Abstract
Tissue-resident memory T (TRM) cells are increasingly associated with the outcomes of health and disease. TRM cells can mediate local immune protection against infections and cancer, which has led to interest in TRM cells as targets for vaccination and immunotherapies. However, these cells have also been implicated in mediating detrimental pro-inflammatory responses in autoimmune skin diseases such as psoriasis, alopecia areata, and vitiligo. Here, we summarize the biology of TRM cells established in animal models and in translational human studies. We review the beneficial effects of TRM cells in mediating protective responses against infection and cancer and the adverse role of TRM cells in driving pathology in autoimmunity. A further understanding of the breadth and mechanisms of TRM cell activity is essential for the safe design of strategies that manipulate TRM cells, such that protective responses can be enhanced without unwanted tissue damage, and pathogenic TRM cells can be eliminated without losing local immunity.
Keywords: human, T cells, tissue-resident, memory, immunity
Subject terms: Immunological memory, Cancer microenvironment
Introduction
Improving the quality and breadth of immunity has been an ongoing challenge for the long-term preservation of human health. Much of the focus has been directed at generating immunological memory, a paradigm demonstrated to be effective at protecting against recurrent pathological challenges in an ever-evolving environment. A critical component of this process is a population of non-recirculating lymphocytes, termed tissue-resident memory T (TRM) cells, which are phenotypically and transcriptionally distinct from circulating memory T cells1 and offer front-line protection against invading microbes and tumor outgrowth.2,3 While the majority of studies detailing the identity and differentiation of TRM cells have been performed in mouse models,4–8 more recent studies of human tissues describe the association of TRM cell density and phenotype with prognosis in several disease settings.9–11 Human TRM cells have heterogeneous developmental and survival requirements, the full extent of which is yet to be completely elucidated, demonstrating a continued need to further dissect fundamental mechanisms of TRM cell biology. Here, we discuss what is known about human TRM cells in health and in disease in the context of infection, cancer, autoimmunity, and transplantation, highlighting knowledge gaps and therapeutic opportunities.
An overview of TRM cells: lessons from mice
TRM cells have been investigated across multiple tissues and in various disease settings. Studies from murine models using transplantation,5,12 parabiosis,13–15 and intravascular labeling15,16 have demonstrated a bona fide resident population of memory T cells that are permanently lodged in the tissue. While the majority of studies have focused on CD8+ TRM cells, there is increasing evidence that CD4+ TRM cells are also present in nonlymphoid tissues in both mice17–21 and humans.22–25 The defining feature of TRM cells is their commitment to the tissue of residence (as opposed to any particular marker), a key feature being their inability to circulate through the bloodstream or lymphatics. TRM cells lack the lymph node homing molecules CD62L and CCR7,6,26 which helps to facilitate tissue residency and downregulate the transcription factor KLF227 and receptors of sphingosine-1-phosphate (S1P), a chemoattractant produced by endothelial cells that promotes egress from lymph nodes and tissue.28 Moreover, the expression of CD69, which is commonly used to define TRM cells, has a role in antagonizing the expression of the egress receptor S1P receptor 1 (S1PR1) and preventing cells from migrating out of the tissue.27,29
The canonical markers associated with CD8+ TRM cells are CD69 and integrin CD103, although TRM cell populations lacking CD103 have also been detected in tissues, including the kidney30 and liver31,32 and the small intestine intraepithelial lymphocytes (SI-IELs) and lamina propria.33,34 The expression of other surface molecules has been associated with CD8+ TRM cells, including CD49a, CD101, PD-1, and CXCR6,10,35–37 some of which are dependent on the local tissue. Following entry into nonlymphoid organs, TRM cells acquire several differentiation markers in a process that involves the action of cytokines, including tumor growth factor-β (TGFβ) and interleukin-15 (IL-15). Using T cells from mice deficient in cytokines or their receptors, it has been demonstrated that TGFβ plays a role in the development of TRM cells in the skin,6,7 lung,7,38 salivary gland,39,40 and SI-IELs34,41 and that IL-15 contributes to TRM cell development in the skin, salivary gland, lung, and liver (but not in SI-IELs8). Importantly, it has been shown that other tissue-resident cells (including natural killer and natural killer T cells) share a common transcriptional signature regulated by the transcription factors Hobit and Blimp-1, which are distinct from the genetic profile of circulating memory T cells.31 Both the Notch family of signaling receptors and the aryl hydrocarbon receptor play a role in the maintenance of mouse TRM cells.6,42 More recently, Runx3 and Bhlhe40 were identified as transcription factors that could regulate TRM cell development and functionality.43,44
The capacity of TRM cells to protect against re-infection and tumor growth has now been shown in numerous settings.45 Using experimental systems where circulating memory T cells are depleted, leaving only tissue-resident cells, it has been shown that TRM cells are capable of “sounding the alarm” against recurrent immunogenic challenges.46 Upon restimulation, TRM cells are capable of proliferating, secreting effector cytokines, such as interferon-γ (IFNγ), tumor necrosis factor-α (TNFα), and granzyme B, and recruiting other immune cells to the site of challenge; therefore, the mucosal recall response is contributed both by pre-existing TRM cells and those recruited from the circulation.47,48 TRM cells prevent and control a wide variety of viral infections.14,49–53 Additionally, TRM cell abundance in tumors positively correlates with patient survival,11,54–58 and they are able to maintain malignant cells in a state of equilibrium to prevent outgrowth.3 All the above illustrate the role of TRM cells as critical players in mediating long-term immunity.
From mice to men: investigating human TRM cells
The understanding of murine TRM cell biology has driven the investigation of TRM cells in an array of human conditions, including in the context of organ donation and transplantation, infectious diseases, and tumor biology. The persistence of donor HLA-mismatched TRM cells following facial,59 lung60 and small-bowel61,62 transplantation provides strong evidence for non-recirculating TRM cells in human tissue. In one study, organ donors were analyzed over six decades of life, and the frequency of CD4+ or CD8+ T cells with a CD69+ or CD69+CD103+ phenotype was found to be the greatest in mucosal sites, including the gut and lung, compared to lymphoid tissue.63 Additionally, the administration of anti-CD52 therapeutic antibody (alemtuzumab) to patients with cutaneous T cell lymphoma was found to deplete circulating T cells, but spare those found in skin, illustrating a population of tissue-resident skin cells in disequilibrium with the blood.64
While CD69 expression is generally used to identify human CD8+ and CD4+ TRM cells, it is likely that additional resident T cells exist that do not express this putative marker, as has been reported in mice.15 For example, while CD69+CD103− and CD69+CD103+ populations of CD8+ and CD4+ TRM cells have been found in tissues, including healthy human lung60 and skin,65 TRM cells lacking the expression of both of these markers have been reported in cells from the female reproductive tract,50,66 pancreas,66 and salivary glands.40,67
Even within the same tissue, the TRM cell pool displays heterogeneity, with distinct transcriptional and phenotypic subsets.23 For example, skin CD8+ TRM cells may be CD49a+ or CD49−,10 with the expression of CD49a facilitating TRM cell retention through its dimerization with CD29 (integrin β1) and the binding of collagen IV and laminin.68 CD49a+ TRM cells have a cytotoxic phenotype based on perforin and IFNγ production, while CD49a− TRM cells can secrete IL-17.10,37 Given the unique microenvironment of various organs, local tissue-specific features are likely associated with the differentiation, homeostasis, and protective functions of TRM cells (reviewed in ref. 69).
It has been demonstrated that the CD69+ memory T cells isolated from human lung tissue share a similar transcriptional profile to that of mouse TRM cells, with the upregulation of the surface markers Itgae (which encodes CD103), Itga1 (which encodes CD49a), and Cxcr6 and the downregulation of migration-associated genes such as S1pr1, Klf2, and Sell (encodes CD62L) when compared to CD69− memory T cell compartments from the lung and spleen.23 The expression of immune checkpoints, including PD-1,23,48 on TRM cells indicates that they may be negatively regulated to prevent aberrant activation, a feature that may be critical to the tumor response following treatment with immune checkpoint inhibitors.
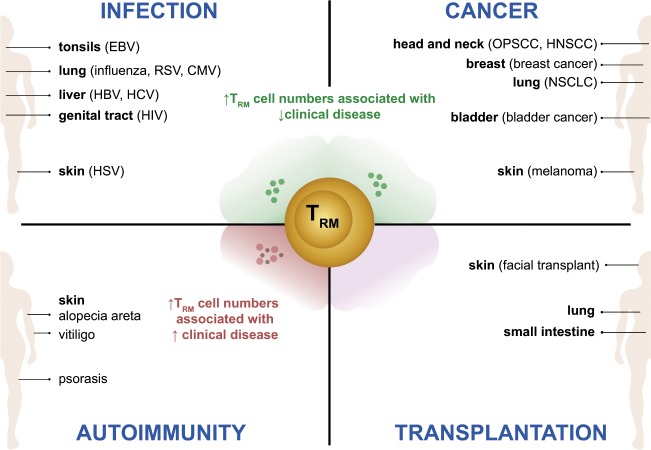
Despite the discovery of TRM cells in various human tissues, there is still a lack of complete understanding surrounding TRM cell identification, development, and functionality. Below, we discuss reports on human TRM cells with a focus on CD8+ TRM cells in a range of disease settings, highlighting their potential to promote tissue immunity in some contexts and to contribute to tissue damage in others (summarized in Fig. 1).
