Abstract
Objective
To establish a model of human implantation that responds to hormonal stimuli and can differentiate between endometrium from fertile women and those with idiopathic infertility.
Design
A trophoblast stem cell (trophectodermal) line (TSC; derived from human pre-implantation embryo) was used to form trophectodermal spheroids (TS). TS attachment to monolayers of endometrial epithelial cell lines or primary endometrial epithelial cells (pHEECs) was determined.
Setting
Independent Medical Research Institute with close clinical linkages
Interventions
Spheroid attachment and outgrowth was determined with added hormones (estradiol 17β (E), E + medroxyprogesterone acetate (MPA) or E + MPA + human chorionic gonadotropin (hCG)). Spheroid attachment to E/MPA treated pHEEC prepared from fertile women or those with idiopathic infertility tested.
Main outcome measure
Firmly attached spheroids counted after co-culture for 6 h. Outgrowth was determined by quantitation of area covered by spheroid after firm adhesion.
Results
Functional adhesion of TS to two endometrial epithelial cell lines, Ishikawa and ECC-1 cells, was hormonally responsive, with adhesion/outgrowth increased by E/MPA (ECC-1; p < 0.01, Ishikawa; p < 0.01) and E/MPA/hCG (ECC-1; p < 0.001, Ishikawa p < 0.01) versus E alone. The same pattern of hormone responsiveness was observed in pHEEC obtained from fertile women (E vs, E/MPA; p < 0.01, E vs. E/MPA/hCG; p < 0.001). TS adhered to 85% of pHEEC obtained from fertile women (11/13) and 11% of pHEEC obtained from women with unexplained infertility (2/18, p < 0.001).
Conclusion
This new model of “embryo” implantation largely discriminates between endometrial epithelial cells obtained from fertile vs. infertile women based on adhesion; this holds potential as an in vitro “diagnostic” tool of endometrial infertility.
Keywords: Receptive endometrium, Implantation model, Trophectodermal spheroid, Fertile, Infertile
Introduction
The molecular pathways and events underlying the first stages of human life are gradually being elucidated. In vitro fertilization (IVF) has provided an elegant system to examine and understand critical interactions between oocyte and sperm at the time of conception. We can also study and manipulate the initial developmental stages after fertilization using excess human embryos [1]. However, the subsequent earliest stages of pregnancy, embryo attachment to, and implantation into the endometrium remain enigmatic. In IVF cycles, the time immediately before and after the embryo is placed into the uterine cavity has been termed the “black box” of reproduction [2]. It is estimated that inadequate endometrial receptivity or failure of the endometrium and the embryo to interact appropriately underlies ~ 40% of implantation failures of euploid embryos [3]. The dynamic molecular changes underpinning the initial adhesion and attachment stage, during which the blastocyst makes first contact with the endometrial epithelium to initiate the implantation cascade, remain largely unknown in humans, due to a lack of effective and accurate models.
In vitro models utilizing cell lines, derived from choriocarcinomas, have facilitated studies of maternal-fetal interactions [4–6] with the cells induced to form spheroids mimicking the blastocyst. However, these cancer cells represent the highly invasive cells of the later stages of placentation and choriocarcinoma and are thus unlikely to closely represent the trophectoderm layer of the blastocyst at implantation.
In vitro models, utilizing both cryopreserved human embryos and primary human endometrial cells in 3-dimensional or monolayer culture, are highly relevant for implantation studies [7–10], but there is uncertainty regarding the quality of the available thawed embryos (generally scored only on morphology), which remain after the best are selected for transfer. Further, embryos available for research are scarce, and the reason they do not consistently attach to hormonally primed endometrial epithelial cells obtained from fertile women is unknown [7–10]. Models examining human embryo attachment to plastic have also been used to mimic the “implantation stage” [11, 12]. Unfortunately, this is not physiological; indeed, the embryo adheres readily to tissues outside the uterus (eye, kidney, spleen, testis) [13–15], reinforced by the high incidence of pregnancies in ectopic sites (1–2% of all pregnancies) [16]. However, embryos will not implant into non-receptive endometrium [17]. This highlights the specificity of receptive endometrium–embryo interactions; thus, this process must be studied within the context of endometrial cells from the same species.
Mouse models of implantation (particularly mice with genetic modifications) have also been widely studied, but substantial species differences exist versus women [18]. These include different modes of endometrial preparation (particularly decidualization), different mechanisms of interaction between blastocyst and luminal epithelium [19], and different modes of placental development [20]. Many findings in genetically modified mice have not translated to the human. For example, the leukemia inhibitory factor (LIF) knockout mouse is infertile due to an endometrial defect [21], but this has not held up in women [22, 23], and an RCT of recombinant human LIF, administered to infertile women subcutaneously for 7 days starting on the day of embryo transfer, demonstrated reduced clinical pregnancy rates [24]. Thus, to understand and monitor human specific events, an appropriate human model for the very early stages of implantation is urgently needed.
A novel trophoblastic spheroid model was recently reported: a human embryonic stem cell line (Val3) was differentiated into trophoblastic cells, under a complex regime of applied factors [25]. The resultant cells formed spheroids following transfer to low attachment plates: most demonstrated a blastocoel-like cavity. These spheroids differentially adhered to human endometrial epithelial cells (Ishikawa cell line or primary cells) with dependence on time in culture and the epithelial cells used. Separately, mouse embryonic stem (ES) cells “coated” with mouse trophoblast stem cells formed a “blastoid” appearing to mimic a blastocyst [26]. Elaboration of the full trophectoderm-like function of these blastoids and their appropriate implantation into the mouse uterus appeared to require ES cells within the structure. However, the relevance of these findings to the human is unknown.
These elegant studies are severely limited by the need to differentiate embryonic stem cell lines, generation of spheroids of different sizes, and differential adhesion depending on duration of spheroid formation. We therefore aimed to develop a highly reproducible human-specific model of “embryo” adhesion, which could be manipulated to increase or inhibit embryo adhesion and utilized to differentiate between potentially receptive and potentially non-receptive endometrium in women embarking upon assisted reproduction. To achieve these aims, we used a human trophoblast stem cell line [27] developed from individual blastomeres of donated human embryos. These have characteristics of trophectodermal cells (TEAD4, CDX2, geminin, HMGA2, LIFR, GDF15, and LGR5 expressions) and can be manipulated to differentiate towards a syncytiotrophoblast or cytotrophoblast fate. Similar models have proven useful for understanding early implantation events [28]; thus, the model developed herein is likely to be physiologically meaningful.
Subject details and methods
Ethics and tissue collection
Ethical approval for tissue collections was provided by the Institutional Ethics Committees at Monash Health and Monash Surgical Private Hospital. Written informed consent was obtained from all subjects prior to tissue collection.
Endometrial tissue collection and patient details
Endometrial biopsies for culture were collected by curettage from normally cycling women (28–32-day cycles) during the late proliferative phase of the cycle (days 10–15). Inclusion criteria includes the following: under 40 years of age, no steroid hormone therapy/contraception in preceding 6 months, and not breastfeeding. Some women were fertile (> 1 parous pregnancy; n = 13) while others were experiencing unexplained infertility (primary or secondary (inability to conceive after previous successful pregnancy), n = 18). Women included in the infertile group had not conceived after > 1 year of unprotected sex. All women were menstruating regularly (28–32-day cycle) and were determined to have normal ovarian appearance and follicular development. The presence of endometrial polyps was the only potential abnormality noted; however, these were also present in women within the “fertile” group. As these tissues are collected via altruistic donation from women consented immediately before entry to operating theatre through a private hospital, only limited patient background data is available (Table 1).
Table 1.
