Abstract
Introduction
Galactose-deficient IgA1 (Gd-IgA1) and related IgA/IgG immune complexes have been identified as the key drivers in the pathogenesis of IgA nephropathy (IgAN). However, their roles in the development of secondary IgAN are still unknown.
Methods
In this study, we measured the plasma Gd-IgA1 level, IgA/IgG complex, and Gd-IgA1 glomerular deposits in 100 patients with various kinds of secondary IgAN. Plasma Gd-IgA1 was measured using a lectin-based enzyme-linked immunosorbent assay, and Gd-IgA1 in glomerular deposits was examined by double immunofluorescent staining using its specific monoclonal antibody KM55.
Results
Patients with secondary IgAN presented with higher plasma Gd-IgA1 levels compared to healthy controls (median, 354.61 U/ml; interquartile range [IQR], 323.93, 395.57 U/ml vs. median, 303.17 U/ml; IQR, 282.24, 337.92 U/ml, P < 0.001) or patients with other kidney diseases (median, 314.61 U/ml; IQR, 278.97, 343.55 U/ml, P < 0.001). A similar trend was observed in plasma IgA/IgG immune complexes or IgA1. There were no differences between secondary and primary IgAN in plasma Gd-IgA1 levels (median, 378.54 U/ml; IQR, 315.96, 398.33 U/ml, P = 0.700) and IgA1-IgG complex levels (median, 18.76 U/ml; IQR, 14.51, 22.83 U/ml vs. median, 19.11 U/ml; IQR, 13.21, 22.37 U/ml, P = 0.888). Co-localized IgA1 and Gd-IgA1 of both secondary and primary IgAN indicated that they both share the feature of Gd-IgA1 deposits on the glomerular mesangium.
Conclusion
Our study strongly suggests that secondary IgAN shares a similar galactose-deficient IgA1-oriented pathogenesis with primary IgAN.
Keywords: galactose-deficient IgA1, IgA, immune complex, nephropathy, secondary IgA nephropathy
IgA nephropathy (IgAN) is the most common primary glomerulonephritis in the world and the leading cause of end-stage renal disease in young adults. A diagnosis is established by an examination of renal tissue showing IgA as the dominant or co-dominant immunoglobulin deposited in the glomerular mesangium, usually accompanied by complement 3.1 IgA deposition is also documented in patients with various comorbidities, ranging from IgA vasculitis, cirrhosis, and autoimmune diseases to chronic infections and neoplasms, which suggest secondary IgAN.2, 3, 4, 5, 6, 7 Among them, IgA vasculitis and chronic liver disease are the leading causes of secondary IgAN.8 IgAN has been found in 9% to 25% of patients who have undergone biopsy at the time of liver transplantation.9,10 Secondary IgAN clinically presents as hematuria with proteinuria, hypertension, and chronic, slowly progressive renal injury. No specific histologic features could differentiate primary from secondary cases.8,11
The IgA deposited in the mesangial zones of patients with primary IgAN is exclusively of the IgA1 subclass.12 IgA1 is one of the very few serum proteins to process O-linked glycans (containing N-acetylgalactosamine, galactose and sialic acid) in the hinge region.13,14 It is now firmly established that serum IgA1 molecules are poorly O-galactosylated in patients with IgAN, and, more importantly, that IgA deposited in the mesangium and eluted directly from glomeruli predominantly comprises galactose-deficientIgA1 (Gd-IgA1).15 Gd-IgA1 and Gd-IgA1−containing circulating immune complexes have been identified among the key effector molecules in the pathogenesis of IgAN.16 However, the pathogenic mechanism of secondary IgAN has not been fully established.
A recent study confirmed that IgA vasculitis with nephritis and primary IgAN have a shared feature regarding Gd-IgA1−oriented pathogenesis17; however, it is still unclear whether Gd-IgA1 is central to these processes in other kinds of secondary IgAN. In this study of 100 patients with various forms of secondary IgAN, we evaluated immune features including circulating Gd-IgA1, IgA/IgG immune complexes (IgA/IgG-IC), and also glomerular Gd-IgA1 deposition.
Methods
Patients
The International Classification of Diseases code of discharge diagnosis was searched to identify patients with secondary IgAN with comorbidities including cirrhosis, chronic inflammatory disease, chronic infections, and neoplasms. We identified patients with IgAN at Peking University First Hospital between January 1, 2000, and May 31, 2018. IgAN patients with hepatic B virus, hepatic C virus, or fatty liver were excluded from our study for their ambiguous illness progression. Complete clinical data, including age, sex, systolic/diastolic blood pressure, serum creatinine, total serum IgA, 24-h total protein excretion, and pathological characteristics were collected at the time of renal biopsy. Participants included 32 patients with primary IgAN, 41 with other renal diseases, and 39 healthy individuals who served as controls. Written informed consent was obtained from all the participants permitting the use of clinical or pathological data in future studies when entering the IgAN cohort. For this study, the protocol was reviewed and approved by the Ethics Committee of the institution without obtaining additional informed consent (approval number: 2019-research-28).
