Abstract
A healthy gut is predominantly occupied by bacteria which play a vital role in nutrition and health. Any change in normal gut homeostasis imposes gut dysbiosis. So far, efforts have been made to mitigate the gastrointestinal symptoms using modern day probiotics. The majority of the probiotics strains used currently belong to the genera Lactobacillus, Clostridium, Bifidobacterium and Streptococcus. Recent advancements in culturomics by implementing newer techniques coupled with the use of gnotobiotic animal models provide a subtle ground to develop novel host specific probiotics therapies. In this review article, the recent advances in the development of microbe-based therapies which can now be implemented to treat a wide spectrum of diseases have been discussed. However, these probiotics are not classified as drugs and there is a lack of stringent law enforcement to protect the end users against the pseudo-probiotic products. While modern probiotics hold strong promise for the future, more rigorous regulations are needed to develop genuine probiotic products and characterize novel probiotics using the latest research and technology. This article also highlights the possibility of reducing antibiotic usage by utilizing probiotics developed using the latest concepts of syn and ecobiotics.
Keywords: Probiotics, Prebiotics, Synbiotics, Ecobiotics, Challenges, Microbiota, Dysbiosis
Introduction
The human gastrointestinal tract (GIT) fosters a plethora of microorganisms in a range of relationships varying from commensal to symbiotic to pathogenic [1]. This collection of bacteria, archaea, eukarya, and viruses collectively form gut microbiota and any changes in the normal microbial homeostasis impose a great threat on gut integrity and cause dysbiosis [2, 3]. So far the exact number of microbes in the human body, especially in the GIT, has not been settled [4, 5]. Even if we agree with the newly revised estimate which suggests that the humans are 50% microbial i.e., the ratio of human (3.0 × 1013) and bacterial (3.8 × 1013) cells are approximately 1:1.3, with maximum abundance in the colon (1014) followed by saliva and dental plaque (1012), still the role of microbes in shaping the regulatory and metabolic networks remain imperative [6, 7]. It is now a well-established fact that the microbiota maintains human health primarily by shaping the gut epithelium [8], increasing efficient energy harvesting potential [9], protecting against pathogens [10] and regulating host immunity [11].
Based on the information available of the normal microbiota and dysbiosis with reference to a particular disease, the normal gut microbiota can be restored to maintain the gut integrity and host health by using modern-day probiotics that include a handful of well-characterized live microbes which are intended to manipulate the gut and provide the health benefits [12]. These benefits include mitigation of gastrointestinal (GI) symptoms, protection against disease, boosting the immune system and providing a sense of wellbeing [13–15]. Currently, the majority of strains in probiotic blends belong to genera Lactobacillus, Bifidobacterium, Clostridium and Streptococcus (Table 1). But in the recent past, due to the extensive culturomics and metagenomics based studies of healthy and diseased human cohorts, our knowledge of the microbial composition at the species level and their specific role in the occupied niche has significantly improved [16–19]. In spite of this, each human being is a novel reservoir that harbors a unique assemblage of microbiota and the functions that microbes perform are still underestimated. That is why the existence of reference genomes of the human gut library has been challenged often [20]. Recently, a single analysis has added 1520 cultivated bacterial genomes, which covers most of the bacterial phyla and genera reported so far [20]. Based on this research, the current reference catalogue has been extended by 264 novel bacterial members that have not been represented so far in the existing reference genome catalogue, suggesting the need of massive functional characterization of human gut microbiota [20].
Table 1.
List of probiotic strains and their potential effects
| S.No. | Probiotic strains | Features | Potential effect | References |
|---|---|---|---|---|
| 1 | Akkermansia muciniphila | Strict anaerobe, mucin degrader, can colonize in ileum, cecum and colon | Reduces body fat, serum triglyceride, fasting glucose levels and enhances insulin sensitivity | [104, 105] |
| 2 | Brevibacillus laterosporus | Spore forming, Antimicrobial peptide producer | Antibacterial and antitumor activity | [106, 107] |
| 3 | Bacteroides xylanisolvens | Strictly anaerobic, resistant to gastric enzymes and low-pH conditions | Increases the level of TFα-specific immunoglobulin M serum antibodies | [108, 109] |
| 4 | Bifidobacterium bifidum | Acetate and propionate producer | Greater cytokine (IL-6) production and active phagocytic property | [110, 111] |
| 5 | Bifidobacterium breve | Acetate producer and resistant to low-pH | Anti-infectious activity | [112] |
| 6 | Bifidobacterium infantis | Acetate and propionate producer | Therapeutic effect against irritable bowel syndrome and inhibits the secretion of allergen-induced IgE | [113–115] |
| 7 | Bifidobacterium lactis | Aerotolerant | Plasminogen binding activity and regulates the co-stimulatory molecules (CD80, CD86, CD40), required for an effective activation of T-cells | [116, 117] |
| 8 | Bifidobacterium longum | Acetate and propionate producer | modulate the immune system through IL-10 production | [118] |
| 9 | Clostridium butyricum | Butyrate producer | Neuroprotective effects against vascular dementia | [119] |
| 10 | Faecalibacterium prausnitzii | Strictly anaerobic | anti-inflammatory effects by blocking NF-kappaB activation and IL-8 production | [120, 121] |
| 11 | Lactobacillus acidophilus | Lactic and acetic acids producer | Reduces the level of cholesterol through reverse transport in macrophages | [122] |
| 12 | Lactobacillus brevis | γ-Aminobutyric acid (GABA) producer |
Antidepressant, antihypertensive, and anti-diabetic effects |
[123] |
| 13 | Lactobacillus bulgaricus | Lactic acid producer | Anti-microbial peptide production | [124] |
| 14 | Lactobacillus casei group (LCG) | Low-pH tolerant and amino acid utilization | Anti-inflammatory response | [125, 126] |
| 15 | Lactobacillus gasseri | Bile tolerant, cholesterol binding ability | Reduces body weight and adipose tissue mass | [127, 128] |
| 16 | Lactobacillus helveticus | Lactic acid producer with high protease activity | Inhibits the proliferation of lymphocytes through a suppression of JNK signaling pathway | [129] |
| 17 | Lactobacillus plantarum | Anti-microbial peptides producer | Cholesterol lowering activity | [130, 131] |
| 18 | Lactobacillus salivarius | Bile and pH tolerant | Reduces pathogenicity of C. albicans by inhibiting the biofilm formation | [132, 133] |
| 19 | Lactococcus lactis | Bacteriocins and lactic acid producer | Used as a vehicle to deliver therapeutics such as cytokines into the human body | [134] |
| 20 | Pediococcus acidilactici | Bacteriocins and lactic acid producer | Antimicrobial activity against several gram-positive and gram-negative food-spoilage and food-borne pathogens | [135] |
| 21 | Streptococcus thermophiles | Lactic acid producer | Suppresses the Th17 response in inflamed intestines, useful in inflammatory bowel disease | [136] |
| 22 | Enterococcus faecium | Bacteriocin producer | Modulate the Th2-mediated pathologic response | [137, 138] |
| 23 | Saccharomyces boulardii (Yeast) | Resistant to gastric enzymes | Beneficial in Clostridium difficile-associated disease, antibiotic-associated diarrheas, and acute infectious diarrheas | [139, 140] |
There is progressively more information available on the human microbiome and recent discoveries during the last decade have opened up new frontiers for the improvement of human health. However, a critical analysis of the current situation in light of recent developments is lacking. The objective of this review is to emphasize on the recent developments of new generation probiotics, the concept of ecobiotics and implementation of new techniques to discover more efficient probiotic strains along with their application to reduce the need of antibiotics. We have also pointed out the lack of stringency at the marketing level due to low regulatory rules for probiotic manufacturing.
Normal Microbiota of Human GIT, Dysbiosis, and Need for Probiotics
The normal gut microbiota in humans is predominantly composed of bacteria and they assume a pivotal role in nutrition and health. The gut microbiota of humans differs on genus, species and strain level due to the marked differences in food habits, age, ethnicity, and lifestyle. This is also responsible for generating interindividual variability. However, the gut microbiota is conserved at the phylum level and has major representation from four phyla viz., Firmicutes, Bacteroides, Actinobacteria, and Proteobacteria. From the information now available, it is becoming increasingly clear that diet and lifestyle are the two major factors that play a central role in maintaining a healthy microbiome and hence human health. For gaining a holistic view, an integrated gene catalogue having close to a complete gene set of human gut microflora was constructed having the most abundant representation from genera such as Lactobacillus, Prevotella, Peptostreptococcus, Enterococcus, and even Helicobacter, predominately H. winghamensis [21]. It is difficult to make a generalized statement because of the lack of data from non-western countries, but the available information reflects that two major enterotype clusters have been reported in the world population. Bacteroides or Clostridiales species are generally found in European and American subjects (western) linked to high protein and high-fat diets. Prevotella is the major genus in non-western subjects associated with vegetarian and plant fiber-rich diets [22]. The resident gut microflora also harbours pathogenic bacteria such as Helicobacter pylori, Clostridium difficile and Escherichia coli [23, 24]. The normal symbiotic bacteria, using the principle of colonization resistance, may prevent the growth of pathogenic bacteria (Table 2) and generate metabolites which cannot be synthesized by the human body. For example, the normal gut microbiota in humans has been presumed to encode over three million genes producing a variety of metabolites and replaces many functions of a host having only about 23,000 genes [25]. Although the composition and functional characterization of a healthy microbiota still remains to be precisely defined, a shift in normal microbiota (dysbiosis) has been shown to generate serious implications on human health [26]. The dysbiosis generated can be restored using supplementation of food with beneficial bacteria. Keeping in mind the beneficial effect of these ‘good bacteria’, the concept of treating various diseases using probiotics emerged [12, 27, 28].