Characteristics of infertile women
| Participant | BMI | Infertility | Endometrial findings | Spheroid adhesion |
|---|---|---|---|---|
| P1 | 21.5 | Primary | Normal | No |
| P2 | 25.5 | Primary | Normal | No |
| P3 | 19.6 | Primary | Polyps | No |
| P4 | 34 | Secondary | Polyp | Yes |
| P5 | 33 | Secondary | Normal | No |
| P6 | 27 | Primary | Normal | No |
| P7 | 23.7 | Primary | Polyp | No |
| P8 | 24.3 | Primary | Normal | Yes |
| P9 | 21 | Primary | Normal | No |
| P10 | 36 | Primary | Normal | No |
| P11 | 22.7 | Primary | Normal | No |
| P12 | 33 | Primary | Normal | No |
| P13 | 19.4 | Primary | Normal | No |
| P14 | 20.8 | Primary | Normal | No |
| P15 | 24.6 | Secondary | Normal | No |
| P16 | 30.9 | Primary | Normal | No |
| P17 | 22.3 | Secondary | Normal | No |
| P18 | 21.4 | Primary | Polyp | No |
Epithelial cell isolation from human endometrial tissue
Performed per previous protocols [29]. In brief, within 16 h of collection, endometrial tissues were washed in phosphate-buffered saline (PBS), finely chopped and incubated with 1200U collagenase type III and 100 mg/ml DNAse in 2 ml of phosphate-buffered saline (PBS) for 45 min at 37 °C with shaking at 130 rpm. Digestion was terminated by addition of 4 volumes of DMEM/F12 containing 1% v/v penicillin/streptomycin (p/s). Digested tissue was passed through a 45-μm filter and retained epithelial fragments washed off, centrifuged, resuspended in DMEM/F12 containing 10% v/v fetal bovine serum (FBS, Gibco, Invitrogen) and 1% p/s, and seeded into 24-well plates (2 cm2 surface area). Epithelial fragments were allowed to attach for 48 h before thorough washing with PBS to remove stromal and other cells. Endometrial epithelial cell preparations were visually assessed for contamination with endometrial stromal fibroblasts and only those with ≥ 95% epithelial cells used for experimental purposes. Primary human endometrial epithelial cells (pHEECs) were not passaged and were used at p0 (i.e., at first seeding after isolation) as, in our experience, this reduces the likelihood of stromal cell contamination of the cultures. pHEECs at p0 were used for experimental purposes within 1 week of isolation. An example of morphologically pure epithelial preparation with characteristic “rounded” morphology, and no contaminating stromal fibroblasts is provided in Fig. 1a. This is the typical appearance of epithelial cultures used in the current study.
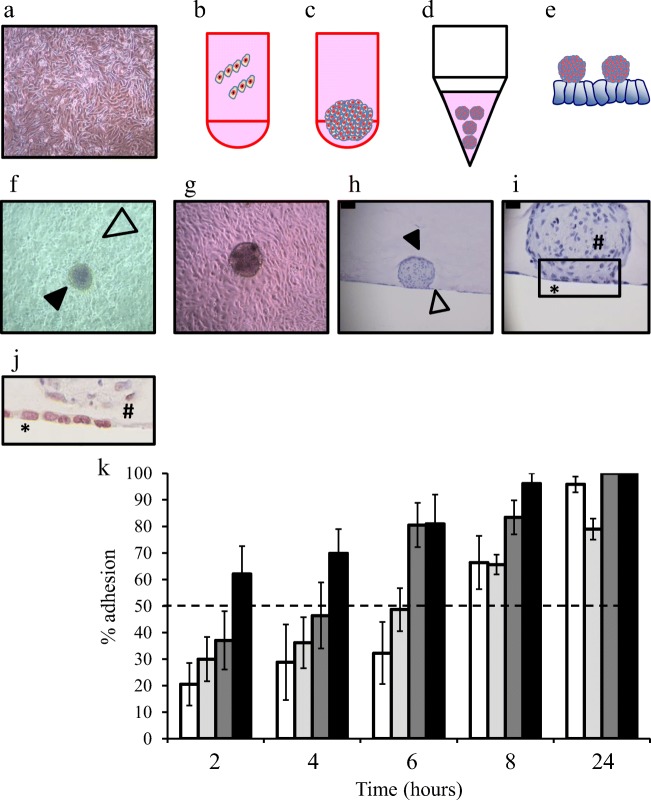
Fig. 1.
Trophectoderm spheroid manufacture, co-culture model development and characterization of adhesion to endometrial epithelial cell lines. Example of a pure preparation of primary human endometrial epithelial cells with characteristic “rounded” morphology (a). TSC trophectodermal cells were seeded into round bottomed well (2500 cells/well) in the presence of methylcellulose (b) for 48 h at which time a compact spheroid was observed (c). Trophectoderm spheroids were collected into conical tubes and centrifuged to pellet (d) before seeding onto monolayers of endometrial epithelial cells (e). The trophectodermal spheroids (black arrowhead, f) can be observed attaching to the endometrial epithelial monolayer (open arrowhead, f). Similar cellular interactions were observed in co-cultures of primary human endometrial epithelial cells and trophectodermal spheroids (G). Fixation of these 3-dimensional structures in agar demonstrates interaction of the trophectoderm spheroid (black arrowhead, h) with the endometrial epithelial monolayer (open arrowhead, h). Closer examination highlights the interaction between these 2 cell-types at the adhesion interface (box, i). Immunostaining demonstrates attachment of the trophectoderm spheroid (#) to the epithelial monolayers (*) but no displacement of the monolayer at 6 h (j). Trophectoderm spheroids demonstrate differential adhesion rates to endometrial epithelial cell lines Hec1a (⎕, k), ECC-1 ( , k), Ishikawa (
, k), Ishikawa ( , k) and RL95-2 (■, k) across a time course of adhesion. Data presented as mean ± SEM of 4 biological replicates
, k) and RL95-2 (■, k) across a time course of adhesion. Data presented as mean ± SEM of 4 biological replicates
Cell culture
The aim of the method development for the trophectoderm spheroid (TS) co-culture model was to identify an endometrial epithelial cell line which (i) facilitated adhesion in a time-span approximating that proposed to occur in vivo in the human (6–24 h) displaying gradually increasing adhesion during this time and (ii) robust response to steroid (estrogen and progesterone) and pregnancy (human chorionic gonadotropin; hCG) hormone stimulation in terms of facilitating TS adhesion. Thus, 4 commonly used endometrial epithelial cell lines were tested for their ability to support TS adhesion.
ECC-1 endometrial epithelial cells (ATCC) are an endometrial cancer cell line with characteristics of the endometrial luminal epithelial layer. They were obtained from the ATCC with validity routinely validated in our laboratory via Short Tandem Repeat (STR) DNA profiling of human cell lines per ATCC guidelines. They were maintained in a 1:1 mix of DMEM:F12 Glutamax (Gibco, Invitrogen) supplemented with 1% p/s/10% v/v FBS.
Ishikawa endometrial epithelial cells were a kind gift of Prof Masato Nishida, National Hospital Organization, Kasumigaura Medical Center, Japan. Ishikawa cells were routinely maintained in phenol red free DMEM supplemented with 1% p/s/1% l-glutamine/10% v/v FBS. HEC-1A endometrial epithelial cells (ATCC) were routinely maintained in McCoy’s 5a modified medium supplemented with 1% p/s/10% FBS. RL95-2 endometrial epithelial cells (ATCC) were routinely maintained in a 1:1 mix of DMEM/F12 Glutamax supplemented with 1% p/s/10% v/v FBS.
All cell lines above were seeded into 24-well plates and used for experimental purposes as described below.
TSC (trophectodermal) cells are human trophoblast stem cells (kind gift of Prof Susan Fisher, UCSF) [27]. They were routinely maintained in a 1:1 mix of DMEM:F12 Glutamax (Gibco, Invitrogen) supplemented with 1% v/v p/s, 10% v/v FBS with addition of 10 ng/ml basic fibroblast growth factor (bFGF, 233-FB-025, R&D systems), and 10 μM SB431542 (#1614, Tocris Bioscience) (referred to as trophectoderm medium throughout). Cells were grown on flasks coated with 0.5% gelatin (G1393, Sigma-Aldrich).