Measurement of Plasma IgA1 and Gd-IgA1
IgA1 and Gd-IgA1 concentrations in plasma were determined by enzyme-linked immunosorbent assay (ELISA) as mentioned previously.18
Briefly, plasma IgA1 levels were determined using capture ELISA. High-absorption polystyrene plates (Thermo Fisher Scientific, Waltham, MA) were coated with 2.5 μg/ml F(ab')2 fragment of goat anti-human IgA (Jackson ImmunoResearch, West Grove, PA), and blocked with 1% bovine serum albumin in phosphate-buffered saline with Tween 20. Diluted (80,000-fold) plasma samples were detected with a monoclonal anti-human IgA1 horseradish peroxidase−conjugated antibody (Bio-Rad, Hercules, CA). The optical density (OD) at 450 nm was measured after tetramethylbenzidine liquid substrate system was applied.
To measure the Gd-IgA1 level, microtitration plates (Thermo Fisher Scientific) were coated with 2.5 μg/ml F(ab')2 fragment of goat anti-human IgA (Jackson ImmunoResearch), and blocked with 1% bovine serum albumin phosphate-buffered saline with Tween 20. Dilutions (4000-fold) of duplicate plasma samples and standards in blocking solution were incubated. The captured IgA was desialylated by neuraminidase (Prozyme, Hayward, CA). Plates were then incubated with biotinylated Helix pomatia lectin (Sigma-Aldrich, St. Louis, MO) and detected by ExtraAvidin-Peroxidase (Sigma-Aldrich, St. Louis, MO). The tetramethylbenzidine liquid substrate system was used for detection and the optical density was measured at 450 nm with wavelength correction at 570 nm. The Gd-IgA1 values were calculated by interpolating the optical density values on calibration curves, constructed using 1:1 mixture of commercial human IgA1 and Gd-IgA1 from plasma exchange buffer of one crescentic IgAN patient.19
The absolute Gd-IgA1 value was multiplied by the paired IgA1 value, and the new value was the adjusted Gd-IgA1 (AD-Gd-IgA1) value for each sample.
Measurement of Plasma IgA/IgG-IC by ELISA
Because IgA/IgG-IC have an important role in the pathogenesis of primary IgAN, the levels of the IgA1-IgG complex and IgA-IgG complex were investigated in patients with secondary IgAN.20,21 Cross-capture ELISA was used to determine plasma IgA/IgG-IC levels as described.22 ELISA plates were coated with 2.5 μg/ml F(ab')2 fragment of goat anti-human IgA (Jackson ImmunoResearch; for cross-capture of IgA-IgG complexes) or 2.5 μg/ml rabbit anti human IgG (H+L) (Abcam, Cambridge, MA; for cross-capture of IgA1-IgG complexes), as described.19 After washing and blocking with 1% bovine serum albumin in phosphate-buffered saline with 0.05% Tween, diluted (1:10,000) fresh plasma was added for incubation with the same buffer. The captured immunoglobulins were then detected with a monoclonal mouse anti human IgG antibody (Abcam; for cross capture of IgA-IgG complexes) or a biotin-labeled mouse anti-human IgA1 antibody (Southern Biotech, Birmingham, AL; for cross-capture of IgA1-IgG complexes). Goat anti-mouse−horseradish peroxidase antibody (Cwbio, Beijing, China) and ExtraAvidin-Peroxidase (Sigma-Aldrich, St. Louis, MO) were used for detection. The reaction was developed as described above. Commercial human IgA1-streptavidin combined with human IgG-biotin was used as a standard for quantification. IgA1-streptavidin was made by combining IgA1 (Abcam) and a streptavidin conjugation kit (Abcam); IgG-biotin was made by combining IgG (Sigma-Aldrich) and a biotinylation kit (Abcam). Then, the IgA1-streptavidin and IgG-biotin proteins were mixed 1:1 at 4 °C overnight as standard IgA1-IgG complexes for quantification.