Table 2.
List of bacterial isolates with inhibitory effect on pathogens in co-culture assay
| S. No. | Bacterial species | Pathogen | References |
|---|---|---|---|
| 1. | Bacteroides ovatus SNUG 40239 | C. difficile | [141] |
| 2. | Bifidobacterium longum IPLA20022 B. breve IPLA20006 | C. difficile | [142] |
| 3. | Clostridium butyricum MIYAIRI 588 | C. difficile | [143] |
| 4. | Lactobacillus delbrueckii ssp. bulgaricus B-30892 | C. difficile | [144] |
| 5. | Lactococcus lactis, Enterococcus faecalis and Enterococcus faecium | Listeria monocytogenes and Salmonella enterica serovar enteritidis | [145] |
| 6. | Enterococcus faecalis EJ97 | Listeria monocytogenes | [146] |
Probiotics as a Nutritional Aid for Human Health
The concept of probiotics (means “for life”) was proposed by Nobel laureate Elie Metchnikoff in the early 20th century, who hypothesized that senility can be delayed and health can be improved by consuming beneficial bacteria from yogurt [29]. With the desire to improve lifestyle and food quality, the requirement of probiotics as a nutritional aid has been progressively raised in the past years. Probiotics are used as a promising food enhancer with quality deliverables mostly opt-in meals combinations [30]. During the process of manufacturing, the beneficial bacterial culture is artificially added into the foodstuff. Commercial production of these cultures generates high concentrations for dietary viable supplements (DVS) either in the form of frozen cultures or in freeze-dried culture powders. Major manufacturers prefer to use these DVS as it is difficult to raise the cultures at production sites. In comparison, the frozen cultures contain more than 1010 CFU g−1, while freeze-dried cultures typically contain more than 1011 CFU g−1 [31, 32]. Production of secondary metabolites, such as acetic acids by Bifidobacterium, may alter the taste and aroma of the food product during the fermentation or storage. Hence, it must be assured that culture inoculation does not adversely affect the foodstuff. Packaging material and storage conditions are the crucial steps in the preparations of probiotic supplements. DVS are specifically designed food supplements that are consumed to obtain a specific result, thus are specifically called functional food. Taking these into account, more than 500 promising probiotic food supplements were introduced worldwide [33]. These product lines include fermented cereals, fruits, vegetables, and meat foodstuffs that are gaining popularity among consumers. Typical successful examples of these products are cheese and dips, mayonnaise, edibles spreads, ice cream, milk, juices, oat, etc. [34, 35]. However, recent advances in microbiome research have revealed that these supplements with only a few bacteria are not sufficient to maintain good human health and there is a further need to identify potential probiotic strains to accomplish the goal of better nutritional status along with combatting disease.
The Evolution of Probiotics: Prebiotics, Probiotics, and Synbiotics
As early as in 1995, Gibson and Roberfroid suggested the use of non-digestible food ingredients that can stimulate the growth of bacteria already inhabiting the colon and they called them prebiotics [36]. Later, they refined the original definition and proposed that “A prebiotic is selectively fermented ingredient that allows specific changes both in composition and/or activity in the gastrointestinal microflora that confers benefits upon host wellbeing and health” [37]. It is now sufficiently clear that the gut harbors microbes that are either facultative or obligatory anaerobes. It has been also been established beyond doubt that the seeding of the microbiome during the early stages of life is largely stochastic and typically the pioneer colonizers are mostly facultative anaerobes [38]. The transition towards the anaerobicity is mediated by these initial colonizers and because of them, the number of strict anaerobes increases with the passage of time [38, 39]. Thus, the survival of these colonizers is crucial for maintaining good health. Due to recent developments in this field, the investigators are now employing a broad range of components to develop new probiotics for improving the survival and effectiveness of these initial inoculums. In the colon, the microbes utilize undigested food mainly comprised of non-digestible oligosaccharides, dietary fibers, and undigested proteins. In order to be considered as a prebiotic, an ingredient must reach the large intestine and can be fermented by the gut bacteria. The most common carbohydrate candidates for prebiotics are inulin, soya-oligosaccharides, fructo-oligosaccharides, galacto-oligosaccharides, xylo-oligosaccharides, pyrodextrin and isomalto-oligosaccharides [40]. In December 2016, the International Scientific Association for Probiotics and Prebiotics (ISAPP) expanded the concept of prebiotics and suggested the inclusion of non-carbohydrate substrates with its application on body sites beyond GIT. Based on this, the prebiotic can be defined as “A substrate that is selectively utilized by host microorganisms conferring a health benefit” [41]. It is always advised to improve gut health by boosting the gut microbes. Potentially, the supplementation of natural prebiotic sources (Table 3) into the diet can alone boost the healthy gut microbiome, as the concept of prebiotics specifies the stimulation of healthy microbiome and reduction of potentially harmful bacteria. Due to the anaerobicity in the colon, most of the microbes derive energy through the process of saccharolytic fermentation by utilizing dietary fibers and producing short-chain fatty acids (SCFA). The SCFA present in the colon are mainly comprised of acetate, propionate, and butyrate (≥ 95%) in the molar ratio of 60:20:20 [9, 42, 43]. Along with these, other metabolites such as lactate, succinate, pyruvate, ethanol, and gases like H2, CO2, CH4, and H2S are also produced [44, 45]. Acetate is the principal SCFA produced in the colon and is mainly utilized by the muscle, kidney, heart, liver and brain cells. After absorption, acetate has been known to increase cholesterol synthesis, whereas propionate which is metabolized by the liver, is known to inhibit cholesterol synthesis [46]. On the other hand, butyrate is largely metabolized by colonocytes and serves as a major source of energy [45]. The SCFA also help in eliminating the gut pathogens by lowering the pH. Thus to increase the efficacy of probiotics, different formulations of probiotics and prebiotic fibers are used and these combinations are known as synbiotics to differentiate from prebiotics and probiotics. As a therapeutic strategy, the synbiotics approach was more successful, as compared to using prebiotics and probiotics alone, in a variety of diseases including irritable bowel syndrome (IBS) symptoms [47], rheumatoid arthritis [48], neonatal sepsis among infants [14], and pregnancy outcomes among gestational diabetic (GDM) women [49]. In clinical trials, symbiotic supplementation has been proven beneficial in lowering fasting blood glucose levels and thus it can also reduce the risk of chronic diseases such as diabetes mellitus, obesity, renal and cardiovascular diseases [50, 51]. In the future, the implementation of synbiotic therapy will not only minimize the use of antibiotics but will also offer potential solutions to fight against the increasing burden of diseases.
Table 3.