Spheroid formation and model development
Manufacture of methylcellulose
Methylcellulose (4000 centipoises, Sigma-Aldrich) at 1.5% (w/v) was dissolved in DMEM medium by stirring at room temperature for 90 min followed by stirring overnight at 4 °C and subsequent centrifugation for 90 min at 3500 rpm to remove insoluble methylcellulose.
Formation of trophectodermal spheroids (illustrated, Fig. 1b, c)
Initial optimization studies found that 2500 trophectoderm cells formed a compact spheroid of approximately the same size as a human blastocyst (0.1–0.2 mm); 1000–4000 cells per spheroid were initially tested (data not shown). A total of 2500 trophectoderm cells in 150 μl of 20% methylcellulose/80% trophectoderm medium [30] were seeded into a round bottomed well with one spheroid forming/well. Any mis-formed spheroids (< 5% of spheroids) were discarded.
Trophectoderm spheroid adhesion: time course optimization
The timing of attachment and implantation of a human embryo to the maternal endometrium is unknown. Based on early hCG measurements, full implantation is predicted to take place 8–10 days after ovulation in natural cycles [31, 32]; in IVF, this corresponds to 24 h after transfer of a day 5 blastocyst into the uterine cavity. Co-culture optimization studies of trophectoderm spheroid attachment therefore focused on the 0–24-h time frame. Furthermore, the adhesion characteristics of these trophectoderm spheroids (TS) to endometrial epithelial cells were completely unknown. Therefore, the time course over which TS adhere to the 4 endometrial epithelial cell lines was examined to determine (i) the optimal cell line and (ii) adhesion time point within the physiologically relevant 0–24-h time frame to be used for subsequent studies. Other considerations included determination of a time point and cell line in which TS adhesion could be increased or decreased in subsequent studies.
ECC-1, Ishikawa, Hec-1a, and RL95-2 cells were seeded to confluence (≥ 95%) and allowed to settle overnight. Monolayers were washed twice with PBS and incubated in 0.5% charcoal stripped (cs) FBS for 16 h. Spheroids were harvested and placed into 15 ml conical tubes (10 spheroids/tube) using wide bore filter tips to prevent damaging the spheroid structure by shear force. Spheroids were centrifuged at 800×g for 8 min to pellet and then resuspended in serum free medium to wash out traces of methylcellulose and trophectoderm media (Fig. 1d). Spheroids were resuspended in media containing 1% FCS (pilot studies found a small amount of serum necessary to support adhesion) and left to adhere for 2, 4, 6, 8, or 24 h (Fig. 1e, f). At the end of each time point, firmly adhered spheroids were determined by the following method:
-
i)
Total spheroids present within the well were counted under an inverted light microscope.
-
ii)
The media was gently removed and co-cultures gently washed with PBS by pipetting slowly down the side of the well. NB: each well underwent media removal and PBS washing individually to prevent cell drying (common when dealing with multiple wells) which could influence the adhesion result.
-
iii)
Firmly attached spheroids clearly visible on epithelial monolayers (example provided in Fig. 1f) were re-counted.
-
iv)
Adhered spheroids were expressed as a percent of total spheroids.
Once the optimal time point for adhesion of TS to endometrial epithelial cell lines has been determined, adhesion to pHEEC (p0) was examined at this time point (example provided in Fig. 1g).
Hormonal treatment of endometrial epithelial cells
ECC-1, Ishikawa (chosen based on time course experiments), and pHEEC were examined for hormone mediated alterations in TS adhesion. Cells were seeded as described above followed by two washes in PBS and incubation in 0.5% charcoal stripped (cs) FBS for 16 h. A specific hormonal treatment paradigm was deployed to mimic hormonal exposure throughout the menstrual cycle. Cells were primed with 10−8 M 17β-estradiol (E: henceforth referred to as estrogen) for 24 h. Cells were then:
-
i.
Treated for a further 24 h with E alone followed by examination of TS adhesion at 6 h (optimized time point)
-
ii.
Treated with combined E plus 10−7 M medroxyprogesterone acetate (MPA; henceforth referred to as progestin) for a further 24 h followed by examination of TS adhesion at 6 h
iii. Treated with combined E/progestin for 24 h, followed by treatment with E/P and 10 IU hCG for 24 h and TS adhesion subsequently examined at 6 h
Hypoxia studies
ECC-1 cells were prepared and treated sequentially with E followed by combined E/progestin as described above and TS spheroid adhesion examined at 6 h. From the time of cell seeding, throughout hormone treatment, and during TS adhesion, the cells were incubated at 1–20% oxygen.
Outgrowth studies
After quantitation of firmly adhered TS after hormonal treatment of monolayers, remaining attached TS were left in treatment media (E alone, E/progestin, or E/progestin/hCG) for a further 18 h (24 h total) followed by imaging and outgrowth quantification (measured in nm) assessed using Motic image 2.0 software.
Statistical analysis
GraphPad Prism version 7 for Windows was used for all statistical analyses. Before analysis, all data was tested for normality. If the data was found to be non-parametric, a Kruskal–Wallis or Mann–Whitney U analysis was performed. If the data was parametric, one-way ANOVA with a Tukey’s or Dunnett’s post-hoc test or an unpaired t test was performed. Significance was given as p < 0.05, and all data presented as the mean plus/minus the standard error of the mean (mean ± SEM). Experiments were performed a minimum of 4 times with 2 technical replicates.
Results
Human endometrial epithelial cell lines exhibit differing adhesive capacity for human trophectoderm spheroids
Overall, trophectoderm spheroids (formation: Fig. 1b–e, closed arrowhead, Fig. 1f) attached to confluent endometrial epithelial cell monolayers (open arrowhead, Fig. 1f “cobblestone” appearance cells, adhesion to primary endometrial epithelial cells, Fig. 1g). Endometrial epithelial–trophectoderm spheroid co-culture cross sections (at 6 h) demonstrated spheroid attachment (closed arrowhead, Fig. 1h) to the monolayer (open arrowhead, Fig. 1h), but there was no dispersal of the monolayer (*, Fig. 1i, j) which remained clearly intact below the trophectoderm spheroid (#, Fig. 1i, j), suggestive of early adhesive but not invasive events during the embryo implantation cascade.
HEC1A cells (Fig. 1k), considered to be non-receptive [33], exhibited low attachment between 2 and 6 h (20%; 2 h, 28%; 4 h, 32%; 6 h) rising to 66% adhesion at 8 h and 95% at 24 h.
ECC-1 cells (Fig. 1k) are more representative of the luminal epithelium [4], the first point of contact between blastocyst and maternal endometrium. ECC-1 cells displayed intermediate levels of adhesion (30%; 2 h, 36%; 4 h, 48%; 6 h, 65%; 8 h, 78%; 24 h).
Ishikawa cells (Fig. 1k) have characteristics of the glandular epithelium; adhesion characteristics were not known. Ishikawa cells exhibited intermediate adhesion between 2 and 4 h (37%; 2 h, 46%; 4 h). A high degree of adhesion occurred at subsequent time points (80%; 6 h, 83%; 8 h, 100%; 24 h).
RL95-2 cells (Fig. 1k) are non-polarized and considered highly receptive [34, 35] and here allowed rapid adhesion of trophectoderm spheroids (62%; 2 h, 69%; 4 h, 80%; 6 h, 96%; 8 h, 100%; 24 h).
For an ideal model, a desirable cell line would display a level of adhesion that could be both improved upon with addition of factors to facilitate endometrial receptivity or inhibited with addition of factors to abrogate receptivity. Thus, the ECC-1 cell line and a 6-h time point were chosen as these cells demonstrated ~ 50% adhesion at this time point and are hormonally responsive [36]. The Ishikawa cell line was also used further as a “positive control” for estrogen/progestin–regulated events as it is known to be hormonally responsive.
