim RB, Khanna R, Al-Judaibi B, Feagan BG. Toward a personalized medicine approach to the management of inflammatory bowel disease. Am J Gastroenterol. (2014) 109:994–1004. 10.1038/ajg.2014.110 [DOI] [PubMed] [Google Scholar]
- 3.Cornillie F, Hanauer SB, Diamond RH, Wang J, Tang KL, Xu Z, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. (2014) 63:1721–7. 10.1136/gutjnl-2012-304094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Vos M, Dewit O, D'Haens G, Baert F, Fontaine F, Vermeire S, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naive patients with ulcerative colitis. J Crohns Colitis. (2012) 6:557–62. 10.1016/j.crohns.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 5.Grover Z, Biron R, Carman N, Lewindon P. Predictors of response to Infliximab in children with luminal Crohn's disease. J Crohns Colitis. (2014) 8:739–46. 10.1016/j.crohns.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 6.Guidi L, Marzo M, Andrisani G, Felice C, Pugliese D, Mocci G, et al. Faecal calprotectin assay after induction with anti-tumour necrosis factor alpha agents in inflammatory bowel disease: prediction of clinical response and mucosal healing at one year. Dig Liver Dis. (2014) 46:974–9. 10.1016/j.dld.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 7.Jurgens M, Mahachie John JM, Cleynen I, Schnitzler F, Fidder H, van Moerkercke W, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn's disease. Clin Gastroenterol Hepatol. (2011) 9:421–7.e1. 10.1016/j.cgh.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Magro F, Rodrigues-Pinto E, Santos-Antunes J, Vilas-Boas F, Lopes S, Nunes A, et al. High C-reactive protein in Crohn's disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis. (2014) 8:129–36. 10.1016/j.crohns.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 9.Molander P, af Bjorkesten CG, Mustonen H, Haapamaki J, Vauhkonen M, Kolho KL, et al. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFalpha blocking agents. Inflamm Bowel Dis. (2012) 18:2011–7. 10.1002/ibd.22863 [DOI] [PubMed] [Google Scholar]
- 10.Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn's disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. (2012) 35:568–76. 10.1111/j.1365-2036.2011.04987.x [DOI] [PubMed] [Google Scholar]
- 11.Barre A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. (2018) 47:896–905. 10.1111/apt.14550 [DOI] [PubMed] [Google Scholar]
- 12.Naviglio S, Giuffrida P, Stocco G, Lenti MV, Ventura A, Corazza GR, et al. How to predict response to anti-tumour necrosis factor agents in inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. (2018) 12:797–810. 10.1080/17474124.2018.1494573 [DOI] [PubMed] [Google Scholar]
- 13.Axelrad JE, Roy A, Lawlor G, Korelitz B, Lichtiger S. Thiopurines and inflammatory bowel disease: current evidence and a historical perspective. World J Gastroenterol. (2016) 22:10103–17. 10.3748/wjg.v22.i46.10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. (2015) 2015:CD000067 10.1002/14651858.CD000067.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon M, Taylor K, Akobeng AK, Thomas AG. Azathioprine and 6-mercaptopurine for maintenance of surgically-induced remission in Crohn's disease. Cochrane Database Syst Rev. (2014) 2016:CD010233 10.1002/14651858.CD010233.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmer A, McDonald JW, Tsoulis DJ, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. (2012) CD000478. 10.1002/14651858.CD000478.pub3 [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. (2010) 362:1383–95. 10.1056/NEJMoa0904492 [DOI] [PubMed] [Google Scholar]
- 18.Cuffari C. A physician's guide to azathioprine metabolite testing. Gastroenterol Hepatol. (2006) 2:58–63. [PMC free article] [PubMed] [Google Scholar]