List of prebiotic sources and their potential effect
| S.No. | Prebiotic sources | Component(s) | Potential effects | References |
|---|---|---|---|---|
| 1 | Chicory Root | Inulin | Potential substrate for gut bacteria, helps in increasing bile production | [147, 148] |
| 2 | Dandelion Greens | Inulin | Diuretic, antioxidant and cholesterol-lowering effects | [149, 150] |
| 3 | Jerusalem Artichoke | Inulin, high in thiamine and potassium | Potential substrate for gut bacteria and promotes proper muscle function | [151, 152] |
| 4 | Garlic | Inulin and fructooligosaccharides (FOS) | Increases the growth of Bifidobacterium and reduces the growth of disease promoting bacteria | [153] |
| 5 | Onions | Inulin and fructooligosaccharides (FOS) | Boosts the immune system by increasing nitric oxide production in cells | [154] |
| 6 | Barley | Beta-glucan | Cholesterol-lowering activities | [155] |
| 7 | Oats | Beta-glucan | Reduces serum cholesterol and LDL cholesterol | [156, 157] |
| 8 | Apples | Pectin | Increases the population of butyrate- and beta-glucuronidase producing Clostridiales | [158] |
| 9 | Konjac Root | Glucomannan | Promotes the growth of lactic acid bacteria and colonic fermentation and reduces low-density lipoprotein (LDL) cholesterol | [159, 160] |
| 10 | Flaxseeds | Cellulose and lignin | Reduces cholesterol level and increase fecal fat excretion | [161, 162] |
| 11 | Yacon Root | fructooligosaccharides (FOS) and inulin | Improves the growth of Bifidobacteria in the colon, enhances mineral absorption and gastrointestinal metabolism and plays a role in the regulation of serum cholesterol | [163] |
| 12 | Wheat Bran | Arabinoxylan oligosaccharides | Increases Bifidobacteria levels relative to total fecal microbiota, and reduces colonic protein fermentation | [164] |
The Future Ahead for Probiotics: Ecobiotics
Based on epidemiological data, it has been shown that the decreasing incidence of infections in developed and developing nations leads to the increasing incidence of both autoimmune and allergic diseases [52]. When a person with depleted gut microbiota (mostly through broad-spectrum antibiotic use), encounters a pathogen such as Clostridium difficile, the pathogen successfully colonizes in the large intestine leading to the onset of a range of symptoms from mild and recurrent diarrhea to life-threatening complications such as pseudomembranous colitis (PMC), toxic megacolon and colonic perforation [53, 54]. C. difficile infection has been one of the most common healthcare-associated problems affecting half a million people worldwide and adds a burden of $4.8 billion each year alone in the United States [55]. Traditionally, to treat non-severe C. difficile infection (CDI), antibiotics such as metronidazole, fidaxomicin, and vancomycin are used [56, 57]. Due to the recurrence of the disease even after antibiotic treatment, fecal microbiota transplantation (FMT) as an alternative treatment has risen in prominence during the recent past [58, 59]. This technique is highly proficient as it rehabilitates the normal gut ecology with diverse microbiome by reintroducing them into the colon and displaces the pathogen through niche exclusion, competitive metabolic interaction, and initiation of host immune response [60]. However, the overall failure rate of FMT is approximately 12% [61] and there is always a risk of transferring unidentified pathogens.
In order to reinstate healthy microbiota, it is essential to supplement the gut with normal and healthy microbiota. Currently, researchers are developing ecobiotics as an alternative to FMT. Ecobiotics entail the science of design and formulation of therapeutic doses of microbes delivered orally based on gut ecology. Clinical studies have shown the efficacy of this concept. It has been augmented as an alternative to FMT under the Seres Health plans (SER) trials to cure recurrent C. difficile infection (SER-109: spore-forming bacteria harvested from fecal donations); ulcerative colitis (SER-287: bacterial spores from biological sources); and primary C. difficile infection to prevent recurrence (SER-262: anaerobic commensal bacterial spores produced by in vitro fermentation) [55, 62]. So far, this advanced therapeutic, SER-109, has been successful in treating 29 out of 30 patients. SER-109 is comprised of 50 well-screened spore-forming bacterial species isolated from healthy FMT donors and has proven to be one of the best alternatives to classical FMT. The spore-based target delivery into the GI tract ensures the stability and shelf life of the therapeutic and has been proven in clinical trials [63]. These microbe-based therapies can be used for the treatment of a wide spectrum of diseases based on the inhibitory effect of beneficial microbes on pathogens in co-culture assays (Table 2). In order to overcome the failure rate, it is necessary to design and develop a personalized formulation by including spore formers as they provide an advantage for transferring the obligate anaerobes.
Major Challenges in Developing Formulations and Their Validation
If synbiotics are to become widespread, useful treatments for disease and improving health, there are several challenges to be overcome. These challenges include isolation and characterization of novel probiotics strains, finding cheap but effective sources for prebiotics, the inclusion of obligate anaerobes as probiotics by overcoming gut transit survival difficulties, and producing synbiotics at a reasonable cost. Post-production challenges include low stringency and poor marketing under the umbrella effect with low regulation of the products because probiotics are not considered a medical product in many countries.
-
A.
Effective identification and characterization of probiotic strains
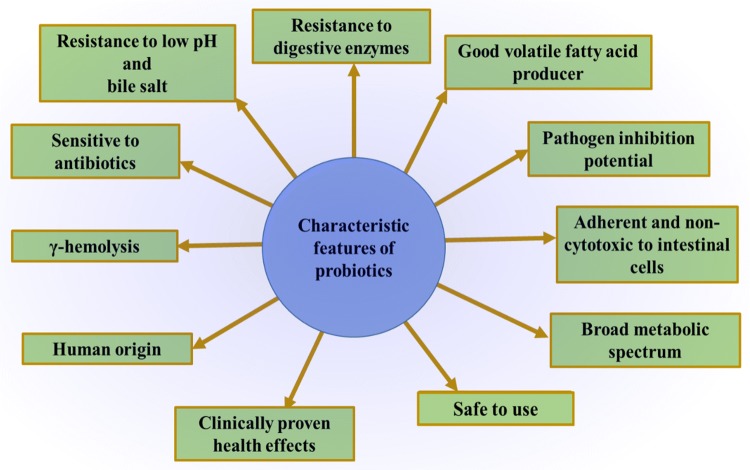
There are a set of features that can be identified to characterize a novel probiotic strain (Fig. 1) [64–66]. Until now, we have characterized only a handful of probiotic bacteria. To overcome this limitation, we need to implement a large-scale culturing and screening method and a robust model system to study the fundamental functions of individual bacteria. In the recent past, a high throughput culturing method known as culturomics was developed. This technique uses multiple culture conditions combined with rapid matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF)) and classical 16S rRNA gene amplification and sequencing identification methods [17, 18, 67]. This approach has proven competent enough to culture the potentially new species [20]. The next step is assessing the potential of these microbes in vitro and in vivo. The use of bioreactors has been beneficial in propagating and establishing complex microbiota populations in vitro by recapitulating the physiological conditions normally present in the GI tract [68]. These bioreactors use the principle of continuous-flow culture models to study the complex microbial interactions by controlling factors such as anaerobicity, pH and nutrient availability [69]. By using bioreactors, we can use a blend of microbes and study the community dynamics using high-throughput sequencing approaches. Once a community establishes itself in the bioreactor, it can be studied in vivo to validate its potential as a probiotic blend. For in vivo studies, we can use germ-free gnotobiotic animal models colonized with known microbiota [70]. These gnotobiotic animal models need some colonized bacteria to develop a normal immune response and gastrointestinal functions [71]. In the mice model, a consortium of eight microbial species known as altered Schaedler flora (ASF) has proven to manifest the relative normal immune response for generations by activating regulatory T-cells and maintaining the normal gut homeostasis [72–74]. These mice models are commercially available for study [75, 76]. Similarly, the gnotobiotic chicken models with known microbiota have been established [77]. All these resources can open new avenues to establish novel probiotic strains.
Fig. 1.
The attributes desired to characterize a human gut isolate into a probiotic strain
All these novel well characterized probiotic strains can be mixed with suitable prebiotics to bump up the health benefits and to do so we need to access suitable prebiotics as well. Currently, the enzyme-mediated production of prebiotics has low yield along with high production cost. To obtain cheap and effective prebiotics, the natural occurring non-dairy oligosaccharides can be explored. A list of rich and natural sources of prebiotics are listed in Table 3.
-
B.
The unregulated market of probiotics: a challenging situation
In 2001, World Health Organization (WHO) and Food and Agriculture Organization (FAO) of United Nations declared that adequate administration of living organisms (probiotics) confers potential health benefits to the individual [78]. Since then, the term probiotics is being misused by producers who market products that do not have beneficial properties. Bacterial products that do not give any beneficial results are called pseudo-probiotic products [79]. These pseudo probiotic products are the consequences of the umbrella concept, where a potential probiotic species is used in many formulations as a minor ingredient, but the product is marketed on that species despite its minor contribution. Manufacturers seek the advantages of this concept and push the results of probiotic products to manufacture other altered and less potential products. This compromises the market of the actual product because pseudo probiotic products have similar specifications but limited or no efficacy, blurring the consumer’s vision due to the unregulated nature of the probiotic market. This leads to health issues and many other problems and questions in terms of quality [80]. Propelled by media coverage and reinforced by marketing heads of probiotic manufacturers, this business has grown from 45.64 billion USD in 2017, to an expected 64.02 billion USD by 2022 globally [81]. Pro-bacterial spp. (Table 1) were accepted to provide gut benefits, however, it was observed that the many of the probiotic effects were species-specific while some are strain specific linked with a specific medical condition. Species-specific probiotics include vitamin production and normalization of disturbed gut community while biochemical, neuronal and immunological effects are considered to be as strain or dose-specific [82]. It has also been reported that a single strain may provide many probiotic effects while only a hand full of strain-specific probiotics are on the market and are medically used for pouchitis, ulcerative colitis and other medical conditions [83]. These probiotics are in the form either mono strain (probiotic with one strain) or multi-strain (more than one species) [84]. A probiotic treatment involves its direct consumption, hence there are two major safety concerns linked with them. First is the assessment of any adverse effect of mono or multi-strain probiotic on consumer health [85]. Second, a stringent standard of quality must be insured during the production of the probiotic to guarantee it is contamination free and the correct strain composition is maintained [86]. Principle risk with the failure of these safeguards are infections and the toxic effect of contaminations which may have immunological implications. Most of the probiotics consumed by humans have an inherent nature to get well conflated and established with the gut microflora by replacing pathogenic bacteria [87]. Thus, the method of administration, nature of the probiotic microbial composition, exposure levels and patient health status, etc. are the critical factors in the clinical trials. A few failures have been reported with sepsis by Lactobacilli and gastrointestinal mucormycosis leading to death due to contamination of the product [88]. In patients with severe and acute pancreatitis, increased mortality was observed due to the consumption of multispecies probiotics [89]. The major threat is in patients with disrupted mucosal lining in the gut and or in immune compromised conditions. A single dose of probiotics creates a load of 109 billion bacteria (CFUs) in the gut, thus a risk–benefit ratio assessment is recommended in patients of HIV, IBD and hepatic encephalopathy [90, 91]. During the synthesis of probiotics, along with the live bacteria, some dead bacteria and their fragments come together which are inseparable, though their number is very few and generally avoided during safety checks. Details of these dead bacteria are not listed on the product labels and are completely overlooked by the manufacturers. Thus, the clinicians are not well informed about these “hidden details” and may miss the real effectiveness of the product. This ratio determines the availability of bacterial DNA (dead + active) for host immune systems. In various patients, gut DNA may trigger inflammatory responses via Toll-like receptors and nuclear kappa B factor activation [92]. This has been reported in Crohn’s disease patients where bacterial DNA translocation into blood increased the relapse risk [93].