Severe, but not moderate, hypoxia alters trophectoderm spheroid adhesion
The microenvironment of embryo implantation is relatively hypoxic, with uterine oxygen concentrations ~ 8% [37, 38]. IVF laboratories conventionally use ~ 5% oxygen in embryo culture [39]. Oxygen concentration approximating those within the human uterine cavity (5–8%) had no significant difference on trophectoderm spheroid adhesion to ECC-1 cells at 6 h compared 20% oxygen (Fig. 2). However, profound hypoxia (1–2.5%) increased trophectoderm spheroid adhesion from ~ 50 to ~ 85% (Fig. 2; *p < 0.05, **p < 0.01). Subsequent experiments were performed under atmospheric oxygen as trophectoderm spheroid adhesion under this condition was highly similar to uterine oxygen conditions (~ 8%).
Fig. 2.
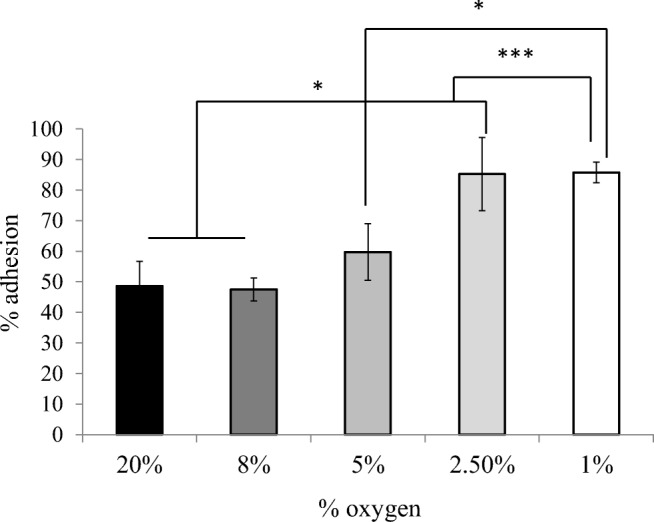
Oxygen tension impacts trophectodermal spheroid adhesion. Trophectodermal spheroids demonstrated significantly increased adhesion to estrogen/progesterone primed endometrial epithelial cells when incubated at 2.5% ( ) and 1% (⎕) oxygen versus incubation at 20% (■), 8% (
) and 1% (⎕) oxygen versus incubation at 20% (■), 8% ( ), and 5% (
), and 5% ( ) oxygen. *p < 0.05; ***p < 0.001. Data presented as mean ± SEM of 4 biological replicates
) oxygen. *p < 0.05; ***p < 0.001. Data presented as mean ± SEM of 4 biological replicates
Reproductive hormone treatments alter trophectoderm spheroid adhesion to human endometrial cells
The necessity of hormonal preparation of the endometrium by estrogen to ensure responsiveness to progesterone has been previously demonstrated [40] as has the requirement for estrogen and progesterone for receptivity changes [40]. A role for hCG in receptivity is well proven, with a large number of in vivo and in vitro studies supporting its role in facilitating receptivity for implantation [29, 41–45]. Furthermore, the proteomes of the ECC1 cells used here differ considerably between cells treated with estrogen alone or estrogen/progestin [46]. Human endometrial epithelial cell lines (ECC1 [Fig. 3a] and Ishikawa [Fig. 3b]) and pHEECs (Fig. 3c) were treated with hormones to mimic the proliferative (estrogen alone) and secretory (estrogen/progestin) phases of non-conception cycles and conception cycles (estrogen/progestin/hCG).
Fig. 3.
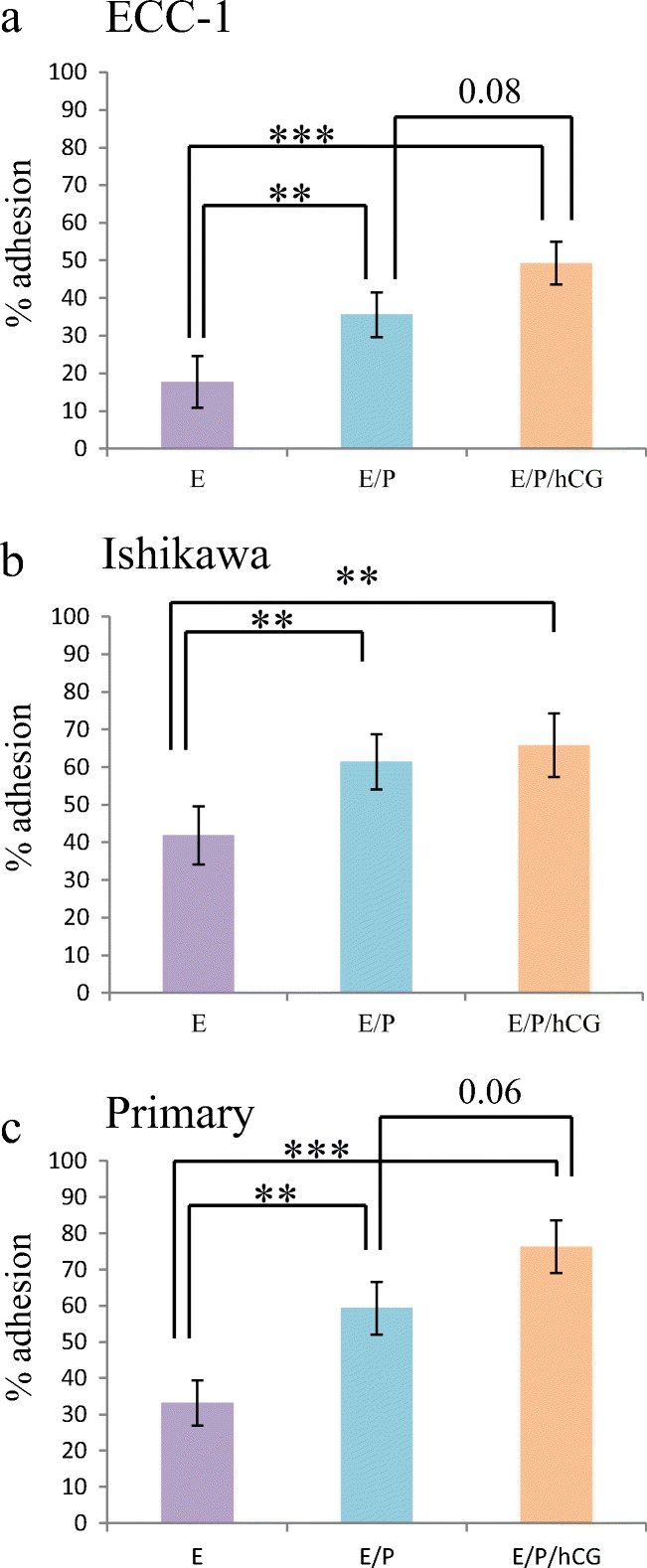
Reproductive hormones alter trophectodermal spheroid adhesion to endometrial epithelial cells. Pre-treatment of endometrial epithelial cell lines ECC-1 (a) and Ishikawa (b) or primary human endometrial epithelial cells (c) with combined estrogen/progestin (E/P,  ) or estrogen/progestin/hCG (E/P/hCG,
) or estrogen/progestin/hCG (E/P/hCG,  ) significantly enhanced trophectodermal spheroid adhesion versus treatment with estrogen alone (E,
) significantly enhanced trophectodermal spheroid adhesion versus treatment with estrogen alone (E,  ). *p < 0.05; **p < 0.01; ***p < 0.001. Data presented as mean ± SEM of 5 biological replicates
). *p < 0.05; **p < 0.01; ***p < 0.001. Data presented as mean ± SEM of 5 biological replicates
ECC-1 cells receiving estrogen alone were minimally adhesive (23%; Fig. 3a), while estrogen/progestin (40% adhesion, **p < 0.01 vs. estrogen) and estrogen/progestin/hCG (54% adhesion, ***p < 0.001 vs. estrogen, p = 0.08 vs. estrogen/progesterone) treatments both increased trophectoderm spheroid adhesion.