- 19.Warner B, Johnston E, Arenas-Hernandez M, Marinaki A, Irving P, Sanderson J. A practical guide to thiopurine prescribing and monitoring in IBD. Frontl Gastroenterol. (2018) 9:10–5. 10.1136/flgastro-2016-100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim SZ, Chua EW. Revisiting the role of thiopurines in inflammatory bowel disease through pharmacogenomics and use of novel methods for therapeutic drug monitoring. Front Pharmacol. (2018) 9:1107. 10.3389/fphar.2018.01107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. Thiopurine pathway. Pharm Genomics. (2010) 20:573–4. 10.1097/FPC.0b013e328334338f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. (2003) 111:1133–45. 10.1172/JCI16432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quéméneur L, Gerland LM, Flacher M, Ffrench M, Revillard JP, Genestier L. Differential control of cell cycle, proliferation, and survival of primary T lymphocytes by purine and pyrimidine nucleotides. J Immunol. (2003) 170:4986–95. 10.4049/jimmunol.170.10.4986 [DOI] [PubMed] [Google Scholar]
- 24.Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, et al. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol. (2006) 176:640–51. 10.4049/jimmunol.176.1.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karim H, Ghalali A, Lafolie P, Vitols S, Fotoohi AK. Differential role of thiopurine methyltransferase in the cytotoxic effects of 6-mercaptopurine and 6-thioguanine on human leukemia cells. Biochem Biophys Res Commun. (2013) 437:280–6. 10.1016/j.bbrc.2013.06.067 [DOI] [PubMed] [Google Scholar]
- 26.Bökkerink JP, De Abreu RA, Bakker MA, Hulscher TW, Van Baal JM, Schretlen ED, et al. Effects of methotrexate on purine and pyrimidine metabolism and cell-kinetic parameters in human malignant lymphoblasts of different lineages. Biochem Pharmacol. (1988) 37:2329–38. 10.1016/0006-2952(88)90359-0 [DOI] [PubMed] [Google Scholar]
- 27.Kaskas BA, Louis E, Hindorf U, Schaeffeler E, Deflandre J, Graepler F, et al. Safe treatment of thiopurine S-methyltransferase deficient Crohn's disease patients with azathioprine. Gut. (2003) 52:140–2. 10.1136/gut.52.1.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coenen MJ, de Jong DJ, van Marrewijk CJ, Derijks LJ, Vermeulen SH, Wong DR, et al. Identification of patients with variants in TPMT and dose reduction reduces hematologic events during thiopurine treatment of inflammatory bowel disease. Gastroenterology. (2015) 149:907–17.e7. 10.1053/j.gastro.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 29.Blaker PA, Arenas-Hernandez M, Smith MA, Shobowale-Bakre EA, Fairbanks L, Irving PM, et al. Mechanism of allopurinol induced TPMT inhibition. Biochem Pharmacol. (2013) 86:539–47. 10.1016/j.bcp.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 30.Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. (2005) 22:441–6. 10.1111/j.1365-2036.2005.02583.x [DOI] [PubMed] [Google Scholar]
- 31.Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis. (2012) 6:905–12. 10.1016/j.crohns.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 32.Leung Y, Sparrow MP, Schwartz M, Hanauer SB. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis. (2009) 3:162–7. 10.1016/j.crohns.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 33.Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. (2007) 5:209–14. 10.1016/j.cgh.2006.11.020 [DOI] [PubMed] [Google Scholar]
- 34.Moreau B, Clement P, Theoret Y, Seidman EG. Allopurinol in combination with thiopurine induces mucosal healing and improves clinical and metabolic outcomes in IBD. Ther Adv Gastroenterol. (2017) 10:819–27. 10.1177/1756283X17733657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. (2017) 153:827–34. 10.1053/j.gastro.2017.07.032 [DOI] [PubMed] [Google Scholar]
- 36.Gisbert JP, Nino P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. (2006) 101:2769–76. 10.1111/j.1572-0241.2006.00843.x [DOI] [PubMed] [Google Scholar]
- 37.Lennard L, Chew TS, Lilleyman JS. Human thiopurine methyltransferase activity varies with red blood cell age. Br J Clin Pharmacol. (2001) 52:539–46. 10.1046/j.0306-5251.2001.01497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. (2016) 48:367–73. 10.1038/ng.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. (2014) 46:1017. 10.1038/ng.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin D, Xia X, Zhang J, Zhang S, Liao F, Zhang G, et al. Impact of NUDT15 polymorphisms on thiopurines-induced myelotoxicity and thiopurines tolerance dose. Oncotarget. (2017) 8:13575–85. 10.18632/oncotarget.14594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. (2005) 42:481–9. 10.1002/hep.20800 [DOI] [PubMed] [Google Scholar]