-
C.
Concealment from the Law enforcement
Another major threat to probiotics is day by day reclassification of bacterial strains. Initially, Lactobacillus and Bifidobacterium, most promising probiotic genera, were taxonomically characterized based on their morphological and biochemical properties [94, 95]. However, they have been extensively reclassified several times based on the current day genomic attributes. Many manufacturers take advantages of these taxonomical muddles and claim the use of genetically equivalent strains in their products due to the presence of iso-functional enzymes. Quality control standards of food quality are therefore not stringent enough to use the probiotics for medical use. Currently, there are not universally stated regulations of probiotics, but in Europe, regulations are being maintained by EFSA under the “regulation 178/2002/EC; directive 2000/13/EU” act. EFSA has released a list of bacterial strains which do not need safety assessment studies to proceed in manufacturing probiotics [96]. On the other hand, EFSA has also rejected all health claims made by any probiotics so far. This limits not only production but seeks no post-marketing scrutiny [97]. In the United States, probiotics are being regulated by 403(a)(1) of the Federal Food, Drug, and Cosmetic Act [98], where a probiotic can be discontinued if found making any false claims. Since they are not drugs and fall into the category of medical foods, they do not face much regulation in the US. These misregulations have been observed in two probiotic samples in the UK for the treatment of inflammatory bowel disease [82]. The products were manufactured in the US and Italy and both were showing divergent results. The Italian product was not attenuated and was having a threefold increase in 1,3-dihydroxyacetones (fructose metabolism intermediate) responsible for increased gut permeability multiple times [99, 100]. This condition is even more complex when probiotics do not have single generic names since medical doctors generally prescribe brands by names. Probiotics are not eligible for pharmaceutical names and there is no control over the trademark terms for manufacturing these products. Terms used by US patents and trademarks office do not require a trademark to be linked with a specific probiotic preparation and so many different preparations can be used under a common trademark. This offers a great possibility of the sale of a totally new probiotic product, not requiring testing, marketed under a well-known trademark. It is important to highlight any change in the composition of any probiotic on its label by law to enable a better decision to be made by consumers and doctors.
In addition to the above-mentioned threats, there are few side effects which can be experienced by some individuals taking probiotics. These include an acute increase in gas and bloating [101], headaches due to biogenic amines in probiotic foods [102], increased histamine levels that can trigger allergy symptoms and trouble breathing [103]. People with allergies should be also careful and must read the labels to avoid ingredients that may induce allergic reactions. However, probiotics are considered safe for the majority of the population but those with compromised immune systems, acute pancreatitis, venous catheters, and prolonged hospitalizations should avoid taking probiotics.
Conclusions
The normal gut microflora plays an important role in maintaining human health. Strains like Lactobacillus and Bifidobacterium are considered probiotic strains which help in maintaining the gut homeostasis through a wide range of functions. Due to the advancement of research tools, more strains with probiotic potential can be characterized. Supplementing the diet with these good bacteria and yeast has improved digestion and health of humans. Though probiotics are not classified as drugs, they certainly provide human health benefits and also sometimes play valuable roles in serious pathological conditions such as IBD, hepatic encephalopathy, etc. In the absenteeism of stringent guidelines, there is a lack of protection through the law in the favor of genuine producers whose objectives are to classify, study and standardize new probiotics using the latest research and development methods. Generally, end users are misled by popular product labelling or pseudo trademarks deprived of true information over the compositions of probiotics. Probiotics are safe for the majority of the population, but side effects can occur. Hence more rigorous regulations are needed in developing and distributing medically beneficial probiotics.
Acknowledgements
We acknowledge Julie Nelson, Veterinary and Biomedical Sciences Department, South Dakota State University for editing and improving this article.
Compliance with Ethical Standards
Conflict of interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Roshan Kumar, Utkarsh Sood and Vipin Gupta have contributed equally to this work.
References
- 1.Group NHW, Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. The NIH human microbiome project. Genome Res. 2009;19:2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bengmark S. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut. 1998;42:2–7. doi: 10.1136/gut.42.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 5.Luckey TD. Introduction to intestinal microecology. Am J Clin Nutr. 1972;25:1292–1294. doi: 10.1093/ajcn/25.12.1292. [DOI] [PubMed] [Google Scholar]
- 6.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmis K, Cavicchioli R, Garcia JL, Nogales B, Chavarria M, Stein L, McGenity TJ, Webster N, Singh B, Handelsman J, de Lorenzo V, Pruzzo C, Timmis J, Martin JLR, Verstraete W, Jetten M, Danchin A, Huang W, Gilbert J, Lal R, Santos H, Lee SY, Sessitsch A, Bonfante P, Gram L, Lin RTP, Ron E, Karahan C, van der Meer JR, Artunkal S, Jahn D, Harper L. The urgent need for microbiology literacy in society. Environ Microbiol. 2019 doi: 10.1111/1462-2920.14611. [DOI] [PubMed] [Google Scholar]
- 8.Natividad JM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69:42–51. doi: 10.1016/j.phrs.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 9.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higginson J. Proportion of cancers due to occupation. Prev Med. 1980;9:180–188. doi: 10.1016/0091-7435(80)90073-0. [DOI] [PubMed] [Google Scholar]
- 11.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isolauri E. Probiotics in human disease. Am J Clin Nutr. 2001;73:1142S–1146S. doi: 10.1093/ajcn/73.6.1142S. [DOI] [PubMed] [Google Scholar]
- 13.Guyonnet D, Schlumberger A, Mhamdi L, Jakob S, Chassany O. Fermented milk containing Bifidobacterium lactis DN-173 010 improves gastrointestinal well-being and digestive symptoms in women reporting minor digestive symptoms: a randomised, double-blind, parallel, controlled study. Br J Nutr. 2009;102:1654–1662. doi: 10.1017/S0007114509990882. [DOI] [PubMed] [Google Scholar]
- 14.Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS, Baccaglini L, Mohapatra A, Mohapatra SS, Misra PR, Chaudhry R, Chen HH, Johnson JA, Morris JG, Paneth N, Gewolb IH. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407–412. doi: 10.1038/nature23480. [DOI] [PubMed] [Google Scholar]
- 15.McKean J, Naug H, Nikbakht E, Amiet B, Colson N. Probiotics and subclinical psychological symptoms in healthy participants: a systematic review and meta-analysis. J Altern Complement Med. 2017;23:249–258. doi: 10.1089/acm.2016.0023. [DOI] [PubMed] [Google Scholar]
- 16.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. 2017 doi: 10.1128/mmbr.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagier J-C, Khelaifia S, Alou MT, Ndongo S, Dione N, Hugon P, Caputo A, Cadoret F, Traore SI, Seck EH, Dubourg G, Durand G, Mourembou G, Guilhot E, Togo A, Bellali S, Bachar D, Cassir N, Bittar F, Delerce J, Mailhe M, Ricaboni D, Bilen M, Dangui Nieko NPM, Dia Badiane NM, Valles C, Mouelhi D, Diop K, Million M, Musso D, Abrahão J, Azhar EI, Bibi F, Yasir M, Diallo A, Sokhna C, Djossou F, Vitton V, Robert C, Rolain JM, La Scola B, Fournier P-E, Levasseur A, Raoult D. Culture of previously uncultured members of the human gut microbiota by culturomics. Nat Microbiol. 2016;1:16203. doi: 10.1038/nmicrobiol.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Lagier JC, Armougom F, Million M, Hugon P, Pagnier I, Robert C, Bittar F, Fournous G, Gimenez G, Maraninchi M, Trape JF, Koonin EV, La Scola B, Raoult D. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin Microbiol Infect. 2012;18:1185–1193. doi: 10.1111/1469-0691.12023. [DOI] [PubMed] [Google Scholar]