Ishikawa cells exhibited similar patterns, with steroid hormone responses similar to those of ECC-1 cells: low adhesion following estrogen treatment (41%; Fig. 3b), increasing significantly with estrogen/progestin treatment (61%, **p < 0.01 vs. estrogen). However, there was no significant increase with addition of hCG (65%, **p < 0.01 vs. estrogen; ns vs. estrogen/progestin).
To establish the physiological relevance of cell line data, hormone experiments were replicated in pHEECs from fertile women. The pattern of trophectoderm spheroid adhesion mirrored that in ECC1 cells: low spheroid adhesion to estrogen-treated cells (33%; Fig. 3c), which increased with estrogen/progestin treatment (59%, **p < 0.01 vs. estrogen), and further with inclusion of hCG (76%, ***p < 0.001 vs. estrogen, p = 0.06 vs. estrogen/progestin). Thus, ECC-1 cells represent a good model for primary endometrial cells in demonstrating functional adhesion responses for trophectoderm spheroids to hormone treatments.
Trophectoderm spheroids discriminate between endometrial epithelial cells derived from fertile and infertile women
Primary endometrial epithelial cells were isolated from 31 endometrial biopsies, blind to the fertility status of each woman, and treated sequentially with estrogen followed by estrogen/progestin to prime the cells for receptivity. Trophectoderm spheroid adhesion assessment was assessed per the optimized protocol. After blinded adhesion assays, the fertility status (fertile/infertile) of each woman was determined from clinical records and adhesion data separated by fertility status. Trophectoderm spheroids adhered to 85% of the fertile tissues assessed (11/13; Fig. 4), whereas adhesion was observed to only 11% of infertile tissues (2/18; Fig. 4, ***p < 0.001) despite appropriate in vitro hormonal (estrogen/progestin) priming. In the infertile group the average BMI was 25.6±5.4, with an average BMI of 24.3 ± 3.8 in the fertile group (ns). A total of 14/18 were experiencing primary infertility while 4/18 had secondary infertility. Polyps were found in 4/18 women within the infertile group while 14/18 women in this group had normal endometrium. Polyps were found in 3/11 women in the fertile group, with 8/11 found to have normal endometrium. Of the 2 infertile women whose endometrial epithelial cells supported adhesion, one had a BMI of 34, secondary infertility, and polyps reported, while the other had a BMI of 24.3, primary infertility, and normal endometrium reported.
Fig. 4.
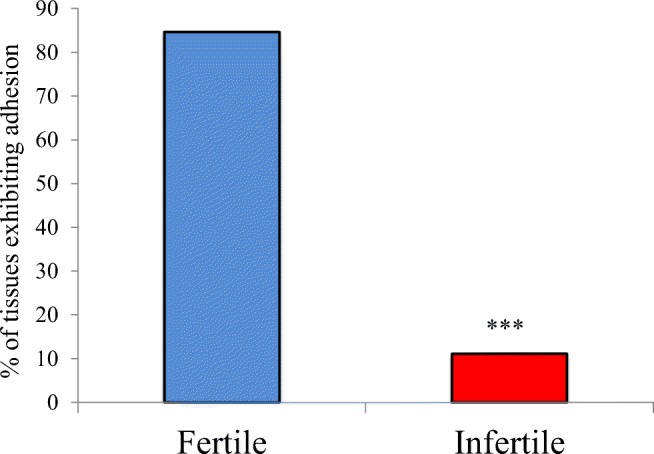
Trophectodermal spheroids discriminate between primary endometrial epithelial cells isolated from fertile and infertile women. Trophectodermal spheroids adhered to 85% of estrogen/progestin primed primary endometrial epithelial cells isolated from fertile women (n = 13) but only 11% of cell preparations treated with the same hormonal priming isolated from infertile women (n = 18). ***p < 0.001
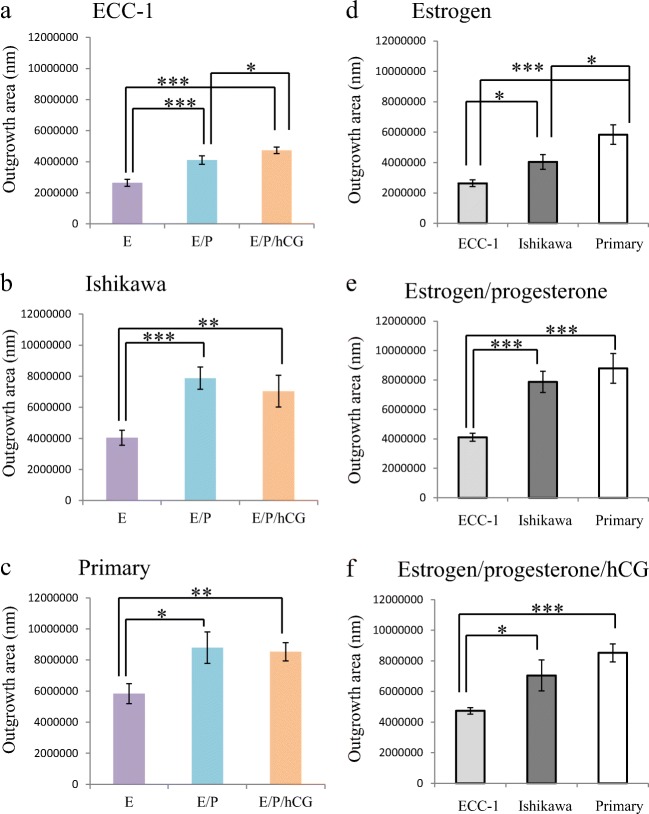
Hormone treatments alter trophectoderm spheroid outgrowth: a proxy indicator of placental development
Outgrowth of trophectoderm spheroids on ECC-1 cells at 24 h (Fig. 5a) progressively increased upon epithelial cell treatment with estrogen/progestin versus estrogen alone (***p < 0.001 vs. estrogen) or estrogen/progestin/hCG (***p < 0.001 vs. estrogen; *p < 0.05 vs. estrogen/progesterone). A similar pattern of increasing outgrowth was also observed in Ishikawa cells (Fig. 5b; ***p < 0.001 vs. estrogen) and pHEECs (Fig. 5c; *p < 0.05 vs. estrogen). In terms of extent of outgrowth, trophectoderm spheroids displayed a greater degree of outgrowth on pHEECs (Fig. 5d–f) compared with outgrowth on ECC-1 or Ishikawa cells.
Fig. 5.
Reproductive hormones alter trophectoderm spheroid outgrowth at 24 h. Pre-treatment of endometrial epithelial cell lines ECC-1 (a) and Ishikawa (b) or primary human endometrial epithelial cells (c) with combined estrogen/progestin (E/P,  ) or estrogen/progestin/hCG (E/P/hCG,
) or estrogen/progestin/hCG (E/P/hCG,  ) significantly enhanced trophectodermal spheroid outgrowth versus treatment with estrogen alone (E,
) significantly enhanced trophectodermal spheroid outgrowth versus treatment with estrogen alone (E,  ). Trophectodermal spheroids consistently demonstrated greater outgrowth on primary human endometrial epithelial cells (⎕) versus ECC-1 (
). Trophectodermal spheroids consistently demonstrated greater outgrowth on primary human endometrial epithelial cells (⎕) versus ECC-1 ( ) and Ishikawa (
) and Ishikawa ( ) after treatment with estrogen (d), while only ECC-1 (
) after treatment with estrogen (d), while only ECC-1 ( ) cells demonstrated significant differences versus primary human endometrial epithelial cells (⎕) after treatment with estrogen/progestin (e) and estrogen/progestin/hCG (f). *p < 0.05; **p < 0.01; ***p < 0.001. Data presented as mean ± SEM of 5 biological replicates
) cells demonstrated significant differences versus primary human endometrial epithelial cells (⎕) after treatment with estrogen/progestin (e) and estrogen/progestin/hCG (f). *p < 0.05; **p < 0.01; ***p < 0.001. Data presented as mean ± SEM of 5 biological replicates
Discussion
This human co-culture model of implantation, whose development is described here, is a promising tool to differentiate between in vivo-derived potentially “receptive” (i.e., able to support adhesion of TS in this context) and potentially “non-receptive” (unable to support adhesion of TS) endometrium, offering promise for identifying and improving endometrial receptivity in women. The model is unique in the use of true trophectodermal spheroids of blastocyst dimensions, while the responses of both the cell lines and the primary endometrial cells, to estrogen, progesterone, and hCG, also mimic in vivo effects.