Double Immunofluorescent Stain of Gd-IgA1 and IgA1
Glomerular Gd-IgA1 and IgA1 deposition of IgAN and was examined by double immunofluorescent staining as described.17 Briefly, dewaxed paraffin sections were subjected to antigen retrieval using 0.05% bacterial protease subtilisin A (Sigma-Aldrich, St. Louis, MO) at room temperature for 30 minutes. Nonspecific binding was blocked with 3% bovine serum albumin in phosphate-buffered saline blocking solution. Sections were incubated for 1 hour at 37 °C with rat monoclonal anti-human Gd-IgA1 antibody (Immuno-Biological Laboratories, Japan), followed by Alexa Fluor 555−conjugated goat anti-rat IgG (Abcam) for 30 minutes at 37 °C. Samples were then incubated with Alexa Fluor 488−conjugated monoclonal mouse anti-human IgA1 antibody (Southern Biotech) for 30 minutes at 37 °C. Slides were sealed with ProLong Gold antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen, Carlsbad, CA). For microscopic observation of immunostained samples, a fluorescence microscope DM2500 (Leica, Wetzlar, Germany), an automated slide scanner Axio Scan Z1 (Zeiss, Oberkochen, Germany), and a confocal microscope LSM780 (Zeiss, Oberkochen, Germany) were used.
Statistical Analyses
Continuous variables are expressed as the mean ± SD, and categorical variables are expressed as percentages. A Student unpaired t test and the Mann-Whitney U test were performed for statistical comparisons. A two-sided P value ≤0.05 was considered statistically significant. All analyses and graphs were conducted using R 3.4.4 software (St. Louis, MO).
Results
Baseline Clinical and Pathological Characteristics
A total of 100 patients with secondary IgAN entered this study, including 20 with cirrhosis, 19 with rheumatoid arthritis, 9 with ankylosing spondylitis, 10 with Sjögren syndrome, 25 with psoriasis, 1 with ulcerative colitis, 3 with neoplasia, 3 with infections, 6 with chronic obstructive bronchiolitis, and 4 with pulmonary fibrosis. Patients with neoplasia did not show pathological character of membranous or any other glomerular lesion. There were 59 (52.2%) male participants, and the mean age was 45.7 ± 14.9 years at the time of kidney biopsy, which was much older than that of the primary IgAN patients (37.9 ± 11.2, P = 0.009) (Table 1). Systolic blood pressure was 131 ± 18 mm Hg, and diastolic blood pressure was 81 ± 13 mm Hg. The baseline characteristics of primary IgAN and other renal disease controls are shown in Table 1. Overall, patients with secondary IgAN had higher serum IgA and IgG levels compared to patients with primary IgAN but had no significant difference in blood pressure and renal function compared to patients with primary IgAN.
Table 1.
Data of the 4 groups of enrolled patients and healthy volunteers
| Secondary IgA nephropathy | IgA nephropathy | Nephropathy control | Healthy control | |
|---|---|---|---|---|
| No. of participants | 100 | 32 | 41 | 39 |
| Age (yr) | 45.67 ± 14.56 | 37.88 ± 11.16 | 39.8 ± 17.56 | 34.95 ± 8.69 |
| Sex (male/female) | 59/41 | 19/13 | 18/23 | 17/22 |
| Proteinuria (g/d) | 2.65 | 2.97 | 5.26 | |
| SBP (mm Hg) | 131 ± 18 | 131 ± 11 | 132 ± 17 | |
| DBP (mm Hg) | 81 ± 13 | 83 ± 9 | 84 ± 14 | |
| MAP (mm Hg) | 98 ± 14 | 99 ± 9 | 100 ± 14 | |
| SCr (mmol/l) | 158 ± 150 | 240 ± 227 | 191 ± 229 | |
| eGFR (ml/min per 1.73 m2) | 64.9 ± 35 | 49.3 ± 33.8 | 71 ± 43 | |
| Serum IgA (g/l) | 4.27 ± 1.60 | 3.30 ± 1.14 | 1.92 ± 0.89 | |
| Serum IgG (g/l) | 11.92 ± 5.21 | 9.96 ± 2.61 | 9.77 ± 6.45 | |
| Serum C3 (g/l) | 0.98 ± 0.27 | 0.87 ± 0.18 | 0.83 ± 0.47 | |
| Serum C4 (g/l) | 0.28 ± 0.35 | 0.21 ± 0.05 | 0.19 ± 0.13 |
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; SBP, systolic blood pressure; SCr, serum creatinine.
In this study, 32 primary IgAN patients, 39 healthy volunteers, 25 glomerulonephritis patients, and 16 lupus nephritis patients were enrolled as controls. Details of the patients’ characteristics are shown in Supplementary Table S1.