- 19.Sood U, Bajaj A, Kumar R, Khurana S, Kalia VC. Infection and microbiome: impact of tuberculosis on human gut microbiome of indian cohort. Indian J Microbiol. 2018;58:123–125. doi: 10.1007/s12088-018-0706-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou Y, Xue W, Luo G, Deng Z, Qin P, Guo R, Sun H, Xia Y, Liang S, Dai Y, Wan D, Jiang R, Su L, Feng Q, Jie Z, Guo T, Xia Z, Liu C, Yu J, Lin Y, Tang S, Huo G, Xu X, Hou Y, Liu X, Wang J, Yang H, Kristiansen K, Li J, Jia H, Xiao L. 1,520 reference genomes from cultivated human gut bacteria enable functional microbiome analyses. Nat Biotechnol. 2019;37:179–185. doi: 10.1038/s41587-018-0008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Jia H, Cai X, Zhong H, Feng Q, Sunagawa S, Arumugam M, Kultima JR, Prifti E, Nielsen T, Juncker AS, Manichanh C, Chen B, Zhang W, Levenez F, Wang J, Xu X, Xiao L, Liang S, Zhang D, Zhang Z, Chen W, Zhao H, Al-Aama JY, Edris S, Yang H, Wang J, Hansen T, Nielsen HB, Brunak S, Kristiansen K, Guarner F, Pedersen O, Dore J, Ehrlich SD, Meta HITC, Bork P, Wang J, Meta HITC. An integrated catalog of reference genes in the human gut microbiome. Nat Biotechnol. 2014;32:834–841. doi: 10.1038/nbt.2942. [DOI] [PubMed] [Google Scholar]
- 22.Gorvitovskaia A, Holmes SP, Huse SM. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome. 2016;4:15. doi: 10.1186/s40168-016-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talebi Bezmin Abadi A. Helicobacter pylori: a beneficial gastric pathogen? Front Med (Lausanne) 2014;1:26. doi: 10.3389/fmed.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conway T, Cohen PS. Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol Spectr. 2015 doi: 10.1128/microbiolspec.mbp-0006-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Abraham BP, Quigley EMM. Probiotics in inflammatory bowel disease. Gastroenterol Clin N Am. 2017;46:769–782. doi: 10.1016/j.gtc.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Schepper JD, Irwin R, Kang J, Dagenais K, Lemon T, Shinouskis A, Parameswaran N, McCabe LR. Probiotics in gut-bone signaling. Adv Exp Med Biol. 2017;1033:225–247. doi: 10.1007/978-3-319-66653-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metchnikoff E. The prolongation of life: optimistic studies. New York: G. P. Putnam’s Sons; 1908. [Google Scholar]
- 30.Markova IuM, Sheveleva SA. Probiotics as functional food products: manufacture and approaches to evaluating of the effectiveness. Vopr Pitan. 2014;83:4–14. [PubMed] [Google Scholar]
- 31.Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotechnol. 2000;84:197–215. doi: 10.1016/S0168-1656(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 32.Berner D, Viernstein H. Effect of protective agents on the viability of Lactococcus lactis subjected to freeze-thawing and freeze-drying. Sci Pharm. 2006;74:137. doi: 10.3797/scipharm.2006.74.137. [DOI] [Google Scholar]
- 33.Begum P, Madhavi G, Rajagopal S, Viswanath B, Razak M, Venkataratnamma V. Probiotics as functional foods: potential effects on human health and its impact on neurological diseases. Int J Nutr Pharmacol Neurol Dis. 2017;7:23–33. doi: 10.4103/ijnpnd.ijnpnd_90_16. [DOI] [Google Scholar]
- 34.Ranadheera CS, Vidanarachchi JK, Rocha RS, Cruz AG, Ajlouni S. Probiotic delivery through fermentation: dairy vs. non-dairy beverages. Fermentation. 2017;3:67. doi: 10.3390/fermentation3040067. [DOI] [Google Scholar]
- 35.Yeung TW, Ucok EF, Tiani KA, McClements DJ, Sela DA. Microencapsulation in alginate and chitosan microgels to enhance viability of Bifidobacterium longum for oral delivery. Front Microbiol. 2016;7:494. doi: 10.3389/fmicb.2016.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 37.Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- 38.Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, Beghini F, Bertorelli R, De Sanctis V, Bariletti I, Canto R, Clementi R, Cologna M, Crifo T, Cusumano G, Gottardi S, Innamorati C, Mase C, Postai D, Savoi D, Duranti S, Lugli GA, Mancabelli L, Turroni F, Ferrario C, Milani C, Mangifesta M, Anzalone R, Viappiani A, Yassour M, Vlamakis H, Xavier R, Collado CM, Koren O, Tateo S, Soffiati M, Pedrotti A, Ventura M, Huttenhower C, Bork P, Segata N. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–145. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Figueroa-Gonzalez I, Quijano G, Ramirez G, Cruz-Guerrero A. Probiotics and prebiotics–perspectives and challenges. J Sci Food Agric. 2011;91:1341–1348. doi: 10.1002/jsfa.4367. [DOI] [PubMed] [Google Scholar]
- 41.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 42.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy. 2007;108:354–358. [PubMed] [Google Scholar]
- 44.Roytio H, Ouwehand AC. The fermentation of polydextrose in the large intestine and its beneficial effects. Benef Microbes. 2014;5:305–313. doi: 10.3920/BM2013.0065. [DOI] [PubMed] [Google Scholar]
- 45.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 46.Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 47.Basturk A, Artan R, Yilmaz A. Efficacy of synbiotic, probiotic, and prebiotic treatments for irritable bowel syndrome in children: a randomized controlled trial. Turk J Gastroenterol. 2016;27:439–443. doi: 10.5152/tjg.2016.16301. [DOI] [PubMed] [Google Scholar]
- 48.Zamani B, Farshbaf S, Golkar HR, Bahmani F, Asemi Z. Synbiotic supplementation and the effects on clinical and metabolic responses in patients with rheumatoid arthritis: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2017;117:1095–1102. doi: 10.1017/S000711451700085X. [DOI] [PubMed] [Google Scholar]
- 49.Karamali M, Nasiri N, Taghavi Shavazi N, Jamilian M, Bahmani F, Tajabadi-Ebrahimi M, Asemi Z. The effects of synbiotic supplementation on pregnancy outcomes in gestational diabetes. Probiotics Antimicrob Proteins. 2018;10:496–503. doi: 10.1007/s12602-017-9313-7. [DOI] [PubMed] [Google Scholar]
- 50.Nikbakht E, Khalesi S, Singh I, Williams LT, West NP, Colson N. Effect of probiotics and synbiotics on blood glucose: a systematic review and meta-analysis of controlled trials. Eur J Nutr. 2018;57:95–106. doi: 10.1007/s00394-016-1300-3. [DOI] [PubMed] [Google Scholar]
- 51.Saez-Lara MJ, Robles-Sanchez C, Ruiz-Ojeda FJ, Plaza-Diaz J, Gil A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: a review of human clinical trials. Int J Mol Sci. 2016 doi: 10.3390/ijms17060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782. doi: 10.1016/0016-5085(78)90457-2. [DOI] [PubMed] [Google Scholar]
- 54.Smits WK, Lyras D, Lacy DB, Wilcox MH, Kuijper EJ. Clostridium difficile infection. Nat Rev Dis Primers. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.https://cdifffoundation.org. Accessed 20 Apr 2019
- 56.Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK, Group OPTCS Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–431. doi: 10.1056/nejmoa0910812. [DOI] [PubMed] [Google Scholar]
- 58.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 59.Sofi AA, Silverman AL, Khuder S, Garborg K, Westerink JM, Nawras A. Relationship of symptom duration and fecal bacteriotherapy in Clostridium difficile infection-pooled data analysis and a systematic review. Scand J Gastroenterol. 2013;48:266–273. doi: 10.3109/00365521.2012.743585. [DOI] [PubMed] [Google Scholar]
- 60.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meighani A, Hart BR, Mittal C, Miller N, John A, Ramesh M. Predictors of fecal transplant failure. Eur J Gastroenterol Hepatol. 2016;28:826–830. doi: 10.1097/MEG.0000000000000614. [DOI] [PubMed] [Google Scholar]