Development of this human “embryo implantation” model utilizing trophectoderm spheroids [27] and their adhesive interaction with endometrial epithelial cells represents a significant advance towards understanding mechanisms underpinning the earliest stages of human embryo implantation. Published studies have utilized in vitro “blastoids” or embryo like structures, developed using differentiated stem cells, to gain greater understanding of pre-implantation blastocyst differentiation and implantation into the endometrium [25, 26, 47]. The model described here is simpler and quicker (spheroid formation within 48 h). Despite its lack of inner cell mass and blastocoel (thus a less accurate representation of a true blastocyst), it does present the main cellular components of the initial implantation interface: the endometrial epithelium and embryonic trophectoderm. This simplicity provides an ideal conduit for medium throughput, cost-effective screening for pro- and anti-implantation compounds, enabling selection of critical molecules for subsequent testing in more complex models, thus optimizing resources. Rivron et al. [26] identified in their mouse model that the presence of cells representing the murine inner cell mass was critical for the appropriate implantation function of the embryo mimics. This does not appear be the case in the present study, as the human trophectoderm spheroids adhered well to hormonally primed human endometrial epithelial cell lines and primary human endometrial epithelial cells obtained from fertile women demonstrating the functional response of these cells to reproductive hormones. However, the trophectoderm spheroids did not adhere to hormonally primed cells obtained from infertile women highlighting the selective nature of this model. Given the human focus of our model, implantation of these spheroids in a mouse model and their induction of implantation-induced decidualization were not examined. This is an important point to emphasize as it is not clear whether the mouse luminal epithelium presents the same “barrier” to implantation as in the human and decidualization is spontaneous in the human as opposed to embryo induced in the mouse; as previously elaborated, physiological differences in implantation are considerable.
Uniquely, this trophectoderm spheroid attachment model largely discriminates between endometrial epithelial cells obtained from fertile versus infertile women. To our knowledge, all previous co-culture studies exclusively utilized tissue biopsies from fertile women. The response of endometrial stromal cells to high- versus low-quality embryos and the alteration in the stromal cell response in women with recurrent pregnancy loss (RPL) has been well documented with stromal cells from women with RPL being too “permissive” to implantation [48]. Here we suggest that a differential response to a high-quality embryo may also be mounted by the endometrial epithelial cells depending on the fertility status of the woman. Indeed, the current study suggests a highly active role for the luminal epithelium in the initial stages of implantation and that in some infertile women, the barrier function of this cell layer cannot be appropriately modulated to enable trophectoderm attachment thus resulting in implantation failure. This model could thus provide a simple, rapid functional test of endometrial receptivity for clinical use in women with no known cause for infertility and/or failed IVF cycles to “diagnose” endometrial infertility in combination with established genetic diagnosis methods of investigating altered receptivity [49] and may also provide an in vitro model to test potential solutions thus leading to personalized infertility treatment. Importantly, to obtain viable epithelial cells, the endometrial tissue is best harvested before ovulation. Nevertheless, the primary epithelial cells used in adhesion assessment following hormonal treatment hold the characteristics of the parent tissue obtained from fertile or infertile women, i.e., capacity for sufficient or insufficient differentiation towards a potentially receptive phenotype. Thus, it appears that potential for the epithelium to subsequently develop receptivity is present even prior to ovulation [50, 51]. Intriguingly, the data presented herein complement the findings of Sebastian-Leon et al. [52] in which retrospective analysis of “receptivity” (window of implantation; WOI) predictions found that up to 76.6% of women with implantation failure had a pathological WOI or a pathological and displaced WOI. The existence of a pathological WOI as a cause for implantation failure may highlight the inability of the endometrial epithelium to mount an appropriate transcriptomic and functional response to an embryo. Herein, we found that pHEEC derived from 11% of women could support TS adhesion, suggesting they may have a displaced WOI but maintain hormonal responsiveness or have a non-endometrial cause of RIF as observed in 4.7% of women [52]. The failure to support TS adhesion after 6 h of co-culture with pHEEC obtained from 89% of infertile women may be indicative of a pathological cause highlighting the need for innovative treatments/interventions or displaced WOI; thus, implantation of these “embryos” (TS spheroids) may occur later. The possibility of later implantation due to displaced WOI/altered receptivity timing was not investigated herein as strict timing criteria were set to enable comparisons and due to limited clinical material. However, novel methods now developed for expanding primary pHEECs (e.g., organoids culture [53–55]) to maximize limited starting material opens opportunities for future expansion on the studies herein and development of personalized therapies.
The increase in trophectoderm spheroid adhesion at extremely low oxygen levels (1–2.5%) is also of interest. This may reflect an increase in lactate production; decreasing oxygen concentration in the microenvironment of human embryos increases glucose metabolism towards lactate production [56] which may facilitate implantation. However, the physiological relevance of these data is unclear in the light of recent findings. Culture of human embryos to the peri- to post-implantation stage in 5% oxygen reduced the expression of pluripotency markers and increased apoptosis, suggesting that peri-implantation, such oxygen concentrations, may be detrimental to embryo health [57]. However, in the current study, 5% oxygen did not significantly alter the rate of spheroid adhesion versus 20% oxygen. Therefore, the increased adhesion at profound levels of hypoxia may be more representative of a stress response rather than of physiological relevance. Furthermore, if this potential test were to be introduced into a clinical setting to examine functional response of the endometrium to a TS (i.e., ability to mount a functional implantation response) combined with complementary transcriptomic analysis (e.g., ERA or ER Map/ER Grade), these assays would be technically more feasible under atmospheric oxygen. Clinically, women with uterine hypoxia are at an increased risk of recurrent pregnancy loss (RPL) [58] while many RPL women are considered “superfertile” and establish another pregnancy within 3 months [59]. These clinical data combined with observations of embryo health [57], and the findings herein may indicate that profound uterine hypoxia favors initial embryo adhesion/implantation but compromises ongoing pregnancy outcomes.
A global understanding of human endometrial receptivity remains elusive, most likely due to the plethora of cell types included in analyses and the variability between women. The current study differs significantly in its specific examination of the functional status of endometrial epithelium. Its strength lies in the unique power of the trophectoderm spheroid model to largely differentiate between epithelial cells derived from “fertile” and “infertile” endometrium rather than reliance on menstrual cycle day or apparent fertility status.
The human implantation co-culture model described here provides a potential new tool for identifying endometrium which is able to support adhesion of a blastocyst, and for testing factors that may modulate this either to enhance fertility in infertile women, or to inhibit receptivity as a contraceptive strategy. Intriguingly, ECC-1 and Ishikawa cells present an adhesion “pattern” in response to steroid and pregnancy hormones similar to that mounted by primary human endometrial epithelial cells, albeit at a lower total adhesion rate. This suggests that ECC-1 or Ishikawa cells may be used for medium throughput screening of compounds which may improve receptivity/adhesion characteristics to select the best potential cohort of compounds to progress into testing using primary endometrial cells for development of personalized therapies. Further work using this model to determine the proteins unique to the receptive state and those regulated at the interface between endometrial and trophectoderm cells, mimicking the implantation interface, is underway and should extend these outcomes.
Acknowledgments
We thank the patients who gave their consent for this study and Sister Judi Hocking for collecting human tissue samples. We thank Prof Susan Fisher for her kind gift of TSC trophectodermal cells.