Fig. 1. Overview of TRM cell function in human disease.
Human TRM cells have been isolated from a broad range of tissues and assessed for their associations with disease activity in the context of infection, cancer, autoimmunity and transplantation as summarized here. Infection: Epstein–Barr virus (EBV)-specific T cells have been identified in the tonsils of infected patients and have shown an increased frequency of CD69 and CD103 coexpression compared to their counterparts in the blood and spleen.125–127 Lung TRM cells from influenza patients195 as well as liver TRM cells from hepatitis B virus (HBV)-infected patients35 are capable of producing strong levels of IFNγ and TNFα ex vivo, and high numbers of these TRM cells were found to correlate with reduced disease severity. This was similarly observed for patients infected with respiratory syncytial virus (RSV),93–95 cytomegalovirus (CMV),117,118,120–122,124 and hepatitis C virus (HCV),35 whereby increased TRM cell numbers were associated with reduced disease activity. A more robust polyfunctional cytokine response by human immunodeficiency virus (HIV)-specific TRM cells is observed in patients who are able to better control infection.131–135 While herpes simplex virus (HSV)-specific TRM cells have shown direct viral control in murine studies,103–105 it has also been predicted that a higher density of HSV-reactive TRM cells in humans can correlate with successful viral containment.108 Cancer: Multiple studies have demonstrated that increased numbers of TRM cells can correlate with an improved survival rate of patients, including those with oropharyngeal squamous cell carcinoma (OPSCC),166–168 head and neck squamous cell carcinoma (HNSCC),154 breast cancer,11 nonsmall-cell lung cancer (NSCLC),54 bladder cancer,56,149 and melanoma.55 Moreover, TRM cells with higher levels of perforin, granzyme B, and other effector proteins were noted in cohorts of patients with better disease prognosis.11,56,149 Autoimmunity: The presence of cytokine-secreting TRM cells in the skin has been shown to be associated with poor outcomes in psoriasis,10 vitiligo,10 and alopecia areata (AA).187 While an increase in IL-17 production has been correlated with psoriasis disease activity,10 the secretion of IFNγ may be linked to AA disease progression,187 and the production of perforin and granzyme B may play a role in vitiligo.10 Transplantation: Studies have described the persistence of donor-derived lymphocytes in allografts of solid organ transplantation. While the correlation of donor-derived TRM cells in both intestinal61,62 and lung60 transplantation has been associated with a reduced incidence of rejection, conflicting studies from facial skin transplant inferred negative outcomes with the use of donor-derived TRM cells.59 Therefore, the contribution of TRM cells to the transplantation field is still in its infancy, and more studies are needed to understand the contribution of donor and recipient cells to allograft rejection.
TRM cells in infectious diseases
It has been well established that classic circulating effector memory and central memory T cells (TEM cells and TCM cells, respectively) provide critical immune memory to infectious agents and protect against re-infection. Circulating naive T cells migrate through the blood and lymphoid tissue until they encounter a cognate antigen on antigen-presenting cells. This leads to cellular activation of the naïve cell, transformation into a memory T cell, and clonal expansion. A subset of TEM will survive the following memory T cell constriction to become TCM cells; these cells traffic through blood and lymph and provide an augmented effector response upon antigen re-encounter that contributes to the prevention and control of disease (reviewed in refs. 70,71). Two phenomena well described in classic circulating T cells are memory inflation and exhaustion; however, it is unknown whether these phenomena similarly occur in TRM cells. Memory T cell inflation describes the development of expanded, functionally distinct antigen-specific CD8+ T cells. These populations occur as the result of restricted contraction, maintain a persisting TEM phenotype without features of exhaustion and have been most characterized in chronic viral infections, including murine and human cytomegalovirus (CMV; reviewed in ref. 72). The repeated antigenic stimulation that occurs in both chronic infection and malignancy leads to an induced hyporesponsiveness of antigen-specific CD8+ T cells known as T cell exhaustion. The terminally differentiated exhausted T cells of chronic infection have a PD-1+CXCR5−TIM3+EOMEShiTBETlo phenotype (reviewed in ref. 73).
More recently, the role of non-recirculating TRM cells in the control of infectious diseases has become appreciated. Poised in front-line niches, TRM cells are opportunely placed to protect against pathogen invasion. Human TRM cells have been most studied in the context of viral immunity, but have also been examined in a number of bacterial, parasitic, and fungal infections (reviewed in ref. 74). Here, we review the contribution of TRM cells to immunity against common viral infections by summarizing evidence derived from primary human tissue and relevant animal models of disease.
Influenza
Influenza-specific TRM cells have been detected in the respiratory tract of infected patients,75–79 and these cells play a role in the control of acute infection and long-term immunity.38 Influenza-specific TRM cells are highly proliferative and polyfunctional,80 demonstrating a rapid and robust IFNγ and TNFα response upon ex vivo stimulation.80 Prior exposure correlates with protection against re-infection,81 possibly due to the accumulation of CD8+ TRM cells in the lungs.77,79 This observation has led to investigation into whether influenza-specific TRM cells play a role in this process and provide long-term heterosubtypic protection.22,51,52,78,82 However, while TRM cells in most tissues are thought to be long lived,5,6,12 it was also recently demonstrated in a mouse model that influenza-specific lung TRM cells can decline during convalescence and increase their expression of apoptotic proteins.83 It is not known whether human lung TRM cells decay in a similar way to mouse lung TRM cells or whether this contributes to the loss of heterosubtypic immunity against influenza infection. Ex vivo T cell receptor (TCR) analysis of influenza-specific clonotypes in the lung is diverse, but certain sequences can also be found in other tissues, such as blood, spleen, and lymph nodes.84 Whether these lymphoid sites act as a source of replenishment for influenza-specific lung TRM cells is unknown. Certainly, it would be of value to assess whether there are preferred clonotypes that form resident and circulating memory T cells.84 The decipherment of clones that favor residency and longevity in human lung TRM cells would provide useful data for vaccine design that could promote the durable protection of lung tissue from repeated exposures through the formation of a persistent influenza-specific lung TRM cell population.85 Currently, live attenuated influenza vaccines (LAIVs) are utilized and have been shown to induce IFNγ-producing T cells in the blood of children and adults,86 and in animal studies, LAIVs provide protection against infection with heterosubtypic strains.79,87,88 While the formation of TRM cells has been reported in patients receiving this vaccine, more information about their protective capacity upon re-infection is required.
Respiratory syncytial virus
Respiratory syncytial virus (RSV) is an acute respiratory infection,89,90 and unlike influenza, natural RSV infection does not elicit complete strain-specific protection upon re-exposure.91 It has been reported that RSV-specific CD8+ T cells are enriched within the lung compared to the blood,79 and these cells remain elevated in tracheal aspirates of children during convalescence following severe RSV infection.92 RSV-specific lung CD8+ TRM cells coexpress CD69 and CD103; however, they were found to have lower levels of granzyme B and perforin than their blood counterparts.93 Higher frequencies of RSV-specific CD8+ TRM cells in the airway correlated with reduced disease severity and viral load. Similarly, low numbers of CD8+ T cells in the airway94,95 (or an increased proportion of activated T cells in the blood) of hospitalized RSV-infected adults have been associated with more severe human RSV disease.96 This may indicate a potential role for lung TRM cells to control and protect against RSV infection,93 as has been demonstrated in animal studies.97–100
Herpes simplex virus
Herpes simplex virus (HSV) types 1 and 2 are neurotropic herpesviruses that establish latency in dorsal root ganglia. HSV reactivation can be asymptomatic or manifest as painful mucocutaneous ulcerations and rarely as encephalitis.101 Episodes of mucocutaneous HSV reactivation are frequent, and effective immune surveillance is required to prevent overt disease.102 The importance of TRM cells in the control of HSV-1 and HSV-2 reactivation is well established in animal models and human ex vivo studies. Mouse HSV-1 and HSV-2 infection studies have demonstrated that localized TRM cells control viral latency through noncytolytic mechanisms and control viral reactivation by readily producing effector cytokines and initiating local proliferative responses.103–105
In humans, HSV-specific CD4+ and CD8+ T cells are enriched in the female genital tract compared to the circulation,103 and HSV-2-specific CD8+ T cells accumulate adjacent to peripheral nerve endings after viral reactivation.106 In samples from the genital tract of HSV-2-infected women, HSV-specific CD8+ T cells expressed the markers of tissue residency, CD69 and CD103.107 A mathematical model has predicted that the density of HSV-2-specific TRM cells in human skin is critical for successful containment of viral reactivation in cells,108 and this has been confirmed directly in mouse HSV infection models.48
Cytomegalovirus
CMV infects the majority of the world’s population and establishes a life-long infection; reactivated CMV can infect many organs, causing retinitis, pneumonitis, colitis, hepatitis, and/or end-organ failure.109 A significant proportion of the host immune system is devoted to the long-term control of CMV. CMV infection in mice and humans can lead to expanded, sustained effector memory CD8+ T cells through the process of memory inflation, which develops in parallel with conventional contracting central memory responses to many epitopes.110 Single-specificity inflationary T cells can constitute up to 30% of the circulating human T cell pool110 and have been shown to provide protection against viral challenge in mouse models.111 Inflationary CMV-specific T cells abundantly express CX3CR1, which recognizes the fractalkine expressed by endothelial cells at intermediate (CX3CR1int) and high levels.112 CX3CR1int CD8+ memory T cells (termed peripheral memory cells) are associated with enhanced self-renewal, proliferative, and tissue-surveying properties,113 suggesting that CX3CR1int CMV-specific T cells may be important for the development of memory inflation.112
Mouse infection models have shown a clear role of CMV-specific TRM cells in controlling viral replication. After primary murine CMV (MCMV) infection, virus-specific TRM cells progressively accumulate in mucosal tissues and salivary glands,114 with MCMV-specific TRM cells providing protection against local re-infection.40 Lung MCMV-specific T cells are maintained in an antigen-independent manner and are long lived compared to effector memory cells induced by acute respiratory viral infections.115 MCMV-specific CD8+ T cells from the gut epithelium show a transcriptome closely linked to a TRM cell phenotype and quite distinct from that of the dominant “inflationary” memory population.116 The capacity of CMV to induce sustained mucosal CD8+ TRM cell responses is of interest to vaccine design, and a number of animal studies have demonstrated that intranasal vaccination with an MCMV vector can generate persistent lung TRM cell responses.99,117
CMV-specific T cells have been detected in a variety of human tissues, including lung, spleen, bone marrow, gut, and lymph nodes.118 A discordance in the frequency of CMV-specific CD8+ T cells in blood compared with tissues is frequently observed.118–124 In the lung and spleen, a significant proportion of CMV-specific CD8+ T cells express CD69 (but not CD103), whereas influenza-specific and RSV-specific memory CD8+ T cells in lung tissue coexpress both CD69 and CD103.79 TCR analysis of CMV-specific CD8+ T cells revealed scant overlap between clones in paired lymph node and blood samples and that there was little clonal overlap between the effector memory T cell and central memory T cell subsets.124 This finding indicates that human CMV-specific CD8+ T cells are anatomically compartmentalized; however, their individual contribution to the control of local CMV replication is currently unknown.