Plasma IgA1 Level and Gd-IgA1 Level
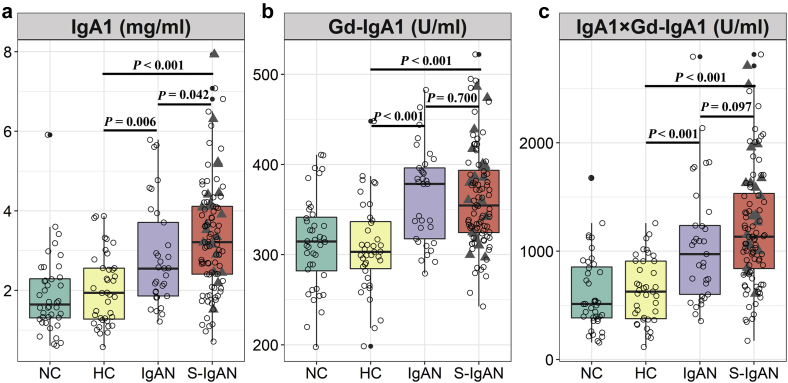
Overall, the average plasma IgA1 levels in patients with secondary IgAN were much higher than those in the primary IgAN group (median, 3.22 mg/ml; IQR, 2.41, 4.11 mg/ml, vs. median, 2.55 mg/ml; IQR, 1.85, 3.83 mg/ml, P = 0.042) and the healthy control group (median, 1.94 mg/ml; IQR, 1.26, 2.56 mg/ml, P < 0.001) (Figure 1). Among the patients with secondary IgAN, the IgA1 levels were highest in patients with concurrent cirrhosis (median, 3.79 mg/ml; IQR, 2.96, 4.44 mg/ml) (Supplementary Figure S1).
Figure 1.
Plasma immunoglobulin A1 (IgA1), galactose-deficient IgA1 (Gd-IgA1), and adjusted galactose-deficient IgA1 (AD-Gd-IgA1) levels in nephropathy controls (NCs), healthy controls (HCs), IgA nephropathy cases (IgANs), and secondary IgA nephropathy cases (S-IgANs). (a) Plasma total IgA1; (b) plasma Gd-IgA1; (c) plasma Ad-Gd-IgA1 (IgA1× Gd-IgA1). Triangles, data for IgANs with concurrent cirrhosis.
The Gd-IgA1 levels showed no difference between patients with secondary and primary IgAN (median, 354.61 U/ml; IQR, 323.93, 395.57 U/ml vs. median = 378.54 U/ml; IQR, 315.96, 398.33 U/ml, P = 0.700). Gd-IgA1 levels in both groups were higher than those in the healthy control group (median, 303.17 U/ml; IQR, 282.24, 337.92 U/ml, P < 0.001) or the other renal disease control group (median, 314.61 U/ml; IQR = 278.97, 343.55 U/ml, P < 0.001). The absolute Gd-IgA1 (AD-Gd-IgA1, every Gd-IgA1 value was multiplied by the paired IgA1 value) levels were highest in the secondary IgAN group (median, 1134.07 U/ml; IQR, 824.53, 1568.31 U/ml), compared to the primary IgAN group (median, 972.88 U/ml; IQR, 592.41, 1299.98 U/ml, P = 0.097), the healthy control group (median, 626.97 U/ml; IQR, 369.21, 908.83 U/ml, P < 0.001), and the other renal disease group (median, 512.97 U/ml; IQR, 377.96, 871.01 U/ml, P < 0.001) (Figure 1).
IgA/IgG-IC Level Between Primary and Secondary IgAN
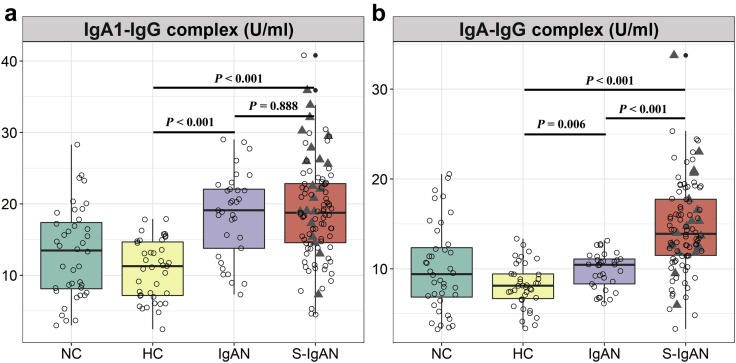
The levels of IgA-IgG complexes were significantly increased in patients with secondary IgAN (median, 13.91 U/ml; IQR, 11.47, 17.74 U/ml) compared to those with primary IgAN (median, 10.44 U/ml; IQR, 8.17, 11.24 U/ml, P = 0.006), healthy controls (median, 8.12 U/ml; IQR, 6.60, 9.45 U/ml, P < 0.001), and other renal disease controls (median, 9.41 U/ml; IQR, 6.59, 12.59 U/ml, P < 0.001).