- 62.Vargason AM, Anselmo AC. Clinical translation of microbe-based therapies: current clinical landscape and preclinical outlook. Bioeng Transl Med. 2018;3:124–137. doi: 10.1002/btm2.10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khanna S, Pardi DS, Kelly CR, Kraft CS, Dhere T, Henn MR, Lombardo MJ, Vulic M, Ohsumi T, Winkler J, Pindar C, McGovern BH, Pomerantz RJ, Aunins JG, Cook DN, Hohmann EL. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J Infect Dis. 2016;214:173–181. doi: 10.1093/infdis/jiv766. [DOI] [PubMed] [Google Scholar]
- 64.Plessas S, Nouska C, Karapetsas A, Kazakos S, Alexopoulos A, Mantzourani I, Chondrou P, Fournomiti M, Galanis A, Bezirtzoglou E. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from feta-type cheese. Food Chem. 2017;226:102–108. doi: 10.1016/j.foodchem.2017.01.052. [DOI] [PubMed] [Google Scholar]
- 65.Munoz-Quezada S, Chenoll E, Vieites JM, Genoves S, Maldonado J, Bermudez-Brito M, Gomez-Llorente C, Matencio E, Bernal MJ, Romero F, Suarez A, Ramon D, Gil A. Isolation, identification and characterisation of three novel probiotic strains (Lactobacillus paracasei CNCM I-4034, Bifidobacterium breve CNCM I-4035 and Lactobacillus rhamnosus CNCM I-4036) from the faeces of exclusively breast-fed infants. Br J Nutr. 2013;109:S51–S62. doi: 10.1017/S0007114512005211. [DOI] [PubMed] [Google Scholar]
- 66.Papadimitriou K, Zoumpopoulou G, Foligne B, Alexandraki V, Kazou M, Pot B, Tsakalidou E. Discovering probiotic microorganisms: in vitro, in vivo, genetic and omics approaches. Front Microbiol. 2015;6:58. doi: 10.3389/fmicb.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sood U, Gupta V, Kumar R, Lal S, Fawcett D, Rattan S, Poinern GEJ, Lal R. Chicken gut microbiome and human health: past scenarios, current perspectives, and futuristic applications. Indian J Microbiol. 2019 doi: 10.1007/s12088-019-00785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guzman-Rodriguez M, McDonald JAK, Hyde R, Allen-Vercoe E, Claud EC, Sheth PM, Petrof EO. Using bioreactors to study the effects of drugs on the human microbiota. Methods. 2018;149:31–41. doi: 10.1016/j.ymeth.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 69.Auchtung JM, Robinson CD, Britton RA. Cultivation of stable, reproducible microbial communities from different fecal donors using minibioreactor arrays (MBRAs) Microbiome. 2015;3:42. doi: 10.1186/s40168-015-0106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faith JJ, Ahern PP, Ridaura VK, Cheng J, Gordon JI. Identifying gut microbe-host phenotype relationships using combinatorial communities in gnotobiotic mice. Sci Transl Med. 2014;6:220ra211. doi: 10.1126/scitranslmed.3008051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brestoff JR, Artis D. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol. 2013;14:676–684. doi: 10.1038/ni.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schaedler RW, Dubs R, Costello R. Association of germfree mice with bacteria isolated from normal mice. J Exp Med. 1965;122:77–82. doi: 10.1084/jem.122.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 74.Wymore Brand M, Wannemuehler MJ, Phillips GJ, Proctor A, Overstreet AM, Jergens AE, Orcutt RP, Fox JG. The altered Schaedler flora: continued applications of a defined murine microbial community. ILAR J. 2015;56:169–178. doi: 10.1093/ilar/ilv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen TC, Albenberg L, Bittinger K, Chehoud C, Chen YY, Judge CA, Chau L, Ni J, Sheng M, Lin A, Wilkins BJ, Buza EL, Lewis JD, Daikhin Y, Nissim I, Yudkoff M, Bushman FD, Wu GD. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest. 2015;125:2841–2850. doi: 10.1172/JCI79214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moghadamrad S, McCoy KD, Geuking MB, Sagesser H, Kirundi J, Macpherson AJ, De Gottardi A. Attenuated portal hypertension in germ-free mice: function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology. 2015;61:1685–1695. doi: 10.1002/hep.27698. [DOI] [PubMed] [Google Scholar]
- 77.Thomas M, Wongkuna S, Ghimire S, Kumar R, Antony L, Doerner KC, Singery A, Nelson E, Woyengo T, Chankhamhaengdecha S, Janvilisri T, Scaria J. Gut microbial dynamics during conventionalization of germfree chicken. mSphere. 2018;4:e00035-19. doi: 10.1128/mSphere.00035-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Probiotics in food: health and nutritional properties and guidelines for evaluation: report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, Cordoba, Argentina, 1–4 October 2001 [and] report of a joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food, London, Ontario, Canada, 30 April–1 May 2002 (2006). FAO food and nutrition paper, 0254-4725; 85., vol Accessed from https://nla.gov.au/nla.cat-vn3788914. Food and Agriculture Organization of the United Nations, World Health Organization, Rome [Italy]
- 79.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 80.Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerg Infect Dis. 2010;16:1661–1665. doi: 10.3201/eid1611.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.https://www.researchandmarkets.com/research/lzfc2k/probiotics_market?w=12. Accessed 20 Apr 2019
- 82.de Simone C. The unregulated probiotic market. Clin Gastroenterol Hepatol. 2019;17:809–817. doi: 10.1016/j.cgh.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Guslandi M. Role of probiotics in Crohn’s disease and in pouchitis. J Clin Gastroenterol. 2015;49:S46–S49. doi: 10.1097/MCG.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 84.Timmerman HM, Koning CJ, Mulder L, Rombouts FM, Beynen AC. Monostrain, multistrain and multispecies probiotics—a comparison of functionality and efficacy. Int J Food Microbiol. 2004;96:219–233. doi: 10.1016/j.ijfoodmicro.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Piotrovskii VK, Veiko NN, Golovanova IV, Gus’kova TA, Polievktov MK. Slow elimination of a prazosin metabolite compared to prazosin kinetics after its intravenous administration to rabbits. Biull Exp Biol Med. 1987;103:73–75. doi: 10.1007/BF00840143. [DOI] [PubMed] [Google Scholar]
- 86.Sanders ME, Merenstein DJ, Ouwehand AC, Reid G, Salminen S, Cabana MD, Paraskevakos G, Leyer G. Probiotic use in at-risk populations. J Am Pharm Assoc. 2006;56:680–686. doi: 10.1016/j.japh.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 87.Bagchi T. Traditional food & modern lifestyle: impact of probiotics. Indian J Med Res. 2014;140:333–335. [PMC free article] [PubMed] [Google Scholar]
- 88.https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6406a6.htm. Accessed 20 Apr 2019
- 89.The Editors of The Lancet Expression of concern—Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;375(9718):875–876. doi: 10.1016/S0140-6736(10)60360-1. [DOI] [PubMed] [Google Scholar]
- 90.Nagpal R, Kumar A, Kumar M, Behare PV, Yadav H, Jain S. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 91.Martin IW, Tonner R, Trivedi J, Miller H, Lee R, Liang X, Rotello L, Isenbergh E, Anderson J, Perl T, Zhang SX. Saccharomyces boulardii probiotic-associated fungemia: questioning the safety of this preventive probiotic’s use. Diagn Microbiol Infect Dis. 2017;87:286–288. doi: 10.1016/j.diagmicrobio.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 92.Oeckinghaus A, Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gutierrez A, Zapater P, Juanola O, Sempere L, Garcia M, Laveda R, Martinez A, Scharl M, Gonzalez-Navajas JM, Such J, Wiest R, Rogler G, Frances R. Gut bacterial DNA translocation is an independent risk factor of flare at short term in patients with Crohn’s disease. Am J Gastroenterol. 2016;111:529–540. doi: 10.1038/ajg.2016.8. [DOI] [PubMed] [Google Scholar]
- 94.Bull M, Plummer S, Marchesi J, Mahenthiralingam E. The life history of Lactobacillus acidophilus as a probiotic: a tale of revisionary taxonomy, misidentification and commercial success. FEMS Microbiol Lett. 2013;349:77–87. doi: 10.1111/1574-6968.12293. [DOI] [PubMed] [Google Scholar]
- 95.Allen AP, Clarke G, Cryan JF, Quigley EMM, Dinan TG. Bifidobacterium infantis 35624 and other probiotics in the management of irritable bowel syndrome. Strain specificity, symptoms, and mechanisms. Curr Med Res Opin. 2017;33:1349–1351. doi: 10.1080/03007995.2017.1322571. [DOI] [PubMed] [Google Scholar]
- 96.https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps. Accessed 20 Apr 2019