Epstein–Barr virus
Epstein–Barr virus (EBV) is a highly prevalent infection that establishes life-long latency. Oropharyngeal exposure to infected saliva leads to transmission with viral replication occurring in epithelial cells and B cells and the clinical presentation of infectious mononucleosis. Following primary infection, EBV establishes latency in the circulating memory B cell pool, and cytotoxic T cells play a major role in controlling EBV-infected cells. EBV is intermittently detected in throat washings of asymptomatic carriers, which has been attributed to the reactivation of EBV in oropharyngeal lymphoid tissue, leading to a lytic oropharyngeal infection.125 Studies have shown that EBV-specific T cells preferentially accumulate in tonsils compared to blood and spleen and that a high proportion express CD69 and CD103.126–128 These data suggest that tonsillar TRM cells may play a role in controlling viral replication in oropharyngeal tissue, thereby preventing reactivation. EBV infection is also associated with a range of cancers, including B cell-derived lymphomas, T cell lymphomas, and nasopharyngeal cancer;129 however, it is not yet known whether EBV-specific TRM cells contribute to the control of EBV-related oncogenesis.
Hepatotropic viruses
Hepatitis B virus (HBV) and hepatitis C virus (HCV) are hepatotropic viruses that can cause chronic infection in the liver, leading to cirrhosis and hepatocellular cancer. In contrast to acute and resolving viral infections (e.g., influenza), the immune response to chronic viral infections is a continuous effort. This requirement may lead to different characteristics of virus-specific TRM cells in chronic infections compared with acute infections. Higher numbers of CD69-expressing CD8+ T cells were observed in the liver of patients with partial immune control of HBV and chronic HCV infection.35,130 In HBV infection, these cells can be distinguished by high expression of the exhaustion markers PD-1 and CD39, but are capable of producing high levels of IL-2, IFNγ, and TNFα upon ex vivo stimulation with HBV epitopes.35 Liver TRM cells obtained from patients with chronic HBV expressed higher levels of granzyme B than those obtained from control patients,130 but whether this contributes to the immunopathology of cirrhosis and hepatocellular cancer is yet to be determined.
Human immunodeficiency virus
Human immunodeficiency virus (HIV) is a chronic noncurable infection that results in acquired immunodeficiency syndrome (AIDS) if left untreated. Although combination anti-retroviral therapy has reduced the incidence of AIDS, HIV/AIDS continues to cause significant morbidity and mortality worldwide. Most HIV replication occurs in tissues, particularly mucosal and lymphoid tissue, and CD8+ T cells are critical for effective immune control. TRM cells are generated in response to HIV infection in multiple locations, including the gastrointestinal tract, female reproductive tract, and lymphoid tissue.131–135 HIV-specific CD8+ TRM cells are found in high frequencies and exhibit robust polyfunctional responses in the gastrointestinal and lymphoid tissue of individuals who naturally control HIV infection (“elite controllers”), suggesting that TRM cells may play an important role in limiting HIV replication.133 Because HIV is frequently transmitted through sexual intercourse, the generation of HIV-specific TRM cells in the genital tract through vaccination is a desirable goal. Several studies have reported success in generating genital HIV (or simian immunodeficiency virus)-specific TRM cells in animals,136–138 offering hope for similar translational studies in humans.
TRM cells in cancer
The immune system plays important roles in controlling and eliminating malignant cells through tumor surveillance. The current model of the tumor immunity cycle suggests that dendritic cells loaded with tumor antigen are presented to naive CD8+ T cells in the lymph node. This results in the activation and expansion of a tumor-specific TEM population that traffics back to tumor sites and engages in cell-mediated tumor lysis.139 How successful tumor-specific memory CD8+ T cells are in penetrating the tumor carries important prognostic value. Memory CD8+ T cells that accumulate at the peripheral margins of tumors are denoted as infiltrated-excluded and are characterized as poorly immunogenic. In contrast, memory CD8+ T cells that further penetrate the tumor are classified as infiltrated-inflamed and are associated with superior responses to therapy (reviewed in ref. 140). Similar to chronic viral infection, repeated exposures of memory T cells to their cognate antigen lead to the upregulation of immune checkpoint molecules during the process of T cell exhaustion, and these cells have been successfully targeted by immune checkpoint inhibitor therapies.141
TRM cells are located in tissues where solid tumors arise, and mounting evidence indicates that CD8+ TRM cells play a role in the inhibition of cancer progression and metastasis. The growing understanding in this field provides an opportunity to improve upon current immunotherapies for solid tumors.
Tumors have distinct microenvironments that host heterogeneous populations, including macrophages, natural killer cells, innate lymphoid cells (ILCs), IELs, γδT cells, and group 1 ILCs. Many studies have shown that a proportion of tumor-infiltrating lymphocytes (TILs) have a TRM cell-like phenotype, defined by surface staining and/or molecular signature.55,142–144 For the purpose of this review, we denote these cells as “TRM-like TILs,” a population that makes up 25–75%142 of all TILs.
In human studies, TRM-like TILs are found in several cancers, including melanoma;55,143,144 non-small-cell lung cancer (NSCLC);54,145 and breast,11,146 colorectal,147,148 bladder,56,149 ovarian,57,150,151 and cervical cancer.152 However, the bona fide residency of these CD69+CD103+ TILs remains to be determined. CD69 expression may be induced by cognate antigen stimulation or oxygen deprivation, and CD103 expression can be induced on circulating CD8+ T cells that recognize antigen in the presence of TGFβ,153,154 all of which are factors that are present in the tumor microenvironment. Whether the expression of CD69 and CD103 induced by the tumor microenvironment is analogous to the formation of TRM cells in nonmalignant tissue is an area of current investigation.
While a high proportion of total TILs is associated with improved cancer survival, the proportion of TRM-like TILs may also be of importance (reviewed in ref. 155). The abundance of TRM-like TILs in the tumor epithelium positively correlates with disease-free and/or overall survival in NSCLC54 and breast,11 melanoma,55 bladder,146 ovarian,57 and endometrial cancer.58 Additionally, the accumulation of TRM-like TILs has been used to predict the clinical response to immune checkpoint inhibitors.11,55
Although CD103+CD8+ TRM-like TILs often have a high expression of immune checkpoint molecules, including PD-1, TIM-3, and LAG-3, in comparison with CD103− TILs, their ability to produce cytotoxic molecules and effector cytokines is maintained.11,54,143,145,152,156 TRM-like TILs exhibit enhanced cytotoxic potential and effector functions compared with CD103− TILs, suggesting that they potentially mediate superior tumor surveillance compared to circulating tumor-specific T cells.157 As expected, TRM-like TILs were capable of mediating a melanoma-immune equilibrium in mouse skin.3 In vitro, CD103+ TRM-like TILs were more efficient at lysing NSCLC cells than their CD103− counterparts, possibly due to these cells being able to form more stable synapses with target cells and to direct cytotoxic granules in a polarized fashion.145,158 Similarly, CD39+CD103+CD8+ TRM-like TILs had the highest tumor reactivity in vitro in tumor specimens obtained from patients with melanoma, lung cancer, HNSCC, ovarian cancer, and rectal cancer.154
A significant proportion of TILs may be TRM-like and potentially do not recirculate, which has implications for divergent treatment responses in metastatic disease.142 TILs are established in each metastasis early, and the TCR repertoires are distinct between individual metastatic sites. The interlesion diversity of TCRs is mostly accounted for by TRM-like TILs,143 even with apparent equal exposure to neoantigens. This suggests that the interlesional diversity of tissue-resident T cells may differentially affect the outcome of checkpoint inhibitor therapy at each site.143
A more comprehensive understanding of the characteristics of tumor-residing TRM cells in different cancer types may reveal novel targets for therapeutic intervention.159 The role of TRM-like TILs in organ-specific malignancies, as elucidated in primary human tissues and animal models of disease, is discussed further below and summarized in Fig. 1.