In contrast, IgA1-IgG complex levels were not significantly different between the secondary and primary IgAN groups (median, 18.76 U/ml; IQR, 14.51, 22.83 U/ml vs. median, 19.11 U/ml; IQR, 13.20, 22.37 U/ml, P = 0.888) but were much higher than those of the healthy controls (median, 11.29 U/ml; IQR, 7.11, 14.75 U/ml, P < 0.001) and other renal disease controls (median, 13.48 U/ml; IQR, 7.90, 17.68 U/ml, P < 0.001) (Figure 2).
Figure 2.
Detection of circulating IgA/IgG immune complex (IgA/IgG-IC) in nephropathy controls (NCs), healthy controls (HCs), IgA nephropathy cases (IgANs), and secondary IgA nephropathy cases (S-IgANs). (a) IgA1-IgG complex; (b) IgA-IgG complex. Triangles, data for IgANs with concurrent cirrhosis.
Among the patients with secondary IgAN, the IgA-IgG and IgA1-IgG complex levels of patients with cirrhosis were both significantly higher than those of patients with primary IgAN (IgA-IgG complexes: median, 14.36 U/ml; IQR, 12.30, 19.94 U/ml, P < 0.001; IgA1-IgG complexes: median, 22.30 U/ml; IQR, 17.69, 29.80 U/ml, P = 0.042) (Supplementary Figure S2).
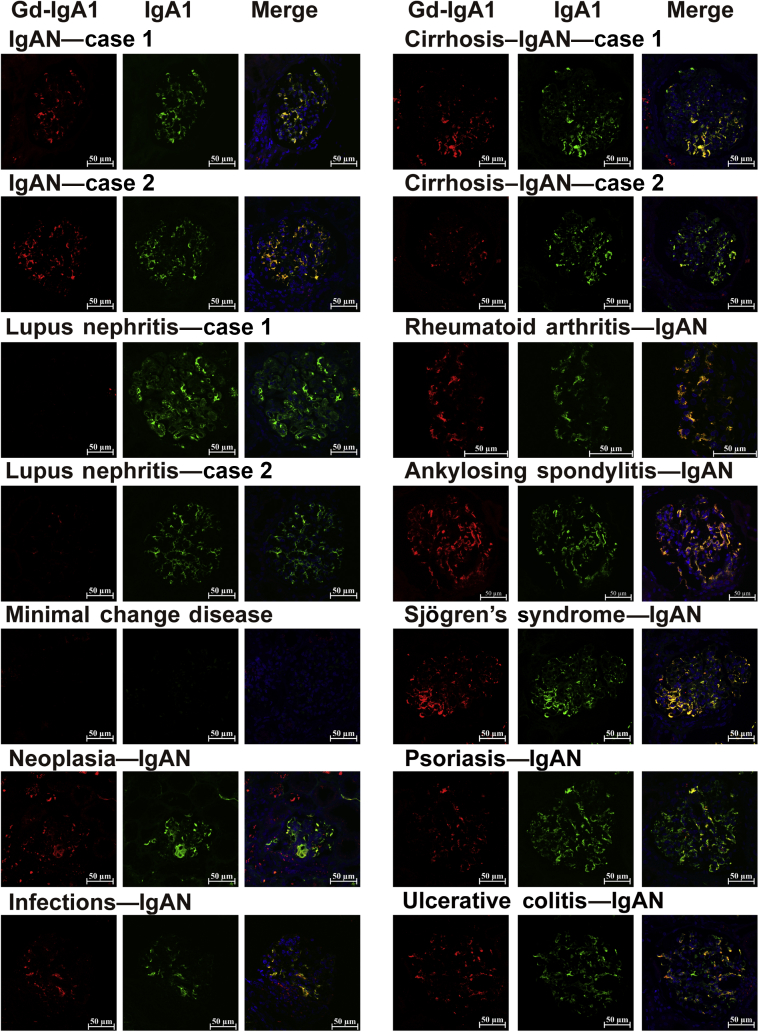
Glomerular Deposition of Gd-IgA1 in Patients With Secondary IgAN
Seventeen patients with IgAN, including 2 primary ones, 5 cases with cirrhosis, 1 rheumatoid arthritis, 1 ankylosing spondylitis, 1 Sjögren syndrome, 1 psoriasis, 1 ulcerative colitis, 1 neoplasia, and all were evaluated with double staining of IgA and Gd-IgA1. Gd-IgA1 in all glomeruli of both primary and secondary IgAN patients was distributed in a pattern similar to that of IgA1 (Figure 3; Supplementary Figure S3). Although the staining pattern and intensity differed among patients, IgA1 and Gd-IgA1 overlapped and were observed mainly in the mesangial and capillary regions. However, in lupus nephritis, only IgA1 was deposited in glomeruli but not Gd-IgA1, the same result as previous data.17,23
Figure 3.