Author contributions
JE conceived the project, performed experimental work, performed data analysis, and wrote the manuscript. KJW and SK performed experimental work. MB helped with model development. LAS helped develop project, wrote, and edited manuscript and provided critical insight.
Funding information
This work was supported by National Health and Medical Research Council of Australia project #1139489 and #1141946 and the Victorian Governments Operational Infrastructure funding. JE supported by a fielding foundation fellowship, Society for Reproductive Investigation Bridge grant, and NHMRC project grant. MB supported by the Ovarian Cancer Research Foundation (OCRF), Fielding Foundation Innovation Award, and the CASS foundation. LAS supported by the Hudson Institute of Medical Research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jemma Evans, Email: Jemma.evans@hudson.org.au.
Kathryn J. Walker, Email: kat.jane.walker@gmail.com
Maree Bilandzic, Email: maree.bilandzic@hudson.org.au.
Sophie Kinnear, Email: sophieckinnear@gmail.com.
Lois A. Salamonsen, Email: lois.salamonsen@hudson.org.au
References
- 1.Fogarty NME, McCarthy A, Snijders KE, Powell BE, Kubikova N, Blakeley P, Lea R, Elder K, Wamaitha SE, Kim D, Maciulyte V, Kleinjung J, Kim JS, Wells D, Vallier L, Bertero A, Turner JMA, Niakan KK. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017;550:67–73. doi: 10.1038/nature24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8:333–343. doi: 10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- 3.Harton GL, Munne S, Surrey M, Grifo J, Kaplan B, McCulloh DH, Griffin DK, Wells D, Group PGDP Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genomic hybridization. Fertil Steril. 2013;100:1695–1703. doi: 10.1016/j.fertnstert.2013.07.2002. [DOI] [PubMed] [Google Scholar]
- 4.Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82:235–245. doi: 10.1095/biolreprod.109.077800. [DOI] [PubMed] [Google Scholar]
- 5.Weimar CH, Post Uiterweer ED, Teklenburg G, Heijnen CJ, Macklon NS. In-vitro model systems for the study of human embryo-endometrium interactions. Reprod BioMed Online. 2013;27:461–476. doi: 10.1016/j.rbmo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Ho H, Singh H, Aljofan M, Nie G. A high-throughput in vitro model of human embryo attachment. Fertil Steril. 2012;97:974–978. doi: 10.1016/j.fertnstert.2012.01.116. [DOI] [PubMed] [Google Scholar]
- 7.Boggavarapu NR, Berger C, von Grothusen C, Menezes J, Gemzell-Danielsson K, Lalitkumar PG. Effects of low doses of mifepristone on human embryo implantation process in a three-dimensional human endometrial in vitro co-culture system. Contraception. 2016;94:143–151. doi: 10.1016/j.contraception.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Galan A, O’Connor JE, Valbuena D, Herrer R, Remohi J, Pampfer S, Pellicer A, Simon C. The human blastocyst regulates endometrial epithelial apoptosis in embryonic adhesion. Biol Reprod. 2000;63:430–439. doi: 10.1093/biolreprod/63.2.430. [DOI] [PubMed] [Google Scholar]
- 9.Lalitkumar PG, Lalitkumar S, Meng CX, Stavreus-Evers A, Hambiliki F, Bentin-Ley U, Gemzell-Danielsson K. Mifepristone, but not levonorgestrel, inhibits human blastocyst attachment to an in vitro endometrial three-dimensional cell culture model. Hum Reprod. 2007;22:3031–3037. doi: 10.1093/humrep/dem297. [DOI] [PubMed] [Google Scholar]
- 10.Lalitkumar S, Boggavarapu NR, Menezes J, Dimitriadis E, Zhang JG, Nicola NA, Gemzell-Danielsson K, Lalitkumar LP. Polyethylene glycated leukemia inhibitory factor antagonist inhibits human blastocyst implantation and triggers apoptosis by down-regulating embryonic AKT. Fertil Steril. 2013;100:1160–1169. doi: 10.1016/j.fertnstert.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Deglincerti A, Croft GF, Pietila LN, Zernicka-Goetz M, Siggia ED, Brivanlou AH. Self-organization of the in vitro attached human embryo. Nature. 2016;533:251–254. doi: 10.1038/nature17948. [DOI] [PubMed] [Google Scholar]
- 12.Shahbazi MN, Jedrusik A, Vuoristo S, Recher G, Hupalowska A, Bolton V, Fogarty NNM, Campbell A, Devito L, Ilic D, Khalaf Y, Niakan KK, Fishel S, Zernicka-Goetz M. Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol. 2016;18:700–708. doi: 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirby DR. The development of mouse blastocysts transplanted to the scrotal and cryptorchid testis. J Anat. 1963;97:119–130. [PMC free article] [PubMed] [Google Scholar]
- 14.Kirby DR. Development of the mouse 9lastocyst transplanted to the spleen. J Reprod Fertil. 1963;5:1–12. doi: 10.1530/jrf.0.0050001. [DOI] [PubMed] [Google Scholar]
- 15.Runner MN. Development of mouse eggs in the anterior chamber of the eye. Anat Rec. 1947;98:1–17. doi: 10.1002/ar.1090980102. [DOI] [PubMed] [Google Scholar]
- 16.Panelli DM, Phillips CH, Brady PC. Incidence, diagnosis and management of tubal and nontubal ectopic pregnancies: a review. Fertil Res Pract. 2015;1:15. doi: 10.1186/s40738-015-0008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valdes CT, Schutt A, Simon C. Implantation failure of endometrial origin: it is not pathology, but our failure to synchronize the developing embryo with a receptive endometrium. Fertil Steril. 2017;108:15–18. doi: 10.1016/j.fertnstert.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Bazer FW, Wu G, Spencer TE, Johnson GA, Burghardt RC, Bayless K. Novel pathways for implantation and establishment and maintenance of pregnancy in mammals. Mol Hum Reprod. 2010;16:135–152. doi: 10.1093/molehr/gap095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Rep. 2015;11:358–365. doi: 10.1016/j.celrep.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 21.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira JB, Vagnini LD, Petersen CG, Renzi A, Oliveira-Pelegrin GR, Mauri AL, Ricci J, Massaro FC, Dieamant F, Cavagna M, Baruffi RL, Franco JG., Jr Association between leukaemia inhibitory factor gene polymorphism and pregnancy outcomes after assisted reproduction techniques. Reprod BioMed Online. 2016;32:66–78. doi: 10.1016/j.rbmo.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 23.Paiva P, Menkhorst E, Salamonsen L, Dimitriadis E. Leukemia inhibitory factor and interleukin-11: critical regulators in the establishment of pregnancy. Cytokine Growth Factor Rev. 2009;20:319–328. doi: 10.1016/j.cytogfr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Brinsden PR, Alam V, de Moustier B, Engrand P. Recombinant human leukemia inhibitory factor does not improve implantation and pregnancy outcomes after assisted reproductive techniques in women with recurrent unexplained implantation failure. Fertil Steril. 2009;91:1445–1447. doi: 10.1016/j.fertnstert.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 25.Lee YL, Fong SW, Chen AC, Li T, Yue C, Lee CL, Ng EH, Yeung WS, Lee KF. Establishment of a novel human embryonic stem cell-derived trophoblastic spheroid implantation model. Hum Reprod. 2015;30:2614–2626. doi: 10.1093/humrep/dev223. [DOI] [PubMed] [Google Scholar]
- 26.Rivron NC, Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivie J, Truckenmuller RK, van Oudenaarden A, van Blitterswijk CA, Geijsen N. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557:106–111. doi: 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- 27.Zdravkovic T, Nazor KL, Larocque N, Gormley M, Donne M, Hunkapillar N, Giritharan G, Bernstein HS, Wei G, Hebrok M, Zeng X, Genbacev O, Mattis A, McMaster MT, Krtolica A, Valbuena D, Simon C, Laurent LC, Loring JF, Fisher SJ. Human stem cells from single blastomeres reveal pathways of embryonic or trophoblast fate specification. Development. 2015;142:4010–4025. doi: 10.1242/dev.122846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghajanova L, Shen S, Rojas AM, Fisher SJ, Irwin JC, Giudice LC. Comparative transcriptome analysis of human trophectoderm and embryonic stem cell-derived trophoblasts reveal key participants in early implantation. Biol Reprod. 2012;86:1–21. doi: 10.1095/biolreprod.111.092775. [DOI] [PubMed] [Google Scholar]