Melanoma
Mouse models have provided strong evidence that TRM cells mediate effective antitumor responses to melanoma and suppress tumor growth,3,160 providing a basis for translational studies in humans. Indications that TRM cells play a role in human melanoma are suggested from reports demonstrating improved survival in patients with greater proportions of TRM-like TILs and the increased expression of tissue residency genes, including Cd69, Itgae, and Cd244.55 TRM-like TILs had significantly higher levels of PD-1 (~75% of CD8+ T cells) than their CD69−CD103− counterparts, and the potential significance of this observation was sustained by longitudinal data that demonstrated that these PD-1+CD69+CD103+ T cells expand early during anti-PD-1 therapy compared to baseline values.55 In the same study, the authors also associated the level of Il-15 transcripts with patient outcome, where improved survival rates were directly proportional to the expression of IL-15 in the tumor.55 While skin TRM cells are known to require IL-15 for their development and longevity, an evaluation of whether this cytokine in the local tumor microenvironment can directly affect TRM-like TILs to promote cancer regression is yet to be determined. An understanding of the contribution of TRM cells in melanoma has the potential to direct the design of anti-melanoma vaccines for long-term protection, which has shown promise in animal models.161
Non-small-cell lung cancer
NSCLC is the most common form of lung cancer162 and is a setting in which the characterization of lung TRM cells is being investigated. Reports have noted the presence of tumor-specific CD103+ TRM-like TILs in NSCLC tissue, and the transcriptional signature of these cells was similar to that of infection-induced lung TRM cells, including a high expression of Itgae, Cxcr6, and Ctla4 with the downregulation of Klf2.54 Moreover, TRM-like TILs had increased levels of genes associated with cell cycling, proliferation, and toxicity.54 Patients with high proportions of TILs had reduced mortality rates, and the risk was further reduced in patients with higher proportions of TRM-like TILs,54 suggesting the potential cytotoxic response of lung TRM cells for controlling NSCLC.
Bladder cancer
The successful use of the therapeutic intravesical bacillus Calmette-Guerin in bladder cancer demonstrates the capacity to stimulate a localized T cell-mediated antitumor response.163 TRM-like TILs were found in local tissue from patients with bladder urothelial cancer,56 and these cells had high expression of perforin, granzyme B, and PD-1 compared with CD8+ T cells from the peripheral blood of healthy volunteers,149 corroborating the licensed use of anti-PD-1 and anti-PDL1 checkpoint inhibitors for the treatment of this cancer.164 An abundance of TRM-like TILs has been associated with improved prognosis in bladder urothelial cancer,56 and TRM-like TILs were the dominant T cell subset of TILs in patients with early (stage I) disease, suggesting a role in disease control.149 In this setting, TRM-like TILs demonstrated a methylation of the perforin gene that was two-fold lower than that in CD8+ T cells from the peripheral blood of healthy volunteers, and this was associated with a corresponding higher perforin protein expression,149 suggesting a potential TRM-like TIL contribution to the control of bladder cancer.
Breast cancer
A high frequency of TILs in breast cancer is a strong predictor for improved patient survival, particularly in triple-negative and HER-2-overexpressing tumors.11 Recently, an in-depth transcriptional analysis of TILs within primary, triple-negative human breast cancer tissue was performed and demonstrated a previously unappreciated level of heterogeneity of T cell subsets within tumors.11 Tumors with a high number of TILs contained CD103+CD8+ T cells with a TRM-like phenotype, and these cells expressed high levels of PD-1 and effector proteins. A CD8+ TRM-like gene signature was significantly associated with improved relapse-free and overall survival following chemotherapy and provided better prognostication than CD8 expression alone.11 These data support the notion that authentic TRM cells exist within tumor microenvironments and contribute to breast cancer immune surveillance.
Oropharyngeal, head and neck cancer
The recent rise in the incidence of oropharyngeal, head and neck cancers has been attributed to high-risk strains of human papilloma virus (HPV), such as HPV16.165 Interestingly, oropharyngeal squamous cell cancer (OPSCC) that occurs independently of HPV infection has a worse prognosis than HPV-associated OPSCC, and it has been postulated that the dense infiltration of CD4+ and CD8+ T cells evident in the latter disease can contribute to a strong antitumor response.166,167 HPV-specific CD4+ T cells were found at a higher concentration in the pro-inflammatory tumor microenvironment of HPV16+ OPSCC than in that of HPV16− OPSCC, and these cells expressed a type 1 T helper (Th1) and type 17 T helper (Th17) phenotype and high levels of CD38, PD-1, and the C-type lectin CD161.168 Improved survival was positively associated with the proportion of CD4+CD161+ TILs as well as greater frequencies of TRM-like TILs.168 The expression of CD161 on TRM cells has been noted in the colon,169 and while its exact function in the setting of malignancy is currently unknown, it may provide an additional correlative marker of patient outcomes.170
In patients with primary head and neck squamous cell carcinoma (HNSCC), a low frequency of CD39+CD103+ CD8+ TRM-like TILs was associated with more advanced disease, and those with a high frequency of CD39+CD103+ CD8+ TRM-like TILs had better survival at 3 years than those with a low frequency (86% survival versus 58%).154 Taken together, many independent studies on diverse cancer types corroborate the consistent observation that improved patient outcomes are strongly correlated with increased frequencies of TRM-like TILs. It is postulated that the enhanced cytotoxic and functional potential of TRM-like TILs, compared with nonresident T cell counterparts, including the increased proficiency of tumor cell lysis, forms the basis of the reported patient survival benefits.
TRM cells in autoimmunity
Recent studies indicate that TRM cell populations residing in the same tissue are highly heterogeneous with distinct phenotypic and functional profiles.65,171,172 This heterogeneity is evident in human disease, and while TRM cells are beneficial against infection and cancer, they may also contribute to the pathology of autoimmune and inflammatory diseases.173 While TRM cells have been implicated in the pathogenesis of multiple sclerosis,174 type 1 diabetes mellitus,175 and inflammatory bowel disease,176 here we focus on the emerging role of pathogenic TRM cells in mediating common autoimmune diseases of the skin, where the evidence is the strongest177 and has been derived both from animal models and primary human tissue (Fig. 1).
Psoriasis
The observation that psoriatic skin lesions reoccur at the same location led to speculation that TRM cells could be the mediator of this site-specific response.178 The pathogenesis of psoriasis is a complex interplay of dysregulated immune cells and cytokines, including IL-17, IL-12, IFNγ, TNFα, and IL-23 (reviewed in ref. 179). Recent investigations have focused on infiltrating CD4+ and/or CD8+ T cells and skin-resident T cells as the drivers of the abnormal regulation of the IL-23/IL-17 axis.10,180 The first indication that TRM cells may have a role in the pathogenesis of psoriasis was the finding that the inhibition of T cell migration from the blood to the skin (by E-selectin blockade) was an ineffective treatment.181 The transfer of nonlesional skin grafts from patients with psoriasis to immunodeficient mice resulted in psoriatic lesions on the mice, indicating that autoreactive pathogenic T cells were present in healthy-appearing skin and that psoriatic skin lesions can develop without the recruitment of cells from blood.182 TCR gene sequencing showed that resolved psoriatic lesions retain IL-17-producing oligoclonal T cell populations, suggesting a mechanism by which disease can reoccur at the same site.183 Subsequent studies demonstrated that IL-22-secreting CD4+ T cells and IL-17-secreting CD8+ T cells remained resident in healed psoriatic skin after treatment with a number of therapies.183,184 Furthermore, the lack of CD49a expression on CD8+ TRM cells from psoriasis lesions defines a TRM cell subset producing IL-17.10 Biological treatments targeting TNFα, IL-17, or IL-23 have revolutionized the treatment of psoriasis and have helped to demonstrate the centrality of T cells to the disorder.178 It is currently unknown whether TRM cells directly contribute to the exacerbation of psoriasis, and the determination of whether TRM cell-mediated cytokine secretion plays a role in disease pathogenesis may encourage the advent of novel therapies aimed at dislodging pathogenic TRM cells while preserving protective counterparts.