- 42.Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. (2013) 144:1419–25. e3. 10.1053/j.gastro.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 43.Björnsson ES, Gu J, Kleiner DE, Chalasani N, Hayashi PH, Hoofnagle JH, et al. Azathioprine and 6-mercaptopurine-induced liver injury: clinical features and outcomes. J Clin Gastroenterol. (2017) 51:63–9. 10.1097/MCG.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simsek M, Meijer B, Mulder CJJ, van Bodegraven AA, de Boer NKH. Analytical pitfalls of therapeutic drug monitoring of thiopurines in patients with inflammatory bowel disease. Ther Drug Monit. (2017) 39:584–8. 10.1097/FTD.0000000000000455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright S, Sanders DS, Lobo AJ, Lennard L. Clinical significance of azathioprine active metabolite concentrations in inflammatory bowel disease. Gut. (2004) 53:1123–8. 10.1136/gut.2003.032896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dujardin RW, Meijer B, de Boer NK, D'Haens GR, Lowenberg M. Usefulness of mean corpuscular volume as a surrogate marker for monitoring thiopurine treatment in inflammatory bowel disease. Eur J Gastroenterol Hepatol. (2016) 28:991–6. 10.1097/MEG.0000000000000675 [DOI] [PubMed] [Google Scholar]
- 47.Park JJ, Cheon JH, Hong SP, Kim TI, Kim WH. Outcome predictors for thiopurine maintenance therapy in patients with Crohn's disease. Dig Dis Sci. (2012) 57:133–41. 10.1007/s10620-011-1955-9 [DOI] [PubMed] [Google Scholar]
- 48.Lemann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, et al. Groupe D'Etude Therapeutique des Affections Inflammatoires du Tube D. A randomized, double-blind, controlled withdrawal trial in Crohn's disease patients in long-term remission on azathioprine. Gastroenterology. (2005) 128:1812–8. 10.1053/j.gastro.2005.03.031 [DOI] [PubMed] [Google Scholar]
- 49.Treton X, Bouhnik Y, Mary JY, Colombel JF, Duclos B, Soule JC, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. (2009) 7:80–85. 10.1016/j.cgh.2008.08.028 [DOI] [PubMed] [Google Scholar]
- 50.Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. (2006) 130:1047–53. 10.1053/j.gastro.2006.01.046 [DOI] [PubMed] [Google Scholar]
- 51.Nguyen TV, Vu DH, Nguyen TM, Lachaux A, Boulieu R. Exploring associations of 6-thioguanine nucleotide levels and other predictive factors with therapeutic response to azathioprine in pediatric patients with IBD using multilevel analysis. Inflamm Bowel Dis. (2013) 19:2404–10. 10.1097/MIB.0b013e3182a508c6 [DOI] [PubMed] [Google Scholar]
- 52.Kwan LY, Devlin SM, Mirocha JM, Papadakis KA. Thiopurine methyltransferase activity combined with 6-thioguanine metabolite levels predicts clinical response to thiopurines in patients with inflammatory bowel disease. Dig Liver Dis. (2008) 40:425–32. 10.1016/j.dld.2008.01.003 [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez-Lama Y, Bermejo F, Lopez-Sanroman A, Garcia-Sanchez V, Esteve M, Cabriada JL, et al. Thiopurine methyl-transferase activity and azathioprine metabolite concentrations do not predict clinical outcome in thiopurine-treated inflammatory bowel disease patients. Aliment Pharmacol Ther. (2011) 34:544–54. 10.1111/j.1365-2036.2011.04756.x [DOI] [PubMed] [Google Scholar]
- 54.Waljee AK, Joyce JC, Wang S, Saxena A, Hart M, Zhu J, et al. Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol. (2010) 8:143–50. 10.1016/j.cgh.2009.09.031 [DOI] [PubMed] [Google Scholar]
- 55.Nakarai A, Kato J, Hiraoka S, Kuriyama M, Akita M, Hirakawa T, et al. Evaluation of mucosal healing of ulcerative colitis by a quantitative fecal immunochemical test. Am J Gastroenterol. (2013) 108:83–9. 10.1038/ajg.2012.315 [DOI] [PubMed] [Google Scholar]