- 97.Kolacek S, Hojsak I, Berni Canani R, Guarino A, Indrio F, Orel R, Pot B, Shamir R, Szajewska H, Vandenplas Y, van Goudoever J, Weizman Z, Probiotics EWGf, Prebiotics Commercial probiotic products: a call for improved quality control. A position paper by the ESPGHAN working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr. 2017;65:117–124. doi: 10.1097/mpg.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 98.https://www.fda.gov/food/guidanceregulation/guidancedocumentsregulatoryinformation/medicalfoods/ucm054048.htm. Accessed 20 Apr 2019
- 99.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol. 2018;68:1063–1075. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rao RK, Samak G. Protection and restitution of gut barrier by probiotics: nutritional and clinical implications. Curr Nutr Food Sci. 2013;9:99–107. doi: 10.2174/1573401311309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Williams NT. Probiotics. Am J Health Syst Pharm. 2010;67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 102.Gezginc Y, Akyol I, Kuley E, Özogul F. Biogenic amines formation in Streptococcus thermophilus isolated from home-made natural yogurt. Food Chem. 2013;138:655–662. doi: 10.1016/j.foodchem.2012.10.138. [DOI] [PubMed] [Google Scholar]
- 103.Pessione E. Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. 2012;2:86. doi: 10.3389/fcimb.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014;8:2116. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit Rev Food Sci Nutr. 2018 doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 106.Hong HA, le Duc H, Cutting SM. The use of bacterial spore formers as probiotics. FEMS Microbiol Rev. 2005;29:813–835. doi: 10.1016/j.femsre.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 107.Jiang H, Ji C, Sui J, Sa R, Wang X, Liu X, Guo TL. Antibacterial and antitumor activity of Bogorol B-JX isolated from Brevibacillus laterosporus JX-5. World J Microbiol Biotechnol. 2017;33:177. doi: 10.1007/s11274-017-2337-z. [DOI] [PubMed] [Google Scholar]
- 108.Ulsemer P, Toutounian K, Schmidt J, Karsten U, Goletz S. Preliminary safety evaluation of a new Bacteroides xylanisolvens isolate. Appl Environ Microbiol. 2012;78:528–535. doi: 10.1128/AEM.06641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ulsemer P, Toutounian K, Kressel G, Goletz C, Schmidt J, Karsten U, Hahn A, Goletz S. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFalpha-specific antibodies in human adults. Benef Microbes. 2016;7:485–500. doi: 10.3920/BM2015.0143. [DOI] [PubMed] [Google Scholar]
- 110.Ku S, Park MS, Ji GE, You HJ. Review on Bifidobacterium bifidum BGN4: functionality and nutraceutical applications as a probiotic microorganism. Int J Mol Sci. 2016 doi: 10.3390/ijms17091544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.LeBlanc JG, Chain F, Martin R, Bermudez-Humaran LG, Courau S, Langella P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb Cell Fact. 2017;16:79. doi: 10.1186/s12934-017-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun. 2004;72:2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yuan F, Ni H, Asche CV, Kim M, Walayat S, Ren J. Efficacy of Bifidobacterium infantis 35624 in patients with irritable bowel syndrome: a meta-analysis. Curr Med Res Opin. 2017;33:1191–1197. doi: 10.1080/03007995.2017.1292230. [DOI] [PubMed] [Google Scholar]
- 114.Liu MY, Yang ZY, Dai WK, Huang JQ, Li YH, Zhang J, Qiu CZ, Wei C, Zhou Q, Sun X, Feng X, Li DF, Wang HP, Zheng YJ. Protective effect of Bifidobacterium infantis CGMCC313-2 on ovalbumin-induced airway asthma and beta-lactoglobulin-induced intestinal food allergy mouse models. World J Gastroenterol. 2017;23:2149–2158. doi: 10.3748/wjg.v23.i12.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merenstein DJ, Tan TP, Molokin A, Smith KH, Roberts RF, Shara NM, Mete M, Sanders ME, Solano-Aguilar G. Safety of Bifidobacterium animalis subsp. lactis (B. lactis) strain BB-12-supplemented yogurt in healthy adults on antibiotics: a phase I safety study. Gut Microbes. 2015;6:66–77. doi: 10.1080/19490976.2015.1005484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ruiz L, Gueimonde M, Ruas-Madiedo P, Ribbera A, de los Reyes-Gavilán CG, Ventura M, Margolles A, Sánchez B. Molecular clues to understand the aerotolerance phenotype of bifidobacterium animalis subsp. lactis. Appl Environ Microbiol. 2012;78:644–650. doi: 10.1128/aem.05455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Celiberto LS, Bedani R, Dejani NN, Ivo de Medeiros A, Sampaio Zuanon JA, Spolidorio LC, Tallarico Adorno MA, Amancio Varesche MB, Carrilho Galvao F, Valentini SR, Font de Valdez G, Rossi EA, Cavallini DCU. Effect of a probiotic beverage consumption (Enterococcus faecium CRL 183 and Bifidobacterium longum ATCC 15707) in rats with chemically induced colitis. PLoS ONE. 2017;12:e0175935. doi: 10.1371/journal.pone.0175935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu J, Sun J, Wang F, Yu X, Ling Z, Li H, Zhang H, Jin J, Chen W, Pang M, Yu J, He Y, Xu J. Neuroprotective Effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. Biomed Res Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Martín R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, Chain F, Berteau O, Azevedo V, Chatel JM, Sokol H, Bermúdez-Humarán LG, Thomas M, Langella P. Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hong YF, Kim H, Kim HS, Park WJ, Kim JY, Chung DK. Lactobacillus acidophilus K301 inhibits atherogenesis via induction of 24 (S), 25-epoxycholesterol-mediated ABCA1 and ABCG1 production and cholesterol efflux in macrophages. PLoS ONE. 2016;11:e0154302. doi: 10.1371/journal.pone.0154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu CH, Hsueh YH, Kuo JM, Liu SJ. Characterization of a potential probiotic Lactobacillus brevis RK03 and efficient production of gamma-aminobutyric acid in batch fermentation. Int J Mol Sci. 2018 doi: 10.3390/ijms19010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moro-García MA, Alonso-Arias R, Baltadjieva M, Fernández Benítez C, Fernández Barrial MA, Díaz Ruisánchez E, Alonso Santos R, Alvarez Sánchez M, Saavedra Miján J, López-Larrea C. Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age (Dordr) 2013;35:1311–1326. doi: 10.1007/s11357-012-9434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hill D, Sugrue I, Tobin C, Hill C, Stanton C, Ross RP. The Lactobacillus casei group: history and health related applications. Front Microbiol. 2018;9:2107. doi: 10.3389/fmicb.2018.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Aktas B, De Wolfe TJ, Tandee K, Safdar N, Darien BJ, Steele JL. The effect of Lactobacillus casei 32G on the mouse cecum microbiota and innate immune response is dose and time dependent. PLoS ONE. 2015;10:e0145784. doi: 10.1371/journal.pone.0145784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Usman Hosono A. Bile tolerance, taurocholate deconjugation, and binding of cholesterol by Lactobacillus gasseri strains. J Dairy Sci. 1999;82:243–248. doi: 10.3168/jds.S0022-0302(99)75229-X. [DOI] [PubMed] [Google Scholar]
- 128.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, Okano M, Kagoshima M, Tsuchida T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–643. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 129.Hosoya T, Sakai F, Yamashita M, Shiozaki T, Endo T, Ukibe K, Uenishi H, Kadooka Y, Moriya T, Nakagawa H, Nakayama Y, Miyazaki T. Lactobacillus helveticus SBT2171 inhibits lymphocyte proliferation by regulation of the JNK signaling pathway. PLoS ONE. 2014;9:e108360. doi: 10.1371/journal.pone.0108360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, Brigidi P, Gibson GR. An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. PLoS ONE. 2017;12:e0187964. doi: 10.1371/journal.pone.0187964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. Biomed Res Int. 2018;2018:9361614. doi: 10.1155/2018/9361614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krzysciak W, Koscielniak D, Papiez M, Vyhouskaya P, Zagorska-Swiezy K, Kolodziej I, Bystrowska B, Jurczak A. Effect of a Lactobacillus salivarius probiotic on a double-species Streptococcus mutans and Candida albicans caries biofilm. Nutrients. 2017 doi: 10.3390/nu9111242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Neville BA, O’Toole PW. Probiotic properties of Lactobacillus salivarius and closely related Lactobacillus species. Future Microbiol. 2010;5:759–774. doi: 10.2217/fmb.10.35. [DOI] [PubMed] [Google Scholar]