Alopecia areata
Alopecia areata (AA) is a chronic immune-mediated disease that results in nonscarring hair loss.185 The pathogenesis of AA is only partially understood, but it is thought that genetic and environmental factors cause an autoimmune reaction in hair follicles.186 MHC class II is normally absent in hair follicle root sheaths and hair matrix, but increased MHC class II expression and the upregulation of adhesion molecules occurs in AA.187 Skin Th1 cells produce IFNγ, which triggers the infiltration of cytotoxic CD8+ T cells to the perifollicular area, leading to hair cycle arrest.187 Early evidence that skin TRM cells may play a pathogenic role in AA was suggested by a study in which T cells isolated from AA scalp explants were injected into severe combined immunodeficiency (SCID) mice, and hair growth and clinical parameters were monitored. When compared to mice that had not received T cells (or had been injected with peripheral blood mononuclear cells), mice with AA skin-derived T cells demonstrated hair loss as a result of fewer hair follicles and presented histological evidence of AA.188 Transcriptional profiling of AA skin revealed gene signatures suggestive of cytotoxic effector CD8+ T cells187 and higher transcripts of Itgae.189 Inhibitors of the Janus kinase (JAK) signaling pathway (e.g., tofacitinib), which predominantly produces IFNγ, have shown promise in treating AA.190 Lesional T cell clones do not completely disappear after tofacitinib administration,191 leading to the relapse of disease after treatment cessation. However, it is yet to be confirmed whether TRM cells are the main source of IFNγ in AA and (if so) the effect of tofacitinib on TRM cell functionality.
Vitiligo
Vitiligo is a disease characterized by the apoptosis of melanocytes or the lack of melanin production, which results in depigmented skin.192 CD8+ TRM cells expressing the cytotoxic molecules perforin and granzyme B are present in the skin of active and stable vitiligo lesions and are responsive to IL-15 stimulation,10 a cytokine with a role in TRM development and survival in skin.7 Both human and mouse TRM cells express the CD122 subunit of the IL-15 receptor, and keratinocytes upregulate CD215, the subunit required to display the cytokine on their surface to promote the activation of T cells.193 In mice, the targeting of IL-15 signaling with an anti-CD122 antibody reversed vitiligo, and the effects of such treatment remain to be confirmed in humans.193 Further work is also needed to determine whether the ablation of IL-15 signaling in TRM cells can directly affect disease outcomes in vitiligo.
TRM cells in transplantation
Donor-derived lymphocytes are transferred within the allograft during solid organ transplantation. The close association between donor-derived TRM cells and host cells may determine whether allografts undergo acute or chronic rejection (Fig. 1).59,194,195 It has been shown that donor-derived CD4+ and CD8+ IELs were dominant in an intestinal allograft for ~400 days post transplant, after which recipient-derived T cells (which adopted a TRM cell phenotype) then outnumbered donor-derived cells.61,62 During rejection, host-versus-graft reactive T cell clones accumulated in the graft, and the infiltration of recipient T cells was accelerated and was associated with a reduced proportion of donor-derived cells. However, in the absence of overwhelming cellular and antibody-mediated rejection, donor-derived TRM cells persisted and even expanded in the graft, delaying complete repopulation by recipient T cells and possibly reducing the risk of rejection.61
While the persistence of donor-derived TRM cells has been associated with a reduced incidence of rejection in both intestinal61 and lung60 transplants, TRM cells have conversely also been implicated in driving allograft rejection. Following a facial transplant, donor-derived CD8+ T cells were predominantly skin-resident (CD8+ CD69+CD103+CLA+) phenotypes, and histological analysis revealed that these donor cells were associated with areas of damaged vasculature and epithelium.59 The results of this report suggest that donor-derived T cells may also play a role in tissue injury that results in graft rejection. Overall, the contribution of tissue-resident cells to the field of transplantation is in its infancy, and more studies are needed to clarify the contribution of TRM cells to graft rejection and stability.
Concluding remarks and future directions
The importance of human TRM cells is increasingly recognized in a wide array of tissue-specific diseases. Progress has been made in identifying surface markers and transcriptional profiles of human TRM cells, although unique human TRM cell phenotypes and signatures still remain to be elucidated. Human studies have shown the beneficial roles of TRM cells in controlling infections, and TRM cells are being targeted in the rational design of T cell vaccines. Additionally, TRM cells appear to play an important role in the control of malignancies and will prove a likely target of cancer immunotherapies. However, given the potentially harmful contribution of TRM cells in autoimmune diseases, understanding both the protective and pathogenic human TRM cell roles is crucial for the safe design of approaches that allow for their manipulation. Many critical questions remain to be answered. For example, do TRM cells contribute to pathology in a direct or indirect manner? What signaling pathways regulate TRM cell differentiation and longevity? How can these processes be manipulated to enhance or eliminate TRM cells in a tissue-specific manner? Much work is required to understand the breadth of TRM cell heterogeneity and function in human tissues, such that adverse effects can be predicted and controlled for; however, there appears enormous potential to harness TRM cells for novel T cell-based vaccines and immunotherapies for a broad range of diseases.
Acknowledgements
S.C.S. is supported by an Oxford-Celgene Postdoctoral Fellowship. C.L.G. is supported by an NHMRC Early Career Fellowship (GNT 1160963). P.K. is supported by an Oxford and NIHR Senior Fellowship (WT10966MA). L.K.M. is a Senior Medical Research Fellow supported by the Sylvia and Charles Viertel Charitable Foundation.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: S. C. Sasson, C. L. Gordon, S. N. Christo
Contributor Information
P. Klenerman, Email: paul.klenerman@ndm.ox.ac.uk
L. K. Mackay, Email: lkmackay@unimelb.edu.au
References
- 1.Mackay L, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. 2017;38:94–103. doi: 10.1016/j.it.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Mueller S, Mackay L. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- 3.Park SL, et al. Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature. 2019;565:366–371. doi: 10.1038/s41586-018-0812-9. [DOI] [PubMed] [Google Scholar]
- 4.Masopust D, Vezys V, Marzo A, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 5.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 6.Mackay L, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 7.Mackay L, et al. T-box transcription factors combine with the cytokines TGF-beta and IL-15 to control tissue-resident memory T cell fate. Immunity. 2015;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Schenkel JM, et al. IL-15-independent maintenance of tissue-resident and boosted effector memory CD8 T cells. J. Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sathaliyawala T, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheuk S, et al. CD49a expression defines tissue-resident CD8(+) T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savas P, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018;24:986–993. doi: 10.1038/s41591-018-0078-7. [DOI] [PubMed] [Google Scholar]
- 12.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/S1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, et al. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinert E, et al. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson K, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J. Immunol. 2012;189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vesely M, et al. Effector TH17 cells give rise to long-lived TRM cells that are essential for an immediate response against bacterial infection. Cell. 2019;178:1176–1188.e15. doi: 10.1016/j.cell.2019.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hondowicz BD, et al. Interleukin-2-dependent allergen-specific tissue-resident memory cells drive asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins N, et al. Skin CD4(+) memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat. Commun. 2016;7:11514. doi: 10.1038/ncomms11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beura LK, et al. CD4+ resident memory T cells dominate immunosurveillance and orchestrate local recall responses. J. Exp. Med. 2019;216:jem.20181365. doi: 10.1084/jem.20181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teijaro J, et al. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol. 2011;187:5510. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar B, et al. Human tissue-resident memory T cells are defined by core transcriptional and functional signatures in lymphoid and mucosal sites. Cell Rep. 2017;20:2921–2934. doi: 10.1016/j.celrep.2017.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oja A, et al. Trigger-happy resident memory CD4(+) T cells inhabit the human lungs. Mucosal Immunol. 2018;11:654–667. doi: 10.1038/mi.2017.94. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschek MA, et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 2009;206:525–534. doi: 10.1084/jem.20081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakim L, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 2012;189:3462–3471. doi: 10.4049/jimmunol.1201305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skon C, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandala S, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 29.Mackay L, et al. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J. Immunol. 2015;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- 30.Ma C, Mishra S, Demel EL, Liu Y, Zhang N. TGF-β controls the formation of kidney-resident T cells via promoting effector T cell extravasation. J. Immunol. 2017;198:749–756. doi: 10.4049/jimmunol.1601500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay L, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Ruiz Daniel, Ng Wei Yi, Holz Lauren E., Ma Joel Z., Zaid Ali, Wong Yik Chun, Lau Lei Shong, Mollard Vanessa, Cozijnsen Anton, Collins Nicholas, Li Jessica, Davey Gayle M., Kato Yu, Devi Sapna, Skandari Roghieh, Pauley Michael, Manton Jonathan H., Godfrey Dale I., Braun Asolina, Tay Szun Szun, Tan Peck Szee, Bowen David G., Koch-Nolte Friedrich, Rissiek Björn, Carbone Francis R., Crabb Brendan S., Lahoud Mireille, Cockburn Ian A., Mueller Scott N., Bertolino Patrick, McFadden Geoffrey I., Caminschi Irina, Heath William R. Liver-Resident Memory CD8 + T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity. 2016;45(4):889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Bergsbaken T, Bevan M. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue-resident CD8(+) T cells responding to infection. Nat. Immunol. 2015;16:406–414. doi: 10.1038/ni.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheridan B, et al. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallett L, et al. IL-2(high) tissue-resident T cells in the human liver: sentinels for hepatotropic infection. J. Exp. Med. 2017;214:1567. doi: 10.1084/jem.20162115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar BV, et al. Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight. 2018;3:e123568. doi: 10.1172/jci.insight.123568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hombrink P, et al. Programs for the persistence, vigilance and control of human CD8(+) lung-resident memory T cells. Nat. Immunol. 2016;17:1467–1478. doi: 10.1038/ni.3589. [DOI] [PubMed] [Google Scholar]