Glomerular deposition of galactose-deficient IgA1 in patients with types of secondary IgA nephropathy (IgANs) and controls. Double staining with anti−Gd-IgA1 mAb (red) and anti-IgA1 mAb (green). Merged figures were also added to the 4′,6-diamidino-2-phenylindole channel. Bars = 50 μm.
Discussion
Although secondary IgAN is observed in many conditions, the pathogenesis is still not fully established. Gd-IgA1- and IgA1-containing circulating immune complexes are among the key effectors in the pathogenesis of primary IgAN.24 In this study, we evaluated the immune characteristics of IgAN that were secondary to cirrhosis, immune diseases, and infections. Our results showed that patients with secondary IgAN showed high levels of circulating Gd-IgA1 and IgA1-IgG complexes that were similar to those of patients with primary IgAN. In addition, using a Gd-IgA1−specific monoclonal antibody, KM55, we showed glomerular deposition of Gd-IgA1 in secondary IgAN. These immune characteristics showed no difference from primary IgAN. However, they were not observed in other glomerular diseases. These findings suggest that secondary IgAN has a shared feature with primary IgAN regarding Gd-IgA1−oriented pathogenesis.
Secondary IgAN is reported mostly in IgA vasculitis, gastrointestinal or liver disorders, and autoimmune diseases. In a recent study on IgA vasculitis nephritis, Suzukiet al. detected Gd-IgA1 in the glomeruli in patients with IgA vasculitis nephritis.17 Cassol et al. also reported that Gd-IgA1 staining was present in 96% of patients with other secondary IgAN (n = 27) diseases, such as liver cirrhosis, psoriasis, and inflammatory bowel disease.23 In a French cohort, galactose deficiency and decreased sialylation of IgA1, as well as increased amounts of abnormally glycosylated polymeric IgA1, were detected in the serum of patients with advanced alcoholic cirrhosis.25 The association of IgAN with multiple autoimmune disorders, including rheumatoid arthritis and psoriasis, has frequently been reported.26,27 Patients with rheumatoid arthritis had elevated levels of serum IgA, and IgG anticitrullinated-protein antibodies displayed less galactose and sialic acid on their Fc portion.28 Whether a similar change in glycosylation occurs for IgA1 remains to be determined. In the Japanese cohort Suzuki et al. did not find kidney Gd-IgA1 deposits in other secondary IgANs except for IgA vasculitis, but this might due to only include 3 hepatitis C virus–related IgANs and one hepatic glomerulosclerosis, and more studies are still needed.17 In this study, with a broader range of secondary IgANs, including cirrhosis, inflammatory bowel disease, autoimmune disorders and neoplasms, we confirmed that high circulating Gd-IgA1 and IgA1-IgG complexes, features of primary IgAN, were also observed in these secondary IgANs. Using a Gd-IgA1−specific monoclonal antibody, we demonstrated that IgA1 deposited on the kidney in secondary IgAN displayed galactose deficiency on O-glycans.
In this study, we found that both primary and secondary IgAN shared the same pathogenesis as the increased circulating IgA1-IgG complex containing Gd-IgA1. However, the reasons for this might be different. As shown in this study, plasma levels of IgA1 in patients with secondary IgAN were 2- to 4-fold higher than those in healthy controls, and 1- to 2-fold higher than those in primary IgAN, especially for patients with concomitant cirrhosis. Hypergammaglobulinemia in patients with cirrhosis may result from increased synthesis and/or decreased clearance of Ig, which may play a role in the development of secondary IgAN.8,21 Asialoglycoprotein receptor on hepatocytes binds desialylated glycoproteins through recognition of glycans with terminal galactose or N-acetylgalactosamine and appears to be the main clearance process of IgA.29,30 The reasons for the overproduction of abnormal glycosylated IgA1 were unclear, but partially relevant to the pathogenic bacteria translocation, especially in patients with cirrhosis.31 There are nearly 100 trillion bacteria in the gastrointestinal tract, usually peacefully coexisting with secretory IgA. Cirrhosis disrupts the homeostasis at multiple interconnected levels, including the pathological bacterial translocation, increased intestinal permeability, and abnormal antimicrobial peptide production and clearance.32 With the disturbed intestinal barrier in patients with cirrhosis, pathogenic bacteria translocation induces a proinflammatory environment of the mucosal system. This environment may stimulate a large amount abnormal glycosylated IgA1. Recent genome-wide association studies strongly indicate that many IgAN-associated loci also affect other autoimmune and infectious diseases, including inflammatory bowel disease (IBD) and rheumatoid arthritis.33 In patients with IBD, the bowel mucosa may also be an important source of Gd-IgA1. However, there was only one patient with IBD in our study, and future study is needed to confirm this association in more patients with IBD. In this study, we confirmed that both circulating IgA1-IgG or IgA-IgG complexes as well as Gd-IgA1 deposits in the kidney showed similar characteristics between primary and secondary IgAN.