- 134.Song AA-L, In LLA, Lim SHE, Rahim RA. A review on Lactococcus lactis: from food to factory. Microb Cell Fact. 2017;16:55. doi: 10.1186/s12934-017-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Abbasiliasi S, Tan JS, Bashokouh F, Ibrahim TAT, Mustafa S, Vakhshiteh F, Sivasamboo S, Ariff AB. In vitro assessment of Pediococcus acidilactici Kp10 for its potential use in the food industry. BMC Microbiol. 2017;17:121. doi: 10.1186/s12866-017-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ogita T, Nakashima M, Morita H, Saito Y, Suzuki T, Tanabe S. Streptococcus thermophilus ST28 ameliorates colitis in mice partially by suppression of inflammatory Th17 cells. J Biomed Biotechnol. 2011;2011:378417. doi: 10.1155/2011/378417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rho MK, Kim YE, Rho HI, Kim TR, Kim YB, Sung WK, Kim TW, Kim DO, Kang H. Enterococcus faecium FC-K derived from kimchi is a probiotic strain that shows anti-allergic activity. J Microbiol Biotechnol. 2017;27:1071–1077. doi: 10.4014/jmb.1611.11020. [DOI] [PubMed] [Google Scholar]
- 138.Vimont A, Fernandez B, Hammami R, Ababsa A, Daba H, Fliss I. Bacteriocin-producing Enterococcus faecium LCW 44: a high potential probiotic candidate from raw camel milk. Front Microbiol. 2017;8:865. doi: 10.3389/fmicb.2017.00865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Buts JP. Twenty-five years of research on Saccharomyces boulardii trophic effects: updates and perspectives. Dig Dis Sci. 2009;54:15–18. doi: 10.1007/s10620-008-0322-y. [DOI] [PubMed] [Google Scholar]
- 140.Czerucka D, Piche T, Rampal P. Review article: yeast as probiotics—Saccharomyces boulardii. Aliment Pharmacol Ther. 2007;26:767–778. doi: 10.1111/j.1365-2036.2007.03442.x. [DOI] [PubMed] [Google Scholar]
- 141.Yoon S, Yu J, McDowell A, Kim SH, You HJ, Ko G. Bile salt hydrolase-mediated inhibitory effect of Bacteroides ovatus on growth of Clostridium difficile. J Microbiol. 2017;55:892–899. doi: 10.1007/s12275-017-7340-4. [DOI] [PubMed] [Google Scholar]
- 142.Valdes-Varela L, Hernandez-Barranco AM, Ruas-Madiedo P, Gueimonde M. Effect of Bifidobacterium upon Clostridium difficile growth and toxicity when co-cultured in different prebiotic substrates. Front Microbiol. 2016;7:738. doi: 10.3389/fmicb.2016.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Woo TD, Oka K, Takahashi M, Hojo F, Osaki T, Hanawa T, Kurata S, Yonezawa H, Kamiya S. Inhibition of the cytotoxic effect of Clostridium difficile in vitro by Clostridium butyricum MIYAIRI 588 strain. J Med Microbiol. 2011;60:1617–1625. doi: 10.1099/jmm.0.033423-0. [DOI] [PubMed] [Google Scholar]
- 144.Banerjee P, Merkel GJ, Bhunia AK. Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog. 2009;1:8. doi: 10.1186/1757-4749-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mariam SH, Zegeye N, Aseffa A, Howe R. Diffusible substances from lactic acid bacterial cultures exert strong inhibitory effects on Listeria monocytogenes and Salmonella enterica serovar enteritidis in a co-culture model. BMC Microbiol. 2017;17:35. doi: 10.1186/s12866-017-0944-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Garcia MT, Marinez Canamero M, Lucas R, Ben Omar N, Perez Pulido R, Galvez A. Inhibition of Listeria monocytogenes by enterocin EJ97 produced by Enterococcus faecalis EJ97. Int J Food Microbiol. 2004;90:161–170. doi: 10.1016/S0168-1605(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 147.Kleessen B, Schwarz S, Boehm A, Fuhrmann H, Richter A, Henle T, Krueger M. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr. 2007;98:540–549. doi: 10.1017/S0007114507730751. [DOI] [PubMed] [Google Scholar]
- 148.Kim M, Shin HK. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats. J Nutr. 1998;128:1731–1736. doi: 10.1093/jn/128.10.1731. [DOI] [PubMed] [Google Scholar]
- 149.Clare BA, Conroy RS, Spelman K. The diuretic effect in human subjects of an extract of Taraxacum officinale folium over a single day. J Altern Complement Med. 2009;15:929–934. doi: 10.1089/acm.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gonzalez-Castejon M, Visioli F, Rodriguez-Casado A. Diverse biological activities of dandelion. Nutr Rev. 2012;70:534–547. doi: 10.1111/j.1753-4887.2012.00509.x. [DOI] [PubMed] [Google Scholar]
- 151.Barszcz M, Taciak M, Skomial J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch Anim Nutr. 2016;70:278–292. doi: 10.1080/1745039X.2016.1184368. [DOI] [PubMed] [Google Scholar]
- 152.Samal L, Chaturvedi VB, Saikumar G, Somvanshi R, Pattanaik AK. Prebiotic potential of Jerusalem artichoke (Helianthus tuberosus L.) in Wistar rats: effects of levels of supplementation on hindgut fermentation, intestinal morphology, blood metabolites and immune response. J Sci Food Agric. 2015;95:1689–1696. doi: 10.1002/jsfa.6873. [DOI] [PubMed] [Google Scholar]
- 153.Ning Zhang XH, Zeng Yanhua, Xiyang Wu, Peng Xichun. Study on prebiotic effectiveness of neutral garlic fructan in vitro. Food Sci Hum Wellness. 2013;2:119–123. doi: 10.1016/j.fshw.2013.07.001. [DOI] [Google Scholar]
- 154.Kolida S, Tuohy K, Gibson GR. Prebiotic effects of inulin and oligofructose. Br J Nutr. 2002;87:S193–S197. doi: 10.1079/BJNBJN/2002537. [DOI] [PubMed] [Google Scholar]
- 155.Delaney B, Nicolosi RJ, Wilson TA, Carlson T, Frazer S, Zheng GH, Hess R, Ostergren K, Haworth J, Knutson N. Beta-glucan fractions from barley and oats are similarly antiatherogenic in hypercholesterolemic Syrian golden hamsters. J Nutr. 2003;133:468–475. doi: 10.1093/jn/133.2.468. [DOI] [PubMed] [Google Scholar]
- 156.Onning G, Wallmark A, Persson M, Akesson B, Elmstahl S, Oste R. Consumption of oat milk for 5 weeks lowers serum cholesterol and LDL cholesterol in free-living men with moderate hypercholesterolemia. Ann Nutr Metab. 1999;43:301–309. doi: 10.1159/000012798. [DOI] [PubMed] [Google Scholar]
- 157.Othman RA, Moghadasian MH, Jones PJ. Cholesterol-lowering effects of oat beta-glucan. Nutr Rev. 2011;69:299–309. doi: 10.1111/j.1753-4887.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- 158.Licht TR, Hansen M, Bergstrom A, Poulsen M, Krath BN, Markowski J, Dragsted LO, Wilcks A. Effects of apples and specific apple components on the cecal environment of conventional rats: role of apple pectin. BMC Microbiol. 2010;10:13. doi: 10.1186/1471-2180-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen HL, Cheng HC, Liu YJ, Liu SY, Wu WT. Konjac acts as a natural laxative by increasing stool bulk and improving colonic ecology in healthy adults. Nutrition. 2006;22:1112–1119. doi: 10.1016/j.nut.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 160.Kaats GR, Bagchi D, Preuss HG. Konjac glucomannan dietary supplementation causes significant fat loss in compliant overweight adults. J Am Coll Nutr. 2015 doi: 10.1080/07315724.2015.1009194. [DOI] [PubMed] [Google Scholar]
- 161.Singh KK, Mridula D, Rehal J, Barnwal P. Flaxseed: a potential source of food, feed and fiber. Crit Rev Food Sci Nutr. 2011;51:210–222. doi: 10.1080/10408390903537241. [DOI] [PubMed] [Google Scholar]
- 162.Kristensen M, Jensen MG, Aarestrup J, Petersen KE, Sondergaard L, Mikkelsen MS, Astrup A. Flaxseed dietary fibers lower cholesterol and increase fecal fat excretion, but magnitude of effect depend on food type. Nutr Metab (Lond) 2012;9:8. doi: 10.1186/1743-7075-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Delgado GT, Tamashiro WM, Marostica Junior MR, Pastore GM. Yacon (Smallanthus sonchifolius): a functional food. Plant Foods Hum Nutr. 2013;68:222–228. doi: 10.1007/s11130-013-0362-0. [DOI] [PubMed] [Google Scholar]
- 164.Francois IE, Lescroart O, Veraverbeke WS, Marzorati M, Possemiers S, Hamer H, Windey K, Welling GW, Delcour JA, Courtin CM, Verbeke K, Broekaert WF. Effects of wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal parameters in healthy preadolescent children. J Pediatr Gastroenterol Nutr. 2014;58:647–653. doi: 10.1097/MPG.0000000000000285. [DOI] [PubMed] [Google Scholar]