- 29.Paiva P, Hannan NJ, Hincks C, Meehan KL, Pruysers E, Dimitriadis E, Salamonsen LA. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod. 2011;26:1153–1162. doi: 10.1093/humrep/der027. [DOI] [PubMed] [Google Scholar]
- 30.Bilandzic M, Stenvers KL. Assessment of ovarian cancer spheroid attachment and invasion of mesothelial cells in real time. J Vis Exp. 2014;(87). 10.3791/51655 [DOI] [PMC free article] [PubMed]
- 31.Nepomnaschy PA, Weinberg CR, Wilcox AJ, Baird DD. Urinary hCG patterns during the week following implantation. Hum Reprod. 2008;23:271–277. doi: 10.1093/humrep/dem397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 33.Thie M, Harrach-Ruprecht B, Sauer H, Fuchs P, Albers A, Denker HW. Cell adhesion to the apical pole of epithelium: a function of cell polarity. Eur J Cell Biol. 1995;66:180–191. [PubMed] [Google Scholar]
- 34.Martin JC, Jasper MJ, Valbuena D, Meseguer M, Remohi J, Pellicer A, Simon C. Increased adhesiveness in cultured endometrial-derived cells is related to the absence of moesin expression. Biol Reprod. 2000;63:1370–1376. doi: 10.1095/biolreprod63.5.1370. [DOI] [PubMed] [Google Scholar]
- 35.Thie M, Denker HW. In vitro studies on endometrial adhesiveness for trophoblast: cellular dynamics in uterine epithelial cells. Cells Tissues Organs. 2002;172:237–252. doi: 10.1159/000066963. [DOI] [PubMed] [Google Scholar]
- 36.Whitby S, Salamonsen LA, Evans J. The endometrial polarity paradox: differential regulation of polarity within secretory-phase human endometrium. Endocrinology. 2018;159:506–518. doi: 10.1210/en.2016-1877. [DOI] [PubMed] [Google Scholar]
- 37.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil. 1993;99:673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 38.Yedwab GA, Paz G, Homonnai TZ, David MP, Kraicer PF. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertil Steril. 1976;27:304–309. doi: 10.1016/s0015-0282(16)41722-x. [DOI] [PubMed] [Google Scholar]
- 39.Bontekoe S, Mantikou E, van Wely M, Seshadri S, Repping S, Mastenbroek S. Low oxygen concentrations for embryo culture in assisted reproductive technologies. Cochrane Database Syst Rev. 2012;(7):CD008950. 10.1002/14651858.CD008950.pub2 [DOI] [PMC free article] [PubMed]
- 40.Graham JD, Clarke CL. Physiological action of progesterone in target tissues. Endocr Rev. 1997;18:502–519. doi: 10.1210/edrv.18.4.0308. [DOI] [PubMed] [Google Scholar]
- 41.Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, Jabbour HN. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fazleabas AT, Donnelly KM, Srinivasan S, Fortman JD, Miller JB. Modulation of the baboon (Papio anubis) uterine endometrium by chorionic gonadotrophin during the period of uterine receptivity. Proc Natl Acad Sci U S A. 1999;96:2543–2548. doi: 10.1073/pnas.96.5.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Licht P, Fluhr H, Neuwinger J, Wallwiener D, Wildt L. Is human chorionic gonadotropin directly involved in the regulation of human implantation? Mol Cell Endocrinol. 2007;269:85–92. doi: 10.1016/j.mce.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 44.Licht P, Russu V, Lehmeyer S, Moll J, Siebzehnrubl E, Wildt L. Intrauterine microdialysis reveals cycle-dependent regulation of endometrial insulin-like growth factor binding protein-1 secretion by human chorionic gonadotropin. Fertil Steril. 2002;78:252–258. doi: 10.1016/s0015-0282(02)03226-0. [DOI] [PubMed] [Google Scholar]
- 45.Sherwin JR, Sharkey AM, Cameo P, Mavrogianis PM, Catalano RD, Edassery S, Fazleabas AT. Identification of novel genes regulated by chorionic gonadotropin in baboon endometrium during the window of implantation. Endocrinology. 2007;148:618–626. doi: 10.1210/en.2006-0832. [DOI] [PubMed] [Google Scholar]
- 46.Greening DW, Nguyen HP, Evans J, Simpson RJ, Salamonsen LA. Modulating the endometrial epithelial proteome and secretome in preparation for pregnancy: The role of ovarian steroid and pregnancy hormones. J Proteome. 2016;144:99–112. doi: 10.1016/j.jprot.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 47.Harrison Sarah Ellys, Sozen Berna, Zernicka-Goetz Magdalena. In vitro generation of mouse polarized embryo-like structures from embryonic and trophoblast stem cells. Nature Protocols. 2018;13(7):1586–1602. doi: 10.1038/s41596-018-0005-x. [DOI] [PubMed] [Google Scholar]
- 48.Macklon NS, Brosens JJ. The human endometrium as a sensor of embryo quality. Biol Reprod. 2014;91:98. doi: 10.1095/biolreprod.114.122846. [DOI] [PubMed] [Google Scholar]
- 49.Diaz-Gimeno P, Horcajadas JA, Martinez-Conejero JA, Esteban FJ, Alama P, Pellicer A, Simon C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril. 2011;95:50–60. doi: 10.1016/j.fertnstert.2010.04.063. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald HC, Evans J, Johnson N, Infusini G, Webb A, Rombauts LJR, Vollenhoven BJ, Salamonsen LA, Edgell TA. Idiopathic infertility in women is associated with distinct changes in proliferative phase uterine fluid proteins. Biol Reprod. 2018;98:752–764. doi: 10.1093/biolre/ioy063. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald HC, Salamonsen LA, Rombauts LJ, Vollenhoven BJ, Edgell TA. The proliferative phase underpins endometrial development: altered cytokine profiles in uterine lavage fluid of women with idiopathic infertility. Cytokine. 2016;88:12–19. doi: 10.1016/j.cyto.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Sebastian-Leon P, Garrido N, Remohi J, Pellicer A, Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum Reprod. 2018;33:626–635. doi: 10.1093/humrep/dey023. [DOI] [PubMed] [Google Scholar]
- 53.Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO, Simons BD, Hemberger M, Koo BK, Moffett A, Burton GJ. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19:568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, Ferrante M, Vankelecom H. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144:1775–1786. doi: 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- 55.Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I, Brems H, Cox B, Ferrante M, Uji IH, Koh KP, D’Hooghe T, Vanhie A, Vergote I, Meuleman C, Tomassetti C, Lambrechts D, Vriens J, Timmerman D, Vankelecom H. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21:1041–1051. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 56.Gardner DK. Lactate production by the mammalian blastocyst: manipulating the microenvironment for uterine implantation and invasion? Bioessays. 2015;37:364–371. doi: 10.1002/bies.201400155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahbazi MN, Scialdone A, Skorupska N, Weberling A, Recher G, Zhu M, Jedrusik A, Devito LG, Noli L, Macaulay IC, Buecker C, Khalaf Y, Ilic D, Voet T, Marioni JC, Zernicka-Goetz M. Pluripotent state transitions coordinate morphogenesis in mouse and human embryos. Nature. 2017;552:239–243. doi: 10.1038/nature24675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X, Jiang L, Wang CC, Huang J, Li TC. Hypoxia inducible factor and microvessels in peri-implantation endometrium of women with recurrent miscarriage. Fertil Steril. 2016;105:1496–1502. doi: 10.1016/j.fertnstert.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]