- 56.Mooiweer E, Fidder HH, Siersema PD, Laheij RJ, Oldenburg B. Fecal hemoglobin and calprotectin are equally effective in identifying patients with inflammatory bowel disease with active endoscopic inflammation. Inflamm Bowel Dis. (2014) 20:307–14. 10.1097/01.MIB.0000438428.30800.a6 [DOI] [PubMed] [Google Scholar]
- 57.Takashima S, Kato J, Hiraoka S, Nakarai A, Takei D, Inokuchi T, et al. Evaluation of mucosal healing in ulcerative colitis by fecal calprotectin vs. fecal immunochemical test. Am J Gastroenterol. (2015) 110:873–80. 10.1038/ajg.2015.66 [DOI] [PubMed] [Google Scholar]
- 58.Estevinho MM, Afonso J, Rosa I, Lago P, Trindade E, Correia L, et al. A systematic review and meta-analysis of 6-thioguanine nucleotide levels and clinical remission in inflammatory bowel disease. J Crohns Colitis. (2017) 11:1381–92. 10.1093/ecco-jcc/jjx089 [DOI] [PubMed] [Google Scholar]
- 59.Thomas CW, Jr., Lowry PW, Franklin CL, Weaver AL, Myhre GM, Mays DC, et al. Erythrocyte mean corpuscular volume as a surrogate marker for 6-thioguanine nucleotide concentration monitoring in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Inflamm Bowel Dis. (2003) 9:237–45. 10.1097/00054725-200307000-00004 [DOI] [PubMed] [Google Scholar]
- 60.Meijer B, Wilhelm AJ, Mulder CJ, Bouma G, Van Bodegraven AA, De Boer NK. Pharmacology of thiopurine therapy in inflammatory bowel disease and complete blood cell count outcomes: a 5-year database study. Ther Drug Monit. (2017) 39:399. 10.1097/FTD.0000000000000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fraser A, Orchard T, Jewell D. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. (2002) 50:485–9. 10.1136/gut.50.4.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bouguen G, Sninsky C, Tang KL, Colombel JF, D'Haens G, Kornbluth A, et al. Change in erythrocyte mean corpuscular volume during combination therapy with azathioprine and infliximab is associated with mucosal healing: a post-hoc analysis from SONIC. Inflamm Bowel Dis. (2015) 21:606–14. 10.1097/MIB.0000000000000302 [DOI] [PubMed] [Google Scholar]
- 63.Musci JO, Cornish JS, Dabritz J. Utility of surrogate markers for the prediction of relapses in inflammatory bowel diseases. J Gastroenterol. (2016) 51:531–47. 10.1007/s00535-016-1191-3 [DOI] [PubMed] [Google Scholar]
- 64.Fengming Y, Jianbing W. Biomarkers of inflammatory bowel disease. Dis Markers. (2014) 2014:710915. 10.1155/2014/710915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beattie RM, Walker-Smith JA, Murch SH. Indications for investigation of chronic gastrointestinal symptoms. Arch Dis Child. (1995) 73:354–5. 10.1136/adc.73.4.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kushner I. C-reactive protein and the acute-phase response. Hosp Pract (Off Ed). (1990) 25:13, 16, 21–8. [PubMed] [Google Scholar]
- 67.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful magic or unnecessary toys? Gut. (2006) 55:426–31. 10.1136/gut.2005.069476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones J, Loftus EV, Jr., Panaccione R, Chen LS, Peterson S, Mcconnell J, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol. (2008) 6:1218–24. 10.1016/j.cgh.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 69.Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, et al. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. (2007) 146:244–55. 10.7326/0003-4819-146-4-200702200-00003 [DOI] [PubMed] [Google Scholar]
- 70.Kim DJ, Jeoun YM, Lee DW, Koo JS, Lee SW. Usefulness of fecal immunochemical test and fecal calprotectin for detection of active ulcerative colitis. Intest Res. (2018) 16:563. 10.5217/ir.2018.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McDonald PJ, Digby J, Innes C, Strachan JA, Carey FA, Steele RJ, et al. Low faecal haemoglobin concentration potentially rules out significant colorectal disease. Colorectal Dis. (2013) 15:e151–9. 10.1111/codi.12087 [DOI] [PubMed] [Google Scholar]