- 38.Pizzolla A, Wakim LM. Memory T cell dynamics in the lung during influenza virus infection. J. Immunol. 2019;202:374–381. doi: 10.4049/jimmunol.1800979. [DOI] [PubMed] [Google Scholar]
- 39.Smith CJ, Caldeira-Dantas S, Turula H, Snyder CM. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep. 2015;13:1137–1148. doi: 10.1016/j.celrep.2015.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thom J, Weber T, Walton S, Torti N, Oxenius A. The salivary gland acts as a sink for tissue-resident memory CD8+ T cells, facilitating protection from local cytomegalovirus infection. Cell Rep. 2015;13:1125. doi: 10.1016/j.celrep.2015.09.082. [DOI] [PubMed] [Google Scholar]
- 41.Zhang N, Bevan MJ. Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaid A, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl Acad. Sci. USA. 2014;111:5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milner J, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, et al. The Transcription factor Bhlhe40 programs mitochondrial regulation of resident CD8+ T cell fitness and functionality. Immunity. 2019;51:491–507.e7. doi: 10.1016/j.immuni.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 2019;37:1–26. doi: 10.1146/annurev-immunol-042617-053214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schenkel J, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol. 2013;14:509. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beura LK, et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 2018;19:173–182. doi: 10.1038/s41590-017-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park S, et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 2018;19:183–191. doi: 10.1038/s41590-017-0027-5. [DOI] [PubMed] [Google Scholar]
- 49.Mackay L, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl Acad. Sci. USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pizzolla A, et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2017;2:eaam6970. doi: 10.1126/sciimmunol.aam6970. [DOI] [PubMed] [Google Scholar]
- 52.Wu T, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol. 2014;95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L, et al. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell–mediated immunity. Nat. Med. 2010;16:224. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ganesan A, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat. Immunol. 2017;18:940–950. doi: 10.1038/ni.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards J, et al. CD103(+) tumor-resident CD8(+) T cells are associated with improved survival in immunotherapy-naive melanoma patients and expand significantly during anti-PD-1 treatment. Clin. Cancer Res. 2018;24:3036–3045. doi: 10.1158/1078-0432.CCR-17-2257. [DOI] [PubMed] [Google Scholar]
- 56.Wang B, et al. CD103+ tumor infiltrating lymphocytes predict a favorable prognosis in urothelial cell carcinoma of the bladder. J. Urology. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- 57.Bösmüller H-C, et al. Combined immunoscore of CD103 and CD3 identifies long-term survivors in high-grade serous ovarian cancer. Int. J. Gynecol. Cancer. 2016;26:671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 58.Workel HH, et al. CD103 defines intraepithelial CD8+ PD1+ tumour-infiltrating lymphocytes of prognostic significance in endometrial adenocarcinoma. Eur. J. Cancer. 2016;60:1–11. doi: 10.1016/j.ejca.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 59.Lian C, et al. Biomarker evaluation of face transplant rejection: association of donor T cells with target cell injury. Mod. Pathol. 2014;27:788–799. doi: 10.1038/modpathol.2013.249. [DOI] [PubMed] [Google Scholar]
- 60.Snyder ME, et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci. Immunol. 2019;4:eaav5581. doi: 10.1126/sciimmunol.aav5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuber J, et al. Bidirectional intragraft alloreactivity drives the repopulation of human intestinal allografts and correlates with clinical outcome. Sci. Immunol. 2016;1:eaah3732. doi: 10.1126/sciimmunol.aah3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bartolomé-Casado Raquel, Landsverk Ole J.B., Chauhan Sudhir Kumar, Richter Lisa, Phung Danh, Greiff Victor, Risnes Louise F., Yao Ying, Neumann Ralf S., Yaqub Sheraz, Øyen Ole, Horneland Rune, Aandahl Einar Martin, Paulsen Vemund, Sollid Ludvig M., Qiao Shuo-Wang, Baekkevold Espen S., Jahnsen Frode L. Resident memory CD8 T cells persist for years in human small intestine. The Journal of Experimental Medicine. 2019;216(10):2412–2426. doi: 10.1084/jem.20190414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thome J, et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark R, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci. Transl. Med. 2012;4:117ra7. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe R, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci. Transl. Med. 2015;7:279ra39–279ra39. doi: 10.1126/scitranslmed.3010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl Acad. Sci. USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topham D, Reilly E. Tissue-resident memory CD8(+) T cells: from phenotype to function. Front. Immunol. 2018;9:515. doi: 10.3389/fimmu.2018.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Ma C, Zhang N. Tissue-specific control of tissue-resident memory T cells. Crit. Rev. Immunol. 2018;38:79–103. doi: 10.1615/CritRevImmunol.2018025653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wherry E, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Remakus S, Sigal LJ. Advances in experimental medicine and biology. Adv. Exp. Med. Biol. 2013;785:77–86. doi: 10.1007/978-1-4614-6217-0_9. [DOI] [PubMed] [Google Scholar]
- 72.Klenerman P. The (gradual) rise of memory inflation. Immunol. Rev. 2018;283:99–112. doi: 10.1111/imr.12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Philip M, Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr. Opin. Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muruganandah V, Sathkumara HD, Navarro S, Kupz A. A systematic review: the role of resident memory T cells in infectious diseases and their relevance for vaccine development. Front. Immunol. 2018;9:1574. doi: 10.3389/fimmu.2018.01574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Bree G, et al. Characterization of CD4+ memory T cell responses directed against common respiratory pathogens in peripheral blood and lung. J. Infect. Dis. 2007;195:1718. doi: 10.1086/517612. [DOI] [PubMed] [Google Scholar]
- 76.Piet B, et al. CD8(+) T cells with an intraepithelial phenotype upregulate cytotoxic function upon influenza infection in human lung. J. Clin. Investig. 2011;121:2254. doi: 10.1172/JCI44675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner D, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koutsakos M, et al. Human CD8+ T cell cross-reactivity across influenza A, B and C viruses. Nat. Immunol. 2019;20:613–625. doi: 10.1038/s41590-019-0320-6. [DOI] [PubMed] [Google Scholar]
- 79.de Bree GJ, et al. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J. Exp. Med. 2005;202:1433–1442. doi: 10.1084/jem.20051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pizzolla A, et al. Influenza-specific lung-resident memory T cells are proliferative and polyfunctional and maintain diverse TCR profiles. J. Clin. Investig. 2018;128:721. doi: 10.1172/JCI96957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sridhar S, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013;19:nm.3350. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 82.Zens K, Chen J, Farber D. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. J. Clin. Invest. Insight. 2016;1:e85832. doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Slütter Bram, Van Braeckel-Budimir Natalija, Abboud Georges, Varga Steven M., Salek-Ardakani Shahram, Harty John T. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Science Immunology. 2017;2(7):eaag2031. doi: 10.1126/sciimmunol.aag2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sant S, et al. Single-cell approach to influenza-specific CD8(+) T cell receptor repertoires across different age groups, tissues, and following influenza virus infection. Front. Immunol. 2018;9:1453. doi: 10.3389/fimmu.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clemens, E., van de Sandt, C., Wong, S., Wakim, L. & Valkenburg, S. Harnessing the power of T cells: the promising hope for a universal influenza vaccine. Vaccines (Basel)6, E18 (2018). [DOI] [PMC free article] [PubMed]
- 86.He X-S, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 2006;80:11756–11766. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng X, et al. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J. Infect. Dis. 2013;208:594. doi: 10.1093/infdis/jit207. [DOI] [PubMed] [Google Scholar]
- 88.Jang Y, et al. Cold-adapted X-31 live attenuated 2009 pandemic H1N1 influenza vaccine elicits protective immune responses in mice and ferrets. Vaccine. 2013;31:1320. doi: 10.1016/j.vaccine.2012.12.072. [DOI] [PubMed] [Google Scholar]