As far as we know, this study is the first to investigate immune characteristics among a wide range of secondary IgANs. The strengths of this study were the inclusion of nearly all known secondary IgANs and the large sample size. The major limitation of this study was that we did not investigate IgA1-specific anti-glycan antibodies; instead, we measured the IgA1-IgG complex. The high level of circulating IgA1-IgG complex suggested that anti-glycan antibodies might also be involved in secondary IgAN. Additional studies are necessary to determine whether anti-glycan autoantibodies are central to these processes, as in primary IgAN. Another limitation is that there was only one patient with secondary IgAN combined with IBD among our patients with secondary IgAN. There has recently been increasing interest in the possibility that the bowel mucosa may be an important source of Gd-IgA1.34
In conclusion, we demonstrated that circulating IgA1 and glomerular IgA1 displayed galactose deficiency of O-glycans in patients with secondary IgAN. In addition, plasma IgA1-IgG complex levels were also high in these patients. These immune characteristics showed no difference between primary and secondary IgAN. Our findings suggest that secondary IgAN shared a similar Gd-IgA1−oriented pathogenesis with primary IgAN.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank Yan Zhang for contributing to research on clinical records. This work was supported by the grant of Clinical Research and Capital Health Development Research Project (CD-2018-2-4073), Peking University Health Sciences Center Joint Institute for Translational, National Key Research and Development Plan (2018YFC1314004), and the National Natural Science Fund (81670649).
Footnotes
Table S1. Various kinds of glomerulonephritis patients.
Figure S1. Plasma immunoglobulin A1 (IgA1), galactose-deficient IgA1 (Gd-IgA1) and adjusted galactose-deficient IgA1 (AD-Gd-IgA1) levels in subgroups of enrolled patients and healthy volunteers.
Figure S2. Detection of circulating IgA/IgG immune complex (IgA/IgG-IC) in sub-groups of enrolled patients and healthy volunteers.
Figure S3. Glomerular deposition of galactose-deficient IgA1 in patients with IgA nephropathy (IgAN) combined cirrhosis. Double staining with anti-Gd-IgA1 mAb (Red) and anti-IgA1 mAb (Green). Merged figures were also added to the 4′,6-diamidino-2-phenylindole channel. (Bars = 50 μm).
Supplementary Material
References
- 1.Wyatt R.J., Julian B.A. IgA nephropathy. N Engl J Med. 2013;368:2402–2414. doi: 10.1056/NEJMra1206793. [DOI] [PubMed] [Google Scholar]
- 2.Endo Y., Hara M. Glomerular IgA deposition in pulmonary diseases. Kidney Int. 1986;29:557–562. doi: 10.1038/ki.1986.34. [DOI] [PubMed] [Google Scholar]
- 3.Pasternack A., Collin P., Mustonen J. Glomerular IgA deposits in patients with celiac disease. Clin Nephrol. 1990;34:56–60. [PubMed] [Google Scholar]
- 4.Axelsen R.A., Crawford D.H., Endre Z.H. Renal glomerular lesions in unselected patients with cirrhosis undergoing orthotopic liver transplantation. Pathology. 1995;27:237–246. doi: 10.1080/00313029500169053. [DOI] [PubMed] [Google Scholar]
- 5.Filiopoulos V., Trompouki S., Hadjiyannakos D. IgA nephropathy in association with Crohn's disease: a case report and brief review of the literature. Ren Fail. 2010;32:523–527. doi: 10.3109/08860221003710554. [DOI] [PubMed] [Google Scholar]
- 6.Ambruzs J.M., Walker P.D., Larsen C.P. The histopathologic spectrum of kidney biopsies in patients with inflammatory bowel disease. Clin J Am Soc Nephrol. 2014;9:265–270. doi: 10.2215/CJN.04660513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollino C., Vischini G., Coppo R. IgA nephropathy and infections. J Nephrol. 2016;29:463–468. doi: 10.1007/s40620-016-0265-x. [DOI] [PubMed] [Google Scholar]
- 8.Saha M.K., Julian B.A., Novak J., Rizk D.V. Secondary IgA nephropathy. Kidney Int. 2018;94:674–681. doi: 10.1016/j.kint.2018.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calmus Y., Conti F., Cluzel P. Prospective assessment of renal histopathological lesions in patients with end-stage liver disease: effects on long-term renal function after liver transplantation. J Hepatol. 2012;57:572–576. doi: 10.1016/j.jhep.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Hommos M.S., El-Zoghby Z.M. Renal outcomes in patients with IgA nephropathy undergoing liver transplant: a retrospective cohort study. Transplant Direct. 2017;3:e193. doi: 10.1097/TXD.0000000000000708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouria S., Barratt J. Secondary IgA nephropathy. Semin Nephrol. 2008;28:27–37. doi: 10.1016/j.semnephrol.