- 72.Mowat C, Digby J, Strachan JA, Wilson R, Carey FA, Fraser CG, et al. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut. (2016) 65:1463. 10.1136/gutjnl-2015-309579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. (1987) 317:1625–9. 10.1056/NEJM198712243172603 [DOI] [PubMed] [Google Scholar]
- 74.Kato J, Hiraoka S, Nakarai A, Takashima S, Inokuchi T, Ichinose M. Fecal immunochemical test as a biomarker for inflammatory bowel diseases: can it rival fecal calprotectin? Intest Res. (2016) 14:5–14. 10.5217/ir.2016.14.1.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Røseth A, Schmidt P, Fagerhol M. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. (1999) 34:50–4. 10.1080/00365529950172835 [DOI] [PubMed] [Google Scholar]
- 76.Sipponen T. Diagnostics and prognostics of inflammatory bowel disease with fecal neutrophil-derived biomarkers calprotectin and lactoferrin. Dig Dis. (2013) 31:336–44. 10.1159/000354689 [DOI] [PubMed] [Google Scholar]
- 77.Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. (2015) 149:1275–85.e2. 10.1053/j.gastro.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 78.Calafat M, Cabré E, Mañosa M, Lobatón T, Marín L, Domènech E. High within-day variability of fecal calprotectin levels in patients with active ulcerative colitis: what is the best timing for stool sampling? Inflamm Bowel Dis. (2015) 21:1072–6. 10.1097/MIB.0000000000000349 [DOI] [PubMed] [Google Scholar]
- 79.Lasson A, Stotzer PO, Ohman L, Isaksson S, Sapnara M, Strid H. The intra-individual variability of faecal calprotectin: a prospective study in patients with active ulcerative colitis. J Crohns Colitis. (2015) 9:26–32. 10.1016/j.crohns.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 80.Ikhtaire S, Shajib MS, Reinisch W, Khan WI. Fecal calprotectin: its scope and utility in the management of inflammatory bowel disease. J Gastroenterol. (2016) 51:434–46. 10.1007/s00535-016-1182-4 [DOI] [PubMed] [Google Scholar]
- 81.Manceau H, Chicha-Cattoir V, Puy H, Peoc'h K. Fecal calprotectin in inflammatory bowel diseases: update and perspectives. Clin Chem Lab Med. (2017) 55:474–83. 10.1515/cclm-2016-0522 [DOI] [PubMed] [Google Scholar]
- 82.Ministro P, Martins D. Fecal biomarkers in inflammatory bowel disease: how, when and why? Expert Rev Gastroenterol Hepatol. (2017) 11:317–28. 10.1080/17474124.2017.1292128 [DOI] [PubMed] [Google Scholar]
- 83.Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. (2016) 9:21–9. 10.2147/CEG.S51902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhulina Y, Cao Y, Amcoff K, Carlson M, Tysk C, Halfvarson J. The prognostic significance of faecal calprotectin in patients with inactive inflammatory bowel disease. Aliment Pharmacol Ther. (2016) 44:495–504. 10.1111/apt.13731 [DOI] [PubMed] [Google Scholar]
- 85.Khanna R, Narula N, Feagan BG. The role of biomarkers in clinical trials of inflammatory bowel disease. Inflamm Bowel Dis. (2018) 24:1619–23. 10.1093/ibd/izy195 [DOI] [PubMed] [Google Scholar]
- 86.Hlavaty T, Pierik M, Henckaerts L, Ferrante M, Joossens S, van Schuerbeek N, et al. Polymorphisms in apoptosis genes predict response to infliximab therapy in luminal and fistulizing Crohn's disease. Aliment Pharmacol Ther. (2005) 22:613–26. 10.1111/j.1365-2036.2005.02635.x [DOI] [PubMed] [Google Scholar]
- 87.Hlavaty T, Ferrante M, Henckaerts L, Pierik M, Rutgeerts P, Vermeire S. Predictive model for the outcome of infliximab therapy in Crohn's disease based on apoptotic pharmacogenetic index and clinical predictors. Inflamm Bowel Dis. (2007) 13:372–9. 10.1002/ibd.20024 [DOI] [PubMed] [Google Scholar]
- 88.Yang SK. Personalizing IBD therapy: the Asian perspective. Dig Dis. (2016) 34:165–74. 10.1159/000443134 [DOI] [PubMed] [Google Scholar]
- 89.D'Haens GR, Sartor RB, Silverberg MS, Petersson J, Rutgeerts P. Future directions in inflammatory bowel disease management. J Crohns Colitis. (2014) 8:726–34. 10.1016/j.crohns.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 90.Fiocchi C. Integrating omics: the future of IBD? Dig Dis. (2014) 32:96–102. 10.1159/000367836 [DOI] [PubMed] [Google Scholar]