- 89.Sommer C, Resch B, Simoes E. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol. J. 2011;5:144. doi: 10.2174/1874285801105010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Falsey A, Hennessey P, Formica M, Cox C, Walsh E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352:1749. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 91.Habibi JozwikA, et al. Impaired antibody-mediated protection and defective IgA B-cell memory in experimental infection of adults with respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2015;191:1040. doi: 10.1164/rccm.201412-2256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heidema J, et al. CD8+ T cell responses in bronchoalveolar lavage fluid and peripheral blood mononuclear cells of infants with severe primary respiratory syncytial virus infections. J. Immunol. (Baltim., Md: 1950) 2007;179:8410. doi: 10.4049/jimmunol.179.12.8410. [DOI] [PubMed] [Google Scholar]
- 93.Jozwik A, et al. RSV-specific airway resident memory CD8+ T cells and differential disease severity after experimental human infection. Nat. Commun. 2015;6:10224. doi: 10.1038/ncomms10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cannon M, Openshaw P, Askonas Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J. Exp. Med. 1988;168:1163. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alwan W, Record F, Openshaw P. CD4+ T cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T cells. Clin. Exp. Immunol. 1992;88:527. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh EE, Peterson DR, Kalkanoglu AE, Lee F, Falsey AR. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J. Infect. Dis. 2013;207:1424–1432. doi: 10.1093/infdis/jit038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H, et al. Respiratory syncytial virus elicits enriched CD8+ T lymphocyte responses in lung compared with blood in African green monkeys. PLoS ONE. 2017;12:e0187642. doi: 10.1371/journal.pone.0187642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kinnear E, et al. Airway T cells protect against RSV infection in the absence of antibody. Mucosal. Immunol. 2018;11:290. doi: 10.1038/mi.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morabito K, et al. Intranasal administration of RSV antigen-expressing MCMV elicits robust tissue-resident effector and effector memory CD8+ T cells in the lung. Mucosal Immunol. 2017;10:545. doi: 10.1038/mi.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang, L. et al. CpG in combination with an inhibitor of notch signaling suppresses formalin-inactivated respiratory syncytial virus-enhanced airway hyperresponsiveness and inflammation by inhibiting Th17 memory responses and promoting tissue-resident memory cells in lungs. J. Virol.91, e021111-16 (2017). [DOI] [PMC free article] [PubMed]
- 101.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 102.Mark KE, et al. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J. Infect. Dis. 2008;198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. Direct recovery of herpes simplex virus (HSV)-specific T lymphocyte clones from recurrent genital HSV-2 lesions. J. Infect. Dis. 1994;169:956–961. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 104.Knickelbein JE, et al. Noncytotoxic lytic granule–mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J. Immunol. 2008;181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 106.Zhu J, et al. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Posavad C, et al. Enrichment of herpes simplex virus type 2 (HSV-2) reactive mucosal T cells in the human female genital tract. Mucosal Immunol. 2017;10:1259. doi: 10.1038/mi.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schiffer JT, et al. A fixed spatial structure of CD8+ T cells in tissue during chronic HSV-2 infection. J. Immunol. (Balt. Md., 1950) 2018;201:1522–1535. doi: 10.4049/jimmunol.1800471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kotton C. CMV: prevention, diagnosis and therapy. Am. J. Transpl. 2013;13:24–40. doi: 10.1111/ajt.12006. [DOI] [PubMed] [Google Scholar]
- 110.Karrer U, et al. Memory inflation: continuous accumulation of antiviral CD8 + T cells over time. J. Immunol. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- 111.Baumann NS, et al. Early primed KLRG1- CMV-specific T cells determine the size of the inflationary T cell pool. PLos Pathog. 2019;15:e1007785. doi: 10.1371/journal.ppat.1007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gordon C, et al. Induction and maintenance of CX3CR1-intermediate peripheral memory CD8 + T cells by persistent viruses and vaccines. Cell Rep. 2018;23:768–782. doi: 10.1016/j.celrep.2018.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gerlach C, et al. The chemokine receptor CX3CR1 defines three antigen-experienced CD8 T cell subsets with distinct roles in immune surveillance and homeostasis. Immunity. 2016;45:1270–1284. doi: 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith C, Caldeira-Dantas S, Turula H, Snyder C. Murine CMV infection induces the continuous production of mucosal resident T cells. Cell Rep. 2015;13:1137. doi: 10.1016/j.celrep.2015.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baumann NS, et al. Tissue maintenance of CMV-specific inflationary memory T cells by IL-15. PLos Pathog. 2018;14:e1006993. doi: 10.1371/journal.ppat.1006993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Highton AJ, et al. Single-cell transcriptome analysis of CD8 + T-cell memory inflation. Wellcome Open Res. 2019;4:78. doi: 10.12688/wellcomeopenres.15115.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morabito KM, et al. Memory inflation drives tissue-resident memory CD8+ T cell maintenance in the lung after intranasal vaccination with murine cytomegalovirus. Front. Immunol. 2018;9:1861. doi: 10.3389/fimmu.2018.01861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gordon CL, et al. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J. Exp. Med. 2017;214:jem.20160758. doi: 10.1084/jem.20160758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ward SM, et al. Virus-specific CD8+ T lymphocytes within the normal human liver. Eur. J. Immunol. 2004;34:1526–1531. doi: 10.1002/eji.200324275. [DOI] [PubMed] [Google Scholar]
- 120.Remmerswaal EB, et al. Human virus-specific effector-type T cells accumulate in blood but not in lymph nodes. Blood. 2012;119:1702–1712. doi: 10.1182/blood-2011-09-381574. [DOI] [PubMed] [Google Scholar]
- 121.Letsch A, et al. CMV-specific central memory T cells reside in bone marrow. Eur. J. Immunol. 2007;37:3063–3068. doi: 10.1002/eji.200636930. [DOI] [PubMed] [Google Scholar]
- 122.Palendira U, et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112:3293–3302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 123.Akulian J, Pipeling M, John E, Orens J, Lechtzin N. High-quality CMV-specific CD4+ memory is enriched in the lung allograft and is associated with mucosal viral control. Am. J. Transpl. 2013;13:146–156. doi: 10.1111/j.1600-6143.2012.04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Remmerswaal EB, et al. Clonal evolution of CD8+ T cell responses against latent viruses: relationship among phenotype, localization, and function. J. Virol. 2015;89:568–580. doi: 10.1128/JVI.02003-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sitki-Green D, Covington M, Raab-Traub N. Compartmentalization and transmission of multiple Epstein–Barr virus strains in asymptomatic carriers. J. Virol. 2003;77:1840–1847. doi: 10.1128/JVI.77.3.1840-1847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woon H, et al. Compartmentalization of total and virus-specific tissue-resident memory CD8+ T cells in human lymphoid organs. PLoS Pathog. 2016;12:e1005799. doi: 10.1371/journal.ppat.1005799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woodberry T, et al. αEβ7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J. Immunol. 2005;175:4355–4362. doi: 10.4049/jimmunol.175.7.4355. [DOI] [PubMed] [Google Scholar]
- 128.Hislop AD, et al. Tonsillar homing of Epstein–Barr virus–specific CD8+ T cells and the virus–host balance. J. Clin. Invest. 2005;115:2546–2555. doi: 10.1172/JCI24810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shannon-Lowe C, Rickinson A. The global landscape of EBV-associated tumors. Front. Oncol. 2019;9:713. doi: 10.3389/fonc.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stelma F, et al. Human intrahepatic CD69 + CD8+ T cells have a tissue resident memory T cell phenotype with reduced cytolytic capacity. Sci. Rep. (UK) 2017;7:6172. doi: 10.1038/s41598-017-06352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibbs Anna, Buggert Marcus, Edfeldt Gabriella, Ranefall Petter, Introini Andrea, Cheuk Stanley, Martini Elisa, Eidsmo Liv, Ball Terry B, Kimani Joshua, Kaul Rupert, Karlsson Annika C, Wählby Carolina, Broliden Kristina, Tjernlund Annelie. Human Immunodeficiency Virus-Infected Women Have High Numbers of CD103−CD8+ T Cells Residing Close to the Basal Membrane of the Ectocervical Epithelium. The Journal of Infectious Diseases. 2017;218(3):453–465. doi: 10.1093/infdis/jix661. [DOI] [PubMed] [Google Scholar]
- 132.Buggert M, et al. Identification and characterization of HIV-specific resident memory CD8+ T cells in human lymphoid tissue. Sci. Immunol. 2018;3:eaar4526. doi: 10.1126/sciimmunol.aar4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kiniry BE, et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8+ T-cells in chronic infection. Mucosal Immunol. 2017;11:909. doi: 10.1038/mi.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moylan DC, et al. Diminished CD103 (aEb7) expression on resident T cells from the female genital tract of HIV-positive women. Pathog. Immun. 2016;1:371–389. doi: 10.20411/pai.v1i2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damouche A, et al. High proportion of PD-1-expressing CD4+ T cells in adipose tissue constitutes an immunomodulatory microenvironment that may support HIV persistence. Eur. J. Immunol. 2017;47:2113–2123. doi: 10.1002/eji.201747060. [DOI] [PubMed] [Google Scholar]
- 136.Tan H-X, et al. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime–boost immunization. Mucosal Immunol. 2017;11:994. doi: 10.1038/mi.2017.89. [DOI] [PubMed] [Google Scholar]
- 137.Zaric M, et al. Long-lived tissue resident HIV-1 specific memory CD8+ T cells are generated by skin immunization with live virus vectored microneedle arrays. J. Control Rel. 2017;268:166–175. doi: 10.1016/j.jconrel.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adnan S, et al. Persistent low-level replication of SIVΔnef drives maturation of antibody and CD8 T cell responses to induce protective immunity against vaginal SIV infection. PLoS Pathog. 2016;12:e1006104. doi: 10.1371/journal.ppat.1006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 140.Farhood B, Najafi M, Mortezaee K. CD8 + cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell Physiol. 2018;234:8509–8521. doi: 10.1002/jcp.27782. [DOI] [PubMed] [Google Scholar]