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Novak J., Barratt J., Julian B.A. Aberrant glycosylation of the IgA1 molecule in IgA nephropathy. Semin Nephrol. 2018;38:461–476. doi: 10.1016/j.semnephrol.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franc V., Rehulka P., Raus M. Elucidating heterogeneity of IgA1 hinge-region O-glycosylation by use of MALDI-TOF/TOF mass spectrometry: role of cysteine alkylation during sample processing. J Proteomics. 2013;92:299–312. doi: 10.1016/j.jprot.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwatani H., Inoue T., Wada Y. Quantitative change of IgA hinge O-glycan composition is a novel marker of therapeutic responses of IgA nephropathy. Biochem Biophys Res Commun. 2012;428:339–342. doi: 10.1016/j.bbrc.2012.10.049. [DOI] [PubMed] [Google Scholar]
- 15.Hiki Y., Odani H., Takahashi M. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 16.Lai K.N. Pathogenesis of IgA nephropathy. Nat Rev Nephrol. 2012;8:275–283. doi: 10.1038/nrneph.2012.58. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H., Yasutake J., Makita Y. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93:700–705. doi: 10.1016/j.kint.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Zhao N., Hou P., Lv J. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82:790–796. doi: 10.1038/ki.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie X., Lv J., Shi S. Plasma exchange as an adjunctive therapy for crescentic IgA nephropathy. Am J Nephrol. 2016;44:141–149. doi: 10.1159/000448767. [DOI] [PubMed] [Google Scholar]
- 20.Roberts I.S. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10:445–454. doi: 10.1038/nrneph.2014.92. [DOI] [PubMed] [Google Scholar]
- 21.Knoppova B., Reily C., Maillard N. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front Immunol. 2016;7:117. doi: 10.3389/fimmu.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matousovic K., Novak J., Yanagihara T. IgA-containing immune complexes in the urine of IgA nephropathy patients. Nephrol Dial Transplant. 2006;21:2478–2484. doi: 10.1093/ndt/gfl240. [DOI] [PubMed] [Google Scholar]
- 23.Cassol CA, Bott C, Nadasdy GM, et al. Immunostaining for galactose-deficient immunoglobulin A is not specific for primary immunoglobulin A nephropathy [e-pub ahead of print]. Nephrol Dial Transplant.https://doi.org/10.1093/ndt/gfz152. Accessed October 20, 2019. [DOI] [PubMed]
- 24.Floege J., Barbour S.J., Cattran D.C. Management and treatment of glomerular diseases (part 1): conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:268–280. doi: 10.1016/j.kint.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Tissandie E., Morelle W., Berthelot L. Both IgA nephropathy and alcoholic cirrhosis feature abnormally glycosylated IgA1 and soluble CD89-IgA and IgG-IgA complexes: common mechanisms for distinct diseases. Kidney Int. 2011;80:1352–1363. doi: 10.1038/ki.2011.276. [DOI] [PubMed] [Google Scholar]
- 26.Nakano M., Ueno M., Nishi S. Determination of IgA- and IgM-rheumatoid factors in patients with rheumatoid arthritis with and without nephropathy. Ann Rheum Dis. 1996;55:520–524. doi: 10.1136/ard.55.8.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grewal S.K., Wan J., Denburg M.R. The risk of IgA nephropathy and glomerular disease in patients with psoriasis: a population-based cohort study. Br J Dermatol. 2017;176:1366–1369. doi: 10.1111/bjd.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rombouts Y., Ewing E., van de Stadt L.A. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis. 2015;74:234–241. doi: 10.1136/annrheumdis-2013-203565. [DOI] [PubMed] [Google Scholar]
- 29.Roggenbuck D., Mytilinaiou M.G., Lapin S.V. Asialoglycoprotein receptor (ASGPR): a peculiar target of liver-specific autoimmunity. Auto Immun Highlights. 2012;3:119–125. doi: 10.1007/s13317-012-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Souza A.A., Devarajan P.V. Asialoglycoprotein receptor mediated hepatocyte targeting─strategies and applications. J Control Release. 2015;203:126–139. doi: 10.1016/j.jconrel.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Wiest R., Lawson M., Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulou A., Agiasotelli D., Vasilieva L.E. Bacterial translocation markers in liver cirrhosis. Ann Gastroenterol. 2017;30:486–497. doi: 10.20524/aog.2017.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiryluk K., Li Y., Scolari F. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. 2014;46:1187–1196. doi: 10.1038/ng.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fellstrom B.C., Barratt J., Cook H. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet. 2017;389:2117–2127. doi: 10.1016/S0140-6736(17)30550-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.