- 141.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dhodapkar K. Role of tissue-resident memory in intra-tumor heterogeneity and response to immune checkpoint blockade. Front Immunol. 2018;9:1655. doi: 10.3389/fimmu.2018.01655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boddupalli C, et al. Interlesional diversity of T cell receptors in melanoma with immune checkpoints enriched in tissue-resident memory T cells. JCI Insight. 2016;1:e88955. doi: 10.1172/jci.insight.88955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Salerno EP, Olson WC, Imming C, Shea S, Slingluff CL. T cells in the human metastatic melanoma microenvironment express site-specific homing receptors and retention integrins. Int. J. Cancer. 2014;134:563–574. doi: 10.1002/ijc.28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Djenidi F, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J. Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 146.Wang Z-Q, et al. CD103 and intratumoral immune response in breast cancer. Clin. Cancer Res. 2016;22:clincanres.0732.2016. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- 147.Quinn E, Hawkins N, Yip Y, Suter C, Ward R. CD103+ intraepithelial lymphocytes—a unique population in microsatellite unstable sporadic colorectal cancer. Eur. J. Cancer. 2003;39:469–475. doi: 10.1016/S0959-8049(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 148.de Vries, N. L. et al. High-dimensional cytometric analysis of colorectal cancer reveals novel mediators of antitumour immunity. Gut gutjnl-2019-318672 (2019). [DOI] [PMC free article] [PubMed]
- 149.Hartana C, et al. Tissue-resident memory T cells are epigenetically cytotoxic with signs of exhaustion in human urinary bladder cancer. Clin. Exp. Immunol. 2018;194:39–53. doi: 10.1111/cei.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Webb JR, et al. Profound elevation of CD8+ T cells expressing the intraepithelial lymphocyte marker CD103 (αE/β7 Integrin) in high-grade serous ovarian cancer. Gynecol. Oncol. 2010;118:228–236. doi: 10.1016/j.ygyno.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 151.Webb J, Milne K, Nelson B. Location, location, location: CD103 demarcates intraepithelial, prognostically favorable CD8(+) tumor-infiltrating lymphocytes in ovarian cancer. Oncoimmunology. 2014;3:e27668. doi: 10.4161/onci.27668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Komdeur FL, et al. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology. 2017;6:00–00. doi: 10.1080/2162402X.2017.1338230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Floc’h A, et al. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Duhen T, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 2018;9:2724. doi: 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Molodtsov A, Turk M. Tissue resident CD8 memory T cell responses in cancer and autoimmunity. Front. Immunol. 2018;9:2810. doi: 10.3389/fimmu.2018.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Clarke James, Panwar Bharat, Madrigal Ariel, Singh Divya, Gujar Ravindra, Wood Oliver, Chee Serena J., Eschweiler Simon, King Emma V., Awad Amiera S., Hanley Christopher J., McCann Katy J., Bhattacharyya Sourya, Woo Edwin, Alzetani Aiman, Seumois Grégory, Thomas Gareth J., Ganesan Anusha-Preethi, Friedmann Peter S., Sanchez-Elsner Tilman, Ay Ferhat, Ottensmeier Christian H., Vijayanand Pandurangan. Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. The Journal of Experimental Medicine. 2019;216(9):2128–2149. doi: 10.1084/jem.20190249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park SL, Gebhardt T, Mackay LK. Tissue-resident memory T cells in cancer immunosurveillance. Trends Immunol. 2019;40:735–747. doi: 10.1016/j.it.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 158.Franciszkiewicz K, et al. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 2013;73:617–628. doi: 10.1158/0008-5472.CAN-12-2569. [DOI] [PubMed] [Google Scholar]
- 159.Amsen D, van Gisbergen K, Hombrink P, van Lier R. Tissue-resident memory T cells at the center of immunity to solid tumors. Nat. Immunol. 2018;19:538–546. doi: 10.1038/s41590-018-0114-2. [DOI] [PubMed] [Google Scholar]
- 160.Malik B, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci. Immunol. 2017;2:eaam6346. doi: 10.1126/sciimmunol.aam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galvez-Cancino F, et al. Vaccination-induced skin-resident memory CD8(+) T cells mediate strong protection against cutaneous melanoma. Oncoimmunology. 2018;7:e1442163. doi: 10.1080/2162402X.2018.1442163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008;83:584–594. doi: 10.1016/S0025-6196(11)60735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-guerin in the treatment of superficial bladder tumors. J. Urol. 1976;116:180–182. doi: 10.1016/S0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 164.Alifrangis C, McGovern U, Freeman A, Powles T, Linch M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat. Rev. Urol. 2019;16:465–483. doi: 10.1038/s41585-019-0208-0. [DOI] [PubMed] [Google Scholar]
- 165.Jou A, Hess J. Epidemiology and molecular biology of head and neck cancer. Oncol. Res. Treat. 2017;40:328–332. doi: 10.1159/000477127. [DOI] [PubMed] [Google Scholar]
- 166.Badoual C, et al. Prognostic value of tumor-infiltrating CD4+ T-Cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 167.Badoual C, et al. PD-1–expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 168.Welters M, et al. Intratumoral HPV16-specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 2018;24:634–647. doi: 10.1158/1078-0432.CCR-17-2140. [DOI] [PubMed] [Google Scholar]
- 169.Fergusson J, et al. CD161intCD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol. 2016;9:401. doi: 10.1038/mi.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gentles AJ, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015;21:nm.3909. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Naik S, et al. Commensal–dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rodriguez R, et al. Memory regulatory T cells reside in human skin. J. Clin. Invest. 2014;124:1027–1036. doi: 10.1172/JCI72932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Park C, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015;21:688. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Machado-Santos J, et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141:2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kuric E, et al. Demonstration of tissue resident memory CD8 T cells in insulitic lesions in adult patients with recent-onset type 1 diabetes. Am. J. Pathol. 2017;187:581–588. doi: 10.1016/j.ajpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 176.Zundler S, et al. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat. Immunol. 2019;20:288–300. doi: 10.1038/s41590-018-0298-5. [DOI] [PubMed] [Google Scholar]
- 177.Clark RA. Resident memory T cells in human health and disease. Sci. Transl. Med. 2015;7:269rv1–269rv1. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ho AW, Kupper TS. T cells and the skin: from protective immunity to inflammatory skin disorders. Nat. Rev. Immunol. 2019;19:490–502. doi: 10.1038/s41577-019-0162-3. [DOI] [PubMed] [Google Scholar]
- 179.Hawkes J, Chan T, Krueger J. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017;140:645. doi: 10.1016/j.jaci.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Teunissen M, et al. The IL-17A-producing CD8+ T-cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J. Invest. Dermatol. 2014;134:2898. doi: 10.1038/jid.2014.261. [DOI] [PubMed] [Google Scholar]
- 181.Bhushan M, et al. Anti-E-selectin is ineffective in the treatment of psoriasis: a randomized trial. Br. J. Dermatol. 2002;146:824. doi: 10.1046/j.1365-2133.2002.04743.x. [DOI] [PubMed] [Google Scholar]
- 182.Boyman O, Hefti H, Conrad C, Nickoloff B, ter, Nestle F. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J. Exp. Med. 2004;199:731. doi: 10.1084/jem.20031482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matos TR, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17–producing αβ T cell clones. J. Clin. Invest. 2017;127:4031–4041. doi: 10.1172/JCI93396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheuk S, et al. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J. Immunol. 2014;192:3111. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gilhar A, Etzioni A, Paus R. Alopecia areata. N. Engl. J. Med. 2012;366:1515. doi: 10.1056/NEJMra1103442. [DOI] [PubMed] [Google Scholar]
- 186.Pratt HC LE, Jr., Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat. Rev. Dis. Prim. 2017;3:nrdp201711. doi: 10.1038/nrdp.2017.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xing L, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014;20:1043. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gilhar A, Ullmann Y, Berkutzki T, Kalish R. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J. Clin. Invest. 1998;101:62–67. doi: 10.1172/JCI551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li J, et al. Laser capture microdissection reveals transcriptional abnormalities in alopecia areata before, during, and after active hair loss. J. Invest. Dermatol. 2016;136:715. doi: 10.1016/j.jid.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 190.Ibrahim O, Bayart C, Hogan S, Piliang M, Bergfeld W. Treatment of alopecia areata with tofacitinib. JAMA Dermatol. 2017;153:600. doi: 10.1001/jamadermatol.2017.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Jong, A. et al. High-throughput T cell receptor sequencing identifies clonally expanded CD8+ T cell populations in alopecia areata. JCI Insight3, e121949 (2018). [DOI] [PMC free article] [PubMed]
- 192.Bishnoi A, Parsad D. Clinical and molecular aspects of vitiligo treatments. Int. J. Mol. Sci. 2018;19:1509. doi: 10.3390/ijms19051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Richmond JM, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci. Transl. Med. 2018;10:eaam7710. doi: 10.1126/scitranslmed.aam7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Prosser AC, Kallies A, Lucas M. Tissue-resident lymphocytes in solid organ transplantation. Transplantation. 2018;102:378–386. doi: 10.1097/01.tp.0000542985.78394.bc. [DOI] [PubMed] [Google Scholar]
- 195.Turner DL, Gordon CL, Farber DL. Tissue-resident T cells, in situ immunity and transplantation. Immunol. Rev. 2014;258:150–166. doi: 10.1111/imr.12149. [DOI] [PubMed] [Google Scholar]